Published online May 25, 2021. doi: 10.5501/wjv.v10.i3.111
Peer-review started: January 10, 2021
First decision: February 15, 2021
Revised: February 21, 2021
Accepted: April 7, 2021
Article in press: April 7, 2021
Published online: May 25, 2021
Processing time: 127 Days and 13.2 Hours
Vitamin D population status may have possible unappreciated consequences to the coronavirus disease 2019 (COVID-19) pandemic. Α significant association between vitamin D sufficiency and reduction in clinical severity and inpatient mortality from COVID-19 disease has recently been shown, while a recent study has claimed lower COVID-19 cases in European countries with a better vitamin D status. Low serum 25-hydroxyvitamin-D [25(OH)D] was identified as an independent risk factor for COVID-19 infection and hospitalization, and administration of 0.532 mg (21280 IU) of calcifediol or 25(OH)D, followed by 0.266 mg on days 3 and 7 and then weekly until discharge or intensive care unit admission significantly reduced the need for intensive care unit treatment.
To elucidate the role of vitamin D European population status in the COVID-19 pandemic, data from the Worldometer were analyzed.
Linear regression explored the correlation between published representative-standardized population vitamin D concentrations and the number of total cases/million (M), recovered/M, deaths/M and serious-critically ill/M from COVID-19 for 26 European countries populated > 4 M (Worldometer). Life expectancy was analyzed with semi-parametric regression. Weighted analysis of variance/analysis of covariance evaluated serious-critical/M and deaths/M by the vitamin D population status: Deficient < 50, insufficient: 50-62.5, mildly insufficient > 62.5-75 and sufficient > 75 nmol/L, while controlling for life expectancy for deaths/M. Statistical analyses were performed in XLSTAT LIFE SCIENCE and R (SemiPar Library).
Linear regression found no correlation between population vitamin D concentrations and the total cases-recovered/M, but negative correlations predicting a reduction of 47%-64%-80% in serious-critical illnesses/M and of 61%-82%-102.4% in deaths/M further enhanced when adapting for life expectancy by 133-177-221% if 25(OH)D concentrations reach 100-125-150 nmol/L, sustained on August 15, 2020, indicating a truthful association. Weighted analysis of variance was performed to evaluate serious-critical/M (r2 = 0.22) by the vitamin D population status and analysis of covariance the deaths/M (r2 = 0.629) controlling for life expectancy (r2 = 0.47). Serious-critical showed a decreasing trend (P < 0.001) from population status deficient (P < 0.001) to insufficient by 9.2% (P < 0.001), to mildly insufficient by 47.6% (P < 0.044) and to sufficient by 100% (reference, P < 0.001). For deaths/M the respective decreasing trend (P < 0.001) was 62.9% from deficient (P < 0.001) to insufficient (P < 0.001), 65.15% to mildly insufficient (P < 0.001) and 78.8% to sufficient (P = 0.041).
Achieving serum 25(OH)D 100-150 nmol/L (40-60 ng/mL) (upper tolerable daily doses followed by maintenance proposed doses not requiring medical supervision, Endocrine Society) may protect from serious-critical illness/death from COVID-19 disease.
Core Tip: To elucidate the role of vitamin D in the coronavirus disease 2019 (COVID-19) pandemic, we examined associations between published representative and standardized European population vitamin D data and the Worldometer COVID-19 data. Linear regression found no correlation between population vitamin D concentrations and the total cases-recovered/million (M), but negative correlations predicting a reduction of 47%-64%-80% in serious-critical illnesses/M and of 61%-82%-102.4% in deaths/M further enhanced when adapting for life expectancy by 133-177-221% if 25-hydroxyvitamin-D concentrations reach 100-125-150 nmol/L. Weighted analysis of variance/analysis of covariance showed a decreasing trend (P < 0.001) evaluating serious-critical/M (r2 = 0.22) and the deaths/M (r2 = 0.629) after controlling for life expectancy (r2 = 0.47), by vitamin D population status, respectively.
- Citation: Papadimitriou DT, Vassaras AK, Holick MF. Association between population vitamin D status and SARS-CoV-2 related serious-critical illness and deaths: An ecological integrative approach. World J Virol 2021; 10(3): 111-129
- URL: https://www.wjgnet.com/2220-3249/full/v10/i3/111.htm
- DOI: https://dx.doi.org/10.5501/wjv.v10.i3.111
Vitamin D deficiency and insufficiency is a global health issue affecting probably many more than 1 billion children and adults worldwide, with institutionalized elderly being at higher risk of exhibiting lower 25-hydroxyvitamin-D [25(OH)D] blood concentrations. According to a systematic review of vitamin D status in populations worldwide, 37.3% of the studies reported 25(OH)D mean concentrations < 50 nmol/L in newborns and institutionalized elderly, who are at higher risk of exhibiting lower 25(OH)D concentrations[1]. Public health policy development is needed to reduce risk for potential health consequences of an inadequate vitamin D status[1], with consequences that should not be underestimated, especially now with this unprecedented pandemic of coronavirus disease 2019 (COVID-19)[2]. The initial universal lockdown for a period of 2-3 mo and the consequent repeated lockdowns along with the social distancing measures would further reduce the incidental solar vitamin D3 production, worsening the population’s vitamin D status[3]. Strong evidence supports the role of vitamin D particularly in preventing rickets and osteomalacia[4]. While circulating 25(OH)D concentrations below 30 nmol/L (12 ng/mL) are associated with an increased risk of rickets/osteomalacia, 25(OH)D concentrations between 50-125 nmol/L (20-50 ng/mL) appear to be safe and sufficient to promote skeletal health in the general population[5]. A serum 25(OH)D concentration of at least ≥ 50 nmol/L at the end of winter (10-20 nmol/L higher at the end of summer, to allow for seasonal decrease) is required for optimal musculoskeletal health[6]. Supplements of vitamin D in low doses together with calcium, alone or in combination with antiresorptive drugs may prevent hip or any type of fracture and have been evaluated in osteoporotic and osteopenic patients for primary as well as secondary prevention[7-9]. However, the role of vitamin D in innate and adaptive immunity remains rather underappreciated, with possible consequences and public health implications, leading to an increased risk for infectious diseases, autoimmune disorders and cancers[10]. Even if a recent randomized control trial (RCT) did not show lower incidence of invasive cancer in men ≥ 50 years or women ≥ 55 receiving 2000 IU of vitamin D3 daily up to 5 years[11], the study did report a statistically significant 25% reduced risk for cancer mortality. The study, however, had several limitations. Only 13% of the participants were vitamin D deficient [25(OH)D < 50 nmol/L], and 42%-45% of the participants were receiving a vitamin D supplement and multivitamins at inclusion. The participants, including the placebo group, were permitted to take up to 800 IU of vitamin D daily. This is the likely explanation why the mean baseline blood concentration of 25(OH)D was 74.5 nmol/L for the participants in this study[11]. The optimal 25(OH)D concentration is at least 75 nmol/L (30 ng/mL), which is what the mean baseline level was for the participants in the VITAL study. Secondary analyses from the VITAL study should be also considered as they indicate that the vitamin D dose was too low, since significant benefits were found for cancer incidence for those with body mass index (BMI) < 25 kg/m2 and almost as significant for blacks. In fact, the authors speculated that the possible trial regimen–associated effects on cancer incidence among normal-weight participants and suggestive effects among black participants, which contrast with the null cardiovascular findings in these groups, may be explained by different vitamin D requirements for these outcomes. The Endocrine Society, which made its recommendations in 2011 for the treatment and prevention of vitamin D deficiency, concluded that to guarantee bone health, a blood level of 25(OH)D of at least 75 nmol/L (30 ng/mL) is required (https://www.endocrine.org/clinical-practice-guidelines/vitamin-d-deficiency)[12]. Beyond musculoskeletal health however, it has been found that vitamin D supplementation significantly reduced the risk of cancer death by 15% in a systematic review and meta-analysis of 52 trials with a total of 75454 participants[13], and it has been suggested that better health outcomes may occur in the range of 100-150 nmol/L[10]. The largest meta-analysis ever conducted of all studies published between January 1, 1966 and January 15, 2013 dealing with all-cause mortality related to serum 25(OH)D showed that 25(OH)D < 75 nmol/L was associated with higher all-cause mortality, its reduction being maintained with 25(OH)D ≥ 175 nmol/L (70 ng/mL), without a U-shaped curve as previously reported[10]. Achieving such concentrations with supplements and sensible sun exposure for a normal weight adult requires 2000–5000 IU daily intake of vitamin D2/D3, practically all year long except maybe during sunny vacations[14]. With vitamin D adequacy relying mainly (80%-90%) on sun exposure rather than on dietary sources (10%-20%), if not on supplementation, these doses should be adapted accordingly during lockdowns. It should also be recognized that sensible sun exposure has many additional health benefits not only in the immune system but also in improving the feeling of well-being[15]. At this time, neither the World Health Organization nor any other public health authority has issued any official advice or recommendation on vitamin D, or any other nutrients, to the best of our knowledge.
A quadratic relationship was found between vitamin D deficiency in countries affected by COVID-19 and the latitudes, implying a possible relation[16]. When mortality/ million (M) is plotted against latitude, all countries below 35 degrees North, above which people do not receive sufficient sunlight to retain adequate 25(OH)D concentrations during winter, have relatively lower mortality, implying a role for vitamin D status in outcomes from COVID-19[17]. Vitamin D is strongly affected by ozone variability, since ozone filters ultraviolet B, an important factor for vitamin D synthesis. A statistically significant link between ozone concentration and incidence of COVID-2019 disease in 34 countries was established[18]. Going back to the 1918-1919 influenza pandemic, substantial correlations were found for associations of July ultraviolet B dose in the United States with case fatality rates and rates of pneumonia[19]. Α significant association between vitamin D sufficiency and reduction in clinical severity and inpatient mortality was very recently shown[20]. Thus, to elucidate further the possible role of vitamin D population status in the COVID-19 pandemic, we examined the associations between published representative and standardized population vitamin D data on European population vitamin D status and the Worldometer COVID-19 data.
Accessing data on European countries at the Worldometer, on June 19, 2020, we analyzed the 28 countries populated > 4 M (Table 1). For months, Swedish public health authorities have defended their controversial decision not to lock down the country in response to the global COVID-19 pandemic, with the country experiencing dramatic casualties. Thus, Sweden was excluded from analysis. The remaining 27 European countries adopted a defensive strategy during the current pandemic, even with delays and hesitations, as in the United Kingdom. Moldova was also excluded as no published vitamin D status data were found. For the remaining 26 countries, we used linear regression to explore the correlation between reported representative and standardized population vitamin D concentrations[21-28] and the number of total cases/M and recovered/M until June 19, 2020 as well as the deaths/M and the serious-critically ill/M from COVID-19 on that date (Table 1). Since mortality of COVID-19 disease has been shown to increase rapidly in respect to age, life expectancy (LE), an age-related index, was analyzed using a semi-parametric regression approach using Worldometer data. Weighted (https://doi.org/10.13094/SMIF-2015-00001) analysis of variance (ANOVA)/analysis of covariance (ANCOVA) was performed to evaluate serious-critical/M and deaths/M by the vitamin D population status - categorized as deficient (D) < 50, insufficient (IN) 50-62.5, mildly insufficient (MIN) > 62.5-75 and sufficient (S) > 75 nmol/L – while controlling for LE for deaths/M. To test whether these correlations withstand at another completely different momentum of this pandemic, which would be an indication of a truthful association, although still not a proof of causality, we also checked the above correlations and the differences between consecutive points of the same variables on August 15, 2020. All statistical analyses were performed in XLSTAT LIFE SCIENCE version April 1, 2020 (copyright Addinsoft 1995-2020) and R (R Core Team 2017), with the use of the SemiPar library.
| Country | Total cases/M | Total recovered | Serious critical | Deaths/M | Life expectancy in yr | Population 25(OH)D in nmol/L | Population, M | |
| 1 | Russia | 3899 | 324406 | 2300 | 54 | 72.99 | 39.7 | 145.93 |
| 2 | Germany | 2273 | 174400 | 396 | 107 | 81.88 | 50.1 | 83.77 |
| 3 | United Kingdom | 4447 | N/A | 379 | 626 | 81.77 | 47.4 | 67.87 |
| 4 | France | 2431 | 73887 | 752 | 454 | 83.13 | 60.0 | 65.26 |
| 5 | Italy | 3939 | 180544 | 168 | 571 | 84.01 | 45.0 | 60.46 |
| 6 | Spain | 6253 | N/A | 617 | 580 | 83.99 | 59.9 | 46.75 |
| 7 | Ukraine | 800 | 16033 | 343 | 23 | 72.50 | 29.0 | 43.74 |
| 8 | Poland | 827 | 15698 | 87 | 35 | 79.27 | 32.0 | 37.84 |
| 9 | Romania | 1216 | 16555 | 184 | 77 | 76.50 | 65.0 | 19.24 |
| 10 | Netherlands | 2885 | N/A | 57 | 355 | 82.78 | 64.7 | 17.13 |
| 11 | Belgium | 5219 | 16751 | 55 | 837 | 82.17 | 49.3 | 11.58 |
| 12 | Czechia | 968 | 7472 | 9 | 31 | 79.85 | 62.5 | 10.70 |
| 13 | Greece | 311 | 1374 | 10 | 18 | 82.80 | 54.3 | 10.42 |
| 14 | Portugal | 3772 | 24477 | 67 | 150 | 82.65 | 55.4 | 9.66 |
| 15 | Sweden | 5550 | N/A | 272 | 500 | 83.33 | 68.7 | 9.44 |
| 16 | Hungary | 422 | 2581 | 15 | 59 | 77.31 | 60.6 | 83.33 |
| 17 | Belarus | 6067 | 35275 | 92 | 36 | 75.20 | 72.0 | 9.00 |
| 18 | Austria | 1918 | 16141 | 7 | 76 | 82.05 | 51.7 | 8.73 |
| 19 | Serbia | 1454 | 11511 | 18 | 30 | 76.47 | 65.7 | 8.65 |
| 20 | Switzerland | 3,608 | 28900 | 17 | 226 | 84.25 | 46.0 | 6.94 |
| 21 | Bulgaria | 529 | 1941 | 13 | 27 | 75.49 | 38.7 | 5.79 |
| 22 | Denmark | 2139 | 11282 | 6 | 104 | 81.40 | 65.0 | 5.54 |
| 23 | Finland | 1287 | 6200 | 2 | 59 | 82.48 | 67.7 | 5.45 |
| 24 | Slovakia | 289 | 1447 | 0 | 5 | 78.00 | 81.5 | 5.41 |
| 25 | Norway | 1,609 | 8138 | 5 | 45 | 82.94 | 71.0 | 4.93 |
| 26 | Ireland | 5137 | 22698 | 28 | 347 | 82.81 | 56.4 | 4.10 |
| 27 | Croatia | 555 | 2142 | 0 | 26 | 79.02 | 46.9 | 4.03 |
| 28 | Moldova | 3249 | 7525 | 455 | 111 | 72.30 | N/A | 10.09 |
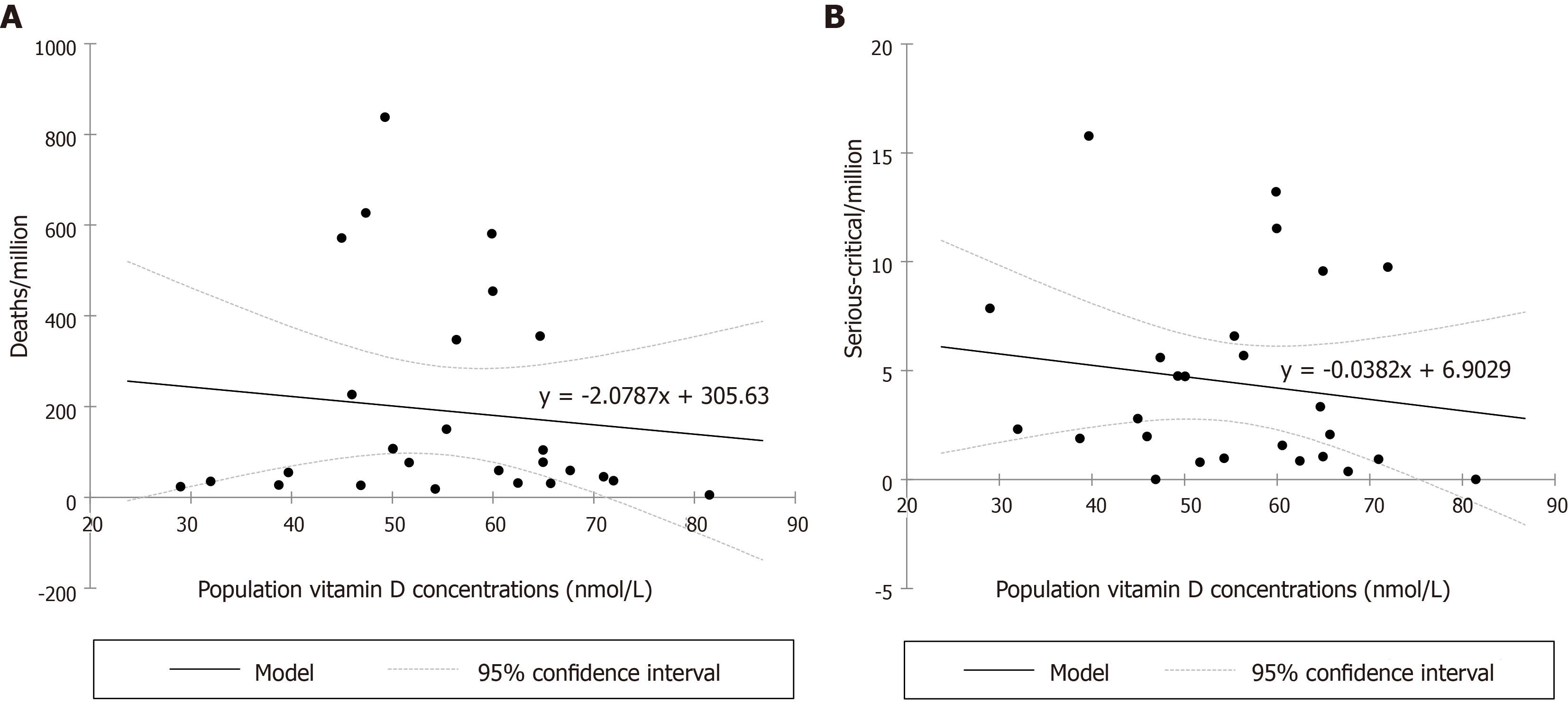
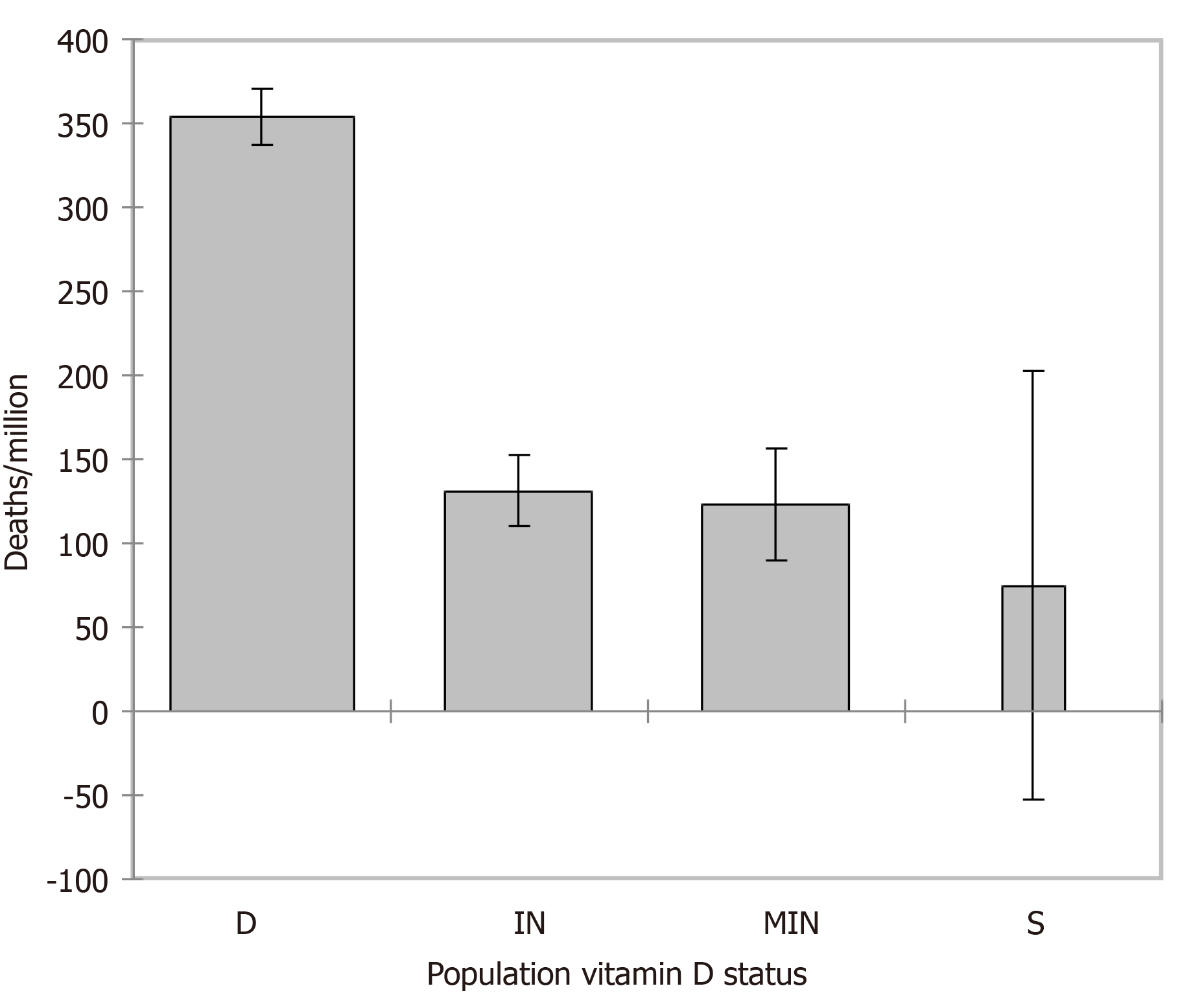
From the 26 European countries included in the analysis, populated 714.661 M in total, nine (54.17%, 387.15 M) had a vitamin D deficient status, eight an insufficient status (33.58%, 240.022 M), eight a mild insufficiency status (11.48%, 82.023 M) and only one country, Slovakia, a sufficient status (0.76%, 5.459 M). There was no correlation between the total cases/M nor the recovered/M and the European population vitamin D concentrations. Negative correlations were recognized regarding the total deaths/M (Figure 1A), predicting a reduction of deaths/M by 20% if the 25(OH)D concentration reaches 50 nmol/L (related to the number calculated at 25), by 40% at 75, by 61% at 100, by 82% at 125 and by 102.4% at 150 nmol/L and the serious-critical/M (Figure 1B), predicting a reduction of serious-critically ill/M by 16% if 25(OH)D concentration reaches 50 nmol/L (related to the number calculated at 25), by 31% at 75, by 47% at 100, by 64% at 125 and by 80% at 150 nmol/L.
