Published online May 25, 2022. doi: 10.5501/wjv.v11.i3.113
Peer-review started: December 14, 2021
First decision: February 15, 2022
Revised: March 3, 2022
Accepted: April 28, 2022
Article in press: April 28, 2022
Published online: May 25, 2022
Processing time: 156 Days and 17.1 Hours
Since December 2019, a novel coronavirus that represents a serious threat to human lives has emerged. There is still no definite treatment for severe cases of the disease caused by this virus, named coronavirus disease 2019 (COVID-19). One of the most considered treatment strategies targets the exaggerated immune regulator, and interleukin (IL)-6 is a crucial pro-inflammatory mediator. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cases show an elevated level of IL-6 related to disease severity. IL-6 activity can be inhibited by the following: IL-6 itself, IL-6 signaling pathways such as Janus kinase and signal transducer and activator of transcription (JAK-STAT), gp130, IL-6R, and downstream activated ILs, such as IL-17 and IL-6 cytokine. Currently, according to these studies and their results, IL-6 blockade with anti-IL-6 or its receptor antibodies such as tocilizumab in COVID-19 is beneficial in severe cases and may reduce the mortality rate. JAK-STAT inhibitors block the cytokine storm by inhibiting several crucial pro-inflammatory mediators such as TNF-α and IL-6 and have shown various results in clinical trials. IL-6 induces IL-17 secretion, and IL-17 is involved in the pathogenesis of inflammatory processes. Clinical trials of anti-IL-17 drugs are currently recruiting, and anti-gp130 antibody is preclinical. However, this agent has shown positive effects in inflammatory bowel disease clinical trials and could be tested for SARS-CoV-2. This study aimed to review the role of IL-6 in the cytokine storm and studies regarding IL-6 and blockade of its inflammatory pathways in COVID-19 to determine if any of these agents are beneficial for COVID-19 patients.
Core Tip: One of the most considered treatment strategies for severe acute respiratory syndrome coronavirus 2 is targeting the immune response and pro-inflammatory cytokines such as interleukin (IL)-6. Patients with severe acute respiratory syndrome coronavirus 2 show elevated levels of IL-6, which is related to disease severity. Current studies have shown that IL-6 blockade by anti-IL-6 or its receptor antibodies such as tocilizumab is beneficial in severe cases and may reduce the mortality rate. Moreover, the combination of anti-inflammatory agents is more effective than single therapy.
- Citation: Bahmani M, Chegini R, Ghanbari E, Sheykhsaran E, Shiri Aghbash P, Leylabadlo HE, Moradian E, Kazemzadeh Houjaghan AM, Bannazadeh Baghi H. Severe acute respiratory syndrome coronavirus 2 infection: Role of interleukin-6 and the inflammatory cascade. World J Virol 2022; 11(3): 113-128
- URL: https://www.wjgnet.com/2220-3249/full/v11/i3/113.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i3.113
In December 2019, an epidemic of secretive pneumonia which started in Wuhan city, Hubei province, China, quickly spread to many other countries and finally resulted in a pandemic[1]. The causative agent, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a single-stranded enveloped RNA virus belonging to Nidovirales and the family Coronaviridae. The analysis of SARS-CoV-2 genome structure has shown that this virus is related to the beta-coronavirus genus, containing bat SARS-identical coronavirus and two previous invasive coronaviruses Middle East Respiratory Syndrome Coronavirus (MERS-CoV) and SARS-CoV[2]. Universally, as of September 2021, there have been 226,844,344 recognized cases of SARS-CoV-2, including 4,666,334 victims[3]. The disease caused by the novel coronavirus, coronavirus disease 2019 (COVID-19), is similar to these previous viruses, which is mainly pulmonary disease[4], and all of them have a zoonotic origin. In addition to pulmonary involvement, various organs such as the kidney, gastrointestinal system, nervous system, liver, and coagulation system, may be targets of the virus, leading to serious complications such as acute kidney injury (AKI), acute pulmonary failure, and disseminated intravascular coagulation (DIC) that may lead to death[5]. Currently, this virus is a serious global concern with enormous social and economic damage to societies worldwide[6].
Moreover, the fatality rate is high in severe cases[7]. At present, we do not have any definite treatment for severe cases of this disease, and the management of severe SARS-CoV-2 patients is still challenging. Therefore, various treatment options have been assumed according to the different levels of viral pathogenesis, including viral entry, replication, and effects of the virus on target cells. The anti-viral agent remdesivir is the only treatment with Food and Drug Administration (FDA) approval for this disease, and dexamethasone is the only drug to reduce mortality in hospitalized patients with decreased oxygen saturation but not in others[8]. However, the World Health Organization (WHO) has suggested mortality trials for some repurposed anti-viral drugs, including lopinavir, interferon beta 1a (INF-β1a), and hydroxychloroquine in hospitalized patients with SARS-CoV-2[9].
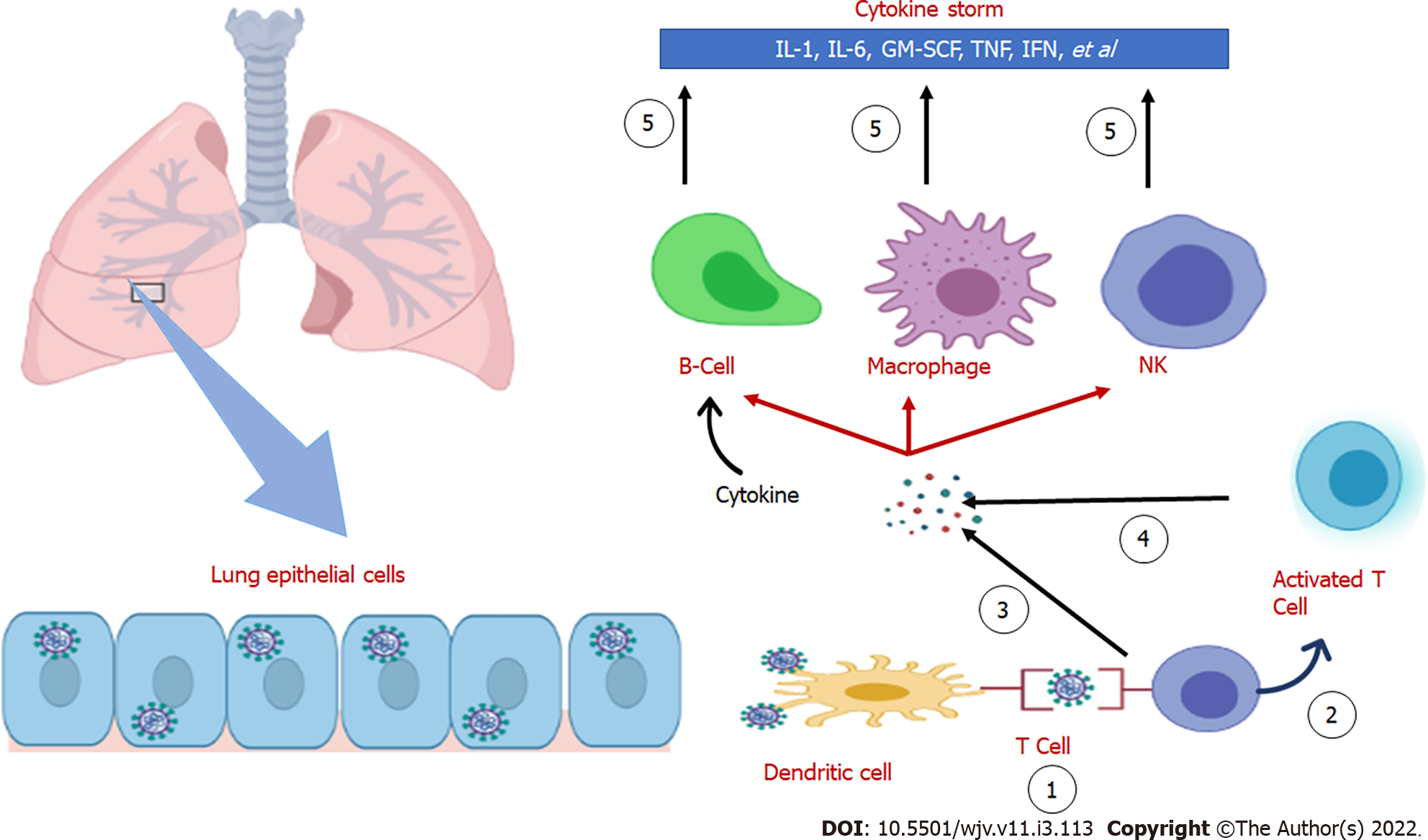
In this regard, IL-6 is known as a crucial inflammatory mediator with essential roles in the pathogenesis of inflammatory diseases in addition to several chronic disorders such as diabetes mellitus[10]. This cytokine is widely expressed by different immune cells and affects immune function[11]. Thus, the disease has a wide range of symptoms. Clinical deterioration in COVID-19 is mainly due to the effects of inflammatory cytokines such as IL-1, IL-6, IFN-α, and tumor necrosis factor (TNF) that are increased in the cytokine storm phase, and the role of immune cells including neutrophils[12-15]. In this process, when a neutrophil encounters a pathogen, the extensive release of cytokines such as IL-1 and IL-6 may become harmful to the body and lead to multi-organ damage[13]. In this rationale, targeting the cytokine release syndrome (CRS) symbolizes a possible therapeutic goal in managing SARS-CoV-2 related cytokine storms and IL-6[16].
In this study, we aim to review the role of IL-6, the rationale of IL-6 blockade in COVID-19, and the results of recent studies on this topic to determine whether any available anti-IL-6 agents or any other drugs with the ability to inhibit inflammatory pathways induced by this cytokine have shown efficacy in improving patient prognosis in SARS-CoV-2 infection.
PubMed, Google Scholar, Scopus, and the Web of Science were searched with the following keywords or their combinations, without any time limits: COVID-19, IL-6, IL-6 receptor, SARS-CoV-2, anti-IL-6, Inflammation. Related articles of any type were selected and reviewed. Extracted information included: SARS-CoV-2 pathophysiology and characteristics, IL-6 activities in the immune system and associated pathways, studies focused on the concept of anti-IL-6 antibodies in the treatment of COVID-19, and other methods of IL-6 inhibition [Janus kinase and signal transducer and activator of transcription (JAK-STAT) inhibition and anti-IL-17 therapies] and are discussed further.
In the last two decades, the third most common coronavirus to cause a pandemic of acute respiratory disease in humans is SARS-CoV-2. These viruses enter the body through respiratory aerosols and are attached to the nasal or paranasal epithelial cells[17]. Angiotensin-converting enzyme 2 (ACE-2) is the major receptor for these viruses to enter host cells, which is expressed in nasal epithelial cells[18,19].
The virus, along with the infection of ciliated cells in the airways, undergoes local replication and dissemination. This stage lasts a few days, and a slight immune response is produced during this process. Despite having a low viral load at this time, infected individuals are highly contagious, and the virus can be identified following a nasal swab[20].
Through its spike (S) protein, the virus enters the host cell by binding to ACE-2 on the cellular surface. Transmembrane serine protease 2 (TMPRSS2), then mediates S protein cleavage, and the virus enters the cell[21]. A high virus infectivity rate is associated with mutations in the binding domain of the receptor and the acquisition of a furin cleavage site in the S protein. The association of the virus with ACE-2 can decrease anti-inflammatory function and increase angiogenic activity[22]. The virus migrates from the nasal epithelium to the upper respiratory tract within the conducting airways[23]. The disease presents various signs and symptoms such as fever and dry cough due to involvement of the upper respiratory tract[24].
At this stage, a higher immune response occurs due to the virus-infected cells and results in the secretion of C-X-C motif chemokine ligand 10 (CXCL-10) and interferons (IFN-β and -λ). As a result of the sufficient immune response to control the spread of infection, the majority of patients do not advance beyond this point[25]. About one-fifth of infected individuals advance to this point and may experience severe symptoms. The virus, via the host receptor ACE-2, targets alveolar epithelial cells type 2 and continues to undergo replication to create more and more viral nucleocapsids[26].
Many distinct cytokines and inflammatory markers are now produced by virus-laden pneumocytes such as ILs (IL-1, IL-6, IL-8, and IL-12), tumor necrosis factor-alpha (TNF-α), IFN-λ and IFN-β, monocyte chemoattractant protein-1 (MCP-1), CXCL-10, and macrophage inflammatory protein-1 alpha (MIP-1α). This 'cytokine storm' serves as a chemoattractant to neutrophils, CD4 helper, and CD8 cytotoxic T cells, and these cells then become sequestered in the pulmonary tissue[27,28]. In addition to being crucial in fighting the virus, these cells cause inflammation and damage to the lungs and other organs. The host cell undergoes apoptosis and releases new viruses, which will then infect the neighboring type 2 alveolar epithelial cells in the same way. Diffuse trauma to the alveoli eventually results in an acute respiratory syndrome and finally respiratory distress, owing to the recurrent injuries triggered by the sequestered immune cells and viral replication, contributing to the annihilation of both type 1 and type 2 pneumocytes[29,30].
COVID-19 spreads mainly by the transmission of respiratory droplets from person to person and occurs when someone is in close contact with an infected individual who is coughing or sneezing violently. This occurs as the host's mucosal surfaces, i.e., the eyes, nose, and mouth, are exposed to the infected respiratory droplets[31]. Virus transmission may also occur by fomites, such as bedsheets, towels, kitchen utensils, thermometers, and stethoscopes, used by or used on the infected person. Airborne transmission of COVID-19 can occur especially in situations where aerosol-generating procedures are conducted, i.e., endotracheal intubation, bronchoscopy, open suction, oxygen nebulization, bronchodilators, or steroids, ventilation using a bag and mask, tracheostomy, and cardiopulmonary resuscitation[32]. In this way, the incubation time for SARS-CoV-2 (between the onset of symptoms and exposure to the virus) is about 5 to 6 d. However, it can be up to 14 d. During this time, also known as the 'pre-symptomatic' phase, the affected individual can be contagious and transmit the virus to the healthy population[33,34]. The most frequent symptoms include fever, muscle aches, shortness of breath, malaise, and a dry cough.
While patients can remain asymptomatic or develop a mild, moderate, or severe illness, gastrointestinal manifestations such as stomach pain, vomiting, and loose stools can also occur. Many of the complications seen in SARS-CoV-2 infected individuals are attributed to the CRS[35,36].
The cytokine storm was historically referred to as an influenza-like syndrome that occurred during systemic diseases such as sepsis and after immunotherapies such as Coley’s toxins. Yersinia pestis (causative agent of plague or black death) infection has led to extreme pandemics; it induces alveolar macrophages to produce disproportionate quantities of cytokines, resulting in the cytokine storm and has subsequently caused massive pandemics[37]. An intensive inflammatory response and fast release of various cytokines (such as TNF-α-1, 2, IL-6, and IFN-γ) to the circulation are activated by pathogen infection (Figure 1). Patients with viral infections are especially vulnerable to acute respiratory failure due to the cytokine storm[38]. For instance, in other coronaviruses (SARS and MERS), cytokine cascades and low lymphocytes are positively linked to the course and severity of the disease. Recent experiments have supported this conclusion in most cases of SARS-CoV-2, indicating low lymphocyte counts and heightened levels of inflammatory mediators[12,39]. Furthermore, it has been shown that pro-inflammatory cytokines such as IL-6 play an essential role in the progression of COVID-19.
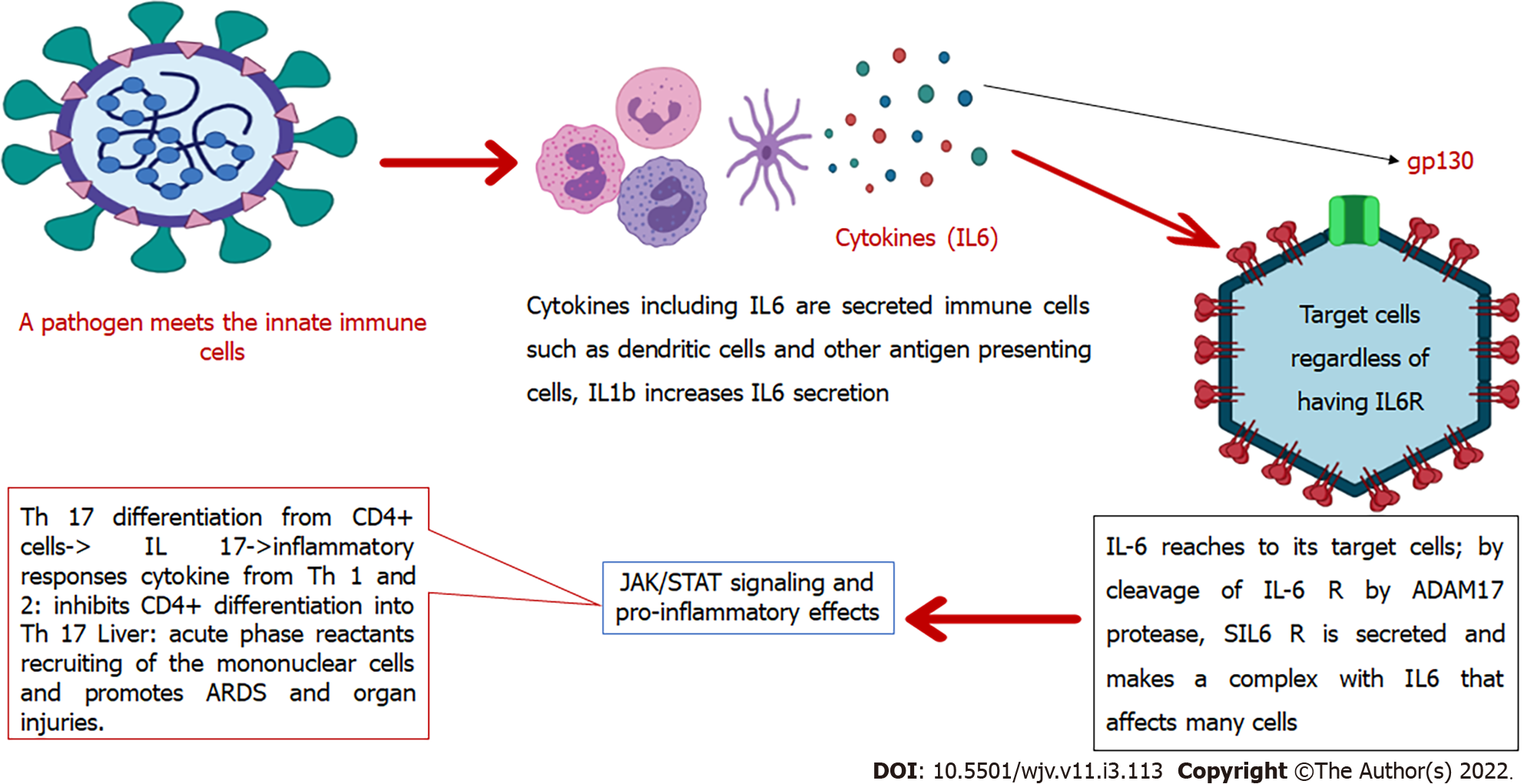
IL-6 is a soluble mediator with various functions in the immune system[40]. For example, controlling the differentiation and migration of immune cells, apoptosis of target cells[41], and assembly of acute-phase proteins such as C-reactive protein (CRP), haptoglobin, and fibrinogen. In contrast, IL-6 reduces the production of other proteins such as albumin. Human IL-6 comprises 212 amino acids (28-amino-acid signal peptide), and its controlling gene is located on chromosome 7p21[40]. This interleukin contributes to hypothalamic-pituitary-adrenal axis regulation and glucose homeostasis. It induces the differentiation of T-helper cells, which secrete IL-17. These cells are related to the pathogenesis of chronic inflammatory diseases[42]. IL-6 is produced in the immune system by various cells including endothelial cells and contributes to the pathogenesis of chronic inflammatory diseases such as rheumatoid arthritis, atherosclerosis, and systemic lupus erythematosus[41]. This cytokine acts by binding to its receptor on the target cells that consist of CD126 (IL-6 Receptor-α) and glycoprotein 130 (gp130). Therefore, it activates signaling pathways such as JAK-STAT[43] and mitogen-activated protein kinase[11]. Conformational alterations in the gp130 cytoplasmic domain when IL-6 binds to the IL-6 receptor induces activation of JAK-STAT[43], and JAK-STAT signaling pathway activation leads to cytokine release[44]. However, these signaling pathways downregulate IL-6 expression[11].
While the membrane-bound receptor (IL-6Rα) is expressed only on the surface of a small number of cells such as leukocytes and hepatocytes (known as IL-6 classic signaling), IL-6 can affect many other cells through its soluble receptor (sIL-6Rα). It was recently discovered that endothelial cells also express IL-6R. This receptor forms a complex with IL-6 that binds to gp130. This complex then mediates a signal known as IL-6 trans-signaling through which pro-inflammatory responses are mainly mediated. In contrast, the classic signaling pathway is related to anti-inflammatory pathways[41]. Furthermore, IL-6 is produced by the innate immune cells after encountering a pathogen and is critical in the body's defense against the respiratory syncytial virus and influenza virus in the early infection phases[45]. However, in CRS, IL-6 and IL-5 can induce coagulation cascade and complement system over-activation, capillary leakage, hypotension, and myocardial dysfunction[46].
In severe SARS-CoV-2 infection, high levels of pro-inflammatory mediators are present, such as IL-6. Although one study showed that monocytes were a source of IL-1β and IL-8, the exact source of IL-6 remains unclear[47]. In the presence of immune dysregulation, in addition to a non-sufficient anti-viral response, there is also a continuous secretion of pro-inflammatory mediators such as IL-6 that resembles the macrophage activation syndrome and lead to multi-organ damage[45]. Also, in COVID-19, multifocal interstitial pneumonia is the chief reason for pulmonary failure and death. In this process, there are inflammatory infiltrates in the interstitial tissue of the lungs, which lead to alveolar damage[48]. These infiltrates consist of mononuclear cells that will be induced after the pro-inflammatory pathways are activated by trans-signal transduction of IL-6[45]. In this way, one study showed that patients with high levels of ACE-2 expression experience more severe tissue damage by IL-6 and the cytokine storm after infection with SARS-CoV-2. These individuals also have a suppressed immune system to fight against the virus[7]. In summary, IL-6 is crucial in both pro-inflammatory and anti-pathogen responses, and trans-signaling is the critical pathway of inflammatory processes conducted by IL-6. A diagram of the significant roles of IL-6 and its location in the immune cascade is summarized in Figure 2.
According to the signaling pathways induced by IL-6 and its components, IL-6 activity can be inhibited by the following: IL-6 itself, IL-6 signaling pathways such as JAK-STAT, gp130, IL-6R, or the IL-6/sIL-6R complex[49]. Two main drugs in the class of IL-6 receptor blockers are tocilizumab (TCZ) and slumab, which are FDA approved monoclonal antibodies for rheumatoid arthritis, and TCZ is also approved for juvenile idiopathic arthritis (JIA) and giant cell arteritis[50].
TCZ blocks both soluble and membrane-bound receptors and accordingly blocks signal transduction via JAK-STAT[51]. JIA, a chimeric antigen receptor (CAR)-T cell-induced CRS, giant cell arteritis, rheumatoid arthritis, and Still’s disease are examples of the conditions in which TCZ has been used to control the disease[52]. Siltuximab is an anti-IL-6 agent that has shown more effectiveness than TCZ in some aspects, and although it is not FDA approved, it is used in refractory CRS cases. Data regarding Siltuximab in COVID-19 are currently restricted[46].
The specific gp130FC named Olamkicept specifically blocks the trans-signaling pathway. In animals, it showed more effectiveness in controlling the hyper-inflammatory status due to sepsis than anti-IL-6 antibodies. Significantly, it did not impair the anti-inflammatory responses of IL-6 via classic signal-transduction[45]. JAK-STAT inhibition is another option. Some of these agents are currently on COVID-19 clinical trials, such as ruxolitinib. A list of these drugs is shown in Table 1.
| Ref. | SARS-CoV-2 clinical trials on Clinicaltrial.gov | Side effects | Examples | Category |
| [93] | NCT04661527, NCT04315298, NCT04357808, NCT04386239, NCT04341870, NCT04359901, NCT04380519 | Cytopenia, intestinal perforation, Hypersensitivity, immunosuppression, and the possibility of infections, impairment of liver enzymes | Sarilumab | The anti-receptor of IL-6 |
| [94,95] | NCT04445272, NCT04331795, NCT04346355, NCT04320615, NCT04356937, NCT04403685, NCT04339712 | Intestinal perforation, Hypersensitivity, immunosuppression, and the possibility of infections, acute liver dysfunction, demyelination, cardiac injury, and hepatitis | Tocilizumab | |
| [96] | NCT04322188, NCT04329650, NCT04330638 | Hypersensitivity disorders, intestinal perforation, risk of infections | Siltuximab | Anti-IL-6 |
| [97] | - | Preclinical; in a phase 2 trial of IBD, it showed effectiveness. Patients in this study who were treated with the drug had hypersensitivity skin reactions and respiratory infections. In animal studies, it did not show serious immunosuppression | Olamkicept | Specific gp130fc |
| [98] | NCT04358614, NCT04401579, NCT04640168, NCT04381936 (RECOVERY Trial), NCT04320277 | Increased risk of infections including reactivation of latent infections, lymphoproliferative disorder, cytopenia, liver enzymes disturbances, clot formation, intestinal perforations | Baricitinib | JAK inhibitors |
| [99] | NCT04348071, NCT04377620, NCT04362137, NCT04366232 | Skin malignancy, exacerbation with drug discontinuation, cytopenia, and immunosuppression, increased risk of infection | Ruxolitinib |
The cytokine storm is associated with disease intensity in SARS-CoV-2, as also shown in SARS-CoV-1 and MERS-CoV. Although the reports from different studies focused on IL-6 blockade in COVID-19 are inconsistent, it was first shown to reduce the mortality rate in critically ill patients[53].
Considering the presence of lymphopenia in SARS-CoV-2 patients, administration of immunosuppressive agents might increase the risk of secondary fungal or bacterial infections[54]. In a previous study, TCZ induced necrotizing fasciitis and candidemia[55]. Accordingly, the exact place for immunosuppression and anti-IL-6 agents in COVID-19 is crucial. The possible effects of TCZ on management of the COVID-19 related cytokine storm first originated from observational studies that showed it to be effective in the clinical improvement of COVID-19 patients[56]. In a recent clinical trial, the effect of a single dose of 8 mg/kg TCZ administration via the intravenous route in addition to the standard of care in the management of COVID-19 was investigated. In this study, 46 adult patients who were positive for SARS-CoV-2 and had multifocal interstitial pneumonia on imaging studies were enrolled soon after showing clinical worsening. The drug was influential in the clinical improvement of severely ill patients and patients in the early clinical worsening state. However, it did not show significant efficacy in reducing the mortality rate and was accompanied by adverse effects[48].
According to a recent observational study, having an IL-6 level of more than 30 pg/mL is related to the disease severity and need for respiratory support in COVID-19 patients. This study showed the positive effects of TCZ in patients with higher IL-6 levels at baseline, but no positive trends were seen in the group with low IL-6 levels[51].
A recent case series showed the efficacy of subcutaneous TCZ in three severely ill COVID-19 patients in reducing inflammatory-related indices and improving the clinical condition[57]. The results of a prospective phase two cohort study (TOCIVID-19) showed that TCZ effectively reduced the mortality rate at 30 d, especially in severe patients who did not require mechanical ventilation. This effect was independent of corticosteroids and was not accompanied by significant adverse events[58].
One of the concerns regarding the use of anti-immune drugs in SARS-CoV-2 is that they may interfere with the proper immune response to the virus. Cytokines, especially IL-6, play a significant role in the host's fight against viruses through the humoral and cellular responses by affecting helper and cytotoxic T cells. Accordingly, a cohort study conducted in Spain found that these drugs do not pose a problem in the body's fight against the virus. Although the study found that patients treated with anti-cytokines had a longer viral clearance time, they initially had higher virus levels, and their disease was more severe[59]. A preprint study that showed an unexpected increase in inflammatory mediators after TCZ administration supports the fact that IL-6 blockade alone may not be effective in the management of COVID-19[60]. Recently, two studies showed a transient elevation in the D-dimer level in SARS-CoV-2 patients receiving TCZ[61,62]. A recent meta-analysis also demonstrated that IL-6 blockade alone does not lower the mortality rate, although it may effectively reduce the risk of respiratory failure in hospitalized patients[63]. According to another study, administration time is another crucial factor, and treatment with TCZ ten days after disease onset is more beneficial[64]. In contrast, other studies, including the RECOVERY trial, have shown that early administration of TCZ in severe cases before intensive care unit (ICU) admission and the need for mechanical ventilation is effective in reducing the mortality rate,[65,66] and when the patient requires mechanical ventilation, it will not have much effect[66,67]. In general, different methods and inclusion criteria in studies do not result in the same conclusions. A list of recent studies in this regard is summarized in Table 2. According to some clinical trials, TCZ, when added to a corticosteroid, markedly reduces the mortality rate compared with corticosteroids (CSs) alone. Treatments that include agents to target more ILs in addition to IL-6 have more efficacy than only IL-6 blockade[63,68]. IFN-γ, granulocyte-macrophage colony-stimulating factor (GM-CSF), TNF, IL-1, and IL-8 are the primary inflammatory mediators that could be targeted in CRS. IL-1 is proximal to IL-6 in the inflammatory cascade, and its blockade has recently been considered. A recent study compared the effectiveness of IL-1 and IL-6 blockade with the standard of care, and it was observed that IL-1 inhibition is more effective in reducing the mortality rate, while positive effects of IL-6 antibodies were restricted to a group of severely ill patients with high CRP levels[50]. Another clinical trial of TCZ in COVID-19 patients with a hyperinflammatory state also stopped recruiting as it failed to reach its primary endpoints (improving the patient's clinical status or reducing the mortality rate)[69]. In general, despite the effect that IL-6 blockade has on the suppression of inflammation, it cannot completely control inflammation as it does not affect the distal inflammatory pathways[70]. However, in severe and critical SARS-CoV-2 patients with a hyperinflammatory state, IL-6 blockade with monoclonal antibodies seems to be effective in reducing the mortality rate, reducing the risk of mechanical ventilation, and improving the clinical condition[67,71-74]. Although all of these studies have been performed in adult patients, the effect of TCZ in the treatment of COVID-19 in children is also being investigated in the RECOVERY trial[67].
| Study design | Inclusion criteria | Interventions | Number of patients | Results | Ref. | ||
| Observational retrospective | Severe SARS-CoV-2 positive ICU admitted patients, with or without respiratory failure | Single 400 mg TCZ dose, without antimicrobialprophylaxis | 55 severe patients were treated,Compared with 41 untreated (non-severe) patients | Lower mortality rate among treated patients against more disease severity, with no serious side effects and no significantly different increased infection rates | [53] | ||
| Quasi-experimental | SARS-CoV-2 positive patients with respiratory failure or a need for supplemental oxygen, with clinical or laboratory signs of acute inflammation | Comparing CSs, and TCZ (8 mg/kg up to 800 mg/dose up to 3 doses) | 33 patients in the TCZ group and 60 in the CS group. | These drugs both reduced the need for supplemental oxygen and ICU stay to the same level, but in the CS group the survival rate was higher, use of TCZ was safe | [100] | ||
| Cohort | COVID-19 patients with respiratory failure and acute inflammatory laboratory findings, such as an elevated CRP level | Anakinra: 5 mg/kg BD until clinical improvement; TCZ: 400 mg single dose, repeated according to the clinical condition; Sarilumab: 400 mg single dose | 62 patients received IL-1 blocker and 55 IL-6 blocker (26 sarilumab and 29 TCZ) (severe patients); 275 without IL blockade (standard of care only) | IL-6 blockade had only limited effectiveness in individuals with high concentrations of CRP, but IL-1 blockade reduced the mortality rate in all patients | [50] | ||
| Retrospective observational | Severe patients | Tocilizumab use was compared with standard of care in ICU patients | 78 severe patients received tocilizumab and were compared with 112 severe patients who received standard of care | Patients on tocilizumab had a longer hospital and ICU stay and more costs with no reduction in the mortality rate | [101] | ||
| Retrospective observational | Severe SARS-CoV-2 patients with respiratory failure | TCZ 8 mg/kg | 30 severe patients with respiratory failure who received TCZ were evaluated for inflammatory markers and clinical condition after treatment | Patients had better oxygenation and inflammatory markers decreased after treatment with TCZ | [102] | ||
| Randomized, double-blindclinical trial | Hospitalized patients without respiratory failure and mechanical ventilation, but with decreased SpO2 in room air | 8 mg/kg up to 800 mg, TCZ; One-two doses | 249 TCZ; 128 SOC | Likelihood ratio of; serious adverse outcomes were significantly lower in the treatment group; But no reduction in all-cause mortality rate | [8] | ||
| Clinical trial | Moderate and severe patients according to the clinical status, with higher IL-6 levels, neither ICU admitted nor on mechanical ventilation | TCZ 400 mg; Single-dose | 29 patients were treated with TCZ and 32 received standard of care only | TCZ was safe but did not show any significant difference in clinical improvement | [103] | ||
| Cross-sectional, observational | Severe patients with high levels of inflammatory markers | TCZ 4 mg/kg | 54 patients were treated with TCZ | Significant reduction in neutrophil count and CRP | [104] | ||
| Clinical trial | Patients with hyper-inflammatory state and acute respiratory failure | TCZ 8 mg/kg (up to 800 mg); After 12 h: second dosage | 66 severe patients received TCZ and were compared with 60 patients who received standard of care | Not effective in decreasing the risk of disease deterioration | [105] | ||
| Open-label clinical trial | Proven SARS-CoV-2 infection, with the need for respiratory support and recent worsening in the clinical condition | TCZ 8 mg/kg | 46 moderate and severe patients were treated with TCZ | Treatment improved respiratory function | [48] | ||
| Clinical trial | High levels of IL-6Moderate and severe disease severity | TCZ 400 mg (second dosage after 24h) | 34 patients were treated and 31 were not | Treatment with TCZ improved respiratory condition without reducing the mortality rate | [106] | ||
| Clinical trial | Severe and critical patients | Sarilumab 400 mg | Total = 416 (Sarilumab 400 mg, n = 173; Placebo, n = 84; Sarilumab 200 mg, n = 159); Primary analysis between 194 severely ill patients who needed respiratory support | Did not meet the primary and secondary endpoints in improving disease progression and the study stopped further recruitment | NCT04315298 [107,108] | ||
| Randomized, double-blindclinical trial | Severe patients with decreased SpO2 without supplemental oxygen | TCZ 8 mg/kg up to 800 mg | 2:1 Placebo+ Standard of care (151); TCZ+ SOC (301) | No significant benefits on mortality rate or clinical improvement, but a positive effect on hospitalization duration was observed with no significant side effects compared with the control group | NCT04320615 [109] | ||
| Retrospective cohort | SARS-CoV-2 positive patients with severe pneumonia | TCZ one to two doses, 400–800 mg every 12 h | n = 62 treated, n = 86 untreated | Treated patients showed significantly lower leukocytosis compared to the control group after 14 d. D-dimer and ferritin initially increased and then decreased in the treated group. The mortality rate at 28 d was statistically lower in the TCZ group. A longer hospital stay was shown in these patients although this was not statistically significant. Ten patients developed an infection during hospitalization | [62] | ||
| Retrospective cohort | Moderate to severe SARS-CoV-2 patients | One to two doses of TCZ 8 mg/kg | 170 treated; 655 untreated | Clinical improvement was significantly better in the treatment group compared with the control group. A significant reduction in the mortality rate at 21 and 28 d was found in patients with respiratory failure and patients with IL-6 levels above 100 pg/mL | [110] | ||
| Randomized clinical trial | Critical patients with respiratory failure who were admitted to the ICU | TCZ one to two doses (8 mg/kg); Sarilumab (a single dose of 400 mg); Other interventions: Anakinra and interferon beta-1a | 350 on TCZ; 45 on sarilumab; 1136 on another immunomodulator; 397 on no immunomodulation | IL-6 blocking agents were effective in reducing the mortality rate. When added to corticosteroids, this effect was stronger compared with IL-6 blockade alone | NCT02735707 [74] | ||
| Randomized, controlled, open-label clinical trial | COVID-19 patients with worsening clinical status or with high CRP levels after 21 d of the first randomization to dexamethasone, lopinavir–ritonavir, hydroxychloroquine, azithromycin, or colchicine or convalescent plasma or a combination of two anti-SARS-CoV-2 spike protein antibodies (REGN-COV2) or aspirin | A single dose of TCZ according to the patient’s weight | 2022 received TCZ; 2094 received standard of care | TCZ group had a significantly lower mortality rate, need for mechanical ventilation, and higher chance of hospital discharge at day 28. This effect was similar in patients randomized less than or more than two days from hospitalization. In patients who were on mechanical ventilation at the time of drug administration, this drug had no significant effect on improving prognosis | [67] | ||
| Randomized, double-blindclinical trial | Severe COVID-19 patients | Sarilumab 200 or 400 mg, single dose | n = 153 sarilumab 400 mg, n = 141 sarilumab 200 mg, n = 75 placebo | No significant effectiveness was found in the treatment groups compared with the control group | [111] | ||
| Randomized, double-Blind, placebo-controlled trial | Patients with COVID-19 in a hyper-inflammatory state | TCZ 8 mg/kg up to 800 mg | TCZ (n = 161); Placebo (n = 81) + standard of care | No significant benefits from early TCZ administration in COVID-19 were observed | [112] | ||
To date, several clinical trials have failed to show the efficiency of TCZ in COVID-19 treatment. However, the RECOVERY trial and some other clinical trials showed positive results[67,75,76]. Although meta-analysis had previously demonstrated an 11% reduction in 28-d mortality following TCZ administration in patients with severe SARS-CoV-2 infection, this reduction was significant when the results of the RECOVERY trial were added[67]. In conclusion, this drug can effectively improve the prognosis in extreme cases.
TCZ inhibits both classic and trans-signal transduction through IL-6, thus interfering with this cytokine's anti- and pro-inflammatory functions. As mentioned previously, IL-6 signaling pathways involve the JAK-STAT that could be targeted with drugs such as ruxolitinib, a JAK 1 and 2 inhibitor. This drug lowers the levels of IL-6 and is currently being evaluated for SARS-CoV-2 and had positive effects in one study[77]. However, RUXCOVID, a phase 3 clinical trial of ruxolitinib, revealed no significant efficacy in reducing the death rate and serious complications[78]. Another JAK inhibitor is baricitinib. A recent clinical trial (ACTT2) that evaluated baricitinib in hospitalized patients with SARS-CoV-2 infection indicated that it reduced the recovery time when added to remdesivir, compared with remdesivir alone[79,80]. Another study also investigated the potency of the anti-myeloproliferative agent ruxolitinib and included the patients requiring supplementary oxygen but not with respiratory failure. This study found that inflammatory mediators significantly reduced after ruxolitinib administration which also improved clinical conditions. These successes were not accompanied by any severe effects[81]. Another effect of JAK inhibitors in hampering the cytokine storm is related to TNF, the other crucial inflammatory mediator in the cytokine storm that uses JAK signaling and can be inhibited by JAK inhibitors. A recent study evaluated the concurrent administration of an IL-1 blocker antibody and ruxolitinib in critical patients with SARS-CoV-2. The preliminary report of this study demonstrated that this combination was beneficial in clinical improvement, and the lymphocyte count increased after this treatment[82]. In addition, no treatment-related severe complications were observed. Tofacitinib is another JAK inhibitor that was shown to reduce adverse outcomes and mortality in COVID-19 patients in a previous retrospective cohort study[83]. Another exciting intervention for IL blockade with positive effects in patients on ECMO in previous research was extracorporeal cytokine adsorption which showed a significant decrease in IL-6 in treated patients[84,85]. Other agents with anti-IL-6 properties have not yet been entered in clinical trials of COVID-19. However, targeting the trans-signaling pathway seems more efficient than non-specific IL-6 blockade with monoclonal antibodies.
