Copyright
©The Author(s) 2016.
World J Virol. Nov 12, 2016; 5(4): 144-154
Published online Nov 12, 2016. doi: 10.5501/wjv.v5.i4.144
Published online Nov 12, 2016. doi: 10.5501/wjv.v5.i4.144
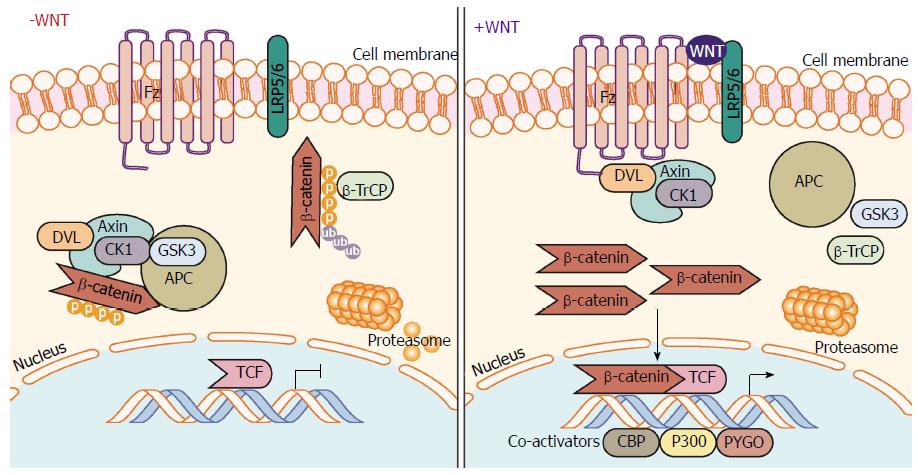
Figure 1 Canonical Wnt/β-catenin signaling pathway.
In the absence of Wnt ligand stimulation, the β-catenin destruction complex - consisting of the proteins Axin, CK1, GSK-3α/β, APC, and DVL - phosphorylate β-catenin allowing β-TrCP to ubiquitinate β-catenin marking it for proteasomal degradation. When stimulated by Wnt ligands, engagement of the Fz receptor and co-receptors LRP5/6, induces signaling through DVL inhibiting the action of the destruction complex. This frees β-catenin from degradation pathways allowing β-catenin to translocate and accumulate in the nucleus. β-catenin mediated interaction with TCF family transcription factors and co-activators (CBP, etc.) and initiates transcription of target genes. APC: Adenomatous polyposis coli; β-TrCP: Beta-transducin repeat containing E3 ubiquitin protein ligase; CBP: CREB-binding protein; CK1: Casein kinase 1; DVL: Dishevelled; Fz: Frizzled receptor; GSK-3: Glycogen synthase kinase 3; LRP: Low-density lipoprotein receptor-related protein; TCF/LEF-1: T-cell factor/lymphoid enhancer-binding factor 1.
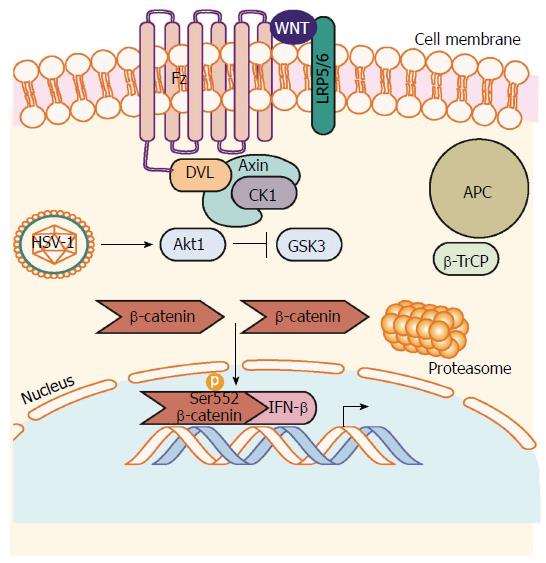
Figure 2 β-catenin mediated antiviral interferon response during herpes simplex virus 1 infection.
HSV-1 infection induces activation of Akt1 activity. Akt1 phosphorylates β-catenin on Serine 552 inhibiting degradation signaling through GSK-3-mediated phosphorylation of β-catenin on Serine 9. β-catenin can then accumulate in the nucleus to induce transcription of β-catenin target genes such as the antiviral cytokine IFN-β. Akt1: Protein kinase B; HSV-1: Herpes simplex virus 1.
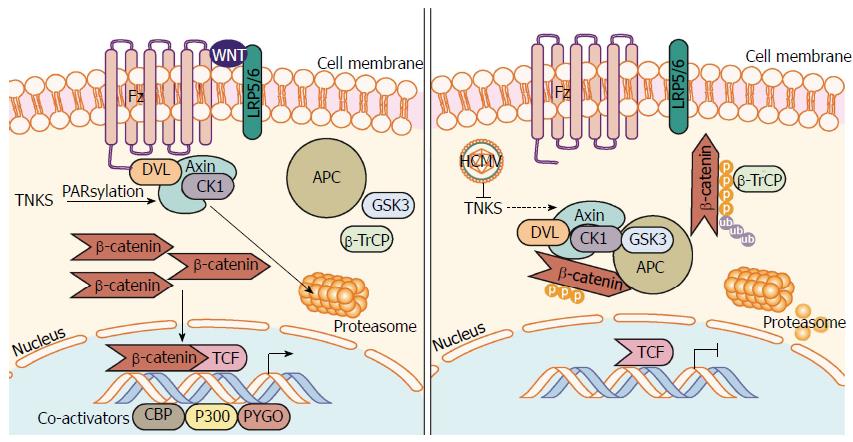
Figure 3 Human cytomegalovirus inhibits PARsylation activity of tankyrase 1 and 2 (PARP5a/b) to enhance infection.
Regulation of Axin is the rate-limiting step in the assembly and function of the β-catenin destruction complex. PARsylation, a process driven by NAD+, marks Axin for proteasomal degradation, inhibiting β-catenin destruction complex formation resulting in β-catenin accumulation and transcription in the nucleus. HCMV infection causes inhibition of TNKS (PARP5a/b) PARsylation activity, which inhibits PARsylation of Axin and increases its stability. Further inhibition of TNKS PARsylation activity or knockdown of TNKS significantly aids in HCMV replication. An increase in stable Axin permits β-catenin destruction complex formation, increased β-catenin degradation, and subsequent inhibition of β-catenin-mediated transcription. HCMV: Human cytomegalovirus; NAD+: Nicotinamide adenine dinucleotide; PARsylation: Poly-ADP ribose modification; PARP5a/b: Poly-ADP ribose polymerase 5a/b; TNKS: Tankyrase 1 and 2 (PARP5a/b).
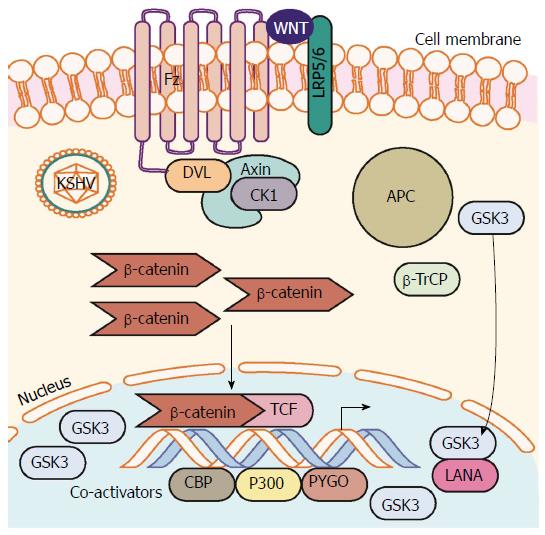
Figure 4 Kaposi’s sarcoma-associated herpesvirus latency upregulates β-catenin.
Establishment of KSHV latency involves expression of the latency associated protein LANA. LANA binds with and translocates GSK-3 into the nucleus after phosphorylation by GSK-3. This translocation of cytoplasmic pools of GSK-3 prevents β-catenin destruction complex formation and stability of cytoplasmic β-catenin. β-catenin translocation to the nucleus occurs resulting in increased β-catenin-mediated transcription. LANA: Latency-associated nuclear antigen; KSHV: Kaposi’s sarcoma-associated herpesvirus.
- Citation: Zwezdaryk KJ, Combs JA, Morris CA, Sullivan DE. Regulation of Wnt/β-catenin signaling by herpesviruses. World J Virol 2016; 5(4): 144-154
- URL: https://www.wjgnet.com/2220-3249/full/v5/i4/144.htm
- DOI: https://dx.doi.org/10.5501/wjv.v5.i4.144