Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.252
Peer-review started: May 18, 2022
First decision: June 16, 2022
Revised: June 25, 2022
Accepted: August 1, 2022
Article in press: August 1, 2022
Published online: September 25, 2022
Processing time: 128 Days and 12.4 Hours
Since the discovery of the coronavirus disease 2019 outbreak, a vast majority of studies have been carried out that confirmed the worst outcome of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in people with preexisting health conditions, including diabetes, obesity, hypertension, cancer, and cardiovascular diseases. Likewise, diabetes itself is one of the leading causes of global public health concerns that impose a heavy global burden on public health as well as socio-economic development. Both diabetes and SARS-CoV-2 infection have their independent ability to induce the pathogenesis and severity of multi-system organ failure, while the co-existence of these two culprits can accelerate the rate of disease progression and magnify the severity of the disease. However, the exact pathophysiology of multi-system organ failure in diabetic patients after SARS-CoV-2 infection is still obscure. This review summarized the organ-specific possible molecular mechanisms of SARS-CoV-2 and diabetes-induced pathophysiology of several diseases of multiple organs, including the lungs, heart, kidneys, brain, eyes, gastrointestinal system, and bones, and sub-sequent manifestation of multi-system organ failure.
Core Tip: There is no therapeutic approach yet that can eradicate diabetes and its complications from human life, as the etiopathology of diabetes is very complex. Before the outbreak of coronavirus disease 2019, it was almost unknown that diabetes is a leading risk factor that could fuel the pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-induced multi-organ dysfunction and subsequent mortality. Additionally, SARS-CoV-2-infected children and young people have been shown to develop diabetes. Therefore, identifying the precise molecular mechanisms of diabetes-induced SARS-CoV-2 susceptibility and subsequent manifestation of multi-organ dysfunction may help us to develop drugs that prevent millions of human lives.
- Citation: Roy B, Runa SA. SARS-CoV-2 infection and diabetes: Pathophysiological mechanism of multi-system organ failure. World J Virol 2022; 11(5): 252-274
- URL: https://www.wjgnet.com/2220-3249/full/v11/i5/252.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i5.252
Diabetes mellitus (DM) is a multi-faceted metabolic syndrome that induces or exacerbates the pathophysiology of several complications, including neuropathy, nephropathy, retinopathy, cardio-vascular diseases, pulmonary dysfunction, gastrointestinal (GI) dysfunction, and osteoporosis[1]. DM and its complications are increasing day by day while decreasing life expectancy and increasing the cost of diagnosis and treatment. According to the most recent Centers for Disease Control and Prevention (CDC) report, 37.3 million Americans have diabetes, and another 96 million US population have prediabetes[2].
Recently, coronavirus disease 2019 (COVID-19) has been the most discussed topic worldwide due to its devastating physiological and socioeconomic impacts since its discovery in December 2019 in Wuhan, China. As of 21 June 2022, the World Health Organization has reported 539119771 globally diagnosed COVID-19 cases, including 6322311 COVID-19-associated mortalities (WHO Coronavirus Dashboard. Available online: https://covid19.who.int). Although the overall COVID-19 positive case numbers declined from the previous years, the post-COVID-19 impact is still increasing. As time goes by, the trend of the pathogenicity for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has shifted from acute to long-term. Many studies determined the physiological consequences of the acute phase of SARS-CoV-2 infection and the post-COVID-19 effects on human health and diseases, including diabetes. However, few studies have been carried out to characterize the long-term risks and burden of diabetes in the post-acute phase of COVID-19. A recent study in a cohort that recruited 181280 participants who tested positive for SARS-CoV-2 and a contemporary control that recruited 4118441 participants showed that people with COVID-19 exhibited an increased risk and excess burden of incident diabetes as well as increased risk of antihyperglycemic use compared with the contemporary control group[3]. Another retrospective cohort study that recruited 126710 participants with one or more nasal swabs positive for SARS-CoV-2 and 2651058 participants with no positive swab showed that SARS-CoV-2 was associated with a higher risk of incident diabetes in men but not in women, compared with no positive tests. This study further demonstrated that among hospitalized COVID-19 patients, SARS-CoV-2 was associated with a higher risk of diabetes at 120 d and the end of follow-up in men but not in women[4]. However, the exact mechanism of SARS-CoV-2-induced increase in diabetic incidence and subsequent multiorgan dysfunction is unknown. This review provides ideas about the organ-specific cellular mechanism through which SARS-CoV-2 and diabetes mellitus results in multi-system organ dysfunction.
We searched our queries using Google Scholar, PubMed Central, ResearchGate, and the CDC databases. For this manuscript, we used articles published recently in standard peer-reviewed journals. We tried to avoid using review articles as much as possible; instead, we used research articles and case studies. We recruited research articles based on the following hierarchy: studies in humans > studies in animals > and studies in cell culture models. We thoroughly read the selected articles and picked the findings supporting our query.
Many studies have been carried out to determine the pathophysiology of diabetes-induced pulmonary dysfunction. For instance, a retrospective, longitudinal cohort study on 1811228 subjects showed that the prevalence of asthma, chronic obstructive pulmonary disease (COPD), fibrosis, and pneumonia was significantly higher in diabetic patients relative to their age and sex-matched non-diabetic controls[5]. Another retrospective cohort study with 1332 patients with concomitant asthma and diabetes showed that the use of metformin, a well-known diabetic drug, significantly reduced the risk of asthma[6]. Findings from this study suggest that diabetes induces the development of asthma in humans. A prospective multicenter study that employed 5334 COPD patients with or without diabetes showed that COPD patients with comorbid diabetes had a more severe profile and higher hospitalization costs[7]. A study in 162 T2DM patients without diabetes complications and 55 healthy control subjects showed that pulmonary function in T2DM patients is negatively correlated with vascular endothelial functional index, e.g., flow-mediated dilation and nitric oxide (NO), whereas positively correlated with endothelin-1 (ET-1) and glycosylated hemoglobinA1c (HbA1c)[8]. This study suggests that T2DM-induced vascular EC dysfunction is an important biomarker of pulmonary dysfunction. Hyperglycemia or insulin resistance (IR)-induced diabetic ketoacidosis (DKA) is associated with reduced serum potassium levels that subsequently cause respiratory muscle weakness and culmination of acute respiratory failure[9]. Additionally, DKA may contribute to the pathogenesis of pulmonary edema results from an acute shift of a large volume of fluid into the extracellular compartment and subsequent elevation of pulmonary venous pressure (hydrostatic pulmonary edema) as well as increased pulmonary capillary permeability (non-hydrostatic pulmonary edema) due to pulmonary microangiopathy[9]. A study in 26 diabetic patients showed a significant increase in angiotensin converting enzyme-2 (ACE2) protein levels in both alveolar tissue and bronchial epithelium compared to the control subjects, independent of smoking, chronic obstructive pulmonary disease, body mass index (BMI), renin-angiotensin-aldosterone system (RAAS) inhibitor use, and other potential confounders[10]. A study in 34239 patients (with or without diabetes) with a pneumonia-related hospitalization and 342390 healthy control subjects showed that poor long-term glycemic control in T1DM and T2DM increases the risk of hospitalization with pneu-monia[11]. In addition to humans, a vast majority of studies have been carried out in rodents to determine the role of diabetes in pulmonary dysfunction. For instance, streptozotocin (STZ)-induced diabetic rats showed increased pulmonary basal membrane thickness, increased inflammatory reaction due to mononuclear cell infiltration in their lungs, and increased levels of protein carbonyl content, a bi-product of oxidative stress, relative to their age-matched controls[12]. Likewise, myocardial ischemia-reperfusion in STZ-induced diabetic rats demonstrated an increase in alveolar wall thickness and lung tissue damage along with increased infiltration or aggregation of neutrophils in lung tissue compared with their age-matched wild-type controls[13]. Another study in STZ-induced diabetic rats showed that the lung tissue and lamellar bodies were significantly collapsed along with a significant reduction in SOD activity and the mRNA expression and protein levels of aldehyde dehydrogenase 2, an alcohol detoxifying mitochondrial enzyme in the lung tissue of diabetic rats[14]. STZ-induced diabetic rats developed pulmonary fibrosis along with increased inflammation in the lung tissue as evaluated by increased expression and levels of several profibrotic and proinflammatory biomarkers, including fibronectin, connective tissue growth factor (CTGF), plasminogen activator inhibitor-1 and tumor necrosis factor (TNF)-α[15]. Additionally, the expression of NADPH oxidase (NOX), an important mediator of oxidative and nitrative stress, significantly increased along with increased protein nitration and upregulation of angiotensin II (Ang II) and its receptor angiotensin II type 1 (AT1) in diabetic lung tissue. This study again reported that chronic administration of Ang II in normal mice induced diabetes-like lung fibrosis and inflammation (Figure 1), and the effects of Ang II were completely abrogated with losartan treatment, a potential AT1 inhibitor[15]. All the studies I stated so far revealed mostly the association between diabetes and different types of lung dysfunction. However, the precise underlying mechanism of diabetes-induced pathophysiology of lung disease is not well understood. Hyper-glycemia and hyperinsulinemia in diabetes increase oxidative stress, non-enzymatic glycation of tissue proteins, activation of protein kinase C (PKC), nuclear factor-κB (NF-ĸB), and polyol pathways, and eventually lead to the development of pulmonary dysfunction through autonomic neuropathy, micro/macroangiopathy in the lung, impairment in pulmonary elastin and collagen content, alteration of pulmonary connective tissue, surfactant dysfunction and malfunctioning of respiratory muscles (Figure 1)[16].
It is well established that ACE-2 is predominantly expressed in pulmonary endothelial cells and airway epithelial cells (AECs), the main cellular entry of SARS-CoV-2 to start the pathogenesis of COVID-19 manifestation. A phenome-wide Mendelian randomization study in 898130 T2DM subjects showed an increase in ACE2 expression in their lung tissue compared with non-diabetic healthy controls[17]. Another study in humans showed an increased expression of ACE2 and transmembrane serine protease 2 (TMPRSS2) in the upper and lower airway tissue in adults than in children. This study further showed an elevated expression of ACE2 and TMPRSS2 in the airway tissue of smokers and COPD patients[18]. A study in 55, 44 COVID-19 post-mortem lung samples showed increased expression of ACE2 along with increased diffuse alveolar damage, acute bronchopneumonia, and acute lung injury in severe COVID-19 patients relative to the lung samples from healthy subjects[19]. There are contradictory findings regarding the prevalence of ACE2 expression in pulmonary vascular endothelial cells vs airway epithelial cells/pneumocytes. Findings from a vast majority of studies confirmed that ACE2 is predominantly expressed in airway epithelial cells. For instance, a study in two cohorts in Australia showed that gene expression and protein levels of ACE2 in the lower AECs were significantly higher in older age and male sex compared with younger age and females, respectively[20]. However, another study in humans suggests that pulmonary endothelial cells express twice as many ACE2 receptors for viral entry than pneumocytes[21].
A study in lungs from patients who died from COVID-19 as well as patients who died from acute respiratory distress syndrome secondary to influenza A (H1N1) infection showed alveolar damage with perivascular T-cell infiltration along with severe endothelial injury associated with the presence of intracellular virus and disrupted cell membranes. Additionally, pulmonary vessels in patients with Covid-19 showed widespread thrombosis with microangiopathy. This study further demonstrated that the prevalence of alveolar-capillary microthrombi was nine times higher in the Covid-19 patients relative to patients with influenza[21]. Another study in the lung tissue from 11 Covid-19 deaths showed an increased loss of alveolar wall integrity, detachment of lung tissue pieces, fibroblast proliferation, and extensive fibrosis[22]. SARS-CoV-2 infected human AECs showed an increased cytopathic effect which was determined through a lack of cilium beating on the surface of AECs after 96 h of inoculation. However, SARS-CoV-2 infected Vero E6, and Huh-7 cell lines did not show any cytopathic effect[23]. Another case series study employing ultrasound-guided minimally invasive autopsy (MIA-US) in 10 fatal cases of COVID-19 showed exudative/proliferative diffuse alveolar damage, intense pleomorphic cytopathic effects on the respiratory epithelium, including airway and alveolar cells, increased fibrinous thrombi in alveolar arterioles and elevation of alveolar megakaryocyte numbers. This study further showed that small thrombi formation was less frequent in other tissues, including the glomeruli, spleen, heart, dermis, testis, and liver sinusoids, compared to the lungs[24]. Findings from this study suggest that COVID-19 is a systemic disease that predominantly infects the lungs through severe epithelial injury and microthrombotic vascular phenomena, along with damage to other organs and tissues. Immunohistochemistry of the lung tissue biopsy from a 72-year-old man with a history of diabetes and hypertension showed denuded alveolar lining cells, increased reactive type II pneumocyte hyperplasia, and increased intra-alveolar fibrinous exudates, along with loose interstitial fibrosis, and chronic inflammatory infiltrates. This study further confirmed the presence of SARS-Cov-2 in alveolar epithelial cells due to the presence of SARS-CoV-2 Rp3 NP protein[25]. A study in 108 individuals showed increased alveolar type II-pneumocyte injury as confirmed by elevated plasma levels of surfactant protein D, a biomarker of alveolar type II-pneumocyte injury, along with increased interleukin (IL)-6 serum levels in critically ill COVID-19 patients[26].
Other studies also showed that the use of ET-1 receptor antagonist, Bosentan has been approved as a drug to treat pulmonary arterial hypertension in New York Heart Association functional classification II-IV and in scleroderma patients, as it decreases the systemic levels of profibrotic and proinflammatory cytokines including IL-2, IL-6, IL-8 and interferon (IFN)-γ in scleroderma patients, as well as slows down the progression of fibrosis and vascular damage[27]. The possible mechanisms of diabetes and SARS-CoV-2-induced pulmonary dysfunction are stated in Figure 1.
The complement system, a complex innate immune surveillance system, contributes to the destruction/neutralization of pathogens, including viruses and bacteria, that invade our body[28]. The complement system is composed of plasma proteins synthesized mainly by the liver or membrane proteins expressed on the cell surface, whose main functions are to promote the opsonization and phagocytosis of microorganisms and apoptotic cells through macrophages and neutrophils to induce their degradation[28,29]. A study showed that hyperglycemia inhibited complement-mediated opsonization of S. aureus in diabetic rats[30]. Another study reported that hyperglycemia caused direct glycosylation of proteins and altered the tertiary Structure of complement proteins, and subsequently inhibited immunoglobulin-mediated opsonization of bacteria[31].