Th17 is related to inflammatory processes. As mentioned in Figure 2, when the IL-6-sIL-6R complex reaches CD4+ T cells, it causes them to differentiate into Th17 cell lineage. This action is mediated through the JAK-STAT signaling pathway (IL-6 recruits JAK 1 and 2). These cells can secrete IL-17, 21, and 22 and GM-CSF, and therefore contribute to the pathogenesis of inflammatory processes and chronic diseases. Viral diseases also promote Th17 related responses, and severe cases show higher Th17-related cytokines. Accordingly, Th17 blockade seems to be another way to fight against COVID-19, especially in extreme cases. One study showed that fedratinib reduced Th17 related cytokines in mouse models. Fedratinib is a JAK 2 inhibitor[86].
It was shown that the Th17 subgroup of T cells is increased relative to the other subgroups in severe COVID-19 cases. The role of these cells in SARS-CoV-2 patients with lung injuries has been revealed. Drugs with anti-IL-17 activities include ixekizumab, secukinumab, and brodalumab, and they are used in moderate to severe cases of psoriasis[87,88]. Ixekizumab is an anti-IL-17 antibody and is currently being evaluated in a COVID-19 clinical trial. Inclusion criteria in this study are those with high serum levels of IL-6 and not admitted to the ICU[89]. When IL-17 is secreted from Th17 cells, it causes target cells to produce inflammatory mediators, including IL-6, TNF-α, chemokine C-C motif 2 (CCL2), and IL-1β. These procedures lead to CRS and clinical worsening in SARS-CoV-2[87]. IL-17 is also related to the cutaneous manifestations of COVID-19[90]. However, recent evidence has shown undetectable quantities of IL-17A expression in COVID-19 patients[91]. In a previous study, secukinumab, an anti-IL-17A selective antibody, resulted in clinical improvement in severe SARS-CoV-2 patients[92].
According to the above-mentioned data, IL-6 blockade alone with anti-IL-6R monoclonal antibodies has no significant benefits in improving the prognosis of patients, except for those in a critical condition and in the hyper-inflammatory state before mechanical ventilation. Many factors are related to a patient's response to IL-6 blockade, such as baseline IL-6 level and disease severity. It may also be associated with some worrying side effects. According to recent data, a combination of anti-inflammatory agents is more effective than any one agent alone. Other ways to inhibit IL-6, such as a selective trans-signaling pathway and JAK-STAT inhibition, should be investigated further.
The authors would like to thank the Clinical Research Development Unit and Tabriz University of Medical Sciences Faculty of Medicine for providing the expertise that greatly assisted in this work.
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Virology
Country/Territory of origin: Iran
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Singh AK, India; Wang H S-Editor: Ma YJ L-Editor: Webster JR P-Editor: Ma YJ
| 1. | Wu YC, Chen CS, Chan YJ. The outbreak of COVID-19: An overview. J Chin Med Assoc. 2020;83:217-220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 878] [Cited by in RCA: 719] [Article Influence: 143.8] [Reference Citation Analysis (0)] |
| 2. | Shahrajabian MH, Sun W, Cheng Q. Product of natural evolution (SARS, MERS, and SARS-CoV-2); deadly diseases, from SARS to SARS-CoV-2. Hum Vaccin Immunother. 2021;17:62-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | World Health Organization. WHO Coronavirus (COVID-19) Dashboard. [cited September 19, 2021] Available from: https://covid19.who.int/. |
| 4. | Castelnovo L, Tamburello A, Lurati A, Zaccara E, Marrazza MG, Olivetti M, Mumoli N, Mastroiacovo D, Colombo D, Ricchiuti E, Vigano' P, Paola F, Mazzone A. Anti-IL6 treatment of serious COVID-19 disease: A monocentric retrospective experience. Medicine (Baltimore). 2021;100:e23582. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Sarkesh A, Daei Sorkhabi A, Sheykhsaran E, Alinezhad F, Mohammadzadeh N, Hemmat N, Bannazadeh Baghi H. Extrapulmonary Clinical Manifestations in COVID-19 Patients. Am J Trop Med Hyg. 2020;103:1783-1796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | IHME COVID-19 Forecasting Team. Modeling COVID-19 scenarios for the United States. Nat Med. 2021;27:94-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 297] [Cited by in RCA: 256] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 7. | Bao Z, Wang LJ, He K, Lin X, Yu T, Li J, Gong J, Xiang G. High expression of ACE2 in the human lung leads to the release of IL6 by suppressing cellular immunity: IL6 plays a key role in COVID-19. Eur Rev Med Pharmacol Sci. 2021;25:527-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 8. | Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, Criner GJ, Kaplan-Lewis E, Baden R, Pandit L, Cameron ML, Garcia-Diaz J, Chávez V, Mekebeb-Reuter M, Lima de Menezes F, Shah R, González-Lara MF, Assman B, Freedman J, Mohan SV. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384:20-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 912] [Cited by in RCA: 922] [Article Influence: 230.5] [Reference Citation Analysis (0)] |
| 9. | WHO Solidarity Trial Consortium; Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, Alejandria MM, Hernández García C, Kieny MP, Malekzadeh R, Murthy S, Reddy KS, Roses Periago M, Abi Hanna P, Ader F, Al-Bader AM, Alhasawi A, Allum E, Alotaibi A, Alvarez-Moreno CA, Appadoo S, Asiri A, Aukrust P, Barratt-Due A, Bellani S, Branca M, Cappel-Porter HBC, Cerrato N, Chow TS, Como N, Eustace J, García PJ, Godbole S, Gotuzzo E, Griskevicius L, Hamra R, Hassan M, Hassany M, Hutton D, Irmansyah I, Jancoriene L, Kirwan J, Kumar S, Lennon P, Lopardo G, Lydon P, Magrini N, Maguire T, Manevska S, Manuel O, McGinty S, Medina MT, Mesa Rubio ML, Miranda-Montoya MC, Nel J, Nunes EP, Perola M, Portolés A, Rasmin MR, Raza A, Rees H, Reges PPS, Rogers CA, Salami K, Salvadori MI, Sinani N, Sterne JAC, Stevanovikj M, Tacconelli E, Tikkinen KAO, Trelle S, Zaid H, Røttingen JA, Swaminathan S. Repurposed Antiviral Drugs for Covid-19 - Interim WHO Solidarity Trial Results. N Engl J Med. 2021;384:497-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1601] [Cited by in RCA: 1744] [Article Influence: 436.0] [Reference Citation Analysis (0)] |
| 10. | Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1352] [Cited by in RCA: 1738] [Article Influence: 173.8] [Reference Citation Analysis (0)] |
| 11. | Jordan SC, Choi J, Kim I, Wu G, Toyoda M, Shin B, Vo A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation. 2017;101:32-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 202] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 12. | Tang Y, Liu J, Zhang D, Xu Z, Ji J, Wen C. Cytokine Storm in COVID-19: The Current Evidence and Treatment Strategies. Front Immunol. 2020;11:1708. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 768] [Cited by in RCA: 724] [Article Influence: 144.8] [Reference Citation Analysis (0)] |
| 13. | Hemmat N, Derakhshani A, Bannazadeh Baghi H, Silvestris N, Baradaran B, De Summa S. Neutrophils, Crucial, or Harmful Immune Cells Involved in Coronavirus Infection: A Bioinformatics Study. Front Genet. 2020;11:641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 14. | Hemmat N, Asadzadeh Z, Karim-ahangar N, Alemohammad H, Najafzadeh B, Derakhshani A, Baghbanzadeh A, Bannazadeh Baghi H, Javadrashid D, Najafi S, Gouilh MA, Baradaran B. The alterations of cellular signaling pathways in the host cell upon the high pathogenic Coronaviruses infection, SARS-CoV and MERS-CoV. What could be expected from the SARS-CoV-2? 2020. Available from: https://www.researchgate.net/publication/344729169_The_alterations_of_cellular_signaling_pathways_in_the_host_cell_upon_the_high_pathogenic_Coronaviruses_infection_SARS-CoV_and_MERS-CoV_What_could_be_expected_from_the_SARS-CoV-2. |
| 15. | Shiri Aghbash P, Eslami N, Shamekh A, Entezari-Maleki T, Bannazadeh Baghi H. SARS-CoV-2 infection: The role of PD-1/PD-L1 and CTLA-4 axis. Life Sci. 2021;270:119124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 16. | Liu B, Li M, Zhou Z, Guan X, Xiang Y. Can we use interleukin-6 (IL-6) blockade for coronavirus disease 2019 (COVID-19)-induced cytokine release syndrome (CRS)? J Autoimmun. 2020;111:102452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 554] [Cited by in RCA: 531] [Article Influence: 106.2] [Reference Citation Analysis (0)] |
| 17. | Wiersinga WJ, Rhodes A, Cheng AC, Peacock SJ, Prescott HC. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2691] [Cited by in RCA: 3169] [Article Influence: 633.8] [Reference Citation Analysis (0)] |
| 18. | Oroojalian F, Haghbin A, Baradaran B, Hemmat N, Shahbazi MA, Bannazadeh Baghi H, Mokhtarzadeh A, Hamblin MR. Novel insights into the treatment of SARS-CoV-2 infection: An overview of current clinical trials. Int J Biol Macromol. 2020;165:18-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271-280.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11946] [Cited by in RCA: 14238] [Article Influence: 2847.6] [Reference Citation Analysis (0)] |
| 20. | Azer SA. COVID-19: pathophysiology, diagnosis, complications and investigational therapeutics. New Microbes New Infect. 2020;37:100738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 70] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 21. | Huang Y, Yang C, Xu XF, Xu W, Liu SW. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141-1149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1433] [Cited by in RCA: 1454] [Article Influence: 290.8] [Reference Citation Analysis (1)] |
| 22. | Li W, Moore MJ, Vasilieva N, Sui J, Wong SK, Berne MA, Somasundaran M, Sullivan JL, Luzuriaga K, Greenough TC, Choe H, Farzan M. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature. 2003;426:450-454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4113] [Cited by in RCA: 4597] [Article Influence: 209.0] [Reference Citation Analysis (0)] |
| 23. | Parasher A. COVID-19: Current understanding of its Pathophysiology, Clinical presentation and Treatment. Postgrad Med J. 2021;97:312-320. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 362] [Cited by in RCA: 402] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 24. | Hassan SA, Sheikh FN, Jamal S, Ezeh JK, Akhtar A. Coronavirus (COVID-19): A Review of Clinical Features, Diagnosis, and Treatment. Cureus. 2020;12:e7355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 25. | Ahmad T, Chaudhuri R, Joshi MC, Almatroudi A, Rahmani AH, Ali SM. COVID-19: The Emerging Immunopathological Determinants for Recovery or Death. Front Microbiol. 2020;11:588409. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Wu J, Deng W, Li S, Yang X. Advances in research on ACE2 as a receptor for 2019-nCoV. Cell Mol Life Sci. 2021;78:531-544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 74] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 27. | Tufan A, Avanoğlu Güler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. 2020;50:620-632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 313] [Cited by in RCA: 289] [Article Influence: 57.8] [Reference Citation Analysis (0)] |
| 28. | Shiri Aghbash P, Hemmat N, Nahand JS, Shamekh A, Memar MY, Babaei A, Bannazadeh Baghi H. The role of Th17 cells in viral infections. Int Immunopharmacol. 2021;91:107331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 29. | Zhang Y, Geng X, Tan Y, Li Q, Xu C, Xu J, Hao L, Zeng Z, Luo X, Liu F, Wang H. New understanding of the damage of SARS-CoV-2 infection outside the respiratory system. Biomed Pharmacother. 2020;127:110195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 191] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 30. | Labbé K, Saleh M. Cell death in the host response to infection. Cell Death Differ. 2008;15:1339-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 292] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 31. | Dhand R, Li J. Coughs and Sneezes: Their Role in Transmission of Respiratory Viral Infections, Including SARS-CoV-2. Am J Respir Crit Care Med. 2020;202:651-659. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 210] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 32. | Noorimotlagh Z, Jaafarzadeh N, Martínez SS, Mirzaee SA. A systematic review of possible airborne transmission of the COVID-19 virus (SARS-CoV-2) in the indoor air environment. Environ Res. 2021;193:110612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 123] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 33. | Hu B, Guo H, Zhou P, Shi ZL. Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol. 2021;19:141-154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2083] [Cited by in RCA: 3149] [Article Influence: 787.3] [Reference Citation Analysis (0)] |
| 34. | Yuki K, Fujiogi M, Koutsogiannaki S. COVID-19 pathophysiology: A review. Clin Immunol. 2020;215:108427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1218] [Cited by in RCA: 1187] [Article Influence: 237.4] [Reference Citation Analysis (0)] |
| 35. | Sharma R, Agarwal M, Gupta M, Somendra S, Saxena SK. Clinical Characteristics and Differential Clinical Diagnosis of Novel Coronavirus Disease 2019 (COVID-19). In: Saxena S (eds). Coronavirus Disease 2019 (COVID-19). Medical Virology: From Pathogenesis to Disease Control. Springer, Singapore. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 36. | Bohn MK, Hall A, Sepiashvili L, Jung B, Steele S, Adeli K. Pathophysiology of COVID-19: Mechanisms Underlying Disease Severity and Progression. Physiology (Bethesda). 2020;35:288-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 151] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 37. | Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. 2020;383:2255-2273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1324] [Cited by in RCA: 2085] [Article Influence: 417.0] [Reference Citation Analysis (0)] |
| 38. | Younan P, Iampietro M, Nishida A, Ramanathan P, Santos RI, Dutta M, Lubaki NM, Koup RA, Katze MG, Bukreyev A. Ebola Virus Binding to Tim-1 on T Lymphocytes Induces a Cytokine Storm. mBio. 2017;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14752] [Article Influence: 2950.4] [Reference Citation Analysis (0)] |
| 40. | Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6:a016295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1881] [Cited by in RCA: 3169] [Article Influence: 288.1] [Reference Citation Analysis (0)] |
| 41. | Ljungberg LU, Zegeye MM, Kardeby C, Fälker K, Repsilber D, Sirsjö A. Global Transcriptional Profiling Reveals Novel Autocrine Functions of Interleukin 6 in Human Vascular Endothelial Cells. Mediators Inflamm. 2020;2020:4623107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (1)] |
| 42. | Jones SA, Scheller J, Rose-John S. Therapeutic strategies for the clinical blockade of IL-6/gp130 signaling. J Clin Invest. 2011;121:3375-3383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 553] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 43. | Moshapa FT, Riches-Suman K, Palmer TM. Therapeutic Targeting of the Proinflammatory IL-6-JAK-STAT Signalling Pathways Responsible for Vascular Restenosis in Type 2 Diabetes Mellitus. Cardiol Res Pract. 2019;2019:9846312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 54] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 44. | Saha A, Sharma AR, Bhattacharya M, Sharma G, Lee SS, Chakraborty C. Tocilizumab: A Therapeutic Option for the Treatment of Cytokine Storm Syndrome in COVID-19. Arch Med Res. 2020;51:595-597. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 76] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 45. | Magro G. SARS-CoV-2 and COVID-19: Is interleukin-6 (IL-6) the 'culprit lesion' of ARDS onset? Cytokine X. 2020;2:100029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 132] [Article Influence: 26.4] [Reference Citation Analysis (0)] |
| 46. | Murthy H, Iqbal M, Chavez JC, Kharfan-Dabaja MA. Cytokine Release Syndrome: Current Perspectives. Immunotargets Ther. 2019;8:43-52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 133] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 47. | Kahn R, Schmidt T, Golestani K, Mossberg A, Gullstrand B, Bengtsson AA, Kahn F. Mismatch between circulating cytokines and spontaneous cytokine production by leukocytes in hyperinflammatory COVID-19. J Leukoc Biol. 2021;109:115-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Pomponio G, Ferrarini A, Bonifazi M, Moretti M, Salvi A, Giacometti A, Tavio M, Titolo G, Morbidoni L, Frausini G, Onesta M, Amico D, Rocchi MLB, Menzo S, Zuccatosta L, Mei F, Menditto V, Svegliati S, Donati A, D'Errico MM, Pavani M, Gabrielli A. Tocilizumab in COVID-19 interstitial pneumonia. J Intern Med. 2021;289:738-746. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 49. | Heo TH, Wahler J, Suh N. Potential therapeutic implications of IL-6/IL-6R/gp130-targeting agents in breast cancer. Oncotarget. 2016;7:15460-15473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 93] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 50. | Cavalli G, Larcher A, Tomelleri A, Campochiaro C, Della-Torre E, De Luca G, Farina N, Boffini N, Ruggeri A, Poli A, Scarpellini P, Rovere-Querini P, Tresoldi M, Salonia A, Montorsi F, Landoni G, Castagna A, Ciceri F, Zangrillo A, Dagna L. Interleukin-1 and interleukin-6 inhibition compared with standard management in patients with COVID-19 and hyperinflammation: a cohort study. Lancet Rheumatol. 2021;3:e253-e261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 126] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 51. | Galván-Román JM, Rodríguez-García SC, Roy-Vallejo E, Marcos-Jiménez A, Sánchez-Alonso S, Fernández-Díaz C, Alcaraz-Serna A, Mateu-Albero T, Rodríguez-Cortes P, Sánchez-Cerrillo I, Esparcia L, Martínez-Fleta P, López-Sanz C, Gabrie L, Del Campo Guerola L, Suárez-Fernández C, Ancochea J, Canabal A, Albert P, Rodríguez-Serrano DA, Aguilar JM, Del Arco C, de Los Santos I, García-Fraile L, de la Cámara R, Serra JM, Ramírez E, Alonso T, Landete P, Soriano JB, Martín-Gayo E, Fraile Torres A, Zurita Cruz ND, García-Vicuña R, Cardeñoso L, Sánchez-Madrid F, Alfranca A, Muñoz-Calleja C, González-Álvaro I; REINMUN-COVID Group. IL-6 serum levels predict severity and response to tocilizumab in COVID-19: An observational study. J Allergy Clin Immunol. 2021;147:72-80.e8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 150] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 52. | Soto GP. Potential therapeutic agents against COVID-19 based on blocking and inhibition of the viral life cycle and the cytokine storm syndrome. An Fac Cienc Méd (Asunción). 2020;53:131-146. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 53. | Huang E, Isonaka S, Yang H, Salce E, Rosales E, Jordan SC. Tocilizumab treatment in critically ill patients with COVID-19: A retrospective observational study. Int J Infect Dis. 2021;105:245-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Deana C, Vetrugno L, Bassi F, De Monte A. Tocilizumab administration in COVID-19 patients: Water on the fire or gasoline? Med Mycol Case Rep. 2021;31:32-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 55. | Setliff E, Kosmisky D, Ngeve R. 683: Necrotizing Fasciitis and Candidemia After Tocilizumab Initiation: A Case Report. Crit Care Med. 2021;49:336. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 56. | Sciascia S, Aprà F, Baffa A, Baldovino S, Boaro D, Boero R, Bonora S, Calcagno A, Cecchi I, Cinnirella G, Converso M, Cozzi M, Crosasso P, De Iaco F, Di Perri G, Eandi M, Fenoglio R, Giusti M, Imperiale D, Imperiale G, Livigni S, Manno E, Massara C, Milone V, Natale G, Navarra M, Oddone V, Osella S, Piccioni P, Radin M, Roccatello D, Rossi D. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in severe patients with COVID-19. Clin Exp Rheumatol. 2020;38:529-532. |
