Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.310
Peer-review started: August 12, 2022
First decision: August 29, 2022
Revised: September 7, 2022
Accepted: September 13, 2022
Article in press: September 13, 2022
Published online: September 25, 2022
Processing time: 42 Days and 20.1 Hours
Pregnant women are among the high-risk population for severe coronavirus disease 2019 (COVID-19) with unfavorable peripartum outcomes and increased incidence of preterm births. Hemolysis, the elevation of liver enzymes, and low platelet count (HELLP) syndrome and severe preeclampsia are among the leading causes of maternal mortality. Evidence supports a higher odd of pre-eclampsia in women with COVID-19, given overlapping pathophysiology. Involvement of angiotensin-converting enzyme 2 receptors by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) for the entry to the host cells and its downregulation cause dysregulation of the renin-angiotensin-aldosterone system. The overexpression of Angiotensin II mediated via p38 Mitogen-Activated Protein Kinase pathways can cause vasoconstriction and uninhibited platelet aggregation, which may be another common link between COVID-19 and HELLP syndrome. On PubMed search from January 1, 2020, to July 30, 2022, we found 18 studies on of SARS-COV-2 infection with HELLP Syndrome. Most of these studies are case reports or series, did not perform histopathology analysis of the placenta, or measured biomarkers linked to pre-eclampsia/HELLP syndrome. Hence, the relationship between SARS-CoV-2 infection and HELLP syndrome is inconclusive in these studies. We intend to perform a mini-review of the published literature on HELLP syndrome and COVID-19 to test the hypothesis on association vs causation, and gaps in the current evidence and propose an area of future research.
Core Tip: Observational studies showed an increased prevalence of preeclampsia and hemolysis, elevated liver enzymes and low platelet (HELLP) syndrome in pregnant women with coronavirus disease 2019 (COVID-19). Despite a possible pathophysiology linkage between COVID-19 and HELLP syndrome, the evidence on temporality to prove a causal association between infection with severe acute respiratory syndrome coronavirus 2 and HELLP syndrome is lacking.
- Citation: Nasa P, Juneja D, Jain R, Nasa R. COVID-19 and hemolysis, elevated liver enzymes and thrombocytopenia syndrome in pregnant women - association or causation? World J Virol 2022; 11(5): 310-320
- URL: https://www.wjgnet.com/2220-3249/full/v11/i5/310.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i5.310
With immense knowledge on the pathogenesis of coronavirus disease 2019 (COVID-19), the viral-host immune interaction plays a critical role in multi-system presentation of the disease. Most of the patients, infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), develop a non-severe illness. However, those patients with specific comorbidities are predisposed to advanced stages of severe COVID-19 infection. Some of the prevalently-reported comorbidities are as follows; age above 75 years, male gender, pre-existing cardiovascular disease, chronic lung, kidney or liver disease, sickle cell disease, diabetes, active cancer, severe obesity and pregnancy[1,2]. The risk factors that aggravate the development of severe COVID-19 among pregnant women include obesity, smoking history, pre-eclampsia and diabetes mellitus[3]. Though pregnancy, per se, does not increase the susceptibility to SARS-CoV-2 infection, pregnant women are highly prone to developing severe illnesses with SARS-CoV-2 infection compared to non-pregnant women. Further, they are also associated with adverse pregnancy and perinatal outcomes[4].
Hemolysis, Elevated Liver enzymes and Low Platelets (HELLP) syndrome is an uncommon yet deadly complication that is associated with severe pre-eclampsia. Early diagnosis and termination of pregnancy only have been proved to be effective in treating HELLP syndrome[5]. A meta-analysis, conducted recently, inferred that COVID-19 infected women recorded high levels of pre-eclampsia and HELLP syndrome odds[6]. However, abnormal liver enzymes, thrombocytopenia and hemolysis are not only associated with HELLP syndrome, but are observed in many of the critically-ill patients, as a component of multi-organ dysfunction. This phenomenon occurs especially in case of certain infectious diseases and other pregnancy-related liver disorders, for instance, acute fatty liver of pregnancy[7]. Substantial evidence infers that some of the viral infections, for instance SARS-CoV-2, tend to mimic HELLP syndrome among women during pregnancy[8,9].
Hence, the overlapping laboratory features of SARS-CoV-2 infection and HELLP syndrome may increase the possibilities of misdiagnosis than a causal association. The current review discusses about the pathogenetic linkage between COVID-19 and HELLP syndrome, reviews the evidences available on association or causation between the variables and proposes novel suggestions for future research.
Pre-eclampsia is a multi-system disorder characterized by de novo hypertension that occurs after 20 wk of gestation. Recently, the International Society for the Study of Hypertension in Pregnancy provided a new definition for pre-eclampsia as given herewith; new onset of hypertension (systolic > 140 mmHg and diastolic > 90 mmHg) accompanied by at least one feature as listed below and is developed either at or after 20 wk of gestation: (1) Proteinuria; (2) Maternal organ dysfunction (like liver, kidney, neurological and haematological); and (3) Evidence of uteroplacental dysfunctions like fetal growth restriction or abnormal Doppler waveform findings of uteroplacental blood flow or stillbirth[10].
