Published online Jul 25, 2022. doi: 10.5501/wjv.v11.i4.176
Peer-review started: February 2, 2022
First decision: April 8, 2022
Revised: April 11, 2022
Accepted: June 27, 2022
Article in press: June 27, 2022
Published online: July 25, 2022
Processing time: 169 Days and 13.4 Hours
Coronavirus disease 2019 (COVID-19) continues to create havoc and may present with myriad complications involving many organ systems. However, the respiratory system bears the maximum brunt of the disease and continues to be most commonly affected. There is a high incidence of air leaks in patients with COVID-19, leading to acute worsening of clinical condition. The air leaks may develop independently of the severity of disease or positive pressure ventilation and even in the absence of any traditional risk factors like smoking and un-derlying lung disease. The exact pathophysiology of air leaks with COVID-19 remains unclear, but multiple factors may play a role in their development. A significant proportion of air leaks may be asymptomatic; hence, a high index of suspicion should be exercised for enabling early diagnosis to prevent further deterioration as it is associated with high morbidity and mortality. These air leaks may even develop weeks to months after the disease onset, leading to acute deterioration in the post-COVID period. Conservative management with close monitoring may suffice for many patients but most of the patients with pneumothorax may require intercostal drainage with only a few requiring surgical interventions for persistent air leaks.
Core Tip: Air leaks are an under-recognized and under-reported complication of coronavirus disease 2019 (COVID-19). Air leaks may also develop in spontaneously breathing patients without any underlying risk factors. Because these leaks may be asymptomatic and may even develop weeks to months after the onset of disease, a high index of suspicion is warranted to ensure early diagnosis and timely intervention. Still, patients with air leaks have poorer overall outcomes with greater need for ventilatory support, longer length of hospitalizations, and higher mortality rates. A better understanding of its pathophysiology may help in preventing the development of air leaks and improve outcomes.
- Citation: Juneja D, Kataria S, Singh O. Air leaks in COVID-19. World J Virol 2022; 11(4): 176-185
- URL: https://www.wjgnet.com/2220-3249/full/v11/i4/176.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i4.176
Coronavirus disease 2019 (COVID-19) is a multisystem disorder that can lead to a myriad of complications. The pathogenesis of respiratory failure is complex and covers different clinical scenarios such as pneumonia, acute respiratory distress syndrome (ARDS) with normal to low lung compliance, pulmonary embolism, and heart failure. Air leak (AL) injury is a well-documented but rare complication of COVID-19, leading to increased morbidity and mortality, particularly in the intensive care unit (ICU) setting[1]. AL is a clinical phenomenon associated with the leakage of air from a cavity that contains air into spaces that usually, under normal circumstances, do not have air[2]. The AL syndrome (ALS) is the presence of AL with associated symptoms of respiratory distress[2]. The AL may be classified as pneumothorax (air within the pleural cavity), pneumomediastinum (air in the mediastinum), pneumopericardium (air within the pericardial sac), pneumoperitoneum (air within the peritoneal cavity), subcutaneous emphysema (air within the subcutaneous tissue), pneumorrhachis (air within the spinal canal), and retroperitoneal emphysema (air within the retroperitoneum area).
Because of the possible inherent component of COVID-19, the patients are more prone to develop AL than other ICU patients. It can be spontaneous, occurring without any precipitating event, or iatrogenic due to invasive or non-invasive mechanical ventilation[1]. Pneumothorax has been reported as the most common cause of AL, followed by pneumomediastinum, and subcutaneous emphysema, with a few case reports of pneumopericardium and pneumoperitoneum[1,3]. However, pneumomediastinum may be under-recognized and under-reported as most patients are asymptomatic, and pneumomediastinum may be easily missed in chest X-rays. Some case series have reported that pneumomediastinum may be the commonest form of AL and may also be a predictive factor for pneumothorax[4,5].
The literature on AL in COVID-19 patients is limited to case reports, case series, and meta-summaries. The data on the guidelines and management of AL does not explicitly address the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected patients. This review aims to examine the breadth of the available literature on this challenging clinical entity concerning the ongoing pandemic, its clinical effects, and its management strategies.
The exact incidence of AL remains uncertain in COVID-19 patients, as most studies on the subject did not have a specific imaging protocol for the diagnosis. The reported incidence of AL in patients with severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome is 12%-30% and 15%-30%, respectively[6-8]. However, the incidence is lower (0.6%-1%) among COVID-19 patients, but a higher incidence (12.8%-28.6%) has been reported in critically ill patients[1,9]. Compared with non-COVID-19 acute respiratory distress syndrome (ARDS), the patients with COVID-19 related ARDS (CARDS) requiring invasive mechanical ventilation (IMV) had a seven times higher incidence of AL, despite using lung-protective mechanical ventilation[10,11].
A retrospective analysis of the SARS-CoV database identified the mean presentation of AL at 19.6 ± 4.6 d from the onset of symptoms[12]. While most of the data show variability in the onset of AL from 9 to 19.6 d from the time of COVID-19 admission[9], it has been seen up to 60 d in some case reports[13]. In patients requiring IMV, it is generally detected after 4-14 d of its initiation[9].
ALs have been shown to occur more commonly in the older population with COVID-19, and there is a higher incidence in males (M:F = 4:1)[3,14,15]. Nevertheless, this age difference could result from the selection bias of elderly patients who tend to run a more severe course of COVID-19.
While pulmonary diseases like asthma, chronic obstructive airway disease (COPD), interstitial lung disease, lung bulla, and a history of smoking are known risk factors for pneumothorax in the general patient population, no such correlation has been observed in COVID-19 patients[3,9,13-15]. In fact, studies have shown that non-smoking COVID-19 patients have a 5.5 times increased risk of developing pneumothorax[15]. Several other risk factors have been reported in different studies, as enumerated in Table 1.
| Risk factors | Probable mechanism |
| Comorbidities like hypertension, diabetes mellitus, and morbid obesity | By increasing the risk of diffuse alveolar damage |
| Persistent cough | Significant strain by causing sudden alveolar distension |
| Time from symptom onset | Increased risk of P-SILI |
| Mode of ventilation | |
| Non-invasive: HFNC and NIV | Increasing the risk of P-SILI |
| Invasive mechanical ventilation | Ventilation associated lung injury |
| Corticosteroids | Weakening the interstitial tissue, lowering immunity, and impairing healing |
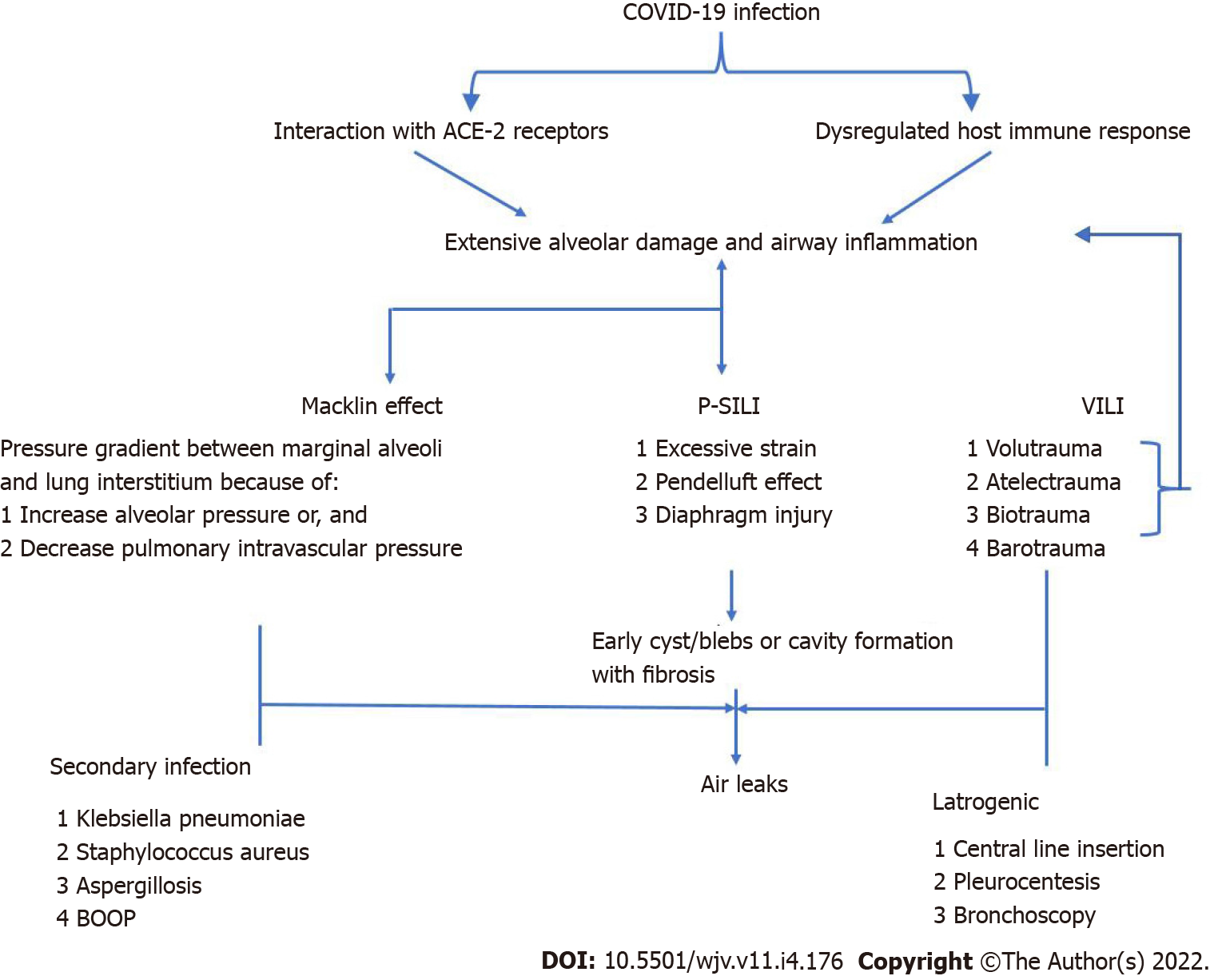
The pathogenesis of SARS-CoV-2 causing AL injuries is complex and not entirely comprehended. Whether CARDS represents a typical or atypical form of ARDS remains a matter of debate. The primary target site of SARS-CoV-2 is angiotensin-converting enzyme-2 (ACE-2) receptors. The higher affinity of ACE-2 receptors for SARS-CoV-2 than that for the SARS coronavirus-1 (SARS-CoV1) may be responsible for the high infectivity of the former[16]. ACE-2 receptors are mainly expressed in type II pneumocytes, besides the vascular endothelium, myocardium, proximal tubules of the kidneys, and intestines[16]. Down-regulation of ACE-2 receptors by SARS-CoV-2 leads to the loss of ACE2 protective function in the local renin-angiotensin system of the lung on inflammation, fibrosis, and pulmonary arterial hypertension. Endothelial dysfunction plays a pivot role in the pathogenesis of SARS-CoV-2 infection by: (1) Unopposed angiotensin II upregulation causing vasoconstriction, increasing the dead space, and producing arterial hypoxemia; (2) coagulation and complement system activation, leading to a thrombotic macro- and micro-angiopathy; and (3) maladaptive immune response and exaggerated inflammatory response[17]. Eventually, these elements cause a lung injury characterized by interstitial inflammatory infiltrates, interstitial alveolar edema, hyaline membrane formation, airway inflammation, and microvascular thrombosis[1,16,17,18]. These factors increase the frailty of airways and alveoli, with early cyst or bullae formation and extensive alveolar destruction, forming cavitary lesions over time, mainly in the non-dependent and caudal region. The peripherally located cysts can either rupture spontaneously or during positive pressure ventilation (PPV) due to increased alveolar pressures, especially in the advanced stages when the lung has undergone fibrotic changes. While PPV may be a contributing factor, data suggest that 30-40% of the patients with COVID-19 who developed ALs were never on invasive ventilation, suggesting that mechanisms apart from barotrauma may play a significant role in the development of AL[3,14].
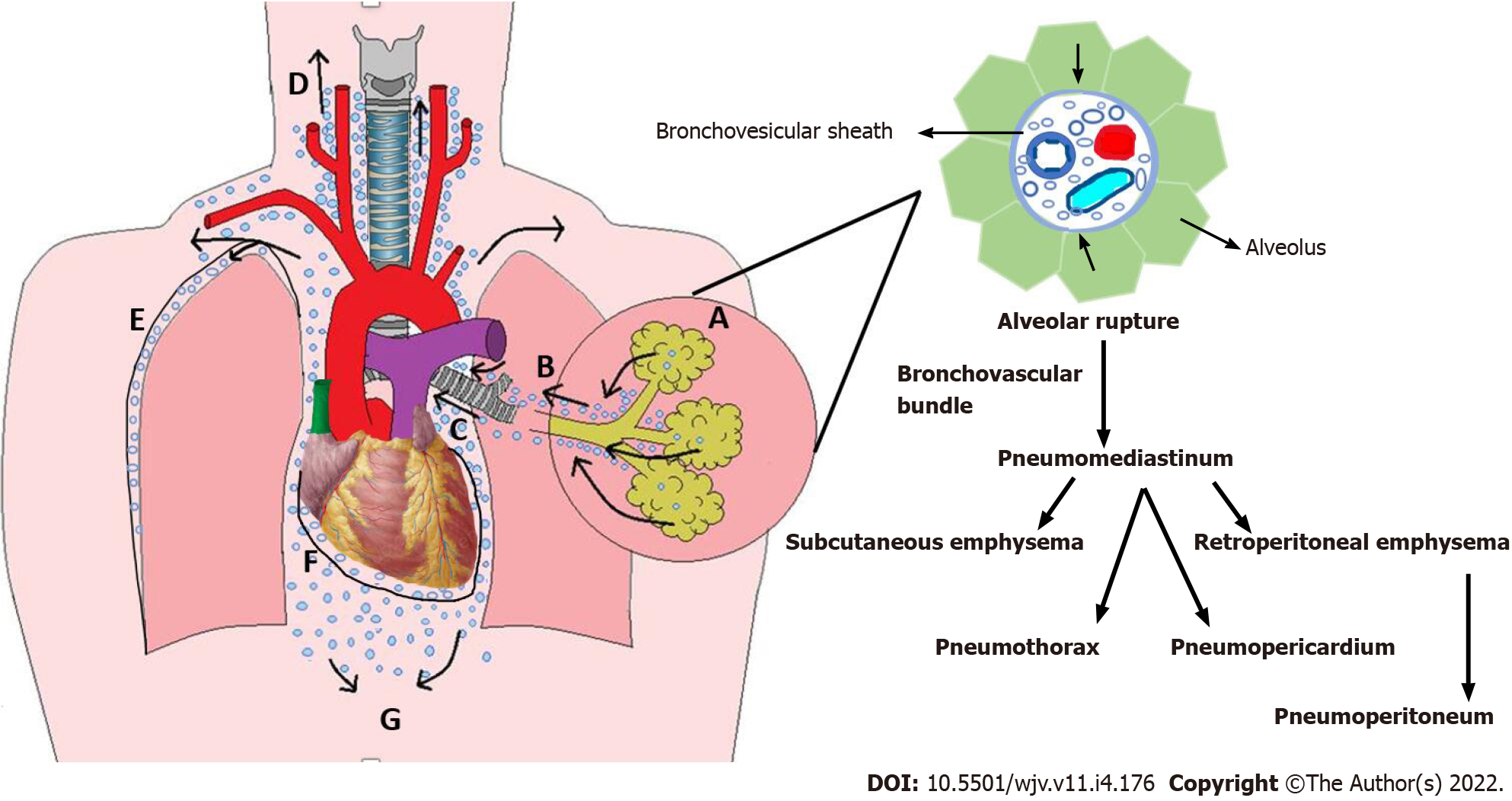
Macklin phenomenon: The marginal alveoli have bases in the bronchi, bronchioles, blood vessels, and pleura separated by a connective tissue layer or interstitium. Increased intrathoracic pressure, by coughing, vomiting, sneezing, defecation, or in cases of asthma or COPD exacerbation, results in increased intra-alveolar pressure and overinflation of the alveoli creating a large pressure gradient between the damaged marginal alveoli and lung interstitium. A pressure gradient may also develop during the Valsalva maneuver by reducing the calibration of pulmonary vasculature without affecting alveolar pressure. This can rupture the marginal alveoli causing the air to leak with centripetal dissection along the bronchovesicular sheath towards the lung's hilum and follow to the low-pressure mediastinum causing spontaneous pneumomediastinum. Pressure in the mediastinum is relieved by the escape of the air into the subcutaneous tissue resulting in subcutaneous emphysema, mainly at the root of the neck, as the cervical fascia is continuous with the mediastinum. Air can then further tract to various cavities causing pneumothorax, pneumopericardium, and retroperitoneal emphysema (Figure 1)[19].
Patient self-inflicted lung injury: Patient self-inflicted lung injury (P-SILI) signifies the possibility of lung injury induced by or worsened by the patient's intense inspiratory effort. P-SILI is a vicious cycle as worsening lung injury increases the respiratory drive, resulting in further strong respiratory efforts. A strong inspiratory effort in a previously injured lung can lead to the following changes[20]: (1) Swings in transpulmonary pressure causing the inflation of large volumes, i.e., excessive strain; (2) abnormal decrease in the alveolar pressure below the positive end-expiratory pressure (PEEP) during assisted ventilation increasing the transvascular hydrostatic pressure, favoring the aggravation of negative-pressure pulmonary edema; (3) significant regional transpulmonary pressure differences in the dependent (posterior) regions than non-dependent (anterior) ones are accompanied by a pendelluft phenomenon, an intrapulmonary shift of gas from non-dependent to dependent lung regions at the very onset of inspiratory effort, even before the start of ventilator insufflation. These effects lead to regional volutrauma and increased cyclic inflation of the dependent regions that were collapsed during expiration (atelectrauma); and (4) diaphragm injury caused by injurious eccentric contractions.
Early intubation was recommended earlier during the pandemic; however, with the increasing incidence of morbidity and mortality associated with IMV, a trial of high-frequency nasal cannula (HFNC) or non-invasive ventilation (NIV) is generally recommended for respiratory support at the outset. Although these strategies might delay IMV, they can still contribute to AL injury by increasing P-SILI. In addition, NIV and HFNC may be associated with a higher incidence of barotrauma than standard low-flow oxygen therapies[21]. Hence, delaying intubation and initiation of IMV may also increase the chances of AL. Therefore, the time from symptom onset to intubation is an independent predictor of AL development[11].
Secondary bacterial infections may enhance the inflammatory mechanism of lung injury triggered by SARS-CoV-2 infection, thus increasing the susceptibility to persistent ALs (PALs). Necrotizing lung infections caused by Klebsiella pneumoniae, Staphylococcus aureus, and Aspergillus spp. may also increase the susceptibility to AL (Figure 2).
As already stated, ALs generally develop later in the disease course, but a minority of patients (less than 1%) have been shown to have AL at the initial presentation[15]. Clinical manifestations can vary from being asymptomatic to having life-threatening conditions. AL may be an incidental finding in 50% of the patients as they may be asymptomatic or have symptoms that might be attributed to disease progression rather than AL[3].
In some studies, pneumothorax in COVID-19 is primarily unilateral and predominately on the right side[1,3]. The most common symptoms of pneumothorax include chest pain and dyspnoea, causing respiratory distress and requiring hospital admission, or worsening of pre-existing respiratory symptoms with increased oxygen requirement. Chest pain is of sudden onset, often sharp, and stabbing type of pleuritic pain, which radiates to the ipsilateral shoulder or arm. The patient might be tachypnoeic and tachycardic, with reduced chest movements and absent breath sounds on examination.
Pneumomediastinum is generally benign; however, retroperitoneal chest pain, dyspnoea, coughing spells, neck pain, or dysphagia can be present[22]. Mediastinal crunching over the cardiac apex and the left sternal border, synchronous with the heartbeat, known as the Hamman's sign, can be heard on auscultation. Subcutaneous emphysema causes painless swelling over the neck and chest, which on palpation gives a feeling of tissue paper in the hands, known as crepitus. This may be the first sign suggestive of an AL. On physical examination, pneumopericardium can be detected by water wheel sound ("bruit de Moulin").
Malignant pneumomediastinum, pneumopericardium, or tension pneumothorax can result in mechanical obstruction, causing a decrease in venous return, hemodynamic instability, and circulatory collapse. This compels a prompt diagnosis and intervention.
Thorough clinical history and physical assessment remains the key to diagnosing ALs. Apart from pulmonary embolism and acute coronary syndrome, a high index of suspicion for ALS is advised in COVID-19 patients with acute onset of hemodynamic instability, worsening hypoxemia, and or hypercapnia.
As such, there is no single laboratory parameter that may assist in making the diagnosis or confirming AL. In patients with SARS-CoV AL, high lactate dehydrogenase (LDH) levels were associated; however, in COVID-19 patients, LDH levels are not significantly high, and mixed results are observed[21,23,24]. Other laboratory parameters associated with increased incidence of AL are increased serum bilirubin and C-reactive protein levels[11]. Arterial blood gases may be helpful to document hypoxemia and sometimes hypercapnia. The resultant respiratory distress or shock may lead to hyperlactatemia.
Chest radiography: Chest radiography is the first investigation performed as it is simple, inexpensive, and rapid. Chest X-ray has a pooled sensitivity of 52-60% and specificity of 88-95% for diagnosing pneumothorax and pneumomediastinum[25]. The best diagnostic film for pneumomediastinum or pneumothorax is a lateral chest X-ray, with the affected side up for the latter. However, the lateral view is challenging to achieve in the ICU setting. Pneumomediastinum can be differentiated from pneumopericardium on chest X-ray as the former shows air around the heart anteriorly (behind the sternum) and superiorly lifting the thymus but not below (diaphragmatic border). In contrast, air surrounds all the heart's borders in pneumopericardium.
Chest ultrasonography: Ultrasonography is a readily available bedside tool in evaluating critically ill patients and is the only imaging modality that allows scouring for reversible causes of non-arrhythmogenic cardiac arrest during ongoing resuscitation. Ultrasound has a pooled sensitivity of 88%-95% and specificity of 100% for diagnosing pneumothorax. However, the presence of subcutaneous emphysema can affect the accuracy of ultrasound[26]. Features of lung ultrasound for the diagnosis of pneumothorax include: Absence of lung sliding (high sensitivity and specificity), absence of comet-tail artifact (high sensitivity and low specificity), and presence of lung point (high specificity and low sensitivity).
Pneumopericardium and pneumomediastinum are arduously diagnosed by ultrasound. In the case of a large pneumopericardium, an echocardiogram shows "no heart" or absent cardiac images, especially during systole as the heart is pushed further away from the transducer by the air and then returns with diastole. This finding is also known as an "air gap sign" found in pneumomediastinum and pneum-opericardium, seen using M-mode[27]. One distinguishing factor between the two is the inability to see the heart in the subxiphoid view in the case of pneumopericardium. In contrast, the heart is usually well visualized in pneumomediastinum due to its direct contact with the diaphragm without an obstructing air artifact[28]. However, similar findings are often seen with respiratory interference, which may develop if the patient is tachypnoeic. Spontaneous or swirling bubbles may be seen in the pericardial space in patients with pneumopericardium. Ultrasonography is heavily operator-dependent, and its sensitivity further drops in patients with ARDS. In addition, it cannot be used to discriminate between a COPD-associated bleb and pneumothorax.
Computed tomography: Computed tomography (CT) is the gold standard in diagnosing ALs and differentiating bullous disease from pneumothorax. Nevertheless, transporting a critically ill patient on mechanical ventilation and vasopressors to the imaging facility could be perilous. Also, the risk-benefit should be contemplated owing to radiation risk with CT. In addition, the risk of spread of infection should also be kept in mind in patients with active COVID-19 disease.
The Macklin effect on lung parenchyma in CT images is a linear collection of air contiguous to the broncho-vascular sheath. CT has a sensitivity of 89.2% (95% confidence interval [CI]: 74.6–96.9), specificity of 95.6% (95%CI: 90.6–98.4), and accuracy of 94.2% (95%CI: 89.6-97.2) to detect the Macklin effect. Macklin's effect on CT can accurately predict AL development in CARDS patients 8.5 d in advance[29].
The most straightforward and widely used technique to quantify AL is asking the patient to cough while observing the water column and the water seal column in the chest tube drainage system. During this maneuver, no air bubbles in the water seal suggest that pleural space is devoid of air. If the intensity of bubbles remains the same on repeated coughs, it is likely to be an active leak. The AL is deemed significant if bubbling is even present during normal breathing or while the patient is talking. However, this method lacks standardization and validation among observers.
The other most commonly used classification is the Cerfolio system[30], which is also based on observation but is less subjective and is a validated classification. It is based on the degree of the leak (measured with an AL meter) and the phase of respiration in which it appears (Table 2). However, there is no specific classification for AL in COVID-19 patients.
| Grade | Description |
| Grade 1, FE | During forced expiration only, typically when asking the patient to cough |
| Grade 2, E | During expiration only |
| Grade 3, I | During inspiration only |
| Grade 4, C | Continuous bubbling both during expiration and inspiration |
Persistent AL (PAL) refers to the continued airflow from the endobronchial tree to the pleural space, which can occur due to an abnormal connection between the pleural space and airways (bronchopleural fistula, BPF) or alveolus. An AL is referred to as a PAL when it persists longer than 5–7 d. This typically used 5-d cut-off to define PAL was initially derived from the expected length of stay following pulmonary resection, where an AL for several days was not uncommon[31,32]. However, some authors suggest that an AL in the setting of secondary spontaneous pneumothorax should be considered persistent after 48 h[31]. Although the exact incidence of PAL is unknown, it may be prevalent in patients with COVID-19. Before diagnosing PAL, one must inspect the chest tube drainage circuit, as a leak in the circuit or malfunctioning three-way stop cock may masquerade as BPF.
The treatment option for COVID-19 associated ALs depends on their type and severity. Currently, there are no guidelines for managing ALs in COVID-19 patients. Many patients with ALs may be managed conservatively, gradually absorbing the air in the following days. The patient should be closely monitored for any clinical deterioration. If possible, PPV should be avoided in such patients, and low-flow oxygen delivery devices for oxygen supplementation or, if required, an HFNC might be preferable over NIV. No independent lung ventilation strategies are consistently effective in expediting the resolution of ALs in a patient on PPV. Although reducing the tidal volume, PEEP, and inspiratory time, if feasible, can promote closure of the pleural defect[33].
Even though most of the COVID-19 patients with pneumomediastinum and around 30% of patients with pneumothorax may be managed conservatively, the remaining patients will require intercostal drainage (ICD), and a few will require further surgical intervention[34]. Tension pneumothorax, pneumomediastinum, or pneumopericardium can be fatal and require immediate decompression. For tension pneumothorax, needle drainage may be performed through the second intercostal space anteriorly in the mid-clavicular line, followed by chest tube drainage. For tension pneumomediastinum drains on the anterior thoracic wall, and for tension pneumopericardium pericardiocentesis may be performed for decompression.
As per British Thoracic Society guidelines, in bubbling chest drains in patients with COVID-19, viral filters should be installed onto the suction port of a chest drain bottle. An alternative approach to reducing the risk of spread of infection through droplets is the use of digital drain circuits (for example, Thopaz+, Medela), though they do not contain a viral filter[35].
The management of PAL can prove to be a challenging task, with the first step being localization of AL. The lack of predictive models to identify patients in whom a resolution of AL is likely to occur conservatively leads to incertitude. As a result, management strategies have been highly variable among different centres. There are reports of 80% of cases having been treated conservatively for 14 d with success; however, a delay in surgery may detrimentally affect surgical outcomes and prolong hospital stay. Therefore, an individualized approach to PAL is suggested to improve patient outcomes[31]. As per the two guidelines on the management of PAL, based on the consensus of expert panels, one should consider early surgery in case that the AL persists beyond 4 d, followed by pleurodesis to prevent recurrence[36,37]. However, surgical repair may not be feasible in critically ill patients with CARDS due to a further increase in morbidity or mortality. In the case of an expected conservative resolution, ICD for a prolonged duration may be preferred[3]. The other promising option is the bronchoscopic placement of a one-way endobronchial valve, which appears to be a reasonable minimally invasive therapeutic option with a high success rate. Again, the risk of spread of infection while performing bronchoscopy should be considered in patients with active COVID-19. Autologous blood pleurodesis, Heimlich valve positioning, and albumin-glutaraldehyde tissue adhesives are additional less invasive options for recurrent and refractory cases[38].
It is common to apply negative suction to chest tubes to enhance pleural apposition. There is no unanimity on whether or not applying suction to the chest tube is beneficial or hazardous. A water seal is usually not helpful or even contraindicated in patients with severe restrictive lung disease and a substantial risk of bleeding. An "alternate suction" protocol with suction pressure of –10 cm H2O during the night and water seal only during daytime appears to be a safe option in such patients. It may decrease AL or chest tube duration in patients without a relevant pneumothorax, progressive subcutaneous emphysema, or cardiorespiratory deterioration[39]. There is no data regarding the use of suction in COVID-19 patients. While managing COVID-19 patients, if no viral filter is attached to the suction port, the drainage system can be placed on suction with a suction canister, and the medical gas vacuum lines exhaust providing negative pressure.
As the development of AL is associated with substantial morbidity and mortality, every measure should be taken to prevent AL. If the CT scan demonstrates the Macklin effect, such patients should take extra care to avoid further damage, e.g., avoiding PPV, avoiding high airway pressure, and favoring extracorporeal technologies instead.
Conceptually, HFNC could limit P-SILI risks compared to NIV, but the tidal volume in the former is difficult to monitor. Also, clinicians should be aware that HFNC may be associated with a higher incidence of barotrauma than the standard, low-flow oxygen therapies, which should be preferred if the patient's condition allows.
In patients on IMV, using a lung-protective ventilation strategy by reducing the alveolar pressure and distension reduces the risk of developing pneumothorax. Judicious use of neuromuscular blockers in patients with high airway pressures or those with patient-ventilator dyssynchrony may also reduce the chances of AL by reducing the negative pressure and shear stress in the pleural cavity.
An excessive and insufficient respiratory effort may result in deleterious anatomical and functional modifications of the diaphragm. Thus, if feasible, using lung and diaphragm protective ventilation simulates a normal inspiratory effort, which also benefits early weaning by reducing the sedation requirement. Transposing this notion into clinical practice needs assessment of the patient's inspiratory effort and potentially perilous patient-ventilator interactions, which may be significantly facilitated by oesophageal pressure (Pes) monitoring. If Pes is unavailable, meticulous clinical assessment and analysis of tidal volume, flow, and airway pressure waveforms from the ventilator can help detect situations at risk of P-SILI. Nonetheless, no clinical study has demonstrated improved patient outcomes by limiting P-SILI risk[20,40].
Lastly, timely application of extracorporeal carbon dioxide removal and extracorporeal membrane oxygenation with lung-protective ventilation strategy may play a key role in preventing pneumothorax in critically ill patients with severe ARDS and refractory hypoxemia.
The overall prognosis of patients with AL is guarded. The development of AL has been associated with a higher need for IMV, prolonged hospitalization, and higher in-hospital mortality[1,9,13]. High mortality rates ranging from 40% to 74% have been reported. Patients with pneumothorax have been reported to have higher mortality than patients with pneumomediastinum. Also, patients developing AL while on PPV may have higher mortality rates[1,3,9,33].
As our clinical knowledge of COVID-19 expands, we must recognize that AL is not an uncommon complication of COVID-19. It is likely a sequela of COVID-19 progression resulting from an inflammatory insult and increase in respiratory effort that may foist changes within the lung parenchyma. A high level of clinical suspicion is merited for an early diagnosis as most patients are asymptomatic, and it should be suspected when there is sudden respiratory or hemodynamic deterioration. One should be vigilant when choosing to continue oxygen therapy via various oxygen delivery devices in patients with a high respiratory drive, as P-SILI can aggravate the disease progression, especially in patients who have evidence of Macklin effect on CT. Patients with AL may be managed conservatively but under strict observation. ALS is associated with increased morbidity and mortality, especially in the elderly and patients on IMV despite lung-protective ventilation.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Munteanu C, Romania; Santulli G, United States A-Editor: Yao QG S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Liu JH
| 1. | Nasa P, Juneja D, Jain R. Air leak with COVID-19 - A meta-summary. Asian Cardiovasc Thorac Ann. 2022;30:237-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Adeyinka A, Pierre L. Air Leak. 2022 May 2. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan. [PubMed] |
| 3. | Martinelli AW, Ingle T, Newman J, Nadeem I, Jackson K, Lane ND, Melhorn J, Davies HE, Rostron AJ, Adeni A, Conroy K, Woznitza N, Matson M, Brill SE, Murray J, Shah A, Naran R, Hare SS, Collas O, Bigham S, Spiro M, Huang MM, Iqbal B, Trenfield S, Ledot S, Desai S, Standing L, Babar J, Mahroof R, Smith I, Lee K, Tchrakian N, Uys S, Ricketts W, Patel ARC, Aujayeb A, Kokosi M, Wilkinson AJK, Marciniak SJ. COVID-19 and pneumothorax: a multicentre retrospective case series. Eur Respir J. 2020;56. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 209] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 4. | Eperjesiova B, Hart E, Shokr M, Sinha P, Ferguson GT. Spontaneous Pneumomediastinum/Pneumothorax in Patients With COVID-19. Cureus. 2020;12:e8996. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Wadhawa R, Thakkar A, Chhanwal HS, Bhalotra A, Rana Y, Wadhawa V. Spontaneous pneumomediastinum and subcutaneous emphysema in patients with COVID-19. Saudi J Anaesth. 2021;15:93-96. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Lew TW, Kwek TK, Tai D, Earnest A, Loo S, Singh K, Kwan KM, Chan Y, Yim CF, Bek SL, Kor AC, Yap WS, Chelliah YR, Lai YC, Goh SK. Acute respiratory distress syndrome in critically ill patients with severe acute respiratory syndrome. JAMA. 2003;290:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 323] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 7. | Peiris JS, Chu CM, Cheng VC, Chan KS, Hung IF, Poon LL, Law KI, Tang BS, Hon TY, Chan CS, Chan KH, Ng JS, Zheng BJ, Ng WL, Lai RW, Guan Y, Yuen KY; HKU/UCH SARS Study Group. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767-1772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1737] [Cited by in RCA: 1786] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 8. | Das KM, Lee EY, Al Jawder SE, Enani MA, Singh R, Skakni L, Al-Nakshabandi N, AlDossari K, Larsson SG. Acute Middle East Respiratory Syndrome Coronavirus: Temporal Lung Changes Observed on the Chest Radiographs of 55 Patients. AJR Am J Roentgenol. 2015;205:W267-W274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Chong WH, Saha BK, Hu K, Chopra A. The incidence, clinical characteristics, and outcomes of pneumothorax in hospitalized COVID-19 patients: A systematic review. Heart Lung. 2021;50:599-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 54] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 10. | Lemmers DHL, Abu Hilal M, Bnà C, Prezioso C, Cavallo E, Nencini N, Crisci S, Fusina F, Natalini G. Pneumomediastinum and subcutaneous emphysema in COVID-19: barotrauma or lung frailty? ERJ Open Res. 2020;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 11. | Belletti A, Palumbo D, Zangrillo A, Fominskiy EV, Franchini S, Dell'Acqua A, Marinosci A, Monti G, Vitali G, Colombo S, Guazzarotti G, Lembo R, Maimeri N, Faustini C, Pennella R, Mushtaq J, Landoni G, Scandroglio AM, Dagna L, De Cobelli F; COVID-BioB Study Group. Predictors of Pneumothorax/Pneumomediastinum in Mechanically Ventilated COVID-19 Patients. J Cardiothorac Vasc Anesth. 2021;35:3642-3651. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 12. | Chu CM, Leung YY, Hui JY, Hung IF, Chan VL, Leung WS, Law KI, Chan CS, Chan KS, Yuen KY. Spontaneous pneumomediastinum in patients with severe acute respiratory syndrome. Eur Respir J. 2004;23:802-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 96] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 13. | Juneja D, Goel A, Singh O, Kataria S, Gupta A, Singh A. Air Leak In Post COVID-19 Patients: Incidence, ICU Course And Outcomes. Med Intensiva. 2022;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Udwadia ZF, Toraskar KK, Pinto L, Mullerpatan J, Wagh HD, Mascarenhas JM, Gandhi BM, Tripathi A, Sunavala A, Agrawal U, Nanda V, Abraham N, Francis B, Zore RR, Pundpal G, Gondse B, Gupta GA. Increased frequency of pneumothorax and pneumomediastinum in COVID-19 patients admitted in the ICU: A multicentre study from Mumbai, India. Clin Med (Lond). 2021;21:e615-e619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Miró Ò, Llorens P, Jiménez S, Piñera P, Burillo-Putze G, Martín A, Martín-Sánchez FJ, García-Lamberetchs EJ, Jacob J, Alquézar-Arbé A, Mòdol JM, López-Díez MP, Guardiola JM, Cardozo C, Lucas Imbernón FJ, Aguirre Tejedo A, García García Á, Ruiz Grinspan M, Llopis Roca F, González Del Castillo J; Spanish Investigators on Emergency Situations Team (SIESTA) Network. Frequency, Risk Factors, Clinical Characteristics, and Outcomes of Spontaneous Pneumothorax in Patients With Coronavirus Disease 2019: A Case-Control, Emergency Medicine-Based Multicenter Study. Chest. 2021;159:1241-1255. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 16. | Sardu C, Gambardella J, Morelli MB, Wang X, Marfella R, Santulli G. Hypertension, Thrombosis, Kidney Failure, and Diabetes: Is COVID-19 an Endothelial Disease? J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 358] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 17. | Quinaglia T, Shabani M, Breder I, Silber HA, Lima JAC, Sposito AC. Coronavirus disease-19: The multi-level, multi-faceted vasculopathy. Atherosclerosis. 2021;322:39-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 18. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5772] [Article Influence: 1154.4] [Reference Citation Analysis (2)] |
| 19. | Iqbal N, Malik A, Chaudhry M. The Macklin Effect in COVID-19. Cureus. 2021;13:e16949. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Carteaux G, Parfait M, Combet M, Haudebourg AF, Tuffet S, Mekontso Dessap A. Patient-Self Inflicted Lung Injury: A Practical Review. J Clin Med. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 21. | Nalewajska M, Feret W, Wojczyński Ł, Witkiewicz W, Wiśniewska M, Kotfis K. Spontaneous Pneumothorax in COVID-19 Patients Treated with High-Flow Nasal Cannula outside the ICU: A Case Series. Int J Environ Res Public Health. 2021;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Adhikary AB, R U, Patel NB, S VP, Boruah P, Chandrakar S. Spectrum of pneumothorax/pneumomediastinum in patients with coronavirus disease 2019. Qatar Med J. 2021;2021:41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Shan S, Guangming L, Wei L, Xuedong Y. Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in COVID-19: case report and literature review. Rev Inst Med Trop Sao Paulo. 2020;62:e76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 24. | Chowdhary A, Nirwan L, Abi-Ghanem AS, Arif U, Lahori S, Kassab MB, Karout S, Itani RM, Abdalla R, Naffaa L, Karout L. Spontaneous Pneumomediastinum in Patients Diagnosed with COVID-19: A Case Series with Review of Literature. Acad Radiol. 2021;28:1586-1598. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Iyer VN, Joshi AY, Ryu JH. Spontaneous pneumomediastinum: analysis of 62 consecutive adult patients. Mayo Clin Proc. 2009;84:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 26. | Elnaem WH, Tammam HM, Zidan MA, Mahmoud MI. The relative efficacy of chest ultrasonography in comparison to other diagnostic modalities in the evaluation of dyspneic patient. Egyptian J Chest Dis Tuber. 2017;66: 165-168. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Reid CL, Chandraratna AN, Kawanishi D, Bezdek WD, Schatz R, Nanna M, Rahimtoola SH. Echocardiographic detection of pneumomediastinum and pneumopericardium: the air gap sign. J Am Coll Cardiol. 1983;1:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Allgood NL, Brownlee JR, Green GA. Inability to view the heart through the subxiphoid echocardiographic window: a harbinger of disaster. Pediatr Cardiol. 1994;15:27-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Palumbo D, Zangrillo A, Belletti A, Guazzarotti G, Calvi MR, Guzzo F, Pennella R, Monti G, Gritti C, Marmiere M, Rocchi M, Colombo S, Valsecchi D, Scandroglio AM, Dagna L, Rovere-Querini P, Tresoldi M, Landoni G, De Cobelli F; COVID-BioB Study Group. A radiological predictor for pneumomediastinum/pneumothorax in COVID-19 ARDS patients. J Crit Care. 2021;66:14-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Cerfolio RJ, Bryant AS. The quantification of postoperative air leaks. Multimed Man Cardiothorac Surg. 2009;2009:mmcts.2007.003129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Lazarus DR, Casal RF. Persistent air leaks: a review with an emphasis on bronchoscopic management. J Thorac Dis. 2017;9:4660-4670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 52] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 32. | Kurman JS. Persistent air leak management in critically ill patients. J Thorac Dis. 2021;13:5223-5231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Singh A, Singh Y, Pangasa N, Khanna P, Trikha A. Risk Factors, Clinical Characteristics, and Outcome of Air Leak Syndrome in COVID-19: A Systematic Review. Indian J Crit Care Med. 2021;25:1434-1445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 34. | Kangas-Dick A, Gazivoda V, Ibrahim M, Sun A, Shaw JP, Brichkov I, Wiesel O. Clinical Characteristics and Outcome of Pneumomediastinum in Patients with COVID-19 Pneumonia. J Laparoendosc Adv Surg Tech A. 2021;31:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 35. | Hallifax R, Evison M, Wrightson JM, Bibby A, Walker S, Stanton A, Bedawi E, Clive A, Latham J, Blyth K, Jackson S, Marshall K, Maskell N, Bhatnagar R, Corcoran J, Belcher E, Rahman N, Munavvar M. Pleural services during the "COVID-19" Pandemic – Revised. V4.0 13 December 2021. Pleural services during COVID-19 pandemic.pdf. Assessed on 2nd February 2022. |
| 36. | Baumann MH, Strange C, Heffner JE, Light R, Kirby TJ, Klein J, Luketich JD, Panacek EA, Sahn SA; AACP Pneumothorax Consensus Group. Management of spontaneous pneumothorax: an American College of Chest Physicians Delphi consensus statement. Chest. 2001;119:590-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 772] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 37. | Havelock T, Teoh R, Laws D, Gleeson F; BTS Pleural Disease Guideline Group. Pleural procedures and thoracic ultrasound: British Thoracic Society Pleural Disease Guideline 2010. Thorax. 2010;65 Suppl 2:ii61-ii76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 473] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 38. | Saha BK, Bonnier A, Chong WH, Chenna P. Successful use of endobronchial valve for persistent air leak in a patient with COVID-19 and bullous emphysema. BMJ Case Rep. 2021;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 39. | Porcel JM. Chest Tube Drainage of the Pleural Space: A Concise Review for Pulmonologists. Tuberc Respir Dis (Seoul). 2018;81:106-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Goligher EC, Jonkman AH, Dianti J, Vaporidi K, Beitler JR, Patel BK, Yoshida T, Jaber S, Dres M, Mauri T, Bellani G, Demoule A, Brochard L, Heunks L. Clinical strategies for implementing lung and diaphragm-protective ventilation: avoiding insufficient and excessive effort. Intensive Care Med. 2020;46:2314-2326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 108] [Article Influence: 21.6] [Reference Citation Analysis (0)] |