Published online Mar 25, 2023. doi: 10.5501/wjv.v12.i2.122
Peer-review started: January 4, 2023
First decision: January 17, 2023
Revised: January 23, 2023
Accepted: February 22, 2023
Article in press: February 22, 2023
Published online: March 25, 2023
Processing time: 75 Days and 12.1 Hours
Understanding the transmission dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection among healthcare workers (HCWs) and their social contacts is crucial to plan appropriate risk-reduction measures.
To analyze the socio-demographic risk factors and transmission of SARS-CoV-2 infection among HCWs in two tertiary care hospitals in Dubai, United Arab Emirates.
The demographic and clinical characteristics were available for all HCWs in both facilities from the human resources department. A cross-sectional survey was conducted from January-April 2022 among HCWs who tested positive through Reverse Transcriptase Polymerase Chain Reaction of the nasopharyngeal swab for SARS-CoV-2 between March 2020 and August 2021 in two tertiary-level hospitals. The survey included questions on demographics, work profile, characteristics of coronavirus disease 2019 (COVID-19), and infection among their household or co-workers. The survey also checked the knowledge and perception of participants on the infection prevention measures related to SARS-CoV-2.
Out of a total of 346 HCWs infected with SARS-CoV-2, 286 (82.7%) HCWs consented to participate in this study. From the sample population, 150 (52.5%) of participants were female, and a majority (230, 80.4%) were frontline HCWs, including 121 nurses (121, 42.4%). Only 48 (16.8%) participants were fully vaccinated at the time of infection. Most infected HCWs (85%) were unaware of any unprotected exposure and were symptomatic at the time of testing (225, 78.7%). Nearly half of the participants (140, 49%) had co-infection among household, and nearly one-third (29.5%) had co-infection among three or more household. Another 108 (37.8%) participants reported cross-infection among co-workers. The frontline HCWs were significantly more infected (25.1% vs 8.6%, P < 0.001) compared to non-frontline HCWs. Another significant risk factor for a high infection rate was male sex (P < 0.001). Among the infected frontline HCWs, a significantly higher proportion were male and shared accommodation with family (P < 0.001). COVID-19 vaccination significantly reduced the infection rate (83.2% vs 16.8, P < 0.001) among HCWs. Most participants (99.3%) were aware about importance of appropriate use of personal protective equipment. However, only 70% agreed with the efficacy of the COVID-19 vaccination in preventing an infection and severe disease.
The risk profiling of the HCWs infected with SARS-CoV-2 found that working at frontline and being male increase the rate of infection. COVID-19 vaccination can effectively reduce the rate of transmission of SARS-CoV-2 among HCWs.
Core Tip: The healthcare workers (HCWs) are vulnerable to infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In the current study, the authors found that the frontline and male HCWs were at higher risk of infection. Among the infected frontline HCWs, a significantly higher proportion were male and staying in a rented accommodation with family. The coronavirus disease 2019 vaccination is effective in preventing the transmission of SARS-CoV-2 among HCWs. This information can be utilised for the healthcare workforce management and to formulate strategies to mitigate the risk of transmission of SARS-CoV-2 to the HCWs.
- Citation: Nasa P, Modi P, Setubal G, Puspha A, Upadhyay S, Talal SH. Demographic and risk characteristics of healthcare workers infected with SARS-CoV-2 from two tertiary care hospitals in the United Arab Emirates. World J Virol 2023; 12(2): 122-131
- URL: https://www.wjgnet.com/2220-3249/full/v12/i2/122.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i2.122
The coronavirus disease 2019 (COVID-19) pandemic has overwhelmed the healthcare resources across the globe. Since the inception of the pandemic, reports have been published on the increased vulnerabilities of healthcare workers (HCWs) compared to the general community for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[1,2]. A prospective cohort study conducted among 99795 HCWs reported that the HCWs are at a threefold higher risk of acquiring COVID-19 compared to the general community. However, the risk of exposure is not uniform and depends on multiple factors, such as the nature of work (frontline), race or ethnicity (Black, Asian, and other ethnic minorities), and access to or reuse of the personal protective equipment (PPE)[1]. Besides the risk of illness, the HCWs are at considerable risk of adverse mental health during the COVID-19 pandemic[3]. Moreover, the social and household contacts of the HCWs are also potentially vulnerable to SARS-CoV-2 infection[4]. On the other hand, the absenteeism of HCWs from work is further detrimental to the already stretched healthcare services during the pandemic[5].
