Published online Nov 25, 2022. doi: 10.5501/wjv.v11.i6.426
Peer-review started: June 5, 2022
First decision: August 1, 2022
Revised: August 22, 2022
Accepted: October 12, 2022
Article in press: October 12, 2022
Published online: November 25, 2022
Processing time: 171 Days and 7.6 Hours
Monkeypox virus (MPXV), which belongs to the orthopoxvirus genus, causes zoonotic viral disease. This review discusses the biology, epidemiology, and evolution of MPXV infection, particularly cellular, human, and viral factors, virus transmission dynamics, infection, and persistence in nature. This review also describes the role of recombination, gene loss, and gene gain in MPXV evol-vement and the role of signal transduction in MPXV infection and provides an overview of the current access to therapeutic options for the treatment and prevention of MPXV. Finally, this review highlighted gaps in knowledge and proposed future research endeavors to address the unresolved questions.
Core Tip: Since May 13, 2022, cases of monkeypox have been reported to the World Health Organization (WHO) from 12 Member States that are not endemic to the monkeypox virus across three WHO regions. This emergent pathogen is a significant concern worldwide after severe acute respiratory syndrome coronavirus 2 and requires epidemiological and other data on the virus. The objective of this review is to report comprehensive data on this virus.
- Citation: Beig M, Mohammadi M, Nafe Monfared F, Nasereslami S. Monkeypox: An emerging zoonotic pathogen. World J Virol 2022; 11(6): 426-434
- URL: https://www.wjgnet.com/2220-3249/full/v11/i6/426.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i6.426
Monkeypox virus (MPXV) is one of the human orthopox viruses (OPVs), which consist of variola virus (VARV), cowpox virus (CPXV), and vaccinia virus (VACV)[1]. Monkeypox has similar clinical manifestations to smallpox, but has a milder rash and a lower fatality rate[2]. The aims of this review are to describe the current data on MPXV evolution, epidemiology, and infection-control mechanisms.
When two smallpox-like illnesses appeared in monkey colonies housed for scientific study, the first cases of monkeypox were discovered in 1958[3]; therefore, the name monkeypox and the first human case of the virus were registered in 1970 in the Democratic Republic of the Congo[4]. Attempts to destroy the MPXV have since been documented in humans in other Central and West African countries[5].
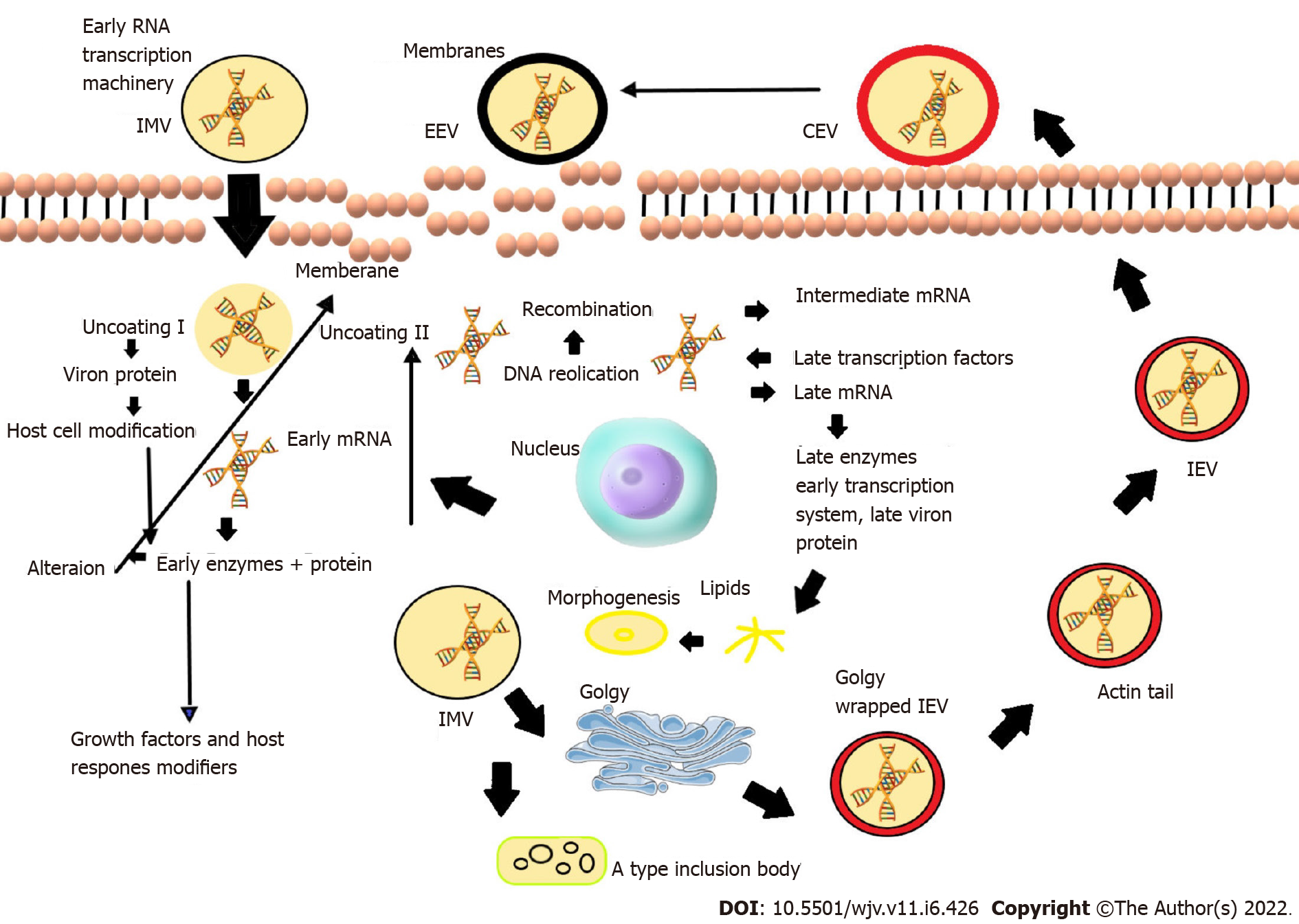
The morphology of MPXV virions has been shown to include brick- or ovoid-shaped particles[6]. Membrane links, a tightly packed core containing enzymes, transcription factors, a double-stranded DNA genome, and an outer membrane protecting the whole structure have been observed[7,8]. Although its whole life cycle occurs in the cytoplasm of infected cells, its genome contains linear double-stranded DNA (197 kb). The genome encodes all the proteins necessary for viral DNA replication, transcription, and virion assembly[6,9]. Cells infected with the poxvirus generate the intracellular mature virus and extracellular enveloped virus, two contagious viruses[10,11] (Figure 1).
An animal model for studying ethnic illness uses a channel of contamination that matches the herbal transmission of the virus or displays development, morbidity, and death similar to those seen during ethnic infection[12,13]. The animal model also has to mirror human instances in at least one or more methods of transmission[14]. Additionally, the red patches on all MPXV-examined animals at the vaccination site showed a decrease in size compared to nearby animals, and beginning around 14 d after the challenge, a continual rise in body size across fully breathing animals in the vaccinated group[15,16]. In a study that examined the sensitivity of 38 inbred strains of mice (32 classical inbreed stresses and six wild strains), only three of the wild-derived strains (CAST/EiJ, PERA/EiJ, and MOLF/EiJ) were highly sensitive to MPXV, whereas all other inbred lines were strong after intranasal MPXV infection[2,17].
Human-to-human and animal-to-human transmission are two potential MPXV transmission pathways[18]. Human-to-human transmission stability is correlated with droplet infection and interactions with body fluids, patient factors, and skin lesions in a contaminated individual[6,18]. The Congo Basin group is more virulent than the West African group and contributes more to interpersonal transport[19]. Direct contact and ingestion of the herbal viral host's food are the two routes by which transmission occurs from animals to humans[20,21]. Furthermore, zoonotic transmission can occur via direct touch, including blood, body fluids, and mucocutaneous lesions on a contaminated animal[22].
Sexual transmission of MPXV: MPXV outbreaks are not typical, as many patients are unrelated to travel to Central or West Africa and episodes of the virus in endemic areas. The MPXV is currently observed among men who have sex with men (MSM) in the United Kingdom. In the studies conducted, a high proportion of simultaneous sexually transmitted diseases and frequent anogenital symptoms were found, which indicates the possibility of transmission during close skin-to-skin or mucous contact during sexual activity[1,23,24].
Transmission by MPXV-contaminated surfaces: Although co-transmission between people and animals was identified as the primary method of infection dissemination in several investigations, transmission in patient care staff via surfaces contaminated with MPXV was seldom recorded. The MPXV may also spread indirectly via contaminated objects. However, the environmental contamination of surfaces with MPXV is not well understood[25].
Phenotypic approaches: Phenotypic methods: According to the clinical diagnosis, in MPXV infection, a prodromal sickness usually accompanies it with a variety of symptoms over 3-5 d, including fever > 38.3°C, back pain, myalgia, headache, acute asthenia, pharyngitis, drenching sweats, malaise, and notably lymphadenopathy[6,26-28]. Vesiculopustular rashes begin on the face during 1-10 d of development, affecting 95% of patients[29], followed by the palms and soles (75%), oral mucosa (70%), genitalia (30%), and conjunctiva (20%). These skin lesions evolve from macules to papules, vesicles, pustules, and finally, scabs or crusts that fall[28]. Lesions in MPXV patients appear monomorphic, pea-sized, and complex, similar to smallpox[30]. The presence of lymphadenopathy in MPXV infection is one of the clinical markers that set it apart from smallpox, along with lesion appearance and limited centrifugal spread[31]. These skin manifestations compromise the skin eruption period of the disease, in which patients are contagious. Before that, patients are not able to transmit the virus. The natural history in patients without complications regularly lasts 2-4 wk[28]. Possible detection of MPXV based on clinical signs is essential to identify suspicious cases during surveillance. Nevertheless, the clinical case definition for MPXV based on unconfirmed studies has high sensitivity (93% to 98%) and low specificity (9% to 26%)[31,32]. Virus transmission occurs by direct bodily contact with pores and skin then skin lesions, along with sexual contact; or contact with contaminated materials, such as clothing, bedding and dishes, within 21 d before signs appear. Laboratory research does not validate the clinical definition, but an epidemiological link, including contact with a proven case does[28].
Genetic methods: It is recommended that genetic techniques, including polymerase chain reaction (PCR) or real-time PCR (RT-PCR), be performed in a biosafety level 3 facility[33].
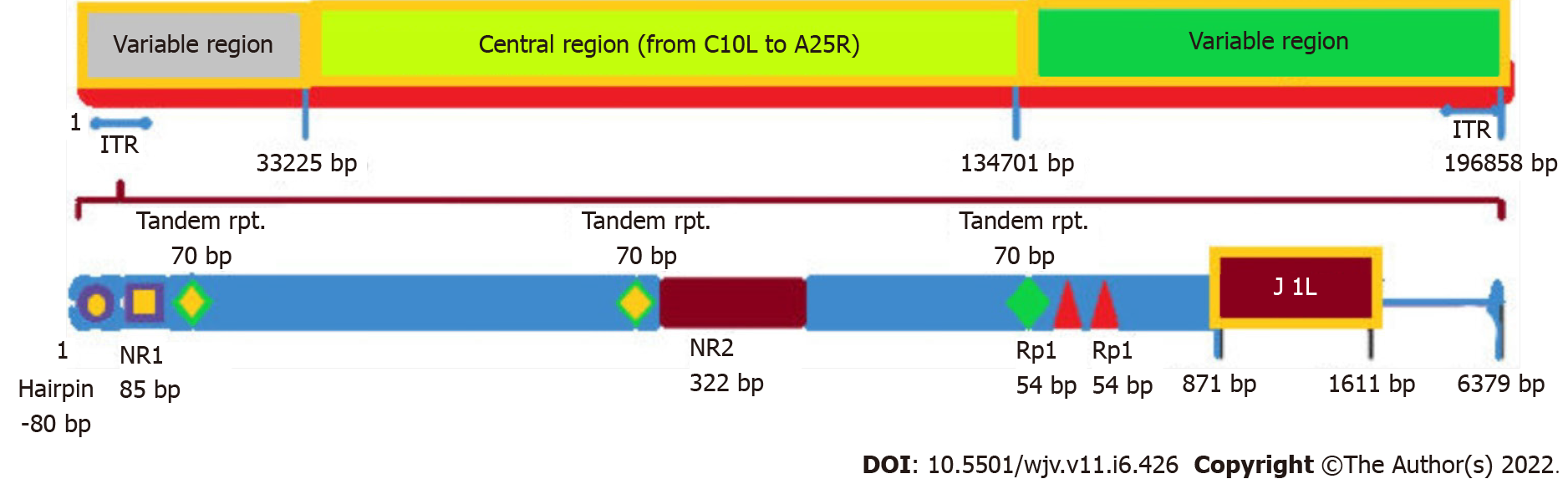
Routine detection of MPXV DNA in clinical and veterinary specimens and cell cultures infected with MPXV is performed by RT-PCR targeting conserved regions of the outer coat protein (B6R) gene, l DNA polymerase E, the DNA-dependent RNA polymerase subunit 18 (rpo18), and the F3L genes[33,34]. Restriction fragment length polymorphism (RFLP) of genes or PCR-amplified gene fragments is also used to detect MPXV DNA, but RFLP is time-consuming and requires viral culture[35]. Additionally, as RFLP of PCR products requires enzymatic digestion after gel electrophoresis, it may not be an appropriate method in a clinical setting where speed, sensitivity, and specificity are essential. Whole genome sequencing (NGS) is valuable in detecting MPXV and OPVs, but this technique is expensive, and downstream sequencing records processing requires extensive computing[36-38]. Therefore, NGS may not be a siutable detection method in resource-poor locations in sub-Saharan Africa. Although RT-PCR remains the optimal method for the identification of MPXV, this must be complemented by genome sequencing technology to provide information on the genome, which is essential for evidence-based epidemiology (Figure 2)[32].
Immunological methods: These methods include enzyme-linked immunosorbent (ELISA) and immunohistochemical assays to determine IgG and IgM antibodies and detect viral antigens[39]. Immunochemical analysis can distinguish poxvirus from herpes virus infection using polyclonal or monoclonal antibodies to all OPVs[11]. It has been shown that antibodies to the virus also have cellular responses and enhancements at the time of disease onset. Approximately 5 d and 8 d or more after the onset of the rash, IgM and IgG are formed in the serum, respectively[40]. Detection of IgM and IgG antibodies in unvaccinated individuals with a history of inflammation and severe illness may increase indirect MPXV discrimination. Despite this, these methods are not specific for MPXV detection and can detect other types of OPVs[32,41]. On the other hand, IgM can assess MPXV infection in people with a history of smallpox vaccination[42]. A positive IgM capture ELISA test indicates recent exposure to OPV (possibly MPXV in endemic areas) in vaccinated individuals.
Conversely, a positive IgG capture ELISA test indicates that a person has been exposed to OPV through vaccination or natural infection. Therefore, IgM and IgG in a sample are strong evidence of recent exposure to an OPV in previously vaccinated or naturally infected individuals. Thus, IgM in individuals vaccinated against smallpox in MPXV-endemic regions reflects recent exposure to MPXV[43,44].
Electron microscopy: MPXV under an electron microscope appears intracytoplasmic brick-shaped with lateral bodies and a central core measuring about 200–300 nm. Although this method is not a definitive diagnostic technique as OPV species cannot be differentiated morphologically, it provides a clue that the virus belongs to the Poxviridae family [45].
Host and tissue tropism: Members of the OPV family are thought to exhibit diverse spectra of host tropisms[46]. Although the reservoir host for MPXV has not been definitively identified, many mammalian species are naturally infected with MPXV[47]. Thus, it is believed that MPXV has a wide host range. Previously, after the challenge with Congo Basin MPXV, large amounts of viral DNA and viable virions died in a variety of animal tissues, suggesting broad tissue tropism. The immunohistochemical and histopathological tests by Falendysz et al[48] found that the MPXV antigen was identified in ovarian, brain, heart, kidney, liver, pancreatic, and lung tissues, and ovarian tissues were susceptible to MPXV[49].
Host responses to the virus: PXVs develop many strategies to escape the host's immune response to infection. Natural killer (NK) cells kill virus-infected cells by secreting cytokines that stimulate the activity of other cell types, such as T cells and dendritic cells[50]. MPXV infection can change lymphocyte numbers, NK cell changes in non-human primates (NHPs), lymphadenopathy, and lymphocyte consumption in MPXV-infected NHPs. Gavin et al[51] using prairie pooches showed a noteworthy increment in the number of all NK subsets (CD16- CD56-, CD16+, CD56+, and CD16+ CD56+) on the seventh day after vaccination. Moreover, the expression of chemokine receptors (CXCR3, CCR5, CCR6, and CCR7) on each NK cell subset suggest that, following the MPXV challenge, receptor expression was delayed or reduced[11,52]. Hammarlund et al[53] anticipated that MPXV has a safe avoidance component such as CPXV. The avoidance process utilized by MPXV ensures the viral store is resistant by repressing the activation of CD4+ and CD8+ T cells after interaction with MPXV-infected cells. Acknowledgment of MPXV-infected monocytes by antiviral CD4+ and CD8+ shows that MPXV does not activate the generation of cytokines (IFN-γ or TNF-α) by virus-specific T cells[52]. Antiviral T-cell responses are substantially increased following contamination with VARV alone. However, T-cell cytokine responses decreased by 95% after co-infection, including MPXV and VARV, and by 80% when low-dose MPXV was added (VARV: MPXV ratio was 10:1)[54].
Vaccination: The smallpox vaccine protects humans against smallpox. The smallpox vaccine incorporates a live vaccinia virus, and not a killed virus[55]. Vaccinated people must take precautions, as the vaccine can result in side effects[56]. Most humans have mild reactions such as flank pain, fever, and body aches[51]. However, some people may react differently, and some side effects can be life-threatening[57]. Although smallpox vaccination can shield humans from smallpox for approximately 3-5 years, its potential to protect humans then decreases, and for long-term protection, additional vaccinations may be needed[58]. Several reviews suggest that smallpox vaccination provides cross-protection against common OPV species and MPXV. Of humans vaccinated against smallpox, 85% did not develop MPXV infection[59]. The smallpox vaccine (ACAM2000TM) was advocated by the Centers for Disease Control and Prevention (CDC)[60].
The attenuated vaccine, IMVAMUNE, is no longer available in MPXV areas[61]. A third-generation modified Ankara vaccine has been selected with the aid of the Food and Drug Administration (FDA) and the European Medicines Agency to prevent varicella or monkeypox in adults (age 18 years) with a high risk of VARV and MPXV infection[61,62]. Unlike the ACAM2000 vaccine, IMVAMUNE is no longer used in humans with immunodeficiency, such as immune disorders and atopic dermatitis. Neither ACAM2000 nor IMVAMUNE is used in specific populations[61,62]. Vaccination is also recommended for sexually high-risk individuals, including MSM, and those with a history of sexually transmitted diseases such as human immunodeficiency virus (HIV), syphilis, and gonorrhea. However, there are no statistics on immunization, including smallpox vaccines JYNNEOS®/IMVANEX® that may confer protection against sexually transmitted MPXV[51,63].
Antivirals: There is no approved, safe remedy for MPXV infection. A 4-trifluoromethylphenol derivative and tecovirimat (ST-246 or TPOXX®), supported by the FDA, have been examined using animal models[64]. These agents have been shown to be beneficial in infected animals. According to a CDC report, clinical trials, including on tecoirimate, show that although the treatment is well tolerated and safe, there are inadequate statistics on its usefulness in treating monkeypox in humans[61,65,66]. Similarly, in vitro studies with cidofovir or brincidofovir (CMX001 or hexadecyloxypropyl-cidofovir) reduced viral DNA polymerase, and is an acyclic nucleoside phosphate conjugate of cidofovir[61,66,67]. However, brincidofovir has increased cytotoxicity and higher antiviral activity than cidofovir towards VARV, MPXV, VACV, and CPXV in vitro.
Brincidofovir has a high selectivity index and is 25-fold greater than cidofovir. Cidofovir is a nucleotide monophosphate analog. Another dynamic agent against poxviruses is NIOCH-14, a precursor of tecovirimat[66-68]. Although the activity of NIOCH-14 towards VARV, MPXV, and ECTV is similar to that of tecovirimat in in vitro studies, its production is less complicated than tecovirimat, and has been recognized as an essential antiviral in the future. Ribavirin and tiazofurin inhibited the activity of every OPV tested including VARV and MPXV[59,61,68,69]. Saquinavir, ritonavir, and nelfinavir are protease inhibitors, and efavirenz, stavudine, and zidovudine are reverse transcriptase inhibitors and have been used against OPVs. In addition, two adenosine analogs (C-ca3-Ado and C3-Npc A) have been shown to have protective activity against OPVs in viral replication assays, and these analogs are also inhibitors of S-adenosylhomocysteine hydrolase (SAH)[59,61,67,68]. These SAH hydrolase inhibitors have broad antiviral activity but had no detectable effect on CPXV in vitro. Using specific mechanisms, cidofovir and N-(2-hydroxypropyl) methacrylamide inhibited viral duplication in PXVs. However, adefovir and dipivoxil showed no sizeable activity against poxviruses.
Furthermore, adenosine oxide N1 had a considerable effect on OPV by inhibiting CPXV viral reproduction in vitro by blocking viral mRNA translation[52,68,70]. Although there is no optimal therapy, MPXV is managed only by supportive than evidential treatment, and is only suitable for symptomatic individuals[66,68]. Thus, environmentally friendly MPXV vaccination and antiviral agents are required to prevent transmission from asymptomatic people.
Biocidal agents and disinfectants: On June 5, 2022, a study was conducted to assess the published data regarding the antiviral effect of biocides and disinfectants against MPXV and orthopoxviruses. Vaccinia viruses must be rendered inactive by at least four log10 using 70% ethanol (70%, 1 min), peracetic acid (0.2%, 10 min), and probiotic cleanser (1%-10%, one h) on contaminated surfaces. These tests also demonstrated the efficacy of glutaraldehyde (2%; 10 min), orthophthalaldehyde (0.55%, 5 min), iodine (0.04%-1%) and sodium hypochlorite (0.25%-2.5%; 1 min). Vaccinia virus was not affected by copper levels (99.9%) but MPXV was at 3 min[71].
As of May 2022, instances of MPXV have been recorded in nations where the infection is not endemic and are still being reported in several endemic nations. As a result, MPXV is no longer restricted to areas where it is endemic as, in recent years, visitors from Africa have brought MPXV to the United States, the United Kingdom, Israel, and Singapore. MPXV is a dangerous reemerging pathogen. MSM males in the United Kingdom have contracted MPXV via community transmission without directly interacting with travelers from endemic nations. In addition, a study reported admission to the Hospital for Infectious and Tropical Diseases in Romania of a 26-year-old HIV-positive male with high fever (up to 39 ℃), chills, rectal pain, vesiculo-pustular rash, dysphagia, and skin lesions primarily in the anogenital area who had developed a mild form of the disease. This was the first MPXV case officially verified in Romania with suspicious epidemiological and clinical symptoms. Excellent knowledge on how to prevent and control MPXV infection, and improve contact tracing is required. This is particularly true in populations with high-risk characteristics. Public health officials and medical professionals should rule out MPXV in all patients who exhibit the typical rash and risky sexual behavior, especially those who have recently had sex with partners who visited countries where MPXV cases have been reported or partners who exhibit the same clinical symptoms even if they do not travel abroad[72]. As a result, it is essential to focus more on national and international research efforts for laboratory diagnosis, infection control, and treatment strategies. These strategies should also support sexual health and other specialized services in managing this condition. For MPXV outbreaks around the world, the Surveillance Outbreak Response Management Analysis System must be established and implemented.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Iran
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Panduro-Correa V, Peru; Wang T, China S-Editor: Zhang H L-Editor: Webster JR P-Editor: Zhang H
| 1. | Girometti N, Byrne R, Bracchi M, Heskin J, McOwan A, Tittle V, Gedela K, Scott C, Patel S, Gohil J, Nugent D, Suchak T, Dickinson M, Feeney M, Mora-Peris B, Stegmann K, Plaha K, Davies G, Moore LSP, Mughal N, Asboe D, Boffito M, Jones R, Whitlock G. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22:1321-1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 275] [Cited by in RCA: 312] [Article Influence: 104.0] [Reference Citation Analysis (0)] |
| 2. | Gong Q, Wang C, Chuai X, Chiu S. Monkeypox virus: a re-emergent threat to humans. Virol Sin. 2022;37:477-482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 151] [Article Influence: 50.3] [Reference Citation Analysis (0)] |
| 3. | Bunge EM, Hoet B, Chen L, Lienert F, Weidenthaler H, Baer LR, Steffen R. The changing epidemiology of human monkeypox-A potential threat? PLoS Negl Trop Dis. 2022;16:e0010141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1103] [Cited by in RCA: 1037] [Article Influence: 345.7] [Reference Citation Analysis (0)] |
| 4. | Guarner J, Del Rio C, Malani PN. Monkeypox in 2022-What Clinicians Need to Know. JAMA. 2022;328:139-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 152] [Article Influence: 50.7] [Reference Citation Analysis (0)] |
| 5. | Diehl MLN, Paes J, Rott MB. Genotype distribution of Acanthamoeba in keratitis: a systematic review. Parasitol Res. 2021;120:3051-3063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 40] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 6. | Luo Q, Han J. Preparedness for a Monkeypox Outbreak. Infect Med. 2022;. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 7. | Hutson CL, Damon IK. Monkeypox virus infections in small animal models for evaluation of anti-poxvirus agents. Viruses. 2010;2:2763-2776. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Kumar S, Subramaniam G, Karuppanan K. Human monkeypox outbreak in 2022. J Med Virol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | Kmiec D, Kirchhoff F. Monkeypox: A New Threat? Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 113] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 10. | Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 11. | Sood A, Sui Y, McDonough E, Santamaría-Pang A, Al-Kofahi Y, Pang Z, Jahrling PB, Kuhn JH, Ginty F. Comparison of Multiplexed Immunofluorescence Imaging to Chromogenic Immunohistochemistry of Skin Biomarkers in Response to Monkeypox Virus Infection. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 12. | Saijo M, Ami Y, Suzaki Y, Nagata N, Iwata N, Hasegawa H, Iizuka I, Shiota T, Sakai K, Ogata M, Fukushi S, Mizutani T, Sata T, Kurata T, Kurane I, Morikawa S. Virulence and pathophysiology of the Congo Basin and West African strains of monkeypox virus in non-human primates. J Gen Virol. 2009;90:2266-2271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 13. | Karumathil S, Raveendran NT, Ganesh D, Kumar Ns S, Nair RR, Dirisala VR. Evolution of Synonymous Codon Usage Bias in West African and Central African Strains of Monkeypox Virus. Evol Bioinform Online. 2018;14:1176934318761368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | Fan C, Wu Y, Rui X, Yang Y, Ling C, Liu S, Wang Y. Animal models for COVID-19: advances, gaps and perspectives. Signal Transduct Target Ther. 2022;7:220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 15. | Mucker EM, Shamblin JD, Raymond JL, Twenhafel NA, Garry RF, Hensley LE. Effect of Monkeypox Virus Preparation on the Lethality of the Intravenous Cynomolgus Macaque Model. Viruses. 2022;14:1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Mucker EM, Golden JW, Hammerbeck CD, Kishimori JM, Royals M, Joselyn MD, Ballantyne J, Nalca A, Hooper JW. A Nucleic Acid-Based Orthopoxvirus Vaccine Targeting the Vaccinia Virus L1, A27, B5, and A33 Proteins Protects Rabbits against Lethal Rabbitpox Virus Aerosol Challenge. J Virol. 2022;96:e0150421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Hutson CL, Kondas AV, Mauldin MR, Doty JB, Grossi IM, Morgan CN, Ostergaard SD, Hughes CM, Nakazawa Y, Kling C, Martin BE, Ellison JA, Carroll DS, Gallardo-Romero NF, Olson VA. Correction for Hutson et al., "Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model". mSphere. 2021;6:e00126-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Jamil H, Tariq W, Tahir MJ, Mahfooz RS, Asghar MS, Ahmed A. Human monkeypox expansion from the endemic to non-endemic regions: Control measures. Ann Med Surg (Lond). 2022;79:104048. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Parvin R, Ali A, Abdou Nagy ZZ, Zhao S, Paul AK, Hafez M. Monkeypox virus: A comprehensive review of taxonomy, evolution, epidemiology, diagnosis, prevention, and control regiments so far. Ger J Microbiol. 2022;. [DOI] [Full Text] |
| 20. | Patrono LV, Pléh K, Samuni L, Ulrich M, Röthemeier C, Sachse A, Muschter S, Nitsche A, Couacy-Hymann E, Boesch C, Wittig RM, Calvignac-Spencer S, Leendertz FH. Monkeypox virus emergence in wild chimpanzees reveals distinct clinical outcomes and viral diversity. Nat Microbiol. 2020;5:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 21. | Peter OJ, Kumar S, Kumari N, Oguntolu FA, Oshinubi K, Musa R. Transmission dynamics of Monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2022;8:3423-3434. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 65] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 22. | Saxena SK, Ansari S, Maurya VK, Kumar S, Jain A, Paweska JT, Tripathi AK, Abdel-Moneim AS. Reemerging human monkeypox: A major public-health debacle. J Med Virol. 2022;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 23. | Zachariou M. Monkeypox: Symptoms seen in London sexual health clinics differ from previous outbreaks, study finds. BMJ. 2022;378:o1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Vallée A, Farfour E, Zucman D. Monkeypox virus: A novel sexually transmitted disease? Travel Med Infect Dis. 2022;49:102394. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 40] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 25. | Nörz D, Pfefferle S, Brehm TT, Franke G, Grewe I, Knobling B, Aepfelbacher M, Huber S, Klupp EM, Jordan S, Addo MM, Schulze Zur Wiesch J, Schmiedel S, Lütgehetmann M, Knobloch JK. Evidence of surface contamination in hospital rooms occupied by patients infected with monkeypox, Germany, June 2022. Euro Surveill. 2022;27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 26. | Samaranayake L, Anil S. The Monkeypox Outbreak and Implications for Dental Practice. Int Dent J. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Bothra A, Maheswari A, Singh M, Pawar M, Jodhani K. Cutaneous manifestations of viral outbreaks. Australas J Dermatol. 2021;62:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Rodriguez-Morales AJ. Monkeypox and the importance of cutaneous manifestations for disease suspicion. Microbes Infect Chemother. 2022;2:e1450. [DOI] [Full Text] |
| 29. | Patel A, Bilinska J, Tam JCH, Da Silva Fontoura D, Mason CY, Daunt A, Snell LB, Murphy J, Potter J, Tuudah C, Sundramoorthi R, Abeywickrema M, Pley C, Naidu V, Nebbia G, Aarons E, Botgros A, Douthwaite ST, van Nispen Tot Pannerden C, Winslow H, Brown A, Chilton D, Nori A. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 303] [Article Influence: 101.0] [Reference Citation Analysis (0)] |
| 30. | Mande G, Akonda I, De Weggheleire A, Brosius I, Liesenborghs L, Bottieau E, Ross N, Gembu GC, Colebunders R, Verheyen E, Ngonda D, Leirs H, Laudisoit A. Enhanced surveillance of monkeypox in Bas-Uélé, Democratic Republic of Congo: the limitations of symptom-based case definitions. Int J Infect Dis. 2022;122:647-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Ortiz-Martínez Y, Rodríguez-Morales AJ, Franco-Paredes C, Chastain DB, Gharamti AA, Vargas Barahona L, Henao-Martínez AF. Monkeypox - a description of the clinical progression of skin lesions: a case report from Colorado, USA. Ther Adv Infect Dis. 2022;9:20499361221117726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Mileto D, Riva A, Cutrera M, Moschese D, Mancon A, Meroni L, Giacomelli A, Bestetti G, Rizzardini G, Gismondo MR, Antinori S. New challenges in human monkeypox outside Africa: A review and case report from Italy. Travel Med Infect Dis. 2022;49:102386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 33. | Isidro J, Borges V, Pinto M, Sobral D, Santos JD, Nunes A, Mixão V, Ferreira R, Santos D, Duarte S, Vieira L, Borrego MJ, Núncio S, de Carvalho IL, Pelerito A, Cordeiro R, Gomes JP. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28:1569-1572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 542] [Cited by in RCA: 468] [Article Influence: 156.0] [Reference Citation Analysis (0)] |
| 34. | Forni D, Molteni C, Cagliani R, Sironi M. Geographic structuring and divergence time frame of monkeypox virus in the endemic region. J Infect Dis. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 35. | Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 186] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 36. | Gul I, Liu C, Xi Y, Du Z, Zhai S, Lei Z, Qun C, Raheem MA, He Q, Zhang H, Zhang C, Wang R, Han S, Ke D, Qin P. Current and perspective sensing methods for monkeypox virus: a reemerging zoonosis in its infancy; 2022. Available from: arXiv: 220805228. |
| 37. | Brinkmann A. Differentialdiagnostik von pockentypischen Hautveränderungen mittels Metagenomanalyse und VAmpSeq. 2021. [DOI] [Full Text] |
| 38. | Bottlender R, Hoff P, Strauss A. Differentialdiagnostik von Paranoia und paranoider Schizophrenie mittels AMDP-Syndromen. In: Möller HJ, Engel RR, Hoff P. Befunderhebung in der Psychiatrie: Lebensqualität, Negativsymptomatik und andere aktuelle Entwicklungen. Springer: Vienna, 1996: 241-248. [DOI] [Full Text] |
| 39. | Shete A, Mohandas S, Jain R, Yadav PD. A qualitative IgG ELISA for detection of SARS-CoV-2-specific antibodies in Syrian hamster serum samples. STAR Protoc. 2021;2:100573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 40. | Adler H, Gould S, Hine P, Snell LB, Wong W, Houlihan CF, Osborne JC, Rampling T, Beadsworth MB, Duncan CJ, Dunning J, Fletcher TE, Hunter ER, Jacobs M, Khoo SH, Newsholme W, Porter D, Porter RJ, Ratcliffe L, Schmid ML, Semple MG, Tunbridge AJ, Wingfield T, Price NM; NHS England High Consequence Infectious Diseases (Airborne) Network. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153-1162. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 686] [Cited by in RCA: 739] [Article Influence: 246.3] [Reference Citation Analysis (0)] |
| 41. | Hraib M, Jouni S, Albitar MM, Alaidi S, Alshehabi Z. The outbreak of monkeypox 2022: An overview. Ann Med Surg (Lond). 2022;79:104069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 87] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 42. | Bragazzi NL, Kong JD, Mahroum N, Tsigalou C, Khamisy-Farah R, Converti M, Wu J. Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. J Med Virol. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 156] [Article Influence: 78.0] [Reference Citation Analysis (0)] |
| 43. | Shafaati M, Zandi M. Monkeypox virus neurological manifestations in comparison to other orthopoxviruses. Travel Med Infect Dis. 2022;49:102414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 44. | Bethineedi LD, Kutikuppala LVS, Kandi V. Monkeypox Epidemic: A Throwback From Smallpox Eradication. Cureus. 2022;14:e26577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 45. | Bano R, Jamil M, Kashif M, Qasim M, Khan M, Ali M, Jabeen N, Ahmad S, Naz R. The Zoonotic Disease Human Monkey Pox: An Insights into Epidemiological, Clinical, and Preventative Features. Pakistan J Med Health Sci. 2022;16:1289. [DOI] [Full Text] |
| 46. | Swain SK, Gadnayak A, Mohanty JN, Sarangi R, Das J. Does enterovirus 71 urge for effective vaccine control strategies? Rev Med Virol. 2022;32:e2322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Mariën J, Laudisoit A, Patrono L, Baelo P, Vredendaal Rv, Musaba P, Gembu G, Mande C, Ngoy S, Mussaw M, Van Houtte N, Van de Perre F, Gryseels S, Bottieau E, Calvignac-Spencer S, Leendertz F, Leirs H, Verheyen E. Muyembe-Tamfum J, Monkeypox viruses circulate in distantly-related small mammal species in the Democratic Republic of the Congo. 2021. [DOI] [Full Text] |
| 48. | Falendysz EA, Londoño-Navas AM, Meteyer CU, Pussini N, Lopera JG, Osorio JE, Rocke TE. Evaluation of monkeypox virus infection of black-tailed prairie dogs (Cynomys ludovicianus) using in vivo bioluminescent imaging. J Wildl Dis. 2014;50:524-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Arndt WD, Cotsmire S, Trainor K, Harrington H, Hauns K, Kibler KV, Huynh TP, Jacobs BL. Evasion of the Innate Immune Type I Interferon System by Monkeypox Virus. J Virol. 2015;89:10489-10499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 50. | Zuo W, Zhao X. Natural killer cells play an important role in virus infection control: Antiviral mechanism, subset expansion and clinical application. Clin Immunol. 2021;227:108727. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 48] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 51. | Gavin RH. The oral apparatus of Tetrahymena pyriformis, strain WH-6. IV. Observations on the organization of microtubules and filaments in the isolated oral apparatus and the differential effect of potassium chloride on the stability of oral apparatus microtubules. J Morphol. 1977;151:239-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 52. | Alakunle E, Moens U, Nchinda G, Okeke MI. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 431] [Article Influence: 86.2] [Reference Citation Analysis (0)] |
| 53. | Hammarlund E, Dasgupta A, Pinilla C, Norori P, Früh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci U S A. 2008;105:14567-14572. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 113] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 54. | Demaria O, Gauthier L, Debroas G, Vivier E. Natural killer cell engagers in cancer immunotherapy: Next generation of immuno-oncology treatments. Eur J Immunol. 2021;51:1934-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 106] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 55. | Kaynarcalidan O, Moreno Mascaraque S, Drexler I. Vaccinia Virus: From Crude Smallpox Vaccines to Elaborate Viral Vector Vaccine Design. Biomedicines. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 56. | Abdelaal A, Reda A, Lashin BI, Katamesh BE, Brakat AM, AL-Manaseer BM, Kaur S, Asija A, Patel NK, Basnyat S, Rabaan AA, Alhumaid S, Albayat H, Aljeldah M, Al Shammari BR, Al-Najjar AH, Al-Jassem AK, AlShurbaji ST, Alshahrani FS, Alynbiawi, Alfaraj ZH, Alfaraj DH, Aldawood AH, Sedhai YR, Mumbo V, Rodriguez-Morales AJ, Sah R. Preventing The Next Pandemic: Is Live Vaccine Efficacious Against Monkeypox, or There is a Need for Killed Virus and mRNA Vaccines? 2022. [DOI] [Full Text] |
| 57. | Nagata N, Saijo M, Kataoka M, Ami Y, Suzaki Y, Sato Y, Iwata-Yoshikawa N, Ogata M, Kurane I, Morikawa S, Sata T, Hasegawa H. Pathogenesis of fulminant monkeypox with bacterial sepsis after experimental infection with West African monkeypox virus in a cynomolgus monkey. Int J Clin Exp Pathol. 2014;7:4359-4370. [PubMed] |
| 58. | Gershon AA, Gershon MD. Widespread Use of Varicella Vaccine Does Not Reduce Immunity to Zoster of Others. J Infect Dis. 2022;225:361-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 59. | Russo AT, Grosenbach DW, Brasel TL, Baker RO, Cawthon AG, Reynolds E, Bailey T, Kuehl PJ, Sugita V, Agans K, Hruby DE. Effects of Treatment Delay on Efficacy of Tecovirimat Following Lethal Aerosol Monkeypox Virus Challenge in Cynomolgus Macaques. J Infect Dis. 2018;218:1490-1499. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 61. | Russo AT, Berhanu A, Bigger CB, Prigge J, Silvera PM, Grosenbach DW, Hruby D. Co-administration of tecovirimat and ACAM2000™ in non-human primates: Effect of tecovirimat treatment on ACAM2000 immunogenicity and efficacy versus lethal monkeypox virus challenge. Vaccine. 2020;38:644-654. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 62. | Hatch GJ, Graham VA, Bewley KR, Tree JA, Dennis M, Taylor I, Funnell SG, Bate SR, Steeds K, Tipton T, Bean T, Hudson L, Atkinson DJ, McLuckie G, Charlwood M, Roberts AD, Vipond J. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol. 2013;87:7805-7815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 63. | Petersen E, Zumla A, Hui DS, Blumberg L, Valdoleiros SR, Amao L, Ntoumi F, Asogun D, Simonsen L, Haider N, Traore T, Kapata N, Dar O, Nachega J, Abbara A, Al Balushi A, Kock R, Maeurer M, Lee SS, Lucey DR, Ippolito G, Koopmans M. Vaccination for monkeypox prevention in persons with high-risk sexual behaviours to control on-going outbreak of monkeypox virus clade 3. Int J Infect Dis. 2022;122:569-571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 64. | Rizk JG, Lippi G, Henry BM, Forthal DN, Rizk Y. Prevention and Treatment of Monkeypox. Drugs. 2022;82:957-963. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 325] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 65. | Realegeno S, Puschnik AS, Kumar A, Goldsmith C, Burgado J, Sambhara S, Olson VA, Carroll D, Damon I, Hirata T, Kinoshita T, Carette JE, Satheshkumar PS. Monkeypox Virus Host Factor Screen Using Haploid Cells Identifies Essential Role of GARP Complex in Extracellular Virus Formation. J Virol. 2017;91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 66. | Hutson CL, Kondas AV, Mauldin MR, Doty JB, Grossi IM, Morgan CN, Ostergaard SD, Hughes CM, Nakazawa Y, Kling C, Martin BE, Ellison JA, Carroll DS, Gallardo-Romero NF, Olson VA. Pharmacokinetics and Efficacy of a Potential Smallpox Therapeutic, Brincidofovir, in a Lethal Monkeypox Virus Animal Model. mSphere. 2021;6:e00927-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 68] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 67. | Davi SD, Kissenkötter J, Faye M, Böhlken-Fascher S, Stahl-Hennig C, Faye O, Sall AA, Weidmann M, Ademowo OG, Hufert FT, Czerny CP, Abd El Wahed A. Recombinase polymerase amplification assay for rapid detection of Monkeypox virus. Diagn Microbiol Infect Dis. 2019;95:41-45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 85] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 68. | Smith SK, Self J, Weiss S, Carroll D, Braden Z, Regnery RL, Davidson W, Jordan R, Hruby DE, Damon IK. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J Virol. 2011;85:9176-9187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 69. | Berhanu A, Prigge JT, Silvera PM, Honeychurch KM, Hruby DE, Grosenbach DW. Treatment with the smallpox antiviral tecovirimat (ST-246) alone or in combination with ACAM2000 vaccination is effective as a postsymptomatic therapy for monkeypox virus infection. Antimicrob Agents Chemother. 2015;59:4296-4300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 70. | Mucker EM, Wollen-Roberts SE, Kimmel A, Shamblin J, Sampey D, Hooper JW. Intranasal monkeypox marmoset model: Prophylactic antibody treatment provides benefit against severe monkeypox virus disease. PLoS Negl Trop Dis. 2018;12:e0006581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 71. | Kampf G. Efficacy of biocidal agents and disinfectants against the monkeypox virus and other orthopoxviruses. J Hosp Infect. 2022;127:101-110. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 72. | Oprea C, Ianache I, Piscu S, Tardei G, Nica M, Ceausu E, Popescu CP, Florescu SA. First report of monkeypox in a patient living with HIV from Romania. Travel Med Infect Dis. 2022;49:102395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |