Peer-review started: September 13, 2022
First decision: October 3, 2022
Revised: October 19, 2022
Accepted: December 6, 2022
Article in press: December 6, 2022
Published online: January 25, 2023
Processing time: 126 Days and 11.3 Hours
Coronavirus disease 2019 (COVID-19) has affected patients with pre-existing chronic liver disease (CLD) in various ways. The maximum impact was seen on patients with underlying cirrhosis who have shown to have poor clinical outcomes in the form of increased risk of hepatic decompensation, acute-on-chronic liver failure, and even mortality. It is of paramount importance to identify various factors which are associated with unfavorable outcomes for prognostication and making informed management strategy. Many factors have been evaluated in different studies in patients with underlying CLD. Some of these factors include the severity of underlying chronic liver disease, comorbid conditions, age, and severity of COVID-19. Overall, the outcomes are not fav-orable in patients with cirrhosis as evidenced by data from various studies. The main purpose of this review is to identify the predictors of adverse clinical outcomes including mortality in patients with CLD for risk stratification, prognostication, and appropriate clinical management.
Core Tip: Coronavirus disease 2019 (COVID-19) has been shown to negatively affect the outcomes of patients with chronic liver disease. Some of the major factors predicting poor outcomes and mortality as shown by various studies include old age (> 60 years) and presence of comorbidities like diabetes, hypertension, and obesity. Apart from these, the most important outcome measure is the severity of underlying chronic liver disease and in some cases the etiology of chronic liver disease. Another major predictor of outcome is the severity of COVID-19, with respiratory failure being a common cause of mortality. Further data is required to draw a definitive relation between these risk factors and outcomes in these patients.
- Citation: Walia D, Saraya A, Gunjan D. COVID-19 in patients with pre-existing chronic liver disease – predictors of outcomes. World J Virol 2023; 12(1): 30-43
- URL: https://www.wjgnet.com/2220-3249/full/v12/i1/30.htm
- DOI: https://dx.doi.org/10.5501/wjv.v12.i1.30
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)–related coronavirus disease 2019 (COVID-19) has wreaked havoc since its outbreak in late 2019. As per the most recent estimates, it has affected more than 575 million people worldwide[1]. It has adversely affected the health care system even in developed nations. Although the most common manifestations of COVID-19 are either asymptomatic infection or mildly symptomatic infection with fever, cough, and generalized weakness, some patients developed severe respiratory failure requiring mechanical ventilation and even death[2].
Patients with chronic liver disease (CLD) have been affected due to the COVID-19 pandemic, such as lack of routine services like variceal and hepatocellular carcinoma (HCC) screening, lack of physical follow-up to monitor the response to treatment like ascites, re-allocation of health care facilities for COVID-19 management, etc. An acute insult in the form of COVID-19 in a background of CLD may lead to further decompensation and increased morbidity and mortality. Therefore, in this review, we will summarize and identify the predictors of adverse outcomes in patients with CLD, which will help in prognostication, risk stratifying, and providing optimal care to the patients.
COVID-19 is a systemic disease affecting multiple organ systems and gastrointestinal system (GI) involvement may be seen in a subset of patients. Studies have shown that nearly 20% of the affected patients had some abnormalities in liver function as reflected by elevated liver enzymes (20%) and elevated bilirubin (16%)[3]. About 35% of patients showed abnormal alanine aminotransferase or bilirubin levels, out of which 77% showed elevation to levels less than 5 times the upper limit of normal[4]. The prevalence of underlying CLD in COVID-19 patients was 3%-6.3% in various studies[5-8]. A meta-analysis of 73 studies with 24299 patients, showed that the prevalence of CLD in COVID-19 positive patients was 3%, which was similar to that among COVID-19 negative patients[3]. The differences in prevalence might be due to admission bias, sampling bias, and retrospective nature of studies.
A recent study showed that mortality rates among alcoholic liver disease (ALD) and non-alcoholic fatty liver disease (NAFLD) affected patients increased significantly during the pandemic while the rate of mortality among patients with viral hepatitis remained similar to the pre-pandemic times[9]. A recent meta-analysis of 40 studies has shown that patients with CLD had a significantly higher risk of severe COVID-19 (pooled odds ratio [OR]: 2.44) and death (pooled OR: 2.35) as compared to COVID-19 patients without[10]. Hence, COVID-19 does affect patients with CLD and in some cases with adverse outcomes.
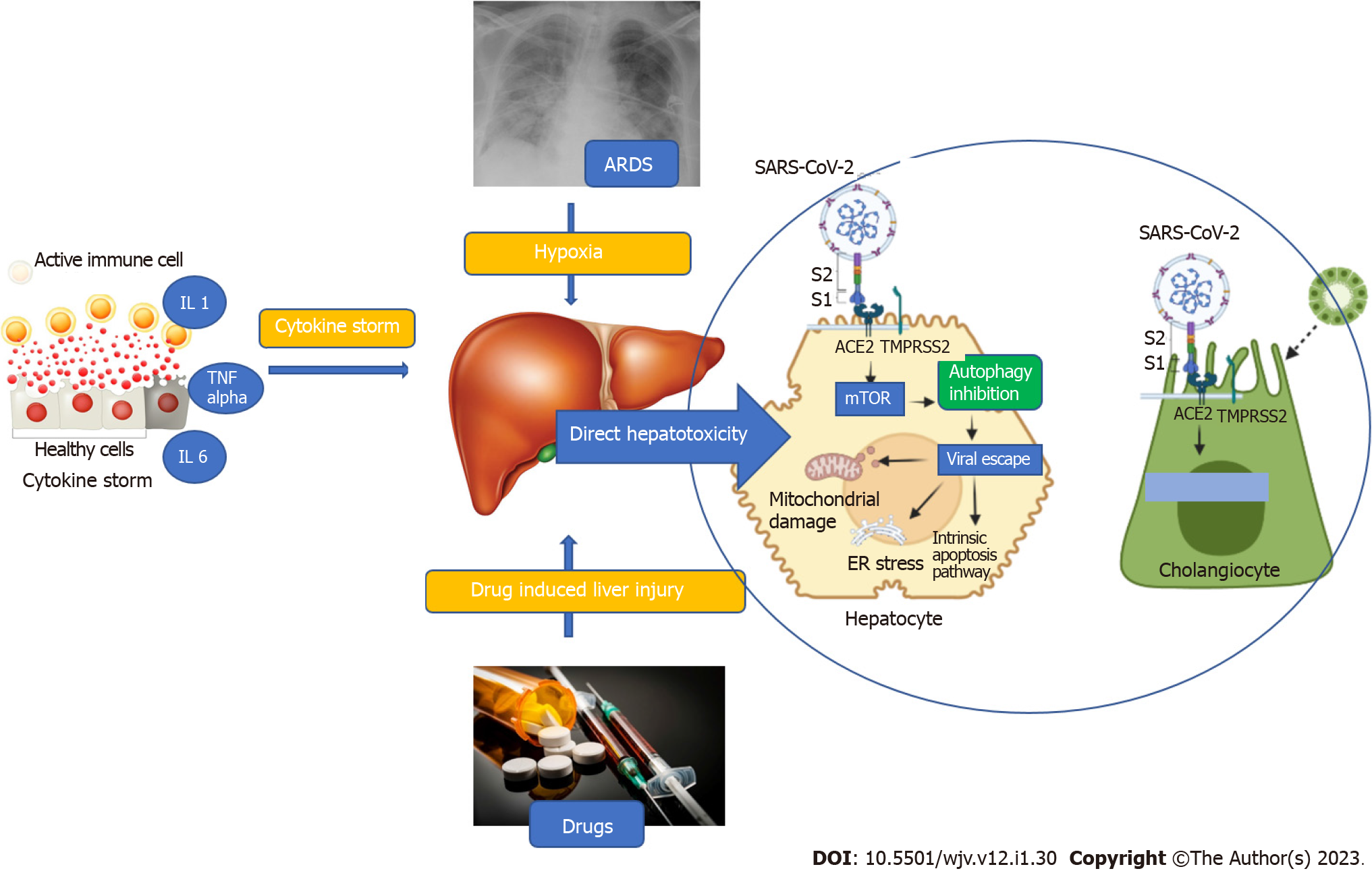
The liver involvement in COVID-19 is multifactorial, including direct viral hepatotoxicity, immune-mediated liver injury, sepsis, hypoxemia, or drug-induced liver injury[11]. Direct hepatotoxicity is due to entry of SARS-CoV-2 into the liver through the binding of viral spike (S) protein to the angiotensin-converting enzyme 2 (ACE2) receptor on cholangiocytes. The expression of ACE2 is highest in cholangiocytes followed by sinusoidal endothelial cells and hepatocytes as shown in healthy livers by single cell RNA sequencing methods[12]. After entering the cell, the S protein is primed by a specialized serine protease, transmembrane serine protease 2 (TMPRSS2), in the host cell[13]. Once inside the cell, SARS-CoV-2 causes activation of the mTOR pathway which inhibits autophagy of the viral particles. Thus, the viral particles evade the immune system and increase in number, exerting direct hepatotoxicity via mitochondrial dysfunction, ER stress, and activation of the intrinsic pathway of apoptosis as depicted in Figure 1[14].