| 57. | Mazzitelli M, Arrighi E, Serapide F, Pelle MC, Tassone B, Lionello R, Marrazzo G, Laganà D, Costanzo FS, Matera G, Trecarichi EM, Torti C. Use of subcutaneous tocilizumab in patients with COVID-19 pneumonia. J Med Virol. 2021;93:32-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 58. | Perrone F, Piccirillo MC, Ascierto PA, Salvarani C, Parrella R, Marata AM, Popoli P, Ferraris L, Marrocco-Trischitta MM, Ripamonti D, Binda F, Bonfanti P, Squillace N, Castelli F, Muiesan ML, Lichtner M, Calzetti C, Salerno ND, Atripaldi L, Cascella M, Costantini M, Dolci G, Facciolongo NC, Fraganza F, Massari M, Montesarchio V, Mussini C, Negri EA, Botti G, Cardone C, Gargiulo P, Gravina A, Schettino C, Arenare L, Chiodini P, Gallo C; TOCIVID-19 investigators, Italy. Tocilizumab for patients with COVID-19 pneumonia. The single-arm TOCIVID-19 prospective trial. J Transl Med. 2020;18:405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 59. | Masiá M, Fernández-González M, Padilla S, Ortega P, García JA, Agulló V, García-Abellán J, Telenti G, Guillén L, Gutiérrez F. Impact of interleukin-6 blockade with tocilizumab on SARS-CoV-2 viral kinetics and antibody responses in patients with COVID-19: A prospective cohort study. EBioMedicine. 2020;60:102999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 60. | Ponthieux F, Dauby N, Maillart E, Fils JF, Smet J, Claus M, Besse-Hammer T, Bels D, Corazza F, Nagant C. Tocilizumab-Induced Unexpected Increase of Several Inflammatory Cytokines in Critically Ill COVID-19 Patients: The Anti-Inflammatory Side of IL-6. Viral Immunol. 2022;35:60-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 61. | Chan KH, Patel B, Podel B, Szablea ME, Shaaban HS, Guron G, Slim J. Tocilizumab and Thromboembolism in COVID-19: A Retrospective Hospital-Based Cohort Analysis. Cureus. 2021;13:e15208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 62. | Al-Baadani A, Eltayeb N, Alsufyani E, Albahrani S, Basheri S, Albayat H, Batubara E, Ballool S, Al Assiri A, Faqihi F, Musa AB, Robert AA, Alsherbeeni N, Elzein F. Efficacy of tocilizumab in patients with severe COVID-19: Survival and clinical outcomes. J Infect Public Health. 2021;14:1021-1027. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Kow CS, Hasan SS. The effect of tocilizumab on mortality in hospitalized patients with COVID-19: a meta-analysis of randomized controlled trials. Eur J Clin Pharmacol. 2021;77:1089-1094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 64. | Moreno Diaz R, Amor García MA, Teigell Muñoz FJ, Saldaña Perez LE, Mateos Gonzalez M, Melero Bermejo JA, López Hernández A, Reyes Marquez L, De Guzman García-Monge MT, Perez Quero JL, Homez Guzman MP. Does timing matter on tocilizumab administration? Eur J Hosp Pharm. 2021;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 65. | Eşkazan AE, Balkan İİ, Demirbaş KC, Ar MC, Karaali R, Sekibağ Y, Mulamahmutoğlu S, Yartaş Dumanlı G, Çakmak F, Özgür Yurttaş N, Kurt F, Aladağ Kurt S, Kuşkucu M, Ürkmez S, Börekçi Ş, Saribal D, Mete B, Bavunoğlu I, Dikmen Y, Aygün G, Midilli K, Tabak F. Tocilizumab in COVID-19: The Cerrahpaşa-PREDICT score. J Infect Chemother. 2021;27:1329-1335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 66. | Li P, Lu Z, Li Q, Wang Z, Guo Y, Cai C, Wang S, Liu P, Su X, Huang Y, Dong Y, Qiu W, Ling Y, Yarmus L, Luo F, Zeng L, Bai C, Zhang W. Administration Timing and Efficacy of Tocilizumab in Patients With COVID-19 and Elevated IL-6. Front Mol Biosci. 2021;8:651662. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 67. | RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 946] [Cited by in RCA: 948] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 68. | Van den Eynde E, Gasch O, Oliva JC, Prieto E, Calzado S, Gomila A, Machado ML, Falgueras L, Ortonobes S, Morón A, Capilla S, Navarro G, Oristrell J, Cervantes M, Navarro M. Corticosteroids and tocilizumab reduce in-hospital mortality in severe COVID-19 pneumonia: a retrospective study in a Spanish hospital. Infect Dis (Lond). 2021;53:291-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 69. | Efficacy of Early Administration of Tocilizumab in COVID-19 Patients - American College of Cardiology. [cited March 28, 2021] Available from: https://www.acc.org/Latest-in-cardiology/clinical-trials/2020/12/31/20/42/rct-tcz-covid-19. |
| 70. | Akinosoglou K, Velissaris D, Ziazias D, Davoulos C, Tousis A, Tsiotsios K, Kalogeropoulou C, Spyridonidis A, Marangos M, Fligkou F, Gogos C. Remdesivir and tocilizumab: Mix or match. J Med Virol. 2021;93:56-58. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 71. | Antony SJ, Davis MA, Davis MG, Almaghlouth NK, Guevara R, Omar F, Del Rey F, Hassan A, Arian MU, Antony N, Prakash BV. Early use of tocilizumab in the prevention of adult respiratory failure in SARS-CoV-2 infections and the utilization of interleukin-6 levels in the management. J Med Virol. 2021;93:491-498. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 72. | Bhandari S, Rankawat G, Singh A. Tocilizumab: An Effective Therapy for Severely and Critically Ill COVID-19 Patients. Indian J Crit Care Med. 2021;25:260-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Chilimuri S, Sun H, Alemam A, Kang KS, Lao P, Mantri N, Schiller L, Sharabun M, Shehi E, Tejada J, Yugay A, Nayudu SK. Tocilizumab use in patients with moderate to severe COVID-19: A retrospective cohort study. J Clin Pharm Ther. 2021;46:440-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 3.0] [Reference Citation Analysis (2)] |
| 74. | REMAP-CAP Investigators, Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, Arabi YM, Annane D, Beane A, van Bentum-Puijk W, Berry LR, Bhimani Z, Bonten MJM, Bradbury CA, Brunkhorst FM, Buzgau A, Cheng AC, Detry MA, Duffy EJ, Estcourt LJ, Fitzgerald M, Goossens H, Haniffa R, Higgins AM, Hills TE, Horvat CM, Lamontagne F, Lawler PR, Leavis HL, Linstrum KM, Litton E, Lorenzi E, Marshall JC, Mayr FB, McAuley DF, McGlothlin A, McGuinness SP, McVerry BJ, Montgomery SK, Morpeth SC, Murthy S, Orr K, Parke RL, Parker JC, Patanwala AE, Pettilä V, Rademaker E, Santos MS, Saunders CT, Seymour CW, Shankar-Hari M, Sligl WI, Turgeon AF, Turner AM, van de Veerdonk FL, Zarychanski R, Green C, Lewis RJ, Angus DC, McArthur CJ, Berry S, Webb SA, Derde LPG. Interleukin-6 Receptor Antagonists in Critically Ill Patients with Covid-19. N Engl J Med. 2021;384:1491-1502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1317] [Article Influence: 329.3] [Reference Citation Analysis (0)] |
| 75. | Furlow B. COVACTA trial raises questions about tocilizumab's benefit in COVID-19. Lancet Rheumatol. 2020;2:e592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 92] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 76. | Hasanin A, Mostafa M. Tocilizumab in patients with COVID-19: which patient, time, and dose? J Anesth. 2021;35:896-902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 77. | Satarker S, Tom AA, Shaji RA, Alosious A, Luvis M, Nampoothiri M. JAK-STAT Pathway Inhibition and their Implications in COVID-19 Therapy. Postgrad Med. 2021;133:489-507. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 78. | Novartis. Novartis provides update on RUXCOVID study of ruxolitinib for hospitalized patients with COVID-19. [cited March 20, 2021] Available from: https://www.novartis.com/news/media-releases/novartis-provides-update-ruxcovid-study-ruxolitinib-hospitalized-patients-covid-19. |
| 79. | Eli Lilly and Company. Baricitinib in Combination with Remdesivir Reduces Time to Recovery in Hospitalized Patients with COVID-19 in NIAID-Sponsored ACTT-2 Trial. [cited March 20, 2021]. Available from: https://investor.lilly.com/news-releases/news-release-details/baricitinib-combination-remdesivir-reduces-time-recovery. |
| 80. | Kalil AC, Patterson TF, Mehta AK, Tomashek KM, Wolfe CR, Ghazaryan V, Marconi VC, Ruiz-Palacios GM, Hsieh L, Kline S, Tapson V, Iovine NM, Jain MK, Sweeney DA, El Sahly HM, Branche AR, Regalado Pineda J, Lye DC, Sandkovsky U, Luetkemeyer AF, Cohen SH, Finberg RW, Jackson PEH, Taiwo B, Paules CI, Arguinchona H, Erdmann N, Ahuja N, Frank M, Oh MD, Kim ES, Tan SY, Mularski RA, Nielsen H, Ponce PO, Taylor BS, Larson L, Rouphael NG, Saklawi Y, Cantos VD, Ko ER, Engemann JJ, Amin AN, Watanabe M, Billings J, Elie MC, Davey RT, Burgess TH, Ferreira J, Green M, Makowski M, Cardoso A, de Bono S, Bonnett T, Proschan M, Deye GA, Dempsey W, Nayak SU, Dodd LE, Beigel JH; ACTT-2 Study Group Members. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2021;384:795-807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1159] [Cited by in RCA: 1327] [Article Influence: 331.8] [Reference Citation Analysis (1)] |
| 81. | Mortara A, Mazzetti S, Margonato D, Delfino P, Bersano C, Catagnano F, Lauriola M, Grosso P, Perseghin G, Ippoliti G. Compassionate use of ruxolitinib in patients with SARS-Cov-2 infection not on mechanical ventilation: Short-term effects on inflammation and ventilation. Clin Transl Sci. 2021;14:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 82. | Kaplanski G, Bontemps D, Esnault P, Blasco V, Carvelli J, Delarbre D, Cauchois R, Forel JM, Papazian L. Combined Anakinra and Ruxolitinib treatment to rescue extremely ill COVID-19 patients: A pilot study. Autoimmun Rev. 2021;20:102726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | Maslennikov R, Ivashkin V, Vasilieva E, Chipurik M, Semikova P, Semenets V, Russkova T, Levshina A, Grigoriadis D, Magomedov S, Efremova I, Dzhakhaya N. Tofacitinib reduces mortality in coronavirus disease 2019 Tofacitinib in COVID-19. Pulm Pharmacol Ther. 2021;69:102039. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (1)] |
| 84. | Rieder M, Wengenmayer T, Staudacher D, Duerschmied D, Supady A. Cytokine adsorption in patients with severe COVID-19 pneumonia requiring extracorporeal membrane oxygenation. Crit Care. 2020;24:435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 85. | Supady A, Duerschmied D, Bode C, Rieder M, Lother A. Extracorporeal cytokine adsorption as an alternative to pharmacological inhibition of IL-6 in COVID-19. Crit Care. 2020;24:514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 86. | Wu D, Yang XO. TH17 responses in cytokine storm of COVID-19: An emerging target of JAK2 inhibitor Fedratinib. J Microbiol Immunol Infect. 2020;53:368-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 523] [Cited by in RCA: 581] [Article Influence: 116.2] [Reference Citation Analysis (0)] |
| 87. | Bulat V, Situm M, Azdajic MD, Likic R. Potential role of IL-17 blocking agents in the treatment of severe COVID-19? Br J Clin Pharmacol. 2021;87:1578-1581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 41] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 88. | Martonik D, Parfieniuk-Kowerda A, Rogalska M, Flisiak R. The Role of Th17 Response in COVID-19. Cells. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 95] [Article Influence: 23.8] [Reference Citation Analysis (1)] |
| 89. | Liu P, Huang Z, Yin M, Liu C, Chen X, Pan P, Kuang Y. Safety and Efficacy of Ixekizumab and Antiviral Treatment for Patients with COVID-19: A structured summary of a study protocol for a Pilot Randomized Controlled Trial. Trials. 2020;21:999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 90. | Carugno A, Gambini DM, Raponi F, Vezzoli P, Robustelli Test E, Arosio MEG, Callegaro A, Sena P. Coronavirus disease 2019 (COVID-19) rash in a psoriatic patient treated with Secukinumab: Is there a role for Interleukin 17? Dermatol Ther. 2020;33:e14011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 91. | Sette A, Crotty S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell. 2021;184:861-880. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1199] [Cited by in RCA: 1299] [Article Influence: 324.8] [Reference Citation Analysis (0)] |
| 92. | Hasan MJ, Rabbani R, Anam AM, Huq SMR. Secukinumab in severe COVID-19 pneumonia: Does it have a clinical impact? J Infect. 2021;83:e11-e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 93. | National Library of Medicine. DailyMed - KEVZARA- sarilumab injection, solution. [cited March 20, 2021] https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=827bc01c-d379-4266-a18c-c7f904b76af3. |
| 94. | National Library of Medicine. DailyMed - ACTEMRA- tocilizumab injection, solution, concentrate ACTEMRA- tocilizumab injection, solution ACTEMRA ACTPEN- tocilizumab injection, solution. [cited March 20, 2021] Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=2e5365ff-cb2a-4b16-b2c7-e35c6bf2de13. |
| 95. | Charan J, Dutta S, Kaur R, Bhardwaj P, Sharma P, Ambwani S, Jahan I, Abubakar AR, Islam S, Hardcastle TC, Rahman NAA, Lugova H, Haque M. Tocilizumab in COVID-19: a study of adverse drug events reported in the WHO database. Expert Opin Drug Saf. 2021;20:1125-1136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | National Library of Medicine. DailyMed - SYLVANT- siltuximab injection, powder, for solution. [cited March 20, 2021] Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8d663642-f52e-49c0-a023-2da083fdfc0b. |
| 97. | National Library of Medicine. DailyMed - OLUMIANT- baricitinib tablet, film coated. [cited March 20, 2021] Available from: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=866e9f35-9035-4581-a4b1-75a621ab55cf#s21. |
| 98. | Schreiber S, Aden K, Bernardes JP, Conrad C, Tran F, Höper H, Volk V, Mishra N, Blase JI, Nikolaus S, Bethge J, Kühbacher T, Röcken C, Chen M, Cottingham I, Petri N, Rasmussen BB, Lokau J, Lenk L, Garbers C, Feuerhake F, Rose-John S, Waetzig GH, Rosenstiel P. Therapeutic Interleukin-6 Trans-signaling Inhibition by Olamkicept (sgp130Fc) in Patients With Active Inflammatory Bowel Disease. Gastroenterology. 2021;160:2354-2366.e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 167] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 99. | National Library of Medicine. DailyMed - JAKAFI- ruxolitinib tablet. [cited March 20, 2021] https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=f1c82580-87ae-11e0-bc84-0002a5d5c51b. |
| 100. | Chachar AZK, Khan KA, Iqbal J, Shahid AH, Asif M, Fatima SA, Khan AA, Younis BB. "Tocilizumab-an option for patients with COVID-19 associated cytokine release syndrome: A single center experience", a retrospective study-original article. Ann Med Surg (Lond). 2021;63:102165. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 101. | Riggs K, Patel V, Pittiglio M, Cavanaugh J, Sullivan J. 309: Evaluation of the Efficacy of Tocilizumab in Critically Ill COVID-19 Patients. Crit Care Med. 2021;49:141. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 102. | Adıyeke E, Coşkun N, Bakan N, Demir S, Cihan M, Yiyit N. Efficacy of Tocilizumab in the treatment of severe COVID-19 patients with respiratory failure. Med Sci Discov. 2021;8:86-90. [DOI] [Full Text] |
| 103. | Chen Y, Zhang X. Preliminary Efficacy of Tocilizumab Treatment in The Patients With COVID-19. 2021. [DOI] [Full Text] |
| 104. | Amin S, Rahim F, Bahadur S, Noor M, Mahmood A, Gul H. The Effect of Tocilizumab on Inflammatory Markers in Survivors and Non-survivors of Severe COVID-19. J Coll Physicians Surg Pak. 2021;31:S7-S10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 105. | Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, Bruzzi P, Boni F, Braglia L, Turrà C, Ballerini PF, Sciascia R, Zammarchi L, Para O, Scotton PG, Inojosa WO, Ravagnani V, Salerno ND, Sainaghi PP, Brignone A, Codeluppi M, Teopompi E, Milesi M, Bertomoro P, Claudio N, Salio M, Falcone M, Cenderello G, Donghi L, Del Bono V, Colombelli PL, Angheben A, Passaro A, Secondo G, Pascale R, Piazza I, Facciolongo N, Costantini M; RCT-TCZ-COVID-19 Study Group. Effect of Tocilizumab vs Standard Care on Clinical Worsening in Patients Hospitalized With COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Intern Med. 2021;181:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 474] [Cited by in RCA: 528] [Article Influence: 132.0] [Reference Citation Analysis (0)] |
| 106. | Wang D, Fu B, Peng Z, Yang D, Han M, Li M, Yang Y, Yang T, Sun L, Li W. Tocilizumab ameliorates the hypoxia in COVID-19 moderate patients with bilateral pulmonary lesions: a randomized, controlled, open-label, multicenter trial. 2020. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 107. | Sanofi. Sanofi provides update on Kevzara® (sarilumab) Phase 3 trial in severe and critically ill COVID-19 patients outside the U.S. (cited March 28, 2021). https://www.sanofi.com/en/media-room/press-releases/2020/2020-09-01-07-00-00. |
| 108. | Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, Patel N, Hagino O. Sarilumab treatment of hospitalised patients with severe or critical COVID-19: a multinational, randomised, adaptive, phase 3, double-blind, placebo-controlled trial. MedRxiv 2021. [DOI] [Full Text] |
| 109. | Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, Skiest D, Aziz MS, Cooper N, Douglas IS, Savic S, Youngstein T, Del Sorbo L, Cubillo Gracian A, De La Zerda DJ, Ustianowski A, Bao M, Dimonaco S, Graham E, Matharu B, Spotswood H, Tsai L, Malhotra A. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med. 2021;384:1503-1516. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 661] [Cited by in RCA: 717] [Article Influence: 179.3] [Reference Citation Analysis (0)] |
| 110. | Flisiak R, Jaroszewicz J, Rogalska M, Łapiński T, Berkan-Kawińska A, Bolewska B, Tudrujek-Zdunek M, Kozielewicz D, Rorat M, Leszczyński P. Tocilizumab Improves the Prognosis of COVID-19 in Patients with High IL-6. J Clin Med. 2021;10. [DOI] [Full Text] |
| 111. | Lescure FX, Honda H, Fowler RA, Lazar JS, Shi G, Wung P, Patel N, Hagino O; Sarilumab COVID-19 Global Study Group. Sarilumab in patients admitted to hospital with severe or critical COVID-19: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Respir Med. 2021;9:522-532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 179] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 112. | Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, Horick NK, Healy BC, Shah R, Bensaci AM, Woolley AE, Nikiforow S, Lin N, Sagar M, Schrager H, Huckins DS, Axelrod M, Pincus MD, Fleisher J, Sacks CA, Dougan M, North CM, Halvorsen YD, Thurber TK, Dagher Z, Scherer A, Wallwork RS, Kim AY, Schoenfeld S, Sen P, Neilan TG, Perugino CA, Unizony SH, Collier DS, Matza MA, Yinh JM, Bowman KA, Meyerowitz E, Zafar A, Drobni ZD, Bolster MB, Kohler M, D'Silva KM, Dau J, Lockwood MM, Cubbison C, Weber BN, Mansour MK; BACC Bay Tocilizumab Trial Investigators. Efficacy of Tocilizumab in Patients Hospitalized with Covid-19. N Engl J Med. 2020;383:2333-2344. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 998] [Cited by in RCA: 1011] [Article Influence: 202.2] [Reference Citation Analysis (0)] |