Peer-review started: December 18, 2020
First decision: January 7, 2021
Revised: January 16, 2021
Accepted: February 19, 2021
Article in press: February 19, 2021
Published online: March 25, 2021
Processing time: 88 Days and 2.7 Hours
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which causes coronavirus disease 2019 (COVID-19), has become a historic pandemic, and dealing with it is one of the most important aspects of infectious disease treatment today. SARS-CoV-2 has been found to have characteristic and powerful infectivity (ability to propagate) and lethality (severity). With influenza, primary influenza pneumonia from the virus itself is known to exist in addition to secondary bacterial pneumonia. With COVID-19, on the other hand, it is important to provide diagnosis and treatment while keeping acute respiratory distress syndrome and pulmonary edema (alveolar flood) from a similar cytokine storm, as well as severe angiopathy, in mind. The importance of complying with hand hygiene and masks in infection control remains the same as in previous general infection control measures and responses to influenza virus infections and others, but in the future, vaccination will likely be the key to infection control in the community.
Core Tip: We are focusing the differences and similarity of coronavirus disease 2019 (COVID-19) and Influenza, and review the characteristic pathophysiology and basic concepts of treatment and prevention for COVID-19. Primary influenza pneumonia is known to exist in addition to secondary bacterial pneumonia, however, pulmonary edema (alveolar flood) from a similar cytokine storm, as well as severe angiopathy should be considered in COVID-19.
- Citation: Seki M. Trends in the management of infectious disease under SARS-CoV-2 era: From pathophysiological comparison of COVID-19 and influenza. World J Virol 2021; 10(2): 62-68
- URL: https://www.wjgnet.com/2220-3249/full/v10/i2/62.htm
- DOI: https://dx.doi.org/10.5501/wjv.v10.i2.62
Coronavirus disease 2019 (COVID-19), which continues to spread around the world, has become the main focus of infectious disease treatment since the 2020 season. We have previously experienced acute epidemic viral infections, with influenza being typical, but a pandemic of this size has not been seen since the “Spanish flu” of 1918[1]. These two virus look similar, but we have found the critical differences between influenza and COVID-19. In this review, the epidemiological and clinical characteristics of COVID-19, compared with influenza, in addition to the trend of treatment and prevention, including anti-viral agents and vaccines, could be described.
The virulence of viral infections is defined mainly by infectivity (ability to propagate) and lethality (severity). Coronavirus infections experienced in recent years include severe acute respiratory syndrome (SARS), which spread globally from Guangdong Province in China in 2002, and Middle East respiratory syndrome, which spread in the Middle East in 2012. Although both SARS and Middle East respiratory syndrome showed a moderate to high level of lethality, their ability to propagate was not as strong, and they came mostly to an end while being fairly limited in duration and geography[2].
Compared with these two serious coronavirus infections, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has somewhat stronger infectivity, but not as high a level of lethality/severity. Initially, COVID-19 was viewed as a viral infection similar to seasonal influenza, but it soon became empirically clear that COVID-19 was much more serious.
While its infectivity on average is about the same as that for influenza, it has been found that under the condition of the “three Cs”-crowded places, close-contact settings, and confined and enclosed spaces-a “cluster” is generated with nearly all people present becoming infected at a speed comparable to that of the measles[3]. This situation may be affected by the fact that there is a subtle mechanism somewhat like the human immunodeficiency viruses in which propagation occurs while evading attack by the human immune system, during which time, people are asymptomatic for about a week after being infected.
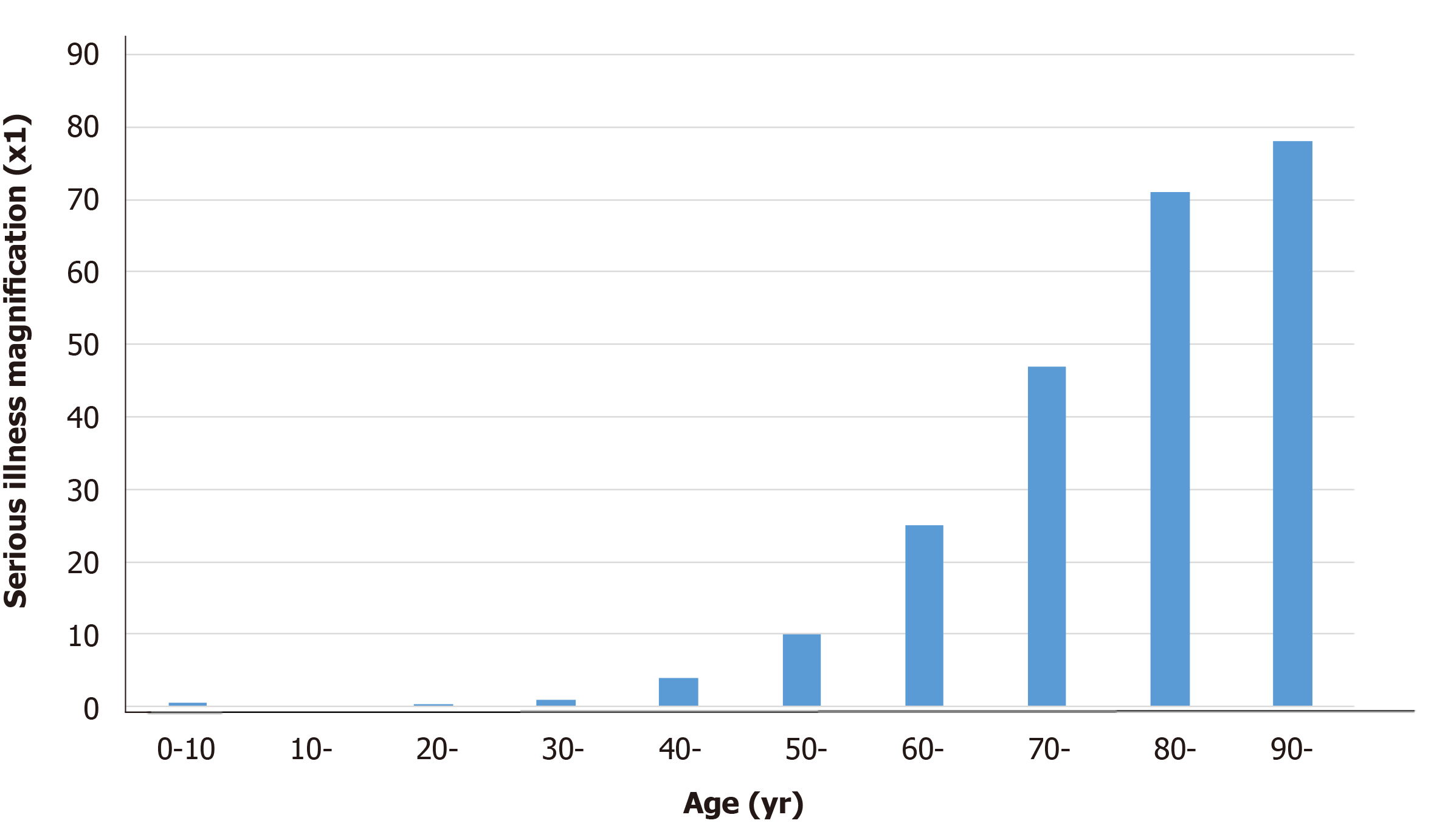
Although the overall fatality rate in Japan is very low, it is still much higher than that for influenza, and there have been many reports of increased severity in the aged; therefore, we cannot let our guard down. While COVID-19 is relatively mild in most young people, chest computed tomography scans have shown pneumonia presenting with characteristic bilateral ground-glass opacity, even in nearly asymptomatic patients. On the other hand, among older people, especially those with preexisting conditions, COVID-19 can become serious and potentially fatal at a very high rate[2]. Consequently, age is one of the most important factors in determining the prognosis of patients infected with SARS-CoV-2 (Figure 1). Therefore, COVID-19 may be viewed as a formidable infectious disease with two distinct manifestations.
Clinically, influenza and COVID-19 are both respiratory illnesses caused by viral infections, but show different symptoms and signs depending on the characteristics of influenza virus and SARS-CoV-2 (Table 1). Compared with influenza, COVID-19 seems to spread more easily and cause a more severe condition. Influenza and COVID-19 share many common signs and symptoms, but the loss of smell and taste is considered specific to COVID-19; therefore, diagnostic testing may be critical to confirm a diagnosis.
| Characteristics | Clinical differences |
| Signs and symptoms | Influenza: Mild to severe illness, including common signs and symptoms. COVID-19: More serious illnesses in some people. Change or loss of taste or smell may be included |
| Incubation period | Flu: 1-4 d after infection. COVID-19: 5 d, but symptoms can appear as early as 2 d or as late as 14 d after infection |
| Duration of the symptoms | Flu: 3-7 d. COVID-19: 2-3 wk |
| Asymptomatic patients | Flu: 10%. COVID-19: A few 60% |
In addition, it is well known that complications from pneumonia in influenza, particularly a high rate of secondary bacterial pneumonia, are a common mechanism in terms of disease severity[4]. However, pulmonary edema-like primary viral pneumonia, which does not occur at a high rate in influenza, has been found in nearly all cases of COVID-19[2,5].
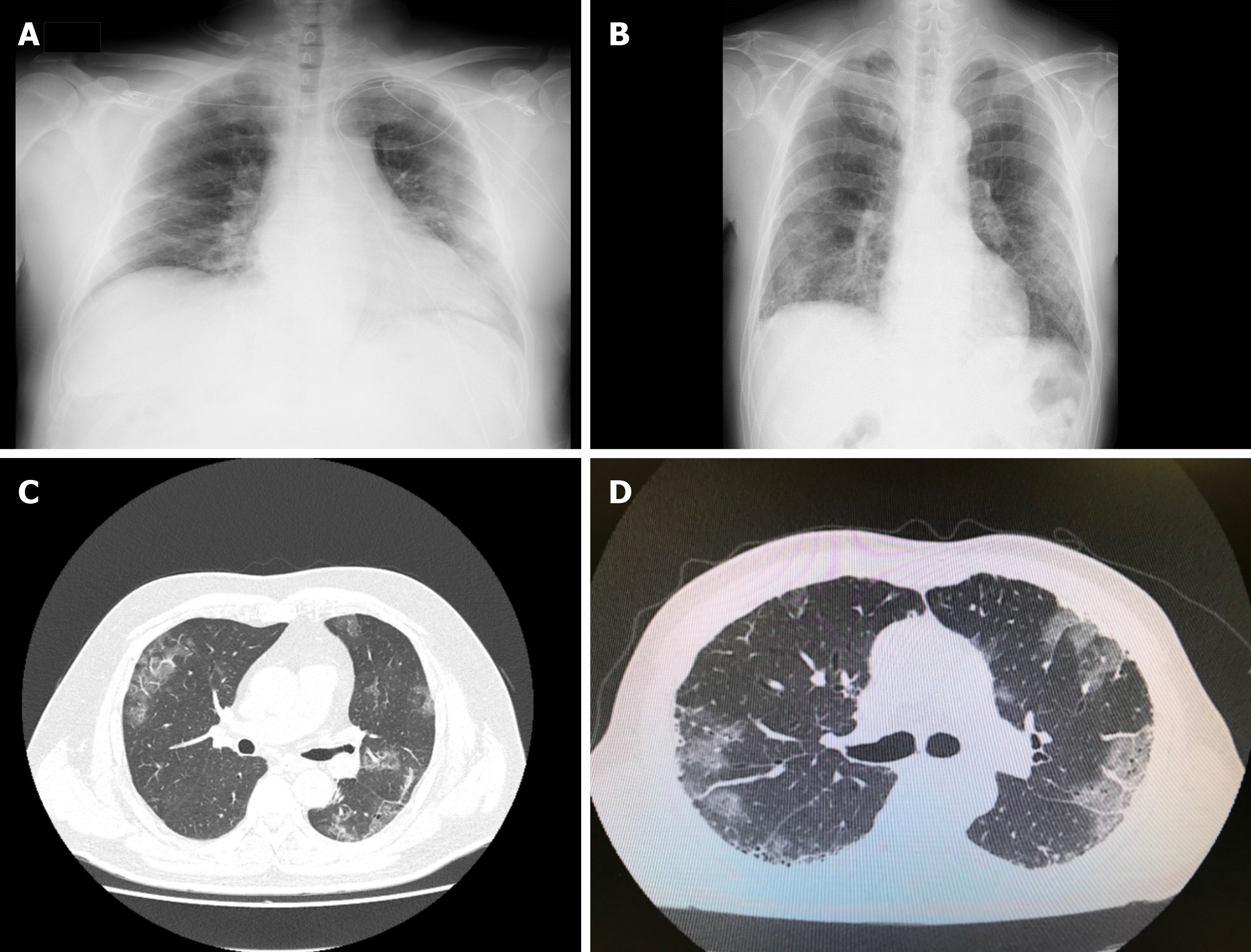
Therefore, with influenza, strong inflammation is induced under relatively rare conditions in which an excessive immune response called a “cytokine storm” may occur, even with infection by the influenza virus alone. It has been shown that pulmonary edema (alveolar flooding) may occur from pneumonia and the breakdown of alveolar epithelial and pulmonary vascular endothelial cells[6]. By contrast, SARS-CoV-2 uses the angiotensin-converting enzyme 2 distributed in the human vascular endothelium as a receptor, and thus has a strong affinity for vascular endothelial cells in particular, which facilitates vascular permeability and makes angiopathy more likely[7]. In fact, pulmonary edema is thought to be significantly more likely to occur than general pneumonia, and typical findings of chest radiographs in patients with COVID-19 are very similar to cases of victims who drowned in freshwater (Figure 2). An abundance of extravascular fluid is found because of changes in osmotic pressure and the junction between alveolar spaces and plasma membranes.
Therefore, it has been suggested that COVID-19 is essentially pulmonary edema and pulmonary microthrombosis due to cytokine disease from viral infection and subsequent vascular destruction. These conditions progress particularly rapidly in older people with comorbidities, ultimately leading to multi-organ failure[8,9].
For the treatment of influenza, a large number of anti-influenza drugs have been developed that target the virus itself. In recent years, the appearance of baloxavir (trade name: Xofluza) has attracted much attention. Infectious disease treatment is based on elimination of the causative agent, and this has been a very effective anti-influenza strategy[10].
Many drugs are currently being developed for the treatment of COVID-19[11]. As of this writing, remdesivir is the first drug to be used that acts against the virus itself, and has shown good efficacy. Therefore, remdesivir has been approved in insurance systems in Japan, and is currently used to treat many severe cases of COVID-19 that require oxygen management, including with artificial ventilation. We also look forward to the use of other antiviral drugs, such as favipiravir.
Favipiravir (T-705; 6-fluoro-3-hydroxy-2-pyrazinecarboxamide) is an antiviral agent that selectively inhibits the ribonucleic acid (RNA)-dependent RNA polymerase of RNA viruses, and has been stockpiled in Japan for the treatment of severe influenza[12]. Since the catalytic domain of RNA-dependent RNA polymerase is conserved among various types of RNA viruses, this mechanism of action suggests a broader virus spectrum, including SARS-CoV-2. In fact, favipiravir has shown significant efficacy for COVID-19 treatment compared with anti- human immunodeficiency viruses drugs in an open-label study[13]. However, its efficacy depends on the severity of the disease, its effects remain unclear, and its treatment strategy remains controversial.
Moreover, as mentioned above, the effects of cytokine storms and hyperimmunity on the clinical pathology of COVID-19 are confirmed to be greater than those in influenza. Sequences of anti-immune drugs and immunomodulators are effective, and these are characteristically proposed as powerful drug candidates. The recommendation of steroids as being efficacious against infectious disease in severe cases of respiratory failure is a first for acute respiratory infections[14]. We are currently waiting for the establishment of infectious disease treatment as an “antiviral + immunomodulator + anti-thrombul” treatment regimen/bundle, and this may provide clues for new treatments for infectious disease (Table 2).
| Drugs | Pathophysiological characteristics |
| Antiviral drugs | |
| Remdesivir for moderate to very severe patients | |
| Favipiravir for mild to severe patients | |
| Immnomodulators | |
| Corticosteroids for moderarte to very severe patients | |
| Tocilizumab for hospitalized COVID-19 patients | |
| Jak/Stat signaling inhibitors for hospitalized COVID-19 patients | |
| Anticoaglnat drugs | |
| Heparin | |
| DOAC |
To combat viral infections, prevention via vaccines or other measures, or infection control so that infections do not spread, are much more important than treatment. The appearance of an effective new SARS-CoV-2 vaccine that is equal or superior to influenza or pneumococcal vaccines, and practical vaccinations that steadily become a reality in general clinical practice, will be considered a breakthrough. Novel vaccines, including mRNA and viral vector-based types, have been in practical use for COVID-19 prevention. By contrast, inactivated vaccines have been available for influenza prevention (Table 3)[15-17]. COVID-19 vaccines to date have been shown to cause mild side effects in small numbers of individuals after the first or second dose, including pain, redness or swelling at the injection site, fever, and headache; however, vaccination might help prevent people from contracting COVID-19 or experiencing a severe case and developing serious complications. In fact, the novel COVID-19 vaccines have shown an efficacy of around 90%, compared with the standard influenza vaccine, which shows an efficacy of around 60%. The COVID-19 pandemic may be said to have in fact brought about dramatic advances in the treatment and management of infectious diseases.
| Vaccine | Clinical trials |
| Pfizer vaccine (Name: BNT 162b2) | |
| Type: mRNA | |
| Age: ≥ 16 years old | |
| Dose: 30 μg (0.3 mL) twice (21 d interval) | |
| Efficiency (95%CI): 95.0% (90.3-97.6) | |
| Moderna vaccine (Name: mRNA-1273) | |
| Type: mRNA | |
| Age: ≥ 18 years old | |
| Dose: 100 μg (0.5 mL) twice (28 d interval) | |
| Efficiency (95%CI): 94.5% (86.5-97.8) | |
| AstraZeneca vaccine (Name: ChAdOx1) | |
| Type: Virus vector | |
| Age: ≥ 18 years old | |
| Dose: Low doss: 2.2 × 1010 virus particle andStandard dose: 5 × 1010 virus particle, twice (28 d interval) | |
| Efficiency (95%CI): 90.0% (67.4-97.0) |
More than previous anti-influenza measures, hand-washing and masks have been demonstrated to be extremely effective against the spread of respiratory viruses, which are mainly transmitted through droplets. Further responses should be advanced based on standard preventive measures and anti-droplet infection measures that have proven effective for other infectious diseases[18].
The term “social distancing” has become well known in dealing with COVD-19. Social distancing is an intervention that arose from measures to prevent “clusters” when the abovementioned special “3C” conditions are met[3]. If the 3Cs can be avoided, the spread of infections can be minimized in many cases. An age has come in which we can advance infectious disease responses that protect ourselves and our communities with a “new lifestyle” that implements these new techniques and ways of thinking.
In conclusion, we described the differences between influenza and COVID-19. SARS-CoV-2 has been found to have epidemiological and clinical characteristics with the pathophysiological conditions, including cytokine storm and severe angiopathy. Novel anti-viral agents and vaccines might be available soon. The responsibilities of doctors and health-care workers who support community medicine and infectious disease treatment are likely to continue to grow.
Manuscript source: Invited manuscript
Specialty type: Infectious diseases
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Balaban DV S-Editor: Zhang L L-Editor: A P-Editor: Xing YX
| 1. | Uyeki TM, Bernstein HH, Bradley JS, Englund JA, File TM, Fry AM, Gravenstein S, Hayden FG, Harper SA, Hirshon JM, Ison MG, Johnston BL, Knight SL, McGeer A, Riley LE, Wolfe CR, Alexander PE, Pavia AT. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin Infect Dis. 2019;68:895-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 231] [Article Influence: 38.5] [Reference Citation Analysis (0)] |
| 2. | Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, Xiang H, Cheng Z, Xiong Y, Zhao Y, Li Y, Wang X, Peng Z. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061-1069. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14113] [Cited by in RCA: 14764] [Article Influence: 2952.8] [Reference Citation Analysis (0)] |
| 3. | Sugano N, Ando W, Fukushima W. Cluster of Severe Acute Respiratory Syndrome Coronavirus 2 Infections Linked to Music Clubs in Osaka, Japan. J Infect Dis. 2020;222:1635-1640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Seki M, Yanagihara K, Higashiyama Y, Fukuda Y, Kaneko Y, Ohno H, Miyazaki Y, Hirakata Y, Tomono K, Kadota J, Tashiro T, Kohno S. Immunokinetics in severe pneumonia due to influenza virus and bacteria coinfection in mice. Eur Respir J. 2004;24:143-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 5. | Seki M, Hashiguchi K, Tanaka A, Kosai K, Kakugawa T, Awaya Y, Kurihara S, Izumikawa K, Kakeya H, Yamamoto Y, Yanagihara K, Tashiro T, Kohno S. Characteristics and disease severity of healthcare-associated pneumonia among patients in a hospital in Kitakyushu, Japan. J Infect Chemother. 2011;17:363-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Seki M, Kohno S, Newstead MW, Zeng X, Bhan U, Lukacs NW, Kunkel SL, Standiford TJ. Critical role of IL-1 receptor-associated kinase-M in regulating chemokine-dependent deleterious inflammation in murine influenza pneumonia. J Immunol. 2010;184:1410-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 7. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4579] [Article Influence: 915.8] [Reference Citation Analysis (0)] |
| 8. | Koo BS, Oh H, Kim G, Hwang EH, Jung H, Lee Y, Kang P, Park JH, Ryu CM, Hong JJ. Transient Lymphopenia and Interstitial Pneumonia with Endotheliitis in SARS-CoV-2-Infected Macaques. J Infect Dis. 2020;222:1596-1600. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Matthay MA, Leligdowicz A, Liu KD. Biological Mechanisms of COVID-19 Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;202:1489-1491. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Hayden FG, Sugaya N, Hirotsu N, Lee N, de Jong MD, Hurt AC, Ishida T, Sekino H, Yamada K, Portsmouth S, Kawaguchi K, Shishido T, Arai M, Tsuchiya K, Uehara T, Watanabe A; Baloxavir Marboxil Investigators Group. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N Engl J Med. 2018;379:913-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 618] [Article Influence: 88.3] [Reference Citation Analysis (0)] |
| 11. | Bhimraj A, Morgan RL, Shumaker AH, Lavergne V, Baden L, Cheng VC, Edwards KM, Gandhi R, Muller WJ, O'Horo JC, Shoham S, Murad MH, Mustafa RA, Sultan S, Falck-Ytter Y. Infectious Diseases Society of America Guidelines on the Treatment and Management of Patients with COVID-19. Clin Infect Dis. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 424] [Cited by in RCA: 586] [Article Influence: 117.2] [Reference Citation Analysis (0)] |
| 12. | Furuta Y, Komeno T, Nakamura T. Favipiravir (T-705), a broad spectrum inhibitor of viral RNA polymerase. Proc Jpn Acad Ser B Phys Biol Sci. 2017;93:449-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 675] [Cited by in RCA: 691] [Article Influence: 86.4] [Reference Citation Analysis (0)] |
| 13. | Cai Q, Yang M, Liu D, Chen J, Shu D, Xia J, Liao X, Gu Y, Cai Q, Yang Y, Shen C, Li X, Peng L, Huang D, Zhang J, Zhang S, Wang F, Liu J, Chen L, Chen S, Wang Z, Zhang Z, Cao R, Zhong W, Liu Y, Liu L. Experimental Treatment with Favipiravir for COVID-19: An Open-Label Control Study. Engineering (Beijing). 2020;6:1192-1198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 774] [Article Influence: 154.8] [Reference Citation Analysis (0)] |
| 14. | Wu C, Chen X, Cai Y, Xia J, Zhou X, Xu S, Huang H, Zhang L, Zhou X, Du C, Zhang Y, Song J, Wang S, Chao Y, Yang Z, Xu J, Zhou X, Chen D, Xiong W, Xu L, Zhou F, Jiang J, Bai C, Zheng J, Song Y. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4960] [Cited by in RCA: 5517] [Article Influence: 1103.4] [Reference Citation Analysis (1)] |
| 15. | Ramasamy MN, Minassian AM, Ewer KJ, Flaxman AL, Folegatti PM, Owens DR, Voysey M, Aley PK, Angus B, Babbage G, Belij-Rammerstorfer S, Berry L, Bibi S, Bittaye M, Cathie K, Chappell H, Charlton S, Cicconi P, Clutterbuck EA, Colin-Jones R, Dold C, Emary KRW, Fedosyuk S, Fuskova M, Gbesemete D, Green C, Hallis B, Hou MM, Jenkin D, Joe CCD, Kelly EJ, Kerridge S, Lawrie AM, Lelliott A, Lwin MN, Makinson R, Marchevsky NG, Mujadidi Y, Munro APS, Pacurar M, Plested E, Rand J, Rawlinson T, Rhead S, Robinson H, Ritchie AJ, Ross-Russell AL, Saich S, Singh N, Smith CC, Snape MD, Song R, Tarrant R, Themistocleous Y, Thomas KM, Villafana TL, Warren SC, Watson MEE, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Faust SN, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979-1993. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1071] [Cited by in RCA: 1072] [Article Influence: 268.0] [Reference Citation Analysis (0)] |
| 16. | Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, Pérez Marc G, Moreira ED, Zerbini C, Bailey R, Swanson KA, Roychoudhury S, Koury K, Li P, Kalina WV, Cooper D, Frenck RW Jr, Hammitt LL, Türeci Ö, Nell H, Schaefer A, Ünal S, Tresnan DB, Mather S, Dormitzer PR, Şahin U, Jansen KU, Gruber WC; C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603-2615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10556] [Cited by in RCA: 10695] [Article Influence: 2139.0] [Reference Citation Analysis (1)] |
| 17. | Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, Angus B, Baillie VL, Barnabas SL, Bhorat QE, Bibi S, Briner C, Cicconi P, Collins AM, Colin-Jones R, Cutland CL, Darton TC, Dheda K, Duncan CJA, Emary KRW, Ewer KJ, Fairlie L, Faust SN, Feng S, Ferreira DM, Finn A, Goodman AL, Green CM, Green CA, Heath PT, Hill C, Hill H, Hirsch I, Hodgson SHC, Izu A, Jackson S, Jenkin D, Joe CCD, Kerridge S, Koen A, Kwatra G, Lazarus R, Lawrie AM, Lelliott A, Libri V, Lillie PJ, Mallory R, Mendes AVA, Milan EP, Minassian AM, McGregor A, Morrison H, Mujadidi YF, Nana A, O'Reilly PJ, Padayachee SD, Pittella A, Plested E, Pollock KM, Ramasamy MN, Rhead S, Schwarzbold AV, Singh N, Smith A, Song R, Snape MD, Sprinz E, Sutherland RK, Tarrant R, Thomson EC, Török ME, Toshner M, Turner DPJ, Vekemans J, Villafana TL, Watson MEE, Williams CJ, Douglas AD, Hill AVS, Lambe T, Gilbert SC, Pollard AJ; Oxford COVID Vaccine Trial Group. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99-111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3551] [Cited by in RCA: 3437] [Article Influence: 859.3] [Reference Citation Analysis (0)] |
| 18. | Wang X, Ferro EG, Zhou G, Hashimoto D, Bhatt DL. Association Between Universal Masking in a Health Care System and SARS-CoV-2 Positivity Among Health Care Workers. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 44.4] [Reference Citation Analysis (0)] |