From the start of COVID-19 pandemic, the experts strongly expressed concerns regarding the nosocomial transmission of SARS-CoV-2[6,7]. HCWs were assumed to play a pivotal role in the transmission chain during a nosocomial outbreak of SARS-CoV-2. However, limited information exists on the transmission characteristics and dynamics of SARS-CoV-2 infection among the HCWs or their social contacts. In this scenario, it is crucial to explore the dynamics of SARS-CoV-2 transmission among the HCWs and their social contacts to develop and implement appropriate risk-reduction measures[6].
In the current study, the authors performed a retrospective analysis of HCWs infected with SARS-CoV-2 to analyze the socio-demographic risk factors and the characteristics of SARS-CoV-2 infection among HCWs and their social contacts.
The demographic and clinical characteristics were available for all HCWs in both facilities from the human resources department. A cross-sectional survey was conducted between January and April 2022 among the HCWs who tested positive for reverse transcriptase polymerase chain reaction (RT-PCR) for SARS-CoV-2 between March 2020 and August 2021 in two multi-specialty tertiary-level hospitals located in Dubai. The cross-sectional survey was conducted to extract further information from the infected HCWs on their social contacts, including household. The survey included Multiple-Choice Questions and questions with 5-point Likert scale. The survey questionnaire, attached in the Supplementary material, has a total of three sections: (1) Demographic details of the participants, including age, gender, department, nature of work, and COVID-19 vaccination status; (2) Details about SARS-CoV-2 infection, the reason for RT-PCR testing, severity and duration of the symptoms, and infection among their household contacts and co-workers; and (3) Knowledge and perception among the participants on PPE and infection prevention measures related to SARS-CoV-2. The human resource department, who were not part of the data analysis, sent the survey questionnaire through e-mail. The identity of the participants was kept confidential. Frontline HCWs were those who provide care for patients with COVID-19 or worked in areas with direct patient contact during the pandemic. As per the local health regulatory requirements, the HCWs were tested with RT-PCR only in case of symptomatic infections, contact tracing, or pre-travel screening during this period. The study considered only the first SARS-CoV-2 infection for further analysis. In the United Arab Emirates (UAE), seven COVID-19 vaccines were approved for use and are made available to the public for free of cost. The data on the average number of new cases in the community was extracted from the website of National Emergency Crisis and Disaster Management Authority, UAE (https://covid19.ncema.gov.ae/en). The study was approved by the scientific and ethical committee of the hospital and Dubai Scientific Research Ethics Committee (DSREC/09/2020_32).
Descriptive statistical analysis was conducted for frequencies, percentages, medians, and ranges. Continuous data was presented as mean [standard deviation (SD)] or median with Interquartile Range (IQR). Two groups were compared in this study using the 2-sample test for equality of proportions with continuity correction (Chi-square). A comparison was made between the categorical paired data with McNemar Test. The authors used Fisher exact test to compare less than five-count cells. All the tests were 2-tailed, and P < 0.05 was considered to be significant. The statistical analyses were conducted using R version 3.4.2 from the Comprehensive R Archive Network (R Core Team, 2020).
Out of a total of 1568 HCWs working in both hospitals, 346 (22.1%) tested positive for SARS-CoV-2 RT-PCR during the study period. Amongst this study population, 16 (4.6%) HCWs were found to be re-infected with SARS-CoV-2. However, as mentioned earlier, only the first infection was considered for the analysis. From the 346 infected HCWs, 286 (82.7%) HCWs agreed to participate in the cross-sectional survey. Amongst the participants, 150 (52.5%) were female, whereas a majority of the participants (230, 80.4%) were frontline HCWs, including 121 nurses (121, 42.4%). Only 48 (16.8%) participants were fully vaccinated at the time of infection.
Most of the participants (225, 78.7%) were symptomatic at the time of RT-PCR testing. Among the asymptomatic HCWs, 35 (12.2%) were tested for close contact tracing. Nearly half of the participants (140, 49%) had a co-infection with their household contacts. Moreover, half (48, 51.6%) of the infection in the households occurred in a single person, while nearly one-third (29.5%) had infection among three or more households. Further, 108 (37.8%) participants reported cross-infection among their co-workers (Table 1).
| Variables | n (%) | |
| Age, yr | 36 (IQR-10) | |
| Female | 150 (52.5) | |
| Staff travelling in hospital accommodation | 454/1467 (29) | |
| Work profile of healthcare workers | Doctor | 34 (11.9) |
| Nurses | 121 (42.4) | |
| Technician | 37 (12.9) | |
| Pharmacy | 11 (3.8) | |
| Paramedical staff | 10 (3.5) | |
| Non-clinical staff | 73 (25.5) | |
| Reason for testing with RT-PCR | Symptomatic | 225 (78.7) |
| Contact tracing | 35 (12.2) | |
| Travel screening | 8 (2.8) | |
| Other | 18 (6.3) | |
| Severity of COVID-19 | Asymptomatic | 36 (12.6) |
| Mild | 220 (76.9) | |
| Moderate | 25 (8.7) | |
| Severe | 5 (1.7) | |
| Place of isolation | Institutional | 50 (17.4) |
| Home isolation | 189 (66.1) | |
| Hospitalization | 49 (17.1) | |
| Symptom duration | < 1 wk | 99 (34.6) |
| 1-2 wk | 128 (44.8) | |
| 2-3 wk | 49 (17.1) | |
| > 3 wk | 10 (3.5) | |
| Pre-existing chronic illness | Diabetes Mellitus | 33 (11.5) |
| Hypertension | 31 (10.8) | |
| Chronic respiratory disease | 6 (2.1) | |
| Chronic kidney disease | 2 (0.7) | |
| Other | 4 (1.4) | |
| None | 210 (73.5) | |
| HCWs with households infected within 14 d | Total | 140 (49) |
| Earlier (3-14 d) | 49 (35) | |
| Same time (within 2 d) | 55 (39.3) | |
| Later (3-14 d) | 36 (25.7) | |
| Number of infected households | 1 | 48 (51.6) |
| 2 | 18 (18.9) | |
| 3 | 16 (16.8) | |
| > 3 | 12 (12.7) | |
| HCWs with co-workers infected within 14 d | Total | 108 (37.8) |
| Earlier (3-14 d) | 38 (35.2) | |
| Same time (within 2 d) | 33 (30.5) | |
| Later (3-14 d) | 37 (34.3) | |
| Number of infected co-workers | 1 | 23 (24.2) |
| 2 | 16 (16.8) | |
| 3 | 3 (3.1) | |
| > 3 | 17 (17.9) |
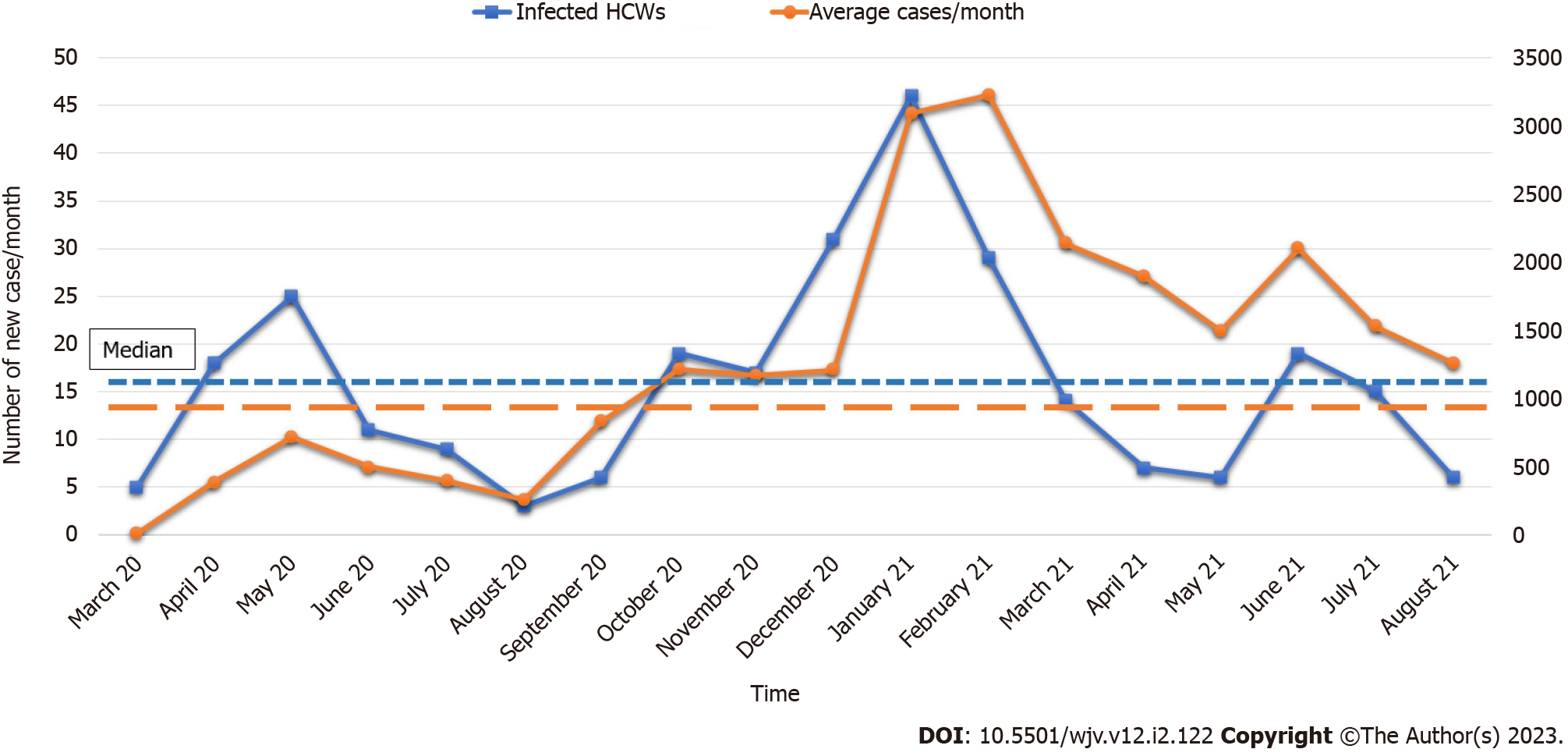
When compared between the infected and the uninfected HCWs, frontline HCWs (25.1% vs 8.6%, P < 0.001), who were males (54% vs 46%, P < 0.001) recorded a significantly high infection rate. The infection rate among the unvaccinated HCWs (83.2% vs 16.8%, P < 0.001) was nearly five times higher than those HCWs who were vaccinated against COVID-19. The study found that the type of accommodation (self-owned vs hospital sponsored) showed no significance effect on the infection rate (Table 2). A significantly high proportion of the infected frontline HCWs were males who stayed in rented accommodation with family (P < 0.001) (Table 3). Finally, the trend chart of a month-wise comparison of the infected HCWs and the average new cases in UAE showed three peaks.
| Uninfected HCWs, n = 1282 | Infected HCWs, n = 346 | P value | |
| Accommodation | |||
| Hospital sponsored | 381 (29.7) | 89 (25.9) | 0.15 |
| Self-owned | 901 (70.3) | 257 (74.1) | (χ2 statistic value, 2.12) |
| Work profile | |||
| Frontline | 689 (53.7) | 278 (80.4) | < 0.001 |
| Non-frontline | 593 (46.3) | 67(19.6) | (χ2 statistic value, 81.19) |
| COVID-19 vaccination | |||
| Vaccinated | 1198 (93.2) | 58 (16.8) | < 0.001 |
| Unvaccinated | 88 (6.8) | 287 (83.2) | (χ2 statistic value, 895.49) |
| Sex | |||
| Male | 511 (39.9) | 187 (54.0) | < 0.001 |
| Female | 771 (60.1) | 159 (46.0) | (χ2 statistic value, 22.38) |
| Frontline healthcare workers, n = 238 | Non-Frontline healthcare workers, n = 48 | P value | |
| Accommodation | |||
| Shared with family | 167 (70.2) | 23 (47.9) | < 0.001 |
| Shared with friends | 60 (25.2) | 24 (50) | (χ2 statistic value, 31.07) |
| Non-shared | 11 (4.6) | 1 (2.1) | |
| Accommodation | |||
| Self-rented | 140 (58.8) | 18 (11.4) | < 0.001 |
| Hospital provided | 49 (20.6) | 25 (33.8) | (χ2 statistic value, 42.68) |
| Others | 49 (20.6) | 5 (9.3) | |
| Sex | |||
| Male | 205 (86.1) | 35 (72.9) | 0.02 |
| Female | 33 (13.9) | 13 (27.1) | (χ2 statistic value, 5.17) |
The survey also tried to assess the knowledge and perception of the participants about safety precaution, vaccination, and the disease. Most of the participants were aware about the appropriate usage of PPE (99.3%) and did not agree to unprotected exposure to a patient with COVID-19 (85%). Around 74% of the participants agreed with the importance of social precautions like face mask, social distancing, and hand hygiene in preventing the SARS-CoV-2 infection. Only 70% agreed on the efficacy of COVID-19 vaccination in preventing infection or progression to the severe disease. The deficiency of PPE at the workplace was reported by 23.4% of the participants, whereas 29.7% participants wanted an improvement in the quality and availability of the PPE (Table 4).
| Are you aware about appropriate personal protective equipment for the care of COVID-19 patients? | Yes: 99.3%; No: 0.7% |
| Have you ever been exposed to a COVID-19 patient without adequate PPE? | Yes: 43 (15); No: 243 (85) |
| There was always enough PPE in my workplace | Agree: 183 (64); Neutral: 36 (12.6); Disagree: 67 (23.4) |
| PPE availability and quality should be improved at my workplace | Agree: 85 (29.7); Neutral 49 (17.1); Disagree: 152 (53.2) |
| Proper precautions (face mask, hand hygiene, social distance) are most important tools to save you from SARS-CoV-2 | Agree: 213 (74.4); Neutral: 12 (4.2); Disagree: 61 (21.3) |
| Vaccines for SARS-CoV-2 can reduce infection rate and can prevent severe disease and hospitalisation | Agree: 201 (70.2); Neutral: 27 (9.6); Disagree: 58 (20.2) |
This cross-sectional analysis of RT-PCR-positive HCWs from two tertiary care hospitals showed that frontline HCWs had a significantly higher infection rate. The study infers that being a male is a significant risk factors for getting infected with COVID-19. Among the infected frontline staff, a significantly higher proportion were male who shared their accommodation with family members. COVID-19 vaccination was effective in reducing the rate of infection among HCWs.
From the start of COVID-19 pandemic, various studies recorded a higher infection rate among the frontline HCWs. The risk was higher due to the reuse or inadequate availability of the PPEs and due to which the studies advocated strategies like access to high-quality PPEs and early COVID-19 vaccination to curb the spread of the virus[2,8]. Nearly one-fourth of the participants in this study reported insufficient access to the PPEs, while most were unaware of any unprotected exposure with COVID-19 patients. Limited access to adequate PPE has been linked with higher odds of infection[9-11]. Hence, ensuring access to high-quality PPEs for HCWs is an important workplace risk-reduction measure. The rate of infection was significantly higher among the male HCWs as found in other studies[2,12].
Around 13% of the study participants had asymptomatic infection. The number of asymptomatic infections could have been higher, if the hospitals had routine surveillance testing for the HCWs. However, the impact of the routine surveillance testing of asymptomatic HCWs in preventing nosocomial transmission of SARS-CoV-2 is unknown[13]. A consensus experts’ panel recommended testing the HCWs to get tested for SARS-CoV-2 only when they are symptomatic or when they encountered unprotected exposure over routine testing[14].
Around 38% of the infected participants agreed to infection among their co-workers within 14 days of their own infection, and nearly one-fifth of them agreed to have three or more infected co-workers. Moreover, sharing accommodation with family or friends was significantly higher among the infected frontline HCWs. In the absence of epidemiological investigation and genomic sequencing, these infections cannot be segregated as an outbreak. However, the absenteeism of multiple HCWs from the same department can disrupt the services of already overwhelmed frontline departments during the pandemic. Despite various published reports on a nosocomial outbreak of SARS-CoV-2, ambiguity exists regarding the role of HCWs in initiating or amplifying the nosocomial outbreaks[6,15].
Most epidemiological research on SARS-CoV-2 infection among the HCWs has focused on transmission dynamics within the hospital setting. However, the research on the impact of social-cultural and demographic factors on the transmission of SARS-CoV-2 among HCWs is lacking. Recently, a large prospective study conducted in the United Kingdom found the effect of socio-demographic characteristics on the risk of infection among the vulnerable HCWs. The study found that amongst the demographic and household risk factors, young age, living with a co-worker, and high religiosity are associated with high infection odds among the HCWs[9]. In another study, high odds of infection were observed among the HCWs from community contact with a suspected or a confirmed COVID-19 individual, instead of the workplace[16]. Socio-demographic risk factors may differ based on the culture and geographical differences, and the availability of resources. The cross-transmission of SARS-CoV-2 among the household is well-established concept and persists even during the low-community transmission[17].
The current study also found a significantly higher proportion of the infected frontline HCWs were staying in shared accommodation. When comparing infected HCWs per month with average new cases in the community, an agreement was observed in the peaks of two trend charts (Figure 1). This pattern reveals a synchronization in the infection rate among the HCWs and the transmission rate of SARS-CoV-2 infection in the community. Hence, the HCWs are vulnerable to contracting the infection from their households and social contacts, especially with a higher rate of SARS-CoV-2 transmission in the community. Hospital leadership can utilize this valuable insight for workforce management and to develop strategies to mitigate the risk of exposure to HCWs. Theoretically, public transport can be another risk factor for transmission. However, as reported in the literature, the current study authors did not find any increased transmission risk with public transport[18].
According to a study conducted earlier, the vaccination of the HCWs effectively reduces the risk of severe disease and the transmission of SARS-CoV-2[19]. Advanced age (≥ 65 years), male sex, and other co-morbidities like diabetes mellitus, chronic respiratory disease, hypertension, chronic kidney disease, and cardiovascular disease are risk factors for severe illness and mortality[20]. COVID-19 vaccination is highly effective in reducing the progression and the severity of disease and intensive care unit (ICU) or hospital admission, especially in the elderly population and patients with co-morbidities[21]. Vaccination is an essential intervention for the HCWs to protect them from getting infected and severe illness that may require hospital or ICU admission. However, the effectiveness of the vaccine in reducing the risk of disease reduces considerably after six months of the last dose. So, a booster dose is recommended for the vulnerable population, including HCWs[19]. Vaccine hesitancy among the HCWs is a major issue in the successful implementation of the COVID-19 vaccination programme. Only 70.2% of the participants have agreed upon the efficacy of the COVID-19 vaccines. Other studies also found more vaccination hesitation among the previously infected people[22,23]. Hospital leadership and infection preventionist should address the issue of vaccine hesitancy strategically and through collaboration.
This is the first study to the best of the author’s knowledge from the UAE or the countries in the Gulf Cooperation Council on risk profiling of RT-PCR-positive HCWs with COVID-19 using socio-demographic factors. The study also evaluated the impact of COVID-19 vaccination on cross-transmission among the HCWs. The current study has a few limitations that are listed herewith. Firstly, the information on social contacts and households was collected through a cross-sectional survey. Hence, there exists a potential recall bias because of the time-gap between the period of infection and data collection. However, to avoid this bias, the data collected from the cross-sectional survey was validated through the human resource records maintained by the hospital.
There is missing data for about 17% of the eligible HCWs who did not participate in the cross-sectional survey due to reasons like resignation and immigration to other countries. Secondly, genomic sequencing was not used to confirm the phylogenetic linkage in infection among co-workers or the household. Thirdly, the small cohort size could have missed portraying the complete statistical correlation of various socio-demographic factors. Finally, the impact of the COVID-19 vaccination booster on transmission dynamics was not assessed.
The risk profiling of the HCWs, infected with SARS-CoV-2 from two tertiary care hospitals showed that the frontline HCWs had a significantly higher infection rate. Another significant risk factor was male sex. COVID-19 vaccination can effectively reduce the rate of SARS-CoV-2 transmission among HCWs.
There is paucity of the research on the transmission dynamics of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among the healthcare workers (HCWs) and their co-workers and household. The current study conducted a retrospective analysis of the infected HCWs to analyze the socio-demographic risk factors and characteristics of SARS-CoV-2 transmission among HCWs and their social contacts.
HCWs are vulnerable to SARS-CoV-2 infection during their work, and the potential risk of transmission of SARS-CoV-2 infection from the household and co-workers of HCWs is unclear. This study provides valuable insights for workforce management and helps formulate strategies to mitigate the risk of exposure to the HCWs.
The current study evaluated the risk factors of SARS-CoV-2 infection among HCWs and explored the potential of transmission of SARS-CoV-2 among the household and co-workers of infected HCWs.
The health records of all infected HCWs between March 2020 and August 2021 were analysed. The information on the coronavirus disease 2019 (COVID-19) vaccination, household and co-workers of the infected HCWs was collected through a cross-sectional survey.
The cross-sectional analysis of health records of 346 reverse transcriptase polymerase chain reaction (RT-PCR)-positive HCWs showed that the risk of infection was significantly higher among frontline HCWs. Being male was a significant risk factor for SARS-CoV-2 infection. Among infected frontline staff, a significantly higher proportion were male, and were staying with their families in rented accommodation. COVID-19 vaccination was effective in reducing the infection rate among HCWs.
Working at the frontline and being male are the significant risk factors for SARS-CoV-2 infection among the HCWs. COVID-19 vaccination is effective in reducing the infection rate among HCWs.
Future research should explore the role of community transmission of SARS-CoV-2 in the infection of HCWs.
The authors acknowledge the scientific guidance of Ms Helen King, Dr Rita Vassena, and Rohit Dusane during this study.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Freund O, Israel; Su C, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH
| 1. | Mutambudzi M, Niedwiedz C, Macdonald EB, Leyland A, Mair F, Anderson J, Celis-Morales C, Cleland J, Forbes J, Gill J, Hastie C, Ho F, Jani B, Mackay DF, Nicholl B, O'Donnell C, Sattar N, Welsh P, Pell JP, Katikireddi SV, Demou E. Occupation and risk of severe COVID-19: prospective cohort study of 120 075 UK Biobank participants. Occup Environ Med. 2020;78:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 349] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 2. | Nguyen LH, Drew DA, Graham MS, Joshi AD, Guo CG, Ma W, Mehta RS, Warner ET, Sikavi DR, Lo CH, Kwon S, Song M, Mucci LA, Stampfer MJ, Willett WC, Eliassen AH, Hart JE, Chavarro JE, Rich-Edwards JW, Davies R, Capdevila J, Lee KA, Lochlainn MN, Varsavsky T, Sudre CH, Cardoso MJ, Wolf J, Spector TD, Ourselin S, Steves CJ, Chan AT; COronavirus Pandemic Epidemiology Consortium. Risk of COVID-19 among front-line health-care workers and the general community: a prospective cohort study. Lancet Public Health. 2020;5:e475-e483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1446] [Cited by in RCA: 1381] [Article Influence: 276.2] [Reference Citation Analysis (0)] |
| 3. | De Kock JH, Latham HA, Leslie SJ, Grindle M, Munoz SA, Ellis L, Polson R, O'Malley CM. A rapid review of the impact of COVID-19 on the mental health of healthcare workers: implications for supporting psychological well-being. BMC Public Health. 2021;21:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 409] [Cited by in RCA: 457] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 4. | Zheng C, Hafezi-Bakhtiari N, Cooper V, Davidson H, Habibi M, Riley P, Breathnach A. Characteristics and transmission dynamics of COVID-19 in healthcare workers at a London teaching hospital. J Hosp Infect. 2020;106:325-329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 55] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 5. | Schneider S, Piening B, Nouri-Pasovsky PA, Krüger AC, Gastmeier P, Aghdassi SJS. SARS-Coronavirus-2 cases in healthcare workers may not regularly originate from patient care: lessons from a university hospital on the underestimated risk of healthcare worker to healthcare worker transmission. Antimicrob Resist Infect Control. 2020;9:192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 6. | Abbas M, Robalo Nunes T, Martischang R, Zingg W, Iten A, Pittet D, Harbarth S. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 199] [Cited by in RCA: 180] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 7. | Asad H, Johnston C, Blyth I, Holborow A, Bone A, Porter L, Tidswell P, Healy B. Health Care Workers and Patients as Trojan Horses: a COVID19 ward outbreak. Infect Prev Pract. 2020;2:100073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Fell A, Beaudoin A, D'Heilly P, Mumm E, Cole C, Tourdot L, Ruhland A, Klumb C, Rounds J, Bailey B, Liverseed G, Peterson M, Mahoehney JP, Ireland M, Bye M, Setty S, Leeds M, Taylor J, Holzbauer S; Minnesota Department of Health COVID-19 HCW Monitoring Response Team; Minnesota Department of Health COVID-19 Response Task Force. SARS-CoV-2 Exposure and Infection Among Health Care Personnel - Minnesota, March 6-July 11, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1605-1610. [PubMed] |
| 9. | Martin CA, Pan D, Nazareth J, Aujayeb A, Bryant L, Carr S, Gray LJ, Gregary B, Gupta A, Guyatt AL, Gopal A, Hine T, John C, McManus IC, Melbourne C, Nellums LB, Reza R, Simpson S, Tobin MD, Woolf K, Zingwe S, Khunti K, Pareek M; UK-REACH Study Collaborative Group. Access to personal protective equipment in healthcare workers during the COVID-19 pandemic in the United Kingdom: results from a nationwide cohort study (UK-REACH). BMC Health Serv Res. 2022;22:867. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Kim H, Hegde S, LaFiura C, Raghavan M, Sun N, Cheng S, Rebholz CM, Seidelmann SB. Access to personal protective equipment in exposed healthcare workers and COVID-19 illness, severity, symptoms and duration: a population-based case-control study in six countries. BMJ Glob Health. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Dzinamarira T, Nkambule SJ, Hlongwa M, Mhango M, Iradukunda PG, Chitungo I, Dzobo M, Mapingure MP, Chingombe I, Mashora M, Madziva R, Herrera H, Makanda P, Atwine J, Mbunge E, Musuka G, Murewanhema G, Ngara B. Risk Factors for COVID-19 Infection Among Healthcare Workers. A First Report From a Living Systematic Review and meta-Analysis. Saf Health Work. 2022;13:263-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 12. | Chatterjee P, Anand T, Singh KJ, Rasaily R, Singh R, Das S, Singh H, Praharaj I, Gangakhedkar RR, Bhargava B, Panda S. Healthcare workers & SARS-CoV-2 infection in India: A case-control investigation in the time of COVID-19. Indian J Med Res. 2020;151:459-467. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 135] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 13. | D'Ettorre G, Pellicani V, Muratore M, Ceccarelli G. Occupational health surveillance of healthcare workers during COVID 19 pandemic: a narrative review. Acta Biomed. 2022;93:e2022007. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 14. | Nasa P, Azoulay E, Chakrabarti A, Divatia JV, Jain R, Rodrigues C, Rosenthal VD, Alhazzani W, Arabi YM, Bakker J, Bassetti M, De Waele J, Dimopoulos G, Du B, Einav S, Evans L, Finfer S, Guérin C, Hammond NE, Jaber S, Kleinpell RM, Koh Y, Kollef M, Levy MM, Machado FR, Mancebo J, Martin-Loeches I, Mer M, Niederman MS, Pelosi P, Perner A, Peter JV, Phua J, Piquilloud L, Pletz MW, Rhodes A, Schultz MJ, Singer M, Timsit JF, Venkatesh B, Vincent JL, Welte T, Myatra SN. Infection control in the intensive care unit: expert consensus statements for SARS-CoV-2 using a Delphi method. Lancet Infect Dis. 2022;22:e74-e87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Lindsey BB, Villabona-Arenas CJ, Campbell F, Keeley AJ, Parker MD, Shah DR, Parsons H, Zhang P, Kakkar N, Gallis M, Foulkes BH, Wolverson P, Louka SF, Christou S, State A, Johnson K, Raza M, Hsu S, Jombart T, Cori A; Sheffield COVID-19 Genomics Group; COVID-19 Genomics UK (COG-UK) consortium; CMMID COVID-19 working group, Evans CM, Partridge DG, Atkins KE, Hué S, de Silva TI. Characterising within-hospitalSARS-CoV-2 transmission events using epidemiological and viral genomic data across two pandemic waves. Nat Commun. 2022;13:671. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 16. | Jacob JT, Baker JM, Fridkin SK, Lopman BA, Steinberg JP, Christenson RH, King B, Leekha S, O'Hara LM, Rock P, Schrank GM, Hayden MK, Hota B, Lin MY, Stein BD, Caturegli P, Milstone AM, Rock C, Voskertchian A, Reddy SC, Harris AD. Risk Factors Associated With SARS-CoV-2 Seropositivity Among US Health Care Personnel. JAMA Netw Open. 2021;4:e211283. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 17. | Madewell ZJ, Yang Y, Longini IM Jr, Halloran ME, Dean NE. Household Secondary Attack Rates of SARS-CoV-2 by Variant and Vaccination Status: An Updated Systematic Review and Meta-analysis. JAMA Netw Open. 2022;5:e229317. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 150] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 18. | Mohammed I, Nauman A, Paul P, Ganesan S, Chen KH, Jalil SMS, Jaouni SH, Kawas H, Khan WA, Vattoth AL, Al-Hashimi YA, Fares A, Zeghlache R, Zakaria D. The efficacy and effectiveness of the COVID-19 vaccines in reducing infection, severity, hospitalization, and mortality: a systematic review. Hum Vaccin Immunother. 2022;18:2027160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 218] [Article Influence: 72.7] [Reference Citation Analysis (0)] |
| 19. | Feikin DR, Higdon MM, Abu-Raddad LJ, Andrews N, Araos R, Goldberg Y, Groome MJ, Huppert A, O'Brien KL, Smith PG, Wilder-Smith A, Zeger S, Deloria Knoll M, Patel MK. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: results of a systematic review and meta-regression. Lancet. 2022;399:924-944. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 823] [Cited by in RCA: 833] [Article Influence: 277.7] [Reference Citation Analysis (0)] |
| 20. | Du P, Li D, Wang A, Shen S, Ma Z, Li X. A Systematic Review and Meta-Analysis of Risk Factors Associated with Severity and Death in COVID-19 Patients. Can J Infect Dis Med Microbiol. 2021;2021:6660930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 21. | Freund O, Tau L, Weiss TE, Zornitzki L, Frydman S, Jacob G, Bornstein G. Associations of vaccine status with characteristics and outcomes of hospitalized severe COVID-19 patients in the booster era. PLoS One. 2022;17:e0268050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 22. | Martin CA, Marshall C, Patel P, Goss C, Jenkins DR, Ellwood C, Barton L, Price A, Brunskill NJ, Khunti K, Pareek M. SARS-CoV-2 vaccine uptake in a multi-ethnic UK healthcare workforce: A cross-sectional study. PLoS Med. 2021;18:e1003823. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 23. | Caspi I, Freund O, Pines O, Elkana O, Ablin JN, Bornstein G. Effect of patient COVID-19 vaccine hesitancy on hospital care team perceptions. World J Clin Cases. 2023;11:821-829. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (1)] |