Thrombotic microangiopathy (TMA) is a pathological condition that is associated with thrombosis in capillaries and arterioles, which leads to microangiopathic hemolytic anemia, thrombocytopenia, and organ damage, such as neurological, renal and cardiac dysfunction[32]. There are several risk factors that can contribute to the pathogenesis of TMA, including viruses, bacteria, drugs, oxidative stress, complement hyperactivation, and congenital predisposing conditions. All these factors directly or indirectly induce vascular endothelial cell damage, followed by the development of TMA. Studies showed that DM is associated with increased activation of the C3 complement component, which is a central factor of complement cascade[33,34]. Another study also showed that Insulin resistance is linked with elevated circulating complement factor C3 levels[35]. Increased C3 levels in plasma contribute to the hyperactivation of complement cascade that may lead to the development of TMA[36]. Oxidative stress-induced vascular endothelial dysfunction is an important hallmark of DM[37] and may contribute to the development of TMA[38]. Von Willebrand factor (VWF) is a clotting factor that is required for the pathogenesis of thrombotic thrombocytopenic purpura (TTP), a fatal blood disorder[39]. In pathological conditions, a multimeric form of VWF and platelets are prone to form aggregates and subsequently cause TTP[40]. A disintegrin and metalloprotease with thrombospondin type I repeats-13 (ADAMTS13), a zinc-containing metalloprotease that cleaves multimeric form of VWF and mitigates the formation of VWF-platelets aggregates[40]. The deficiency of plasma ADAMTS13 contributes to the progression of TTP[41]. A study in human subjects showed that T2DM-induced oxidative stress modifies the amino acid sequences of VWF and thereby prevents its proteolytic cleavage by ADAMTS13[42]. Another study showed that ADAMTS13 activity is significantly lower in T2DM patients compared with healthy control people[43]. Several virus strains, including SARS-CoV-2, HIV, MCV, EBV, parvovirus, rubella, and measles, have been recognized so far that can cause TTP by modulating the autoimmune process in human and animal species[44,45]. However, the exact molecular mechanism of the novel coronavirus, SARS-CoV-2-induced TTP, is completely unknown. Rapidly emerging data from clinical observations, autopsy-based findings, extrapolations from in vitro and in vivo studies, and dynamic modeling are not well enough to provide the exact pathophysiology of secondary complications associated with SARS-COV-2 infection[46]. However, a large number of patients with severe COVID-19 demonstrated TMA-like systemic coagulopathy that led to an increased number of deaths[47]. Findings from several studies reported that coagulopathy in COVID-19 patients was confirmed through the presence of elevated D-dimer, elevated lactate dehydrogenase, elevated total bilirubin, and decreased platelets[46,48]. As DM and SARS-COV-2 both are associated with TMA-like symptoms, the mortality rate should be higher in DM patients when infected with SARS-COV-2. A single-center cross-sectional study that employed 68 patients with COVID-19, including 48 ICU and 20 non-ICU patients, as well as 13 non-hospitalized, asymptomatic controls, showed that the levels of endothelial cell and platelet activation markers, including VWF antigen and soluble P-selectin. This study further demonstrated that the mortality of ICU patients was positively correlated with concentrations of VWF antigen and soluble throm-bomodulin[49].
Hemophagocytic lymphohistiocytosis (HLH) is a cytokine storm-induced inflammatory syndrome that is associated with substantial morbidity and mortality due to multiorgan failure[50,51]. Several case reports demonstrated that diabetes insipidus is associated with secondary HLH (sHLH)[52,53]. Accumulating evidence suggests that several patients with severe COVID-19 demonstrated sHLH due to cytokine storm, which is characterized by increased interleukin IL-2, IL-7, granulocyte colony-stimulating factor, interferon-γ inducible protein 10, monocyte chemoattractant protein 1, macrophage inflammatory protein 1-α, and tumor necrosis factor-α[51]. Based on the findings stated above, it can be surmised that diabetes patients infected with SARS-COV-2 possess a higher risk of fatality compared with SARS-COV-2 infected non-diabetic subjects.
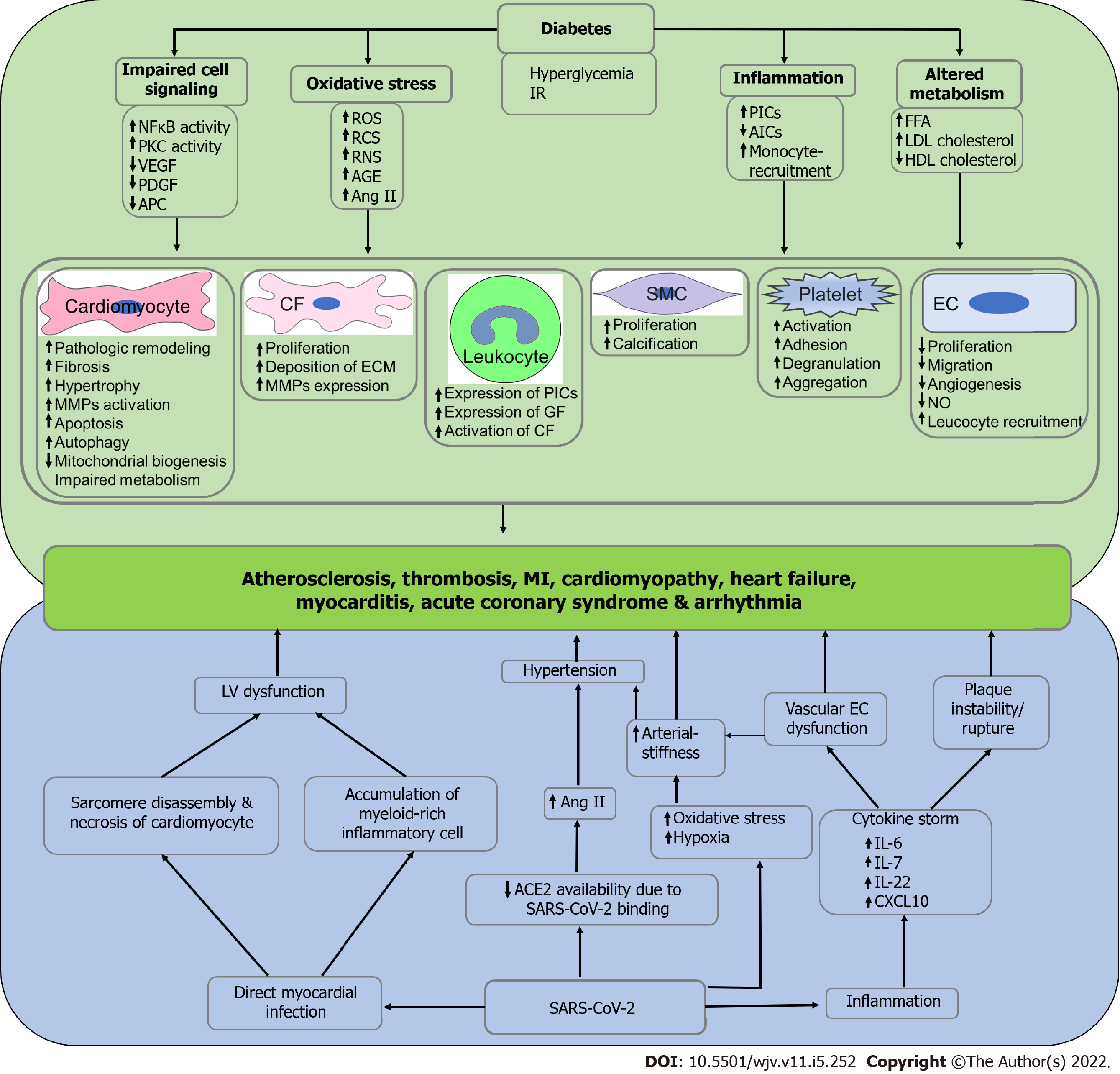
The prevalence of atrial fibrillation (AF), an important hallmark of arrhythmia, is higher in DM patients. In an observational, age- and sex-matched cohort, a longitudinal study that included 34744 patients with and without diabetes showed that AF was 44% more prevalent and 38% more likely to develop in T2DM[54]. A cohort study with 421855 T2DM patients showed that there is a 35% increased risk of developing AF in T2DM patients compared with age- and sex-matched controls from the general population. This study also showed that the risk of developing AF is exacerbated in T2DM patients with poor glycemic control and renal complications[55]. There are several mechanisms that may lead to the development of AF in DM. Oxidative stress in diabetes is associated with increased formation of reactive oxygen species, carbonyl species, nitrogen species, and AGE, which in turn predisposes to the development of AF through endothelial dysfunction, increased atherogenesis and reduced coronary angiogenesis. T2DM is a leading cause of cardiovascular diseases (CVDs), including atherosclerosis, MI, HF, and cardiomyopathy. There are several mechanisms that result in the pathogenesis of diabetic cardiomyopathy, including impaired insulin signaling, mitochondrial dysfunction, increased oxidative stress, reduced NO levels, elevated AGEs levels, stiffness of extracellular matrix, impaired handling of Ca2+ by cardiomyocytes, inflammation, RAAS over activation, cardiac autonomic neuropathy, endoplasmic reticulum stress, microvascular dysfunction, and several cardiac metabolic abnormalities[56].
Several studies have demonstrated that hospitalization and mortality rate are significantly higher in COVID-19 patients who has preexisting arrhythmia. A study in a cohort of 153760 US veterans who survived in the first 30 d of COVID-19 has experienced different types of CVDs, including dys-rhythmias, ischemic and non-ischemic heart disease, pericarditis, myocarditis, heart failure, and thromboembolic disease[57]. A multicenter study with 696 hospitalized covid-19 patients developed acute HF and multiorgan failure as well as increased mortality which had a history of AF[58]. A retrospective observational study also showed that iCOVID-19 patients with a myocardial injury (determined by increased systemic C-reactive protein levels) are positively correlated with inflammation and coagulopathy and increased hospitalization[59]. Several possible mechanisms may contribute to the pathogenesis of acute myocardial injury in COVID-19 subjects, including micro-vascular injury, hypoxemia, preexisting CVDs, ventricular/atrial arrhythmias, hypotension, viral myocarditis, cytokine storm, and stress-induced cardiomyopathy[60]. A systematic pathological analysis that included 40 hearts from hospitalized patients dying of COVID-19 showed that the most common pathological cause of cardiomyocyte necrosis in COVID-19 patients was microthrombi. This study further demonstrated that the composition of intramyocardial microthrombi was different between COVID-19-negative and positive subjects[61].
A study using the samples from right atrial appendage biopsies in 57 diabetics and 22 non-diabetic subjects who underwent coronary artery bypass graft surgery showed that ACE2 mRNA expression and protein levels in heart tissue, as well as serum ACE2 levels, were significantly higher in diabetic patients relative to the non-diabetic control subjects. Additionally, ACE2 levels were positively correlated with glycosylated hemoglobin (HbA1c) levels, BMI, and activation of RAAS, and negatively correlated with ejection fraction. This study further demonstrated that the expression of TMPRSS2, metalloprotease ADAM10, and ADAM17 that facilitate viral-ACE2 complex entry and degradation were increased in diabetic hearts[62]. Diabetes is associated with increased activation of RAAS and subsequent elevation of systemic Ang II levels. However, the direct association of T2DM and SARS-CoV-2 susceptibility to the human heart is still unclear. STZ-induced diabetic mice developed severe cardiovascular complications after influenza virus infection as evaluated with increased circulatory levels of serum cardiac troponin I and creatine-kinase MB, left ventricular structural changes, and right ventricular functional alterations[63]. A prospective cohort study in Germany demonstrated an elevation of myocardial SARS–CoV-2 RNA in 5 out of 12 COVID-19–positive deaths[64]. Another study by Wenzel et al[65] showed an increased myocardial SARS-CoV-2 RNA in patients with clinically suspected myocarditis who were tested negative for COVID-19 in nasopharyngeal swabs. A case study in a 72-years-old male patient who died due to severe COVID-19 reported the presence of SARS-COV-2 RNA as well as SARS-COV-2 antigen in his cardiac tissue and cardiomyocyte, respectively[66]. Another study that employed endomyocardial biopsy samples from four SARS-CoV-2 infected dead patients who were diagnosed with myocarditis showed left ventricular systolic dysfunction due to cardiomyocyte injury and degenerative vacuolization of cardiomyocyte cytoplasm along with myeloid-rich inflammatory cell infiltrate. Additionally, the myocardium of each COVID-19 myocarditis subject also showed an increased expression of SARS-CoV-2 spike and nucleocapsid RNAs as well as nucleocapsid protein levels. This study further demonstrated that SARS-CoV-2 selectively infects hPSC-derived cardiomyocytes through an ACE2 and endosomal cysteine protease-dependent pathway and subsequent production of the infectious virus with peak titers on day three post-inoculation. Infecting engineered heart tissues with SARS-CoV-2 confirmed that cytokine production, myocardial sarcomere disassembly, and cardiomyocyte death were a direct consequence of cardiomyocyte infection[67]. The possible mechanisms of diabetes and SARS-CoV-2-induced cardiovascular dysfunction are stated in Figure 2.
According to the most recent data from the CDC, around 37 million people in the united states are estimated to have chronic kidney disease (CKD)[68], and approximately 1 in 3 adults with diabetes has CKD, which is also known as diabetic kidney disease (DKD) or diabetic nephropathy. Additionally, the increased rate of mortality and associated socioeconomic and medical burden due to CKD-mediated development of end-stage renal disease is receiving more attention as a leading cause of death around the world[69]. DKD is associated with several structural changes in the kidney, including mesangial expansion, thickening of the glomerular and tubular basement membrane, glomerular sclerosis that manifests clinical symptoms including elevated blood pressure, sustained reduction in glomerular filtration rate (GFR), persistent albuminuria, increased cardiovascular events and associated mortality.
There are several possible mechanisms that contribute to the pathogenesis of DKD in diabetes, including impairment in renal hemodynamics, inflammation, abnormal glucose metabolism, oxidative stress, and overactive RAAS. Diabetes is associated with increased dilatation of afferent arteriole in the kidney due to increased generation of important vasoactive peptides, including prostaglandin and NO. A cohort study with 171 subjects showed that plasma prostaglandin E2 levels were increased in 136 T2DM patients compared with 35 non-diabetic controls[70]. Studies in humans and animals showed that poor glycemic control is associated with increased generation of NO and subsequent inhibition of tubuloglomerular feedback–mediated vasoconstriction of afferent arterioles in diabetic kidneys[71,72]. Additionally, T2DM is associated with elevated circulatory Ang II levels due to the overactivation of RAAS, which constricts efferent arteriole and subsequently results in glomerular hypertension and impaired autoregulation. A study in a cohort with COVID-19 patients (n = 89) demonstrated that regardless of their severity, circulatory PEG2 levels increased significantly in SARS-CoV-2 infection compared with age and sex-matched healthy controls. Additionally, this study showed that the entire COVID-19 cohort had an increased rate of diabetes, BMI, as well as elevated circulatory C-reactive protein levels[73], an important prognostic biomarker of COVID-19[74] and CVDs[75].
Similarly, T2DM contributes to the development of renal fibrosis, podocyte injury, and inflammation through an increased generation of renal ET-1, an important vasoconstrictor. Studies showed that pulmonary infection and hypoxia cause an elevation of circulatory ET-1 levels in humans and animals[76]. A study in a cohort showed that plasma levels of the stable precursor protein of endothelin-1, proET-1 were significantly higher in non-survivor COVID-19 patients relative to the survivor COVID-19 patients. This study also showed that plasma proET-1 levels were significantly higher in patients with community-acquired pneumonia compared with both survivors and non-survivors of COVID-19 patients. Additionally, data from this study showed that there is no significant association between proET-1 levels and mortality in a regression model adjusted for age, gender, creatinine level, diastolic blood pressure, as well as cancer and coronary artery disease[77].
Hyperglycemia contributes to the generation of ROS through mitochondrial overload and subsequently leads to podocyte dysfunction and apoptosis. Hyperglycemia and oxidative stress in diabetes mellitus increase intrarenal AGE levels that may cause morphological and functional impairments in the kidney, including modification of basement membranes, glomerulosclerosis, interstitial fibrosis, and tubular atrophy. Studies in humans and animals showed that in most cases, AGE exerts its role through the activation of the receptor for AGE (RAGE) in the kidney[78]. Studies in rodents showed that inhibition of AGE binding with RAGE using RAGE-aptamers attenuates the development and progression of diabetic nephropathy in streptozotocin-induced diabetic rats. This study further showed that continuous administration of RAGE-aptamer significantly suppressed the AGE-induced oxidative stress generation and inflammatory and fibrotic reactions in human cultured mesangial cells[79].
A single-center observational cohort study in Germany showed that serum levels of soluble RAGE (sRAGE) increased significantly with COVID-19 severity, the need for dialysis, and catecholamine support[80].
Being a pleiotropic receptor, RAGE also interacts with a wide range of ligands in the S100 family, including S100A8/MRP8, S100A9/MRP14, S100A11, S100A12, S100B, high-mobility group box 1 (HMGB1). A study in Wuhan, China, showed that expression of S100A8, S100A9, S100A11, and S100A12 are significantly elevated in the lung tissue and serum of fatal COVID-19 patients compared to less severe cases of COVID-19[81]. Some other studies also demonstrated a positive association between COVID-19 severity/fatality and plasma levels of S100A8[82], S100A9[82], HMGB1[82], S100A12[83] and S100B[84]. Additionally, an in vitro study revealed that HMGB1 epigenetically upregulates the expression of ACE2 in Vero-E6 cells and subsequently increases the susceptibility to SARS-CoV-1, SARS-CoV-2, and NL63 infection[85].
Prolonged hyperglycemia in diabetes is a renowned risk factor for kidney injury leading to proteinuria in humans[86,87]. A prospective, multicenter study in France showed that 60% of COVID-19 patients developed proteinuria as determined by urinary protein to creatine ratio. Additionally, this study also showed that proteinuria was significantly elevated in severe COVID-19 patients who required ICU admission[88].
The prevalence of obstructive sleep apnea (OSA) is relatively high in T2DM[89]. A multicenter, observational, cross-sectional study using 214 DKD patients showed that UACR was higher in DKD with severe OSA relative to moderate OSA, mild OSA, or non-OSA subjects. Additionally, this study showed that the estimated GFR (eGFR) decreased in an OSA severity-dependent manner[90]. OSA-induced intermittent hypoxia and increased sympathetic nerve activity are associated with increased vascular complications, including endothelial damage and hypertension that leads to renal dysfunction[91]. A study using 445 COVID-19 patients where 8.5% had OSA showed that OSA is an independent risk factor of severe COVID-19 that requires hospitalization[92]. Findings from the studies above, it can be surmised that T2DM-induced OSA may contribute to the pathogenesis of DKD and subsequent fatality in severe COVID-19 patients.
A retrospective study in 2345 children having both type-1 diabetes and albuminuria demonstrated that the development of acute kidney injury is positively associated with the episodes of DKA[93]. A study that included 658 hospitalized patients with confirmed COVID-19 showed an increase in ketoacidosis in both diabetic and non-diabetic COVID-19 patients regardless of their age and sex[94]. In an observational study with 3993 hospitalized COVID-19 patients without any history of end-stage kidney disease end-stage kidney disease (ESKD) prior to admission, 1835 (46%) patients developed AKI[95]. Another retrospective cohort study that employed 89216 patients who were 30-d survivors of COVID-19 and 1637467 non-infected controls showed that 30-d survivors of COVID-19 exhibited a higher risk of AKI, declined eGFR, ESKD and major adverse kidney events[96].
Hyperglycemic osmotic diuresis in DKA contributes to the progression of dehydration, hypovolemia, and, ultimately, a reduction in the GFR[97]. Sepsis and hypovolemia are two of the major risk factors that contribute to the pathogenesis of AKI[98]. A vast majority of COVID-19 patients experienced several complications, including fever, malaise, nausea, vomiting, and diarrhea for several days before seeking medical care and subsequently developed hypovolemia[99]. A prospective case-control study showed that COVID-19 patients with AKI are associated with reduced renal blood flow compared with healthy controls, which are independent of left/right cardiac dysfunction[100]. Reduced renal blood flow is a common pathophysiological mechanism of subsequent reduction of GFR and culmination of AKI[101]. Hypercoagulability is a common feature of diabetes. A study in humans showed significantly elevated platelet activity as well as more severe blood clots in patients with concomitant diabetes and renal dysfunction compared with healthy controls and patients with renal dysfunction but no diabetes[102]. The possible mechanisms of diabetes and SARS-CoV-2-induced renal dysfunction are stated in Figure 3.
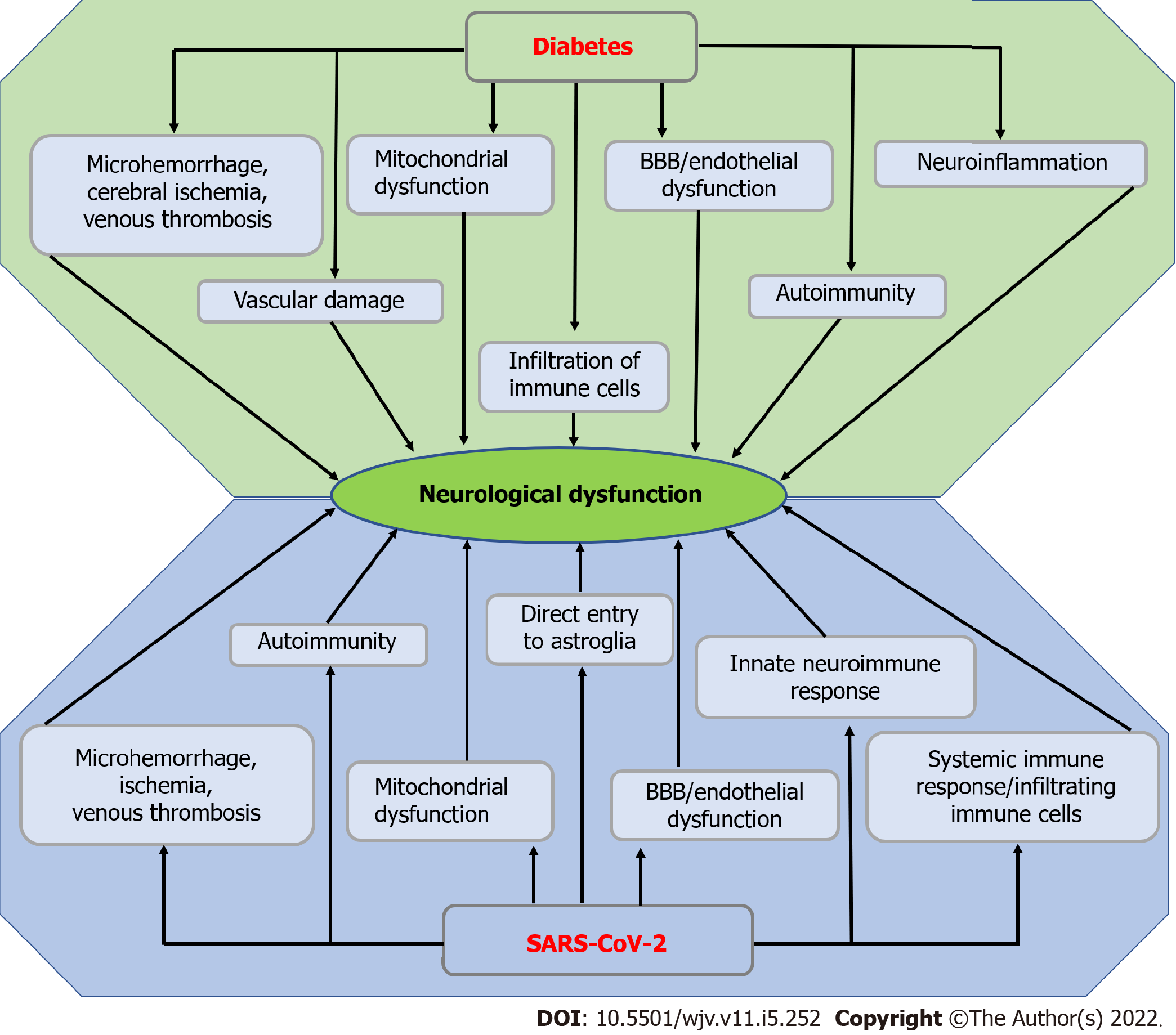
Approximately one-third of COVID-19 patients have been shown to develop neurological symptoms, including headache, disturbed consciousness, paresthesias, brain tissue edema, stroke, neuronal degeneration, and neuronal encephalitis[103]. Plenty of studies determined the association between COVID-19 and brain dysfunction. However, very little is known about the exact pathophysiology of SARS-CoV-2-induced neurological dysfunction. A recent study by Douaud et al[104] recruited 785 participants of UK Biobank who went through the magnetic resonance imaging (MRI) twice, including 401 cases which tested positive for SARS-CoV-2 infection on an average of 141 d before the second scan and 384 controls. Contrary to the first scan, data from the second scan of the SARS-CoV-2 positive cases revealed several striking features associated with brain dysfunction, including a reduction in grey matter thickness and tissue contrast in the orbitofrontal cortex and parahippocampal gyrus, significant alteration in the presence of tissue damage markers in regions that are functionally connected to the primary olfactory cortex; and a significant reduction in global brain size in the SARS-CoV-2 cases[104]. A study by Reiken et al[103] showed that SARS-CoV-2 infection is associated with Alzheimer's disease-like phenotypes, which are characterized by the upregulation of TGF-β signaling and hyperphosphorylation of tau protein and leaky phenotype of the ryanodine receptor in the brain. STZ-induced diabetic mice exhibited mild hyperphosphorylation of tau protein after 10, 20, and 30 d of STZ injection, and massive hyperphosphorylation of tau protein was observed after 40 d of STZ injection[105]. There are several studies that confirmed the direct involvement/ entry of SARS-CoV-2 in the brain. For instance, a study using the autopsy samples of olfactory nervous tracts and defined CNS regions from 33 individuals with COVID-19 showed the presence of SARS-CoV-2 RNA in olfactory mucosa, its nervous projections, and distinct CNS regions[106].
Encephalopathy refers to brain disorders that alter brain function or Structure. Acute encephalopathy is a rare but fatal complication caused by several factors, including metabolic diseases (e.g., diabetes) and pathogen infection. The pathogenesis of encephalopathy caused by diabetes-induced microvascular dysfunction in the brain is called diabetic encephalopathy (DE). DE is a chronic microvascular complication of diabetes mellitus that is characterized by impaired cognitive functions and electrophysiological, neurochemical, and structural abnormalities[107,108]. A case study showed that there is a possible association between T1D and autoimmune neurologic disorders due to elevated systemic levels of glutamic acid decarboxylase antibody, an important biomarker of limbic encephalopathy[109].
One of the most frequent neurological complications in COVID-19 is acute encephalopathy. Several studies have been conducted to understand the neuropathogenesis of COVID-19-induced acute encephalopathy. For instance, a study by Uginet et al[110] that recruited 707 COVID-19 hospitalized patients showed that the severity of the pneumonia was not associated with the severity of the COVID-19 encephalopathy. Additionally, increased MRI abnormalities, intracranial vessel gadolinium enhancement, and disruption of BBR disruption were observed in maximum COVID-19 patients who had no history of acute encephalopathy and other neurological disorders. A case study that employed a SARS-CoV-2 positive middle-aged woman who presented to the emergency department of a tertiary care hospital with an episode of generalized tonic-clonic seizures primarily showed neuropsychiatric manifestations, including viral encephalitis rather than the most common COVID-19 symptoms[111]. Another single-center retrospective study that comprised 1683 patients with COVID-19 showed that 23 (1.4%) patients developed cerebrovascular disease. Out of these 23 patients, 17 developed cerebral ischemia, five developed intracerebral hemorrhages, and one developed leukoencephalopathy of posterior reversible encephalopathy type. This study further showed that elevated ferritin levels were observed in hemorrhagic patients at the time of stroke along with subarachnoid hemorrhage, parieto-occipital leukoencephalopathy, microbleeds, and single or multiple focal hematomas, thrombotic microangiopathy, and endothelial injury, with no evidence of vasculitis or necrotizing encephalitis[112]. A study that employed both the human and animal brains showed that the hypothalamus and associated regions express ACE2 and transmembrane proteinase, serine 2, which mediate SARS-CoV-2 cellular entry, along with several genes or pathways involved in physiological functions or viral pathogenesis[113]. A multicenter study employing 25 COVID-19 patients with encephalitis developed acute demyelinating encephalomyelitis and limbic encephalitis along with hyper proteinopathies and/or pleocytosis in the CSF[114]. Another study that recruited 13 encephalitis patients with COVID-19, 21 encephalitis patients without COVID-19, and 18 healthy controls, showed that CSF from the encephalitis patients with COVID-19 was negative for SARS-CoV-2, whereas the levels of IL-6, IL-8, TNF-α, β2-microglobulin and glial markers including glial fibrillary acidic protein, soluble triggering receptor expressed on myeloid cells 2, and chitinase-3-like protein 1 (YKL-40) were significantly elevated compared with the encephalitis patients without COVID-19[115].
The blood-brain barrier (BBB) is a highly selective semipermeable border that mediates the communication between the periphery and the central nervous system (CNS), composed of endothelial cells, neurons, astrocytic end-feet, pericytes, and a thick basement membrane[116]. This BBB allows the transport of various nutrients, ions, glucose, water, amino acids, and hydrophobic molecules, including O2, CO2, and hormones[116], whereas it restricts the entry of pathogens, peripheral inflammatory mediators (e.g., cytokines and antibodies) as well as large or hydrophilic molecules into the CNS[117]. Tight junctions (TJs) form a diffusion barrier between cerebral endothelial cells and prevent blood-borne substances from entering the brain[118].
DM-induced hyperglycemia upregulates the expression and activation of proangiogenic factors, including hypoxia-inducible factor-1α (HIF-1α) and vascular endothelial growth factor (VRGF), and subsequently increases capillary formation at the BBB. Additionally, hyperglycemia downregulates the expression of inter-endothelial tight junction proteins, including occludin, claudin-5, ZO-1, and JAM-1, and subsequently increases tight junction malfunctioning[119]. DM-induced formation of advanced glycation end-products contributes to the loss of BBB integrity through the upregulation of matrix metalloproteinases 2 in BBB ECs. Oxidative stress-induced formation of ROS[120] may disrupt the BBB through increasing systemic inflammation in diabetes[121]. Increased hypoxia associated with severe COVID-19 may increase capillary density in the BBB through the upregulation of HIF-1α and VRGF. Increased capillary formation and malfunctioning/disruption of TJs facilitate the invasion of inflammatory factors, neurotoxins, and pathogens into the CNS[122]. Since the BBB is the only route for the pathogens and systemic proinflammatory cytokines/chemokines to enter inside the brain, pathogens including SARS, MERS, SARS-CoV, and SARS-CoV-2 and proinflammatory cytokines in the systemic circulation generated due to cytokine storm may easily penetrate inside the CNS of a diabetic person through the damaged BBB. Since the human brain tissue is known to express ACE2 receptors[123], SARS-CoV-2 may infect the brain tissue, followed by the expression of several pathophysiological symptoms associated with the CNS infection. For instance, a study that recruited 8 COVID-19 patients exhibited an elevation of anti-SARS-CoV-2 antibodies in the CSF of comatose or encephalopathic patients suggesting intrathecal IgG synthesis or BBB disruption. BBB disruption may facilitate the entry of proinflammatory cytokines and inflammatory mediators into the CNS and subsequent neuroinflammation and neurodegeneration[124]. A study that recruited 15 hospitalized COVID-19 patients with neurological manifestations exhibited lymphocytic pleocytosis, cranial neuropathy with meningo-polyradiculitis, brainstem encephalitis, and delirium[125]. The possible mechanisms of diabetes and SARS-CoV-2-induced neurological dysfunction are stated in Figure 4.
Diabetic retinopathy (DR) is prevalent in diabetic patients and is one of the leading causes of blindness worldwide[126]. In 2020, the number of adults worldwide with DR, vision-threatening DR, and clinically significant macular edema was estimated to be 103.12 million, 28.54 million, and 18.83 million, respectively, and projection speculated that this number would increase to 160.50 million, 44.82 million, and 28.61 million, respectively in 2045[127]. The manifestation of DR is characterized by microaneurysms, retinal hemorrhages, intraretinal microvascular abnormalities, preretinal neovascularization, venous caliber changes, and lipid exudates from the damaged vasculature, capillary nonperfusion with accompanying neuronal infarcts and diabetic macular edema[128]. There are several possible mechanisms that may contribute to the pathogenesis of DR, including hyperglycemia-induced microangiopathy, inflammation, and retinal neurodegeneration[129]. Hyperglycemia has been implicated in the pathogenesis of retinal microvascular dysfunction through the impairment of several metabolic pathways, including the polyol pathway, formation of AGEs, the PKC pathway, and the hexosamine pathway[129]. Hyperglycemia is a well-known factor in pericyte and endothelial dysfunction mediated microaneurysm formation, impairment of blood-retinal barrier (BRB), capillary occlusion, and ischemia in DR[126]. Additionally, ischemia in diabetic eyes upregulates the expression of VEGF through the activation of HIF1[130] and phospholipase A2[131] and subsequently induces the pathogenesis of proliferative DR and diabetic macular edema by increasing vascular permeability. An in vitro cell culture study showed that VEGF-A mediates the early glucose-induced damage in human retinal endothelial cells through the activation of the ERK1/2/PLA2 signaling pathway[132]. A retrospective cohort study comprising 241196 DM patients showed that the prevalence of retinal artery occlusion is 2.30-times higher in DM patients compared to their age and sex-match healthy controls[133]. Leucocyte plays an important role in the pathogenesis of DR through the leukostasis-mediated retinal occlusion. A study in humans showed that subjects with central retinal vein occlusion were characterized by elevated levels of monocyte chemotactic protein-1, macrophage inflammatory protein-1alpha (MIP-1α), and MIP-1β that regulate the activation and binding of leukocytes[134]. STZ-induced diabetic mice showed retinal inflammation, which was characterized by leukostasis, increased expression of ICAM-1 on the luminal surface of the vascular endothelium, elevated retinal IL-6, CXCL1 expression, and superoxide generation[135]. Another preclinical study in STZ-induced diabetic rats showed that leukocytes lead to the pathogenesis of DME through Fas-FasL-dependent retinal endothelial cell apoptosis and subsequent dysfunction of BRB[136]. A prospective study that employed 22 DR patients and 28 non-diabetic subjects showed that inflammatory cytokines such as TNF-α, IL-6, IL-8, and IL-1β were significantly upregulated in the vitreous samples from DR patients and their levels were proportional to the severity of DR[137]. Some other studies demonstrated that retinal Müller glial cells and microglia as the initiators of retinal inflammation and subsequent pathogenesis of DR. For instance, a study by Portillo et al[138] showed that STZ-induced diabetic mice with overexpressed CD40 in Müller cells upregulated retinal expression of TNF-α, IL-1β, ICAM-1, and nitric oxide synthase (NOS2), developed leukostasis and capillary degeneration. This study further showed that overexpression of CD40 did not cause TNF-α or IL-1β secretion in Müller cells. Rather, TNF-α was upregulated in macrophages/microglia in the retina. The CD40 overexpressing Müller cells induced PLC–dependent ATP release and subsequent P2X7-dependent production of TNF-α and IL-1β by macrophages. Findings from this study suggest that CD40 in Müller cells is sufficient to upregulate retinal inflammatory markers and appears to promote experimental DR through the activation of the CD40-ATP-P2X7 pathway[138]. Hyperglycemia in diabetes is also implicated in mitochondrial dysfunction mediated apoptosis of retinal neurons and subsequent pathogenesis of DR. An in vitro cell culture study showed that rat retinal Müller cells grown in a high-glucose medium developed mitochondrial dysfunction that may contribute to retinal Müller cell loss and subsequent pathogenesis of DR[139]. Retinal neurodegeneration is a hallmark of the pathogenesis of early DR. Recent studies have reported that vascular changes are preceded by the damage and loss of retinal neurons due to apoptosis or autophagy. According to Silva et al[140] STZ-induced diabetic rats started to show DR symptoms after one month of STZ injection.
STZ-induced diabetic mice showed loss of rod cells, reduced thickness of the outer and inner synaptic layers along with the upregulation of autophagic proteins, including Beclin-1 and Atg5. Findings from this study suggest that the pathogenic pathways leading to cell death develop with the initial dysregulation of autophagy and subsequent vascular damage[141]. For instance, STZ-induced diabetic mice increased ERK activation and subsequent reduction of synaptophysin and depletion of a brain-derived neurotrophic factor in the diabetic retina after one month of STZ injection[142].
Although the respiratory tract is considered the predominant route of SARS-CoV-2 infection, several studies hypothesized that the conjunctiva could be contaminated by SARS-CoV-2 droplets and dirty hands, thereby initiating the viral entry into the body[143]. A study using 14 retinal biopsies (RB) samples and 13 optic nerve biopsy (ONB) samples collected from COVID-19 deaths showed that 7 out of 14 RB samples and 10 out of 13 ONB samples contained the SARS-CoV-2 RNA[144]. Similarly, a study in 91 hospitalized COVID-19 patients showed the presence of SARS-CoV-2 RNA on the ocular surfaces of 52 patients (57.1%). This study further showed that the virus was detected on the ocular surface in 10 out of 17 patients whose nasopharyngeal swab was negative[145]. However, the mechanism of direct eye infection by SARS-CoV-2 is still unknown. A study using human post-mortem eyes and surgical specimens showed the expression of both ACE2 and TMPRSS2 in conjunctiva, limbus, and cornea[146]. A study by Menuchin-Lasowski et al[147] showed that SARS-CoV-2 infected human stem cell-derived retinal organoids increased the production of several inflammatory genes associated with acute COVID-19 and retinal degeneration, including IL-33, CXCL2, and CXCL10. This study further showed that the inhibition of ACE2 activity with antibody significantly reduced SARS-CoV-2 infection of retinal organoids, indicating that SARS-CoV-2 infects retinal cells in an ACE2-dependent manner[147].
Conjunctivitis is the most common ophthalmic manifestation documented in COVID-19 patients[148]. A retrospective cross-sectional, single-center study using 127 COVID-19 patients with mild symptoms showed that 11 out of 127 (8.66%) patients had conjunctivitis[149]. Another study that recruited 535 COVID-19 patients showed that 27 patients (5.0%) presented with conjunctival congestion, which may result from direct hand contact with the eyes[150]. However, the mechanism of SARS-CoV-2-induced conjunctivitis is poorly understood. A case study in a 53-year-old man showed viral conjunctivitis along with the presence of SARS-CoV-2 RNA in the left eye after ten days of COVID-19 onset. The symptoms of the left eye conjunctivitis were completely cured in 5 d with proper medications. However, the patients experienced viral keratoconjunctivitis with progressive spot staining observed at the periphery of the corneal epithelium in both eyes; after five days, the symptoms in the left eye were completely cured. At this stage, the patient also showed an elevation of IL-6 levels in both eyes as well as in the circulation[151]. Findings from this study suggest that SARS-CoV-2-induced cytokine storm may contribute to the pathogenesis of conjunctivitis and keratoconjunctivitis. A population-based case-control study in 16 193 adults showed that diabetes is a risk factor for acute infectious conjunctivitis[152].
DM is an important risk factor for osteoporosis. A single-center cross-sectional study that enrolled 388 Japanese patients with a history of T1D showed that long-term hypoglycemia is significantly associated with a higher risk of bone fracture[153]. A prospective and retrospective cohort study demonstrated that DM patients had a greater risk of total hip, upper arm, and ankle fractures, and this risk was pronounced in T1DM patients than T2DM patients[154]. Several mechanisms may contribute to the pathogenesis of DM-induced osteoporosis. Hyperglycemia and/or IR in DM are associated with increased production of proinflammatory cytokines, including IL-1, IL-6, and TNF-α, and vasoactive peptides, including Ang II. In contrast, it decreases the levels of vitamin D, which may downregulate osteoblast number/activity and upregulate osteoclast number/activity. Decrease in osteo-blast/osteoclast ratio results in increased bone resorption and subsequent osteoporosis[155].
COVID-19 has been recognized to induce osteo-metabolic complications that are characterized by hypocalcemia, chronic hypovitaminosis D, and a high prevalence of bone fragility[156,157]. The presence of SARS-CoV-2 in bone cells has not been identified so far; however, the expression of ACE2 in the bone cells has been identified as a positive regulator of bone health. For instance, cell culture and human gingival bone samples have been shown to express ACE2 in osteoblast and osteoclast. Using both in vitro and in vivo models, this study further demonstrated that pharmacological activation of ACE2 with diminazene aceturate, an essential activator of ACE 2, significantly decreased alveolar bone loss through the improvement of osteoblast/osteoclast ratio[158]. Since SARS-CoV-2 bindings with the ACE2 receptors result in a decrease in ACE2 numbers, SARS-CoV-2 infection may increase bone loss. A study by Awosanya et al[159] has shown that human ACE2 expressing mice (TG) developed severe health problems and a significant reduction in trabecular bone volume due to an increase in the number and surface area of osteoclasts after 14 d of SARS-CoV-2 infection. However, more studies are required to confirm this finding. Cytokine storm upon SARS-CoV-2 infection is associated with increased circulatory levels of CXCL-10, TNF-α, IL-1β, and IL-6[160], whereas decreased reduced vitamin D levels are associated with increased infection and severity of COVID-19[161]. Many COVID-19 patients have experienced conjunctivitis in their eyes[148]. Therefore, it can be surmised that the possibility of fall-mediated bone fracture in COVID-19 patients who has conjunctivitis should be higher than the healthy people.
A study in a cohort including 59 patients with COVID-19 showed that 15 patients had GI dysfunction, and nine patients had stool containing SARS-CoV-2 RNA. This study also conducted a meta-analysis comprising 4243 COVID-19 patients showed that the prevalence of GI symptoms in COVID-19 patients was 17.6%, and 48.1% of COVID-19 patients exhibited the presence of SARS-CoV-2 RNA in their stool samples, although 70.3% of those samples were collected after the loss of virus from respiratory specimens tested positive for the virus[162]. The expression of ACE2 in the human GI tract has been confirmed through several studies[163,164]. Several studies demonstrated the direct infection of SARS-CoV-2 in the GI tract. For instance, an in vitro study using gastric organoids from fetal, pediatric, and adult biopsies showed that pediatric and late fetal gastric organoids are susceptible to SARS-CoV-2 infection, while viral replication is significantly lower in undifferentiated organoids of early fetal and adult origin. Through transcriptomic analysis, they further showed that SARS-CoV-2 infected stomach sample elicits a moderate innate antiviral response and a lack of differentially expressed genes belonging to the interferon family. Findings from this study suggest that SARS-CoV-2 can efficiently infect the gastric epithelium, suggesting that the stomach might have an active role in fecal-oral SARS-CoV-2 transmission[165]. A retrospective, single-center study comprising 95 cases with SARS-CoV-2 infection demonstrated that GI symptoms, including diarrhea, anorexia, and nausea, were observed in 58 cases[166]. Findings from another retrospective cohort study comprising 104 patients with COVID-19 demonstrated that GI infection with SARS-CoV-2 prolongs the duration of SARS-CoV-2 shedding and hospitalization in the patients with COVID-19[167].
Diabetic patients are implicated in developing several GI complications, including gastroparesis, intestinal enteropathy, non-alcoholic fatty liver disease (NAFLD), pancreatitis, and peptic ulcer disease. There are clinical and preclinical studies that confirmed the association of diabetes with GI abnormalities. For instance, a study that recruited 50 DM patients and 20 non-DM healthy controls showed that patients with long-term DM exhibited lower maximal squeeze pressure, a higher mean threshold of minimal rectal sensation, and enhanced features of dyssynergic defection compared with the control group. Findings from this study suggest that DM patients demonstrated an impaired function of the external anal sphincter, enhanced features of dyssynergic defecation as well as impaired visceral sensation[168]. A case study that comprised ten patients with maternally inherited diabetes and deafness syndrome (MIDD) showed that GI symptoms, including constipation and diarrhea along with the mucosal accumulation of normal mitochondria and lipid droplets, are frequent in MIDD[169]. A study using the GI mucosal biopsy samples from subjects with and without type 2 diabetes exhibited that taste signaling molecules that modulate the upper GI function and energy intake are decreased in diabetic subjects with elevated blood glucose concentration and decreased by luminal glucose in mice[170]. A cohort study that recruited 5699 T2DM patients and 11226 age and sex-matched non-diabetic controls showed that in a 7-year follow-up period, T2DM patients had a significantly higher cumulative hazard of peptic ulcer bleeding than the controls with adjusted age, sex, and comorbidities[171]. Another study that recruited healthy subjects and peptic ulcer patients with or without T2DM showed that the number of circulating EPCs and their colony-forming ability, essential prerequisites for vascular repair and angiogenesis, was significantly reduced in peptic ulcer patients with T2DM[172]. There are findings from many preclinical and clinical studies that confirm diabetes as a significant risk factor for NAFLD[173-175]. A 14-years follow-up study by Han et al[176] that recruited 3047 subjects without underlying DM showed that NAFLD could be used as a biomarker better than BMI in predicting incident DM.
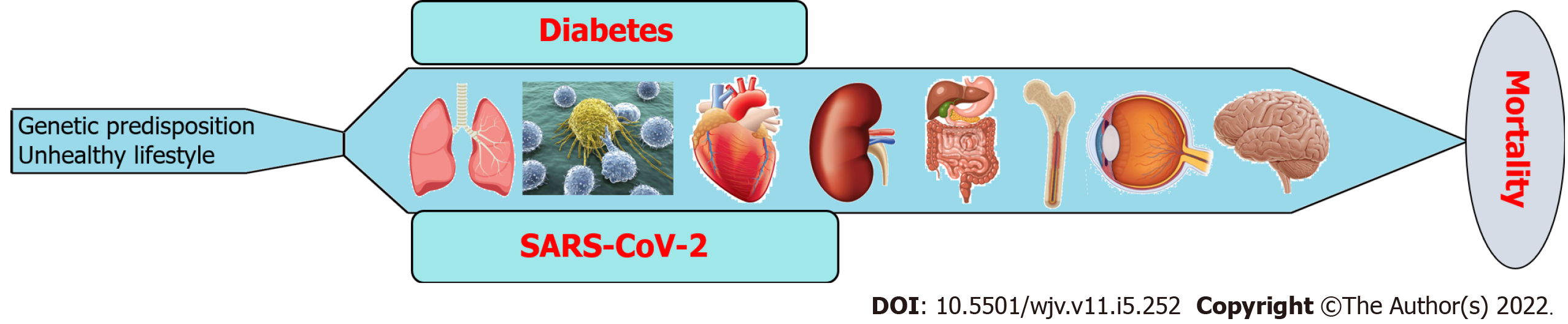
Diabetes is a chronic metabolic disease that differentially induces the pathogenesis of several complications associated with different organs, including the brain, eyes, bone, GI tract, kidneys, heart, immune system, and lungs (Figure 5). On the other hand, SARS-CoV-2 infection has both acute and chronic effects on the manifestation of all diseases (Figure 5). In addition to their independent mechanisms for the pathogenesis of any disease, the coexistence of diabetes and SARS-CoV-2 infection exacerbates the disease severity and subsequent fatality (Figure 5). There are several approaches, including medications, diet and exercise that can reduce the blood glucose levels in both type 1 and type 2 DM patients. Insulin therapy and amylinomimetic drugs are used to reduce bold glucose levels in type 1 DM patients. Similarly, biguanides (e.g., metformin), dopamine agonist (e.g., bromocriptine), dipeptidyl peptidase-4 inhibitors (e.g., alogliptin), glucagon-like peptide-1 receptor agonists (e.g., albiglutide), meglitinides (e.g., nateglinide), sodium-glucose transporter 2 inhibitors (e.g., dapagliflozin), sulfonylureas (e.g., glimepiride), and thiazolidinediones (e.g., rosiglitazone) are well known type 2 DM medications[177]. However, there is no effective treatment that can completely cure diabetes or diabetic complications. On the other hand, there are several approaches that can prevent the transmission as well as the severity of SARS-CoV-2 infection, including mRNA vaccines (e.g., Pfizer-BioNTech covid-19 vaccine), and antiviral drugs (e.g., remdesivir) and monoclonal antibodies (e.g., bebtelovimab)[178]. However, there is no effective therapy yet that can completely prevent the transmission of SARS-CoV-2 and cure COVID-19 without any side effects. Since SARS-CoV-2 is still circulating among the community, new variants like delta and omicron are evolving that can be even more transmissible and lethal than the existing variants. Because of these new mutant variants, COVID-19 is out of control despite widespread vaccination in the United States as well as other countries. There are some drugs that can prevent viral entry into the host cells as well as decrease blood glucose levels. For instance, Camostat mesylate, a serine protease inhibitor used primarily for treating postoperative reflux esophagitis and chronic pancreatitis. However, studies showed that blocking TMPRSS2 with Camostat mesylate and its metabolite 4-(4-guanidinobenzoyloxy) phenylacetic acid can prevent upper respiratory tract infection by SARS-CoV-2[179]. Chloroquine and hydroxychloroquine are glucose-lowering drugs and have been used extensively to treat COVID-19 due to their antiviral properties. However, these drugs have adverse health effects. Therefore, patients with DM and/or other underlying health conditions should be aware that SARS-CoV-2 infection can elevate blood glucose levels, and, as such, they should follow clinical guidelines for the management of DM more strictly.
People with diabetes possess a higher risk of SARS-CoV-2 infection and subsequent severe COVID-19 manifestation. On the other hand, the prevalence of SARS-CoV-2 infection-mediated manifestation of diabetes is also increasing. It is more likely to develop severe consequences due to the global increase in diabetic patients and the co-existence of diabetes and SARS-CoV-2. Still, we need to wait longer, and more research should be conducted to see the long-term effects of post-COVID-19 manifestation. To prevent or cure the long-term coexistence of diabetes and COVID-19 in the human body, we should more adhere to standardized prevention and control, cutting the transmission chain of the virus and blocking it to a minimum. Maintaining a healthy lifestyle with a healthy diet, regular exercise, and proper hygiene can reduce the risk of developing diabetes as well as the number of SARS-CoV-2 infection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single-blind
Specialty type: Pathology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mukhopadhyay A, India; Munteanu C, Romania S-Editor: Gong ZM L-Editor: A P-Editor: Gong ZM
| 1. | Beckman JA, Creager MA. Vascular Complications of Diabetes. Circ Res. 2016;118:1771-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 258] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 2. | Centers for Disease Control and Prevention. National Diabetes Statistics Report. [cited January 18, 2022] Available from: https://www.cdc.gov/diabetes/data/statistics-report/index.html. |
| 3. | Xie Y, Al-Aly Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. 2022;10:311-321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 350] [Cited by in RCA: 351] [Article Influence: 117.0] [Reference Citation Analysis (0)] |
| 4. | Wander PL, Lowy E, Beste LA, Tulloch-Palomino L, Korpak A, Peterson AC, Kahn SE, Boyko EJ. The Incidence of Diabetes Among 2,777,768 Veterans With and Without Recent SARS-CoV-2 Infection. Diabetes Care. 2022;45:782-788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 5. | Ehrlich SF, Quesenberry CP Jr, Van Den Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, chronic obstructive pulmonary disease, pulmonary fibrosis, and pneumonia but not lung cancer. Diabetes Care. 2010;33:55-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 6. | Forno E. Asthma and diabetes: Does treatment with metformin improve asthma? Respirology. 2016;21:1144-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Mao X, Liang C, Niu H, Dong F, Huang K, Chen Y, Zhan Q, Zhang Y, Huang Y, Yang T, Wang C. Outcomes associated with comorbid diabetes among patients with COPD exacerbation: findings from the ACURE registry. Respir Res. 2021;22:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 8. | Tai H, Jiang XL, Yao SC, Liu Y, Wei H, Li LB, Jiao ZJ, Wang TQ, Kuang JS, Jia LQ. Vascular Endothelial Function as a Valid Predictor of Variations in Pulmonary Function in T2DM Patients Without Related Complications. Front Endocrinol (Lausanne). 2021;12:622768. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Gallo de Moraes A, Surani S. Effects of diabetic ketoacidosis in the respiratory system. World J Diabetes. 2019;10:16-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (13)] |
| 10. | Wijnant SRA, Jacobs M, Van Eeckhoutte HP, Lapauw B, Joos GF, Bracke KR, Brusselle GG. Expression of ACE2, the SARS-CoV-2 Receptor, in Lung Tissue of Patients With Type 2 Diabetes. Diabetes. 2020;69:2691-2699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Kornum JB, Thomsen RW, Riis A, Lervang HH, Schønheyder HC, Sørensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: a population-based case-control study. Diabetes Care. 2008;31:1541-1545. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 196] [Cited by in RCA: 231] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 12. | Eren G, Cukurova Z, Hergunsel O, Demir G, Kucur M, Uslu E, Dalo E, Uhri M, Tugcu V. Protective effect of the nuclear factor kappa B inhibitor pyrrolidine dithiocarbamate in lung injury in rats with streptozotocin-induced diabetes. Respiration. 2010;79:402-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Alkan M, Çelik A, Bilge M, Kiraz HA, Kip G, Özer A, Şıvgın V, Erdem Ö, Arslan M, Kavutçu M. The effect of levosimendan on lung damage after myocardial ischemia reperfusion in rats in which experimental diabetes was induced. J Surg Res. 2015;193:920-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Hu JF, Zhang GJ, Wang L, Kang PF, Li J, Wang HJ, Gao Q, Chen YQ. Ethanol at low concentration attenuates diabetes induced lung injury in rats model. J Diabetes Res. 2014;2014:107152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Yang J, Tan Y, Zhao F, Ma Z, Wang Y, Zheng S, Epstein PN, Yu J, Yin X, Zheng Y, Li X, Miao L, Cai L. Angiotensin II plays a critical role in diabetic pulmonary fibrosis most likely via activation of NADPH oxidase-mediated nitrosative damage. Am J Physiol Endocrinol Metab. 2011;301:E132-E144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Zheng H, Wu J, Jin Z, Yan LJ. Potential Biochemical Mechanisms of Lung Injury in Diabetes. Aging Dis. 2017;8:7-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 17. | Rao S, Lau A, So HC. Exploring Diseases/Traits and Blood Proteins Causally Related to Expression of ACE2, the Putative Receptor of SARS-CoV-2: A Mendelian Randomization Analysis Highlights Tentative Relevance of Diabetes-Related Traits. Diabetes Care. 2020;43:1416-1426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 170] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 18. | Saheb Sharif-Askari N, Saheb Sharif-Askari F, Alabed M, Temsah MH, Al Heialy S, Hamid Q, Halwani R. Airways Expression of SARS-CoV-2 Receptor, ACE2, and TMPRSS2 Is Lower in Children Than Adults and Increases with Smoking and COPD. Mol Ther Methods Clin Dev. 2020;18:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 19. | Gheware A, Ray A, Rana D, Bajpai P, Nambirajan A, Arulselvi S, Mathur P, Trikha A, Arava S, Das P, Mridha AR, Singh G, Soneja M, Nischal N, Lalwani S, Wig N, Sarkar C, Jain D. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci Rep. 2022;12:4058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 54] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 20. | Wark PAB, Pathinayake PS, Kaiko G, Nichol K, Ali A, Chen L, Sutanto EN, Garratt LW, Sohal SS, Lu W, Eapen MS, Oldmeadow C, Bartlett N, Reid A, Veerati P, Hsu AC, Looi K, Iosifidis T, Stick SM, Hansbro PM, Kicic A. ACE2 expression is elevated in airway epithelial cells from older and male healthy individuals but reduced in asthma. Respirology. 2021;26:442-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, Vanstapel A, Werlein C, Stark H, Tzankov A, Li WW, Li VW, Mentzer SJ, Jonigk D. Pulmonary Vascular Endothelialitis, Thrombosis, and Angiogenesis in Covid-19. N Engl J Med. 2020;383:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4313] [Cited by in RCA: 4064] [Article Influence: 812.8] [Reference Citation Analysis (0)] |
| 22. | Valdebenito S, Bessis S, Annane D, Lorin de la Grandmaison G, Cramer-Bordé E, Prideaux B, Eugenin EA, Bomsel M. COVID-19 Lung Pathogenesis in SARS-CoV-2 Autopsy Cases. Front Immunol. 2021;12:735922. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 23. | Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W; China Novel Coronavirus Investigating and Research Team. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18987] [Cited by in RCA: 17646] [Article Influence: 3529.2] [Reference Citation Analysis (0)] |
| 24. | Duarte-Neto AN, Monteiro RAA, da Silva LFF, Malheiros DMAC, de Oliveira EP, Theodoro-Filho J, Pinho JRR, Gomes-Gouvêa MS, Salles APM, de Oliveira IRS, Mauad T, Saldiva PHN, Dolhnikoff M. Pulmonary and systemic involvement in COVID-19 patients assessed with ultrasound-guided minimally invasive autopsy. Histopathology. 2020;77:186-197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 219] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 25. | Zhang H, Zhou P, Wei Y, Yue H, Wang Y, Hu M, Zhang S, Cao T, Yang C, Li M, Guo G, Chen X, Chen Y, Lei M, Liu H, Zhao J, Peng P, Wang CY, Du R. Histopathologic Changes and SARS-CoV-2 Immunostaining in the Lung of a Patient With COVID-19. Ann Intern Med. 2020;172:629-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 308] [Cited by in RCA: 376] [Article Influence: 75.2] [Reference Citation Analysis (0)] |
| 26. | Kerget B, Kerget F, Koçak AO, Kızıltunç A, Araz Ö, Uçar EY, Akgün M. Are Serum Interleukin 6 and Surfactant Protein D Levels Associated with the Clinical Course of COVID-19? Lung. 2020;198:777-784. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 27. | Bellisai F, Morozzi G, Scaccia F, Chellini F, Simpatico A, Pecetti G, Galeazzi M. Evaluation of the effect of Bosentan treatment on proinflammatory cytokine serum levels in patients affected by Systemic Sclerosis. Int J Immunopathol Pharmacol. 2011;24:261-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Merle NS, Church SE, Fremeaux-Bacchi V, Roumenina LT. Complement System Part I - Molecular Mechanisms of Activation and Regulation. Front Immunol. 2015;6:262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 695] [Cited by in RCA: 1127] [Article Influence: 112.7] [Reference Citation Analysis (0)] |
| 29. | Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16 Suppl 1:S27-S36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 377] [Cited by in RCA: 515] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 30. | Mauriello CT, Hair PS, Rohn RD, Rister NS, Krishna NK, Cunnion KM. Hyperglycemia inhibits complement-mediated immunological control of S. aureus in a rat model of peritonitis. J Diabetes Res. 2014;2014:762051. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Jafar N, Edriss H, Nugent K. The Effect of Short-Term Hyperglycemia on the Innate Immune System. Am J Med Sci. 2016;351:201-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 281] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 32. | Gavriilaki E, Brodsky RA. Severe COVID-19 infection and thrombotic microangiopathy: success does not come easily. Br J Haematol. 2020;189:e227-e230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 141] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 33. | McMillan DE. Elevation of complement components in diabetes mellitus. Diabete Metab. 1980;6:265-270. [PubMed] |
| 34. | Engström G, Hedblad B, Eriksson KF, Janzon L, Lindgärde F. Complement C3 is a risk factor for the development of diabetes: a population-based cohort study. Diabetes. 2005;54:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 35. | Wlazlo N, van Greevenbroek MM, Ferreira I, Feskens EJ, van der Kallen CJ, Schalkwijk CG, Bravenboer B, Stehouwer CD. Complement factor 3 is associated with insulin resistance and with incident type 2 diabetes over a 7-year follow-up period: the CODAM Study. Diabetes Care. 2014;37:1900-1909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 36. | Park MH, Caselman N, Ulmer S, Weitz IC. Complement-mediated thrombotic microangiopathy associated with lupus nephritis. Blood Adv. 2018;2:2090-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 37. | Shi Y, Vanhoutte PM. Macro- and microvascular endothelial dysfunction in diabetes. J Diabetes. 2017;9:434-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 368] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 38. | Goldberg RJ, Nakagawa T, Johnson RJ, Thurman JM. The role of endothelial cell injury in thrombotic microangiopathy. Am J Kidney Dis. 2010;56:1168-1174. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 90] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Zheng XL. ADAMTS13 and von Willebrand factor in thrombotic thrombocytopenic purpura. Annu Rev Med. 2015;66:211-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 173] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 40. | Tsai HM. Pathophysiology of thrombotic thrombocytopenic purpura. Int J Hematol. 2010;91:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 41. | Zander CB, Cao W, Zheng XL. ADAMTS13 and von Willebrand factor interactions. Curr Opin Hematol. 2015;22:452-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 42. | Oggianu L, Lancellotti S, Pitocco D, Zaccardi F, Rizzo P, Martini F, Ghirlanda G, De Cristofaro R. The oxidative modification of von Willebrand factor is associated with thrombotic angiopathies in diabetes mellitus. PLoS One. 2013;8:e55396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 43. | Skeppholm M, Kallner A, Kalani M, Jörneskog G, Blombäck M, Wallén HN. ADAMTS13 and von Willebrand factor concentrations in patients with diabetes mellitus. Blood Coagul Fibrinolysis. 2009;20:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Han H, Yang L, Liu R, Liu F, Wu KL, Li J, Liu XH, Zhu CL. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 788] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 45. | Magdi M, Rahil A. Severe Immune Thrombocytopenia Complicated by Intracerebral Haemorrhage Associated with Coronavirus Infection: A Case Report and Literature Review. Eur J Case Rep Intern Med. 2019;6:001155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 46. | Becker RC. COVID-19 update: Covid-19-associated coagulopathy. J Thromb Thrombolysis. 2020;50:54-67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 372] [Cited by in RCA: 436] [Article Influence: 87.2] [Reference Citation Analysis (1)] |
| 47. | Levi M, Thachil J, Iba T, Levy JH. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7:e438-e440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 1062] [Article Influence: 212.4] [Reference Citation Analysis (0)] |
| 48. | Campbell CM, Kahwash R. Will Complement Inhibition Be the New Target in Treating COVID-19-Related Systemic Thrombosis? Circulation. 2020;141:1739-1741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 205] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 49. | Goshua G, Pine AB, Meizlish ML, Chang CH, Zhang H, Bahel P, Baluha A, Bar N, Bona RD, Burns AJ, Dela Cruz CS, Dumont A, Halene S, Hwa J, Koff J, Menninger H, Neparidze N, Price C, Siner JM, Tormey C, Rinder HM, Chun HJ, Lee AI. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575-e582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 605] [Cited by in RCA: 802] [Article Influence: 160.4] [Reference Citation Analysis (0)] |
| 50. | Ahmed A, Merrill SA, Alsawah F, Bockenstedt P, Campagnaro E, Devata S, Gitlin SD, Kaminski M, Cusick A, Phillips T, Sood S, Talpaz M, Quiery A, Boonstra PS, Wilcox RA. Ruxolitinib in adult patients with secondary haemophagocytic lymphohistiocytosis: an open-label, single-centre, pilot trial. Lancet Haematol. 2019;6:e630-e637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 214] [Article Influence: 35.7] [Reference Citation Analysis (0)] |
| 51. | Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ; HLH Across Speciality Collaboration, UK. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033-1034. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6366] [Cited by in RCA: 6751] [Article Influence: 1350.2] [Reference Citation Analysis (0)] |
| 52. | Pajvani U, Lipton ML, Grajower MM. Diabetes insipidus associated with hemophagocytic lymphohistiocytosis: first case report. Endocr Pract. 2011;17:e118-e122. [PubMed] [DOI] [Full Text] |
| 53. | Machaczka M. Hemophagocytic lymphohistiocytosis in adults. Ups J Med Sci. 2013;118:201-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | Nichols GA, Reinier K, Chugh SS. Independent contribution of diabetes to increased prevalence and incidence of atrial fibrillation. Diabetes Care. 2009;32:1851-1856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 55. | Seyed Ahmadi S, Svensson AM, Pivodic A, Rosengren A, Lind M. Risk of atrial fibrillation in persons with type 2 diabetes and the excess risk in relation to glycaemic control and renal function: a Swedish cohort study. Cardiovasc Diabetol. 2020;19:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 56. | Jia G, Hill MA, Sowers JR. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ Res. 2018;122:624-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 689] [Cited by in RCA: 1247] [Article Influence: 207.8] [Reference Citation Analysis (0)] |
| 57. | Xie Y, Xu E, Bowe B, Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. 2022;28:583-590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1452] [Cited by in RCA: 1194] [Article Influence: 398.0] [Reference Citation Analysis (0)] |
| 58. | Paris S, Inciardi RM, Lombardi CM, Tomasoni D, Ameri P, Carubelli V, Agostoni P, Canale C, Carugo S, Danzi G, Di Pasquale M, Sarullo F, La Rovere MT, Mortara A, Piepoli M, Porto I, Sinagra G, Volterrani M, Gnecchi M, Leonardi S, Merlo M, Iorio A, Giovinazzo S, Bellasi A, Zaccone G, Camporotondo R, Catagnano F, Dalla Vecchia L, Maccagni G, Mapelli M, Margonato D, Monzo L, Nuzzi V, Pozzi A, Provenzale G, Specchia C, Tedino C, Guazzi M, Senni M, Metra M. Implications of atrial fibrillation on the clinical course and outcomes of hospitalized COVID-19 patients: results of the Cardio-COVID-Italy multicentre study. Europace. 2021;23:1603-1611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 59. | Arévalos V, Ortega-Paz L, Rodríguez-Arias JJ, Calvo M, Castrillo L, Salazar A, Roque M, Dantas AP, Sabaté M, Brugaletta S. Myocardial Injury in COVID-19 Patients: Association with Inflammation, Coagulopathy and In-Hospital Prognosis. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 60. | Hendren NS, Drazner MH, Bozkurt B, Cooper LT Jr. Description and Proposed Management of the Acute COVID-19 Cardiovascular Syndrome. Circulation. 2020;141:1903-1914. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 395] [Cited by in RCA: 405] [Article Influence: 81.0] [Reference Citation Analysis (0)] |
| 61. | Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A, Nasr A, Kutys R, Guo L, Cornelissen A, Faggi L, Mori M, Sato Y, Pescetelli I, Brivio M, Romero M, Virmani R, Finn AV. Microthrombi as a Major Cause of Cardiac Injury in COVID-19: A Pathologic Study. Circulation. 2021;143:1031-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 197] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 62. | Herman-Edelstein M, Guetta T, Barnea A, Waldman M, Ben-Dor N, Barac YD, Kornowski R, Arad M, Hochhauser E, Aravot D. Expression of the SARS-CoV-2 receptorACE2 in human heart is associated with uncontrolled diabetes, obesity, and activation of the renin angiotensin system. Cardiovasc Diabetol. 2021;20:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 63. | Sinclair JE, Bloxham CJ, Chiu H, Chew KY, Russell J, Yoshikawa Y, Bielefeldt-Ohmann H, Steele LE, Hulme KD, Verzele NA, Noye EC, Wu M, Reichelt ME, Thomas WG, Gallo LA, Redd MA, Short KR. Type I Diabetes Mellitus Increases the Cardiovascular Complications of Influenza Virus Infection. Front Cell Infect Microbiol. 2021;11:714440. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 64. | Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A, Heinrich F, Mushumba H, Kniep I, Schröder AS, Burdelski C, de Heer G, Nierhaus A, Frings D, Pfefferle S, Becker H, Bredereke-Wiedling H, de Weerth A, Paschen HR, Sheikhzadeh-Eggers S, Stang A, Schmiedel S, Bokemeyer C, Addo MM, Aepfelbacher M, Püschel K, Kluge S. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268-277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1577] [Cited by in RCA: 1749] [Article Influence: 349.8] [Reference Citation Analysis (0)] |
| 65. | Wenzel P, Kopp S, Göbel S, Jansen T, Geyer M, Hahn F, Kreitner KF, Escher F, Schultheiss HP, Münzel T. Evidence of SARS-CoV-2 mRNA in endomyocardial biopsies of patients with clinically suspected myocarditis tested negative for COVID-19 in nasopharyngeal swab. Cardiovasc Res. 2020;116:1661-1663. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 66. | Nakamura Y, Katano H, Nakajima N, Sato Y, Suzuki T, Sekizuka T, Kuroda M, Izutani Y, Morimoto S, Maruyama J, Koie M, Kitamura T, Ishikura H. SARS-CoV-2 is localized in cardiomyocytes: a postmortem biopsy case. Int J Infect Dis. 2021;111:43-46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 67. | Bailey AL, Dmytrenko O, Greenberg L, Bredemeyer AL, Ma P, Liu J, Penna V, Winkler ES, Sviben S, Brooks E, Nair AP, Heck KA, Rali AS, Simpson L, Saririan M, Hobohm D, Stump WT, Fitzpatrick JA, Xie X, Zhang X, Shi PY, Hinson JT, Gi WT, Schmidt C, Leuschner F, Lin CY, Diamond MS, Greenberg MJ, Lavine KJ. SARS-CoV-2 Infects Human Engineered Heart Tissues and Models COVID-19 Myocarditis. JACC Basic Transl Sci. 2021;6:331-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 120] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 68. | Centers for Disease Control and Prevention. Chronic Kidney Disease in the United States, 2019. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention. |
| 69. | Li S, Wang F, Sun D. The renal microcirculation in chronic kidney disease: novel diagnostic methods and therapeutic perspectives. Cell Biosci. 2021;11:90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 70. | Fenske R, Weeks A, Brill A, Nall R, Pabitch S, Punt M, Daniels M, Blaha S, Davis DB, Kimple M. Prostaglandin E2 (PGE2) Levels As a Predictor of Type 2 Diabetes Control in Human Subjects: A cross-sectional view of initial cohort study data. FASEB J. 2018;31:675.6. [DOI] [Full Text] |
| 71. | Ito S, Ren Y. Evidence for the role of nitric oxide in macula densa control of glomerular hemodynamics. J Clin Invest. 1993;92:1093-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 135] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 72. | Schneider MP, Ott C, Schmidt S, Kistner I, Friedrich S, Schmieder RE. Poor glycemic control is related to increased nitric oxide activity within the renal circulation of patients with type 2 diabetes. Diabetes Care. 2013;36:4071-4075. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 73. | Ricke-Hoch M, Stelling E, Lasswitz L, Gunesch A, Kasten M, Zapatero-Belinchón F, Brogden G, Gerold G, Battmer K, Pietschmann T, Montiel V, Balligand J, Facciotti F, Hirsch E, Elbahesh H, Rimmelzwaan G, Hoefer A, Kühnel M, Jonigk D, Eigendorf J, Tegtbur U, Mink L, Scherr M, Illig T, Schambach A, Pfeffer T, Andrée B, Hilfiker A, Haverich A, Hilfiker-Kleiner D. SARS-CoV-2-induced impaired immune response by Prostaglandin E2 is accelerated by age, male sex and air pollution. Research Square. 2021;. [DOI] [Full Text] |
| 74. | Ali N. Elevated level of C-reactive protein may be an early marker to predict risk for severity of COVID-19. J Med Virol. 2020;92:2409-2411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 214] [Article Influence: 42.8] [Reference Citation Analysis (0)] |
| 75. | Emerging Risk Factors Collaboration, Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, Gao P, Walker M, Thompson A, Sarwar N, Caslake M, Butterworth AS, Amouyel P, Assmann G, Bakker SJ, Barr EL, Barrett-Connor E, Benjamin EJ, Björkelund C, Brenner H, Brunner E, Clarke R, Cooper JA, Cremer P, Cushman M, Dagenais GR, D'Agostino RB Sr, Dankner R, Davey-Smith G, Deeg D, Dekker JM, Engström G, Folsom AR, Fowkes FG, Gallacher J, Gaziano JM, Giampaoli S, Gillum RF, Hofman A, Howard BV, Ingelsson E, Iso H, Jørgensen T, Kiechl S, Kitamura A, Kiyohara Y, Koenig W, Kromhout D, Kuller LH, Lawlor DA, Meade TW, Nissinen A, Nordestgaard BG, Onat A, Panagiotakos DB, Psaty BM, Rodriguez B, Rosengren A, Salomaa V, Kauhanen J, Salonen JT, Shaffer JA, Shea S, Ford I, Stehouwer CD, Strandberg TE, Tipping RW, Tosetto A, Wassertheil-Smoller S, Wennberg P, Westendorp RG, Whincup PH, Wilhelmsen L, Woodward M, Lowe GD, Wareham NJ, Khaw KT, Sattar N, Packard CJ, Gudnason V, Ridker PM, Pepys MB, Thompson SG, Danesh J. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 770] [Cited by in RCA: 836] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 76. | Carpenter TC, Schomberg S, Stenmark KR. Endothelin-mediated increases in lung VEGF content promote vascular leak in young rats exposed to viral infection and hypoxia. Am J Physiol Lung Cell Mol Physiol. 2005;289:L1075-L1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 77. | Gregoriano C, Damm D, Kutz A, Koch D, Wolfisberg S, Haubitz S, Conen A, Bernasconi L, Hammerer-Lercher A, Fux CA, Mueller B, Schuetz P. Association of endothelial activation assessed through endothelin-I precursor peptide measurement with mortality in COVID-19 patients: an observational analysis. Respir Res. 2021;22:148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 78. | Tanji N, Markowitz GS, Fu C, Kislinger T, Taguchi A, Pischetsrieder M, Stern D, Schmidt AM, D'Agati VD. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J Am Soc Nephrol. 2000;11:1656-1666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 359] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 79. | Matsui T, Higashimoto Y, Nishino Y, Nakamura N, Fukami K, Yamagishi SI. RAGE-Aptamer Blocks the Development and Progression of Experimental Diabetic Nephropathy. Diabetes. 2017;66:1683-1695. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 101] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 80. | Lim A, Radujkovic A, Weigand MA, Merle U. Soluble receptor for advanced glycation end products (sRAGE) as a biomarker of COVID-19 disease severity and indicator of the need for mechanical ventilation, ARDS and mortality. Ann Intensive Care. 2021;11:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 81. | Wu M, Chen Y, Xia H, Wang C, Tan CY, Cai X, Liu Y, Ji F, Xiong P, Liu R, Guan Y, Duan Y, Kuang D, Xu S, Cai H, Xia Q, Yang D, Wang MW, Chiu IM, Cheng C, Ahern PP, Liu L, Wang G, Surana NK, Xia T, Kasper DL. Transcriptional and proteomic insights into the host response in fatal COVID-19 cases. Proc Natl Acad Sci U S A. 2020;117:28336-28343. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 82. | Chen L, Long X, Xu Q, Tan J, Wang G, Cao Y, Wei J, Luo H, Zhu H, Huang L, Meng F, Wang N, Zhou X, Zhao L, Chen X, Mao Z, Chen C, Li Z, Sun Z, Zhao J, Wang D, Huang G, Wang W, Zhou J. Elevated serum levels of S100A8/A9 and HMGB1 at hospital admission are correlated with inferior clinical outcomes in COVID-19 patients. Cell Mol Immunol. 2020;17:992-994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 197] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 83. | Arunachalam PS, Wimmers F, Mok CKP, Perera RAPM, Scott M, Hagan T, Sigal N, Feng Y, Bristow L, Tak-Yin Tsang O, Wagh D, Coller J, Pellegrini KL, Kazmin D, Alaaeddine G, Leung WS, Chan JMC, Chik TSH, Choi CYC, Huerta C, Paine McCullough M, Lv H, Anderson E, Edupuganti S, Upadhyay AA, Bosinger SE, Maecker HT, Khatri P, Rouphael N, Peiris M, Pulendran B. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science. 2020;369:1210-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 661] [Cited by in RCA: 860] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 84. | Aceti A, Margarucci LM, Scaramucci E, Orsini M, Salerno G, Di Sante G, Gianfranceschi G, Di Liddo R, Valeriani F, Ria F, Simmaco M, Parnigotto PP, Vitali M, Romano Spica V, Michetti F. Serum S100B protein as a marker of severity in Covid-19 patients. Sci Rep. 2020;10:18665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 77] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 85. | Wei J, Alfajaro MM, DeWeirdt PC, Hanna RE, Lu-Culligan WJ, Cai WL, Strine MS, Zhang SM, Graziano VR, Schmitz CO, Chen JS, Mankowski MC, Filler RB, Ravindra NG, Gasque V, de Miguel FJ, Patil A, Chen H, Oguntuyo KY, Abriola L, Surovtseva YV, Orchard RC, Lee B, Lindenbach BD, Politi K, van Dijk D, Kadoch C, Simon MD, Yan Q, Doench JG, Wilen CB. Genome-wide CRISPR Screens Reveal Host Factors Critical for SARS-CoV-2 Infection. Cell. 2021;184:76-91.e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 410] [Cited by in RCA: 391] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 86. | Gross JL, de Azevedo MJ, Silveiro SP, Canani LH, Caramori ML, Zelmanovitz T. Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care. 2005;28:164-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1112] [Article Influence: 55.6] [Reference Citation Analysis (0)] |
| 87. | Liang H, Kennedy C, Manne S, Lin JH, Dolin P. Monitoring for proteinuria in patients with type 2 diabetes mellitus. BMJ Open Diabetes Res Care. 2015;3:e000071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 88. | Ouahmi H, Courjon J, Morand L, François J, Bruckert V, Lombardi R, Esnault V, Seitz-Polski B, Demonchy E, Dellamonica J, Boyer-Suavet S. Proteinuria as a Biomarker for COVID-19 Severity. Front Physiol. 2021;12:611772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Trinh MD, Plihalova A, Gojda J, Westlake K, Spicka J, Lattova Z, Pretl M, Polak J. Obstructive sleep apnoea increases lipolysis and deteriorates glucose homeostasis in patients with type 2 diabetes mellitus. Sci Rep. 2021;11:3567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 90. | Zamarrón E, Jaureguizar A, García-Sánchez A, Díaz-Cambriles T, Alonso-Fernández A, Lores V, Mediano O, Rodríguez-Rodríguez P, Cabello-Pelegrín S, Morales-Ruíz E, Ramírez-Prieto MT, Valiente-Díaz MI, Gómez-García T, García-Río F; Spanish Sleep Network. Obstructive sleep apnea is associated with impaired renal function in patients with diabetic kidney disease. Sci Rep. 2021;11:5675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 91. | Lin CH, Perger E, Lyons OD. Obstructive sleep apnea and chronic kidney disease. Curr Opin Pulm Med. 2018;24:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 92. | Strausz S, Kiiskinen T, Broberg M, Ruotsalainen S, Koskela J, Bachour A; FinnGen, Palotie A, Palotie T, Ripatti S, Ollila HM. Sleep apnoea is a risk factor for severe COVID-19. BMJ Open Respir Res. 2021;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 93. | Huang JX, Casper TC, Pitts C, Myers S, Loomba L, Ramesh J, Kuppermann N, Glaser N. Association of Acute Kidney Injury During Diabetic Ketoacidosis With Risk of Microalbuminuria in Children With Type 1 Diabetes. JAMA Pediatr. 2022;176:169-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 94. | Li J, Wang X, Chen J, Zuo X, Zhang H, Deng A. COVID-19 infection may cause ketosis and ketoacidosis. Diabetes Obes Metab. 2020;22:1935-1941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 392] [Article Influence: 78.4] [Reference Citation Analysis (0)] |
| 95. | Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, Somani S, Richter F, Miotto R, Lala A, Kia A, Timsina P, Li L, Freeman R, Chen R, Narula J, Just AC, Horowitz C, Fayad Z, Cordon-Cardo C, Schadt E, Levin MA, Reich DL, Fuster V, Murphy B, He JC, Charney AW, Böttinger EP, Glicksberg BS, Coca SG, Nadkarni GN; Mount Sinai COVID Informatics Center (MSCIC). AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol. 2021;32:151-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 483] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 96. | Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney Outcomes in Long COVID. J Am Soc Nephrol. 2021;32:2851-2862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 234] [Article Influence: 58.5] [Reference Citation Analysis (0)] |
| 97. | Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases. 2014;2:488-496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 170] [Cited by in RCA: 192] [Article Influence: 17.5] [Reference Citation Analysis (2)] |
| 98. | Montomoli J, Donati A, Ince C. Acute Kidney Injury and Fluid Resuscitation in Septic Patients: Are We Protecting the Kidney? Nephron. 2019;143:170-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 99. | Kazory A, Ronco C, McCullough PA. SARS-CoV-2 (COVID-19) and intravascular volume management strategies in the critically ill. Proc (Bayl Univ Med Cent). 2020;0:1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 100. | Watchorn J, Huang DY, Joslin J, Bramham K, Hutchings SD. Critically Ill COVID-19 Patients With Acute Kidney Injury Have Reduced Renal Blood Flow and Perfusion Despite Preserved Cardiac Function: A Case-Control Study Using Contrast-Enhanced Ultrasound. Shock. 2021;55:479-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 101. | Matejovic M, Ince C, Chawla LS, Blantz R, Molitoris BA, Rosner MH, Okusa MD, Kellum JA, Ronco C; ADQI XIII Work Group. Renal Hemodynamics in AKI: In Search of New Treatment Targets. J Am Soc Nephrol. 2016;27:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 102. | Brophy DF, Martin RJ, Gehr TW, Carr ME Jr. A hypothesis-generating study to evaluate platelet activity in diabetics with chronic kidney disease. Thromb J. 2005;3:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 103. | Reiken S, Sittenfeld L, Dridi H, Liu Y, Liu X, Marks AR. Alzheimer's-like signaling in brains of COVID-19 patients. Alzheimers Dement. 2022;18:955-965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 128] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 104. | Douaud G, Lee S, Alfaro-Almagro F, Arthofer C, Wang C, McCarthy P, Lange F, Andersson JLR, Griffanti L, Duff E, Jbabdi S, Taschler B, Keating P, Winkler AM, Collins R, Matthews PM, Allen N, Miller KL, Nichols TE, Smith SM. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 897] [Cited by in RCA: 953] [Article Influence: 317.7] [Reference Citation Analysis (0)] |
| 105. | Planel E, Tatebayashi Y, Miyasaka T, Liu L, Wang L, Herman M, Yu WH, Luchsinger JA, Wadzinski B, Duff KE, Takashima A. Insulin dysfunction induces in vivo tau hyperphosphorylation through distinct mechanisms. J Neurosci. 2007;27:13635-13648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 106. | Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, Laue M, Schneider J, Brünink S, Greuel S, Lehmann M, Hassan O, Aschman T, Schumann E, Chua RL, Conrad C, Eils R, Stenzel W, Windgassen M, Rößler L, Goebel HH, Gelderblom HR, Martin H, Nitsche A, Schulz-Schaeffer WJ, Hakroush S, Winkler MS, Tampe B, Scheibe F, Körtvélyessy P, Reinhold D, Siegmund B, Kühl AA, Elezkurtaj S, Horst D, Oesterhelweg L, Tsokos M, Ingold-Heppner B, Stadelmann C, Drosten C, Corman VM, Radbruch H, Heppner FL. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 799] [Cited by in RCA: 945] [Article Influence: 236.3] [Reference Citation Analysis (0)] |
| 107. | Díaz-Gerevini GT, Daín A, Pasqualini ME, López CB, Eynard AR, Repossi G. Diabetic encephalopathy: beneficial effects of supplementation with fatty acids ω3 and nordihydroguaiaretic acid in a spontaneous diabetes rat model. Lipids Health Dis. 2019;18:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 108. | Cai XJ, Xu HQ, Lu Y. C-peptide and diabetic encephalopathy. Chin Med Sci J. 2011;26:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 109. | Akın O, Kılınç Uğurlu A, Akbaş ED, Döğer E, Akbaş Y, Bideci A, Yüce Ö, Gücüyener K, Çamurdan MO, Karabacak N, Cinaz P. Autoimmune Limbic Encephalitis Associated with Type 1 Diabetes Mellitus. J Clin Res Pediatr Endocrinol. 2017;9:387-388. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 110. | Uginet M, Breville G, Assal F, Lövblad KO, Vargas MI, Pugin J, Serratrice J, Herrmann FR, Lalive PH, Allali G. COVID-19 encephalopathy: Clinical and neurobiological features. J Med Virol. 2021;93:4374-4381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 111. | Siddiqui AF, Saadia S, Ejaz T, Mushtaq Z. COVID-19 encephalopathy: an unusual presentation with new-onset seizure causing convulsive status epilepticus. BMJ Case Rep. 2022;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 112. | Hernández-Fernández F, Sandoval Valencia H, Barbella-Aponte RA, Collado-Jiménez R, Ayo-Martín Ó, Barrena C, Molina-Nuevo JD, García-García J, Lozano-Setién E, Alcahut-Rodriguez C, Martínez-Martín Á, Sánchez-López A, Segura T. Cerebrovascular disease in patients with COVID-19: neuroimaging, histological and clinical description. Brain. 2020;143:3089-3103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 208] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 113. | Nampoothiri S, Sauve F, Ternier G, Fernandois D, Coelho C, Imbernon M, Deligia E, Perbet R, Florent V, Baroncini M, Pasquier F, Trottein F, Maurage CA, Mattot V, Giacobini P, Rasika S, Prevot V. The hypothalamus as a hub for SARS-CoV-2 brain infection and pathogenesis. bioRxiv. 2020;. [DOI] [Full Text] |
| 114. | Pilotto A, Masciocchi S, Volonghi I, Crabbio M, Magni E, De Giuli V, Caprioli F, Rifino N, Sessa M, Gennuso M, Cotelli MS, Turla M, Balducci U, Mariotto S, Ferrari S, Ciccone A, Fiacco F, Imarisio A, Risi B, Benussi A, Premi E, Focà E, Caccuri F, Leonardi M, Gasparotti R, Castelli F, Zanusso G, Pezzini A, Padovani A; SARS-CoV-2 related encephalopaties (ENCOVID) Study Group. Clinical Presentation and Outcomes of Severe Acute Respiratory Syndrome Coronavirus 2-Related Encephalitis: The ENCOVID Multicenter Study. J Infect Dis. 2021;223:28-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 115. | Pilotto A, Masciocchi S, Volonghi I, De Giuli V, Caprioli F, Mariotto S, Ferrari S, Bozzetti S, Imarisio A, Risi B, Premi E, Benussi A, Focà E, Castelli F, Zanusso G, Monaco S, Stefanelli P, Gasparotti R, Zekeridou A, McKeon A, Ashton NJ, BlennoW K, Zetterberg H, Padovani A. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Encephalitis Is a Cytokine Release Syndrome: Evidences From Cerebrospinal Fluid Analyses. Clin Infect Dis. 2021;73:e3019-e3026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 124] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 116. | Rhea EM, Banks WA. Role of the Blood-Brain Barrier in Central Nervous System Insulin Resistance. Front Neurosci. 2019;13:521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 157] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 117. | Małkiewicz MA, Szarmach A, Sabisz A, Cubała WJ, Szurowska E, Winklewski PJ. Blood-brain barrier permeability and physical exercise. J Neuroinflammation. 2019;16:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 166] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 118. | Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol Dis. 2010;37:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2891] [Cited by in RCA: 3475] [Article Influence: 217.2] [Reference Citation Analysis (0)] |
| 119. | Prasad S, Sajja RK, Naik P, Cucullo L. Diabetes Mellitus and Blood-Brain Barrier Dysfunction: An Overview. J Pharmacovigil. 2014;2:125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 120. | Hussain T, Tan B, Yin Y, Blachier F, Tossou MC, Rahu N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid Med Cell Longev. 2016;2016:7432797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 764] [Cited by in RCA: 1264] [Article Influence: 140.4] [Reference Citation Analysis (0)] |
| 121. | Dénes A, Ferenczi S, Kovács KJ. Systemic inflammatory challenges compromise survival after experimental stroke via augmenting brain inflammation, blood- brain barrier damage and brain oedema independently of infarct size. J Neuroinflammation. 2011;8:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 121] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 122. | Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 668] [Cited by in RCA: 767] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 123. | Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1028] [Cited by in RCA: 1068] [Article Influence: 213.6] [Reference Citation Analysis (0)] |
| 124. | Alexopoulos H, Magira E, Bitzogli K, Kafasi N, Vlachoyiannopoulos P, Tzioufas A, Kotanidou A, Dalakas MC. Anti-SARS-CoV-2 antibodies in the CSF, blood-brain barrier dysfunction, and neurological outcome: Studies in 8 stuporous and comatose patients. Neurol Neuroimmunol Neuroinflamm. 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 107] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 125. | Guilmot A, Maldonado Slootjes S, Sellimi A, Bronchain M, Hanseeuw B, Belkhir L, Yombi JC, De Greef J, Pothen L, Yildiz H, Duprez T, Fillée C, Anantharajah A, Capes A, Hantson P, Jacquerye P, Raymackers JM, London F, El Sankari S, Ivanoiu A, Maggi P, van Pesch V. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J Neurol. 2021;268:751-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 138] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 126. | Eshaq RS, Aldalati AMZ, Alexander JS, Harris NR. Diabetic retinopathy: Breaking the barrier. Pathophysiology. 2017;24:229-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (1)] |
| 127. | Teo ZL, Tham YC, Yu M, Chee ML, Rim TH, Cheung N, Bikbov MM, Wang YX, Tang Y, Lu Y, Wong IY, Ting DSW, Tan GSW, Jonas JB, Sabanayagam C, Wong TY, Cheng CY. Global Prevalence of Diabetic Retinopathy and Projection of Burden through 2045: Systematic Review and Meta-analysis. Ophthalmology. 2021;128:1580-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 1022] [Article Influence: 255.5] [Reference Citation Analysis (1)] |
| 128. | Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 674] [Article Influence: 84.3] [Reference Citation Analysis (76)] |
| 129. | Wang W, Lo ACY. Diabetic Retinopathy: Pathophysiology and Treatments. Int J Mol Sci. 2018;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 745] [Article Influence: 106.4] [Reference Citation Analysis (0)] |
| 130. | Huang H, He J, Johnson D, Wei Y, Liu Y, Wang S, Lutty GA, Duh EJ, Semba RD. Deletion of placental growth factor prevents diabetic retinopathy and is associated with Akt activation and HIF1α-VEGF pathway inhibition. Diabetes. 2015;64:200-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 111] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 131. | Lupo G, Motta C, Giurdanella G, Anfuso CD, Alberghina M, Drago F, Salomone S, Bucolo C. Role of phospholipases A2 in diabetic retinopathy: in vitro and in vivo studies. Biochem Pharmacol. 2013;86:1603-1613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 132. | Giurdanella G, Lupo G, Gennuso F, Conti F, Furno DL, Mannino G, Anfuso CD, Drago F, Salomone S, Bucolo C. Activation of the VEGF-A/ERK/PLA2 Axis Mediates Early Retinal Endothelial Cell Damage Induced by High Glucose: New Insight from an In Vitro Model of Diabetic Retinopathy. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 133. | Chang YS, Ho CH, Chu CC, Wang JJ, Tseng SH, Jan RL. Risk of retinal artery occlusion in patients with diabetes mellitus: A retrospective large-scale cohort study. PLoS One. 2018;13:e0201627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 134. | Suzuki Y, Nakazawa M, Suzuki K, Yamazaki H, Miyagawa Y. Expression profiles of cytokines and chemokines in vitreous fluid in diabetic retinopathy and central retinal vein occlusion. Jpn J Ophthalmol. 2011;55:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 135. | Tang J, Allen Lee C, Du Y, Sun Y, Pearlman E, Sheibani N, Kern TS. MyD88-dependent pathways in leukocytes affect the retina in diabetes. PLoS One. 2013;8:e68871. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 136. | Joussen AM, Poulaki V, Mitsiades N, Cai WY, Suzuma I, Pak J, Ju ST, Rook SL, Esser P, Mitsiades CS, Kirchhof B, Adamis AP, Aiello LP. Suppression of Fas-FasL-induced endothelial cell apoptosis prevents diabetic blood-retinal barrier breakdown in a model of streptozotocin-induced diabetes. FASEB J. 2003;17:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 136] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 137. | Boss JD, Singh PK, Pandya HK, Tosi J, Kim C, Tewari A, Juzych MS, Abrams GW, Kumar A. Assessment of Neurotrophins and Inflammatory Mediators in Vitreous of Patients With Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017;58:5594-5603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 159] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 138. | Portillo JC, Lopez Corcino Y, Miao Y, Tang J, Sheibani N, Kern TS, Dubyak GR, Subauste CS. CD40 in Retinal Müller Cells Induces P2X7-Dependent Cytokine Expression in Macrophages/Microglia in Diabetic Mice and Development of Early Experimental Diabetic Retinopathy. Diabetes. 2017;66:483-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 139. | Tien T, Zhang J, Muto T, Kim D, Sarthy VP, Roy S. High Glucose Induces Mitochondrial Dysfunction in Retinal Müller Cells: Implications for Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 2017;58:2915-2921. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 82] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 140. | Silva VAO, André ND, Sousa TAE, Alves VM, Kettelhut IDC, De Lucca FL. Nuclear PKR in retinal neurons in the early stage of diabetic retinopathy in streptozotocininduced diabetic rats. Mol Med Rep. 2021;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 141. | Piano I, Novelli E, Della Santina L, Strettoi E, Cervetto L, Gargini C. Involvement of Autophagic Pathway in the Progression of Retinal Degeneration in a Mouse Model of Diabetes. Front Cell Neurosci. 2016;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 142. | Sasaki M, Ozawa Y, Kurihara T, Kubota S, Yuki K, Noda K, Kobayashi S, Ishida S, Tsubota K. Neurodegenerative influence of oxidative stress in the retina of a murine model of diabetes. Diabetologia. 2010;53:971-979. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 216] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 143. | Loffredo L, Pacella F, Pacella E, Tiscione G, Oliva A, Violi F. Conjunctivitis and COVID-19: A meta-analysis. J Med Virol. 2020;92:1413-1414. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 112] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 144. | Casagrande M, Fitzek A, Spitzer M, Püschel K, Glatzel M, Krasemann S, Aepfelbacher M, Nörz D, Lütgehetmann M, Pfefferle S, Schultheiss M. Detection of SARS-CoV-2 genomic and subgenomic RNA in retina and optic nerve of patients with COVID-19. Br J Ophthalmol. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 145. | Azzolini C, Donati S, Premi E, Baj A, Siracusa C, Genoni A, Grossi PA, Azzi L, Sessa F, Dentali F, Severgnini P, Minoja G, Cabrini L, Chiaravalli M, Veronesi G, Carcano G, Maffioli LS, Tagliabue A. SARS-CoV-2 on Ocular Surfaces in a Cohort of Patients With COVID-19 From the Lombardy Region, Italy. JAMA Ophthalmol. 2021;139:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 146. | Zhou L, Xu Z, Castiglione GM, Soiberman US, Eberhart CG, Duh EJ. ACE2 and TMPRSS2 are expressed on the human ocular surface, suggesting susceptibility to SARS-CoV-2 infection. Ocul Surf. 2020;18:537-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 250] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 147. | Menuchin-Lasowski Y, Schreiber A, Lecanda A, Mecate-Zambrano A, Brunotte L, Psathaki OE, Ludwig S, Rauen T, Schöler HR. SARS-CoV-2 infects and replicates in photoreceptor and retinal ganglion cells of human retinal organoids. Stem Cell Reports. 2022;17:789-803. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 148. | Sen M, Honavar SG, Sharma N, Sachdev MS. COVID-19 and Eye: A Review of Ophthalmic Manifestations of COVID-19. Indian J Ophthalmol. 2021;69:488-509. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 182] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 149. | Sindhuja K, Lomi N, Asif MI, Tandon R. Clinical profile and prevalence of conjunctivitis in mild COVID-19 patients in a tertiary care COVID-19 hospital: A retrospective cross-sectional study. Indian J Ophthalmol. 2020;68:1546-1550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 150. | Chen L, Deng C, Chen X, Zhang X, Chen B, Yu H, Qin Y, Xiao K, Zhang H, Sun X. Ocular manifestations and clinical characteristics of 535 cases of COVID-19 in Wuhan, China: a cross-sectional study. Acta Ophthalmol. 2020;98:e951-e959. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 194] [Article Influence: 38.8] [Reference Citation Analysis (0)] |
| 151. | Guo D, Xia J, Wang Y, Zhang X, Shen Y, Tong JP. Relapsing viral keratoconjunctivitis in COVID-19: a case report. Virol J. 2020;17:97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 152. | Kruse A, Thomsen RW, Hundborg HH, Knudsen LL, Sørensen HT, Schønheyder HC. Diabetes and risk of acute infectious conjunctivitis--a population-based case-control study. Diabet Med. 2006;23:393-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 153. | Komorita Y, Minami M, Maeda Y, Yoshioka R, Ohkuma T, Kitazono T. Prevalence of bone fracture and its association with severe hypoglycemia in Japanese patients with type 1 diabetes. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 154. | Wang H, Ba Y, Xing Q, Du JL. Diabetes mellitus and the risk of fractures at specific sites: a meta-analysis. BMJ Open. 2019;9:e024067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 119] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 155. | Roy B. Biomolecular basis of the role of diabetes mellitus in osteoporosis and bone fractures. World J Diabetes. 2013;4:101-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 81] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 156. | Lauwers M, Au M, Yuan S, Wen C. COVID-19 in Joint Ageing and Osteoarthritis: Current Status and Perspectives. Int J Mol Sci. 2022;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 157. | di Filippo L, Frara S, Giustina A. The emerging osteo-metabolic phenotype of COVID-19: clinical and pathophysiological aspects. Nat Rev Endocrinol. 2021;17:445-446. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 158. | Queiroz-Junior CM, Santos ACPM, Galvão I, Souto GR, Mesquita RA, Sá MA, Ferreira AJ. The angiotensin converting enzyme 2/angiotensin-(1-7)/Mas Receptor axis as a key player in alveolar bone remodeling. Bone. 2019;128:115041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 159. | Awosanya OD, Dalloul CE, Blosser RJ, Dadwal UC, Carozza M, Boschen K, Klemsz MJ, Johnston NA, Bruzzaniti A, Robinson CM, Srour EF, Kacena MA. Osteoclast-mediated bone loss observed in a COVID-19 mouse model. Bone. 2022;154:116227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 160. | Tripathy AS, Vishwakarma S, Trimbake D, Gurav YK, Potdar VA, Mokashi ND, Patsute SD, Kaushal H, Choudhary ML, Tilekar BN, Sarje P, Dange VS, Abraham P. Pro-inflammatory CXCL-10, TNF-α, IL-1β, and IL-6: biomarkers of SARS-CoV-2 infection. Arch Virol. 2021;166:3301-3310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 47] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 161. | Demir M, Demir F, Aygun H. Vitamin D deficiency is associated with COVID-19 positivity and severity of the disease. J Med Virol. 2021;93:2992-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 162. | Cheung KS, Hung IFN, Chan PPY, Lung KC, Tso E, Liu R, Ng YY, Chu MY, Chung TWH, Tam AR, Yip CCY, Leung KH, Fung AY, Zhang RR, Lin Y, Cheng HM, Zhang AJX, To KKW, Chan KH, Yuen KY, Leung WK. Gastrointestinal Manifestations of SARS-CoV-2 Infection and Virus Load in Fecal Samples From a Hong Kong Cohort: Systematic Review and Meta-analysis. Gastroenterology. 2020;159:81-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1113] [Cited by in RCA: 1132] [Article Influence: 226.4] [Reference Citation Analysis (1)] |
| 163. | Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homologue of angiotensin converting enzyme. FEBS Lett. 2002;532:107-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 655] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 164. | Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science. 2020;367:1444-1448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3274] [Cited by in RCA: 3676] [Article Influence: 735.2] [Reference Citation Analysis (0)] |
| 165. | Giobbe GG, Bonfante F, Jones BC, Gagliano O, Luni C, Zambaiti E, Perin S, Laterza C, Busslinger G, Stuart H, Pagliari M, Bortolami A, Mazzetto E, Manfredi A, Colantuono C, Di Filippo L, Pellegata AF, Panzarin V, Thapar N, Li VSW, Eaton S, Cacchiarelli D, Clevers H, Elvassore N, De Coppi P. SARS-CoV-2 infection and replication in human gastric organoids. Nat Commun. 2021;12:6610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 12.5] [Reference Citation Analysis (1)] |
| 166. | Lin L, Jiang X, Zhang Z, Huang S, Fang Z, Gu Z, Gao L, Shi H, Mai L, Liu Y, Lin X, Lai R, Yan Z, Li X, Shan H. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut. 2020;69:997-1001. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 630] [Cited by in RCA: 658] [Article Influence: 131.6] [Reference Citation Analysis (0)] |
| 167. | Xu Z, Tang M, Chen P, Cai H, Xiao F. SARS-CoV-2 Gastrointestinal Infection Prolongs the Time to Recover From COVID-19. Front Med (Lausanne). 2021;8:683551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 168. | Reszczyńska M, Kempiński R. The Prevalence of Enteropathy Symptoms from the Lower Gastrointestinal Tract and the Evaluation of Anorectal Function in Diabetes Mellitus Patients. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 169. | Narbonne H, Paquis-Fluckinger V, Valero R, Heyries L, Pellissier JF, Vialettes B. Gastrointestinal tract symptoms in Maternally Inherited Diabetes and Deafness (MIDD). Diabetes Metab. 2004;30:61-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 170. | Young RL, Sutherland K, Pezos N, Brierley SM, Horowitz M, Rayner CK, Blackshaw LA. Expression of taste molecules in the upper gastrointestinal tract in humans with and without type 2 diabetes. Gut. 2009;58:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 171. | Peng YL, Leu HB, Luo JC, Huang CC, Hou MC, Lin HC, Lee FY. Diabetes is an independent risk factor for peptic ulcer bleeding: a nationwide population-based cohort study. J Gastroenterol Hepatol. 2013;28:1295-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 172. | Nie Z, Xu L, Li C, Tian T, Xie P, Chen X, Li B. Association of endothelial progenitor cells and peptic ulcer treatment in patients with type 2 diabetes mellitus. Exp Ther Med. 2016;11:1581-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 173. | Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic Fatty liver disease: a pathogenic duo. Endocr Rev. 2013;34:84-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 174. | Dharmalingam M, Yamasandhi PG. Nonalcoholic Fatty Liver Disease and Type 2 Diabetes Mellitus. Indian J Endocrinol Metab. 2018;22:421-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 134] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 175. | Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do Diabetologists stand? Clin Diabetes Endocrinol. 2020;6:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 73] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 176. | Han JE, Shin HB, Ahn YH, Cho HJ, Cheong JY, Park B, Kim SS. Relationship between the dynamics of non-alcoholic fatty liver disease and incident diabetes mellitus. Sci Rep. 2022;12:2538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 177. | Kuzulugil D, Papeix G, Luu J, Kerridge RK. Recent advances in diabetes treatments and their perioperative implications. Curr Opin Anaesthesiol. 2019;32:398-404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 178. | Dixit SB, Zirpe KG, Kulkarni AP, Chaudhry D, Govil D, Mehta Y, Jog SA, Khatib KI, Pandit RA, Samavedam S, Rangappa P, Bandopadhyay S, Shrivastav O, Mhatre U. Current Approaches to COVID-19: Therapy and Prevention. Indian J Crit Care Med. 2020;24:838-846. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 179. | Hoffmann M, Hofmann-Winkler H, Smith JC, Krüger N, Arora P, Sørensen LK, Søgaard OS, Hasselstrøm JB, Winkler M, Hempel T, Raich L, Olsson S, Danov O, Jonigk D, Yamazoe T, Yamatsuta K, Mizuno H, Ludwig S, Noé F, Kjolby M, Braun A, Sheltzer JM, Pöhlmann S. Camostat mesylate inhibits SARS-CoV-2 activation by TMPRSS2-related proteases and its metabolite GBPA exerts antiviral activity. EBioMedicine. 2021;65:103255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 240] [Cited by in RCA: 246] [Article Influence: 61.5] [Reference Citation Analysis (0)] |