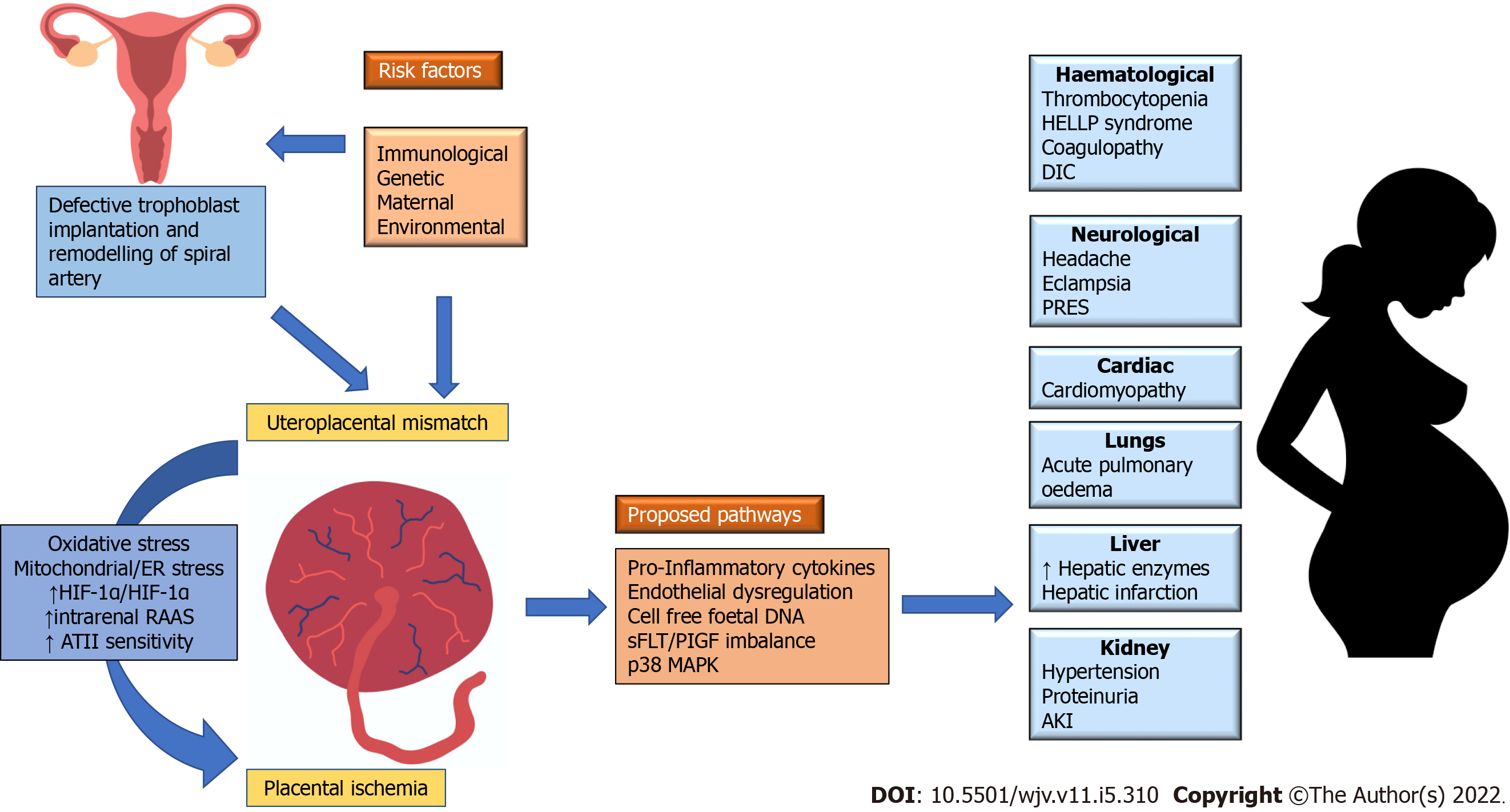
The exact pathogenesis of pre-eclampsia remains uncertain. However, the termination of pregnancy by removing the placenta seems to be an effective therapeutic measure. This method confirms the importance of placenta in the pathophysiology of pre-eclampsia. Two pathogenic phenotypes are established such as early and late pre-eclampsia. The major cause of early pre-eclampsia is placental in nature whereas the late pre-eclampsia is a result of interactions that occur between placental senescence and other factors such as genetics, obesity and nutrition or environmental factors. The oxidative stress upon syncytiotrophoblast, a cell that covers the placental villi on the maternal side, plays a crucial role by getting released into maternal circulation factors like inflammatory cytokines, cell-free fetal DNA, exosomes, and anti-angiogenic agents. This results in the endothelial dysfunction and hypertensive syndrome[11].
Oxidative stress occurs as a result of either uteroplacental hypoperfusion from the defective remodelling of uterine spiral arteries (i.e., early pre-eclampsia) or due to a mismatch between supply and demand in maternal perfusion and placental or foetus requirements (i.e., late pre-eclampsia). Placental stress results in the dysfunction of vascular endothelium which in turn releases the placental factors that cause systemic manifestations of pre-eclampsia. The pathways proposed earlier for the above discussed phenomenon include an increased release of pro-inflammatory cytokines, cell-free fetal DNA, p38 Mitogen-Activated Protein Kinase (MAPK), placental apoptotic debris, soluble receptor for Vascular Endothelial Growth Factor, and soluble fms like tyrosine kinase (sFlt-1)/Placental Growth Factor (PlGF) ratio (Figure 1)[11,12].
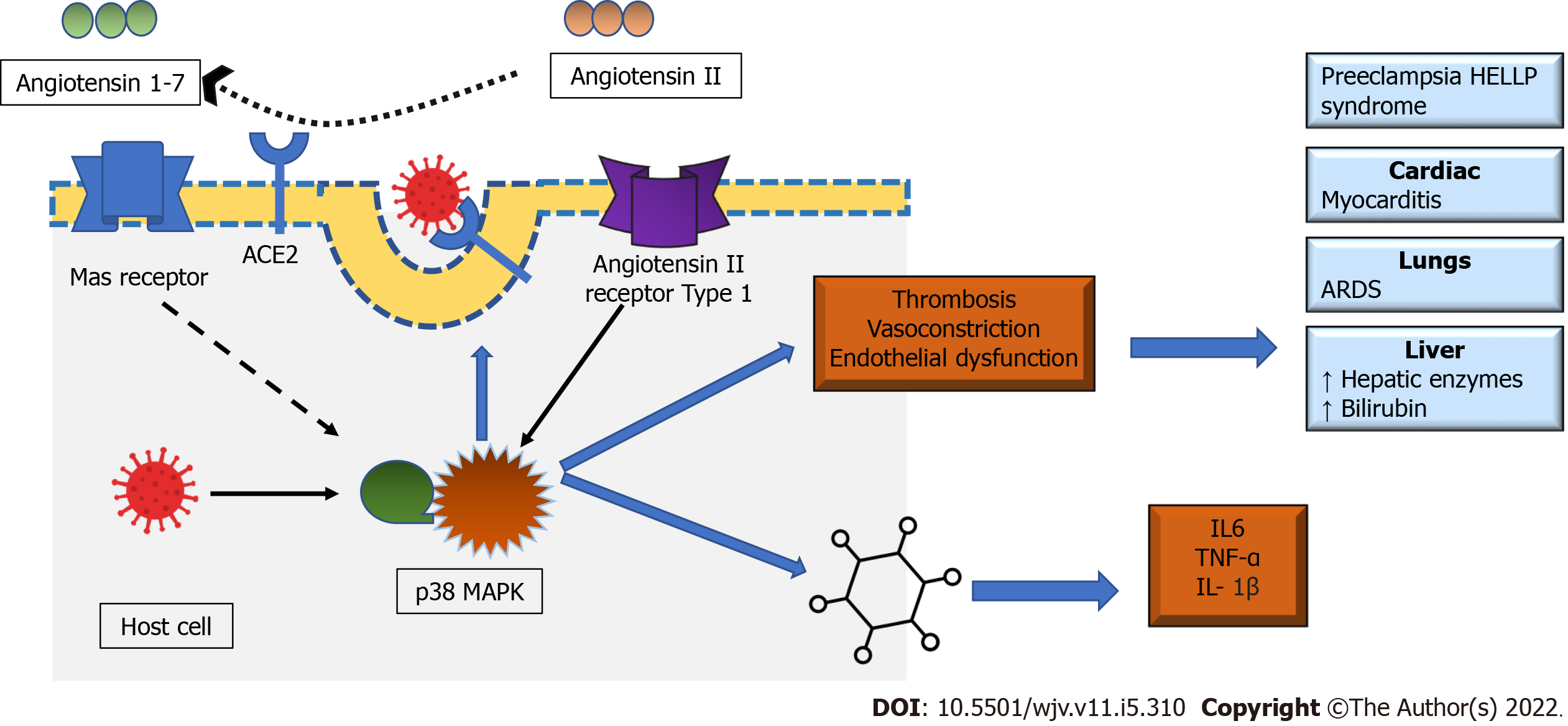
The role played by Renin-Angiotensin-Aldosterone System (RAAS) in placenta homeostasis is crucial since it regulates the proliferation of trophoblasts, angiogenesis and blood flow. When RASS is not regulated, it creates an imbalance of vasoactive peptides due to high production of angiotensin II (ATII) and low vasodilatory angiotensin 1-7. ATII is a pro-inflammatory, pro-thrombotic element that induces vascular constriction, endothelial injury and vascular smooth cell proliferation which altogether contribute to pre-eclampsia[13]. Recent evidence suggests that ATII actions are mediated through the MAPK pathway. MAPK is a cellular signaling pathway existing in three forms, p38 MAPK, extracellular signal-regulated kinase, and Janus kinase. p38 MAPK critical component in immune functions as well as stress response pathways, mediates the cellular response to pathogenic microbes, pro-inflammatory cytokines and environmental stress (oxidate stress). p38 MAPK can be stimulated by intrauterine oxidate stress, with exact function unknown. Available evidence supports p38 MAPK is linked to normal embryonic development and maintaining parturition, and premature activation, or overexpression may lead to adverse perinatal and pregnancy outcomes[14]. The upregulated p38 MAPK pathway is linked with increased pro-inflammatory cytokines like NF-κB, Tumour Necrosis Factor (TNF)-α, interleukin (IL)-6 and IL-1β, and COX-2. The activation of NF-κB with p38 MAPK overexpression is found in various tissues, but in uterine tissue, its role is unclear. On the other hand, Angiotensin 1-7 is vasodilatory, attenuate this inflammation, atrophy, and fibrosis by simulating the Mas receptor. Hence, the dysregulation of RASS and high ATII levels lead to uninhibited feedback loop to p38 MAPK pathway which in turn causes untamed inflammation observed in pre-eclampsia[15,16].
