Published online Mar 25, 2023. doi: 10.5501/wjv.v12.i2.100
Peer-review started: October 11, 2022
First decision: November 15, 2022
Revised: November 23, 2022
Accepted: January 23, 2023
Article in press: January 23, 2023
Published online: March 25, 2023
Processing time: 160 Days and 9.8 Hours
Liver injury secondary to vaccination is a rare adverse event that has recently come under attention thanks to the continuous pharmacovigilance following the widespread implementation of coronavirus disease 2019 (COVID-19) vaccination protocols. All three most widely distributed severe acute respiratory syndrome coronavirus 2 vaccine formulations, e.g., BNT162b2, mRNA-1273, and ChAdOx1-S, can induce liver injury that may involve immune-mediated pathways and result in autoimmune hepatitis-like presentation that may require therapeutic intervention in the form of corticosteroid administration. Various mechanisms have been proposed in an attempt to highlight immune checkpoint inhibition and thus establish causality with vaccination. The autoimmune features of such a reaction also prompt an in-depth investigation of the newly employed vaccine technologies. Novel vaccine delivery platforms, e.g., mRNA-containing lipid nanoparticles and adenoviral vectors, contribute to the inflammatory background that leads to an exaggerated immune response, while patterns of molecular mimicry between the spike (S) protein and prominent liver antigens may account for the autoimmune presentation. Immune mediators triggered by vaccination or vaccine ingredients per se, including autoreactive antibodies, cytokines, and cytotoxic T-cell populations, may inflict hepatocellular damage through well-established pathways. We aim to review available data associated with immune-mediated liver injury associated with COVID-19 vaccination and elucidate potential mechanisms underlying its pathogenesis.
Core Tip: Following the worldwide implementation of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccination protocols, several reports suggest an increase in the occurrence of autoimmune phenomena involving the liver. Studies on vaccine-induced liver injury point to a specific pattern of hepatocellular injury that involves immune-mediated pathways. This minireview explores the underlying pathophysiology of immune-mediated liver injury following SARS-CoV-2 vaccination and examines the most widely distributed vaccine formulations’ autoimmune and hepatotoxic potential.
- Citation: Schinas G, Polyzou E, Dimakopoulou V, Tsoupra S, Gogos C, Akinosoglou K. Immune-mediated liver injury following COVID-19 vaccination. World J Virol 2023; 12(2): 100-108
- URL: https://www.wjgnet.com/2220-3249/full/v12/i2/100.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i2.100
As of August 4, 2022, approximately 5.3 billion people around the world have received at least one dose of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. Widespread implementation of vaccination protocols has successfully contained the spread of the pandemic and has reduced the disease burden for patients and health systems alike[1]. Newly employed vaccination platforms, e.g., mRNA- and adenovirus (AdV)- based formulations, have achieved high efficacy rates combined with a good safety profile[2]. SARS-CoV-2 vaccines have undergone the most intensive safety monitoring in the history of mankind. Both active and passive monitoring systems have been employed in order to timely detect and properly identify adverse events related to vaccination[3,4]. This worldwide vigilance has proved fruitful for epidemiological purposes and has been instrumental in ensuring public support for vaccination. Most frequently reported adverse events have been mild in nature and local in character. They primarily concern injection site-related reactions, e.g., topical pain and redness or generalized systemic symptoms, like fever and fatigue[5,6]. As far as serious, organ-specific adverse events are concerned, a very low risk of myocarditis mainly in younger individuals has been linked to vaccination with an mRNA vaccine, whereas adenoviral vector vaccines have been associated with incidents of thrombosis accompanied by thrombocytopenia and possibly Guillain–Barré Syndrome (GBS) cases. A rather rare side effect that has recently come under attention is that of liver injury following vaccination with a SARS-CoV-2 vaccine.
Drug-induced liver injury (DILI), under the umbrella of which such a clinical syndrome would initially be examined, is characterized by new-onset, profound increases in liver function enzyme levels. According to the latest expert panel update, this is defined as a ≥ 5 × upper limit of normal (ULN) elevation of alanine (ALT) or aspartate aminotransferase (AST) and/or ≥2× ULN increase in alkaline phosphatase (ALP) levels or ALT/AST ≥ 3 × UNL and bilirubin ≥ 2 × ULN[7]. Upon removal of the offending agent, most cases of DILI are usually self-contained; corticosteroids are sometimes added to the therapeutic regimen if autoimmune features are demonstrated. In fact, most of the reported cases’ clinical and histological features closely resemble those encountered in autoimmune hepatitis (AIH)[8,9], steering the focus of the causality investigation onto the immune-mediated background of the reaction. However, it remains unclear whether the reported association of AIH with vaccination is coincidental, represents unique SARS-CoV-2-induced antigen-specific immune activation or is associated with transient drug-induced liver injury. In this study, we aim to review underlying mechanisms driving immune-mediated liver injury following COVID-19 vaccination and discuss potential implications
We carried out broad searches of PubMed, Scopus, and Embase between 1 January 2021 and 1 September 2022 to identify literature describing immune-mediated liver injury or autoimmune hepatitis following COVID-19 vaccination. Relevant publications were identified based on the titles and abstracts. No restriction on the type of paper or language was set, even though the main focus was put on underlying mechanisms. Two reviewers independently screened all titles/abstracts and hand-searched references of retrieved articles. Data were assessed for their quality based on overall judgement and not aggregate scores. Disagreements were discussed and resolved and duplicates were removed.
Throughout the vaccine rollout period, there have been reported cases of presumed AIH that were attributed to COVID-19 vaccination because they were observed shortly after either the first or second dose of the vaccine, with the initial case described as early as January 2021[10]. AIH is part of a diverse group of chronic inflammatory liver conditions that include primary biliary cholangitis and primary sclerosing cholangitis, and its complex pathophysiology involves underlying genetic predisposition and interactions with environmental triggers[11]. Viral infections, drug exposure, and vaccinations have been implicated in the pathogenesis of AIH[12]. AIH had fallen under the radar during the phase 3 clinical trials of all vaccines, and like every other rare adverse event, much debate has ensued over its association with the vaccination. New onset autoimmune reactions following vaccination have previously been described in the literature[13]. Both Hepatitis A and Hepatitis B vaccines have been linked to the development of AIH-like conditions[14]. Human papillomavirus, Hepatitis B, and Influenza vaccines have been held accountable for autoimmune reactions[15,16]. Molecular mimicry theory has been the platform upon which causality with vaccination has been determined[17]. It has been hypothesized that individuals with a genetic predisposition to autoimmunity undergo vaccination, and similarly to other environmental inputs, e.g., smoking and nutrition, their immune tolerance becomes compromised. Reportedly, susceptible groups include those with systemic lupus erythematosus, GBS, multiple sclerosis, and narcolepsy. Concerning SARS-CoV-2 vaccination, immune-based phenomena such as GBS, IgA nephropathy, immune thrombotic thrombocytopenia, and myocarditis have been linked to both novel vaccine platforms, i.e., mRNA- and AdV-based formulations[18].
As of early 2021, case reports of documented AIH following COVID-19 vaccination have begun to emerge[19-21]. We estimate that AIH related to COVID-19 is almost 1 in 14 million, even though we do acknowledge that many cases remain undocumented[19]. Data mostly deriving from comprehensive case-series reporting liver injury following vaccination with SARS-CoV-2 vaccines, point to the fact that most cases are in fact, immune-related, with 57% of all patients displaying both autoantibody presence and IgG hyperglobulinemia. They mostly affected elderly females, with most of the reports originating from European countries, followed by the United States[20]. The mean time of symptom onset is close to three weeks following the first vaccination, with some individuals presenting as early as 3 d after and others coming in as late as a month later, suggesting some heterogeneity in the underlying response mechanism. The mean duration between receiving the first or second vaccine dose and subsequent onset of liver injury was 17.3 (11.2–23.4) days and was mostly associated with mRNA vaccines, possibly to their stronger immunogenic potency[20]. The presence of underlying autoimmune diseases (e.g, Hashimoto thyroiditis, primary sclerosing cholangitis) is evident in approximately 25% of patients and could explain temporal and spatial differences in manifestations and prevalence, respectively, according to genetic predispositions[22]. Antinuclear antibody (ANA) was by far the most prevalent autoantibody, followed by spinal muscular atrophy and anti-myocardial antibody (AMA), resembling a type 1 AIH pattern. Biopsy findings were also consistent with AIH in most individuals. Around 1/3 of those who did not undergo the diagnostic procedures fit the clinical profile of AIH. Icteric manifestations, including jaundice, choluria, and pruritus, account for around 2/3 of all presentations. Outcomes were similar in all three vaccine products, i.e., BNT162b2, mRNA-1273 and ChAdOx1 nCoV-19. Although recovery time varied greatly among study populations, the mean time for transaminase normalization was calculated at 46 d[21]. Corticosteroid treatment proved safe and effective for all those who were prescribed. No relapse was noted in the subgroup of patients whose immunosuppressant treatment was discontinued and remission was maintained in all those who spontaneously recovered.
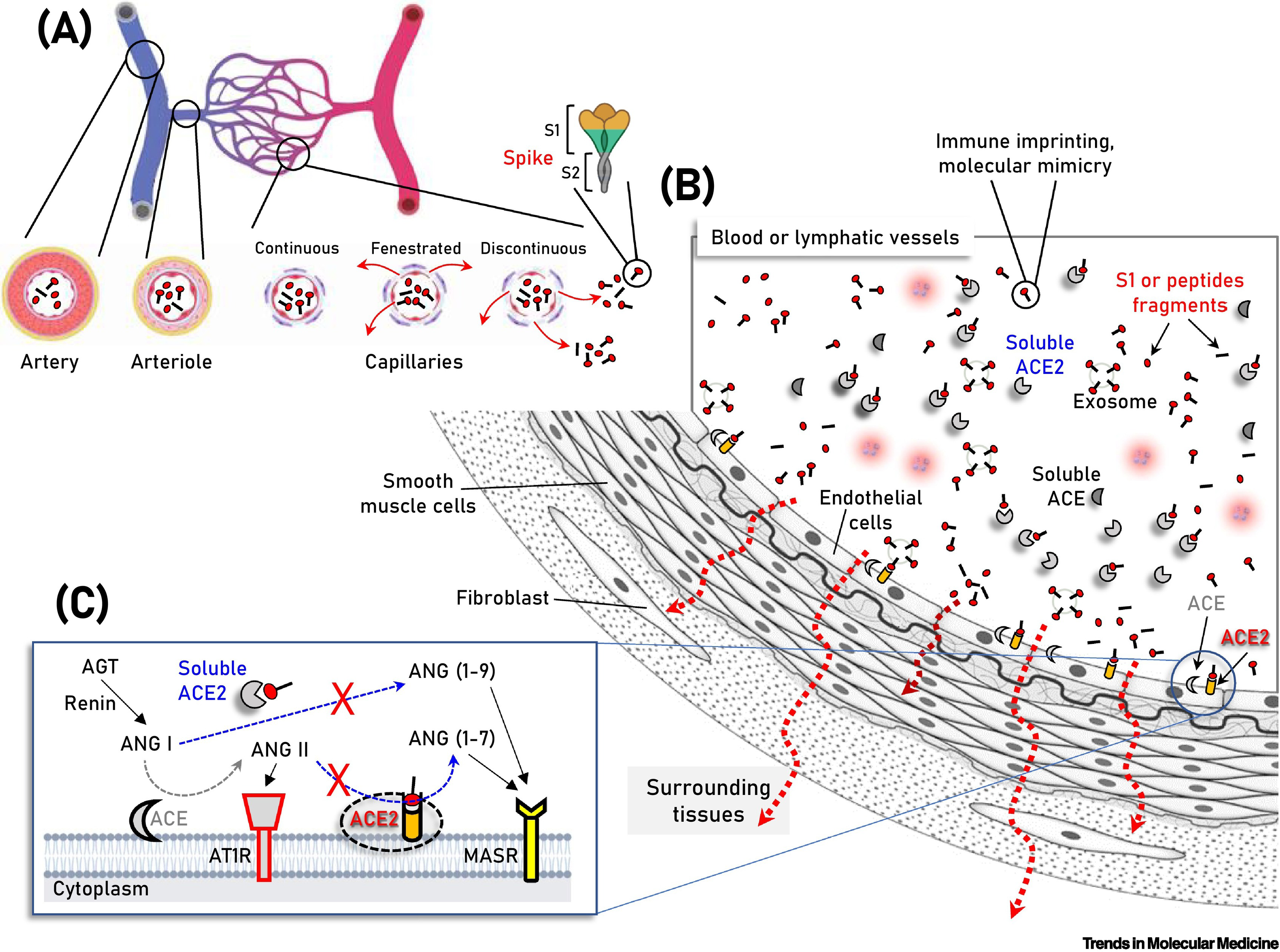
Establishing causality is by definition, a difficult task, while the mechanism of action of such a reaction remains elusive. Several theories have been proposed in an attempt to link clinical manifestations of hepatocellular injury to patterns of immune mediation involving vaccine ingredients and products. Molecular mimicry-based reactivity and pro-inflammatory interactions involving the SARS-CoV-2 spike protein have been explored (Figure 1)[23]. The vaccine adjuvants have also come under scrutiny. The BNT162b2 and mRNA-1273 vaccines employ lipid nanoparticle (LNP) coated mRNA technology, whereas the ChAdOx1 nCoV-19 vaccine is deoxyribonucleic acid based and utilizes AdV vectors. Both AdV and mRNA vaccine platforms are newly licensed; hence, many rare in vivo interactions are to be explored and clarified. A deeper look into the active ingredients of the vaccines may provide us with a plausible mechanism. The mRNA itself has been carefully designed and tested as to its immunogenic properties[24,25]; however, prior to translation, it may still be recognized by cytosolic and endosomal toll-like receptors. The encoded S-spike protein elicits a strong immune reaction that involves the activation of the innate inflammatory cascade, as well as that of the adaptive humoral response. Regarding the former, SARS-CoV-2 vaccines employ the type I interferon pathway in particular, in order to maintain an adequate and effective immune response, that in turn, may increase the probability of an autoimmune occurrence in certain individuals[26]. Concerning the latter, reactivity between anti-S protein antibodies and human tissue antigens has been confirmed by a recent report[27]. The systemic distribution of the spike protein has also been postulated as a mechanism to explain adverse events by mRNA-based vaccines as well. Its interaction with soluble Angiotensin-converting enzyme 2 (ACE-2) and ACE-2-ligands may point to an organ-specific pattern of insult[28]. The presentation and/or production of the spike protein by the hepatocytes may induce the activation of cytotoxic T-cell subsets. Under this scope, the formation of immune complexes cannot be excluded. Their subsequent deposition on the liver may cause inflammation or exacerbation of the underlying autoimmune disease. Matyushkina et al[27] have identified the susceptibility of human leukocyte antigen (HLA) B15:01 and HLA B39:01 allele carriers to autoimmunity following COVID-19. HLA B15 has been strongly associated with the development of infliximab-induced liver injury[29], while HLA B39 has been recorded as one of the most prevalent alleles in AIH patients in Pakistan[30]. In the same report, although most autoreactive antibodies were associated with nuclear products, cross-reactivity with cytokeratin 18 (CK18), a prominent liver disease biomarker, was noted. Elevated anti-CK18 antibody titers have been described in AIH patients[31], and their relationship to the soluble liver antigen (SLA) has been established in the literature. CK18’s immunoreactivity has even been proposed but disproved as a potential diagnostic marker for the SLA subgroup of AIH patients[32]. The presence of SLA antibodies has been reported twice following vaccination with the mRNA-1273 vaccine, a fact that prompted investigators to conduct a genomic sequence analysis study which revealed, however, no homology between the SARS-CoV-2 spike protein and soluble liver antigen[33].
As far as AdV vectors are concerned, their clinical application as potential gene therapy delivery particles has been hindered by their hepatotoxic properties since the start of the century[34]. Recent reports have attributed this to their inherent liver tropism[34]. It should also be noted that the recombinant ChAdOx1-S AdV used in Vaxzervria formulations is likely hepatotropic since it is derived from a subset of non-human, Y25-coded adenoviruses that have been linked to viral hepatitis outbreaks in chimpanzees in the past[34]. In the same report, the authors build a case for a post-transcriptional modification taking place inside the nucleus of AdV-transduced host cells, resulting in alternate gene splicing and subsequent truncation of produced S-protein proteins that may in turn, be released in circulation. In addition to that, they demonstrated that in ChAdOx1-S-transduced hepatocytes, the truncated S-protein is the main splicing product, thereby providing us with another plausible mechanism to explain liver injury by AdV-based formulations. Of note, the spike protein produced by Vaxzervria has comparable receptor binding selection and affinity to its original counterpart[35]. Regarding common vaccine adjuvants, CpG 1018 and Aluminum, although widely used for immune response enhancement purposes and deemed safe by clinical trials[36] and regulatory authorities alike, have the potential to induce liver injury[37] and likely precipitate the development of auto-immune disease in a small percentage of the population[38]. Reportedly, none of the vaccines discussed in this review contain the aforementioned adjuvants, but future formulations may include them. Furthermore, we need to consider other vaccine formulation specificities, like the active ingredient’s delivery system. The immunogenicity of the mRNA-containing LNPs has recently come under question, despite the fact that prior to their use in COVID-19 vaccines, they were being hailed as a potential genetic treatment platform for inherited liver disease[39]. The mRNA delivery particles have been linked to the development of allergic reactions[40], suggesting a plausible, if not definite, role as immune mediators. The LNP platform mounts a strong immune response, which relies on the medium’s pro-inflammatory properties for its efficacy. Such an immune response-provoking environment could potentiate a loss in self-tolerance. LNPs have been known to act as adjuvants to vaccine-induced immune reactions[41,42]. In particular, LNPs seem to trigger the NLRP3 inflammasome pathway that has been implicated in the pathogenesis of other autoimmune phenomena, like pericarditis, rheumatoid arthritis and AIH[43-46]. The intense immunogenic character of LNPs has been demonstrated both histochemically and graphically in animal models through multiple route administration of its purified form, e.g., intramuscularly and intranasally.
The hepatocellular type of injury that is predominantly associated with post-vaccination liver injury can also be attributed to the direct action of cytotoxic T-lymphocytes, as rapid and sustained activation of this cell subset has been confirmed in the context of SARS-CoV-2 vaccination[47]. In a recent report, vaccination with the BNT162b2 vaccine resulted in a CD8+ rich lymphocytic infiltrate in the liver of a patient that presented with probable AIH. The clonal expansion and peripheral activation state of this particular subset of lymphocytes correlated closely with the clinical course of hepatitis in this individual, suggesting T-cells’ involvement in the development and resolution of the disease[48]. It has also been demonstrated, in animal models, that cytokine-activated, “bystander” CD8+ lymphocytes may cause hepatocellular injury even in the absence of a direct antigen[49]. Accumulation of cytotoxic infiltrates in the liver has been reported in the literature following acute infection in influenza pneumonia[50]. All the plausible mechanisms resulting in immune-mediated liver injury discussed above are presented concisely in Table 1.
| Mechanism of injury | Liver antigens | Immune mediators |
| Molecular mimicry | SLA | Autoreactive antibodies |
| CK-18 | ||
| Cytotoxicity/Humoral response | S protein (membrane expression) | Activated CD8 + clone/protective anti-S antibodies |
| Humoral response | ACE-2 transmembrane receptor | Protective anti-S antibodies |
| Immune complex deposition | S protein (soluble) | |
| Soluble ACE-2/ACE-2 ligand + S protein | ||
| “Bystander” toxicity | Activated CD8 + clone | |
| Loss of self-tolerance/Fibrosis | Type I IFN | |
| NLRP3 inflammasome activation | LNPs | |
| TLR-mediated innate immune response | mRNA |
The immune-mediated mechanism of a clinical syndrome involving hepatocellular injury would most likely be highlighted by an elevation in ANA and/or AMA titers in a similar fashion to AIH. A report from early on in the pandemic noted the presence of elevated autoimmunity markers, including ANA and AMA, in SARS-CoV-2 antibody-rich plasma, thereby suggesting their self-reactive potential[51]. However, otherwise typical AIH auto-antibodies may be present in the acute phase of liver injury by multiple causes[52]. In order to distinguish between them, a biopsy is the preferred option, with fibrosis being the prime differentiating factor[53]. Features of widespread fibrosis would be evident in an AIH-stricken liver[54], whereas evidence of acute or chronic inflammation with eosinophilic infiltration between or within the portal triads is to be expected in the case of direct liver toxicity, i.e., DILI[55]. It is important to note that centrilobular necrosis is not a pathognomonic clue and should not be interpreted as such[56]. All in all, a definitive diagnosis of AIH may be challenging to make, as it relies on a constellation of clinical, serological and histological findings. Response to treatment with immunosuppressants is the only way to confirm a diagnosis[57]. AIH is a chronic condition with a high relapse rate if immunosuppression is withdrawn, whereas causes closely resembling AIH do not usually relapse[58].
Immune-mediated liver injury remains an elusive but rare entity following COVID-19 vaccination. It is the responsibility of investigators and scientists worldwide to maintain a vigilant eye and continue reporting rare incidents related to vaccination with a high index of suspicion. However, adverse events as such, are significantly less frequent than potentially serious complications of COVID-19 disease[59] and should by no means discourage vaccination programs worldwide.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Immunology
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Li Y, China; Rekabi A, Egypt S-Editor: Xing YX L-Editor: A P-Editor: Xing YX
| 1. | Tenforde MW, Patel MM, Ginde AA, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, Gaglani M, McNeal T, Ghamande S, Shapiro NI, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Exline MC, Gong MN, Mohamed A, Henning DJ, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CT, Busse L, Lohuis CCT, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Gershengorn HB, Babcock HM, Kwon JH, Halasa N, Chappell JD, Lauring AS, Grijalva CG, Rice TW, Jones ID, Stubblefield WB, Baughman A, Womack KN, Lindsell CJ, Hart KW, Zhu Y, Olson SM, Stephenson M, Schrag SJ, Kobayashi M, Verani JR, Self WH; Influenza and Other Viruses in the Acutely Ill (IVY) Network. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. medRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 2. | Sharif N, Alzahrani KJ, Ahmed SN, Dey SK. Efficacy, Immunogenicity and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis. Front Immunol. 2021;12:714170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 156] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 3. | Barda N, Dagan N, Ben-Shlomo Y, Kepten E, Waxman J, Ohana R, Hernán MA, Lipsitch M, Kohane I, Netzer D, Reis BY, Balicer RD. Safety of the BNT162b2 mRNA Covid-19 Vaccine in a Nationwide Setting. N Engl J Med. 2021;385:1078-1090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 669] [Cited by in RCA: 721] [Article Influence: 180.3] [Reference Citation Analysis (0)] |
| 4. | Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, Gilbert P, Janes H, Follmann D, Marovich M, Mascola J, Polakowski L, Ledgerwood J, Graham BS, Bennett H, Pajon R, Knightly C, Leav B, Deng W, Zhou H, Han S, Ivarsson M, Miller J, Zaks T; COVE Study Group. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N Engl J Med. 2021;384:403-416. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7073] [Cited by in RCA: 7568] [Article Influence: 1892.0] [Reference Citation Analysis (1)] |
| 5. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10701] [Article Influence: 2140.2] [Reference Citation Analysis (1)] |
| 6. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3551] [Cited by in RCA: 3439] [Article Influence: 859.8] [Reference Citation Analysis (0)] |
| 7. | Hayashi PH, Lucena MI, Fontana RJ, Bjornsson ES, Aithal GP, Barnhart H, Gonzalez-Jimenez A, Yang Q, Gu J, Andrade RJ, Hoofnagle JH. A revised electronic version of RUCAM for the diagnosis of DILI. Hepatology. 2022;76:18-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 88] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 8. | Mungmunpuntipantip R, Wiwanitkit V. Letter to the editor: "Autoimmune hepatitis after COVID-19 vaccination". Hepatology. 2022;75:756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Palla P, Vergadis C, Sakellariou S, Androutsakos T. Letter to the editor: Autoimmune hepatitis after COVID-19 vaccination: A rare adverse effect? Hepatology. 2022;75:489-490. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 10. | Bril F, Al Diffalha S, Dean M, Fettig DM. Autoimmune hepatitis developing after coronavirus disease 2019 (COVID-19) vaccine: Causality or casualty? J Hepatol. 2021;75:222-224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 11. | Liaskou E, Hirschfield GM, Gershwin ME. Mechanisms of tissue injury in autoimmune liver diseases. Semin Immunopathol. 2014;36:553-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 12. | Lammert C, Chalasani SN, Atkinson EJ, McCauley BM, Lazaridis KN. Environmental risk factors are associated with autoimmune hepatitis. Liver Int. 2021;41:2396-2403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Watad A, Bragazzi NL, McGonagle D, Adawi M, Bridgewood C, Damiani G, Alijotas-Reig J, Esteve-Valverde E, Quaresma M, Amital H, Shoenfeld Y. Autoimmune/inflammatory syndrome induced by adjuvants (ASIA) demonstrates distinct autoimmune and autoinflammatory disease associations according to the adjuvant subtype: Insights from an analysis of 500 cases. Clin Immunol. 2019;203:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 14. | van Gemeren MA, van Wijngaarden P, Doukas M, de Man RA. Vaccine-related autoimmune hepatitis: the same disease as idiopathic autoimmune hepatitis? Scand J Gastroenterol. 2017;52:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Pellegrino P, Carnovale C, Pozzi M, Antoniazzi S, Perrone V, Salvati D, Gentili M, Brusadelli T, Clementi E, Radice S. On the relationship between human papilloma virus vaccine and autoimmune diseases. Autoimmun Rev. 2014;13:736-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 16. | Wraith DC, Goldman M, Lambert PH. Vaccination and autoimmune disease: what is the evidence? Lancet. 2003;362:1659-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 17. | Segal Y, Shoenfeld Y. Vaccine-induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15:586-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 288] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Xu Z, Wang P, Li XM, Shuai ZW, Ye DQ, Pan HF. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165:386-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 323] [Article Influence: 80.8] [Reference Citation Analysis (0)] |
| 19. | Chow KW, Pham NV, Ibrahim BM, Hong K, Saab S. Autoimmune Hepatitis-Like Syndrome Following COVID-19 Vaccination: A Systematic Review of the Literature. Dig Dis Sci. 2022;67:4574-4580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 20. | Roy A, Verma N, Singh S, Pradhan P, Taneja S, Singh M. Immune-mediated liver injury following COVID-19 vaccination: A systematic review. Hepatol Commun. 2022;6:2513-2522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Efe C, Kulkarni AV, Terziroli Beretta-Piccoli B, Magro B, Stättermayer A, Cengiz M, Clayton-Chubb D, Lammert C, Bernsmeier C, Gül Ö, la Tijera FH, Anders M, Lytvyak E, Akın M, Purnak T, Liberal R, Peralta M, Ebik B, Duman S, Demir N, Balaban Y, Urzua Á, Contreras F, Venturelli MG, Bilgiç Y, Medina A, Girala M, Günşar F, Londoño MC, Androutsakos T, Kisch A, Yurci A, Güzelbulut F, Çağın YF, Avcı E, Akyıldız M, Dindar-Demiray EK, Harputluoğlu M, Kumar R, Satapathy SK, Mendizabal M, Silva M, Fagiuoli S, Roberts SK, Soylu NK, Idilman R, Yoshida EM, Montano-Loza AJ, Dalekos GN, Ridruejo E, Schiano TD, Wahlin S. Liver injury after SARS-CoV-2 vaccination: Features of immune-mediated hepatitis, role of corticosteroid therapy and outcome. Hepatology. 2022;76:1576-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 72] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 22. | Alhumaid S, Al Mutair A, Rabaan AA, ALShakhs FM, Choudhary OP, Yong SJ, Nainu F, Khan A, Muhammad J, Alhelal F, Al Khamees MH, Alsouaib HA, Al Majhad AS, Al-Tarfi HR, ALyasin AH, Alatiyyah YY, Alsultan AA, Alessa ME, Alissa MA, Alsayegh EH, Alshakhs HN, Al Samaeel HA, AlShayeb RA, Alnami DA, Alhassan HA, Alabdullah AA, Alhmed AH, AlDera FH, Hajissa K, Al-Tawfiq JA, Al-Omari A. New-onset and relapsed liver diseases following COVID-19 vaccination: a systematic review. BMC Gastroenterol. 2022;22:433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 23. | Trougakos IP, Terpos E, Alexopoulos H, Politou M, Paraskevis D, Scorilas A, Kastritis E, Andreakos E, Dimopoulos MA. Adverse effects of COVID-19 mRNA vaccines: the spike hypothesis. Trends Mol Med. 2022;28:542-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 164] [Article Influence: 54.7] [Reference Citation Analysis (0)] |
| 24. | Pelka K, Shibata T, Miyake K, Latz E. Nucleic acid-sensing TLRs and autoimmunity: novel insights from structural and cell biology. Immunol Rev. 2016;269:60-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 25. | Karikó K, Muramatsu H, Welsh FA, Ludwig J, Kato H, Akira S, Weissman D. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol Ther. 2008;16:1833-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1401] [Cited by in RCA: 1228] [Article Influence: 72.2] [Reference Citation Analysis (0)] |
| 26. | Teijaro JR, Farber DL. COVID-19 vaccines: modes of immune activation and future challenges. Nat Rev Immunol. 2021;21:195-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 532] [Article Influence: 133.0] [Reference Citation Analysis (0)] |
| 27. | Matyushkina D, Shokina V, Tikhonova P, Manuvera V, Shirokov D, Kharlampieva D, Lazarev V, Varizhuk A, Vedekhina T, Pavlenko A, Penkin L, Arapidi G, Pavlov K, Pushkar D, Kolontarev K, Rumyantsev A, Rumyantsev S, Rychkova L, Govorun V. Autoimmune Effect of Antibodies against the SARS-CoV-2 Nucleoprotein. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, Cao Y, Yousif AS, Bals J, Hauser BM, Feldman J, Muus C, Wadsworth MH 2nd, Kazer SW, Hughes TK, Doran B, Gatter GJ, Vukovic M, Taliaferro F, Mead BE, Guo Z, Wang JP, Gras D, Plaisant M, Ansari M, Angelidis I, Adler H, Sucre JMS, Taylor CJ, Lin B, Waghray A, Mitsialis V, Dwyer DF, Buchheit KM, Boyce JA, Barrett NA, Laidlaw TM, Carroll SL, Colonna L, Tkachev V, Peterson CW, Yu A, Zheng HB, Gideon HP, Winchell CG, Lin PL, Bingle CD, Snapper SB, Kropski JA, Theis FJ, Schiller HB, Zaragosi LE, Barbry P, Leslie A, Kiem HP, Flynn JL, Fortune SM, Berger B, Finberg RW, Kean LS, Garber M, Schmidt AG, Lingwood D, Shalek AK, Ordovas-Montanes J; HCA Lung Biological Network. HCA Lung Biological Network. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell. 2020;181:1016-1035.e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1890] [Cited by in RCA: 1794] [Article Influence: 358.8] [Reference Citation Analysis (0)] |
| 29. | Bruno CD, Fremd B, Church RJ, Daly AK, Aithal GP, Björnsson ES, Larrey D, Watkins PB, Chow CR. HLA associations with infliximab-induced liver injury. Pharmacogenomics J. 2020;20:681-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Hassan N, Siddiqui AR, Abbas Z, Hassan SM, Soomro GB, Mubarak M, Anis S, Muzaffar R, Zafar MN. Clinical Profile and HLA Typing of Autoimmune Hepatitis From Pakistan. Hepat Mon. 2013;13:e13598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 31. | Murota M, Nishioka M, Fujita J, Dobashi N, Wu F, Ohtsuki Y, Hojo S, Takahara J, Kuriyama S. Anti-cytokeratin antibodies in sera of the patients with autoimmune hepatitis. Clin Exp Immunol. 2001;125:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Martin L, Bäurle A, Fiehn W, Volkmann M. Immunoreactivity to cytokeratin 8/18 in patients with soluble liver antigen (SLA) positive autoimmune hepatitis (AIH type 3) is not sufficient for diagnostic use. Clin Lab. 2000;46:339-344. [PubMed] |
| 33. | Londoño MC, Gratacós-Ginès J, Sáez-Peñataro J. Another case of autoimmune hepatitis after SARS-CoV-2 vaccination - still casualty? J Hepatol. 2021;75:1248-1249. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 75] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 34. | Kowarz E, Krutzke L, Külp M, Streb P, Larghero P, Reis J, Bracharz S, Engler T, Kochanek S, Marschalek R. Vaccine-induced COVID-19 mimicry syndrome. Elife. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 35. | Watanabe Y, Mendonça L, Allen ER, Howe A, Lee M, Allen JD, Chawla H, Pulido D, Donnellan F, Davies H, Ulaszewska M, Belij-Rammerstorfer S, Morris S, Krebs AS, Dejnirattisai W, Mongkolsapaya J, Supasa P, Screaton GR, Green CM, Lambe T, Zhang P, Gilbert SC, Crispin M. Native-like SARS-CoV-2 Spike Glycoprotein Expressed by ChAdOx1 nCoV-19/AZD1222 Vaccine. ACS Cent Sci. 2021;7:594-602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 119] [Cited by in RCA: 102] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 36. | Hsieh SM, Liu MC, Chen YH, Lee WS, Hwang SJ, Cheng SH, Ko WC, Hwang KP, Wang NC, Lee YL, Lin YL, Shih SR, Huang CG, Liao CC, Liang JJ, Chang CS, Chen C, Lien CE, Tai IC, Lin TY. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir Med. 2021;9:1396-1406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 37. | Zhang N, Li K, Liu Z, Nandakumar KS, Jiang S. A Perspective on the Roles of Adjuvants in Developing Highly Potent COVID-19 Vaccines. Viruses. 2022;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Cerpa-Cruz S, Paredes-Casillas P, Landeros Navarro E, Bernard-Medina AG, Martínez-Bonilla G, Gutiérrez-Ureña S. Adverse events following immunization with vaccines containing adjuvants. Immunol Res. 2013;56:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 39. | Truong B, Allegri G, Liu XB, Burke KE, Zhu X, Cederbaum SD, Häberle J, Martini PGV, Lipshutz GS. Lipid nanoparticle-targeted mRNA therapy as a treatment for the inherited metabolic liver disorder arginase deficiency. Proc Natl Acad Sci USA. 2019;116:21150-21159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 40. | Moghimi SM. Allergic Reactions and Anaphylaxis to LNP-Based COVID-19 Vaccines. Mol Ther. 2021;29:898-900. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 41. | Igyártó BZ, Jacobsen S, Ndeupen S. Future considerations for the mRNA-lipid nanoparticle vaccine platform. Curr Opin Virol. 2021;48:65-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 42. | Pardi N, Hogan MJ, Naradikian MS, Parkhouse K, Cain DW, Jones L, Moody MA, Verkerke HP, Myles A, Willis E, LaBranche CC, Montefiori DC, Lobby JL, Saunders KO, Liao HX, Korber BT, Sutherland LL, Scearce RM, Hraber PT, Tombácz I, Muramatsu H, Ni H, Balikov DA, Li C, Mui BL, Tam YK, Krammer F, Karikó K, Polacino P, Eisenlohr LC, Madden TD, Hope MJ, Lewis MG, Lee KK, Hu SL, Hensley SE, Cancro MP, Haynes BF, Weissman D. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J Exp Med. 2018;215:1571-1588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 382] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 43. | Ndeupen S, Qin Z, Jacobsen S, Estanbouli H, Bouteau A, Igyártó BZ. The mRNA-LNP platform's lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. bioRxiv. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 44. | Mauro AG, Bonaventura A, Vecchié A, Mezzaroma E, Carbone S, Narayan P, Potere N, Cannatà A, Paolini JF, Bussani R, Montecucco F, Sinagra G, Van Tassel BW, Abbate A, Toldo S. The Role of NLRP3 Inflammasome in Pericarditis: Potential for Therapeutic Approaches. JACC Basic Transl Sci. 2021;6:137-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 60] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 45. | Guo C, Fu R, Wang S, Huang Y, Li X, Zhou M, Zhao J, Yang N. NLRP3 inflammasome activation contributes to the pathogenesis of rheumatoid arthritis. Clin Exp Immunol. 2018;194:231-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 217] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 46. | Luan J, Zhang X, Wang S, Li Y, Fan J, Chen W, Zai W, Wang Y, Chen M, Meng G, Ju D. NOD-Like Receptor Protein 3 Inflammasome-Dependent IL-1β Accelerated ConA-Induced Hepatitis. Front Immunol. 2018;9:758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 103] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 47. | Oberhardt V, Luxenburger H, Kemming J, Schulien I, Ciminski K, Giese S, Csernalabics B, Lang-Meli J, Janowska I, Staniek J, Wild K, Basho K, Marinescu MS, Fuchs J, Topfstedt F, Janda A, Sogukpinar O, Hilger H, Stete K, Emmerich F, Bengsch B, Waller CF, Rieg S, Sagar, Boettler T, Zoldan K, Kochs G, Schwemmle M, Rizzi M, Thimme R, Neumann-Haefelin C, Hofmann M. Rapid and stable mobilization of CD8(+) T cells by SARS-CoV-2 mRNA vaccine. Nature. 2021;597:268-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 288] [Cited by in RCA: 279] [Article Influence: 69.8] [Reference Citation Analysis (0)] |
| 48. | Boettler T, Csernalabics B, Salié H, Luxenburger H, Wischer L, Salimi Alizei E, Zoldan K, Krimmel L, Bronsert P, Schwabenland M, Prinz M, Mogler C, Neumann-Haefelin C, Thimme R, Hofmann M, Bengsch B. SARS-CoV-2 vaccination can elicit a CD8 T-cell dominant hepatitis. J Hepatol. 2022;77:653-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 91] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 49. | Bowen DG, Warren A, Davis T, Hoffmann MW, McCaughan GW, Fazekas de St Groth B, Bertolino P. Cytokine-dependent bystander hepatitis due to intrahepatic murine CD8 T-cell activation by bone marrow-derived cells. Gastroenterology. 2002;123:1252-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 70] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 50. | Belz GT, Altman JD, Doherty PC. Characteristics of virus-specific CD8(+) T cells in the liver during the control and resolution phases of influenza pneumonia. Proc Natl Acad Sci USA. 1998;95:13812-13817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 424] [Article Influence: 84.8] [Reference Citation Analysis (0)] |
| 52. | Bernal W, Ma Y, Smith HM, Portmann B, Wendon J, Vergani D. The significance of autoantibodies and immunoglobulins in acute liver failure: a cohort study. J Hepatol. 2007;47:664-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 77] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 53. | Febres-Aldana CA, Alghamdi S, Krishnamurthy K, Poppiti RJ. Liver Fibrosis Helps to Distinguish Autoimmune Hepatitis from DILI with Autoimmune Features: A Review of Twenty Cases. J Clin Transl Hepatol. 2019;7:21-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 54. | Suzuki A, Brunt EM, Kleiner DE, Miquel R, Smyrk TC, Andrade RJ, Lucena MI, Castiella A, Lindor K, Björnsson E. The use of liver biopsy evaluation in discrimination of idiopathic autoimmune hepatitis versus drug-induced liver injury. Hepatology. 2011;54:931-939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 276] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 55. | Weiler-Normann C, Schramm C. Drug induced liver injury and its relationship to autoimmune hepatitis. J Hepatol. 2011;55:747-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 56. | Hofer H, Oesterreicher C, Wrba F, Ferenci P, Penner E. Centrilobular necrosis in autoimmune hepatitis: a histological feature associated with acute clinical presentation. J Clin Pathol. 2006;59:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 91] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM; American Association for the Study of Liver Diseases. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1039] [Cited by in RCA: 1010] [Article Influence: 67.3] [Reference Citation Analysis (0)] |
| 58. | Björnsson ES, Bergmann OM, Björnsson HK, Kvaran RB, Olafsson S. Incidence, presentation, and outcomes in patients with drug-induced liver injury in the general population of Iceland. Gastroenterology. 2013;144:1419-1425, 1425.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 535] [Cited by in RCA: 583] [Article Influence: 48.6] [Reference Citation Analysis (0)] |
| 59. | Wong CKH, Mak LY, Au ICH, Lai FTT, Li X, Wan EYF, Chui CSL, Chan EWY, Cheng WY, Cheng FWT, Yuen MF, Wong ICK. Risk of acute liver injury following the mRNA (BNT162b2) and inactivated (CoronaVac) COVID-19 vaccines. J Hepatol. 2022;77:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 38] [Article Influence: 12.7] [Reference Citation Analysis (0)] |