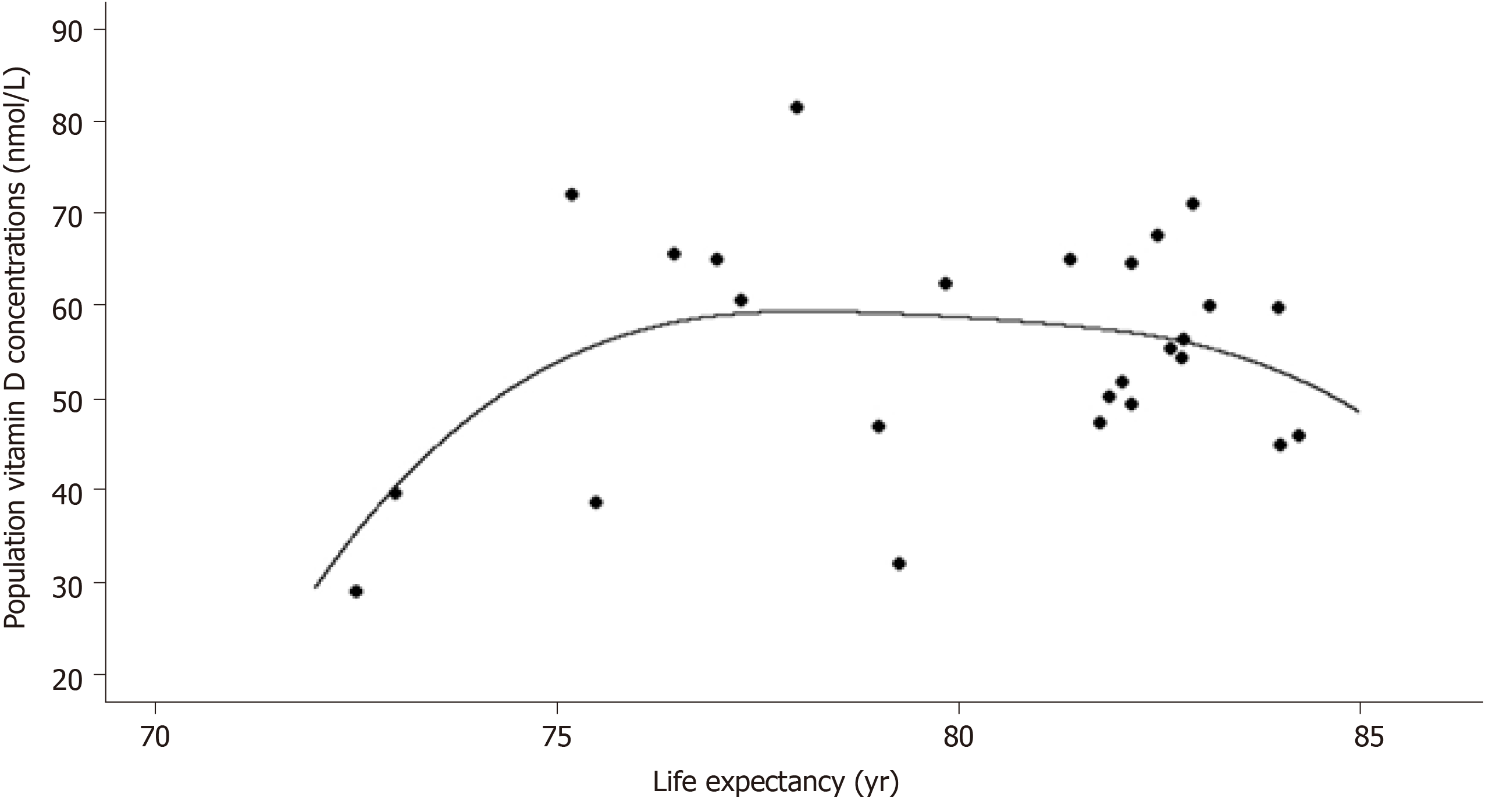
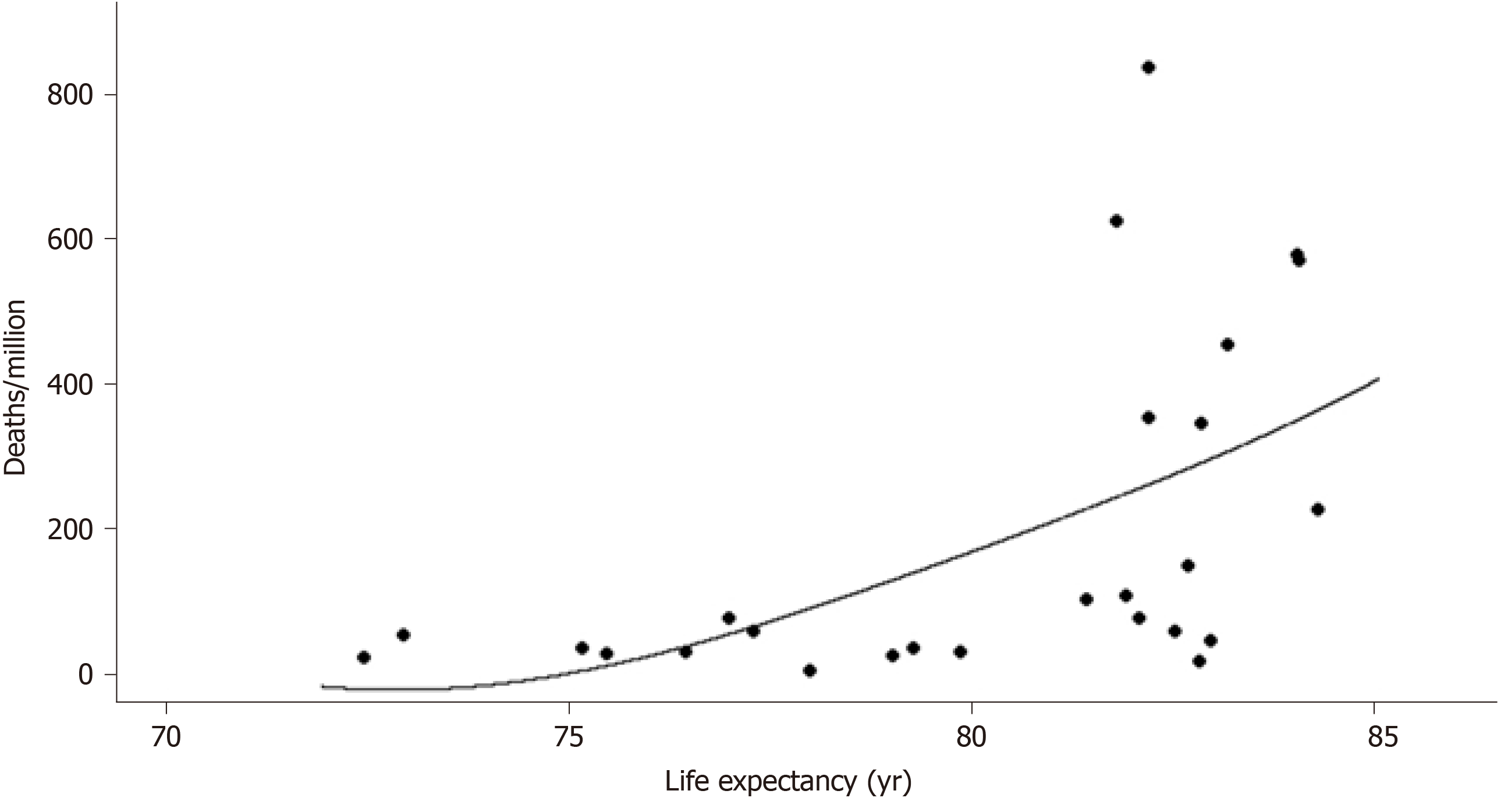
Population vitamin D concentrations vs life expectancy exhibits a non-linear relationship (Figure 2): Higher life expectancy until approximately 77 years of age is characterized by better vitamin D concentrations, while practically reaching a plateau at 82 years, and then by a decline as expected in the elderly. There is a non-linear relationship between life expectancy and deaths/M with a dramatic increase in deaths/M after approximately 80 years (Figure 3). LE (i.e. age) seems to interfere with the effect of a better vitamin D concentration to the total number of deaths/M, rendering the vitamin D benefit even more important than the unadjusted one: A reduction in total deaths/M by 44% if 25(OH)D concentration reaches 50 nmol/L (related to the number calculated at 25), by 88% at 75, by 133% at 100, by 177% at 125 and by 221% at 150 nmol/L. The analytical form for the model on the deaths/M accounting for a potential non-linear effect of LE is year = -2675-4.111*vitamin D + f(LE), where f(.) is a non-linear smooth function of life expectancy. The P value for the term f(LE) was estimated via likelihood ratio test to be P = 0.042, indicating a statistically significant effect of life expectancy on deaths/M after adjusting for vitamin D concentration.