The second hit in the pathogenesis of liver injury is secondary damage caused by cytokine storm. Infection with SARS-CoV-2 has been postulated to cause a massive surge in proinflammatory cytokine levels. The predominant cytokines implicated include interleukin (IL)-1, IL-6, tumor necrosis factor (TNF)-α, and elevated levels of ferritin and CRP[15]. These proinflammatory cytokines result in cholestatic liver injury by causing downregulation of proteins and channels involved in uptake and secretion of bilirubin and bile salts similar to what is seen in sepsis-related cholestasis. Another postulated mechanism is decreased albumin synthesis due to IL-6 mediated suppression of C/EBP pathway[14]. TNF-α and IL-1 also activate and recruit macrophages to the liver and induce apoptosis of hepatocytes. These inflammatory cytokines also cause hypoxic liver injury by causing endothelial damage and inducing microvascular thrombosis[14]. Thus, various cytokines act in concert to cause liver injury reflected as biochemical alterations in liver function. Other mechanisms include hypoxemic injury secondary to type 1 hypoxemic respiratory failure and drug induced liver injury.
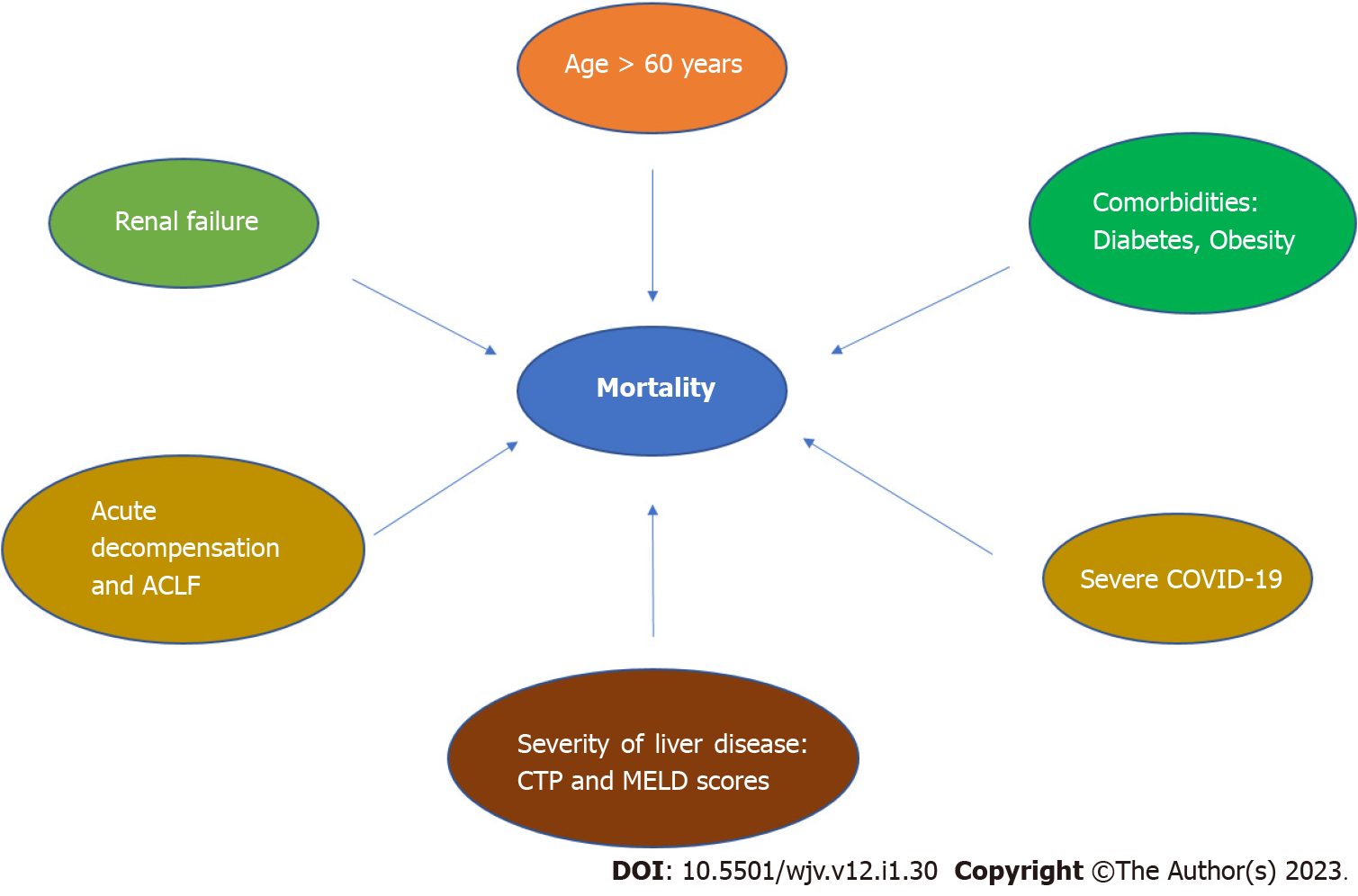
Numerous studies across the globe have tried to identify the predisposing factors for poor outcomes of COVID-19 in patients with CLD as summarized in Figure 2 and Table 1. A summary of evidence available and the possible risk factors has been enumerated below.
| Ref. | Type | Clinical outcomes | Predictors of outcomes |
| Iavarone et al[53] | Multicentric retrospective study of 50 cirrhotics | ACLF and de novo acute liver injury: 28%; 30-d mortality: 34% | Predictors of mortality: CLIF-OF (HR: 1.426); Moderate/severe respiratory failure (HR: 1.608) |
| Marjot et al[22] | Retrospective data from United Kingdom hospital network including 745 patients with CLD (386 with and 359 without cirrhosis) | Acute hepatic decompensation: 46%; ACLF: 50%; Mortality in cirrhosis, ACLF, and non-cirrhotics: 32%, 65%, and 8% | Predictors of mortality: ALD (OR: 1.79); Child-Pugh class: Child-Pugh A +2.0%, Child-Pugh B +20.0%, Child-Pugh C +38.1%. Predictors of decompensation: Child-Pugh class |
| Ge et al[16] | Data from the National COVID Cohort Collaborative (N3C) dataset of 6.4 million cases | 3.31 times adjusted hazard of death in cirrhotics at 30 d than non-cirrhotics | Predictors of 30-d mortality: Age (aHR: 1.05 per year); Hispanic ethnicity (aHR: 1.20); Chronic hepatitis C (aHR: 1.27); ALD (aHR: 1.40); Modified CCI (aHR: 1.07 per point) |
| Elhence et al[24] | Retrospective analysis of 221 cirrhosis patients | Compensated cirrhosis: 8.1%; Acute decompensation: 62.9%; ACLF: 29.0%; MODS: 55.6%; Type 1 respiratory failure: 20.0%; Sudden cardiac arrest: 6.7%; GI bleeding: 3.3% | Predictors of mortality: Higher TLC [HR: 1.054]; Elevated creatinine [HR: 1.184]; MELD score [HR: 1.038]; Alkaline phosphatase [HR: 1.003]; COVID-19 severity [HR: 2.573]; ACLF on presentation (HR: 2.573) |
| Xiao et al[52] | Medical records collected from 23 Chinese hospitals | Decompensated cirrhosis: 57.5%; Mortality: 28.9% | Factors associated with mortality: Child-Pugh class (OR: 5.71); CURB65 (OR: 5.88) |
| Grgurevic et al[48] | 4014 patients | Four times higher risk of 30-d mortality in cirrhosis | Predictor of 30-d mortality: Cirrhosis (HR: 2.95) |
| Mendizabal et al[17] | Prospective cohort of 96 cirrhosis patients | Mortality in cirrhotic: 47% vs 16% in non-cirrhotics; Acute decompensation: 61.4%; ACLF: 55.2% | Factors associated with mortality: Age > 65 yr (OR: 7.2); Male gender (OR: 1.8); BMI > 30 (OR: 1.7); Cirrhosis (OR: 3.1) |
| Kim et al[18] | Multicentre observational cohort study in 21 institutes in United States with 867 CLD cases (227 with cirrhosis) | Mortality: 25%; Hepatic decompensation: 7.7%; Hepatic encephalopathy: 34.3%; Ascites: 16.4%; Variceal bleed: 10.4% | Predictors of all-cause mortality: ALD (HR: 2.42); Hepatic decompensation at baseline (HR: 2.91); HCC (HR: 3.31); Increasing age (HR:1.44 per 10 yr); Diabetes (HR: 1.59); Hypertension (HR:1.77); COPD (HR:1.77); Current smoking (HR: 2.48) |
| Sarin et al[37] | Retrospective data from 13 Asian countries with228 patients [185 CLD without cirrhosis and 43 with cirrhosis] | ACLF: 11.6%; Acute decompensation: 9%; Mortality rate: 43% among decompensated cirrhotics | Predictors of sever liver injury: In CLD without cirrhosis, diabetes [57.7% vs 39.7%, OR: 2.1 (1.1-3.7)]; In cirrhotics, obesity [64.3% vs 17.2%, OR: 8.1 (1.9-38.8). Predictor of mortality: CTP score of 9 or more at presentation [AUROC 0.94, HR:19.2] |
| Xiang et al[54] | Retrospective cohort study of 267 patients | Severe COVID-19: 15%; High-flow oxygen support: 14%; Mechanical ventilator support: 4%; Death: 1 | Predictor of severity: FIB-4 > 3.25 |
Age: Increasing age is associated with a blunted immune response and multiple comorbidities, and thus may have an impact on the outcomes in patients with pre-existing CLD. Age more than 60 years had an adjusted hazard ratio (aHR) of 1.05 for mortality in cirrhotics with COVID-19[16]. Another study showed that the mortality increased from 6.1% in patients aged less than 50 years to 33.9% among those who were more than 65 years with an adjusted OR (aOR) of 7.2 in this group of patients[17]. Therefore, increasing age especially more than 60 years has been associated with increased mortality and risk of liver decompensation[16-18].