The association between pre-eclampsia and HELLP syndrome is unclear. According to a few experts, HELLP syndrome is nothing but an extended manifestation of severe pre-eclampsia. However, a few others argue that HELLP syndrome is an independent entity since it exists without the classical features of pre-eclampsia like proteinuria and oedema. A few resemblances exist between the pathogenesis of pre-eclampsia and HELLP syndrome such as endothelial dysfunction, platelet aggregation and consumption, vasospasm, and end-organ ischemia. However, immune dysregulation with maternal immunological intolerance to fetal tissues is considered as a prominent pathway in HELLP syndrome. This immunological maladaptation has been proved in literature via the high levels of fetal mRNA and HLA-DR in the blood of women with HELLP syndrome, who was compared with women with pre-eclampsia[16,17]. One of the recent studies demonstrated that those patients with HELLP syndrome, had a high titer of agonist antibodies to Type I ATII receptor (AT1r-AA), when compared with patients with pre-eclampsia. The agonist antibodies can simulate the ATII effect upon the receptor[18].
Women with HELLP syndrome possess high levels of other types of anti-angiogenesis factors such as endoglin and Fas ligand than the women with pre-eclampsia. These two factors are responsible for vascular endothelial injury and intense inflammation in HELLP syndrome. The role played by p38 MAPK pathway, in the pathogenesis of HELLP syndrome, is hypothesized to be an angiogenic response for environmental hypoxia. The elevated serum levels of p38 MAPK increase the serum vascular permeability and it has the potential to aggravate edema in different tissues including the brain. A recent study that compared the serum levels of p38 MAPK among patients with HELLP syndrome and pre-eclampsia found that the serum levels were significantly higher in HELLP syndrome patients than their counterpart. The authors also recommended to use serum p38 MAPK in the diagnosis of HELLP syndrome[19]. As per the literature, patients with HELLP syndrome exhibit high serum levels of p38 MAPK and low expression in placental p38 MAPK[20,21]. The future researchers must explore this relationship which may shed more insights about the role played by p38 MAPK in the pathophysiology of HELLP syndrome. Furthermore, the activation of immune complexes, C5b-9 complement pathway, anaphylatoxins like C3a and C5a and the release of inflammatory cytokines, TNF-α and active von Willebrand factor from leucocytes, macrophages and platelets also cause endothelial injury. In turn, endothelial injury contributes to multiple activities such as hemolysis, platelet aggregation and consumption (causing thrombocytopenia), intraluminal fibrin deposition, vasospasm and end-organ ischemia (causing hepatitis) that are generally observed in HELLP syndrome[21].
Conventional pre-eclampsia screening includes a periodic assessment and an early detection of hypertension and proteinuria. But, the precision of pre-eclampsia screening has increased tremendously, thanks to the measurement of circulating biomarkers and Doppler assessment of uteroplacental circulation. sFlt-1/PlGF ratio is a potential and a highly-accurate marker that can be used in the prediction of pre-eclampsia and fetal growth restriction[22]. In the prediction of early pre-eclampsia and the complications associated with it, a combination of multiple factors such as demographic risk factors with periodic blood pressure measurement, doppler assessment of uterine artery and the measurements of biomarkers is found to be highly accurate[23].
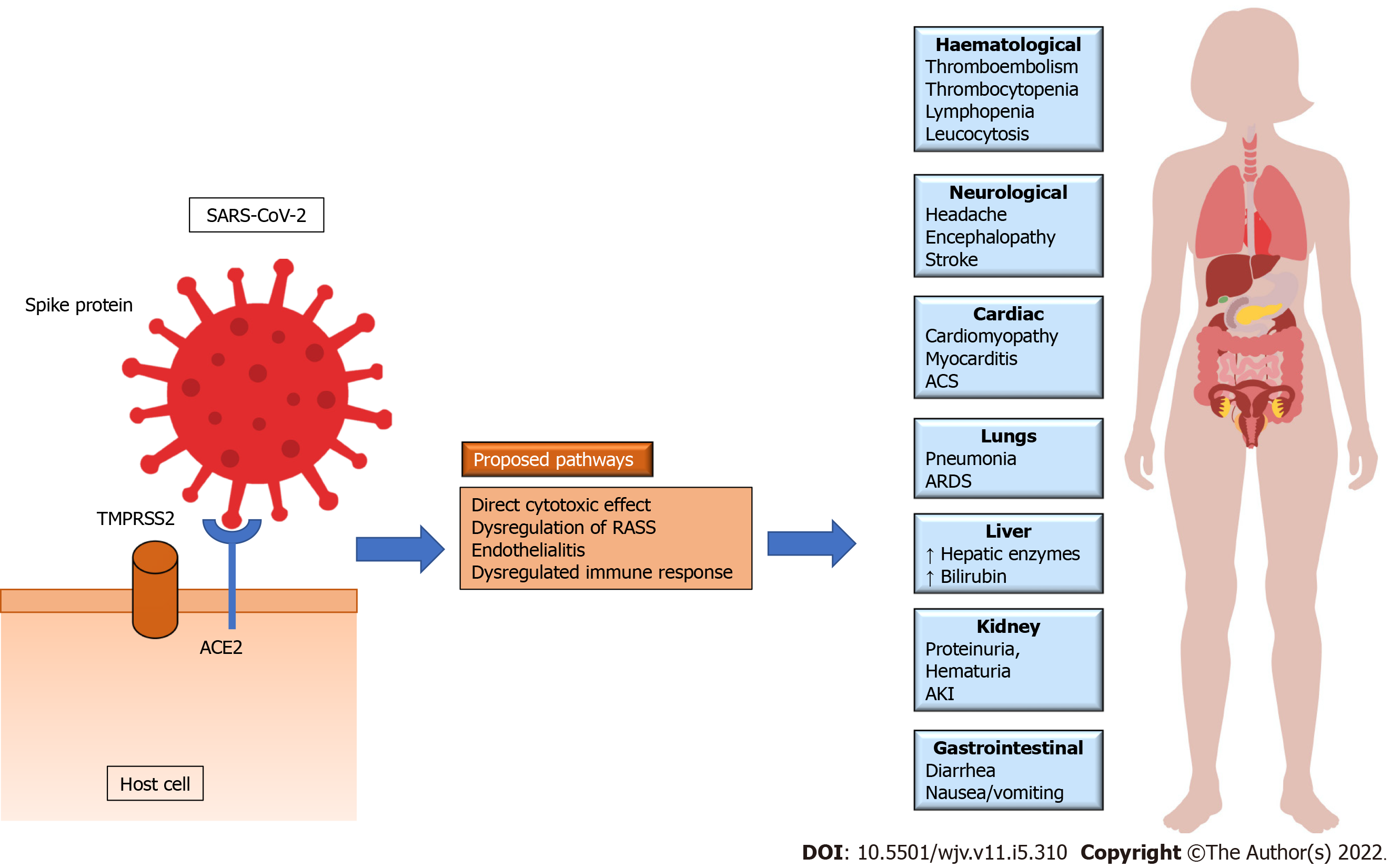
The internalization of SARS-CoV-2, within the host cell, occurs by binding the S-spike protein of the virus with Angiotensin-Converting-Enzyme 2 (ACE2) present on the cell surface and is supplemented by Transmembrane Serine Protease 2 (TMPRSS2) on the host cell. Though ACE2 is found in multiple tissues, it is predominantly expressed in lung and heart tissues. This phenomenon may explain the high incidence of acute respiratory distress syndrome and myocarditis among patients with COVID-19 and the primary cause behind the high mortality rate. ACE2 is an integral part of RAAS and is directly associated in the conversion of ATII to Angiotensin 1-7. Like SARS-CoV, when SARS-CoV-2 interacts with ACE2 receptor, the receptor gets downregulated, thus potentiating RAAS and ATII. All three MAPK pathways are involved in the pathogenesis of COVID-19. The interaction between SARS-CoV-2 and ACE2, like many other viruses, is associated with upregulation of p38 MAPK through the interaction with ACE2 receptors and its direct activation[16,24]. The upregulated ATII through its effect on ATII Type 1 receptor causes an intense vasoconstriction and inflammation. As discussed earlier, the effect of ATII in heart and lung tissues are mediated by p38 MAPK pathway. The crosstalk between p38 MAPK and NF-κB is also found to be involved in the pathophysiology of COVID-19. SARS-CoV and SARS-CoV-2 infection activates p38 MAPK pathway and induces phosphorylation of various down-stream proteins involved in the transcription of various inflammatory cytokines. The upregulation of p38 MAPK is linked with excessive vasoconstriction, production of pro-inflammatory cytokines such as IL6, TNF-α and IL-1β. Hence, an unrestrained p38 MAPK results in hyperinflammation, vasoconstriction and thrombosis, a hallmark of COVID-19 (Figure 2)[16]. Recently, various agents like emetine, chelerythrine and papaverine regulating the p38 MAPK signaling pathway are found to have therapeutic potential in the management of COVID-19[25-27].
The role played by virus-host immune interplays is crucial in the pathogenesis of COVID-19. Various pro-inflammatory cytokines like IL-6, IL-10, TNF-α, granulocyte-colony stimulating factors and monocyte chemoattract protein 1 mediate lungs and other systemic manifestations of SARS-CoV-2 infection. Though respiratory system is the primary target site of SARS-CoV-2 infection, COVID-19 can be characterized as a multi-system disease that affects heart, kidneys, brain, liver, gastrointestinal and haematological systems and skin (Figure 2)[28].
COVID-19 patients generally exhibit different biochemical manifestations of pre-eclampsia and HELLP syndrome such as thrombocytopenia, raised liver enzymes, proteinuria, coagulopathy, acute kidney injury, and increased lactate dehydrogenase[8,29]. Mild thrombocytopenia (the count of platelets stands at 100-150 × 109/L) is observed among 20%-36% patients with COVID-19 whereas severe thrombocytopenia (< 50 × 109/L) is uncommon[30].
A total of 11 studies was found by the authors when PubMed database was mined using the following keywords; “COVID-19” OR “SARS-CoV-2” AND “HELLP syndrome” between 01st January 2020 to 30th July 2022. When a broader keyword i.e., “HELLP syndrome” was used within the same period, a total of 361 studies was found. Out of the total studies filtered, 18 studies were finalized and critically analyzed after excluding non-COVID-19 studies and non-English literature (Table 1)[6, 31-47].
| Ref. | Type of study | Age (yr), gestation (wk) | Number of patients | Main results | Conclusion |
| Mendoza et al[31], 2020 | Case series | 5 cases with severe PE and/or HELLP syndrome | Out of 8 cases with severe COVID-19, 5 developed PE, proteinuria, elevated liver enzymes and hypertension. One developed platelet less than 150000. However, only one patient had PE based on the uterine artery pulsatility index, sFlt-1/PlGF ratio and LDH | PE like clinical features can develop with severe COVID-19. It can be distinguished from true by PE by sFlt-1/PlGF, LDH and UtAPI measurement | |
| Braga et al[32], 2020 | Case report | 31, 31 | 1 | Multiple pregnancy (dichorionic twins) with PE and partial HELLP syndrome. Moderate COVID-19 with HRCT showing ground-glassing. Underwent caesarean delivery for HELLP syndrome. One of the foetus died on day 16 due to intracranial hemorrhage. Both women and other foetus survived | There is a possible synergism between the pathophysiology of COVID-19 and PE/HELLP syndrome |
| Federici et al[33], 2020 | Case report | 33, 23.5 | 1 | Multigravida, severe COVID-19 with ARDS requiring mechanical ventilation develop features of PE and HELLP syndrome. The serum sFlt-1/PlGF ratio was normal. Pregnancy continued and laboratory abnormalities resolved spontaneously with removal of mechanical ventilation after 10 d and discharge on day 19. Mother delivered spontaneously a live foetus at 33.4 wk | Severe COVID-19 can mimic PE and HELLP syndrome. Pregnancy can be continued in absence of complications with strict surveillance |
| Ahmed et al[34], 2020 | Case report | 26, 37 | 1 | Family history of PE, atypical HELLP syndrome with acute kidney injury. Vaginal delivery with induction Postpartum day 3, developed abdominal hematoma requiring laparotomy and blood transfusions. Moderate respiratory symptoms with foetus and mother survived | Severe SARS-CoV-2 infection may be a risk factor for hypertensive disorders of pregnancy |
| Ronnje et al[35], 2020 | Case report | 26, 32.6 | 1 | Underwent emergency caesarean. Both mother and foetus survived | Possible association of HELLP syndrome and COVID-19 was proposed |
| Coronado-Arroyo et al[36], 2021 | Case series | Mean: 29 yr, gestation 31 wk | 14 out of 20 patients with severe PE including 5 with HELLP syndrome | One out of 5 women was multipara. Two were asymptomatic and remaining had mild severity COVID-19. Four required caesarean delivery and two had still-birth. No maternal mortality | SARS-CoV-2 infection, can predisposes pregnant female to a greater severity of PE, irrespective of the severity of respiratory symptoms |
| Norooznezhad et al[37], 2021 | Case report | 24, 29 | 1 | Primigravida, emergency caesarean for HELLP syndrome. Ostelmavir, lopinavir/ritonavir, chloroquine and 0.5 gm/d of methylprednisolone was used. Moderate respiratory symptoms. Both foetus and mother survived | Association between COVID-19 and HELLP syndrome cannot be concluded but deliver and methylprednisolone caused improvement in the condition |
| Farahani et al[38], 2021 | Case report | 28, 38 | 1 | Multigravida, vaginal delivery for HELLP syndrome. Postpartum developed seizure, lopinavir/ritonavir and dexamethasone was used for treatment. Moderate respiratory symptoms. Both mother and foetus survived | COVID-19 in pregnant women can resemble PE and with possible CNS involvement |
| Aydın et al[39], 2021 | Observational retrospective study | Case 1: 22, 31 Case 2: 25, 28 | 167 pregnant with COVID-19. 20 patients had PE and two (1.2%) had HELLP syndrome. | Case 1: Pregnancy with IVF. Need invasive mechanical ventilation, underwent caesarean delivery for HELLP syndrome and postpartum developed arterial thrombosis. Case 2: Vaginal delivery with preterm foetus. Both patients survived | No significant difference was observed in adverse pregnancy outcomes such as PE, preterm birth, and foetal growth restriction, gestational diabetes mellitus and HELLP syndrome according to the gestational age |
| Vaezi et al[40], 2021 | Case series | 36, 28 | 24 patients, 1 with HELLP syndrome | Delivery by caesarean section, performed for HELLP syndrome, preterm foetus admitted to NICU. Both mother and foetus survived | - |
| Jering et al[41], 2021 | Retrospective cohort study | 406 446 women hospitalized for childbirth. Among women with HELLP syndrome, 989 (0.2%) were without COVID-19 and 33 (0.5%) with COVID-19 | Unadjusted and adjusted OR for HELLP syndrome with COVID-19 was 2.10 (95%CI- 1.48-2.97) and 1.96 (1.36-2.81), P < 0.001 | In large US cohort of women admitted for childbirth during the pandemic, patients with COVID-19 had higher risk of in-hospital mortality, pre-eclampsia, VTE and HELLP syndrome | |
| Bhardwaj et al[42], 2022 | Case report | 33, 36 | 1 | Underwent caesarean delivery. Both mother and foetus survived | COVID-19 and HELLP overlap and associations are puzzling to clinicians |
| Conde-Agudelo et al[6], 2022 | Meta-analysis of observational studies | 28 studies, 790954 patients including One study for HELLP syndrome | SARS-CoV-2 infection during pregnancy was associated with significant increase in the odd ratio of PE (1.58, 95%CI- 1.39-1.8), severe PE (1.76, 95%CI 1.18-2.63), eclampsia (1.97, 95%CI 1.01-3.84) and HELLP syndrome (2.76, 95%CI 1.48-2.97) | SARS-CoV-2 infection during pregnancy is associated with significantly higher odds of PE | |
| Madaan et al[43], 2022 | Case series | Case 1: 32, 34 Case 2: 29, 37 Case 3: 26, 39 | 3 | All three cases had HELLP syndrome and ground glassing opacities on HRCT with RT-PCR positive for SARS-COV-2. Case 1: Severe COVID-19, mother survived, baby still born by caesarean section. Case 2: Patient developed eclampsia and required mechanical ventilation, died on day -8, baby delivered vaginally Case 3: Patient survived and discharged day 15, baby delivered alive by caesarean section due to transverse lie | Authors proposed a synergism in the pathophysiology of COVID-19 and HELLP Syndrome. and combination of both can cause morbidity or mortality risk to fetus and the mother |
| Takahashi et al[44], 2022 | Case report | 27, 37 | 1 | Underwent caesarean delivery for infection control measures. Postpartum HELLP syndrome. Both mother and foetus survived | Overlap of clinical features with COVID-19 and HELLP syndrome is plausible explanation |
| Guida et al[45], 2022 | Nested case-control analysis | - | 203 women with COVID-19, including 21 with PE and 2 HELLP syndrome | There was no difference in the rate of PE and HELLP syndrome in women with or without COVID-19. However, imminent eclampsia was more frequent complication and overall maternal perinatal outcomes were worse with patients with PE and COVID-19 | Prevalence of PE among women with COVID-19 was around 10%. Chronic hypertension and obesity were more likely associated with PE. High caesarean rate and NICU admissions due to prematurity in women with COVID-19 |
| Snelgrove et al[46], 2022 | Retrospective cohort study | - | 157779 patients during the pandemic compared to 563859 patients delivered between March 2015-september 2019 (historical group) | There was no difference in the rate of PE/HELLP (879, 0.6%) syndrome and severe maternal morbidity (SMM) between the pandemic and historical group (3119, 0.6%). No difference between primiparous and multiparous on severe maternal morbidity and risk of PE/HELLP syndrome. Maternal age, rurality, preexisting comorbidities and use of artificial reproduction therapy were associated with increased risk of PE/HELLP syndrome | Changes in obstetrical care during the pandemic have not increased the risk the PE/HELLP syndrome and adverse maternal outcomes |
| Arslan[47], 2022 | Case report | 30, 32 | 1 | Mutigravida pregnancy, emergency Caeserian delivery. Foetus tested positive for SARS-CoV-2 and died 5 d after delivery. Mother had severe COVID-19, required invasive mechanical ventilation and died, 10 d after delivery | Severe COVID-19 as etiological causation of HELLP syndrome is presumptive |
Out of the 18 studies considered for final analysis, 13 were case reports or series in which 23 patients were included[31-38,40,42,44,45,47]. Maternal and fetal mortality rates were 8.6% (2) and 21.7% (5) respectively, with the development of severe COVID-19 in three patients. Mendoza et al[31] authored a case series in which five patients were suspected with pre-eclampsia and HELLP syndrome whereas only one had actual pre-eclampsia features based on the Doppler assessment of uterine artery pulsatility index, sFlt-1/PlGF ratio and lactate dehydrogenase. However, another case report failed to find the elevated sFlt-1/PlGF ratio in a patient who exhibited the biochemical features of HELLP syndrome. The patient was managed conservatively and her biochemical abnormalities were resolved spontaneously while the patient achieved a good perinatal outcome[33]. Most of the studies confirmed the existence of a linkage between HELLP syndrome and COVID-19. However, the inference from individual cases without a case-control remains highly biased. Two retrospective cohort studies, in which women with and without COVID-19 were compared, reported conflicting results on the increased incidence of HELLP syndrome with COVID-19[41,46]. In a population-based study authored by Snelgrove JW et al[46], no increased incidence of pre-eclampsia and HELLP syndrome was observed among women infected with SARS-CoV-2 compared to historical controls. On the other hand, in a large registry developed upon hospitalized women for childbirth in the United States, highly-adjusted odds of pre-eclampsia [1.21, 95% confidence interval (CI) 1.11-1.33] and HELLP syndrome (1.96, 95%CI 1.36-2.81) were found in pregnant women with COVID-19 compared to those without COVID-19, during the same duration[41]. A recent meta-analysis, in which 28 studies were included which covered a total of 790954 pregnant women, reported a significantly-high risk of pre-eclampsia (pooled odd ratio (OR) 1.62, 95%CI 1.45-1.82, P < 0.00001, 26 studies) with SARS-CoV-2 infection compared to non-infected individuals[6]. A single study outcomes from Jering et al[41], reported highly-unadjusted odds of HELLP syndrome (2.10, 95%CI 1.48-2.97), in pregnant women with SARS-CoV-2 infection.
Recent evidences confirm the worst clinical outcomes for pregnant women with COVID-19 in terms of high incidence of pre-eclampsia, preterm birth and the need for caesarean delivery[48,49].
ACE2 receptors and TMPRSS2, which are required for the entry of SARS-CoV-2 into human cells, are expressed in placental components including villous cytotrophoblasts, syncytiotrophoblasts and extravillous trophoblasts[50]. This makes the placenta, predisposed to SARS-CoV-2 infection. When S-spike protein of SARS-CoV-2 binds with ACE2 receptor, it results in the downregulation of the receptor, dysfunction of RAAS and triggering of local placental inflammation. Further, ATII type I -receptor and sFlt-1 are also heavily produced from the infected placenta. The increased serum levels of AT1r-AA, found in cases of SARS-CoV-2 infection, can be observed in pre-eclampsia and HELLP syndrome too[7].
Some evidence supports the presence of high levels of placental ACE2 in women with COVID-19. This may explain the increased association between pre-eclampsia and preterm birth[51]. Another study showed that ACE2 receptors and the expression of protease are dependent upon each other during gestational age. The increased levels of expression is prevalent during the first trimester compared to the rest of the trimesters in pregnancy[52]. In a molecular linkage study by Beys-da-Silva et al[53], SARS-CoV-2 infection was found to interact with multiple pathways that are involved in pre-eclampsia and HELLP syndrome pathogenesis like upregulation of sFlt-1 and endoglin, angiogenesis, the balance between vasoconstrictive peptides and nitric oxide modulators, hypoxia and inflammation and prothrombotic-related molecules.
There exist a few similarities in the pathophysiology of COVID-19 and HELLP syndrome. The interaction between ATII and p38 MAPK is a plausible linkage among COVID-19, preeclampsia and HELLP (Figure 3)[16]. The upregulation of p38 MAPK pathway is also linked with endothelial injury which in turn causes platelet aggregation and arterial thrombosis. This scenario reveals the systemic manifestations of COVID-19 like thrombocytopenia and raised liver enzymes[54]. However, it is still unclear whether the above-discussed biochemical abnormalities are manifestations of COVID-19 or HELLP syndrome. There is a lack of temporal studies in this domain that can establish a causal relationship between COVID-19 and HELLP syndrome. The studies conducted earlier that can prove that exposure occurred before the outcome (HELLP syndrome) establishing the temporality are missing. So, it is crucial to identify the causal association since immediate termination of the pregnancy is the only successful treatment used for HELLP syndrome, a predominant placental pathology, so far. However, an expectant and a watchful continuation of pregnancy with better perinatal outcomes may be considered in selected cases of COVID-19[33].
Future studies should explore this linkage using the principle of temporality and circulatory biomarkers like serum p38 MAPK, sFlt-1/PIGF ratio and/or doppler assessment of uteroplacental hypoxia to identify any causal association between COVID-19 and HELLP syndrome.
There exists an association among SARS-CoV-2 infection during pregnancy, pre-eclampsia and HELLP syndrome. Evidence accepts the plausible overlap in the pathogenesis of COVID-19 and HELLP syndrome through ACE2 and RAAS dysregulation that involve ATII and p38 MAPK pathways. However, no prospective studies are available based on screening biomarkers and temporality to prove the causal relationship in this domain. Future studies should establish a temporal relationship between SARS-CoV-2 infection and the development of HELLP syndrome including circulatory biomarkers and tissue or radiological documentation of uteroplacental insufficiency.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: United Arab Emirates
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D, D
Grade E (Poor): 0
P-Reviewer: Mehri-Ghahfarrokhi A, Iran; Mukhopadhyay A, India; Shariati MBH, Iran; Valipour M, Iran S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
| 1. | Gao YD, Ding M, Dong X, Zhang JJ, Kursat Azkur A, Azkur D, Gan H, Sun YL, Fu W, Li W, Liang HL, Cao YY, Yan Q, Cao C, Gao HY, Brüggen MC, van de Veen W, Sokolowska M, Akdis M, Akdis CA. Risk factors for severe and critically ill COVID-19 patients: A review. Allergy. 2021;76:428-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 885] [Article Influence: 221.3] [Reference Citation Analysis (0)] |
| 2. | Booth A, Reed AB, Ponzo S, Yassaee A, Aral M, Plans D, Labrique A, Mohan D. Population risk factors for severe disease and mortality in COVID-19: A global systematic review and meta-analysis. PLoS One. 2021;16:e0247461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 361] [Article Influence: 90.3] [Reference Citation Analysis (0)] |
| 3. | Lassi ZS, Ana A, Das JK, Salam RA, Padhani ZA, Irfan O, Bhutta ZA. A systematic review and meta-analysis of data on pregnant women with confirmed COVID-19: Clinical presentation, and pregnancy and perinatal outcomes based on COVID-19 severity. J Glob Health. 2021;11:05018. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 4. | Allotey J, Stallings E, Bonet M, Yap M, Chatterjee S, Kew T, Debenham L, Llavall AC, Dixit A, Zhou D, Balaji R, Lee SI, Qiu X, Yuan M, Coomar D, Sheikh J, Lawson H, Ansari K, van Wely M, van Leeuwen E, Kostova E, Kunst H, Khalil A, Tiberi S, Brizuela V, Broutet N, Kara E, Kim CR, Thorson A, Oladapo OT, Mofenson L, Zamora J, Thangaratinam S; for PregCOV-19 Living Systematic Review Consortium. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020;370:m3320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1213] [Cited by in RCA: 1339] [Article Influence: 267.8] [Reference Citation Analysis (0)] |
| 5. | van Lieshout LCEW, Koek GH, Spaanderman MA, van Runnard Heimel PJ. Placenta derived factors involved in the pathogenesis of the liver in the syndrome of haemolysis, elevated liver enzymes and low platelets (HELLP): A review. Pregnancy Hypertens. 2019;18:42-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Conde-Agudelo A, Romero R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am J Obstet Gynecol. 2022;226:68-89.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 171] [Article Influence: 57.0] [Reference Citation Analysis (0)] |
| 7. | Sathiya R, Rajendran J, Sumathi S. COVID-19 and Preeclampsia: Overlapping Features in Pregnancy. Rambam Maimonides Med J. 2022;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Talwani R, Gilliam BL, Howell C. Infectious diseases and the liver. Clin Liver Dis. 2011;15:111-130. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Nasa P, Alexander G. COVID-19 and the liver: What do we know so far? World J Hepatol. 2021;13:522-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 10. | Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S; International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertensive Disorders of Pregnancy: ISSHP Classification, Diagnosis, and Management Recommendations for International Practice. Hypertension. 2018;72:24-43. [PubMed] |
| 11. | Burton GJ, Redman CW, Roberts JM, Moffett A. Pre-eclampsia: pathophysiology and clinical implications. BMJ. 2019;366:l2381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 651] [Article Influence: 108.5] [Reference Citation Analysis (0)] |
| 12. | Yagel S, Cohen SM, Goldman-Wohl D. An integrated model of preeclampsia: a multifaceted syndrome of the maternal cardiovascular-placental-fetal array. Am J Obstet Gynecol. 2022;226:S963-S972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 13. | Lumbers ER, Delforce SJ, Arthurs AL, Pringle KG. Causes and Consequences of the Dysregulated Maternal Renin-Angiotensin System in Preeclampsia. Front Endocrinol (Lausanne). 2019;10:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 14. | Sheller-Miller S, Richardson L, Martin L, Jin J, Menon R. Systematic review of p38 mitogen-activated kinase and its functional role in reproductive tissues. Am J Reprod Immunol. 2018;80:e13047.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 15. | Crowley SD, Rudemiller NP. Immunologic Effects of the Renin-Angiotensin System. J Am Soc Nephrol. 2017;28:1350-1361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 16. | Grimes JM, Grimes KV. p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 195] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 17. | Bu S, Wang Y, Sun S, Zheng Y, Jin Z, Zhi J. Role and mechanism of AT1-AA in the pathogenesis of HELLP syndrome. Sci Rep. 2018;8:279. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Petca A, Miron BC, Pacu I, Dumitrașcu MC, Mehedințu C, Șandru F, Petca RC, Rotar IC. HELLP Syndrome-Holistic Insight into Pathophysiology. Medicina (Kaunas). 2022;58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 19. | Efendi L, Chalid MT, Upik AM, Syakib B. Comparison of p38 MAPK, soluble endoglin and endothelin-1 Level in severe preeclampsia and HELLP syndrome patients. Asian Pac J Reprod. 2019;8:83-88. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Corradetti A, Saccucci F, Emanuelli M, Vagnoni G, Cecati M, Sartini D, Giannubilo SR, Tranquilli AL. The role of p38alpha mitogen-activated protein kinase gene in the HELLP syndrome. Cell Stress Chaperones. 2010;15:95-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Abildgaard U, Heimdal K. Pathogenesis of the syndrome of hemolysis, elevated liver enzymes, and low platelet count (HELLP): a review. Eur J Obstet Gynecol Reprod Biol. 2013;166:117-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 22. | Liu N, Guo YN, Gong LK, Wang BS. Advances in biomarker development and potential application for preeclampsia based on pathogenesis. Eur J Obstet Gynecol Reprod Biol ×. 2021;9:100119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 23. | McLaughlin K, Zhang J, Lye SJ, Parker JD, Kingdom JC. Phenotypes of Pregnant Women Who Subsequently Develop Hypertension in Pregnancy. J Am Heart Assoc. 2018;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 24. | Griego SD, Weston CB, Adams JL, Tal-Singer R, Dillon SB. Role of p38 mitogen-activated protein kinase in rhinovirus-induced cytokine production by bronchial epithelial cells. J Immunol. 2000;165:5211-5220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 104] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 25. | Valipour M, Irannejad H, Emami S. Application of emetine in SARS-CoV-2 treatment: regulation of p38 MAPK signaling pathway for preventing emetine-induced cardiac complications. Cell Cycle. 2022;1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 26. | Valipour M, Zarghi A, Ebrahimzadeh MA, Irannejad H. Therapeutic potential of chelerythrine as a multi-purpose adjuvant for the treatment of COVID-19. Cell Cycle. 2021;20:2321-2336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 27. | Valipour M, Irannejad H, Emami S. Papaverine, a promising therapeutic agent for the treatment of COVID-19 patients with underlying cardiovascular diseases (CVDs). Drug Dev Res. 2022;10.1002/ddr.21961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Azer SA. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020;37:100738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 29. | Agbuduwe C, Basu S. Haematological manifestations of COVID-19: From cytopenia to coagulopathy. Eur J Haematol. 2020;105:540-546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 30. | Fan BE, Chong VCL, Chan SSW, Lim GH, Lim KGE, Tan GB, Mucheli SS, Kuperan P, Ong KH. Hematologic parameters in patients with COVID-19 infection. Am J Hematol. 2020;95:E131-E134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 396] [Article Influence: 79.2] [Reference Citation Analysis (0)] |
| 31. | Mendoza M, Garcia-Ruiz I, Maiz N, Rodo C, Garcia-Manau P, Serrano B, Lopez-Martinez RM, Balcells J, Fernandez-Hidalgo N, Carreras E, Suy A. Pre-eclampsia-like syndrome induced by severe COVID-19: a prospective observational study. BJOG. 2020;127:1374-1380. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 228] [Article Influence: 45.6] [Reference Citation Analysis (0)] |
| 32. | Braga LFB, Sass N. Coronavirus 2019, Thrombocytopenia and HELLP Syndrome: Association or Coincidence? Rev Bras Ginecol Obstet. 2020;42:669-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Federici L, Picone O, Dreyfuss D, Sibiude J. Successful continuation of pregnancy in a patient with COVID-19-related ARDS. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Ahmed I, Eltaweel N, Antoun L, Rehal A. Severe pre-eclampsia complicated by acute fatty liver disease of pregnancy, HELLP syndrome and acute kidney injury following SARS-CoV-2 infection. BMJ Case Rep. 2020;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Ronnje L, Länsberg JK, Vikhareva O, Hansson SR, Herbst A, Zaigham M. Complicated COVID-19 in pregnancy: a case report with severe liver and coagulation dysfunction promptly improved by delivery. BMC Pregnancy Childbirth. 2020;20:511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 36. | Coronado-Arroyo JC, Concepción-Zavaleta MJ, Zavaleta-Gutiérrez FE, Concepción-Urteaga LA. Is COVID-19 a risk factor for severe preeclampsia? Eur J Obstet Gynecol Reprod Biol. 2021;256:502-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 37. | Norooznezhad AH, Nurzadeh M, Darabi MH, Naemi M. Coronavirus disease 2019 (COVID-19) in a pregnant women with treatment resistance thrombocytopenic purpura with and suspicion to HELLP syndrome: a case report. BMC Pregnancy Childbirth. 2021;21:567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Farahani M, Azadi K, Hashemnejad M, Agoushi A, Nirouei M. Ruled out of preeclampsia-like syndrome due to COVID-19: A case study. Clin Case Rep. 2021;9:e05195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 39. | Aydın GA, Ünal S, Özsoy HGT. The effect of gestational age at the time of diagnosis on adverse pregnancy outcomes in women with COVID-19. J Obstet Gynaecol Res. 2021;47:4232-4240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 40. | Vaezi M, Mirghafourvand M, Hemmatzadeh S. Characteristics, clinical and laboratory data and outcomes of pregnant women with confirmed SARS-CoV-2 infection admitted to Al-Zahra tertiary referral maternity center in Iran: a case series of 24 patients. BMC Pregnancy Childbirth. 2021;21:378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 41. | Jering KS, Claggett BL, Cunningham JW, Rosenthal N, Vardeny O, Greene MF, Solomon SD. Clinical Characteristics and Outcomes of Hospitalized Women Giving Birth With and Without COVID-19. JAMA Intern Med. 2021;181:714-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 170] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 42. | Bhardwaj Y, Chakole V, Singam A, Madaan S. Anesthetic Management in a Post-COVID Hemolysis, Elevated Liver Enzymes, and Low Platelet Count (HELLP) Patient in Rural Central India: A Close Shave. Cureus. 2022;14:e24196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Madaan S, Talwar D, Kumar S, Jaiswal A, Acharya N, Acharya S. HELLP Syndrome and COVID-19; association or accident: A case series. J Family Med Prim Care. 2022;11:802-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Takahashi K, Sato T, Kamide T, Hoshina T, Kanuka H, Kumazawa K, Tanabe Y, Samura O, Okamoto A. Perinatal management of a pregnant woman with COVID-19: A case report from Japan. Taiwan J Obstet Gynecol. 2022;61:378-381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 45. | Guida JP, Cecatti JG, Souza RT, Pacagnella RC, Ribeiro-do-Valle CC, Luz AG, Lajos GJ, Surita FG, Nobrega GM, Griggio TB, Charles CM, Miele MJ, Ferreira SB, Tedesco RP, Fernandes KG, Martins-Costa SHA, Ramos JGL, Peret FJA, Feitosa FE, Traina E, Cunha-Filho EV, Vettorazzi J, Haddad SM, Andreucci CB, Correa-Junior MD, Mayrink J, Dias MAB, Oliveira LG, Melo-Junior EF, da Luz MGQ, Costa ML; REBRACO Study Group. Preeclampsia among women with COVID-19 during pregnancy and its impact on maternal and perinatal outcomes: Results from a national multicenter study on COVID in Brazil, the REBRACO initiative. Pregnancy Hypertens. 2022;28:168-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 46. | Snelgrove JW, Simpson AN, Sutradhar R, Everett K, Liu N, Baxter NN. Preeclampsia and Severe Maternal Morbidity During the COVID-19 Pandemic: A Population-Based Cohort Study in Ontario, Canada. J Obstet Gynaecol Can. 2022;44:777-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Arslan E. COVID-19: A Cause of HELLP Syndrome? Int J Womens Health. 2022;14:617-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Jafari M, Pormohammad A, Sheikh Neshin SA, Ghorbani S, Bose D, Alimohammadi S, Basirjafari S, Mohammadi M, Rasmussen-Ivey C, Razizadeh MH, Nouri-Vaskeh M, Zarei M. Clinical characteristics and outcomes of pregnant women with COVID-19 and comparison with control patients: A systematic review and meta-analysis. Rev Med Virol. 2021;31:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 171] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 49. | Gesaka SR, Obimbo MM, Wanyoro A. Coronavirus disease 2019 and the placenta: A literature review. Placenta. 2022;126:209-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 50. | Verma S, Joshi CS, Silverstein RB, He M, Carter EB, Mysorekar IU. SARS-CoV-2 colonization of maternal and fetal cells of the human placenta promotes alteration of local renin-angiotensin system. Med (N Y). 2021;2:575-590.e5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 51. | Lu-Culligan A, Chavan AR, Vijayakumar P, Irshaid L, Courchaine EM, Milano KM, Tang Z, Pope SD, Song E, Vogels CBF, Lu-Culligan WJ, Campbell KH, Casanovas-Massana A, Bermejo S, Toothaker JM, Lee HJ, Liu F, Schulz W, Fournier J, Muenker MC, Moore AJ; Yale IMPACT Team, Konnikova L, Neugebauer KM, Ring A, Grubaugh ND, Ko AI, Morotti R, Guller S, Kliman HJ, Iwasaki A, Farhadian SF. Maternal respiratory SARS-CoV-2 infection in pregnancy is associated with a robust inflammatory response at the maternal-fetal interface. Med (N Y). 2021;2:591-610.e10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 107] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 52. | Bloise E, Zhang J, Nakpu J, Hamada H, Dunk CE, Li S, Imperio GE, Nadeem L, Kibschull M, Lye P, Matthews SG, Lye SJ. Expression of severe acute respiratory syndrome coronavirus 2 cell entry genes, angiotensin-converting enzyme 2 and transmembrane protease serine 2, in the placenta across gestation and at the maternal-fetal interface in pregnancies complicated by preterm birth or preeclampsia. Am J Obstet Gynecol. 2021;224:298.e1-298.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 67] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 53. | Beys-da-Silva WO, da Rosa RL, Santi L, Tureta EF, Terraciano PB, Guimarães JA, Passos EP, Berger M. The risk of COVID-19 for pregnant women: Evidences of molecular alterations associated with preeclampsia in SARS-CoV-2 infection. Biochim Biophys Acta Mol Basis Dis. 2021;1867:165999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Chen X, Tao T, Wang H, Zhao H, Lu L, Wu F. Arterial Thrombosis Is Accompanied by Elevated Mitogen-Activated Protein Kinase (MAPK) and Cyclooxygenase-2 (COX-2) Expression via Toll-Like Receptor 4 (TLR-4) Activation by S100A8/A9. Med Sci Monit. 2018;24:7673-7681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 18] [Article Influence: 2.6] [Reference Citation Analysis (0)] |