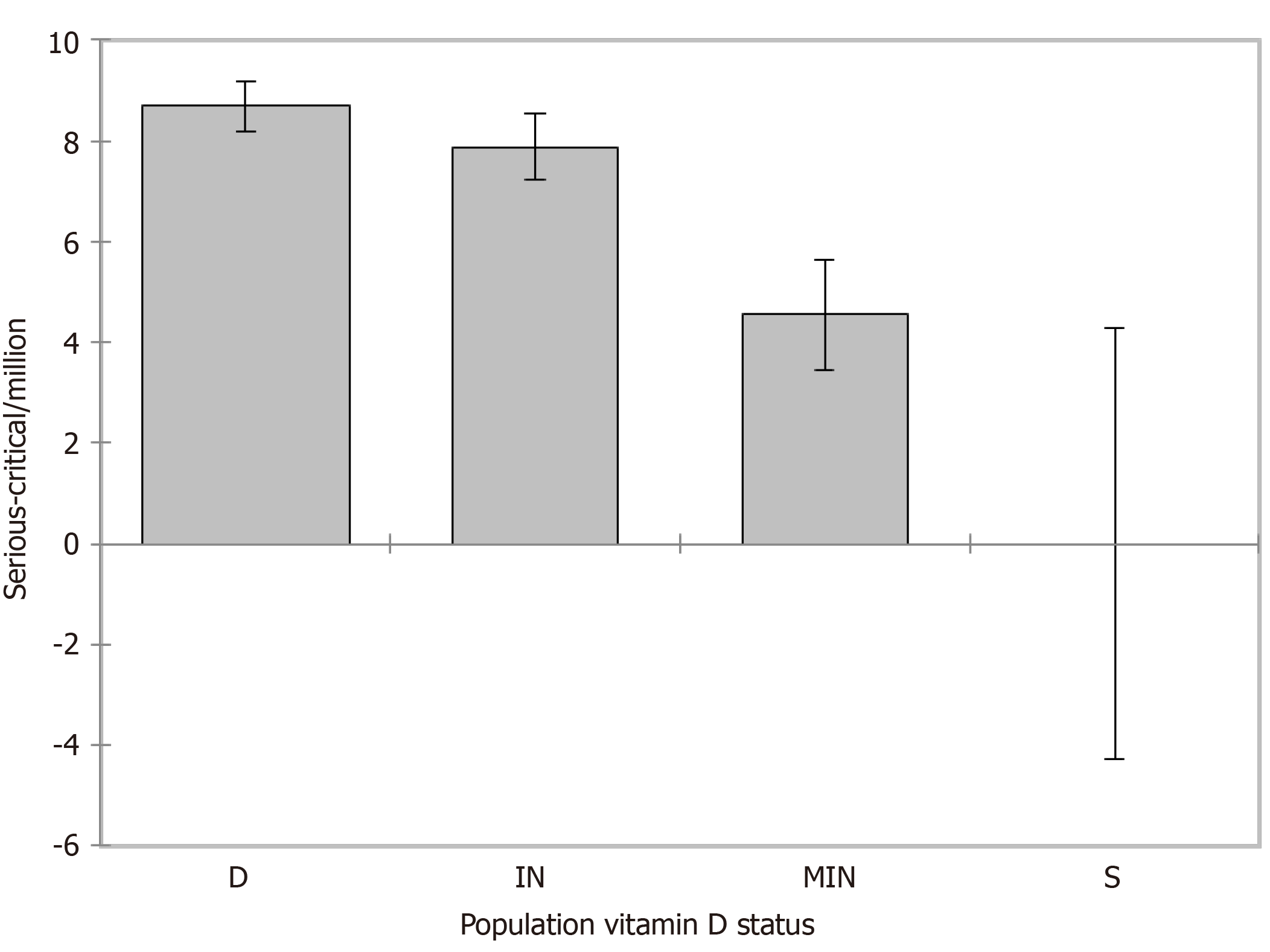
Weighted (https://doi.org/10.13094/SMIF-2015-00001) ANOVA was performed to evaluate serious-critical/M and ANCOVA for deaths/M by the population vitamin D status while controlling for LE. Given the r2, about 22% of the variability of the dependent variable serious-critical/M could be explained by the population vitamin D status. A decreasing trend from population status D [β = 8.684, standard error (SE) = 2.196, 95% confidence interval (CI): 4.372/12.996, P < 0.001], IN (β = 7.883, SE = 2.205, 95%CI: 3.553/12.213, P < 0.001), MIN (β = 4.548, SE = 2.252, 95%CI: 0.126/8.169, P = 0.044) to S (LE mean 0.0, SE 2.181, 95%CI: -4.282/4.282, P < 0.001) was found with an average reduction of serious-critical/M of 9.2% from vitamin D status deficient to insufficient, of 47.6% from deficient to mildly insufficient and 100% from deficient to sufficient (reference, Figure 4). Regarding deaths/M (Figure 5), given the r2, about 63% of the variability of the dependent variable deaths/M could be explained by the two variables, LE alone accounting for 47%. A decreasing trend from population status deficient (β = 150.375, SE = 8.859, 95%CI: 132.982/167.768, P < 0.001), insufficient (β =
On August 15, 2020, the above correlations were sustained and the differences between consecutive points for the two variables serious-critical/M and deaths/M in the two time points were correlated, not proving causality but suggesting a truthful association.
We explored any possible correlation between the population vitamin D status - influenced by various factors - and COVID-19 disease, in particular total cases, serious-critical illness and deaths. In contrast to a recently published study[29], we found no association between the vitamin D status of the European populations and the total confirmed cases/M of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections when we analyzed data from the Worldometer on June 19, 2020 on 26 European countries populated > 4 M. However, the negative correlations that we found between population vitamin D status and serious-critical/M and deaths/M show a clear tendency, even if they do not prove causality, namely after adjusting for LE, underlining the importance of an optimal vitamin D status especially in the elderly[30]. On August 15, 2020, at a completely different time point of this pandemic, before the second wave even had started, the above associations were sustained, suggesting a truthful correlation. Since the risk of COVID-19 disease increases rapidly with respect to age, an age-related index, such as LE, was found, as expected, to be a more important predictor of death rates. Thus, according to our results, a higher 25(OH)D concentration may protect from serious-critical illness and death from COVID-19 disease even more in the elderly but does not seem to prevent SARS-CoV-2 from spreading, in contrast to a recent study[29], which however reported also a negative correlation between the mean population vitamin D concentrations of 20 European countries and deaths/M from COVID-19 on April 8, 2020. Our findings also coincide with a recent study from Maghbooli et al[20] showing that vitamin D sufficiency [a serum 25(OH)D > 75 nmol/L (30 ng/mL)] reduced risk for adverse clinical outcomes in patients with COVID-19 infection: 6.3% of the patients who had a blood 25(OH)D concentration of at least 100 nmol/L (40 ng/mL) succumbed to the infection compared to 9.7% and 20% who died and had a circulating blood level above and below 75 nmol/L (30 ng/mL), respectively[20,31], suggesting that a blood level of at least 100 nmol/L (40 ng/mL) may be optimal for obtaining vitamin D’s immunomodulatory benefit.
Various parameters played a significant role in the spread of the current pandemic. Among them, air travel and direct connections with China and particularly Wuhan, where the epidemic started. Then, health policymaking with mass quarantine was instituted in most countries, influencing the course of the disease, but with no central coordination of the measures taken during the first wave of the pandemic, not even in the core of the European Union itself. Timing of the lockdowns, at least in the first wave, seemed to have been the main factor affecting the number of the cumulative deaths – although this has been strongly debated (https://thefatemperor.com/published-papers-and-data-on-lockdown-weak-efficacy-and-lockdown-huge-harms/), along with travel and border restrictions. Recent research emphasizes the importance of face masks while self-protection measures seem to be better implemented by populations with higher educational levels. Temperature also appears to have a small but statistically significant impact on the viral transmission rate as countries with daily average temperatures below 20 °C had a faster transmission rate. Most probably, genetic predisposition must have played a fundamental role in the susceptibility in SARS-CoV-2 infection[32,33]. The recent discovery of robust genetic signals relating to key host antiviral defense mechanisms and mediators of inflammatory organ damage in COVID-19 may lead to targeted treatment with existing drugs[33]. Most recent evidence show that angiotensin-I converting enzyme-2 (ACE2) expression and/or polymorphism could also influence both the individual susceptibility to SARS-CoV-2 infection and the outcome of the COVID-19 disease[34]. Thus, the integrity of our immune system and its ability to fight back with a coordinated way, keeping asymptomatic or within the subclinical spectrum most of the people infected and saving the lives of the severely infected, is a crucial factor. And there is significant evidence that vitamin D deficiency may compromise both innate and acquired immunity responses, leading to increased vulnerability to infections as to autoimmune responses and disorders[35].
The vitamin D status of a population is dependent on a variety of factors including supplementation and food fortification strategies, latitude of the country, season as well as on the local nutritional and sun exposure habits, especially in the non-institutionalized elderly[36]. The vitamin D status in the winter is even lower[1,37,38], with underappreciated consequences to the immune function[39,40]. Ideally, we should be able to analyze data on vitamin D status of the elderly in winter. Thus, a major limitation of our ecological approach is that we had to rely on published - but perhaps not always completely representative - data on the vitamin D status of the populations in Europe. However, data analyzed are based mainly on “Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: A position statement of the European Calcified Tissue Society” recently published in the European Journal of Endocrinology[21] – presenting not only representative nationally or regionally as possible but also standardized population vitamin D concentrations -, a systematic review of vitamin D status in southern European countries[22], and a very important study applying the protocols developed by the National Institutes of Health-led international Vitamin D Standardization Program to serum 25(OH)D data from representative childhood/teenage and adult/older (we chose data from older adults) European populations, representing a sizable geographical footprint, to better quantify the prevalence of vitamin D deficiency in Europe[28]. Keeping in mind that the population vitamin D status reflects that of the elderly, which by default will be worse, we tried to analyze the most recently validated and representative data possible, whereas from the available data for each country we chose data from older adults in winter where provided, and in any case from Caucasian descent. Ideally, we should be able to analyze data on 25(OH)D concentrations of the patients as in an interesting recent report from Switzerland, which found significantly lower circulating 25(OH)D concentrations [27.75 nmol/L (11.1 ng/mL), P = 0.004] in polymerase chain reaction-positive for SARS-CoV-2 patients compared with negative patients [61.5 nmol/L (24.6 ng/mL)], even after stratifying patients according to age > 70 years[41]. Another important issue would be the differences in assessment mainly of the COVID-19 deaths in the various European countries. However, the World Health Organization had already issued the “International guidelines for certification and classification (coding) of COVID-19 as cause of death, April 20, 2020” 2 mo earlier to our analysis, allowing us to assume that they must had already been adopted by the European Countries responsible public health authorities. Furthermore, Worldometer.info mainly collects data from official reports, directly from governmental communication channels. An additional important limitation is the true evaluation of the number of affected subjects in the variable countries: Since not all patients infected with COVID-19 are symptomatic, the cases/M are dependent upon the percentage of the population tested and the consistency of the frequency of testing during the disease period evaluated, not to mention that several patients or carriers have been tested several times. Furthermore, the definition of case includes a carrier as well as a patient. Unfortunately, this limitation could not be overcome with the publicly available COVID-19 data at the time of our analysis. However, we had to report the absence of any correlation between total cases/M and the population vitamin D status in the sample we analyzed, in contrast to a recently published study with the opposite results[29]. Assessment of serious-critical cases in the European countries may also have been limited at some points by the shortcoming of intensive care unit (ICU) beds as well as the introduction of different drugs and “cocktail” treatments from country to country. Albeit, on June 19, 2020, the first wave of the pandemic in Europe was kind of winding down, and not particularly effective new or repurposed medication had at least been qualified at that point as such to change significantly the clinical course of the serious-critical patients, other than the accumulated experience of the health workers fighting on the frontline.
Independent researchers increasingly call for optimization of vitamin D status for enhanced immune protection against COVID-19 at least in older adults, hospital inpatients, nursing home residents and other vulnerable groups, extending this recommendation to the general population[42]. The elderly (> 65 years) have a higher risk for vitamin D deficiency due to decreased sun exposure and reduced ability for cutaneous synthesis[38], whereas aging exerts significant effects on all cells of the innate immune system[40], making vitamin D sufficiency even more valuable in this group. Early nutritional supplementation in non-critically ill patients hospitalized for COVID-19 has been implemented in hospital protocols providing 50000 UI/wk if 25(OH)D < 50 nmol/L and 25000 UI/wk if 25(OH)D < 75 nmol/L aiming at improved immunologic recovery with reduced levels of inflammation, immune activation, and increased immunity against pathogens[43].
The COVID-19 pandemic presents a puzzling challenge without specific treatment yet with timely administration being crucial for all current regimens on clinical trial or use. This is also the case for vitamin D, and this might be the reason why in a recent RCT, a single enteral dose of 540000 IU of vitamin D3 or matched placebo started late within 12 h after the decision to admit the critically ill (unrelated to COVID- 19) vitamin D deficient patient to an intensive care unit, had no benefit at a 90-d all-cause, all-location mortality[44]. Regarding vitamin D, we know that respiratory viruses downregulate vitamin D receptor expression in human bronchial epithelial cells, while improvement in vitamin D status increases antiviral defenses via cathelicidins and innate interferon pathways[45]. Vitamin D has a 12% overall protective effect against bacterial and viral acute respiratory tract infection, increased to 19% in those individuals on daily or weekly regimen compared to those on monthly boluses and up to 70% when vitamin D deficiency is corrected with daily supplementation[46]. Bioavailable 25(OH)D is inversely associated with illness severity in critically ill ICU patients associated with increased mortality and morbidity[47]. Calcitriol [1,25(OH)2D3] alleviates lipopolysaccharide induced acute lung injury and prevents the adult respiratory distress syndrome by minimizing the alveolar damage[48]. Vitamin D is also a negative endocrine regulator of the renin-angiotensin system. The mechanism for SARS-CoV-2 infection is the requisite binding of the virus to the membrane-bound form of ACE2 and internalization of the complex by the host cell. Recognition that ACE2 is the main host receptor by SARS-CoV-2 to infect human has prompted new therapeutic approaches to block the enzyme or reduce its expression to prevent cellular entry of SARS-CoV-2 in tissues expressing ACE2 (lung, heart, kidney, brain, and gut). Thus, it seems that both stimulation of the immune system and inhibition of renin-angiotensin system are mechanisms by which vitamin D may play a beneficial role in COVID-19 infection[49]. Vitamin D repletion in critical illness with a more aggressive dosing is showing similarly promising results with vitamin C repletion in septic shock[50] and may be able to prevent the cytokine storm that seems to be killing people rather than the virus itself[51]. C-reactive protein is a surrogate marker for unregulated inflammation and cytokine storm and is associated with vitamin D deficiency. Retrospective data and indirect evidence also show a possible role for vitamin D in reducing complications attributed to and the cytokine storm itself[52]. Moreover, recent research revealed that vitamin D receptor signaling in macrophages regulates a shift between proinflammatory and anti-inflammatory activation during ER stress-induced inflammation[53]. Thus, supplementation within recommended upper safety limits, for specific nutrients such as vitamins C and D, warrants optimal nutritional status to insure a well-functioning immune system protecting against viral infections[54].
Recent research demonstrated that low serum 25(OH)D was an independent risk factor for COVID-19 infection and hospitalization analyzing data from 14,000 members of Leumit Health Services in Israel[55]. A very recent pilot randomized clinical study demonstrated that administration of a relatively high dose (0.532 mg-21280 IU) of calcifediol or 25(OH)D, followed by 0.266 mg on days 3 and 7, and then weekly until discharge or ICU admission, significantly reduced the need for ICU treatment of patients requiring hospitalization due to proven COVID-19 disease[56]. In a single-center, retrospective cohort study concerning 489 patients, likely deficient vitamin D status was associated with increased COVID-19 risk[57].
Our analysis took place at two completely different time points during the beginning and the end of the first wave of this pandemic. We needed to confirm our first results at a completely different time point of the first wave. One could not attempt to extend this type of approach to the second wave or the third wave, which is now hitting Europe, first because the virus has significantly spread into the European populations. Secondly, after extended lockdowns and limited - if any - summer vacations and with no public health authority having officially advised supple
At this time and despite the ongoing debate on “The Big Vitamin D Mistake”[15], referring to the statistical error in the estimation of the Recommended Dietary Allowance of vitamin D discovered by Veugelers and Ekwaru[58] in 2014 and confirmed by Heaney et al[59]: About 4000 IU/d (3385) are needed to ensure 50 nmol/L in 97.5% of the population, about 6000 IU/d (6201) are needed to achieve the Endocrine Society’s recommendation of 75 nmol/L and about 9000 IU/d (9122) to reach 100 nmol/L, and even if the vitamin D deficiency pandemic is still being questioned[60], no one should confuse the global consensus on the minimum vitamin D doses needed to prevent nutritional rickets[61], with the doses needed to exert all of its extra-skeletal health benefits[62], particularly those related to our immune system. Apart from the known disagreement between the Endocrine Society and the Institute of Medicine (IOM) but also the discrepancy between the IOM and the Scientific Advisory Committee on Nutrition in Great Britain, two equally respectable government advisory committees, who after reviewing the same evidence, ended up with a twofold difference in target concentrations in serum 25(OH)D and similarly divergent conclusions for intakes of vitamin D[12], one can notice that differences concerning upper tolerable limits for vitamin D administration are limited. The more conservative IOM advises up to (upper tolerable limit) 1500 IU daily in infants < 1 year, 2500 IU in children 1-3 years, 3000 IU in children 4-8 years and up to 4000 IU for everybody after 9 years of age; where the Endocrine Society advices are up to 2000 IU for infants < 2 years, up to 4000 IU for children 1-18 years and up to 10000 IU for adults, adult pregnant and lactating women as well as the elderly, underlining that obese people may need up to two to three times more, as it may be needed to correct vitamin D deficiency or to treat specific conditions such as rickets, osteomalacia, hyperparathyroidism, malabsorption syndromes or if on medications affecting vitamin D’s metabolism. However, the doses that the Endocrine Society practice committee characterizes as not requiring medical supervision are practically identical to the IOM’s upper tolerable limits. Thus, supplementation with vitamin D within recommended safety limits, with doses that do not require prior measurement of the 25(OH)D concentration or medical supervision, apart from the already established protective role in bone mineral density[63], may also assure a well-functioning immune system[64].
In 2011, the Endocrine Society published the Endocrine Society Practice Guidelines on vitamin D, recommending how to treat and prevent vitamin D deficiency in children and adults. Based on the literature these recommendations were related to maximizing musculoskeletal health. However, in 2011 there was not enough scientific evidence for the Committee to recommend improvement in vitamin D status for reducing risk of many chronic illnesses or improving immune function. During the past decade, however, numerous studies have been conducted demonstrating that improvement in vitamin D status reduces risk for upper respiratory tract viral infections as well as having a wide variety of effects on both innate and acquired immunity[39,65]. A recent randomized controlled double-blind clinical trial assessed the impact of vitamin D supplementation on calcium metabolism and non-calcemic broad gene expression by relating them to the individual’s responsiveness to varying doses of vitamin D3[66]. Thirty healthy adults were randomized to receive 600, 4000 or 10000 IU/d of vitamin D3 for 6 mo. Circulating parathyroid hormone (PTH), 25(OH)D, calcium and peripheral white blood cells broad gene expression were evaluated. The investigators reported dose-dependent increase in circulating 25(OH)D concentrations, decreased PTH concentrations and no change in serum calcium levels. A plateau in circulating PTH levels was achieved at 16 wk in the 4000 and 10000 IU/d groups. There was a dose-dependent 25(OH)D alteration in broad gene expression with 162, 320 and 1289 genes up- or down-regulated in their white blood cells, respectively. Thus, improvement in vitamin D status does have a dramatic effect on immune cell activity. However, can it therefore be expected that everyone who improves their vitamin D status would experience the same genomic influences on their immune system if they raised their blood level of 25(OH)D to the same degree? Carlberg and Haq[67] gave daily 3200 IU of vitamin D3 to 71 prediabetic patients for 5 mo and found robust changes in total gene expression in peripheral blood mononuclear cells only in about half the subjects. Shirvani et al[66] observed in healthy adults who were vitamin D deficient and who received this same dose of vitamin D and raised their blood concentrations of 25(OH)D to the same degree, marked differences in the level of expression of the same genes. They reported that 60% of the healthy vitamin D deficient adults who received 10000 IU daily for 6 mo had a robust response in gene expression compared to the other 40% who had minimum to modest responses even though these subjects raised their blood concentrations of 25(OH)D in the same range of 60-90 ng/mL (150-225 nmol/L).
With all of this compelling information, it is reasonable for all responsible Public Health Authorities to consider advising their populations to enhance their immune system by improving their vitamin D status by encouraging sensible sun exposure and by taking vitamin D supplements (if not already on adequate supplementation or medically prohibited due to a vitamin D hypersensitivity disorder) at the doses which, as proposed by the Endocrine Society Guideline Committee in 2011, do not require previous laboratory testing nor medical supervision. To prevent nutritional rickets, daily doses of 400-1000 IU in infants, 600-1000 in children and 1500-2000 in teenagers (should be treated as adults) and adults, are needed. However, to achieve higher circulating concentrations of 25(OH)D at the range of 100-150 nmol/L (40-60 ng/ml), appearing according to our analysis to be necessary for substantially improving immune function and protect from COVID-19 disease, without any risk of toxicity[68], higher doses can be used. As mentioned above, the Endocrine Society Practice Guidelines recommends the safe upper limit for infants < 1 year is 2000 IU daily, children 1-18 years 4000 and adults (including elderly and adult pregnant-lactating women) 10000 IU, unless they are obese, requiring two to three times more. Thus, after a necessary initial repletion for up to 2 mo with these upper tolerable doses, the Endocrine Society’s Committee’s maintenance proposed doses, which can be safely given without medical supervision to prevent vitamin D deficiency and are practically identical with the IOM’s upper tolerable limits, can be continued: i.e. up to 1000 IU/d for infants aged < 6 m, 1500 for age 6 m - 1 year, 2500 for 1-3 years, 3000 for children 4-8 years and 4000 for children > 8 years, with adults, pregnant/lactating women and adolescents requiring a daily intake of 4000-5000 (8000-10000 if obese) to maintain circulating concentrations of 25(OH)D at the range of 100-150 nmol/L. For teenagers and adults on a weekly scheme, these doses translate to about 50000 or if obese 100,000 IU, this being equivalent to approximately 6000 IU daily and 12000 IU for obese, respectively.
These doses will achieve blood concentrations of 25(OH)D of at least 75 nmol/L (30 ng/mL) aiming at the preferred range of 100-150 nmol/L (40-60 ng/mL), without any risk of toxicity[68]. It has been estimated that once a blood concentration of 25(OH)D reaches 50 nmol/L (20 ng/mL) that for every 100 IU ingested, the blood concentration increases by approximately 0.6-1 ng/mL. A good example of this dosing was reported by Shirvani et al[66] who demonstrated that circulating concentrations of 25(OH)D were maintained in the range of 24.3 ± 4.1, 40.8 ± 3.8 and 78.6 ± 13.5 ng/mL, in vitamin D deficient adults who ingested 600, 4000 and 10000 IU daily for 6 mo. These data are supported by a population based Canadian study demonstrating that some adults taking up to 20000 IU daily for more than a year maintained a blood concentration of 25(OH)D in the range of 60-80 ng/mL without any evidence of toxicity[69]. This study also nicely demonstrated the effect of BMI on vitamin D status. The authors observed that those who had a BMI > 30 kg/m2 needed to ingest 2.5 times more vitamin D to maintain the same blood level as a normal weight adult.