Ethnicity: African Americans with CLD were twice more likely to develop COVID-19 than Caucasians in a study[19]. Another study found that non-Hispanic blacks and Hispanics had higher chances of contracting COVID-19 in patients with CLD; however, they did not find any difference in outcomes of disease in different ethnicities[20]. Hispanics had more severe COVID-19 infection in patients with CLD[18]. Henceforth, Hispanics and blacks have been shown to have higher risk of contracting COVID-19 disease and having a severe course likely due to lower socioeconomic status, poverty, overcrowding, and inadequate access to health care services.
Etiology of CLD: Several studies have tried to relate the etiology of CLD with outcomes in COVID-19 patients. In the following sections, the determinants of outcomes are described according to etiology of CLD.
Alcohol is one of the most common etiologies of CLD. The pandemic led to a situation of social isolation and unemployment which lead to an increased consumption of alcohol in higher quantities[21]. A study found a higher rate of mortality among alcohol-related liver disease with an aHR of 1.79[22]. Kim et al[18] showed an aHR of 2.42 of mortality among alcohol-related liver disease. There was a three times increase in the monthly percent change of crude ALD-related mortality after February 2020 as compared to January 2017 to December 2017 in one study[23]. However, another study did not find alcohol as a poor outcome variable on multivariate analysis of retrospective data[24,25]. A recent study showed that the mortality among ALD patients was declining in the pre-pandemic era but increased fivefold during the pandemic[9]. Therefore, it seems that alcohol as an etiology increases the risk of adverse outcomes and mortality[26] in patients with COVID-19 and CLD as summarized in Table 2 and Suppl
| Ref. | Study | Outcomes |
| Deutsch-Link et al[23] | Retrospective analysis - pre (January 2017 to December 2017) and post-COVID era (February 2020) | Increase in the monthly percent change of crude ALD-related mortality: Males: 3.18 vs 0.96; Females: 3.8 vs 1.18 |
| Yeo et al[26] | 16813 patients with ALD before and 11625 during the pandemic | OR of death in ALD - 18.7 during the pandemic vs 0.995 in the pre-pandemic era |
NAFLD is rapidly becoming the most common cause of CLD across the world. It is considered to be the hepatic manifestation of metabolic syndrome and usually coexists with other components of metabolic syndrome. The COVID-19 pandemic showed a bidirectional relationship with NAFLD. Lockdown during the pandemic and lack of exercise lead to an increase in sedentary behavior and thus metabolic syndrome including NAFLD. Such patients had a more severe COVID-19 infection as evidenced by higher requirement of oxygen, mechanical ventilation, and prolonged intensive care unit (ICU) stay[27-30] (Table 3). Studies have demonstrated that patients with features of metabolic syndrome including higher body mass index (BMI) and waist circumference and presence of diabetes and hypertension had adverse effects on outcomes[29-31].
| Ref. | Type of study | Patients included | Outcomes | Predictors |
| Chang et al[27] | Retrospective study | 3122 COVID-19 cases [FLI (fatty liver index) was calculated] | Severe disease: 223 (7.14%); Mechanical ventilation: 82 (2.63%); ICU admission: 126 (4.04%) High-flow oxygen therapy: 75 (2.40%); Death: 94 (3.01%) | FLI associated with severe complications from COVID-19 (aOR: 1.77) |
| Vrsaljko et al[28] | Prospective observational study | 120 NAFLD patients (of 216 COVID-19 patients) | Patients with NAFLD had more high-flow nasal cannula or non-invasive ventilation (21.66%, vs 10.42%), longer duration of hospitalization (10 d vs 9 d), and more pulmonary thromboembolism risk (26.66% vs 13.54%) | Delayed time to recovery (HR: 0.64); Increased pulmonary thrombosis (OR: 2.15) among NAFLD patients |
| Velazquez et al[29] | Retrospective cohort study | 359 NAFLD patients as per Dallas steatosis index (DSI) out of total 470 cases | Lower oxygen saturation levels; Higher D-dimer; Elevated LDH; Higher lymphocyte count among NAFLD | On multivariable analysis, NAFLD is a predictor of mortality (OR: 2.13) |
| Madan et al[36] | Retrospective observational case control study | 289 NAFLD patients among 446 cases | Similar in-hospital mortality, ICU requirement, ventilatory support, and duration of ICU and hospital stay | Predictors of in-hospital mortality: High total leukocyte count (OR: 1.082); High FIB-4 (OR: 1.606) |
| Chen et al[34] | Retrospective single centre cohort study | 172 patients with hepatic steatosis (HS) among 342 cases | 19% of patients expired; > 50% required ICU admission | Increased intubation (aOR: 2.75); Vasopressor requirements (aOR: 1.22); ALT > 5 x ULN (aOR: 7.09) |
| Sarin et al[37] | Retrospective multinational cohort | 113 NAFLD cases out of 228 cases (185 without cirrhosis and 43 with cirrhosis) | Higher risk of acute liver injury in obese cirrhotics vs normal weight patients (OR: 8.9) | Higher risk of liver injury: In non-cirrhotics, diabetes [57.7% vs 39.7%, OR: 2.1]; In cirrhotics, obesity, [64.3% vs 17.2%, OR: 8.1] |
| Li et al[31] | Observational study | Genome-wide meta-analysis (GWMA) of 3711 NAFLD cases and 426252 controls from United Kingdom Biobank data | No significant association of NAFLD and severe COVID-19 after adjusting for confounders | Predictors of severity: Body mass index (OR: 1.73); Waist circumference (OR: 1.76); Hip circumference (OR: 1.33) |
| Yao et al[30] | Retrospective study in China | 86 COVID-19 patients with NAFLD | NAFLD patients with advanced fibrosis (NFS > -1.5) had more fever (81.6% vs 50%), shortness of breath (18.4% vs 0%), and severe disease (28.9% vs 2.1) | Predictors of severe disease: Diabetes (OR: 8.264); Advanced liver fibrosis [NFS > -1.5] (OR: 11.057) |
| Targher et al[35] | Retrospective study | 94 NAFLD cases among 310 patients | Factors associated with severity: Increasing FIB-4 (aOR: 1.90); Increasing NFS (aOR: 2.57) |
Patients with COVID-19 and underlying NAFLD have multiple associated comorbidities including diabetes, hypertension, dyslipidemia, and obesity. These factors have been independently associated with poor outcomes in patients with COVID-19[32,33]. Different studies have estimated different prevalence of comorbidities in NAFLD. The prevalence of obesity, diabetes mellitus, and hypertension was 47%, 27%, and 31%, respectively, in NAFLD patients in a study, in which 27% of patients required non-invasive mechanical ventilation, 44% required ICU admission, and 27% were expired[29]. Another study showed that 69% of patients had hypertension, 43% had diabetes, 47% had dyslipidemia, 85% were overweight, and 52% were obese, and out of total 342 patients, > 50% required ICU admission and 19% were expired[34]. Thus, the presence of comorbidities is associated with poor outcomes in patients with NAFLD and COVID-19.
Advanced fibrosis in patients with NAFLD was associated with more severe COVID-19 and adverse outcomes with an almost two-fold increased risk of severity in patients with an FIB-4 score of more than 2.67[35,36]. Another study showed that presence of cirrhosis with diabetes was associated with poor outcomes in COVID-19 patients with higher risk of liver injury (OR: 2) and NAFLD was the most common cause of CLD in this study[37]. The rate of severe illness was significantly higher in patients with advanced fibrosis [NAFLD fibrosis score (NFS) > -1.5] compared to those with non-advanced fibrosis with an NFS < -1.5 (28.9% vs 2.1%, P < 0.001)[30]. Therefore, the underlying degree of fibrosis in NAFLD patients and various components of metabolic syndrome has been associated with poor outcomes as compared to those without significant fibrosis.
Patients with chronic hepatitis B (CHB) had a higher rate of ICU admission (HR: 1.86) and increase risk of mortality (HR: 3.19) among hepatitis B virus “e” antigen positive (HBeAg+) CHB cases as shown in Table 4[8,38,39]. Another study showed that although the mortality rate was higher among patients with hepatitis B, it was not statistically significant after adjusting for other factors[40]. The study had shown that patients with COVID-19 had a lower positive rate for CHB[41,42]. One of the postulated reasons for this finding is that patients with CHB mount a reduced T cell mediated immune response termed as ‘immune exhaustion’ which may reduce the extent of cytokine storm seen in patients with COVID-19[41]. The major predictor of poor outcomes was positivity for HBeAg suggestive of active viral replication and ongoing liver injury in addition to liver injury inflicted by SARS-CoV-2[38]. Reactivation of hepatitis B virus (HBV) with anti-IL6 therapy (tocilizumab) was found to be 3.3% in a systematic review[43]. In short, patients with HBeAg positive CHB are more likely to have a poor outcome in terms of hospitalization requirement and mortality[44] and some specific treatment for COVID-19 will lead to reactivation of HBV like anti-IL6 antibody (tocilizumab).
| Ref. | Study | Patients | Results | Predictors |
| Yang et al[38] | Single centre retrospective study | Patients with HBV infection out of 2899 COVID patients. Resolved hepatitis B (n = 503); HBeAg (-) CHB/infection (n = 44); HBeAg (+) CHB/infection (n = 55); HBV reactivation (n = 6) | HBeAg (+) CHB/infection and HBV reactivation were associated with more abnormal liver function, severe disease, longer ICU stay, and death | Increased ICU admission (HR: 1.86) and mortality (HR: 3.19) in HBeAg (+) CHB/infection |
| Choe et al[40] | Nationwide population-based cohort study | 676 chronic HBV infection cases (19160 COVID-19 cases) | Mortality in HBV infected vs non-infected patients with COVID-19: 8.2% vs 13.5% | No difference in mortality, ICU admission, or organ failure |
| Wang et al[8] | Multicentre retrospective cohort study | 109 CHB and 327 non-CHB patients with COVID-19 | CHB vs non-CHB patients: Severe disease (27.5% vs 12.84%) and more dyspnoea (55.05% vs 43.12%) and mechanical ventilation requirement (22.49% vs 7.95%) in CHB | Increased mortality in CHB patients (OR: 3.748). Predictors of mortality: AST; ALT; ALP; Bilirubin; LDH; Elevated D-dimer. Protective effect: ALB (HR: 0.13); ALB/GLO (HR: 0.123) |
| Yip et al[44] | Retrospective cohort study | Current (353) and past HBV infection (359) out of total 5639 COVID cases | Mortality in current HBV vs past HBV vs non-HBV infection: 2.3% vs 5.8% vs 2.2% | Acute liver injury associated with mortality (aHR: 2.45), more than current (aHR: 1.29) or past (aHR: 0.90) HBV infection |
| Kang et al[42] | Nationwide cohort study | 7723 COVID-19 cases and 46231 controls | Lower SARS-CoV-2 positivity rate in CHB, after adjusting for comorbidities (aOR: 0.65) | Reduced SARS-CoV-2 positivity (aOR: 0.49) on antivirals |
| Liu et al[39] | Retrospective cohort study | 347 COVID-19 patients (21 vs 326 with or without chronic HBV infection) | Severe COVID-19 in 30% vs 31.4% in the HBV vs non-HBV group | Similar SARS-CoV-2 clearance and severe COVID-19 |
Hepatitis C predominantly causes a chronic indolent infection. Various management aspects of hepatitis C have been impacted during the COVID-19 pandemic. The impact of COVID-19 on chronic hepatitis C depends on the extent and severity of underlying CLD as discussed previously. A study by Ronderos et al[45] showed an increased mortality among hepatitis C virus (HCV)-infected patients, and increasing age, elevated D-dimer, ferritin, and FIB-4 score were identified as predictors on multivariate analysis. However, more data are required to draw a conclusion regarding the effect of HCV infection on COVID-19, excluding the severity of liver disease.
Autoimmune hepatitis (AIH) patients are a vulnerable group due to underlying liver disease, use of immunomodulators, and associated systemic diseases. Different studies have tried to identify the risk factors of severity and outcomes in these patients including those on immunosuppressants. Di Giorgio et al[46] demonstrated that the predictors of outcomes were same in AIH as in the general population, including increasing age and presence of comorbidities. Cirrhosis was the most important predictor of mortality among patients with underlying autoimmune liver diseases (OR: 17.46) in a study[47,48]. Among cirrhotics, outcomes worsened with progressive underlying liver dysfunction measured by increasing Child-Pugh-Turcotte (CTP) scores with an OR of mortality increasing from 42 to 69 in Child-Pugh classes B and C, respectively[49] (Table 5).
| Ref. | Study | Patients | Results | Predictors of outcomes |
| Efe et al[47] | Multicentre retrospective study from 34 centres in Europe and the Americas | 110 AIH patients | Acute liver injury: 37.1% | Predictor of severe COVID-19: cirrhosis (OR: 17.46); Immunosuppression not associated with severe COVID-19 (OR: 0.26) |
| Di Giorgio et al[46] | Phone based survey in tertiary centre | adult AIH patients: AIH (n = 97, 96%); PSC/AIH overlap (n = 2, 2%); PBC/AIH (n = 2, 2%); 4 patients had confirmed COVID | Severe COVID: 1; Death: 1 | No difference in risk factors of mortality |
| Marjot et al[49] | Retrospective data from three international registries | 70 AIH cases among 932 patients with CLD with COVID-19 | No differences between AIH and non-AIH related CLD in Hospitalization (76% vs 85%); ICU admission (29% vs 23%); Death (23% vs 20%) | Factors predicting mortality in AIH: Age (OR: 2.16/10 yr); Child-Pugh class [B (OR: 42.48) and C (OR: 69.30)] cirrhosis |
| Efe et al[50] | Retrospective data from 15 countries | 254 AIH patients | Hospitalization: 94 (37%); Death: 18 (7.1%) | Factors associated with COVID-19 severity: Systemic glucocorticoids (aOR: 4.73); Thiopurines (aOR: 4.78); Mycophenolate mofetil (aOR: 3.56); Tacrolimus (aOR: 4.09) |
The effect of immunosuppressive treatment on outcomes in COVID-19 patients has shown some diverging results. A study by Efe et al[50] in 254 AIH patients showed that systemic glucocorticoids (aOR: 4.73), thiopurines (aOR: 4.78), mycophenolate mofetil (MMF) (aOR: 3.56), and tacrolimus (aOR: 4.09) were associated with a more severe COVID-19 course. The study showed that outcomes were worse in patients on steroids at a prednisolone equivalent dose of > 5 mg/d. Similarly, another study showed that baseline treatment with steroids, thiopurines, MMF, and tacrolimus were associated with a severe disease course[51]. Therefore, patients with AIH having cirrhosis and stage of cirrhosis reflected by CTP score are predictors of adverse outcomes. The use of immunosuppressive drugs is also associated with worse outcomes among COVID-19 patients with AIH.
Severity of CLD: One of the most important determinants of clinical outcomes is the presence and severity of underlying cirrhosis as shown in various studies. There was an increasing risk of mortality with increasing CTP score ranging from additional +2% in Child-Pugh A to +20% in Child-Pugh B and +38% in Child-Pugh C. Similarly, another study showed that Child-Pugh score was associated with mortality[52]. CTP score of more than 9 was associated with a high mortality (HR: 19) in another study[37]. Mortality in cirrhotics increased with worsening chronic liver failure consortium score (HR: 1.42), which is an indicator of hepatic and extrahepatic organ failures[53]. Model for end stage liver disease (MELD) score more than 25 is associated with a two-fold increase in mortality as demonstrated by univariate analysis in a study from India[24]. Similarly, a study from Italy showed that MELD score more than 15 was associated with a higher mortality (HR: 5.18) at 30 d[53]. Various factors associated with adverse outcomes in cirrhosis are summarized in Table 1. Therefore, the currently available evidence suggests that increasing severity of underlying CLD is associated with poor outcomes including mortality.
Degree of liver fibrosis: A study from China showed that higher FIB-4 score, which is a marker of liver fibrosis, was associated with a more severe COVID-19 disease[36] with greater requirement of high flow oxygen, prolonged hospitalization, and even death[54]. They postulated that FIB-4 score could be a prognostic marker of disease outcomes but more data is required to increase external validity. Similar findings were seen in a meta-analysis which showed that elevated FIB-4 score was associated with severe COVID-19 and mortality[54]. Hence, the degree of liver fibrosis is an important determinant of disease outcome with higher degree of fibrosis being negatively associated with outcomes[55].
Comorbidities: Another predictor of mortality was the presence of comorbidities, with the most common being diabetes, obesity, dyslipidemia, and hypertension[16-18,37]. This may be due to a more severe COVID-19 infection seen in this subgroup of patients irrespective of the presence of cirrhosis. A prospective study showed that a BMI of more than 30 was associated with mortality[17]. Another study showed that diabetes and hypertension were predictors of mortality[18]. A study from Asia showed that diabetes was associated with severe liver injury without cirrhosis (OR: 2.1), as was obesity in cirrhotics (OR: 8.1)[37]. Therefore, the presence of comorbidities increases the severity of liver disease and has unfavorable outcomes.
Severity of COVID-19: Some studies showed that respiratory failure was the main cause of mortality among cirrhotic COVID-19 patients. Outcomes were poor for patients with higher CURB-65 (confusion, uremia, respiratory rate, blood pressure, age > 65) scores, substantiating the fact that respiratory failure was associated with mortality[44]. In this study, they observed that CURB-65 score was associated with a 5-fold increased risk of mortality[52]. Severe COVID-19 with respiratory failure was a significant predictor of mortality (HR: 2.5) in patients with chronic liver disease in another study[24]. Henceforth, more severe COVID-19 is associated with an increased risk of liver injury and mortality.
Biomarkers: A recent study evaluated the role of inflammatory biomarkers in risk-stratifying the patients with regard to liver injury and mortality in 221 COVID-19 patients[56]. They included CRP, IL6, D-dimer, and blood lymphocyte counts as inflammatory biomarkers, which were all significantly elevated in the patients who subsequently expired as compared to survivors. They found that patients who showed rising aspartate transaminase and alkaline phosphatase over time, as markers of liver injury, had a higher mortality. These correlations attenuated with age. Thus, inflammatory biomarkers may serve as predictors of poor outcomes, but more studies are required for identification of biomarkers and their validation.
Miscellaneous factors: Some other factors affecting the severity and outcomes of COVID-19 have also been seen in some studies. As discussed previously, obesity and physical inactivity have been associated with worse outcomes. Recently, it has been shown that obesity, physical inactivity, and diet rich in simple sugars predisposes to chronic low-grade inflammation in the mucosal barrier along with microbial dysbiosis. This state of chronic inflammation has been shown to be associated with worse clinical outcomes in COVID-19 patients[57]. Similarly, obese individuals with excessive visceral fat have excessive proinflammatory adipokines that have been postulated to be associated with poor outcomes[57]. Extrapolating the role of inflammation, patients with CLD have low level endotoxemia with increased gut permeability which may be associated with unfavorable outcomes; however, concrete evidence for the same is lacking[58]. A summary of risk factors associated with poor outcomes is shown in Table 6.
| Demographics | Etiology | Clinical parameters | Underlying disease severity | Biochemical parameters |
| Age > 60 yr; Hispanic and black ethnicity; Diabetes mellitus; Hypertension; Obesity | Alcohol; HBeAg positivity among CHB; AIH on immunosuppressants | Respiratory failure: CURB-65 score; Decompensation at baseline; ACLF at presentation | CTP score; MELD score; FIB-4 index | Elevated creatinine; Leucocytosis; AST levels; ALT levels; CRP |
In patients with CLD, acute decompensation and acute-on-chronic liver failure (ACLF) usually develop due to a precipitating factor, with infections being the most common such factor. COVID-19 may act as a trigger for such decompensation. Marjot et al[22] observed in their study that the major predictor of decompensation was Child-Pugh class, with a rate of decompensation of 30%, 56%, and 64% observed in Child-Pugh A, B, and C patients, respectively. Hepatic decompensation at baseline was associated with an increased mortality (HR: 2.91) in another study[18]. Acute decompensation developed in 9% and ACLF in 11.6% among 43 cirrhotic patients, and CTP score was the major predictor of mortality, with a CTP score of 9 or more at presentation associated with high mortality (HR, 19.2)[37]. Another study showed that acute decompensation developed in 62.9% and ACLF in 29% with a mortality as high as 72% among ACLF patients, with major predictors of mortality being MELD score, leukocytosis, elevated creatinine, and COVID-19 severity on multivariate analysis[24]. The mortality in grades 1, 2, and 3 ACLF patients was 56.3%, 50%, and 93.3%, respectively (P = 0.001) in the same study. In a prospective study of 96 cirrhotic patients, 61.4% developed acute decompensation and ACLF in 55% according to CLIF-C criteria. The major predictors of mortality were CLIF-C organ failure score (AUROC: 0.85) and MELD Na score (AUROC: 0.70)[17].
These observations suggest that SARS-CoV-2 infection may be a triggering factor for decompensation and subsequent ACLF in cirrhotic patients by triggering a pro-inflammatory cascade as discussed earlier. Possible factors that can be postulated could be a proinflammatory milieu, multi-organ dysfunction due to severe COVID-19, direct hepatotoxicity to a compromised liver, or sepsis. In summary, COVID-19 patients with underlying CLD are more prone to develop acute decompensation and may progress to ACLF. The major predictors of these outcomes are the baseline severity of liver disease reflected by CTP and MELD score, and the severity of hepatic and extra-hepatic organ failure as indicated by CLIF-C scores.
The data on the rate and risk factors of variceal bleed in patients with COVID-19 positive patients with underlying CLD is scarce. Upper gastrointestinal (UGI) bleeding developed in 24/1342 (1.8%) of all patients admitted with COVID-19[24]. Most of bleeding episodes (88%) were variceal bleeding in patients with cirrhosis with no rebleeding or death at 5 d with medical management alone[24]. The same group also observed that the initial control of UGI bleeding was achieved in all patients with no one requiring an emergency endoscopy. Thus, emphasizing the utility of conservative management of variceal bleeding with endoscopic therapy is only needed on a case-to-case basis[25].
Another study from Hong Kong showed that although peptic ulcer bleeding was the most common cause of UGI bleeding both before (66.0%) and during (66.1%) the COVID-19 pandemic, there was a significant increase in the proportion of patients with UGI bleeding with variceal bleeding after COVID-19 (5.3% vs 10.5%, P < 0.01)[51]. Patients had significantly lower hemoglobin (7.5 vs baseline 8.3 g/dL) and higher requirement for blood transfusion (64.5% vs baseline 50.4%) but had similar rates of all-cause mortality (6.9% vs 7.1%) and rebleeding (6.7% vs 5.1%)[59]. There was no significant difference in the timing of endoscopy after admission or the percentage of patients requiring endoscopic hemostasis (77.3% vs 76.3%) before and during the COVID-19 pandemic[59]. Thus, patients with variceal bleeding in the COVID-19 pandemic have similar management principles as the pre-COVID-19 era.
The major impact of the COVID-19 pandemic on patients with HCC was multifactorial. A decline of 26.7% in new HCC cases was reported during the pandemic compared to the pre-pandemic era[60]. Advanced BCLC stage and higher tumor burden at diagnosis were due to resource limitation and lack of physical appointments, and were associated with a higher incidence of spontaneous tumoral hemorrhage[61]. Delayed treatment initiation longer than 1 month (21.5% vs 9.5%; P < 0.001) due to re-allocation of services for the pandemic was reported[62]. Muñoz-Martínez et al[63] reported an increase in mortality rate proportional to advanced BCLC stage. Thus, the impact of COVID-19 on patients with HCC is predominantly due to delayed diagnosis, delayed presentation, delay in initiating treatment, and availability of imaging and locoregional or transplant facilities.
Some studies identified the impact of different waves of the pandemic on liver disease. The waves of COVID-19 occurred due to mutations and spread of newer variants of the virus that evaded the immune response. The second wave was predominantly caused by delta variant[64]. Nawghare et al[65] showed that the second wave had more number of acute decompensations and the factors predicting outcomes were renal dysfunction and elevated D-dimer. Elhence et al[24] compared outcomes in the first wave to those in the second wave and reported that although the disease severity was more during the second wave but the mortality rate and duration of hospital stay were similar with no significant differences.
COVID-19 has a major impact on patients with pre-existing CLD in the form of severe COVID infections and worsening of underlying hepatic disease. The predictors of poor outcomes of COVID-19 patients with underlying CLD are multiple and have been different in numerous studies across the globe, with the most important predictor being presence of cirrhosis with outcomes progressively deteriorating with increasing severity of underlying liver dysfunction estimated by CTP and MELD scores. These are the subgroups of patients who are more prone to risk of decompensation, further decompensation, and ACLF.
The predictors may be related to demographic factors, with increasing age and black and Hispanic ethnicity being associated with poor outcomes. Another major predictor of the severity of COVID-19 is cytokine storm, which may even lead to multiorgan failure, with the liver being one of the organs involved. Other predictors include the presence of comorbidities, whose prevalence is estimated to be around 30%-50% in various studies, and these have been associated with poor outcomes even in the absence of underlying liver disease. Major comorbidities found in studies that are negatively associated with outcomes include diabetes mellitus, hypertension, and obesity. The COVID-19 pandemic also adversely affected routine services for patients with hepatitis B, hepatitis C, and HCC, which will have long-term impacts in the form of increased disease burden, delayed implementation of eradication programs, and poor outcomes in the times yet to come.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Enichen E, United States; Jiang W, China S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | WHO Coronavirus (COVID-19) Dashboard. (accessed 2 August 2022). Available from: https://covid19.who.int. |
| 2. | Berlin DA, Gulick RM, Martinez FJ. Severe Covid-19. N Engl J Med. 2020;383:2451-2460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1027] [Article Influence: 205.4] [Reference Citation Analysis (0)] |
| 3. | Kovalic AJ, Satapathy SK, Thuluvath PJ. Prevalence of chronic liver disease in patients with COVID-19 and their clinical outcomes: a systematic review and meta-analysis. Hepatol Int. 2020;14:612-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Elmunzer BJ, Spitzer RL, Foster LD, Merchant AA, Howard EF, Patel VA, West MK, Qayed E, Nustas R, Zakaria A, Piper MS, Taylor JR, Jaza L, Forbes N, Chau M, Lara LF, Papachristou GI, Volk ML, Hilson LG, Zhou S, Kushnir VM, Lenyo AM, McLeod CG, Amin S, Kuftinec GN, Yadav D, Fox C, Kolb JM, Pawa S, Pawa R, Canakis A, Huang C, Jamil LH, Aneese AM, Glamour BK, Smith ZL, Hanley KA, Wood J, Patel HK, Shah JN, Agarunov E, Sethi A, Fogel EL, McNulty G, Haseeb A, Trieu JA, Dixon RE, Yang JY, Mendelsohn RB, Calo D, Aroniadis OC, LaComb JF, Scheiman JM, Sauer BG, Dang DT, Piraka CR, Shah ED, Pohl H, Tierney WM, Mitchell S, Condon A, Lenhart A, Dua KS, Kanagala VS, Kamal A, Singh VK, Pinto-Sanchez MI, Hutchinson JM, Kwon RS, Korsnes SJ, Singh H, Solati Z, Willingham FF, Yachimski PS, Conwell DL, Mosier E, Azab M, Patel A, Buxbaum J, Wani S, Chak A, Hosmer AE, Keswani RN, DiMaio CJ, Bronze MS, Muthusamy R, Canto MI, Gjeorgjievski VM, Imam Z, Odish F, Edhi AI, Orosey M, Tiwari A, Patwardhan S, Brown NG, Patel AA, Ordiah CO, Sloan IP, Cruz L, Koza CL, Okafor U, Hollander T, Furey N, Reykhart O, Zbib NH, Damianos JA, Esteban J, Hajidiacos N, Saul M, Mays M, Anderson G, Wood K, Mathews L, Diakova G, Caisse M, Wakefield L, Nitchie H, Waljee AK, Tang W, Zhang Y, Zhu J, Deshpande AR, Rockey DC, Alford TB, Durkalski V; North American Alliance for the Study of Digestive Manifestations of COVID-19. Digestive Manifestations in Patients Hospitalized With Coronavirus Disease 2019. Clin Gastroenterol Hepatol. 2021;19:1355-1365.e4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 5. | Mantovani A, Beatrice G, Dalbeni A. Coronavirus disease 2019 and prevalence of chronic liver disease: A meta-analysis. Liver Int. 2020;40:1316-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 120] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 6. | Guerra Veloz MF, Cordero Ruiz P, Ríos-Villegas MJ, Del Pino Bellido P, Bravo-Ferrer J, Galvés Cordero R, Cadena Herrera ML, Vías Parrado C, Bellido Muñoz F, Vega Rodríguez F, Caunedo Álvarez Á, Rodríguez-Baño J, Carmona Soria I. Liver manifestations in COVID-19 and the influence of pre-existing liver disease in the course of the infection. Rev Esp Enferm Dig. 2021;113:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30112] [Article Influence: 6022.4] [Reference Citation Analysis (3)] |
| 8. | Wang J, Lu Z, Jin M, Wang Y, Tian K, Xiao J, Cai Y, Zhang X, Chen T, Yao Z, Yang C, Deng R, Zhong Q, Deng X, Chen X, Yang XP, Wei G, Wang Z, Tian J, Chen XP. Clinical characteristics and risk factors of COVID-19 patients with chronic hepatitis B: a multi-center retrospective cohort study. Front Med. 2022;16:111-125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Kim D, Alshuwaykh O, Dennis BB, Cholankeril G, Ahmed A. Trends in Etiology-based Mortality From Chronic Liver Disease Before and During COVID-19 Pandemic in the United States. Clin Gastroenterol Hepatol. 2022;20:2307-2316.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 10. | Nagarajan R, Krishnamoorthy Y, Rajaa S, Hariharan VS. COVID-19 Severity and Mortality Among Chronic Liver Disease Patients: A Systematic Review and Meta-Analysis. Prev Chronic Dis. 2022;19:E53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 575] [Article Influence: 115.0] [Reference Citation Analysis (0)] |
| 12. | Pirola CJ, Sookoian S. SARS-CoV-2 virus and liver expression of host receptors: Putative mechanisms of liver involvement in COVID-19. Liver Int. 2020;40:2038-2040. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 13. | Dufour JF, Marjot T, Becchetti C, Tilg H. COVID-19 and liver disease. Gut. 2022;71:2350-2362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 14. | Nardo AD, Schneeweiss-Gleixner M, Bakail M, Dixon ED, Lax SF, Trauner M. Pathophysiological mechanisms of liver injury in COVID-19. Liver Int. 2021;41:20-32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 271] [Article Influence: 67.8] [Reference Citation Analysis (2)] |
| 15. | Pedersen SF, Ho YC. SARS-CoV-2: a storm is raging. J Clin Invest. 2020;130:2202-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 853] [Article Influence: 170.6] [Reference Citation Analysis (0)] |
| 16. | Ge J, Pletcher MJ, Lai JC; N3C Consortium. Outcomes of SARS-CoV-2 Infection in Patients With Chronic Liver Disease and Cirrhosis: A National COVID Cohort Collaborative Study. Gastroenterology. 2021;161:1487-1501.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 17. | Mendizabal M, Ridruejo E, Piñero F, Anders M, Padilla M, Toro LG, Torre A, Montes P, Urzúa A, Gonzalez Ballerga E, Silveyra MD, Michelato D, Díaz J, Peralta M, Pages J, García SR, Gutierrez Lozano I, Macias Y, Cocozzella D, Chavez-Tapia N, Tagle M, Dominguez A, Varón A, Vera Pozo E, Higuera-de la Tijera F, Bustios C, Conte D, Escajadillo N, Gómez AJ, Tenorio L, Castillo Barradas M, Schinoni MI, Bessone F, Contreras F, Nazal L, Sanchez A, García M, Brutti J, Cabrera MC, Miranda-Zazueta G, Rojas G, Cattaneo M, Castro-Narro G, Rubinstein F, Silva MO. Comparison of different prognostic scores for patients with cirrhosis hospitalized with SARS-CoV-2 infection. Ann Hepatol. 2021;25:100350. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Kim D, Adeniji N, Latt N, Kumar S, Bloom PP, Aby ES, Perumalswami P, Roytman M, Li M, Vogel AS, Catana AM, Wegermann K, Carr RM, Aloman C, Chen VL, Rabiee A, Sadowski B, Nguyen V, Dunn W, Chavin KD, Zhou K, Lizaola-Mayo B, Moghe A, Debes J, Lee TH, Branch AD, Viveiros K, Chan W, Chascsa DM, Kwo P, Dhanasekaran R. Predictors of Outcomes of COVID-19 in Patients With Chronic Liver Disease: US Multi-center Study. Clin Gastroenterol Hepatol. 2021;19:1469-1479.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 176] [Cited by in RCA: 184] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 19. | Wang Q, Davis PB, Xu R. COVID-19 risk, disparities and outcomes in patients with chronic liver disease in the United States. EClinicalMedicine. 2021;31:100688. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (1)] |
| 20. | Adeniji N, Carr RM, Aby ES, Catana AM, Wegermann K, Dhanasekaran R. Socioeconomic Factors Contribute to the Higher Risk of COVID-19 in Racial and Ethnic Minorities With Chronic Liver Diseases. Gastroenterology. 2021;160:1406-1409.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Kim JU, Majid A, Judge R, Crook P, Nathwani R, Selvapatt N, Lovendoski J, Manousou P, Thursz M, Dhar A, Lewis H, Vergis N, Lemoine M. Effect of COVID-19 lockdown on alcohol consumption in patients with pre-existing alcohol use disorder. Lancet Gastroenterol Hepatol. 2020;5:886-887. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 153] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 22. | Marjot T, Moon AM, Cook JA, Abd-Elsalam S, Aloman C, Armstrong MJ, Pose E, Brenner EJ, Cargill T, Catana MA, Dhanasekaran R, Eshraghian A, García-Juárez I, Gill US, Jones PD, Kennedy J, Marshall A, Matthews C, Mells G, Mercer C, Perumalswami PV, Avitabile E, Qi X, Su F, Ufere NN, Wong YJ, Zheng MH, Barnes E, Barritt AS 4th, Webb GJ. Outcomes following SARS-CoV-2 infection in patients with chronic liver disease: An international registry study. J Hepatol. 2021;74:567-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 384] [Article Influence: 96.0] [Reference Citation Analysis (0)] |
| 23. | Deutsch-Link S, Jiang Y, Peery AF, Barritt AS, Bataller R, Moon AM. Alcohol-Associated Liver Disease Mortality Increased From 2017 to 2020 and Accelerated During the COVID-19 Pandemic. Clin Gastroenterol Hepatol. 2022;20:2142-2144.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 53] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 24. | Elhence A, Vaishnav M, Biswas S, Anand A, Gunjan D, Kedia S, Mahapatra SJ, Nayak B, Sheikh S, Soni KD, Trikha A, Goel A, Shalimar. Predictors of in-hospital Outcomes in Patients With Cirrhosis and Coronavirus Disease-2019. J Clin Exp Hepatol. 2022;12:876-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 25. | Shalimar, Elhence A, Vaishnav M, Kumar R, Pathak P, Soni KD, Aggarwal R, Soneja M, Jorwal P, Kumar A, Khanna P, Singh AK, Biswas A, Nischal N, Dar L, Choudhary A, Rangarajan K, Mohan A, Acharya P, Nayak B, Gunjan D, Saraya A, Mahapatra S, Makharia G, Trikha A, Garg P. Poor outcomes in patients with cirrhosis and Corona Virus Disease-19. Indian J Gastroenterol. 2020;39:285-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 26. | Yeo YH, Zou B, Cheung R, Nguyen MH. Increased mortality of patients with alcohol-related liver diseases during the COVID-19 pandemic in the United States. J Intern Med. 2022;292:837-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Chang Y, Jeon J, Song TJ, Kim J. Association between the fatty liver index and the risk of severe complications in COVID-19 patients: a nationwide retrospective cohort study. BMC Infect Dis. 2022;22:384. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Vrsaljko N, Samadan L, Viskovic K, Mehmedović A, Budimir J, Vince A, Papic N. Association of Nonalcoholic Fatty Liver Disease With COVID-19 Severity and Pulmonary Thrombosis: CovidFAT, a Prospective, Observational Cohort Study. Open Forum Infect Dis. 2022;9:ofac073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 29. | Moctezuma-Velázquez P, Miranda-Zazueta G, Ortiz-Brizuela E, Garay-Mora JA, González-Lara MF, Tamez-Torres KM, Román-Montes CM, Díaz-Mejía BA, Pérez-García E, Villanueva-Reza M, Chapa-Ibargüengoitia M, Uscanga-Domínguez L, Sifuentes-Osornio J, Ponce-de-León A, Kershenobich-Stalnikowitz D, Mota-Ayala B, Moctezuma-Velázquez C. NAFLD determined by Dallas Steatosis Index is associated with poor outcomes in COVID-19 pneumonia: a cohort study. Intern Emerg Med. 2022;17:1355-1362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Yao R, Zhu L, Wang J, Liu J, Xue R, Xue L, Liu L, Li C, Zhao H, Cheng J, Huang S, Li Y, Zhao XA, Zhu C, Li M, Huang R, Wu C. Risk of severe illness of COVID-19 patients with NAFLD and increased NAFLD fibrosis scores. J Clin Lab Anal. 2021;35:e23880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Li J, Tian A, Zhu H, Chen L, Wen J, Liu W, Chen P. Mendelian Randomization Analysis Reveals No Causal Relationship Between Nonalcoholic Fatty Liver Disease and Severe COVID-19. Clin Gastroenterol Hepatol. 2022;20:1553-1560.e78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Jayaswal SK, Singh S, Malik PS, Venigalla SK, Gupta P, Samaga SN, Hota RN, Bhatia SS, Gupta I. Detrimental effect of diabetes and hypertension on the severity and mortality of COVID-19 infection: A multi-center case-control study from India. Diabetes Metab Syndr. 2021;15:102248. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 33. | de Almeida-Pititto B, Dualib PM, Zajdenverg L, Dantas JR, de Souza FD, Rodacki M, Bertoluci MC; Brazilian Diabetes Society Study Group (SBD). Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta-analysis. Diabetol Metab Syndr. 2020;12:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 216] [Cited by in RCA: 207] [Article Influence: 41.4] [Reference Citation Analysis (0)] |
| 34. | Chen VL, Hawa F, Berinstein JA, Reddy CA, Kassab I, Platt KD, Hsu CY, Steiner CA, Louissaint J, Gunaratnam NT, Sharma P. Hepatic Steatosis Is Associated with Increased Disease Severity and Liver Injury in Coronavirus Disease-19. Dig Dis Sci. 2021;66:3192-3198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 35. | Targher G, Mantovani A, Byrne CD, Wang XB, Yan HD, Sun QF, Pan KH, Zheng KI, Chen YP, Eslam M, George J, Zheng MH. Risk of severe illness from COVID-19 in patients with metabolic dysfunction-associated fatty liver disease and increased fibrosis scores. Gut. 2020;69:1545-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 161] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 36. | Madan K, Rastogi R, Bhargava R, Dagar V, Singla V, Sahu A, Singh P, Garg P, Aggarwal B, Singh RK. Is Fatty Liver Associated with Increased Mortality and Morbidity in Coronavirus Disease 2019 (COVID-19) Pneumonia? J Clin Exp Hepatol. 2022;12:1320-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Sarin SK, Choudhury A, Lau GK, Zheng MH, Ji D, Abd-Elsalam S, Hwang J, Qi X, Cua IH, Suh JI, Park JG, Putcharoen O, Kaewdech A, Piratvisuth T, Treeprasertsuk S, Park S, Wejnaruemarn S, Payawal DA, Baatarkhuu O, Ahn SH, Yeo CD, Alonzo UR, Chinbayar T, Loho IM, Yokosuka O, Jafri W, Tan S, Soo LI, Tanwandee T, Gani R, Anand L, Esmail ES, Khalaf M, Alam S, Lin CY, Chuang WL, Soin AS, Garg HK, Kalista K, Batsukh B, Purnomo HD, Dara VP, Rathi P, Al Mahtab M, Shukla A, Sharma MK, Omata M; APASL COVID Task Force, APASL COVID Liver Injury Spectrum Study (APCOLIS Study-NCT 04345640). Pre-existing liver disease is associated with poor outcome in patients with SARS CoV2 infection; The APCOLIS Study (APASL COVID-19 Liver Injury Spectrum Study). Hepatol Int. 2020;14:690-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 216] [Article Influence: 43.2] [Reference Citation Analysis (1)] |
| 38. | Yang S, Wang S, Du M, Liu M, Liu Y, He Y. Patients with COVID-19 and HBV Coinfection are at Risk of Poor Prognosis. Infect Dis Ther. 2022;11:1229-1242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 39. | Liu J, Wang T, Cai Q, Sun L, Huang D, Zhou G, He Q, Wang FS, Liu L, Chen J. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol Res. 2020;50:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 63] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 40. | Choe JW, Jung YK, Yim HJ, Seo GH. Clinical Effect of Hepatitis B Virus on COVID-19 Infected Patients: A Nationwide Population-Based Study Using the Health Insurance Review & Assessment Service Database. J Korean Med Sci. 2022;37:e29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 20] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 41. | Anugwom CM, Aby ES, Debes JD. Inverse Association Between Chronic Hepatitis B Infection and Coronavirus Disease 2019 (COVID-19): Immune Exhaustion or Coincidence? Clin Infect Dis. 2021;72:180-182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Kang SH, Cho DH, Choi J, Baik SK, Gwon JG, Kim MY. Association between chronic hepatitis B infection and COVID-19 outcomes: A Korean nationwide cohort study. PLoS One. 2021;16:e0258229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 43. | Campbell C, Andersson MI, Ansari MA, Moswela O, Misbah SA, Klenerman P, Matthews PC. Risk of Reactivation of Hepatitis B Virus (HBV) and Tuberculosis (TB) and Complications of Hepatitis C Virus (HCV) Following Tocilizumab Therapy: A Systematic Review to Inform Risk Assessment in the COVID-19 Era. Front Med (Lausanne). 2021;8:706482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Yip TC, Wong VW, Lui GC, Chow VC, Tse YK, Hui VW, Liang LY, Chan HL, Hui DS, Wong GL. Current and Past Infections of HBV Do Not Increase Mortality in Patients With COVID-19. Hepatology. 2021;74:1750-1765. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 45. | Ronderos D, Omar AMS, Abbas H, Makker J, Baiomi A, Sun H, Mantri N, Choi Y, Fortuzi K, Shin D, Patel H, Chilimuri S. Chronic hepatitis-C infection in COVID-19 patients is associated with in-hospital mortality. World J Clin Cases. 2021;9:8749-8762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 46. | Di Giorgio A, Nicastro E, Speziani C, De Giorgio M, Pasulo L, Magro B, Fagiuoli S, D' Antiga L. Health status of patients with autoimmune liver disease during SARS-CoV-2 outbreak in northern Italy. J Hepatol. 2020;73:702-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 47. | Efe C, Dhanasekaran R, Lammert C, Ebik B, Higuera-de la Tijera F, Aloman C, Rıza Calışkan A, Peralta M, Gerussi A, Massoumi H, Catana AM, Torgutalp M, Purnak T, Rigamonti C, Gomez Aldana AJ, Khakoo N, Kacmaz H, Nazal L, Frager S, Demir N, Irak K, Ellik ZM, Balaban Y, Atay K, Eren F, Cristoferi L, Batıbay E, Urzua Á, Snijders R, Kıyıcı M, Akyıldız M, Ekin N, Carr RM, Harputluoğlu M, Hatemi I, Mendizabal M, Silva M, Idilman R, Silveira M, Drenth JPH, Assis DN, Björnsson E, Boyer JL, Invernizzi P, Levy C, Schiano TD, Ridruejo E, Wahlin S. Outcome of COVID-19 in Patients With Autoimmune Hepatitis: An International Multicenter Study. Hepatology. 2021;73:2099-2109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (1)] |
| 48. | Grgurevic I, Lucijanić M, Pastrovic F, Barisic Jaman M, Tjesic Drinkovic I, Zelenika M, Milosevic M, Medic B, Kardum D, Bokun T, Luksic I, Piskac Zivkovic N, Keres T, Grabovac V, Persec J, Barsic B. Short-term outcomes of patients with chronic liver disease hospitalised with COVID-19. Intern Med J. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 49. | Marjot T, Buescher G, Sebode M, Barnes E, Barritt AS 4th, Armstrong MJ, Baldelli L, Kennedy J, Mercer C, Ozga AK, Casar C, Schramm C; contributing Members and Collaborators of ERN RARE-LIVER/COVID-Hep/SECURE-Cirrhosis, Moon AM, Webb GJ, Lohse AW. SARS-CoV-2 infection in patients with autoimmune hepatitis. J Hepatol. 2021;74:1335-1343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 89] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 50. | Efe C, Lammert C, Taşçılar K, Dhanasekaran R, Ebik B, Higuera-de la Tijera F, Calışkan AR, Peralta M, Gerussi A, Massoumi H, Catana AM, Purnak T, Rigamonti C, Aldana AJG, Khakoo N, Nazal L, Frager S, Demir N, Irak K, Melekoğlu-Ellik Z, Kacmaz H, Balaban Y, Atay K, Eren F, Alvares-da-Silva MR, Cristoferi L, Urzua Á, Eşkazan T, Magro B, Snijders R, Barutçu S, Lytvyak E, Zazueta GM, Demirezer-Bolat A, Aydın M, Heurgue-Berlot A, De Martin E, Ekin N, Yıldırım S, Yavuz A, Bıyık M, Narro GC, Kıyıcı M, Akyıldız M, Kahramanoğlu-Aksoy E, Vincent M, Carr RM, Günşar F, Reyes EC, Harputluoğlu M, Aloman C, Gatselis NK, Üstündağ Y, Brahm J, Vargas NCE, Güzelbulut F, Garcia SR, Aguirre J, Anders M, Ratusnu N, Hatemi I, Mendizabal M, Floreani A, Fagiuoli S, Silva M, Idilman R, Satapathy SK, Silveira M, Drenth JPH, Dalekos GN, N Assis D, Björnsson E, Boyer JL, Yoshida EM, Invernizzi P, Levy C, Montano-Loza AJ, Schiano TD, Ridruejo E, Wahlin S. Effects of immunosuppressive drugs on COVID-19 severity in patients with autoimmune hepatitis. Liver Int. 2022;42:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 51. | Vuppalanchi V, Gelow K, Green K, Vuppalanchi R, Lammert C. Behaviors, symptoms, and outcomes of North American patients with autoimmune hepatitis during the COVID-19 pandemic. J Investig Med. 2021;69:1426-1433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 52. | Xiao Y, Wu D, Shi X, Liu S, Hu X, Zhou C, Tian X, Liu H, Long H, Li Z, Wang J, Tan T, Xu Y, Chen B, Liu T, Zhang H, Zheng S, Hu S, Song J, Tang J, Cheng Z, Xu W, Shen Y, Yu W, Li J, Zhou J, Wang F, Chen M. High Child-Pugh and CRUB65 scores predict mortality of decompensated cirrhosis patients with COVID-19: A 23-center, retrospective study. Virulence. 2021;12:1199-1208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 53. | Iavarone M, D'Ambrosio R, Soria A, Triolo M, Pugliese N, Del Poggio P, Perricone G, Massironi S, Spinetti A, Buscarini E, Viganò M, Carriero C, Fagiuoli S, Aghemo A, Belli LS, Lucà M, Pedaci M, Rimondi A, Rumi MG, Invernizzi P, Bonfanti P, Lampertico P. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J Hepatol. 2020;73:1063-1071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (2)] |
| 54. | Xiang F, Sun J, Chen PH, Han P, Zheng H, Cai S, Kirk GD. Early Elevation of Fibrosis-4 Liver Fibrosis Score Is Associated With Adverse Outcomes Among Patients With Coronavirus Disease 2019. Clin Infect Dis. 2021;73:e594-e601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Pranata R, Yonas E, Huang I, Lim MA, Nasution SA, Kuswardhani RAT. Fibrosis-4 index and mortality in coronavirus disease 2019: a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:e368-e374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 56. | Diaz-Louzao C, Barrera-Lopez L, Lopez-Rodriguez M, Casar C, Vazquez-Agra N, Pernas-Pardavila H, Marques-Afonso A, Vidal-Vazquez M, Montoya JG, Andrade AH, Fernandez-Castro I, Varela P, Gonzalez-Quintela A, Otero E, Gude F, Cadarso-Suarez C, Tome S. Longitudinal relationship of liver injury with inflammation biomarkers in COVID-19 hospitalized patients using a joint modeling approach. Sci Rep. 2022;12:5547. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 57. | Enichen E, Harvey C, Demmig-Adams B. COVID-19 Spotlights Connections between Disease and Multiple Lifestyle Factors. Am J Lifestyle Med 2022; 15598276221123004.. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 58. | Martínez-Esparza M, Tristán-Manzano M, Ruiz-Alcaraz AJ, García-Peñarrubia P. Inflammatory status in human hepatic cirrhosis. World J Gastroenterol. 2015;21:11522-11541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 59. | Lui TKL, Tsui VWM, Leung WK. Impact of first wave of COVID-19 on outcomes of hospitalization for upper gastrointestinal bleeding in Hong Kong: a population-based study. Endosc Int Open. 2021;9:E284-E288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 60. | Gandhi M, Ling WH, Chen CH, Lee JH, Kudo M, Chanwat R, Strasser SI, Xu Z, Lai SH, Chow PK. Impact of COVID-19 on Hepatocellular Carcinoma Management: A Multicountry and Region Study. J Hepatocell Carcinoma. 2021;8:1159-1167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 61. | Geh D, Watson R, Sen G, French JJ, Hammond J, Turner P, Hoare T, Anderson K, McNeil M, McPherson S, Masson S, Dyson J, Donnelly M, MacDougal L, Patel P, Hudson M, Anstee QM, White S, Robinson S, Pandanaboyana S, Walker L, McCain M, Bury Y, Raman S, Burt A, Parkinson D, Haugk B, Darne A, Wadd N, Asghar S, Mariappan L, Margetts J, Stenberg B, Scott J, Littler P, Manas DM, Reeves HL. COVID-19 and liver cancer: lost patients and larger tumours. BMJ Open Gastroenterol. 2022;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 62. | Amaddeo G, Brustia R, Allaire M, Lequoy M, Hollande C, Regnault H, Blaise L, Ganne-Carrié N, Séror O, Larrey E, Lim C, Scatton O, El Mouhadi S, Ozenne V, Paye F, Balladur P, Dohan A, Massault PP, Pol S, Dioguardi Burgio M, Vilgrain V, Sepulveda A, Cauchy F, Luciani A, Sommacale D, Leroy V, Roudot-Thoraval F, Bouattour M, Nault JC; Paris Liver Cancer Group. Impact of COVID-19 on the management of hepatocellular carcinoma in a high-prevalence area. JHEP Rep. 2021;3:100199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 58] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 63. | Muñoz-Martínez S, Sapena V, Forner A, Nault JC, Sapisochin G, Rimassa L, Sangro B, Bruix J, Sanduzzi-Zamparelli M, Hołówko W, El Kassas M, Mocan T, Bouattour M, Merle P, Hoogwater FJH, Alqahtani SA, Reeves HL, Pinato DJ, Giorgakis E, Meyer T, Villadsen GE, Wege H, Salati M, Mínguez B, Di Costanzo GG, Roderburg C, Tacke F, Varela M, Galle PR, Alvares-da-Silva MR, Trojan J, Bridgewater J, Cabibbo G, Toso C, Lachenmayer A, Casadei-Gardini A, Toyoda H, Lüdde T, Villani R, Matilla Peña AM, Guedes Leal CR, Ronzoni M, Delgado M, Perelló C, Pascual S, Lledó JL, Argemi J, Basu B, da Fonseca L, Acevedo J, Siebenhüner AR, Braconi C, Meyers BM, Granito A, Sala M, Rodríguez-Lope C, Blaise L, Romero-Gómez M, Piñero F, Gomez D, Mello V, Pinheiro Alves RC, França A, Branco F, Brandi G, Pereira G, Coll S, Guarino M, Benítez C, Anders MM, Bandi JC, Vergara M, Calvo M, Peck-Radosavljevic M, García-Juárez I, Cardinale V, Lozano M, Gambato M, Okolicsanyi S, Morales-Arraez D, Elvevi A, Muñoz AE, Lué A, Iavarone M, Reig M. Assessing the impact of COVID-19 on liver cancer management (CERO-19). JHEP Rep. 2021;3:100260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 64. | Song H, Fan G, Liu Y, Wang X, He D. The Second Wave of COVID-19 in South and Southeast Asia and the Effects of Vaccination. Front Med (Lausanne). 2021;8:773110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 65. | Nawghare P, Jain S, Chandnani S, Bansal S, Patel S, Debnath P, Rane S, Deshmukh R, Rathi P, Contractor Q. Predictors of Severity and Mortality in Chronic Liver Disease Patients With COVID-19 During the Second Wave of the Pandemic in India. Cureus. 2022;14:e20891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |