Copyright
©The Author(s) 2022.
World J Virol. Sep 25, 2022; 11(5): 252-274
Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.252
Published online Sep 25, 2022. doi: 10.5501/wjv.v11.i5.252
Figure 1 Possible mechanism of diabetes and severe acute respiratory syndrome coronavirus 2-induced pulmonary dysfunction.
Hyperglycemia and insulin resistance in diabetes is associated with impaired cell signaling, oxidative stress, inflammation, and altered metabolism and subsequently lead to the manifestation of pulmonary dysfunction due to increased airway hyperresponsiveness, fibrosis, autonomic neuropathy, T helper 2, mast cell degranulation, Inflammatory macrophage, airway smooth muscle cell proliferation, deposition of extracellular matrix in the lung tissue, epithelial-mesenchymal transition, whereas reduced mucociliary clearance, respiratory muscle strength and synthesis of SP-A and SP-D. On the other hand, platelet or complement activation, endothelial damage, and inflammation in severe acute respiratory syndrome coronavirus 2 infection lead to the pathogenesis of pulmonary dysfunction due to elevated thrombosis, IL-6, IL-8, procoagulants, fibrosis, vasoconstriction, pulmonary edema, and angiogenesis. IR: Insulin resistance; NFκB: Nuclear factor-κB; PKC: Protein kinase C; PI3K: Phosphoinositide 3-kinases; STAT3: Signal transducer and activator of transcription 3; CTGF: Connective tissue growth factor; TGFβ: Transforming growth factor beta; Rho/RocK: Ras homologous/Rho-associated coiled-coil kinase; ROS: Reactive oxygen species; RNS: Reactive nitrogen species; RCS: Reactive carbonyl species; AGE: Advanced glycation end products; NOX: Nitrogen oxides; Ang II: Angiotensin II; Th2: T helper 2; SMC: Smooth muscle cell; ECM: Extracellular matrix; SP-A: Surfactant proteins A; SP-D: Surfactant proteins D; FFA: Free fatty acid; LDL: low-density lipoprotein; NADPH: Reduced nicotinamide adenine dinucleotide phosphate; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
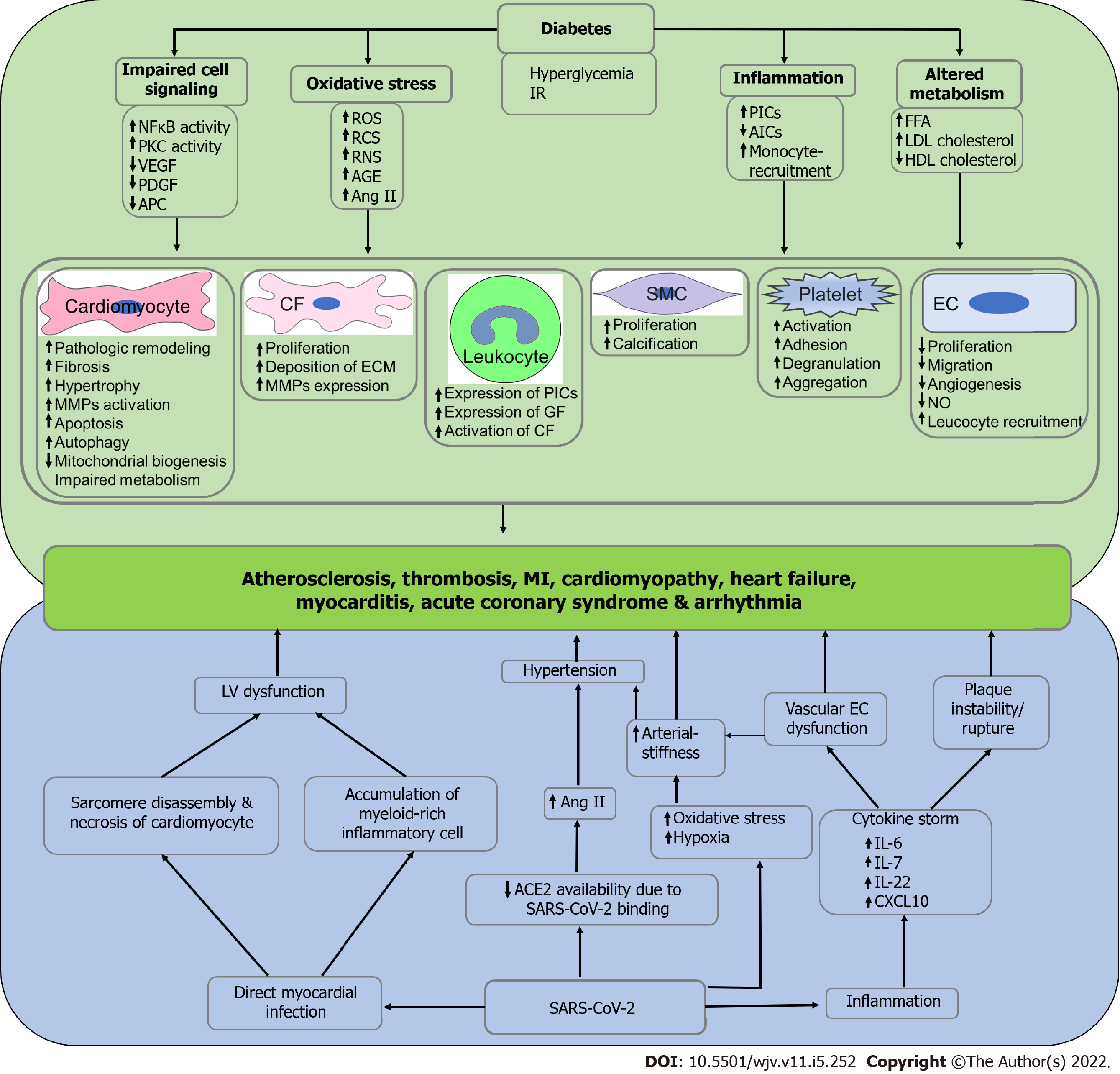
Figure 2 Possible mechanism of diabetes and severe acute respiratory syndrome coronavirus 2-induced cardiovascular complications.
Hyperglycemia and insulin resistance in diabetes is associated with impaired cell signaling, oxidative stress, inflammation, and altered metabolism and subsequently induce the pathogenesis of cardiovascular diseases, including atherosclerosis, thrombosis, myocardial infarction, cardiomyopathy, heart failure, myocarditis, acute coronary syndrome and arrhythmia due to impaired functioning of cardiomyocytes, cardiac fibroblasts, smooth muscle cells, endothelial cells and endothelial cells, leukocytes, and platelets. On the other hand, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) directly infects the myocardium and subsequently induces left ventricular dysfunction due to sarcomere disassembly, necrosis of cardiomyocytes, and infiltration of myeloid-rich inflammatory cells. Additionally, SARS-CoV-2 infection reduces the availability of angiotensin converting enzyme-2 numbers, which results in elevated angiotensin II levels and subsequent manifestation of hypertension. SARS-CoV-2 infection is also associated with increased oxidative stress and hypoxia that may manifest hypertension due to arterial stiffness. Finally, inflammation in SARS-CoV-2 infection leads to cytokine storm and eventually induces vascular endothelial cell dysfunction and thrombotic plaque instability. Left ventricular dysfunction, hypertension, arterial stiffness, vascular endothelial cell dysfunction, and plaque instability induce the pathophysiology of cardiovascular diseases. IR: Insulin resistance; NFκB: Nuclear factor-κB; PKC: Protein kinase C; VEGF: Vascular endothelial growth factor; PDGF: Platelet-derived growth factor; APC: Adenomatous polyposis coli; ROS: Reactive oxygen species; RNS: Reactive nitrogen species; RCS: Reactive carbonyl species; AGE: Advanced glycation end products; Ang II: Angiotensin II; PICs: Pro-inflammatory cytokines; AICs: Anti-inflammatory cytokines; FFA: Free fatty acid; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; CF: Cardiac fibroblast; ECM: Extracellular matrix; GF: Growth factor; SMC: Smooth muscle cell; EC: Endothelial cell; NO: Nitric oxide; LV: Left ventricle; ACE2: Angiotensin converting enzyme-2; IL-6: Interleukin-6; IL-7: Interleukin-7; IL-22: Interleukin-22; CXCL10: C-X-C motif chemokine ligand 10; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
Figure 3 Possible mechanism of diabetes and severe acute respiratory syndrome coronavirus 2-induced renal dysfunction.
Hyperglycemia in diabetes is associated with increased oxidative stress mediated endothelial cell (EC) dysfunction, hypovolemia, and diabetic ketoacidosis that subsequently induces renal dysfunction. Likewise, inflammation in diabetes is associated with increased oxidative stress mediated EC dysfunction and hypercoagulability mediated cardiac dysfunction that subsequently leads to renal dysfunction. On the other hand, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection reduces the availability of angiotensin converting enzyme-2 numbers, which results in elevated serum angiotensin II and subsequent kidney injury and fibrosis due to hypertension. Additionally, hypovolemia in SARS-CoV-2 infection induces renal dysfunction. Finally, inflammation in SARS-CoV-2 infection is associated with cytokine storm mediated endothelial cell dysfunction and upregulation of damage-associated molecular patterns, hypercoagulability mediated cardiac dysfunction results in renal dysfunction. EC: Endothelial cell; Ang II: Angiotensin II; ACE2: Angiotensin converting enzyme-2; DAMPs: Damage-associated molecular patterns; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
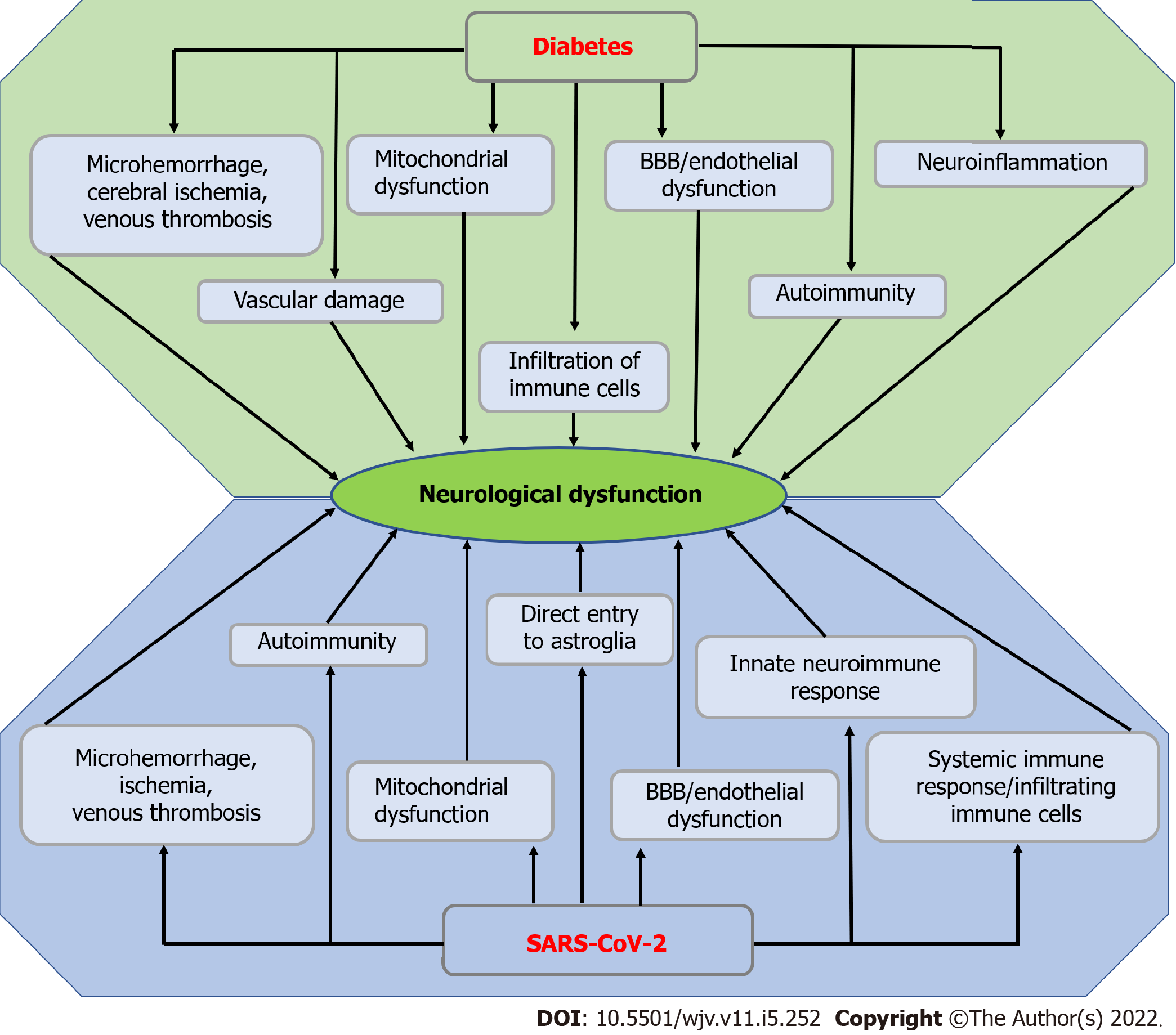
Figure 4 Possible mechanism of diabetes and severe acute respiratory syndrome coronavirus 2-induced neurological dysfunction.
Microhemorrhage, cerebral ischemia, venous thrombosis, mitochondrial dysfunction, endothelial or blood-brain barrier dysfunction, vascular damage, autoimmunity, infiltration of immune cells, and neuroinflammation in diabetes result in neurological dysfunction. On the other hand, microhemorrhage, ischemia, venous thrombosis, autoimmunity, mitochondrial dysfunction, endothelial or blood-brain barrier dysfunction, innate neuroimmune response, systemic immune response or infiltration of immune cells, and direct entry to astroglia lead to neurological dysfunction. BBB: Blood-brain barrier; SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
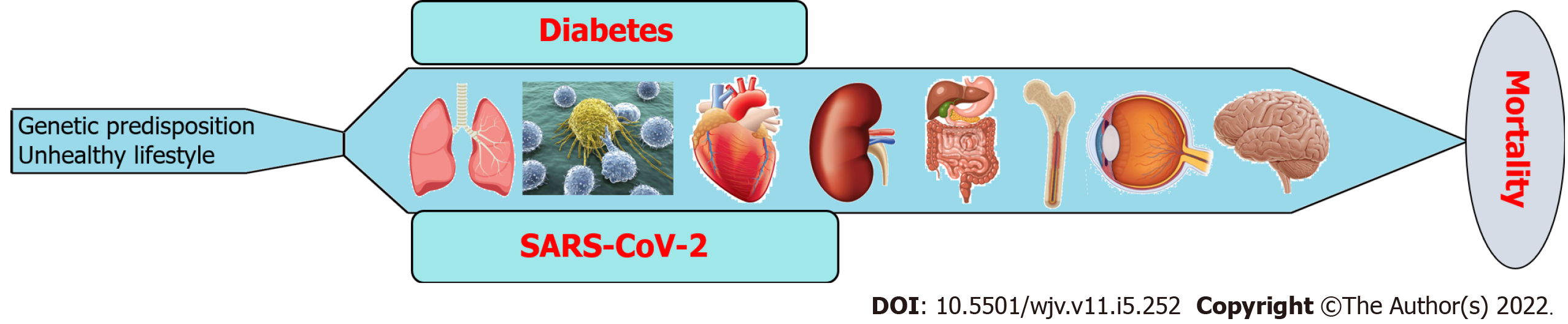
Figure 5 Role of diabetes and severe acute respiratory syndrome coronavirus 2 co-existence in multi-system organ failure.
SARS-CoV-2: Severe acute respiratory syndrome coronavirus 2.
- Citation: Roy B, Runa SA. SARS-CoV-2 infection and diabetes: Pathophysiological mechanism of multi-system organ failure. World J Virol 2022; 11(5): 252-274
- URL: https://www.wjgnet.com/2220-3249/full/v11/i5/252.htm
- DOI: https://dx.doi.org/10.5501/wjv.v11.i5.252