Achieving circulating concentrations of 25(OH)D in the range of 100-150 nmol/L (40-60 ng/mL) appears to optimize vitamin D’s effect on improving immune function, thereby substantially reducing the risk for serious-critical infections, particularly from SARS-CoV-2 according to our study, and possibly modulating the immune response, helping to prevent the dangerous cytokine storm often leading to COVID-19 related deaths. The COVID-19 pandemic is an unprecedented medical emergency for the modern world, and we may not possess the luxury, the time nor even the ethical argument to wait the definite results on RCTs while people are dying[70], while prospective well designed studies are needed to conclude on the impact of the vitamin D status on COVID-19 morbidity and mortality[71]. These trials are hopefully awaited, but before a medical emergency of this magnitude we need to remember that Evidence Based Medicine is not necessarily synonymous to RCTs. We do know that vitamin D enhances immune function. We know the extent of vitamin D deficiency, and we know that restrictions and lockdowns have probably worsened the populations’ vitamin D status. Thus, until then, decisions are taken based on and adapted to the best available evidence. And, as far as vitamin D, the evidence is there[51], justifying even the use of vitamin D as a possible adjuvant therapy for COVID-19 disease[72]. A preponderance of evidence does suggest that vitamin D deficiency increases mortality. Our findings predict a striking reduction of serious-critical illness and deaths from COVID-19 if 25(OH)D concentrations reach 100-150 nmol/L (40-60 ng/ml), and very recently SARS-CoV-2 positivity was found to be strongly and inversely associated with circulating 25(OH)D concentrations irrespective of latitudes, races/ethnicities, both sexes and age ranges[73]. Slovakia, at five deaths/M, having the lowest mortality rate in Europe from COVID-19 disease at the time of our analysis, a 125-fold lower than in the UK where official advice remains that 25(OH)D deficiency is < 25 nmol/L (https://www.nice.org.uk/advice/es28/evidence/evidence-review-pdf-8777674477), is a characteristic paradigm, being practically the only country in Europe with a 25(OH)D status meeting the Endocrine Society’s recommended level of sufficiency > 75 nmol/L (30 ng/mL).
From a public health perspective, given the established safety of even high doses, and the potential benefits in enhancing innate and adaptive immunity[74], mitigating also the inflammatory response[3], the recommendation of intensive supplementation with vitamin D as possible prophylaxis with safe doses that do not require prior measurement or medical supervision, must be seriously considered, especially now that the world is facing the third deadly wave of this pandemic, forcing populations into repeated new lockdowns without the broad availability of specific medications yet and while awaiting for vaccinations to be widely available and plausible.
There is no need to require a measurement of serum 25(OH)D before recom
There is essentially no vitamin D naturally occurring in the diet apart from oily fish, cod liver oil and sun-dried mushrooms. The modern way of life deprives us from sun exposure together with the warning to avoid all direct sun exposure by the national and international Dermatology Societies contributing to the worldwide vitamin D deficiency pandemic: Approximately 40% of the world’s population is vitamin D deficient, i.e. 25(OH)D < 50 nmol/L (20 ng/mL) and 60% or insufficient i.e. 50-79 nmol/L (20-29 ng/mL). Therefore, we also need to consider worldwide recommendations for vitamin D food fortification that is practiced in several countries including the United States, Canada, and Finland to name a few. Most other countries either do not encourage or forbid food fortification with vitamin D. Recently, in 2017, India implemented fortification of milk and cooking oil with vitamin D2 as a means of reducing vitamin D deficiency that is common in both children and adults in this sunny Asian subcontinent.
Vitamin D is safe, not toxic and inexpensive. In the “shade” of the modern way of life, the human body cannot produce enough vitamin D from sun exposure, as our hunter gatherer forefathers did and as Maasai herders and the Hazda continue to do. Vitamin D may improve and modulate immune response against SARS-CoV-2. With all the above data, the limitations and the perspectives discussed, the possible benefit in the fight against SARS-CoV-2 should the protection against COVID-19 serious-critical illnesses and death with vitamin D prove truthful, and this without any risk of toxicity, the gain for humanity as well global public health might be just invaluable.
Recent studies have claimed lower coronavirus disease 2019 (COVID-19) cases in European countries with a better vitamin D status and a significant association between vitamin D sufficiency and reduction in clinical severity and inpatient mortality from COVID-19 disease. Low serum 25(OH)D was identified as an independent risk factor for COVID-19 infection and hospitalization, and administration of calcifediol or 25(OH)D significantly reduced the need for intensive care unit treatment.
Vitamin D population status may indeed have possible unappreciated consequences to the COVID-19 pandemic, a hypothesis that needed to be further elucidated.
Following an ecological integrative approach, we examined the associations between published representative and standardized European population vitamin D data and the Worldometer COVID-19 data at two completely different time points of the first wave of this pandemic. If any sustained correlations were to be found, they would be an indication of a truthful association, even though they could not prove causality.
Using linear regression, we explored the correlation between published representative and standardized population vitamin D concentrations and the number of total cases/million (M), recovered/M, deaths/M and serious-critically ill/M from COVID-19 for 26 European countries populated > 4 M. Life expectancy (LE) was also analyzed with semi-parametric regression. Weighted analysis of variance/analysis of covariance evaluated serious-critical/M and deaths/M by the vitamin D population status: deficient < 50, insufficient: 50-62.5, mildly insufficient > 62.5-75 and sufficient > 75 nmol/L, while controlling for LE for deaths/M. Statistical analyses were performed in XLSTAT LIFE SCIENCE and R (SemiPar library).
No correlation was found between population vitamin D concentrations and the total cases-recovered/M, but negative correlations were depicted predicting a reduction of 47%-64%-80% in serious-critical illnesses/M and of 61%-82%-102.4% in deaths/M, further enhanced when adapting for LE by 133%-177%-221% if 25(OH)D concentrations reach 100-125-150 nmol/L. Weighted analysis of variance evaluated serious-critical/M (r2 = 0.22) by the vitamin-D population status and analysis of covariance the deaths/M (r2= 0.629) while controlling for LE (r2 = 0.47). Serious-critical showed a decreasing trend (P < 0.001) from population status deficient (P < 0.001) to insufficient by 9.2% (P < 0.001), to mildly insufficient by 47.6% (P = 0.044) and to sufficient by 100% (reference, P < 0.001). For deaths/M the respective decreasing trend (P < 0.001) was 62.9% from deficient to insufficient (P < 0.001), 65.15% to mildly insufficient (P < 0.001) and 78.8% to sufficient (P = 0.041).
A higher 25(OH)D concentration may protect from serious-critical illness and death from COVID-19 disease - even more in the elderly - but does not seem to prevent severe acute respiratory syndrome coronavirus 2 from spreading.
Considering the ongoing pandemic situation, the presented results are useful for public health systems to advise their populations to enhance their immune system by improving their vitamin D status. Specifically, achieving a serum 25(OH)D concentration of 100-150 nmol/L (40-60 ng/mL) with vitamin D2/D3 supplementation using the upper tolerable daily doses for up to 2 mo (infants < 1 year 2000 IU daily, children 1-18 years 4000 and adults including elderly and adult pregnant-lactating women 10000 IU, unless they are obese requiring 2-3 times more) followed by the maintenance proposed doses not requiring medical supervision, as proposed by the Endocrine Society and being practically identical with the Institute of Medicine’s upper tolerable limits (up to 1000 IU/d for infants aged < 6 mo, 1500 for age 6 mo - 1 year, 2500 for 1-3 years, 3000 for children 4-8 years and 4000 IU for children > 8 years, with adults, pregnant-lactating women and adolescents requiring a daily intake of 4000-5000 unless they are obese requiring two to three times more) may protect from serious-critical illness and death from COVID-19 disease.
We thank the experts in biostatistics Alexandros Gryparis and Arash Shirvani for guiding us in performing the statistical analysis.
Manuscript source: Unsolicited manuscript
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jahromi R S-Editor: Liu M L-Editor: Filipodia P-Editor: Xing YX
| 1. | Hilger J, Friedel A, Herr R, Rausch T, Roos F, Wahl DA, Pierroz DD, Weber P, Hoffmann K. A systematic review of vitamin D status in populations worldwide. Br J Nutr. 2014;111:23-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 487] [Cited by in RCA: 535] [Article Influence: 44.6] [Reference Citation Analysis (0)] |
| 2. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14751] [Article Influence: 2950.2] [Reference Citation Analysis (0)] |
| 3. | Somasundaram NP, Ranathunga I, Ratnasamy V, Wijewickrama PSA, Dissanayake HA, Yogendranathan N, Gamage KKK, de Silva NL, Sumanatilleke M, Katulanda P, Grossman AB. The Impact of SARS-Cov-2 Virus Infection on the Endocrine System. J Endocr Soc. 2020;4:bvaa082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 49] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 4. | Bolland MJ, Grey A, Avenell A. Effects of vitamin D supplementation on musculoskeletal health: a systematic review, meta-analysis, and trial sequential analysis. Lancet Diabetes Endocrinol. 2018;6:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 278] [Article Influence: 39.7] [Reference Citation Analysis (0)] |
| 5. | Sempos CT, Heijboer AC, Bikle DD, Bollerslev J, Bouillon R, Brannon PM, DeLuca HF, Jones G, Munns CF, Bilezikian JP, Giustina A, Binkley N. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br J Clin Pharmacol. 2018;84:2194-2207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 213] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 6. | Nowson CA, McGrath JJ, Ebeling PR, Haikerwal A, Daly RM, Sanders KM, Seibel MJ, Mason RS; Working Group of Australian and New Zealand Bone and Mineral Society; Endocrine Society of Australia and Osteoporosis Australia. Vitamin D and health in adults in Australia and New Zealand: a position statement. Med J Aust. 2012;196:686-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 238] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 7. | Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;CD000227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 8. | Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 9. | Cesareo R, Iozzino M, D'onofrio L, Terrinoni I, Maddaloni E, Casini A, Campagna G, Santonati A, Palermo A. Effectiveness and safety of calcium and vitamin D treatment for postmenopausal osteoporosis. Minerva Endocrinol. 2015;40:231-237. [PubMed] |
| 10. | Garland CF, Kim JJ, Mohr SB, Gorham ED, Grant WB, Giovannucci EL, Baggerly L, Hofflich H, Ramsdell JW, Zeng K, Heaney RP. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. Am J Public Health. 2014;104:e43-e50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 143] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Manson JE, Cook NR, Lee IM, Christen W, Bassuk SS, Mora S, Gibson H, Gordon D, Copeland T, D'Agostino D, Friedenberg G, Ridge C, Bubes V, Giovannucci EL, Willett WC, Buring JE; VITAL Research Group. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N Engl J Med. 2019;380:33-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1127] [Cited by in RCA: 1133] [Article Influence: 188.8] [Reference Citation Analysis (0)] |
| 12. | Vieth R, Holick MF. The IOM—Endocrine Society Controversy on Recommended Vitamin D Targets. In: Vitamin D. 4th ed. Feldman D, editor. Academic Press, 2018: 1091-1107. [DOI] [Full Text] |
| 13. | Zhang Y, Fang F, Tang J, Jia L, Feng Y, Xu P, Faramand A. Association between vitamin D supplementation and mortality: systematic review and meta-analysis. BMJ. 2019;366:l4673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 247] [Cited by in RCA: 216] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 14. | Papadimitriou DT. The Big Vitamin D Mistake. J Prev Med Public Health. 2017;50:278-281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Mead MN. Benefits of sunlight: a bright spot for human health. Environ Health Perspect. 2008;116:A160-A167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Kara M, Ekiz T, Ricci V, Kara Ö, Chang KV, Özçakar L. 'Scientific Strabismus' or two related pandemics: coronavirus disease and vitamin D deficiency. Br J Nutr. 2020;124:736-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 17. | Rhodes JM, Subramanian S, Laird E, Kenny RA. Editorial: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;51:1434-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 31.4] [Reference Citation Analysis (0)] |
| 18. | Alipio MM. Do latitude and ozone concentration predict Covid-2019 cases in 34 countries? medRxiv. 2020 Preprint. [DOI] [Full Text] |
| 19. | Grant WB, Giovannucci E. The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918-1919 influenza pandemic in the United States. Dermatoendocrinol. 2009;1:215-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Maghbooli Z, Sahraian MA, Ebrahimi M, Pazoki M, Kafan S, Tabriz HM, Hadadi A, Montazeri M, Nasiri M, Shirvani A, Holick MF. Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One. 2020;15:e0239799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 187] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 21. | Lips P, Cashman KD, Lamberg-Allardt C, Bischoff-Ferrari HA, Obermayer-Pietsch B, Bianchi ML, Stepan J, El-Hajj Fuleihan G, Bouillon R. Current vitamin D status in European and Middle East countries and strategies to prevent vitamin D deficiency: a position statement of the European Calcified Tissue Society. Eur J Endocrinol. 2019;180:P23-P54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 426] [Article Influence: 71.0] [Reference Citation Analysis (0)] |
| 22. | Manios Y, Moschonis G, Lambrinou CP, Tsoutsoulopoulou K, Binou P, Karachaliou A, Breidenassel C, Gonzalez-Gross M, Kiely M, Cashman KD. A systematic review of vitamin D status in southern European countries. Eur J Nutr. 2018;57:2001-2036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 90] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 23. | Vidovic N, Faid F, Pantovic A, Nikolic M, Debeljak-Martacic J, Zekovic M, Milesevic J, Drah MM, Zec M. Vitamin D and cardio-metabolic biomarkers: small-scale comparative study between Libyan migrants and resident women in Serbia. Libyan J Med. 2019;14:1622364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Vojdeman FJ, Madsen CM, Frederiksen K, Durup D, Olsen A, Hansen L, Heegaard AM, Lind B, Tjønneland A, Jørgensen HL, Schwarz P. Vitamin D levels and cancer incidence in 217,244 individuals from primary health care in Denmark. Int J Cancer. 2019;145:338-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 25. | Bettencourt A, Boleixa D, Reis J, Oliveira JC, Mendonça D, Costa PP, Silva BMD, Marinho A, Silva AMD. Serum 25-hydroxyvitamin D levels in a healthy population from the North of Portugal. J Steroid Biochem Mol Biol. 2018;175:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 26. | Borissova AM, Shinkov A, Vlahov J, Dakovska L, Todorov T, Svinarov D, Kassabova L. Vitamin D status in Bulgaria--winter data. Arch Osteoporos. 2013;8:133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 27. | Elmadfa I, Meyer AL, Wottawa D, Wagner K, Hasenegger V. Vitamin D intake and status in Austria and its effects on some health indicators. Austin J Nutr Metab. 2017;4:1050. |
| 28. | Cashman KD, Dowling KG, Škrabáková Z, Gonzalez-Gross M, Valtueña J, De Henauw S, Moreno L, Damsgaard CT, Michaelsen KF, Mølgaard C, Jorde R, Grimnes G, Moschonis G, Mavrogianni C, Manios Y, Thamm M, Mensink GB, Rabenberg M, Busch MA, Cox L, Meadows S, Goldberg G, Prentice A, Dekker JM, Nijpels G, Pilz S, Swart KM, van Schoor NM, Lips P, Eiriksdottir G, Gudnason V, Cotch MF, Koskinen S, Lamberg-Allardt C, Durazo-Arvizu RA, Sempos CT, Kiely M. Vitamin D deficiency in Europe: pandemic? Am J Clin Nutr. 2016;103:1033-1044. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 874] [Cited by in RCA: 900] [Article Influence: 100.0] [Reference Citation Analysis (0)] |
| 29. | Ilie PC, Stefanescu S, Smith L. The role of vitamin D in the prevention of coronavirus disease 2019 infection and mortality. Aging Clin Exp Res. 2020;32:1195-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 434] [Cited by in RCA: 467] [Article Influence: 93.4] [Reference Citation Analysis (0)] |
| 30. | Tramontana F, Napoli N, El-Hajj Fuleihan G, Strollo R. The D-side of COVID-19: musculoskeletal benefits of vitamin D and beyond. Endocrine. 2020;69:237-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 31. | PLOS ONE Editors. Expression of Concern: Vitamin D sufficiency, a serum 25-hydroxyvitamin D at least 30 ng/mL reduced risk for adverse clinical outcomes in patients with COVID-19 infection. PLoS One. 2020;15:e0240965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Darbeheshti F, Rezaei N. Genetic predisposition models to COVID-19 infection. Med Hypotheses. 2020;142:109818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 33. | Pairo-Castineira E, Clohisey S, Klaric L, Bretherick AD, Rawlik K, Pasko D, Walker S, Parkinson N, Fourman MH, Russell CD, Furniss J, Richmond A, Gountouna E, Wrobel N, Harrison D, Wang B, Wu Y, Meynert A, Griffiths F, Oosthuyzen W, Kousathanas A, Moutsianas L, Yang Z, Zhai R, Zheng C, Grimes G, Beale R, Millar J, Shih B, Keating S, Zechner M, Haley C, Porteous DJ, Hayward C, Yang J, Knight J, Summers C, Shankar-Hari M, Klenerman P, Turtle L, Ho A, Moore SC, Hinds C, Horby P, Nichol A, Maslove D, Ling L, McAuley D, Montgomery H, Walsh T, Pereira A, Renieri A, Openshaw PJM, Ponting C, Meikle J, Finernan P, McMaster E, Law A, Baillie JK, Paterson T, Wackett T, Armstrong R, Clark R, Coutts A, Donnelly L, Gilchrist T, Hafezi K, Macgillivray L, Maclean A, McCafferty S, Morrice K, Weaver J, Boz C, Golightly A, Ward M, Mal H, Szoor-McElhinney H, Brown A, Hendry R, Stenhouse A, Cullum L, Law D, Law S, Law R, Swets M, Day N, Taneski F, Duncan E, Collier D, Wood S, Zak A, Borra C, Matharu M, May P, Alldis Z, Mitchelmore O, Bowles R, Easthorpe A, Bibi F, Lancoma-Malcolm I, Gurasashvili J, Pheby J, Shiel J, Bolton M, Patel M, Taylor M, Zongo O, Ebano P, Harding P, Astin-Chamberlain R, Choudhury Y, Cox A, Kallon D, Burton M, Hall R, Blowes S, Prime Z, Biddle J, Prysyazhna O, Newman T, Tierney C, Kassam J, Ostermann M, Campos S, Bociek A, Lim R, Grau N, Jones TO, Whitton C, Marotti M, Arbane G, Bonner S, Hugill K, Reid J, Welters I, Waugh V, Williams K, Shaw D, Roman JF, Martinez ML, Johnson E, Waite A, Johnson B, Hamilton O, Mulla S, McPhail M, Smith J, Barclay L, Hope D, McCulloch C, McQuillan L, Clark S, Singleton J, Priestley K, Rea N, Callaghan M, Campbell R, Andrew G, Marshall L, McKechnie S, Hutton P, Bashyal A, Davidson N, Polgarova P, Stroud K, Pathan N, Elston K, Agrawal S, Battle C, Newey L, Rees T, Harford R, Brinkworth E, Williams M, Murphy C, White I, Croft M, Bandla N, Gellamucho M, Tomlinson J, Turner H, Davies M, Quinn A, Hussain I, Thompson C, Parker H, Bradley R, Griffiths R, Scriven J, Gill J, Puxty A, Cathcart S, Salutous D, Turner L, Duffy K, Puxty K, Joseph A, Herdman-Grant R, Simms R, Swain A, Naranjo A, Crowe R, Sollesta K, Loveridge A, Baptista D, Morino E, Davey M, Golden D, Jones J, Cuesta JM, Haldeos A, Bakthavatsalam D, Vincent R, Elhassan M, Xavier K, Ganesan A, Abdelrazik DPM, Morgan J, Akeroyd L, Bano S, Warren D, Bromley M, Sellick K, Gurr L, Wilkinson B, Nagarajan V, Szedlak P, Cupitt J, Stoddard E, Benham L, Preston S, Slawson N, Bradshaw Z, brown J, Caswell M, Melling S, Bamford P, Faulkner M, Cawley K, Jeffrey H, London E, Sainsbury H, Nagra I, Nasir F, Dunmore C, Jones R, Abraheem A, Al-Moasseb M, Girach R, Brantwood C, Alexander P, Bradley-Potts J, Allen S, Felton T, Manna S, Farnell-Ward S, Leaver S, Queiroz J, Maccacari E, Dawson D, Delgado CC, Saluzzio RP, Ezeobu O, Ding L, Sicat C, Kanu R, The Gen OI, Gen OC, Gen Oc-i, Central m, laboratory t, Data analysis t, Barts Health Nhs Trust LUK, Guys, St Thomas’ Hospital LUK, James Cook University Hospital MUK, The Royal Liverpool University Hospital LUK, King’s College Hospital LUK, Royal Infirmary of Edinburgh EUK, John Radcliffe Hospital OUK, Addenbrooke’s Hospital CUK, Morriston Hospital SUK, Ashford, St Peter’s Hospital SUK, Royal Stoke University Hospital SUK, Queen Elizabeth Hospital BUK, Glasgow Royal Infirmary GUK, Kingston Hospital SUK, The Tunbridge Wells H, Maidstone Hospital KUK, North Middlesex University Hospital Nhs Trust LUK, Bradford Royal Infirmary BUK, Blackpool Victoria Hospital BUK, Countess of Chester Hospital CUK, Wythenshawe Hospital MUK, St George’s Hospital LUK. Nature. 2021;92-98 Genetic mechanisms of critical illness in Covid-19.. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1045] [Cited by in RCA: 931] [Article Influence: 232.8] [Reference Citation Analysis (0)] |
| 34. | Devaux CA, Rolain JM, Raoult D. ACE2 receptor polymorphism: Susceptibility to SARS-CoV-2, hypertension, multi-organ failure, and COVID-19 disease outcome. J Microbiol Immunol Infect. 2020;53:425-435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 361] [Cited by in RCA: 345] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 35. | Egro FM. Why is type 1 diabetes increasing? J Mol Endocrinol. 2013;51:R1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 36. | van der Wielen RP, Löwik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 529] [Cited by in RCA: 485] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 37. | de Groot LC, Verheijden MW, de Henauw S, Schroll M, van Staveren WA; SENECA Investigators. Lifestyle, nutritional status, health, and mortality in elderly people across Europe: a review of the longitudinal results of the SENECA study. J Gerontol A Biol Sci Med Sci. 2004;59:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 111] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 38. | Meehan M, Penckofer S. The Role of Vitamin D in the Aging Adult. J Aging Gerontol. 2014;2:60-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 39. | Martens PJ, Gysemans C, Verstuyf A, Mathieu AC. Vitamin D's Effect on Immune Function. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 40. | Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43:718-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 41. | D'Avolio A, Avataneo V, Manca A, Cusato J, De Nicolò A, Lucchini R, Keller F, Cantù M. 25-Hydroxyvitamin D Concentrations Are Lower in Patients with Positive PCR for SARS-CoV-2. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 279] [Article Influence: 55.8] [Reference Citation Analysis (0)] |
| 42. | McCartney DM, Byrne DG. Optimisation of Vitamin D Status for Enhanced Immuno-protection Against Covid-19. Ir Med J. 2020;113:58. [PubMed] |
| 43. | Caccialanza R, Laviano A, Lobascio F, Montagna E, Bruno R, Ludovisi S, Corsico AG, Di Sabatino A, Belliato M, Calvi M, Iacona I, Grugnetti G, Bonadeo E, Muzzi A, Cereda E. Early nutritional supplementation in non-critically ill patients hospitalized for the 2019 novel coronavirus disease (COVID-19): Rationale and feasibility of a shared pragmatic protocol. Nutrition. 2020;74:110835. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 162] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 44. | Ginde AA, Brower RG, Caterino JM, Finck L, Banner-Goodspeed VM, Grissom CK, Hayden D, Hough CL, Hyzy RC, Khan A, Levitt JE, Park PK, Ringwood N, Rivers EP, Self WH, Shapiro NI, Thompson BT, Yealy DM, Talmor D, National Heart, Lung, and Blood Institute PETAL Clinical Trials Network. Early High-Dose Vitamin D3 for Critically Ill, Vitamin D-Deficient Patients. N Engl J Med. 2019;381:2529-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 196] [Article Influence: 32.7] [Reference Citation Analysis (0)] |
| 45. | Telcian AG, Zdrenghea MT, Edwards MR, Laza-Stanca V, Mallia P, Johnston SL, Stanciu LA. Vitamin D increases the antiviral activity of bronchial epithelial cells in vitro. Antiviral Res. 2017;137:93-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 115] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 46. | Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, Esposito S, Ganmaa D, Ginde AA, Goodall EC, Grant CC, Griffiths CJ, Janssens W, Laaksi I, Manaseki-Holland S, Mauger D, Murdoch DR, Neale R, Rees JR, Simpson S Jr, Stelmach I, Kumar GT, Urashima M, Camargo CA Jr. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1094] [Cited by in RCA: 1207] [Article Influence: 150.9] [Reference Citation Analysis (0)] |
| 47. | Madden K, Feldman HA, Chun RF, Smith EM, Sullivan RM, Agan AA, Keisling SM, Panoskaltsis-Mortari A, Randolph AG. Critically Ill Children Have Low Vitamin D-Binding Protein, Influencing Bioavailability of Vitamin D. Ann Am Thorac Soc. 2015;12:1654-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Dancer RC, Parekh D, Lax S, D'Souza V, Zheng S, Bassford CR, Park D, Bartis DG, Mahida R, Turner AM, Sapey E, Wei W, Naidu B, Stewart PM, Fraser WD, Christopher KB, Cooper MS, Gao F, Sansom DM, Martineau AR, Perkins GD, Thickett DR. Vitamin D deficiency contributes directly to the acute respiratory distress syndrome (ARDS). Thorax. 2015;70:617-624. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 49. | Mansur JL. Letter: low population mortality from COVID-19 in countries south of latitude 35 degrees North supports vitamin D as a factor determining severity. Aliment Pharmacol Ther. 2020;52:411-412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Amrein K, Papinutti A, Mathew E, Vila G, Parekh D. Vitamin D and critical illness: what endocrinology can learn from intensive care and vice versa. Endocr Connect. 2018;7:R304-R315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 51. | Grant WB, Lahore H, McDonnell SL, Baggerly CA, French CB, Aliano JL, Bhattoa HP. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1018] [Cited by in RCA: 1094] [Article Influence: 218.8] [Reference Citation Analysis (0)] |
| 52. | Daneshkhah A, Agrawal V, Eshein A, Subramanian H, Roy HK, Backman V. The Role of Vitamin D in Suppressing Cytokine Storm and Associated Mortality in COVID-19 Patients. MedRxiv. 2020 Preprint. [DOI] [Full Text] |
| 53. | Zhou Y, Dong B, Kim KH, Choi S, Sun Z, Wu N, Wu Y, Scott J, Moore DD. Vitamin D Receptor Activation in Liver Macrophages Protects Against Hepatic Endoplasmic Reticulum Stress in Mice. Hepatology. 2020;71:1453-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 54. | Calder PC, Carr AC, Gombart AF, Eggersdorfer M. Optimal Nutritional Status for a Well-Functioning Immune System Is an Important Factor to Protect against Viral Infections. Nutrients. 2020;12:1181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 565] [Cited by in RCA: 491] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 55. | Merzon E, Tworowski D, Gorohovski A, Vinker S, Golan Cohen A, Green I, Frenkel-Morgenstern M. Low plasma 25(OH) vitamin D level is associated with increased risk of COVID-19 infection: an Israeli population-based study. FEBS J. 2020;287:3693-3702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 275] [Article Influence: 55.0] [Reference Citation Analysis (0)] |
| 56. | Entrenas Castillo M, Entrenas Costa LM, Vaquero Barrios JM, Alcalá Díaz JF, López Miranda J, Bouillon R, Quesada Gomez JM. "Effect of calcifediol treatment and best available therapy vs best available therapy on intensive care unit admission and mortality among patients hospitalized for COVID-19: A pilot randomized clinical study". J Steroid Biochem Mol Biol. 2020;203:105751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 493] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 57. | Meltzer DO, Best TJ, Zhang H, Vokes T, Arora V, Solway J. Association of Vitamin D Status and Other Clinical Characteristics With COVID-19 Test Results. JAMA Netw Open. 2020;3:e2019722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 58. | Veugelers PJ, Ekwaru JP. A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients. 2014;6:4472-4475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 59. | Heaney R, Garland C, Baggerly C, French C, Gorham E. Letter to Veugelers, P.J. and Ekwaru, J.P., A statistical error in the estimation of the recommended dietary allowance for vitamin D. Nutrients 2014, 6, 4472-4475; doi:10.3390/nu6104472. Nutrients. 2015;7:1688-1690. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 60. | Manson JE, Brannon PM, Rosen CJ, Taylor CL. Vitamin D Deficiency - Is There Really a Pandemic? N Engl J Med. 2016;375:1817-1820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 199] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 61. | Munns CF, Shaw N, Kiely M, Specker BL, Thacher TD, Ozono K, Michigami T, Tiosano D, Mughal MZ, Mäkitie O, Ramos-Abad L, Ward L, DiMeglio LA, Atapattu N, Cassinelli H, Braegger C, Pettifor JM, Seth A, Idris HW, Bhatia V, Fu J, Goldberg G, Sävendahl L, Khadgawat R, Pludowski P, Maddock J, Hyppönen E, Oduwole A, Frew E, Aguiar M, Tulchinsky T, Butler G, Högler W. Global Consensus Recommendations on Prevention and Management of Nutritional Rickets. J Clin Endocrinol Metab. 2016;101:394-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 584] [Cited by in RCA: 700] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 62. | Wacker M, Holick MF. Vitamin D - effects on skeletal and extraskeletal health and the need for supplementation. Nutrients. 2013;5:111-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 437] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 63. | Napoli N, Strollo R, Sprini D, Maddaloni E, Rini GB, Carmina E. Serum 25-OH Vitamin D in relation to Bone Mineral Density and Bone Turnover. Int J Endocrinol. 2014;2014:487463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Rondanelli M, Miccono A, Lamburghini S, Avanzato I, Riva A, Allegrini P, Faliva MA, Peroni G, Nichetti M, Perna S. Self-Care for Common Colds: The Pivotal Role of Vitamin D, Vitamin C, Zinc, and Echinacea in Three Main Immune Interactive Clusters (Physical Barriers, Innate and Adaptive Immunity) Involved during an Episode of Common Colds-Practical Advice on Dosages and on the Time to Take These Nutrients/Botanicals in order to Prevent or Treat Common Colds. Evid Based Complement Alternat Med. 2018;2018:5813095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 65. | Koivisto O, Hanel A, Carlberg C. Key Vitamin D Target Genes with Functions in the Immune System. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 66. | Shirvani A, Kalajian TA, Song A, Holick MF. Disassociation of Vitamin D's Calcemic Activity and Non-calcemic Genomic Activity and Individual Responsiveness: A Randomized Controlled Double-Blind Clinical Trial. Sci Rep. 2019;9:17685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 84] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 67. | Carlberg C, Haq A. The concept of the personal vitamin D response index. J Steroid Biochem Mol Biol. 2018;175:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 118] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 68. | Vieth R. Vitamin D toxicity, policy, and science. J Bone Miner Res. 2007;22 Suppl 2:V64-V68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 173] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 69. | Ekwaru JP, Zwicker JD, Holick MF, Giovannucci E, Veugelers PJ. The importance of body weight for the dose response relationship of oral vitamin D supplementation and serum 25-hydroxyvitamin D in healthy volunteers. PLoS One. 2014;9:e111265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 176] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 70. | National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Board on Health Sciences Policy; Board on Global Health; Committee on Clinical Trials During the 2014-2015 Ebola Outbreak; Busta ER; Mancher M, Cuff PA, et al. Integrating Clinical Research into Epidemic Response: The Ebola Experience. Washington (DC): National Academies Press (US); 2017 Jun 26. [PubMed] [DOI] [Full Text] |
| 71. | Braiman M. Latitude Dependence of the COVID-19 Mortality Rate—A Possible Relationship to Vitamin D Deficiency? SSRN. 2020;. [RCA] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 72. | Jakovac H. COVID-19 and vitamin D-Is there a link and an opportunity for intervention? Am J Physiol Endocrinol Metab. 2020;318:E589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 73. | Kaufman HW, Niles JK, Kroll MH, Bi C, Holick MF. SARS-CoV-2 positivity rates associated with circulating 25-hydroxyvitamin D levels. PLoS One. 2020;15:e0239252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 292] [Cited by in RCA: 257] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 74. | Bilezikian JP, Bikle D, Hewison M, Lazaretti-Castro M, Formenti AM, Gupta A, Madhavan MV, Nair N, Babalyan V, Hutchings N, Napoli N, Accili D, Binkley N, Landry DW, Giustina A. MECHANISMS IN ENDOCRINOLOGY: Vitamin D and COVID-19. Eur J Endocrinol. 2020;183:R133-R147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 239] [Cited by in RCA: 259] [Article Influence: 51.8] [Reference Citation Analysis (0)] |
| 75. | Demetriou ET, Travison TG, Holick MF. Treatment with 50,000 IU vitamin D₂ every other week and effect on serum 25-hydroxyvitamin D₂, 25-hydroxyvitamin D₃, and total 25-hydroxyvitamin D in a clinical setting. Endocr Pract. 2012;18:399-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |