Revised: February 16, 2012
Accepted: March 5, 2012
Published online: April 12, 2012
West Nile virus (WNV), a flavivirus of the Flaviviridae family, is maintained in nature in an enzootic transmission cycle between avian hosts and ornithophilic mosquito vectors, although the virus occasionally infects other vertebrates. WNV causes sporadic disease outbreaks in horses and humans, which may result in febrile illness, meningitis, encephalitis and flaccid paralysis. Until recently, its medical and veterinary health concern was relatively low; however, the number, frequency and severity of outbreaks with neurological consequences in humans and horses have lately increased in Europe and the Mediterranean basin. Since its introduction in the Americas, the virus spread across the continent with worrisome consequences in bird mortality and a considerable number of outbreaks among humans and horses, which have resulted in the largest epidemics of neuroinvasive WNV disease ever documented. Surprisingly, its incidence in human and animal health is very different in Central and South America, and the reasons for it are not yet understood. Even though great advances have been obtained lately regarding WNV infection, and although efficient equine vaccines are available, no specific treatments or vaccines for human use are on the market. This review updates the most recent investigations in different aspects of WNV life cycle: molecular virology, transmission dynamics, host range, clinical presentations, epidemiology, ecology, diagnosis, control, and prevention, and highlights some aspects that certainly require further research.
- Citation: Martín-Acebes MA, Saiz JC. West Nile virus: A re-emerging pathogen revisited. World J Virol 2012; 1(2): 51-70
- URL: https://www.wjgnet.com/2220-3249/full/v1/i2/51.htm
- DOI: https://dx.doi.org/10.5501/wjv.v1.i2.51
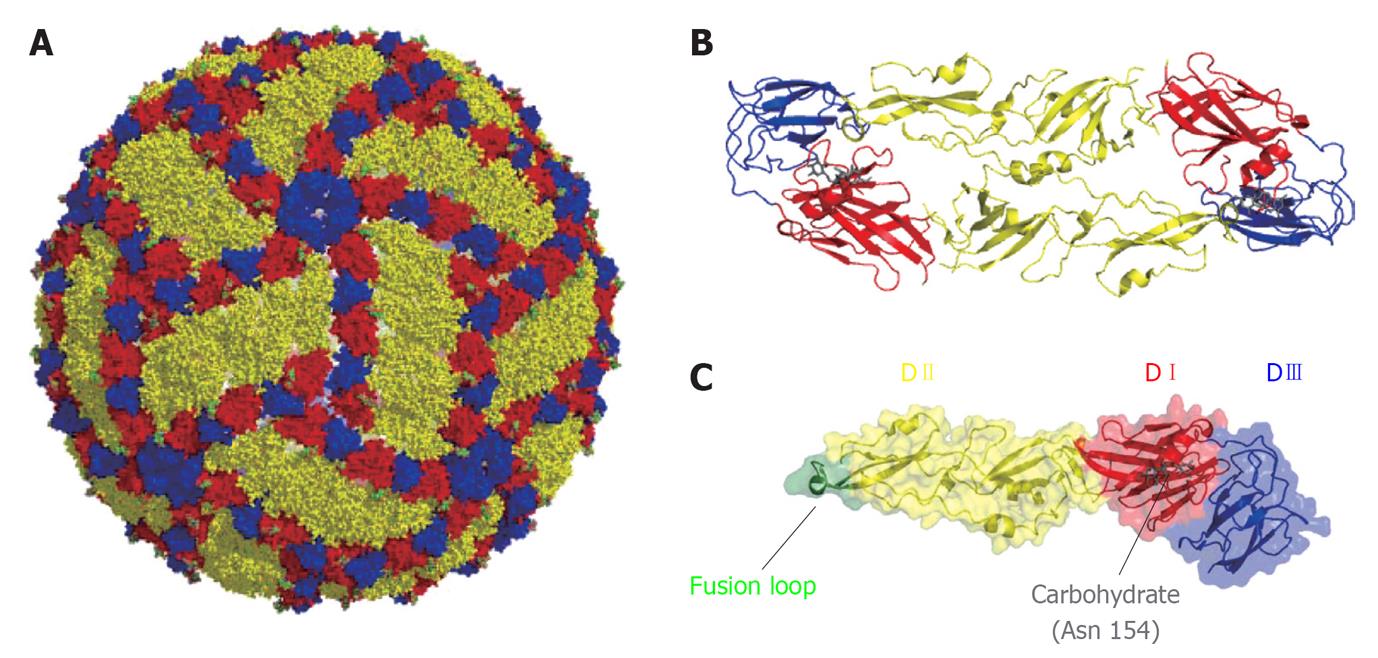
West Nile virus (WNV) is a small enveloped virus about 50 nm in diameter. The genomic RNA is enclosed within a nucleocapsid formed by the capsid (C) protein that constitutes the core of the virion and is enveloped by a lipid bilayer derived from the host cell. Mature virions display a smooth outer surface with no projections or spikes. This outer shell is constituted by 180 copies of the small membrane (M) protein and an equal number of copies of the E glycoprotein disposed as 90 anti-parallel homodimers arranged in three distinct symmetry environments (Figure 1), thus resulting in a particle of icosahedral symmetry[1].
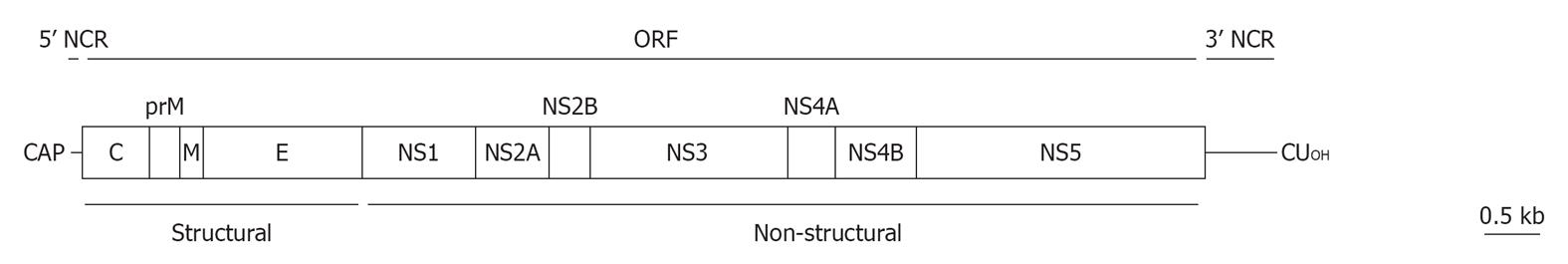
The WNV genome is constituted by a single-stranded RNA molecule of positive polarity (Figure 2). This RNA molecule of about 11 000 nucleotides in length encodes a polyprotein in a single open reading frame that is flanked by two non-coding regions (NCRs) located at the 5’ and 3’ ends of the genome (about 96 and 635 nucleotides in length, respectively)[2,3]. The polyprotein is proteolytically processed by viral and cellular proteases rendering ten major viral proteins: three structural (C, prM and E) and seven non-structural, NS (NS1, 2A, 2B, 3, 4A, 4B y 5)[3]. WNV genome has a cap structure at 5’ end, but it lacks a 3’ poly(A) tract and ends with CUOH[3]. Proper methylation of the cap structure at the guanine N-7 and the ribose 2’-OH positions of the first transcribed adenine is necessary for optimal infectivity of WNV RNA. Viruses defective in the N7 methylation mechanism are non-replicative, and recently the 2’-OH methylation has been related to evasion of innate immunity by evading certain components of interferon response, therefore WNV defective in this methylation mechanism can replicate but is attenuated in vivo[4,5].
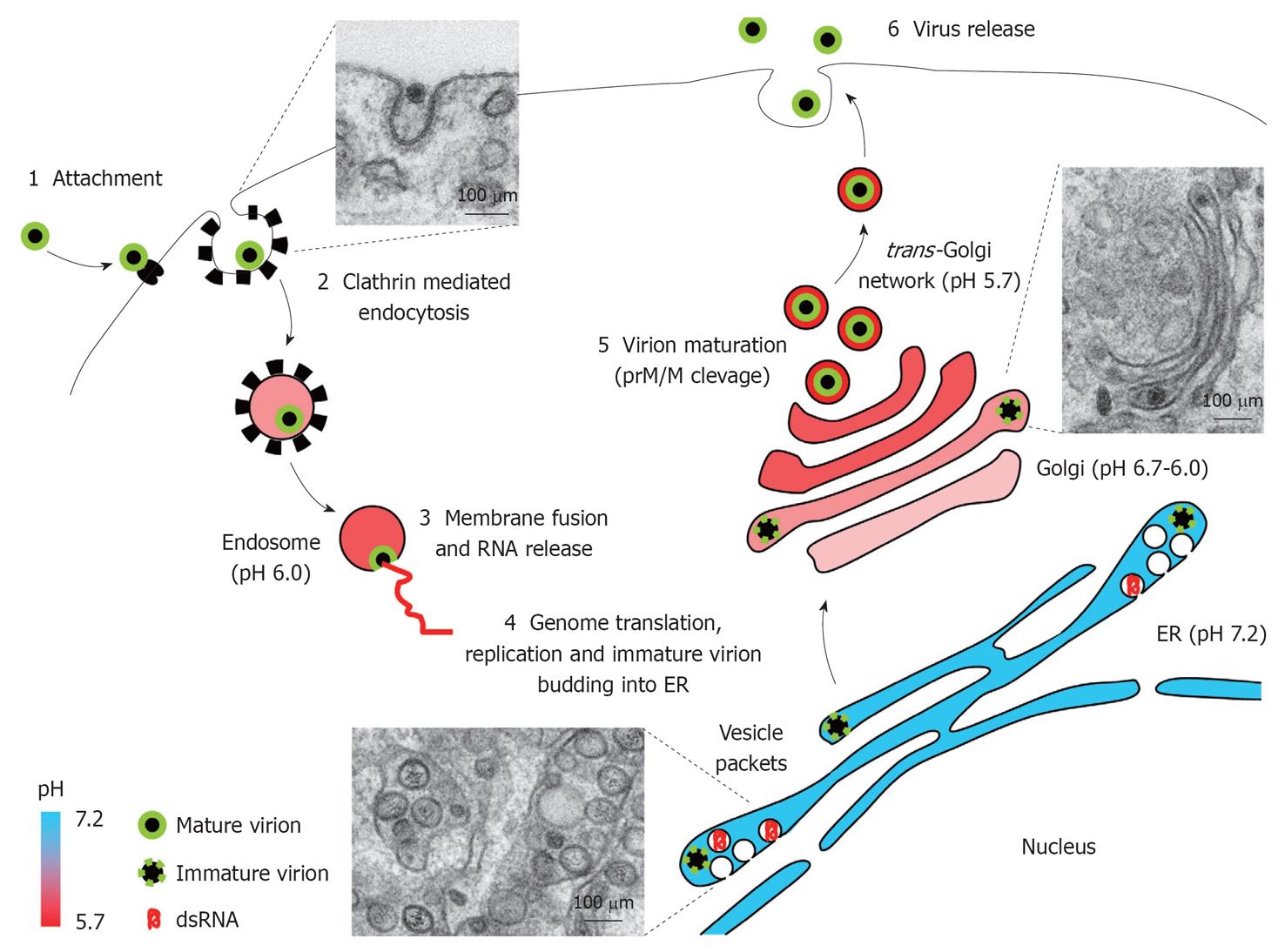
Early steps: Attachment, entry and fusion: Infection by WNV is initiated by binding of virions to its cellular receptor (Figure 3). Glycosaminoglycans, c-type lectins, and, although still controversial, integrin αvβ3 have been proposed as the cellular receptors for WNV[6-9]. Viral particles are internalized into host cells via a clathrin dependent mechanism and are transported to endosomal compartments with the involvement of cellular actin and microtubules[10,11]. Entry of WNV has been suggested to be dependent on cellular protein Cas-Br-M (murine) ecotropic retroviral transforming sequence-like 1[12], although a role of this protein on viral replication rather than in WNV entry has been recently proposed[13]. Transport of WNV particles to early endosomes is dependent on the activity of small GTPase Rab5[14]. Inside endosome, acid pH triggers rapid conformational changes on the envelope protein that result in its fusion with endosomal membrane, thus allowing nucleocapsid release to the cytoplasm for genome uncoating. The optimal pH for conformational rearrangements and viral fusion is 6.3-6.4, and this fusion process is dependent on the presence of cholesterol in the target membrane[15,16]. In this way, exposure of viral particles to acid pH in absence of target membranes induces conformational rearrangements that resemble those induced inside endosomes and causes loss of fusion capacity, thus reducing its infectivity[15]. Freezing virus particles in a pre-fusion intermediate by antibodies that neutralize WNV infection after attachment, likely by impairing acid-induced fusion within the endosome, have been described[17,18].
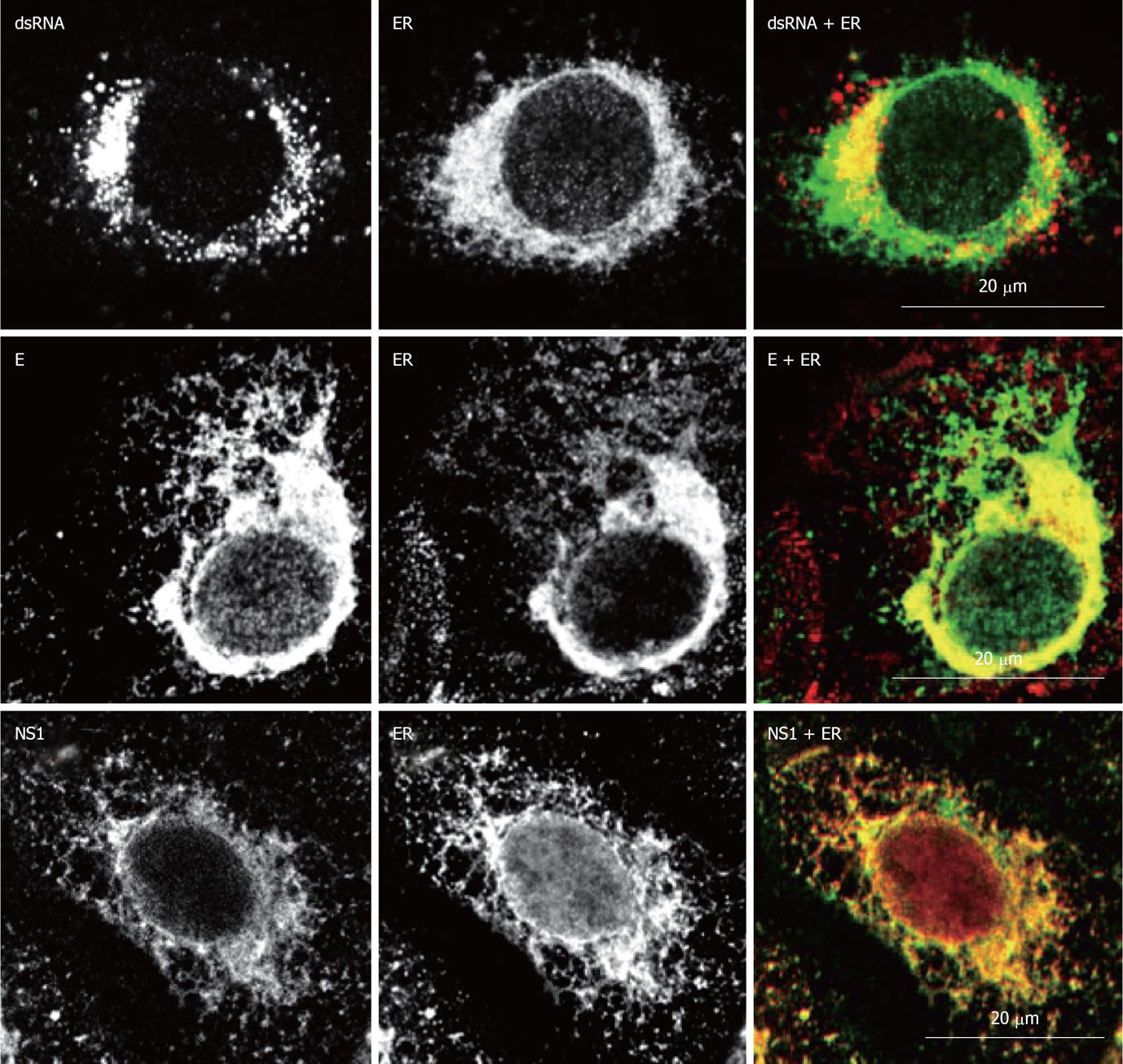
Replication complex assembly: Once viral RNA reaches the host cell cytoplasm it is translated to raise structural and non-structural proteins involved in viral replication and virion assembly (Figure 3). The genome is translated into a single RNA polyprotein that is processed to render mature viral proteins. RNA replication requires the synthesis of a minus-strand RNA which acts as template[3]. Cells infected by WNV undergo notable intracellular membrane remodelling. Major membrane reorganizations leading to different well defined structures aimed to establish the viral replication complex have been described. The primary membrane source for these structures is provided by the endoplasmic reticulum (ER)[19]. The presence of markers from organelles involved in the endocytic pathway (endosomes/lysosomes) or from the Golgi complex have been also suggested[20]. However, recent data did not support this hypothesis[19,21]. These structures are termed convoluted membranes (CM), paracrystalline arrays (PC) and vesicle packets (VP)[22,23]. Viral replication takes place at VPs, which are generated as invaginations of the membrane of ER and contact by pores with the cell cytoplasm[19]. VPs contain dsRNA replication intermediates, and assembled virions bud into the ER[19] (Figure 4). A specific role of cholesterol and fatty acids in WNV-induced membrane structures has been proposed[21,24,25], and proteasome activity seems to be also important for viral replication[13,26]. Apart from providing the adequate platform for viral replication, these membrane rearrangements may also play a role for the evasion of innate immune response by interfering with the interferon signalling machinery[24,27]. Replication of WNV and accumulation of non-structural proteins at the ER induces ER stress and activates the unfolded protein response[28,29]. In addition, replication of WNV also induces apoptosis of infected cells[30].
Particle maturation and viral egress: To render infectious virions, immature viral particles assembled at the ERs traffic through Golgi complex for maturation (Figure 3). This maturation process requires the cleavage of Premembrane/membrane protein (prM/M) by a furin-like protease located at the acidic environment of the trans-Golgi network[3]. After maturation, viral particles are released from infect cells following the secretory pathway.
The NCRs located at 5’ and 3’ ends of viral genome contain conserved secondary RNA structures that play important roles in genome replication, may function as protein translation enhancers and, in the case of 3’NCR, display translational regulatory properties similar to those of a poly(A) tail[3]. Different cellular proteins that interact with both 5’ and 3’ NCRs have been involved in viral replication, including elongation factor 1α which binds 5’ NCR, T cell-restricted intracellular antigen (TIA)-related protein (TIAR), and a closely related protein TIA-1 which bind to 3’ NCR in minus-sense strand[3,31-33]. Several short conserved sequences located at both NCR are important for establishment of two pairs of long-distance RNA interactions that mediate genome cyclization and replication[34]. Functional analyses have confirmed that mutations that abolish these interactions affect viral replication, but not RNA translation[35]. As the RNA dependent RNA polymerase binds to a stem loop within the 5’ NCR, genome cyclization should facilitate binding of the polymerase to the 3’ end of the genome and, thus, to the initiation of minus strand synthesis[34,36]. The balance between circular and lineal forms of flavivirus genomes are essential for efficient RNA replication and control of translation initiation, as well as for switch between translation and RNA synthesis during the viral life cycle[37].
Apart from the involvement in genome cyclization, 5’ NCR acts as a template for recognition of the enzyme responsible for methylation reactions required for cap assembly[36]. Mutational analysis of six terminal nucleotides of the 3’ NCR have revealed that it displays a conserved RNA structure, which may function as contact sites for specific assembly of the replication complex or for efficient initiation of minus-sense RNA synthesis[38].
Capsid (C): The capsid, or core, (C) protein contains a large number of scattered charged amino acids[39] and is implicated in viral assembly and replication[40]. The protein dimerizes and tetramerizes to build the nucleocapsid that, together with viral RNA, forms the electron-dense core of the virion that is enveloped by the lipid bilayer. The N and C-terminal parts of the protein are intrinsically disordered regions and may play a role in RNA folding during viral replication by conferring RNA chaperoning activity to the C protein[41]. X-ray crystallography analysis of the central part of the C protein structure showed the presence of four α-helices[39]. In WNV-infected cells, capsid protein can be detected in the cytoplasm, nuclei and the nucleolus of the cell, and it has been related to the induction of apoptosis[42]. Nuclear location of the C protein is mediated by a bipartite nuclear location signal and requires specific interaction with cellular importins[43]. The capsid protein also interacts with other cellular factors, as the inhibitor of the serine/threonine phosphatase PP2A, I(2) (PP2A), Hsp70 and Jab1[44-46]. The phosphorilation status of the protein and Jab1 can regulate nuclear location and RNA binding activity[46-48]. The C protein has been also implicated in degradation of claudin proteins and disruption of epithelia barrier, thus helping to virus dissemination[49].
prM/M: The prM/M is a short transmembrane glycosylated protein associated to the lipid bilayer of the virion. The cleavage of this protein by a furin-like protease occurs within the trans-Golgi network and is necessary for particle maturation[3]. This protein protects virions from fusion inside acidic vesicles of the Golgi complex[50]. The furin-like protease cleaves the prM/M membrane protein, enabling a conformational rearrangement in the viral particle from immature particles[51] to mature ones[1]. Modulation of the proportion of prM/M cleavage can also modulate the sensitivity of antibody-mediated neutralization[52].
Envelope: The envelope (E) is a transmembrane protein anchored to the lipid envelope by a C-terminal α-helical hairpin. It is the most immunogenic protein of the virus and the target for most neutralizing antibodies. The protein is glycosylated on position 154 on most WNV strains[53]. Glycosylation is important for efficient transmission in mosquito and birds[54,55] and may be related to neuroinvasiveness[56]. The atomic structure of the E glycoprotein as a soluble ectodomain has been solved by X-ray crystallography[57,58], showing that it presents the typical folding of the flavivirus E glycoproteins and is organized in three domains (Figure 1): DI, DII, that contains a hydrophobic peptide responsible for virus fusion termed fusion loop, and DIII, an immunoglobulin-like domain. DII mediates the homodimerization of the protein on the surface of the virion (Figure 1). DIII is involved in receptor binding and contains multiple epitopes that are recognized by neutralizing antibodies. Upon acid exposure, the E glycoprotein undergoes conformational rearrangements and changes from dimers to trimers, exposing the fusion loop to enable viral fusion of the virion with cellular endosomal target membranes. For other flaviviruses, as tick-borne encephalitis virus, this process is triggered by protonation of an individual His residue on E glycoprotein[59] that should act as a critical pH sensor. However, this hypothesis has not been validated for WNV[60], although point mutations can modulate the fusion threshold[15].
NS1: This viral glycoprotein, which can be secreted from infected cells[61], may vary its oligomeric state between monomers, dimers (the primary form) and hexamers, and this seems to be related to its cellular retention or secretion stage[3,53]. Intracellular NS1 functions as an essential cofactor for viral replication, and it localizes to WNV replication sites[62], whilst cell surface and secreted NS1 act as immunomodulators. NS1 antagonizes complement activation[63] and inhibits Toll-like receptor 3 (TLR3) signalling[64]. Recently, a larger NS1-related protein (termed NS1’), produced by a ribosomal frameshift near the beginning of the NS2A gene, has been detected in infected cells and related to neuroinvasiveness[65].
NS2A: This is a small hydrophobic transmembrane protein involved in the production of intracellular virus-induced membranous structures and virion assembly[66,67]. In fact, NS2A has been detected by immunogold labelling primarily within VP, associated with labelled dsRNA[62]. In addition, NS2A seems to have an immunomodulatory role because it inhibits α/β interferon production[68], and mutations in this protein result in viral attenuation in vivo[68,69].
NS2B: It is also a small hydrophobic protein that acts as a cofactor of NS3 protease and may function as a membrane anchor for viral protease[3,53,70]. Alanine scanning approaches of NS2B has revealed two sites critical for regulation of the proteolytic activity of NS2B-NS3 complex[71]. The interaction between NS2B and NS3 may also confer specificity for RNA unwinding of NS3 discriminating from DNA[72].
NS3: This is a highly conserved protein, which N-terminal encodes the viral trypsin-like serine protease. However, it is not active unless tethered to its cofactor, NS2B[70]. This protease cleaves the viral polyprotein to release structural and non-structural proteins and, thus, disruption of its activity is lethal for virus replication. The NS3 protein also encodes other enzymatic activities within its sequence (helicase, nucleoside triphosphatase, RNA triphosphatase) important for viral replication[3,53,70]. NS3 (and also its cofactor NS2B) has been localized within PC or CM, suggesting that these membranes are the sites of proteolytic cleavage[62]. The structure of the helicase domain has been solved by X-ray crystallography[73] and a single substitution in this domain has been related to increased virogenesis in American crows[74]. All these properties of NS3 made of this protein and its active form, NS2B-NS3, a promising antiviral target.
NS4A: It is also a small hydrophobic protein with several transmembrane domains that has been localized to the viral replication complex in virus induced membranes (VP, CM and PC)[62]. It may be responsible of membrane rearrangements in infected cells upon cleavage of its C-terminal region (designated 2K fragment) that, in dengue virus, acts as a signal sequence for translocation into the ER of the adjacent NS4B protein[75]. NS4A has been also related, together with NS2A and NS4B, to the inhibition of interferon signalling in flavivirus infected cells[76]. Accumulation of NS4A (and also NS4B) into ER of infected cells seems to be involved in induction of the unfolded protein response upon WNV infection[29]. Mutations in the 2K fragment have been related to resistance against the antiviral action of the interferon-inducible 2’, 5’-oligoadenylate synthetase 1b protein[77], and also to resistance against the flavivirus inhibitor lycorine thanks to the enhancement of RNA replication[78]. NS4A has been proposed to also act as a cofactor regulating ATPase activity of the NS3 helicase[79].
NS4B: This small hydrophobic protein plays a major role on inhibition of WNV interferon signalling[80,81]. Mutations in NS4B can result in attenuation of WNV in vivo[82,83]. NS4B has been found in perinuclear membranes and in the nucleus of WNV infected cells[84] and it may be involved in the formation of viral replication complex.
NS5: It is the largest protein encoded by the virus. NS5 localizes to virus induced membranes in infected cells and colocalizes with dsRNA at viral replication complexes[85]. It has two different enzymatic activities: the N-terminal encodes the methyltransferase[86], while the C-terminal encodes the viral RNA-dependent RNA polymerase for replication of viral genome[87]. Due to the lack of proof-reading activity of NS5, WNV populations display a variable level of sequence diversity that favours selection of variants in response to selective pressures. The methyltransferase activity is necessary for capping the 5’ end of the viral RNA, which is performed by sequential methylation reactions[88]. NS5 is also a potent antagonist of interferon signalling[89]. The methyltransferase activities together with the polymerase activities made of NS5 also a promising antiviral target.
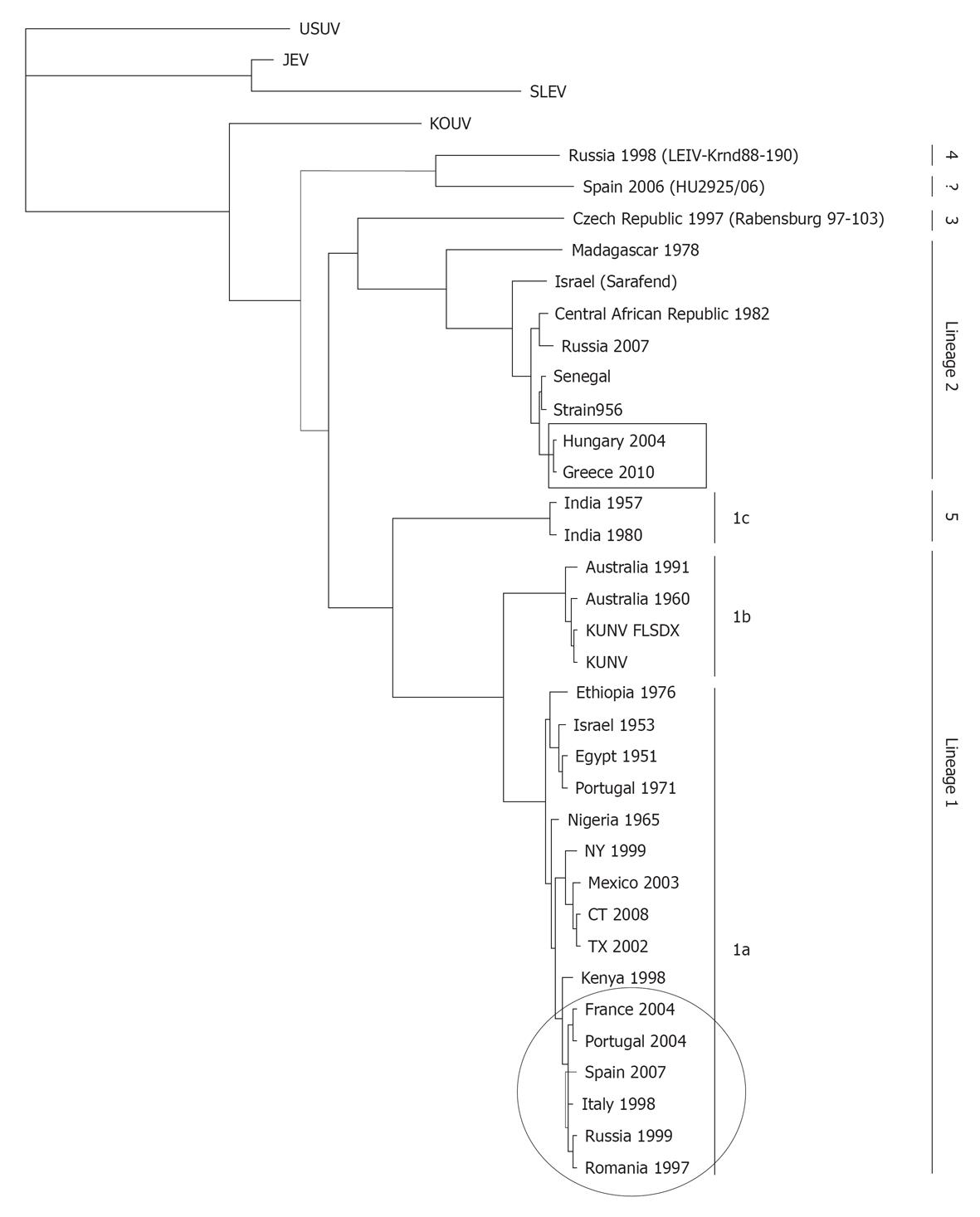
First classifications of WNV were based on cross-neutralization reactions and revealed that WNV is a member of the Japanese encephalitis virus serocomplex. This complex includes also other neurovirulent viruses such as Murray Valley encephalitis virus, St. Louis encephalitis virus, or Usutu virus[53,90]. Recent advances on molecular phylogeny support this antigenic classification and reveal the existence of up to five distinct genetic lineages of WNV[91-94].
Lineage 1 is subdivided into three clades (Figure 5). Clade 1a includes African, European and American isolates; clade 1b groups the Australian Kunjin virus (KUNV), which has been shown to be a subtype of WNV[95]; and clade 1c clusters isolates from India[53,92]. Interestingly, clade 1a displays close genetic relationships between geographically distant areas which are supposed to be the result of WNV introductions via migratory birds (Figure 5). WNV inside clade 1a can be further grouped into different clusters[96]. The fact that only one endemic genotype has been detected in India (1c) and one in Australia (1b), suggests that WNV was successfully introduced into these locations only once, as well as it was the case in the American continent, where WNV was introduced in 1999 in the East Cost of the US[2,96]. The first North American WNV isolate was most closely related to a strain isolated from a dead goose in Israel (lineage 1) during the 1998 outbreak, suggesting that North American WNV was derived from this epidemic[2]. However, recent data suggest that the epidemic in Israel in 1998 was not the direct progenitor of North American epidemics, but rather that both epidemics originated from the same (unknown) location[96].
Lineage 2 initially included WNV strains only detected in Africa and Madagascar, which have been speculated to be less neurovirulent than those included in lineage 1[92]. However, recent outbreaks in Europe (Austria, Hungary and Greece) have been associated to lineage 2 strains[97]. Other lineages of WNV of unknown human pathogenicity are lineage 3 (Rabensburg isolate 97-103), isolated from Culex pipiens mosquitoes in the Czech Republic in 1997[93], and Lineage 4 (LEIVKrnd88-190), isolated from Dermacentor marginatus ticks in 1998 in Russia[94]. It has been proposed that WNV Indian isolates that were grouped as linage 1c make a new cluster termed lineage 5[91]. A putative new lineage of WNV (strain HU2925/06) that forms a common evolutionary branch with lineage 4 has been recently reported in Spain[98]. In addition to these minor lineages, an unusual KUNV isolate from Malaysia (KUN MP502-66) and the African virus Koutango (KOUV) could also constitute additional lineages of WNV[95].
WNV is maintained in nature in an enzootic transmission cycle between avian hosts and ornithophilic mosquito vectors. At least 60 species of mosquitoes from 11 different genera have been described as competent vectors in North America, being those of the Culex species the most efficient ones (Cx. pippiens, Cx. quinquefasciatus, Cx. restuans, Cx. salinarus, Cx. tarsalis, and Cx. nigripalpus), although other species such as Aedes albopictus, Aedes vexans, Ochlerotatus japonicus and Ochlerotatus triseriatus may also play a role on viral transmission as bridging vectors that can transmit the virus to mammals[99]. In Europe, the virus has been isolated from more than 40 different species, being again those of the Culex species the main vectors[100]. Several other species have been also described as competent vectors in other geographical areas, Cx. univittatus in Africa, Cx. annulirostris in Australia, and Cx. vishnui and Cx. tritaeniorhynchus in Asia[99,101,102].
Laboratory analyses have shown that Cx. tarsalis mosquitoes become infected after consumption of blood meals with viral concentrations over 107 PFU/mL, whilst only up to 30% do it if the concentration is in the 105 PFU/mL range[103]. On the other hand, different species of mosquitoes inoculate quite variable doses of WNV (103.4 PFU to 106.1 PFU) into vertebrate hosts during natural feeding, of which around 102 PFU are directly inoculated into the blood[104].
Vertical transmission, overwintering, and survival of adult mosquitoes with some low activity during winter time have been observed in temperate regions[105,106]. It should be also noted that the minimal infection rate (MIR), expressed as the number of infected mosquito pools per 1000 mosquitoes tested, is a good indicator of the intensity of viral transmission in a given area, and is often related to the human risk of disease, so that, a MIR ≥ 1.0 seems to be needed for a mosquito species to represent a high risk for human transmission[107].
Beside from mosquitoes, WNV has been sporadically isolated in other arthropods such as soft and hard ticks, mites and hippoboscid flies[108-110] but, although their implications on WNV transmission still need to be analyzed in detail, it seems unlikely that they play a substantial role on the viral transmission cycle in nature.
Birds are the natural reservoir of WNV. Hundreds of avian species representing over 20 birds families from North America have been described as susceptible to WNV infection after its first introduction in 1999. However, the most affected ones have been the Passeriformes, mostly those of the Corvidae family, in many of which the virus replicates to high titers before the birds become moribund and die a few days after being infected[53]. Up to 100% mortality has been described in experimentally infected crows[111,112], and an estimated 45% decrease in crow population has been reported since the introduction of the virus in US[113]; however, recent data indicate that this mortality rate is decreasing[114]. A study carried out with several different avian orders from America reported that blue jay, common grackle, house finch, American crow, and specially house sparrow are among the most important WNV amplifying avian species, while many other did not show any evident sign of WN disease (WND)[112]. For instance, no significant mortality has been reported in chickens, beside they showed titers in blood of 105 PFU/mL and virus can be isolated from several organs after experimental infection[115].
Many avian species shed large quantities of virus in their feces or oral secretions when infected[112], allowing direct transmission form bird-to-bird and even from bird-to-human. Experimental oral infection of birds has been demonstrated[116] and prey-to-predator infection has been suggested[117].
Although high bird mortality has been a common feature of WNV activity in US and Israel[118], no such trait has been observed in other regions of the world[100,101,118-120].
WNV infection in humans, horses and other mammals are generally considered as “dead end” infections because viral replication does not yield significant viremia to allow transmission from vertebrate hosts to feeding mosquitoes, which is very dependent on the level of viremia in the host. For instance, in experimentally infected horses viremia levels are around 103 PFU/mL[121], thus being usually insufficient to sustain infectivity cycles.
Several other animal species have been described as susceptible to WNV infection, with or without clear evidence of disease, including domestic and wild mammals, such as dogs, cats, sheep, pigs, cows, rabbits, llamas, alpacas, deers, reindeers, raccoons, bears, wolfs, squirrels, chipmunks, and bats, among others[53,122]. As it has been described for humans and horses, in most cases the viremia raised in these animals is low and probably not enough to initiate a new transmission cycle.
Apart from mammals, several reptiles and amphibians, such as snakes, crocodiles, alligators, iguanas and frogs[123-126] have been also described as susceptible to WNV infection and some of them raise high viremia, but the real contribution of animals other than birds and mosquitoes in maintaining WNV cycle in nature is still uncertain.
WNV transmission to vertebrates usually involves mosquitoes; however sporadic reports of non-vector-borne transmission have been documented in humans throughout liver and kidney transplantation, dialysis, needle-stick injury, breast-feeding, by transplacental route, and by blood transfusion, which drove to the consequent screening of blood donors[127-130].
These transmission routes seem to be mainly anecdotic and have minor effects on disease burden. For instance, no fatalities were recorded among 71 WNV infected pregnant women included in a retrospective study, most of whom gave birth healthy children with only a few cases of newborns presenting malformations, though no conclusive association to WNV infection could be established in any of them[130]. In any case, assessment of the fetus or child is recommended when mothers are infected by WNV and clinicians are encouraged to report known or suspected cases of WNV infection during pregnancy.
WNV experimental transmission by oral, intrauterine and lactation routes has been described in mice without evidence of mice-to-mice infection[131,132] and, contrary to what has been observed in humans, it has been reported that WNV infection increases mortality rates in pregnant mice[131]. Direct or experimental oral exposure to the virus resulted in infection of cats, dogs, hamsters, mice, fox squirrels, alligators, crocodiles, and snakes[53,102,122]. Transmission from experimentally infected alligators to uninfected[124], and from infected hamster to uninfected cage-mates has been also reported[133,134].
A primary role of migratory birds in WNV introduction, reintroduction, and spread across Europe[135-139] and the Americas[140,141] is well documented. However, comparison of the levels of neutralizing antibodies in migratory and resident birds in the south of Spain[135,136], the seroconversion observed in some recaptured resident birds of the same area[137], and the significant number of positive resident birds found in Great Britain[142] and Italy[143] support that there is also local circulation of the virus. Contribution of resident birds to the initiation of annual infection cycles in the USA has also been suggested[144].
Since viral cycle may persist in a given geographical region from year to year without reports of human or horses cases, epidemics seem to be more related to the concomitant profusion of birds and mosquitoes populations in the same area, generally wastelands and mashes where migratory birds are abundant, than to other factors. Thus, in addition to the viral load present on the blood of the hosts[103], the efficiency with which mosquitoes transmit WNV seems to be clearly dependent upon various environmental and climate factors, particularly temperature, humidity and rainfall[122,145].
RNA viruses have the capability to replicate at elevate temperature, particularly arboviruses present a wide range of temperatures at which they replicate in order to be propagated within different hosts, vertebrates and invertebrates. In vitro experiments have demonstrated that selection of viruses with increased capability to replicate in vertebrates resulted in a lower capacity to replicate in mosquito[146]. WNV replication in mosquitoes is constrained below 14 °C[147] and strains isolated in 2002 in the USA disseminate more rapidly and efficiently at elevated temperatures than the original strain isolated in New York in 1999[148]. All these data strongly suggest that temperature is a key factor on WNV evolution and dissemination.
Climate changes have a direct effect on WNV amplification and transmission. Lately, mosquitoes expanded to more extreme latitudes and elevations, thus reaching new host populations to feed on and sharing new ecological niches with different vector species. Heavy rainfall as well as warm and dry temperatures benefit the increase of mosquitoes population and positively correlate with WNV transmission[149,150].
Although the way in which the virus causes sporadic outbreaks in humans and horses is still unclear, it is quite reasonable to think that overabundance of birds that share urban and rural areas with ubiquitous fastidious mosquitoes that feed in birds and mammals plays an important role on it. In fact, in two of the major urban outbreaks reported, the ones that took place in Bucharest (Romania)[151] and Volgograd and Volzhskiy (Russia)[152], the regions were heavily infected by potentially mosquito vectors. Hence, new human and animal behaviors and climate changes are facilitating contact between vectors and hosts.
WNV was first detected in the blood of a febrile woman in the West Nile district of Uganda, currently the Arua district, in 1937[153]. Further studies showed that the virus was widely distributed across Africa, Europe, Asia and Australia[100-102,108] but, since only sporadic outbreaks with low clinical incidence were reported until recently, WNV infection was not considered a serious animal or human health treat.
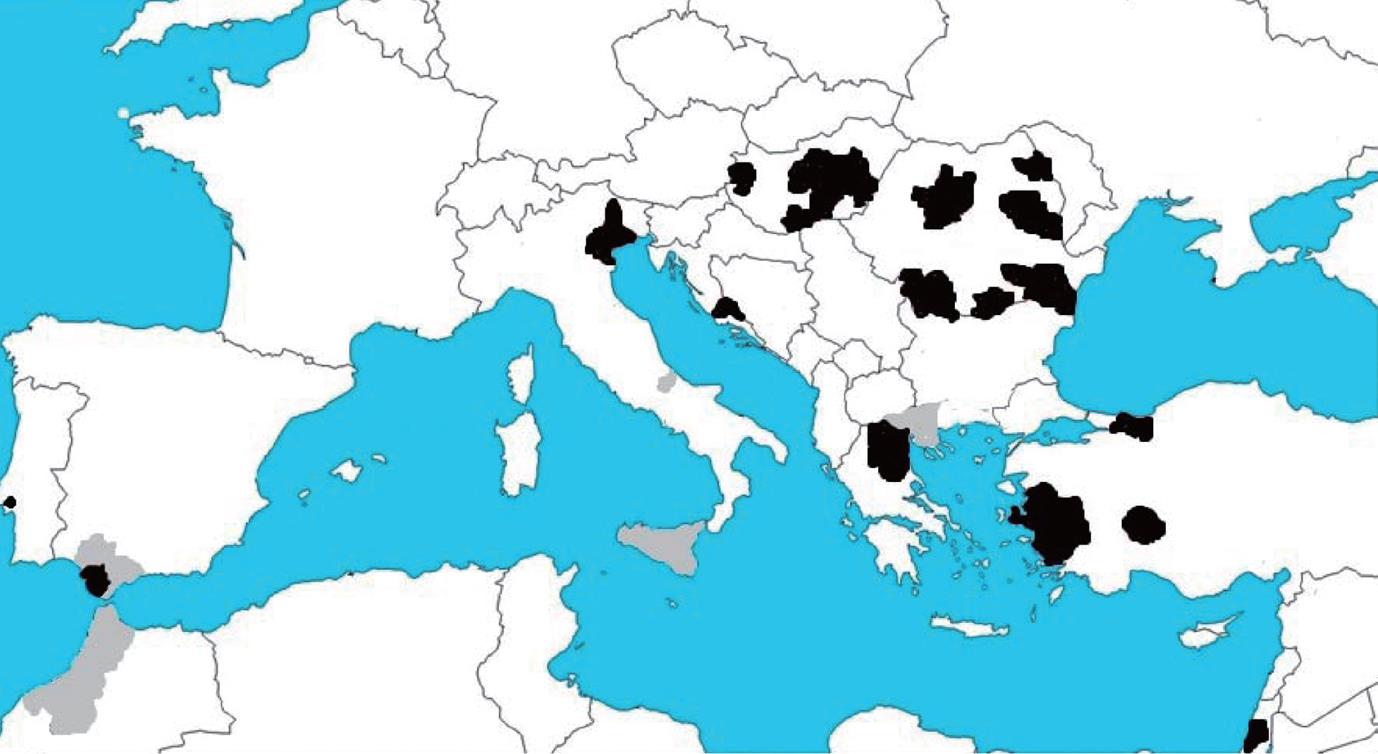
The first recorded human WNV outbreak took place in Israel in 1950s[154,155]. Since then, there were some sporadic reports of WNV circulation in Albania, Bulgaria, Belarus, Ukraine, and Moldavia[108], but viral activity was low until the 1990s, when the virus re-emerged with a worrisome increase in the number, frequency and severity of the outbreaks reported in human and horses. In fact, evidence of WNV activity, with or without recorded human or horse clinical cases, have been lately reported in Algeria, Morocco, Tunisia, Egypt, Israel, Romania, Russia, Poland, Czech Republic, Hungary, Croatia, Serbia, France, Portugal, Spain, and Italy, which, overall, have accounted for hundreds of cases and dozens of deaths[100,156].
Early serological studies in Greece had detected anti-WNV antibodies in mammals and humans, but later intensive serosurveillance studies in blood donor in 2006-2007 failed to do so[157]. Along 2010 (Figure 6) WNV outbreaks were reported in Greece and neighboring countries with more than 250 laboratory confirmed cases and 27 deaths[158]. Analysis of the circulating virus has shown that it was genetically closely related to the virus that emerged in Hungary in 2004[158]. An increasing activity of WNV in horses has also been observed lately in Europe[156,159,160], including a large outbreak that took place in northeast Italy in 2008 involving 251 stables with 794 cases and 5 deaths[156,161]. At the same time, nine human cases of West Nile fever (WNF) were reported in the area[161]. In southern Spain, during summer 2010, the first outbreaks of severe WND in horses were reported with 41 diagnosed cases and 10 deaths, and 2 laboratory confirmed human cases (http://www.oie.int/wahis/). Similar figures have been reported during 2011. The strains responsible for the Italian and Spanish outbreaks are closely similar to those previously circulating in the western-Mediterranean region (Figure 5) and differ from the one that circulate in Greece[162]. All these data suggest that new epidemiological scenarios are being developed in Europe and, thus, that assessment of WNV activity and implementation of coordinated surveillance programs are necessary across the continent.
Since the first detection of WNV in Uganda[153], little information has been available about its activity there. Studies conducted in several sub-Saharan countries have reported a quite variable seroprevalence in humans[120,163-165], with few documented clinical cases, but these data should be taken carefully since most studies have not addressed possible cross-reactivity with other circulating flaviviruses, and clinical cases may have been attributed to other diseases that present similar symptoms. Serological evidence of WNV infection has also been described in horses in the continent[165].
WNV is frequently detected in southern Asia, where some human cases have been reported, and sporadically in South-East Asia[166]. In Australia, the lineage 1 circulating WNV strain is known as KUNV[101] and, although may cause febrile illness and even encephalitis, its clinical effects are usually mild[101,166].
WNV was first detected in North America in the New York metropolitan area in 1999[2], but the way the virus was introduced is still unknown, and several options have been proposed: transport of infected mosquitoes by airplane or other means, arrival of an infected person, or, what it seems more probable, carried by infected migratory or imported birds.
During the first outbreak in New York the virus caused 62 human cases with seven deaths, 25 equine cases with nine deaths, and an enormous mortality of birds, particularly among corvids. Since then, the virus has spread quickly across the country, being, so far, responsible for over 1100 fatalities, over 12 000 cases of meningitis/encephalitis, and more than 30 000 diagnosed human infections (http://www.cdc.gov). In addition, more than 25 000 accumulated cases were reported in horses until the introduction of veterinary vaccines that have greatly contributed to reduce equine mortality and morbidity in the country[167].
Several studies conducted on experimental animal models and birds have demonstrated that the strain introduced, belonging to lineage 1, was highly virulent[111,168]. Further analyses have identified possible determinants in the structural and non-structural proteins of the virus that may account for its neurovirulence[169,170]. Then, the combination of the introduction of highly virulent strains acting over a naïve population in the presence of competent amplifying vectors may have been responsible for the devastating consequences that WNV had in the US early after its introduction.
The virus has also expanded to neighboring regions, and serological evidence of WNV activity has been reported in Canada, Central America, the Caribbean, and South America in mosquitoes, humans, horses, birds, and other animals, from some of which the virus has been occasionally isolated[53,122]. Sequencing analysis of the Mexican strains suggests that the virus was introduced into Mexico in two different events, once in the northern states and another in the Yucatan region, more probably by migratory birds. However, WNV pathogenicity in these regions seems to be lower than in the US, as very few human or horse cases have been reported[122]. In fact, only 5 and 7 human cases have been reported in the Caribbean and in Mexican states bordered to the US, none of them fatal. These differences are intriguing and could be due to a combination of several factors, like to the wide distribution of other related and potentially cross-protective flaviviruses in the region (because individuals that had previously been in contact with them may have natural acquired immunity against WNV), to the species composition, abundance and/or susceptibility of hosts and vectors in the regions, or to a different virulence of the circulating WNV strains that could also contribute to the apparent paucity of the disease there[171,172]. In fact, although slightly divergent in their genomes, strains with attenuated phenotypes in cell culture and/or lower virulence have been isolated after the first introduction in America[173,174]. Even more, although stasis of WNV evolution was initially described in the US[175], recent data suggest that the virus is continuously evolving[176].
Most of WNV infections in humans are asymptomatic, but approximately 20% of the infected people develop clinical symptoms, although severe neurological diseases are observed in less than 1% of them. WNF is characterized by several non-specific flu-like symptoms, such as fatigue, fever, headache, muscle pain, weakness, rash, and neck-pain. Symptoms usually develop between 2 to 15 d after infection and last for 2 to 5 d[177]; however, in some instances, they can result in hospitalization and many patients with WNV encephalitis have movement disorders, including severe tremors and parkinsonism[178,179]. Severe WND is associated with neurological involvement that varies from meningitis and/or encephalitis to poliomyelitis-like condition with acute flaccid paralysis that can result in respiratory failure, with an estimated 10% of the neuroinvasive cases resulting fatal[180]. Analysis of the long-term outcomes of WND has estimated that more than 50% of survivors that presented severe symptoms reported physical and cognitive deficits up to 2 years after being diagnosed[180,181].
Older age use to be associated with a worst prognosis, but neuroinvasive diseases have been also reported in young people and children[177,182]. Hypertension and diabetes have been suggested to be a potential risk factor for severe WND, but the most important factor, beside old age, seem to be immunosuppression, such as that of transplanted people or human immunodeficiency virus infected patients[177]. In fact, a fully functional immune (innate and adaptive) response (humoral and cellular) has been shown to be essential to fight WNV infection in animal models[183-186]. It is known that, overall, humoral immune response is capable of control viral load and dissemination; whereas T-cell mediated response is required for clearance of the virus from the central nervous system (CNS). A TLR3 mediated inflammatory response has been implicated in a higher neuroinvasiveness of the virus in the mouse model[187], but it has not yet been confirmed in humans. Nevertheless, WNV is capable to directly infect neurons, brain stem and spinal cord[188].
The pathogenesis of WNV infection is similar to that of other Flaviviruses. After primary inoculation, WNV is believed to replicate in resident skin Langerhans dendritic cells before it traffics to the lymph nodes and blood stream from where it reaches the spleen and kidneys and, finally penetrates the CNS resulting in inflammation of the medulla, brain stem and spinal cord[184]. In any case, the mechanisms by which the virus enters the CNS are still to be fully elucidated. The proposed routes include: via leukocytes, direct entry across the brain barrier, and by retrograde axonal transport via the peripheral nervous system[189,190].
As many viruses, WNV has developed different strategies to block the action of type I interferon (IFN) and, thus, to evade the host antiviral activity of IFN-stimulating genes, ISG[184,186]. Different reports indicate that non-structural proteins NS1, NS2A, NS4B and NS5 contribute to control IFN α/β signaling by different ways[186,190]. On the other hand, genetic polymorphism of the IFN-inducible 2’5-Oligodenylate synthetases has been implicated in the host innate resistance to WNV infection in horses[191], humans[192], and mice[193].
As in humans, the majority of WNV infections in horses are usually not accompanied by presentation of clinical signs, which are only observed in around 10% of the animals[121] and are characterized by fever and, sometimes, by ataxia and muscular weakness; however, some others signs like dysmetria, somnolence, or hyperexcitation have also been observed[159]. Illness usually last for 3 wk[194]. Even though outbreaks resulted in 25% to 45% of mortality rates among affected horses[194,195], implementation of vaccination campaigns has strongly reduced these mortality rates[167], and treatment guidelines for horses with WND have been published. Lesions by WNV infection in horses are mainly limited to the CNS such as polioencephalomielitis and, in the most severe cases, neuronal degeneration[159]. Even though the underlying mechanism is uncertain, it has been suggested that there is an immunopathological component involved, because inflammatory changes were present in the absence of abundant viral antigens. Contrary to other Flavivirus infections, clinical cases of WND in horses do not precede that in humans, discarding their use as sentinels[196].
Typical clinical signs in birds are neurological, such as ataxia and paralysis, as well as non-neurological, such as depression, lethargy, ruffled feathers, weight loss and myocarditis[112]. When birds die from WND, they use to do it in the first 24 h after the onset of clinical signs. WNV infection causes damage in multiple bird organs, as brain, kidney, heart, lungs, liver, gonads, spleen, intestines, esophagus, and skin. As mentioned early, crows and blue jays are highly susceptible to WNV infection[112] and, thus, monitoring of crow mortality has been successfully adopted as an epidemiological indicator for tracking WNV activity in the USA, predicting an increase of the risk for human infections[197,198].
Beside birds, horses and humans, WND is unusual in other vertebrates, but neurological manifestations have been reported in squirrels[199], dogs and cats[200], and alligators[124]. Hamsters[134,201] and mice[131,132,168] are frequently used as experimental models, as they present some disease signs that parallel those exhibited by humans with severe neuroinvasive disease, such as confusion, tremor of extremities and paralysis.
Persistent infection was early described in rhesus monkeys in which virus could be recovered post-mortem from different tissues of sacrificed animals that did not show any sign of WND and presented specific antibodies in serum[202]. Later on, persistent infection of brains and kidneys has been reported in the hamster model with viral shedding in urine for up to 8 mo[133,134]. Chronic infection has also been recently described in immunocompetent mice without any clinical signs of disease[203]. All these animal experimental data suggest that persistent infections may occur in humans and should be carefully monitored. In fact, a recent study has detected WNV-RNA in the urine of 20% of a study cohort 7 years after being infected, even though no infectious virus could be recovered[181]. Similarly, WNV-RNA and antigens were also detected in the CNS of an encephalitis patient with B cell lymphoma 99 d after symptoms onset[204].
Laboratory diagnosis relies on isolation of virus, detection of viral antigens or RNA in blood or tissues, or detection of virus-specific IgM antibody that should be further confirmed by detection of IgG antibody in the same or a subsequent sample.
Cross-reactivity between Flavivirus antigens is the greatest drawback for proper serological diagnosis and epidemiological studies and, thus, sera have to be tested against different related viruses and results have to be subsequently confirmed by different assays, namely hemagglutination inhibition, immunoflourescence or plaque reduction neutralization test (PRNT), considered as the gold-standard[205]. A 4-fold increase in PRNT titers between 2 sequential serum samples collected 2-3 wk apart usually confirms an acute WNV infection, and WNV neutralizing titers 4-fold higher than titers to other related-flavivirus is usually taken as a probe of the specificity of the infection.
Initially, serological testing was based on IgM antibody capture assays (MAC-ELISA) and in indirect IgG ELISAs, followed by retesting of positive samples by PRNT. Later on, ELISAs using monoclonal antibody blocking assays were set up. Presence of IgM in the cerebrospinal fluid is indicative of infection of the CNS, because IgM does not cross the blood-brain barrier; however, data should be taken with caution since IgM may persist for extended period of time[206]. All these assays have been extensively used for detection of anti-WNV antibodies in human and animal samples[53].
ELISAs, both commercial and in-house, were mainly based in the use of inactivated whole virus as antigen, either produced in mammalian cells or suckling mice; however, its production implies risks for laboratory personnel and needs highly sophisticated biosafety level 3 (BSL-3) containment facilities to grow the virus. For these reasons, several ELISAs have been developed using recombinant viral proteins, mainly the envelope E protein or parts of it, because this protein is highly exposed to the host immune system and bears most of the neutralizing epitopes described[206]. These recombinant antigens have been expressed in a variety of systems, including bacteria[207], mammalian cells[208,209], insect cells[209-211], and larvae[211]. Other formats, such as microsphere particles in conjunction with fluorescent labelled antibodies and lateral-flow assays have also been recently assayed[53].
Virus isolation in susceptible cell culture is the gold standard for virus detection, but it is usually hampered by the typical short duration and low levels of viremia and by the need of BSL-3 facilities, which has lead to the development of alternative methods. Detection of viral antigens is based on antigen-capture ELISAs, dipstick assays, or immunohistochemical methods[212,213].
Several methods for detection of viral RNA have been applied for WNV surveillance and diagnosis, mainly reverse transcription polymerase chain reaction (RT-PCR) assays, quantitative real-time RT-PCR and nucleic acid sequenced-based amplification[214]. All these assays have been extensively used in mosquito pools, and animal and human samples (blood and/or CFS), although the latter are usually collected after the onset of clinical signs, when virus is unlikely to be present on them.
Although great effort on the understanding of the molecular biology of WNV has been paid recently, to date no vaccine or specific therapy has been approved for humans, and clinical treatment is only supportive[186]. However, promising approaches are developing, which can be summarized as the search for antivirals, therapeutic antibodies and the design of vaccines.
The search for antiviral compounds active against WNV infection includes the identification of those targeting distinct aspects of the viral replication cycle. The candidate may be targeted against the viral particle itself, impairing its entry and/or infection, or it may block multiplication of WNV within infected cells. This search has lead to the identification of multiple promising compounds interfering with these processes. However, long way remains to be completed before these novel compounds could to be administered to humans or animals, as they should be first tested for antiviral activity in animal models and adverse effects should be ruled out. The availability of multiple approaches based on sub-genomic replicons and viruses encoding reporter genes has contributed to the initial identification of novel putative antiviral compounds, including those that interfere with viral entry and membrane fusion[215], compounds directed against NS5 enzymatic activities as polymerase or methyltransferase inhibitors[5,216], and inhibitors of the NS2B/NS3 protease complex[70]. Candidates for antiviral molecules include a wide panel of options that range from small molecules (either developed by rational design, identified by structure-based approaches, or by high throughput screening of libraries of chemical substances), peptide inhibitors, antisense oligonucleotides, small interference RNAs, or imino sugars that inhibit glycosylation of WNV proteins[186].
The search for antiviral compounds against WNV not only includes development of novel molecules, but also repositioning of drugs approved for other purposes that have also shown antiviral activities against WNV. Ribavirin, a widely used antiviral drug, has been tested against WNV and, albeit promising anti-viral effect was derived from in vitro assays and also from animal models, treatment of infected patients has not rendered satisfactory results[217]. Mycophenolic acid, an immunosupresant used to prevent rejection of transplanted organs, also inhibits WNV replication in vitro, however antiviral properties in vivo have not been observed[186]. Sodium valproate, a drug that is currently used in humans for treatment of epilepsy and bipolar disorder, has antiviral effect against WNV in cultured cells[218], but no significant anti-WNV activity in experimental animal models has been observed (Saiz JC, Martín-Acebes MA, Vázquez A, and Sobrino F, unpublished results).
Other interesting antiviral tools for the treatment of WNV are drugs that help the immune system to fight the infection. These immunomodulators comprise interferon derivatives or compounds that induce interferon expression[53]. In fact, infection by WNV is highly sensitive to interferon and, when administered to a limited number of WNV-infected patients, help to reduce disease complications[186]. Progress in WNV therapies should thus pass through a combination of drug strategies that targets viral replication, boosts protective immune responses, minimizes neuronal injury, and limits the development of resistant variants.
Therapeutic antibodies that either specifically target WNV particles (mainly directed against E glycoprotein and a minor proportion against prM/M) and neutralize infection or recognize NS1 protein and contribute to protection have also been assayed[219]. Passive administration of anti-WNV antibodies is both protective and therapeutic, and does not cause adverse effects related to the immune enhancement observed for other flaviviruses[186]. Therapeutic antibody administration should be useful for elderly and immunocompromised people, two sectors of the population with major risks of WNV neuroinvasive disease. Treatment with intravenous immunoglobulins obtained from pooled human sera help recovery of infected patients[186,220,221]. These antibodies, which are naturally raised by infected patients, can also be produced in the laboratories, thus reducing the problems associated with their purification from human plasma and the presence of other pathogens, and increasing their availability and reproducible antiviral efficacy. Currently, monoclonal antibodies, either from human origin or humanized, are being tested and it is expected that they should be available for human therapy within a reasonable period of time[222].
Even though notable progress for WNV vaccine development has been made, no approved vaccines exist for human use[205,220,223], and their cost-effectiveness for human treatment is still uncertain. On the other hand, there are effective, licensed vaccines for horses that had greatly contributed to the decrease incidence of equine cases in the US, whilst the number of human cases still remains growing[205,224].
Several experimental vaccines strategies have been tested in preclinical models and some have undergone clinical trials[223,225,226], including classical development of vaccines based on the use of live attenuated or chemically inactivated virus obtained from infected cell cultures or from inoculated suckling mouse brains. Recombinant DNA technology has been also applied for engineering DNA and recombinant vaccines based on the use of viral proteins (or fragments of them) have been synthesized in diverse systems (from bacteria to insect cells and larvae)[205,220,223]. Vertical transfer of acquired maternal immunity to the offspring has been demonstrated in mice immunized with recombinant proteins[227].
For horses, three vaccines are commercialized in the US: one based on formalin-inactivated WNV (Innovator®, FortDodge, Princeton, NJ, US), a recombinant live canarypox vaccine that express prM and E genes formulated with Carbopol adjuvant (Recombitek®, MerialLtd., Athens, GA, US), and a plasmid DNA vaccine, pCBWN, that encodes WNV structural antigens (prM-E) (Fort Dodge and Center for Disease Control and Prevention). Additionally, a commercially available formaldehyde inactivated vaccine derived from infected suckling mice brains and live attenuated vaccines have been administered to domestic geese in Israel[228,229]. The success of veterinary vaccines has encouraged the development of human vaccines that should induce a good response on higher risk groups and achieve an affordable cost/benefit ratio.
Prevention and control of WND require an integrated approach that includes vaccination, enhanced mosquito control and improved clinical management. Preventive tools other than drugs and vaccines can also help to combat the spread of the infection by avoiding mosquito bites responsible for disease transmission. These simple measures can be summarized under the topic ‘fight the bite!’ and include the use of insect repellents, the minimization of skin surface exposed to mosquito bites, the elimination of standing water where mosquitoes can lay eggs, the installation of window and door screens, the minimization of outdoor activities coincident with the maximum activity of mosquitoes, reporting dead birds to local authorities and supporting mosquito control programs (http://www.cdc.gov/).
The recent emergence and expansion of WNV in America and the increase in the number, frequency and severity of outbreaks in Europe represent one of the major zoonotic treats in recent years, and indicate that new epidemiological and ecological scenarios are being developed around the world. Although our knowledge about WNV infection has greatly increased during recent years, some aspects of WNV activity still need to be further addressed: the ways WNV colonizes new ecological niches and the role that climate (temperature, humidity, etc.) and anthropogenic factors play; the differences in WNV disease manifestations between the US and other regions of the world, mainly Central and South America; a better understanding of WNV immunity, pathogenicity, and the molecular basis of virulence; the long-term manifestations and sequelae of WNV infection and the outcome of persistent infections; the development of national and international surveillance programs to monitor WNV spread and to take appropriate measures to control it; the search for more efficient, rapid, and specific diagnostic assays that can be easily adopted worldwide; and the search for cost-effective human vaccines for high-risk targeted populations and for new antiviral targets for therapeutic usage. Advances on our current knowledge on WNV infection will greatly help to fight not only future expansion of WNV to new niches around the world, but also of other arboviruses.
Peer reviewer: Parin Chaivisuthangkura, PhD, Associate Professor, Department of Biology, Faculty of Science, Srinakharinwirot University, Sukhumvit 23, Wattana, Bangkok 10110, Thailand
S- Editor Zhang SS L- Editor A E- Editor Zheng XM
| 1. | Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG, Kuhn RJ. Structure of West Nile virus. Science. 2003;302:248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (1)] |
| 2. | Lanciotti RS, Roehrig JT, Deubel V, Smith J, Parker M, Steele K, Crise B, Volpe KE, Crabtree MB, Scherret JH. Origin of the West Nile virus responsible for an outbreak of encephalitis in the northeastern United States. Science. 1999;286:2333-2337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1158] [Cited by in RCA: 1085] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 3. | Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 277] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 4. | Daffis S, Szretter KJ, Schriewer J, Li J, Youn S, Errett J, Lin TY, Schneller S, Zust R, Dong H. 2'-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452-456. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 756] [Cited by in RCA: 699] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 5. | Dong H, Zhang B, Shi PY. Flavivirus methyltransferase: a novel antiviral target. Antiviral Res. 2008;80:1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 6. | Chu JJ, Ng ML. Interaction of West Nile virus with alpha v beta 3 integrin mediates virus entry into cells. J Biol Chem. 2004;279:54533-54541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 172] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 7. | Medigeshi GR, Hirsch AJ, Streblow DN, Nikolich-Zugich J, Nelson JA. West Nile virus entry requires cholesterol-rich membrane microdomains and is independent of alphavbeta3 integrin. J Virol. 2008;82:5212-5219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Lee E, Hall RA, Lobigs M. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and West Nile viruses. J Virol. 2004;78:8271-8280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 9. | Davis CW, Nguyen HY, Hanna SL, Sánchez MD, Doms RW, Pierson TC. West Nile virus discriminates between DC-SIGN and DC-SIGNR for cellular attachment and infection. J Virol. 2006;80:1290-1301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 266] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 10. | Chu JJ, Leong PW, Ng ML. Analysis of the endocytic pathway mediating the infectious entry of mosquito-borne flavivirus West Nile into Aedes albopictus mosquito (C6/36) cells. Virology. 2006;349:463-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Chu JJ, Ng ML. Infectious entry of West Nile virus occurs through a clathrin-mediated endocytic pathway. J Virol. 2004;78:10543-10555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 249] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, Brass AL, Adametz R, Tsui M, Qian F. RNA interference screen for human genes associated with West Nile virus infection. Nature. 2008;455:242-245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 452] [Cited by in RCA: 423] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 13. | Fernandez-Garcia MD, Meertens L, Bonazzi M, Cossart P, Arenzana-Seisdedos F, Amara A. Appraising the roles of CBLL1 and the ubiquitin/proteasome system for flavivirus entry and replication. J Virol. 2011;85:2980-2989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Krishnan MN, Sukumaran B, Pal U, Agaisse H, Murray JL, Hodge TW, Fikrig E. Rab 5 is required for the cellular entry of dengue and West Nile viruses. J Virol. 2007;81:4881-4885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 185] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 15. | Martín-Acebes MA, Saiz JC. A West Nile virus mutant with increased resistance to acid-induced inactivation. J Gen Virol. 2011;92:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Moesker B, Rodenhuis-Zybert IA, Meijerhof T, Wilschut J, Smit JM. Characterization of the functional requirements of West Nile virus membrane fusion. J Gen Virol. 2010;91:389-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 17. | Kaufmann B, Chipman PR, Holdaway HA, Johnson S, Fremont DH, Kuhn RJ, Diamond MS, Rossmann MG. Capturing a flavivirus pre-fusion intermediate. PLoS Pathog. 2009;5:e1000672. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Thompson BS, Moesker B, Smit JM, Wilschut J, Diamond MS, Fremont DH. A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes. PLoS Pathog. 2009;5:e1000453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Gillespie LK, Hoenen A, Morgan G, Mackenzie JM. The endoplasmic reticulum provides the membrane platform for biogenesis of the flavivirus replication complex. J Virol. 2010;84:10438-10447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 305] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 20. | Mackenzie JM, Jones MK, Westaway EG. Markers for trans-Golgi membranes and the intermediate compartment localize to induced membranes with distinct replication functions in flavivirus-infected cells. J Virol. 1999;73:9555-9567. [PubMed] |
| 21. | Martín-Acebes MA, Blázquez AB, Jiménez de Oya N, Escribano-Romero E, Saiz JC. West Nile virus replication requires fatty acid synthesis but is independent on phosphatidylinositol-4-phosphate lipids. PLoS One. 2011;6:e24970. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Mackenzie JM, Khromykh AA, Westaway EG. Stable expression of noncytopathic Kunjin replicons simulates both ultrastructural and biochemical characteristics observed during replication of Kunjin virus. Virology. 2001;279:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Westaway EG, Mackenzie JM, Kenney MT, Jones MK, Khromykh AA. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J Virol. 1997;71:6650-6661. [PubMed] |
| 24. | Mackenzie JM, Khromykh AA, Parton RG. Cholesterol manipulation by West Nile virus perturbs the cellular immune response. Cell Host Microbe. 2007;2:229-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 241] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Heaton NS, Perera R, Berger KL, Khadka S, Lacount DJ, Kuhn RJ, Randall G. Dengue virus nonstructural protein 3 redistributes fatty acid synthase to sites of viral replication and increases cellular fatty acid synthesis. Proc Natl Acad Sci USA. 2010;107:17345-17350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 364] [Cited by in RCA: 434] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 26. | Gilfoy F, Fayzulin R, Mason PW. West Nile virus genome amplification requires the functional activities of the proteasome. Virology. 2009;385:74-84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Hoenen A, Liu W, Kochs G, Khromykh AA, Mackenzie JM. West Nile virus-induced cytoplasmic membrane structures provide partial protection against the interferon-induced antiviral MxA protein. J Gen Virol. 2007;88:3013-3017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Medigeshi GR, Lancaster AM, Hirsch AJ, Briese T, Lipkin WI, Defilippis V, Früh K, Mason PW, Nikolich-Zugich J, Nelson JA. West Nile virus infection activates the unfolded protein response, leading to CHOP induction and apoptosis. J Virol. 2007;81:10849-10860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 189] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 29. | Ambrose RL, Mackenzie JM. West Nile virus differentially modulates the unfolded protein response to facilitate replication and immune evasion. J Virol. 2011;85:2723-2732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 169] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 30. | Parquet MC, Kumatori A, Hasebe F, Morita K, Igarashi A. West Nile virus-induced bax-dependent apoptosis. FEBS Lett. 2001;500:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Davis WG, Blackwell JL, Shi PY, Brinton MA. Interaction between the cellular protein eEF1A and the 3'-terminal stem-loop of West Nile virus genomic RNA facilitates viral minus-strand RNA synthesis. J Virol. 2007;81:10172-10187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 32. | Emara MM, Brinton MA. Interaction of TIA-1/TIAR with West Nile and dengue virus products in infected cells interferes with stress granule formation and processing body assembly. Proc Natl Acad Sci USA. 2007;104:9041-9046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 251] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 33. | Shi PY, Li W, Brinton MA. Cell proteins bind specifically to West Nile virus minus-strand 3' stem-loop RNA. J Virol. 1996;70:6278-6287. [PubMed] |
| 34. | Zhang B, Dong H, Stein DA, Iversen PL, Shi PY. West Nile virus genome cyclization and RNA replication require two pairs of long-distance RNA interactions. Virology. 2008;373:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 35. | Lo MK, Tilgner M, Bernard KA, Shi PY. Functional analysis of mosquito-borne flavivirus conserved sequence elements within 3' untranslated region of West Nile virus by use of a reporting replicon that differentiates between viral translation and RNA replication. J Virol. 2003;77:10004-10014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 152] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 36. | Dong H, Zhang B, Shi PY. Terminal structures of West Nile virus genomic RNA and their interactions with viral NS5 protein. Virology. 2008;381:123-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 37. | Villordo SM, Alvarez DE, Gamarnik AV. A balance between circular and linear forms of the dengue virus genome is crucial for viral replication. RNA. 2010;16:2325-2335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 38. | Tilgner M, Shi PY. Structure and function of the 3' terminal six nucleotides of the west nile virus genome in viral replication. J Virol. 2004;78:8159-8171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 39. | Dokland T, Walsh M, Mackenzie JM, Khromykh AA, Ee KH, Wang S. West Nile virus core protein; tetramer structure and ribbon formation. Structure. 2004;12:1157-1163. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 40. | Schrauf S, Mandl CW, Bell-Sakyi L, Skern T. Extension of flavivirus protein C differentially affects early RNA synthesis and growth in mammalian and arthropod host cells. J Virol. 2009;83:11201-11210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 41. | Ivanyi-Nagy R, Lavergne JP, Gabus C, Ficheux D, Darlix JL. RNA chaperoning and intrinsic disorder in the core proteins of Flaviviridae. Nucleic Acids Res. 2008;36:712-725. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 42. | Yang MR, Lee SR, Oh W, Lee EW, Yeh JY, Nah JJ, Joo YS, Shin J, Lee HW, Pyo S. West Nile virus capsid protein induces p53-mediated apoptosis via the sequestration of HDM2 to the nucleolus. Cell Microbiol. 2008;10:165-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 43. | Bhuvanakantham R, Chong MK, Ng ML. Specific interaction of capsid protein and importin-alpha/beta influences West Nile virus production. Biochem Biophys Res Commun. 2009;389:63-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Hunt TA, Urbanowski MD, Kakani K, Law LM, Brinton MA, Hobman TC. Interactions between the West Nile virus capsid protein and the host cell-encoded phosphatase inhibitor, I2PP2A. Cell Microbiol. 2007;9:2756-2766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 45. | Oh WK, Song J. Hsp70 functions as a negative regulator of West Nile virus capsid protein through direct interaction. Biochem Biophys Res Commun. 2006;347:994-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 46. | Oh W, Yang MR, Lee EW, Park KM, Pyo S, Yang JS, Lee HW, Song J. Jab1 mediates cytoplasmic localization and degradation of West Nile virus capsid protein. J Biol Chem. 2006;281:30166-30174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Cheong YK, Ng ML. Dephosphorylation of West Nile virus capsid protein enhances the processes of nucleocapsid assembly. Microbes Infect. 2011;13:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 48. | Bhuvanakantham R, Cheong YK, Ng ML. West Nile virus capsid protein interaction with importin and HDM2 protein is regulated by protein kinase C-mediated phosphorylation. Microbes Infect. 2010;12:615-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 49. | Medigeshi GR, Hirsch AJ, Brien JD, Uhrlaub JL, Mason PW, Wiley C, Nikolich-Zugich J, Nelson JA. West nile virus capsid degradation of claudin proteins disrupts epithelial barrier function. J Virol. 2009;83:6125-6134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 50. | Tan TT, Bhuvanakantham R, Li J, Howe J, Ng ML. Tyrosine 78 of premembrane protein is essential for assembly of West Nile virus. J Gen Virol. 2009;90:1081-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Zhang Y, Kaufmann B, Chipman PR, Kuhn RJ, Rossmann MG. Structure of immature West Nile virus. J Virol. 2007;81:6141-6145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 113] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 52. | Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS. Maturation of West Nile virus modulates sensitivity to antibody-mediated neutralization. PLoS Pathog. 2008;4:e1000060. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 144] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 53. | Beasley DW. Recent advances in the molecular biology of west nile virus. Curr Mol Med. 2005;5:835-850. [PubMed] |
| 54. | Moudy RM, Zhang B, Shi PY, Kramer LD. West Nile virus envelope protein glycosylation is required for efficient viral transmission by Culex vectors. Virology. 2009;387:222-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 55. | Murata R, Eshita Y, Maeda A, Maeda J, Akita S, Tanaka T, Yoshii K, Kariwa H, Umemura T, Takashima I. Glycosylation of the West Nile Virus envelope protein increases in vivo and in vitro viral multiplication in birds. Am J Trop Med Hyg. 2010;82:696-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 56. | Shirato K, Miyoshi H, Goto A, Ako Y, Ueki T, Kariwa H, Takashima I. Viral envelope protein glycosylation is a molecular determinant of the neuroinvasiveness of the New York strain of West Nile virus. J Gen Virol. 2004;85:3637-3645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 135] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Kanai R, Kar K, Anthony K, Gould LH, Ledizet M, Fikrig E, Marasco WA, Koski RA, Modis Y. Crystal structure of west nile virus envelope glycoprotein reveals viral surface epitopes. J Virol. 2006;80:11000-11008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 179] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 58. | Nybakken GE, Nelson CA, Chen BR, Diamond MS, Fremont DH. Crystal structure of the West Nile virus envelope glycoprotein. J Virol. 2006;80:11467-11474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 207] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 59. | Fritz R, Stiasny K, Heinz FX. Identification of specific histidines as pH sensors in flavivirus membrane fusion. J Cell Biol. 2008;183:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 137] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 60. | Nelson S, Poddar S, Lin TY, Pierson TC. Protonation of individual histidine residues is not required for the pH-dependent entry of west nile virus: evaluation of the "histidine switch" hypothesis. J Virol. 2009;83:12631-12635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 61. | Macdonald J, Tonry J, Hall RA, Williams B, Palacios G, Ashok MS, Jabado O, Clark D, Tesh RB, Briese T. NS1 protein secretion during the acute phase of West Nile virus infection. J Virol. 2005;79:13924-13933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 62. | Mackenzie JM, Khromykh AA, Jones MK, Westaway EG. Subcellular localization and some biochemical properties of the flavivirus Kunjin nonstructural proteins NS2A and NS4A. Virology. 1998;245:203-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 250] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 63. | Avirutnan P, Fuchs A, Hauhart RE, Somnuke P, Youn S, Diamond MS, Atkinson JP. Antagonism of the complement component C4 by flavivirus nonstructural protein NS1. J Exp Med. 2010;207:793-806. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 235] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 64. | Wilson JR, de Sessions PF, Leon MA, Scholle F. West Nile virus nonstructural protein 1 inhibits TLR3 signal transduction. J Virol. 2008;82:8262-8271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 65. | Melian EB, Hinzman E, Nagasaki T, Firth AE, Wills NM, Nouwens AS, Blitvich BJ, Leung J, Funk A, Atkins JF. NS1' of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J Virol. 2010;84:1641-1647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 147] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 66. | Leung JY, Pijlman GP, Kondratieva N, Hyde J, Mackenzie JM, Khromykh AA. Role of nonstructural protein NS2A in flavivirus assembly. J Virol. 2008;82:4731-4741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 67. | Liu WJ, Chen HB, Khromykh AA. Molecular and functional analyses of Kunjin virus infectious cDNA clones demonstrate the essential roles for NS2A in virus assembly and for a nonconservative residue in NS3 in RNA replication. J Virol. 2003;77:7804-7813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Liu WJ, Wang XJ, Clark DC, Lobigs M, Hall RA, Khromykh AA. A single amino acid substitution in the West Nile virus nonstructural protein NS2A disables its ability to inhibit alpha/beta interferon induction and attenuates virus virulence in mice. J Virol. 2006;80:2396-2404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 198] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 69. | Rossi SL, Fayzulin R, Dewsbury N, Bourne N, Mason PW. Mutations in West Nile virus nonstructural proteins that facilitate replicon persistence in vitro attenuate virus replication in vitro and in vivo. Virology. 2007;364:184-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 70. | Chappell KJ, Stoermer MJ, Fairlie DP, Young PR. West Nile Virus NS2B/NS3 protease as an antiviral target. Curr Med Chem. 2008;15:2771-2784. [PubMed] |
| 71. | Chappell KJ, Stoermer MJ, Fairlie DP, Young PR. Mutagenesis of the West Nile virus NS2B cofactor domain reveals two regions essential for protease activity. J Gen Virol. 2008;89:1010-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 72. | Chernov AV, Shiryaev SA, Aleshin AE, Ratnikov BI, Smith JW, Liddington RC, Strongin AY. The two-component NS2B-NS3 proteinase represses DNA unwinding activity of the West Nile virus NS3 helicase. J Biol Chem. 2008;283:17270-17278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Mastrangelo E, Milani M, Bollati M, Selisko B, Peyrane F, Pandini V, Sorrentino G, Canard B, Konarev PV, Svergun DI. Crystal structure and activity of Kunjin virus NS3 helicase; protease and helicase domain assembly in the full length NS3 protein. J Mol Biol. 2007;372:444-455. [PubMed] |
| 74. | Brault AC, Huang CY, Langevin SA, Kinney RM, Bowen RA, Ramey WN, Panella NA, Holmes EC, Powers AM, Miller BR. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet. 2007;39:1162-1166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 254] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 75. | Miller S, Kastner S, Krijnse-Locker J, Bühler S, Bartenschlager R. The non-structural protein 4A of dengue virus is an integral membrane protein inducing membrane alterations in a 2K-regulated manner. J Biol Chem. 2007;282:8873-8882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 361] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 76. | Muñoz-Jordan JL, Sánchez-Burgos GG, Laurent-Rolle M, García-Sastre A. Inhibition of interferon signaling by dengue virus. Proc Natl Acad Sci USA. 2003;100:14333-14338. [PubMed] |
| 77. | Mertens E, Kajaste-Rudnitski A, Torres S, Funk A, Frenkiel MP, Iteman I, Khromykh AA, Desprès P. Viral determinants in the NS3 helicase and 2K peptide that promote West Nile virus resistance to antiviral action of 2',5'-oligoadenylate synthetase 1b. Virology. 2010;399:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 78. | Zou G, Puig-Basagoiti F, Zhang B, Qing M, Chen L, Pankiewicz KW, Felczak K, Yuan Z, Shi PY. A single-amino acid substitution in West Nile virus 2K peptide between NS4A and NS4B confers resistance to lycorine, a flavivirus inhibitor. Virology. 2009;384:242-252. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 120] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 79. | Shiryaev SA, Chernov AV, Aleshin AE, Shiryaeva TN, Strongin AY. NS4A regulates the ATPase activity of the NS3 helicase: a novel cofactor role of the non-structural protein NS4A from West Nile virus. J Gen Virol. 2009;90:2081-2085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 80. | Evans JD, Seeger C. Differential effects of mutations in NS4B on West Nile virus replication and inhibition of interferon signaling. J Virol. 2007;81:11809-11816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 81. | Muñoz-Jordán JL, Laurent-Rolle M, Ashour J, Martínez-Sobrido L, Ashok M, Lipkin WI, García-Sastre A. Inhibition of alpha/beta interferon signaling by the NS4B protein of flaviviruses. J Virol. 2005;79:8004-8013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 418] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 82. | Welte T, Xie G, Wicker JA, Whiteman MC, Li L, Rachamallu A, Barrett A, Wang T. Immune responses to an attenuated West Nile virus NS4B-P38G mutant strain. Vaccine. 2011;29:4853-4861. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 83. | Wicker JA, Whiteman MC, Beasley DW, Davis CT, Zhang S, Schneider BS, Higgs S, Kinney RM, Barrett AD. A single amino acid substitution in the central portion of the West Nile virus NS4B protein confers a highly attenuated phenotype in mice. Virology. 2006;349:245-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 84. | Westaway EG, Khromykh AA, Kenney MT, Mackenzie JM, Jones MK. Proteins C and NS4B of the flavivirus Kunjin translocate independently into the nucleus. Virology. 1997;234:31-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (1)] |
| 85. | Mackenzie JM, Kenney MT, Westaway EG. West Nile virus strain Kunjin NS5 polymerase is a phosphoprotein localized at the cytoplasmic site of viral RNA synthesis. J Gen Virol. 2007;88:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 86. | Zhou Y, Ray D, Zhao Y, Dong H, Ren S, Li Z, Guo Y, Bernard KA, Shi PY, Li H. Structure and function of flavivirus NS5 methyltransferase. J Virol. 2007;81:3891-3903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 301] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 87. | Malet H, Egloff MP, Selisko B, Butcher RE, Wright PJ, Roberts M, Gruez A, Sulzenbacher G, Vonrhein C, Bricogne G. Crystal structure of the RNA polymerase domain of the West Nile virus non-structural protein 5. J Biol Chem. 2007;282:10678-10689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 212] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 88. | Ray D, Shah A, Tilgner M, Guo Y, Zhao Y, Dong H, Deas TS, Zhou Y, Li H, Shi PY. West Nile virus 5'-cap structure is formed by sequential guanine N-7 and ribose 2'-O methylations by nonstructural protein 5. J Virol. 2006;80:8362-8370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 333] [Cited by in RCA: 310] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 89. | Laurent-Rolle M, Boer EF, Lubick KJ, Wolfinbarger JB, Carmody AB, Rockx B, Liu W, Ashour J, Shupert WL, Holbrook MR. The NS5 protein of the virulent West Nile virus NY99 strain is a potent antagonist of type I interferon-mediated JAK-STAT signaling. J Virol. 2010;84:3503-3515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 187] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 90. | Poidinger M, Hall RA, Mackenzie JS. Molecular characterization of the Japanese encephalitis serocomplex of the flavivirus genus. Virology. 1996;218:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 104] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 91. | Bondre VP, Jadi RS, Mishra AC, Yergolkar PN, Arankalle VA. West Nile virus isolates from India: evidence for a distinct genetic lineage. J Gen Virol. 2007;88:875-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 92. | Lanciotti RS, Ebel GD, Deubel V, Kerst AJ, Murri S, Meyer R, Bowen M, McKinney N, Morrill WE, Crabtree MB. Complete genome sequences and phylogenetic analysis of West Nile virus strains isolated from the United States, Europe, and the Middle East. Virology. 2002;298:96-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 280] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 93. | Hubálek Z, Halouzka J, Juricová Z, Sebesta O. First isolation of mosquito-borne West Nile virus in the Czech Republic. Acta Virol. 1998;42:119-120. [PubMed] |
| 94. | Lvov DK, Butenko AM, Gromashevsky VL, Kovtunov AI, Prilipov AG, Kinney R, Aristova VA, Dzharkenov AF, Samokhvalov EI, Savage HM. West Nile virus and other zoonotic viruses in Russia: examples of emerging-reemerging situations. Arch Virol Suppl. 2004;18:85-96. [PubMed] |
| 95. | Hall RA, Scherret JH, Mackenzie JS. Kunjin virus: an Australian variant of West Nile. Ann N Y Acad Sci. 2001;951:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 96. | May FJ, Davis CT, Tesh RB, Barrett AD. Phylogeography of West Nile virus: from the cradle of evolution in Africa to Eurasia, Australia, and the Americas. J Virol. 2011;85:2964-2974. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 201] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 97. | Bakonyi T, Ivanics E, Erdélyi K, Ursu K, Ferenczi E, Weissenböck H, Nowotny N. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12:618-623. [PubMed] |
| 98. | Vazquez A, Sanchez-Seco MP, Ruiz S, Molero F, Hernandez L, Moreno J, Magallanes A, Tejedor CG, Tenorio A. Putative new lineage of west nile virus, Spain. Emerg Infect Dis. 2010;16:549-552. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 99. | Brault AC. Changing patterns of West Nile virus transmission: altered vector competence and host susceptibility. Vet Res. 2009;40:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 100. | Zeller HG, Schuffenecker I. West Nile virus: an overview of its spread in Europe and the Mediterranean basin in contrast to its spread in the Americas. Eur J Clin Microbiol Infect Dis. 2004;23:147-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 269] [Cited by in RCA: 261] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 101. | Hall RA, Broom AK, Smith DW, Mackenzie JS. The ecology and epidemiology of Kunjin virus. Curr Top Microbiol Immunol. 2002;267:253-269. [PubMed] |
| 102. | Hayes EB, Komar N, Nasci RS, Montgomery SP, O'Leary DR, Campbell GL. Epidemiology and transmission dynamics of West Nile virus disease. Emerg Infect Dis. 2005;11:1167-1173. [PubMed] |
| 103. | Goddard LB, Roth AE, Reisen WK, Scott TW. Vector competence of California mosquitoes for West Nile virus. Emerg Infect Dis. 2002;8:1385-1391. [PubMed] |
| 104. | Styer LM, Kent KA, Albright RG, Bennett CJ, Kramer LD, Bernard KA. Mosquitoes inoculate high doses of West Nile virus as they probe and feed on live hosts. PLoS Pathog. 2007;3:1262-1270. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 197] [Cited by in RCA: 186] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 105. | Anderson JF, Main AJ. Importance of vertical and horizontal transmission of West Nile virus by Culex pipiens in the Northeastern United States. J Infect Dis. 2006;194:1577-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 106. | Nasci RS, Savage HM, White DJ, Miller JR, Cropp BC, Godsey MS, Kerst AJ, Bennett P, Gottfried K, Lanciotti RS. West Nile virus in overwintering Culex mosquitoes, New York City, 2000. Emerg Infect Dis. 2001;7:742-744. [PubMed] |
| 107. | Bernard KA, Maffei JG, Jones SA, Kauffman EB, Ebel G, Dupuis AP, Ngo KA, Nicholas DC, Young DM, Shi PY. West Nile virus infection in birds and mosquitoes, New York State, 2000. Emerg Infect Dis. 2001;7:679-685. [PubMed] |
| 108. | Hubálek Z, Halouzka J. West Nile fever--a reemerging mosquito-borne viral disease in Europe. Emerg Infect Dis. 1999;5:643-650. [PubMed] |
| 109. | Farajollahi A, Crans WJ, Nickerson D, Bryant P, Wolf B, Glaser A, Andreadis TG. Detection of West Nile virus RNA from the louse fly Icosta americana (Diptera: Hippoboscidae). J Am Mosq Control Assoc. 2005;21:474-476. [PubMed] |
| 110. | Mumcuoglu KY, Banet-Noach C, Malkinson M, Shalom U, Galun R. Argasid ticks as possible vectors of West Nile virus in Israel. Vector Borne Zoonotic Dis. 2005;5:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 111. | Brault AC, Langevin SA, Bowen RA, Panella NA, Biggerstaff BJ, Miller BR, Komar N. Differential virulence of West Nile strains for American crows. Emerg Infect Dis. 2004;10:2161-2168. [PubMed] |
| 112. | Komar N, Langevin S, Hinten S, Nemeth N, Edwards E, Hettler D, Davis B, Bowen R, Bunning M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311-322. [PubMed] |
| 113. | LaDeau SL, Kilpatrick AM, Marra PP. West Nile virus emergence and large-scale declines of North American bird populations. Nature. 2007;447:710-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 292] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 114. | Reed LM, Johansson MA, Panella N, McLean R, Creekmore T, Puelle R, Komar N. Declining mortality in American crow (Corvus brachyrhynchos) following natural West Nile virus infection. Avian Dis. 2009;53:458-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 115. | Senne DA, Pedersen JC, Hutto DL, Taylor WD, Schmitt BJ, Panigrahy B. Pathogenicity of West Nile virus in chickens. Avian Dis. 2000;44:642-649. [PubMed] |
| 116. | McLean RG, Ubico SR, Docherty DE, Hansen WR, Sileo L, McNamara TS. West Nile virus transmission and ecology in birds. Ann N Y Acad Sci. 2001;951:54-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 117. | Garmendia AE, Van Kruiningen HJ, French RA, Anderson JF, Andreadis TG, Kumar A, West AB. Recovery and identification of West Nile virus from a hawk in winter. J Clin Microbiol. 2000;38:3110-3111. [PubMed] |
| 118. | Roehrig JT, Layton M, Smith P, Campbell GL, Nasci R, Lanciotti RS. The emergence of West Nile virus in North America: ecology, epidemiology, and surveillance. Curr Top Microbiol Immunol. 2002;267:223-240. [PubMed] |
| 119. | Murgue B, Zeller H, Deubel V. The ecology and epidemiology of West Nile virus in Africa, Europe and Asia. Curr Top Microbiol Immunol. 2002;267:195-221. [PubMed] |
| 120. | Jupp PG. The ecology of West Nile virus in South Africa and the occurrence of outbreaks in humans. Ann N Y Acad Sci. 2001;951:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 86] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 121. | Bunning ML, Bowen RA, Cropp CB, Sullivan KG, Davis BS, Komar N, Godsey MS, Baker D, Hettler DL, Holmes DA. Experimental infection of horses with West Nile virus. Emerg Infect Dis. 2002;8:380-386. [PubMed] |
| 122. | Blitvich BJ. Transmission dynamics and changing epidemiology of West Nile virus. Anim Health Res Rev. 2008;9:71-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 123. | Kostiukov MA, Gordeeva ZE, Bulychev VP, Nemova NV, Daniiarov OA. [The lake frog (Rana ridibunda)--one of the food hosts of blood-sucking mosquitoes in Tadzhikistan--a reservoir of the West Nile fever virus]. Med Parazitol (Mosk). 1985;3:49-50. [PubMed] |
| 124. | Klenk K, Snow J, Morgan K, Bowen R, Stephens M, Foster F, Gordy P, Beckett S, Komar N, Gubler D. Alligators as West Nile virus amplifiers. Emerg Infect Dis. 2004;10:2150-2155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 90] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 125. | Steinman A, Banet-Noach C, Tal S, Levi O, Simanov L, Perk S, Malkinson M, Shpigel N. West Nile virus infection in crocodiles. Emerg Infect Dis. 2003;9:887-889. [PubMed] |
| 126. | Steinman A, Banet-Noach C, Simanov L, Grinfeld N, Aizenberg Z, Levi O, Lahav D, Malkinson M, Perk S, Shpigel NY. Experimental infection of common garter snakes (Thamnophis sirtalis) with West Nile virus. Vector Borne Zoonotic Dis. 2006;6:361-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 127. | Iwamoto M, Jernigan DB, Guasch A, Trepka MJ, Blackmore CG, Hellinger WC, Pham SM, Zaki S, Lanciotti RS, Lance-Parker SE. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348:2196-2203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 520] [Cited by in RCA: 454] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 128. | Paisley JE, Hinckley AF, O'Leary DR, Kramer WC, Lanciotti RS, Campbell GL, Hayes EB. West Nile virus infection among pregnant women in a northern Colorado community, 2003 to 2004. Pediatrics. 2006;117:814-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 129. | Pealer LN, Marfin AA, Petersen LR, Lanciotti RS, Page PL, Stramer SL, Stobierski MG, Signs K, Newman B, Kapoor H. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349:1236-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 434] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 130. | Hayes EB, O'Leary DR. West Nile virus infection: a pediatric perspective. Pediatrics. 2004;113:1375-1381. [PubMed] |
| 131. | Córdoba L, Escribano-Romero E, Garmendia A, Saiz JC. Pregnancy increases the risk of mortality in West Nile virus-infected mice. J Gen Virol. 2007;88:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 132. | Blázquez AB, Sáiz JC. West Nile virus (WNV) transmission routes in the murine model: intrauterine, by breastfeeding and after cannibal ingestion. Virus Res. 2010;151:240-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 133. | Tonry JH, Xiao SY, Siirin M, Chen H, da Rosa AP, Tesh RB. Persistent shedding of West Nile virus in urine of experimentally infected hamsters. Am J Trop Med Hyg. 2005;72:320-324. [PubMed] |
| 134. | Tesh RB, Siirin M, Guzman H, Travassos da Rosa AP, Wu X, Duan T, Lei H, Nunes MR, Xiao SY. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192:287-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 135. | López G, Jiménez-Clavero MA, Tejedor CG, Soriguer R, Figuerola J. Prevalence of West Nile virus neutralizing antibodies in Spain is related to the behavior of migratory birds. Vector Borne Zoonotic Dis. 2008;8:615-621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 136. | Figuerola J, Jiménez-Clavero MA, López G, Rubio C, Soriguer R, Gómez-Tejedor C, Tenorio A. Size matters: West Nile Virus neutralizing antibodies in resident and migratory birds in Spain. Vet Microbiol. 2008;132:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 137. | Figuerola J, Soriguer R, Rojo G, Gómez Tejedor C, Jimenez-Clavero MA. Seroconversion in wild birds and local circulation of West Nile virus, Spain. Emerg Infect Dis. 2007;13:1915-1917. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 70] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 138. | Figuerola J, Jiménez-Clavero MA, Rojo G, Gómez-Tejedor C, Soriguer R. Prevalence of West Nile virus neutralizing antibodies in colonial aquatic birds in southern Spain. Avian Pathol. 2007;36:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 139. | Malkinson M, Banet C, Weisman Y, Pokamunski S, King R, Drouet MT, Deubel V. Introduction of West Nile virus in the Middle East by migrating white storks. Emerg Infect Dis. 2002;8:392-397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 198] [Cited by in RCA: 175] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 140. | Dusek RJ, McLean RG, Kramer LD, Ubico SR, Dupuis AP, Ebel GD, Guptill SC. Prevalence of West Nile virus in migratory birds during spring and fall migration. Am J Trop Med Hyg. 2009;81:1151-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 141. | Reisen WK, Wheeler SS, Garcia S, Fang Y. Migratory birds and the dispersal of arboviruses in California. Am J Trop Med Hyg. 2010;83:808-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 142. | Buckley A, Dawson A, Moss SR, Hinsley SA, Bellamy PE, Gould EA. Serological evidence of West Nile virus, Usutu virus and Sindbis virus infection of birds in the UK. J Gen Virol. 2003;84:2807-2817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | Calistri P, Giovannini A, Savini G, Monaco F, Bonfanti L, Ceolin C, Terregino C, Tamba M, Cordioli P, Lelli R. West Nile virus transmission in 2008 in north-eastern Italy. Zoonoses Public Health. 2010;57:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 60] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 144. | Shelite TR, Rogers CM, Litzner BR, Johnson RR, Schneegurt MA. West Nile virus antibodies in permanent resident and overwintering migrant birds in south-central Kansas. Vector Borne Zoonotic Dis. 2008;8:321-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 145. | Epstein PR. West Nile virus and the climate. J Urban Health. 2001;78:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 146. | Weaver SC, Brault AC, Kang W, Holland JJ. Genetic and fitness changes accompanying adaptation of an arbovirus to vertebrate and invertebrate cells. J Virol. 1999;73:4316-4326. [PubMed] |
| 147. | Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae). J Med Entomol. 2006;43:309-317. [PubMed] |
| 148. | Kilpatrick AM, Meola MA, Moudy RM, Kramer LD. Temperature, viral genetics, and the transmission of West Nile virus by Culex pipiens mosquitoes. PLoS Pathog. 2008;4:e1000092. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 341] [Cited by in RCA: 303] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 149. | Reisen WK, Cayan D, Tyree M, Barker CM, Eldridge B, Dettinger M. Impact of climate variation on mosquito abundance in California. J Vector Ecol. 2008;33:89-98. [PubMed] |
| 150. | Shaman J, Day JF, Stieglitz M. Drought-induced amplification and epidemic transmission of West Nile virus in southern Florida. J Med Entomol. 2005;42:134-141. [PubMed] |
| 151. | Tsai TF, Popovici F, Cernescu C, Campbell GL, Nedelcu NI. West Nile encephalitis epidemic in southeastern Romania. Lancet. 1998;352:767-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 492] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 152. | Platonov AE, Shipulin GA, Shipulina OY, Tyutyunnik EN, Frolochkina TI, Lanciotti RS, Yazyshina S, Platonova OV, Obukhov IL, Zhukov AN. Outbreak of West Nile virus infection, Volgograd Region, Russia, 1999. Emerg Infect Dis. 2001;7:128-132. [PubMed] |
| 153. | Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med. 1940;20:471-492. |
| 154. | Goldblum N, Sterk VV, Paderski B. West Nile fever; the clinical features of the disease and the isolation of West Nile virus from the blood of nine human cases. Am J Hyg. 1954;59:89-103. [PubMed] |
| 155. | Bernkopf H, Levine S, Nerson R. Isolation of West Nile virus in Israel. J Infect Dis. 1953;93:207-218. [PubMed] |
| 156. | Calistri P, Giovannini A, Hubalek Z, Ionescu A, Monaco F, Savini G, Lelli R. Epidemiology of west nile in europe and in the mediterranean basin. Open Virol J. 2010;4:29-37. [PubMed] |
| 157. | Kantzanou MN, Moschidis ZM, Kremastinou G, Levidiotou S, Karafoulidou A, Politis C, Marantidou O, Kavallierou L, Kaperoni A, Veneti C. Searching for West Nile virus (WNV) in Greece. Transfus Med. 2010;20:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 158. | Papa A, Danis K, Baka A, Bakas A, Dougas G, Lytras T, Theocharopoulos G, Chrysagis D, Vassiliadou E, Kamaria F. Ongoing outbreak of West Nile virus infections in humans in Greece, July-August 2010. Euro Surveill. 2010;15:pii=19951. [PubMed] |
| 159. | Castillo-Olivares J, Wood J. West Nile virus infection of horses. Vet Res. 2004;35:467-483. [PubMed] |
| 160. | Lupulovic D, Martín-Acebes MA, Lazic S, Alonso-Padilla J, Blázquez AB, Escribano-Romero E, Petrovic T, Saiz JC. First serological evidence of West Nile virus activity in horses in Serbia. Vector Borne Zoonotic Dis. 2011;11:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 161. | Monaco F, Lelli R, Teodori L, Pinoni C, Di Gennaro A, Polci A, Calistri P, Savini G. Re-emergence of West Nile virus in Italy. Zoonoses Public Health. 2010;57:476-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 162. | Rossini G, Carletti F, Bordi L, Cavrini F, Gaibani P, Landini MP, Pierro A, Capobianchi MR, Di Caro A, Sambri V. Phylogenetic analysis of West Nile virus isolates, Italy, 2008-2009. Emerg Infect Dis. 2011;17:903-906. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 163. | Nur YA, Groen J, Heuvelmans H, Tuynman W, Copra C, Osterhaus AD. An outbreak of West Nile fever among migrants in Kisangani, Democratic Republic of Congo. Am J Trop Med Hyg. 1999;61:885-888. [PubMed] |
| 164. | Pourrut X, Nkoghé D, Paweska J, Leroy E. First serological evidence of West Nile virus in human rural populations of Gabon. Virol J. 2010;7:132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 165. | Venter M, Swanepoel R. West Nile virus lineage 2 as a cause of zoonotic neurological disease in humans and horses in southern Africa. Vector Borne Zoonotic Dis. 2010;10:659-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 166. | Mackenzie JS, Williams DT. The zoonotic flaviviruses of southern, south-eastern and eastern Asia, and Australasia: the potential for emergent viruses. Zoonoses Public Health. 2009;56:338-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 143] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 167. | Ward MP. Spread of equine West Nile virus encephalomyelitis during the 2002 Texas epidemic. Am J Trop Med Hyg. 2006;74:1090-1095. [PubMed] |
| 168. | Beasley DW, Li L, Suderman MT, Barrett AD. Mouse neuroinvasive phenotype of West Nile virus strains varies depending upon virus genotype. Virology. 2002;296:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 185] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 169. | Davis CT, Beasley DW, Guzman H, Siirin M, Parsons RE, Tesh RB, Barrett AD. Emergence of attenuated West Nile virus variants in Texas, 2003. Virology. 2004;330:342-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 72] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 170. | Beasley DW, Whiteman MC, Zhang S, Huang CY, Schneider BS, Smith DR, Gromowski GD, Higgs S, Kinney RM, Barrett AD. Envelope protein glycosylation status influences mouse neuroinvasion phenotype of genetic lineage 1 West Nile virus strains. J Virol. 2005;79:8339-8347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 244] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 171. | Estrada-Franco JG, Navarro-Lopez R, Beasley DW, Coffey L, Carrara AS, Travassos da Rosa A, Clements T, Wang E, Ludwig GV, Cortes AC. West Nile virus in Mexico: evidence of widespread circulation since July 2002. Emerg Infect Dis. 2003;9:1604-1607. [PubMed] |
| 172. | Alonso-Padilla J, Loza-Rubio E, Escribano-Romero E, Córdoba L, Cuevas S, Mejía F, Calderón R, Milián F, Travassos Da Rosa A, Weaver SC. The continuous spread of West Nile virus (WNV): seroprevalence in asymptomatic horses. Epidemiol Infect. 2009;137:1163-1168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 173. | Beasley DW, Davis CT, Estrada-Franco J, Navarro-Lopez R, Campomanes-Cortes A, Tesh RB, Weaver SC, Barrett AD. Genome sequence and attenuating mutations in West Nile virus isolate from Mexico. Emerg Infect Dis. 2004;10:2221-2224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 174. | Davis CT, Ebel GD, Lanciotti RS, Brault AC, Guzman H, Siirin M, Lambert A, Parsons RE, Beasley DW, Novak RJ. Phylogenetic analysis of North American West Nile virus isolates, 2001-2004: evidence for the emergence of a dominant genotype. Virology. 2005;342:252-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 186] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 175. | Beasley DW, Davis CT, Guzman H, Vanlandingham DL, Travassos da Rosa AP, Parsons RE, Higgs S, Tesh RB, Barrett AD. Limited evolution of West Nile virus has occurred during its southwesterly spread in the United States. Virology. 2003;309:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 83] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 176. | McMullen AR, May FJ, Li L, Guzman H, Bueno R, Dennett JA, Tesh RB, Barrett AD. Evolution of new genotype of West Nile virus in North America. Emerg Infect Dis. 2011;17:785-793. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 177. | Campbell GL, Marfin AA, Lanciotti RS, Gubler DJ. West Nile virus. Lancet Infect Dis. 2002;2:519-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 600] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 178. | Hayes EB, Sejvar JJ, Zaki SR, Lanciotti RS, Bode AV, Campbell GL. Virology, pathology, and clinical manifestations of West Nile virus disease. Emerg Infect Dis. 2005;11:1174-1179. [PubMed] |
| 179. | Sejvar JJ, Leis AA, Stokic DS, Van Gerpen JA, Marfin AA, Webb R, Haddad MB, Tierney BC, Slavinski SA, Polk JL. Acute flaccid paralysis and West Nile virus infection. Emerg Infect Dis. 2003;9:788-793. [PubMed] |
| 180. | Sejvar JJ. The long-term outcomes of human West Nile virus infection. Clin Infect Dis. 2007;44:1617-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 170] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 181. | Murray K, Walker C, Herrington E, Lewis JA, McCormick J, Beasley DW, Tesh RB, Fisher-Hoch S. Persistent infection with West Nile virus years after initial infection. J Infect Dis. 2010;201:2-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 182. | Watson JT, Pertel PE, Jones RC, Siston AM, Paul WS, Austin CC, Gerber SI. Clinical characteristics and functional outcomes of West Nile Fever. Ann Intern Med. 2004;141:360-365. [PubMed] |
| 183. | Diamond MS, Shrestha B, Mehlhop E, Sitati E, Engle M. Innate and adaptive immune responses determine protection against disseminated infection by West Nile encephalitis virus. Viral Immunol. 2003;16:259-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 184. | Samuel MA, Diamond MS. Pathogenesis of West Nile Virus infection: a balance between virulence, innate and adaptive immunity, and viral evasion. J Virol. 2006;80:9349-9360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 247] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 185. | Diamond MS. Virus and host determinants of West Nile virus pathogenesis. PLoS Pathog. 2009;5:e1000452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 186. | Diamond MS. Progress on the development of therapeutics against West Nile virus. Antiviral Res. 2009;83:214-227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 91] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 187. | Wang T, Town T, Alexopoulou L, Anderson JF, Fikrig E, Flavell RA. Toll-like receptor 3 mediates West Nile virus entry into the brain causing lethal encephalitis. Nat Med. 2004;10:1366-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 844] [Article Influence: 40.2] [Reference Citation Analysis (0)] |
| 188. | Ceccaldi PE, Lucas M, Despres P. New insights on the neuropathology of West Nile virus. FEMS Microbiol Lett. 2004;233:1-6. [PubMed] |
| 189. | King NJ, Getts DR, Getts MT, Rana S, Shrestha B, Kesson AM. Immunopathology of flavivirus infections. Immunol Cell Biol. 2007;85:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 136] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 190. | Murray KO, Mertens E, Despres P. West Nile virus and its emergence in the United States of America. Vet Res. 2010;41:67. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 131] [Cited by in RCA: 136] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 191. | Rios JJ, Fleming JG, Bryant UK, Carter CN, Huber JC, Long MT, Spencer TE, Adelson DL. OAS1 polymorphisms are associated with susceptibility to West Nile encephalitis in horses. PLoS One. 2010;5:e10537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 192. | Lim JK, Lisco A, McDermott DH, Huynh L, Ward JM, Johnson B, Johnson H, Pape J, Foster GA, Krysztof D. Genetic variation in OAS1 is a risk factor for initial infection with West Nile virus in man. PLoS Pathog. 2009;5:e1000321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 173] [Cited by in RCA: 175] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 193. | Mashimo T, Lucas M, Simon-Chazottes D, Frenkiel MP, Montagutelli X, Ceccaldi PE, Deubel V, Guenet JL, Despres P. A nonsense mutation in the gene encoding 2'-5'-oligoadenylate synthetase/L1 isoform is associated with West Nile virus susceptibility in laboratory mice. Proc Natl Acad Sci USA. 2002;99:11311-11316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 212] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 194. | Salazar P, Traub-Dargatz JL, Morley PS, Wilmot DD, Steffen DJ, Cunningham WE, Salman MD. Outcome of equids with clinical signs of West Nile virus infection and factors associated with death. J Am Vet Med Assoc. 2004;225:267-274. [PubMed] |
| 195. | Murgue B, Murri S, Triki H, Deubel V, Zeller HG. West Nile in the Mediterranean basin: 1950-2000. Ann N Y Acad Sci. 2001;951:117-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 161] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 196. | Corrigan RL, Waldner C, Epp T, Wright J, Whitehead SM, Bangura H, Young E, Townsend HG. Prediction of human cases of West Nile virus by equine cases, Saskatchewan, Canada, 2003. Prev Vet Med. 2006;76:263-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 197. | Eidson M, Komar N, Sorhage F, Nelson R, Talbot T, Mostashari F, McLean R. Crow deaths as a sentinel surveillance system for West Nile virus in the northeastern United States, 1999. Emerg Infect Dis. 2001;7:615-620. [PubMed] |
| 198. | Julian KG, Eidson M, Kipp AM, Weiss E, Petersen LR, Miller JR, Hinten SR, Marfin AA. Early season crow mortality as a sentinel for West Nile virus disease in humans, northeastern United States. Vector Borne Zoonotic Dis. 2002;2:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 42] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 199. | Padgett KA, Reisen WK, Kahl-Purcell N, Fang Y, Cahoon-Young B, Carney R, Anderson N, Zucca L, Woods L, Husted S. West Nile virus infection in tree squirrels (Rodentia: Sciuridae) in California, 2004-2005. Am J Trop Med Hyg. 2007;76:810-813. [PubMed] |
| 200. | Austgen LE, Bowen RA, Bunning ML, Davis BS, Mitchell CJ, Chang GJ. Experimental infection of cats and dogs with West Nile virus. Emerg Infect Dis. 2004;10:82-86. [PubMed] |
| 201. | Sbrana E, Tonry JH, Xiao SY, da Rosa AP, Higgs S, Tesh RB. Oral transmission of West Nile virus in a hamster model. Am J Trop Med Hyg. 2005;72:325-329. [PubMed] |
| 202. | Pogodina VV, Frolova MP, Malenko GV, Fokina GI, Koreshkova GV, Kiseleva LL, Bochkova NG, Ralph NM. Study on West Nile virus persistence in monkeys. Arch Virol. 1983;75:71-86. [PubMed] |
| 203. | Appler KK, Brown AN, Stewart BS, Behr MJ, Demarest VL, Wong SJ, Bernard KA. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One. 2010;5:e10649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 204. | Penn RG, Guarner J, Sejvar JJ, Hartman H, McComb RD, Nevins DL, Bhatnagar J, Zaki SR. Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis. 2006;42:680-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 205. | Dauphin G, Zientara S. West Nile virus: recent trends in diagnosis and vaccine development. Vaccine. 2007;25:5563-5576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 121] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 206. | Roehrig JT, Nash D, Maldin B, Labowitz A, Martin DA, Lanciotti RS, Campbell GL. Persistence of virus-reactive serum immunoglobulin m antibody in confirmed west nile virus encephalitis cases. Emerg Infect Dis. 2003;9:376-379. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 181] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 207. | Wang T, Magnarelli LA, Anderson JF, Gould LH, Bushmich SL, Wong SJ, Fikrig E. A recombinant envelope protein-based enzyme-linked immunosorbent assay for West Nile virus serodiagnosis. Vector Borne Zoonotic Dis. 2002;2:105-109. [PubMed] |
| 208. | Davis BS, Chang GJ, Cropp B, Roehrig JT, Martin DA, Mitchell CJ, Bowen R, Bunning ML. West Nile virus recombinant DNA vaccine protects mouse and horse from virus challenge and expresses in vitro a noninfectious recombinant antigen that can be used in enzyme-linked immunosorbent assays. J Virol. 2001;75:4040-4047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 318] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 209. | Muerhoff AS, Dawson GJ, Dille B, Gutierrez R, Leary TP, Gupta MC, Kyrk CR, Kapoor H, Clark P, Schochetman G. Enzyme-linked immunosorbent assays using recombinant envelope protein expressed in COS-1 and Drosophila S2 cells for detection of West Nile virus immunoglobulin M in serum or cerebrospinal fluid. Clin Diagn Lab Immunol. 2004;11:651-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 210. | Bonafé N, Rininger JA, Chubet RG, Foellmer HG, Fader S, Anderson JF, Bushmich SL, Anthony K, Ledizet M, Fikrig E. A recombinant West Nile virus envelope protein vaccine candidate produced in Spodoptera frugiperda expresSF+ cells. Vaccine. 2009;27:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 211. | Alonso-Padilla J, Jiménez de Oya N, Blázquez AB, Loza-Rubio E, Escribano JM, Saiz JC, Escribano-Romero E. Evaluation of an enzyme-linked immunosorbent assay for detection of West Nile virus infection based on a recombinant envelope protein produced in Trichoplusia ni larvae. J Virol Methods. 2010;166:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 212. | Ellis AE, Mead DG, Allison AB, Gibbs SE, Gottdenker NL, Stallknecht DE, Howerth EW. Comparison of immunohistochemistry and virus isolation for diagnosis of west nile virus. J Clin Microbiol. 2005;43:2904-2908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 213. | Hunt AR, Hall RA, Kerst AJ, Nasci RS, Savage HM, Panella NA, Gottfried KL, Burkhalter KL, Roehrig JT. Detection of West Nile virus antigen in mosquitoes and avian tissues by a monoclonal antibody-based capture enzyme immunoassay. J Clin Microbiol. 2002;40:2023-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 214. | Lanciotti RS. Molecular amplification assays for the detection of flaviviruses. Adv Virus Res. 2003;61:67-99. [PubMed] |
| 215. | Perera R, Khaliq M, Kuhn RJ. Closing the door on flaviviruses: entry as a target for antiviral drug design. Antiviral Res. 2008;80:11-22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 216. | Malet H, Massé N, Selisko B, Romette JL, Alvarez K, Guillemot JC, Tolou H, Yap TL, Vasudevan S, Lescar J. The flavivirus polymerase as a target for drug discovery. Antiviral Res. 2008;80:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 153] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 217. | Chowers MY, Lang R, Nassar F, Ben-David D, Giladi M, Rubinshtein E, Itzhaki A, Mishal J, Siegman-Igra Y, Kitzes R. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis. 2001;7:675-678. [PubMed] |
| 218. | Vázquez-Calvo A, Saiz JC, Sobrino F, Martín-Acebes MA. Inhibition of enveloped virus infection of cultured cells by valproic acid. J Virol. 2011;85:1267-1274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 219. | Diamond MS, Pierson TC, Fremont DH. The structural immunology of antibody protection against West Nile virus. Immunol Rev. 2008;225:212-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 220. | Beasley DW. Vaccines and immunotherapeutics for the prevention and treatment of infections with West Nile virus. Immunotherapy. 2011;3:269-285. [PubMed] |
| 221. | Nathan DB, Samina I, Orr N. High titer human immunoglobulin as a specific therapy against West Nile virus encephalitis. Hum Vaccin. 2010;6:279-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 222. | Beigel JH, Nordstrom JL, Pillemer SR, Roncal C, Goldwater DR, Li H, Holland PC, Johnson S, Stein K, Koenig S. Safety and pharmacokinetics of single intravenous dose of MGAWN1, a novel monoclonal antibody to West Nile virus. Antimicrob Agents Chemother. 2010;54:2431-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 223. | Rossi SL, Ross TM, Evans JD. West Nile virus. Clin Lab Med. 2010;30:47-65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 224. | Ward MP, Schuermann JA, Highfield LD, Murray KO. Characteristics of an outbreak of West Nile virus encephalomyelitis in a previously uninfected population of horses. Vet Microbiol. 2006;118:255-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 225. | Ledgerwood JE, Pierson TC, Hubka SA, Desai N, Rucker S, Gordon IJ, Enama ME, Nelson S, Nason M, Gu W. A West Nile virus DNA vaccine utilizing a modified promoter induces neutralizing antibody in younger and older healthy adults in a phase I clinical trial. J Infect Dis. 2011;203:1396-1404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 226. | Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M. A West Nile virus DNA vaccine induces neutralizing antibody in healthy adults during a phase 1 clinical trial. J Infect Dis. 2007;196:1732-1740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 227. | Alonso-Padilla J, de Oya NJ, Blázquez AB, Escribano-Romero E, Escribano JM, Saiz JC. Recombinant West Nile virus envelope protein E and domain III expressed in insect larvae protects mice against West Nile disease. Vaccine. 2011;29:1830-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 228. | Malkinson M, Banet C, Khinich Y, Samina I, Pokamunski S, Weisman Y. Use of live and inactivated vaccines in the control of West Nile fever in domestic geese. Ann N Y Acad Sci. 2001;951:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 229. | Samina I, Khinich Y, Simanov M, Malkinson M. An inactivated West Nile virus vaccine for domestic geese-efficacy study and a summary of 4 years of field application. Vaccine. 2005;23:4955-4958. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 230. | Kaufmann B, Vogt MR, Goudsmit J, Holdaway HA, Aksyuk AA, Chipman PR, Kuhn RJ, Diamond MS, Rossmann MG. Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354. Proc Natl Acad Sci USA. 2010;107:18950-18955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 231. | Dereeper A, Guignon V, Blanc G, Audic S, Buffet S, Chevenet F, Dufayard JF, Guindon S, Lefort V, Lescot M. Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 2008;36:W465-W469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3313] [Cited by in RCA: 3451] [Article Influence: 203.0] [Reference Citation Analysis (0)] |