Published online Oct 28, 2019. doi: 10.5500/wjt.v9.i6.134
Peer-review started: July 21, 2019
First decision: August 2, 2019
Revised: September 17, 2019
Accepted: October 15, 2019
Article in press: October 15, 2019
Published online: October 28, 2019
Processing time: 101 Days and 10.5 Hours
Novel oral anticoagulants (NOACs) were developed as alternatives to vitamin K antagonists, primarily warfarin, as they do not require routine monitoring and have limited drug-drug and drug-food interactions. However, the efficacy and safety of these agents in kidney transplantation are not well studied.
To assess the profile and safety of NOACs for patients who had kidney transplantation, and to provide recommendations and guidelines on therapeutic strategies in these patients.
This was a retrospective study carried out among adult patients who were actively on the following NOACs (apixaban, rivaroxaban or dabigatran) in our renal transplantation program from December 2015 to December 2016. The patients were identified primarily through electronic medical record system (patient data linkage). Data on the clinical and laboratory profile of the patients were retrieved and analyzed with SPSS 22.0.
Complete data on 42 renal transplant patients were retrieved: 59.5% males, 90.5% were whites and 66.7% were older than 60 years old. The mean duration since renal transplantation of the patients was 8.8 ± 7.4 years. The most common risk factors for the development of end-stage renal disease in the subjects were hypertension (19.0%), polycystic kidney disease (19.0%), followed by diabetic nephropathy (16.7%) and chronic glomerulonephritis (16.7%). The main indications for NOACs use in the cohort were atrial fibrillation in 25 patients (59.5%) and venous thromboembolism in 10 patients (23.8%). Overall, 29 patients (69%) were treated with apixaban, 10 patients (23.8%) with rivaroxaban and 3 patients (7.14%) with dabigatran. No (0%) thromboembolic events were observed during the one-year period, but 3 (7.1%) bleeding events occurred in the cohort consisting of 1 patient treated with rivaroxaban 15 mg daily and 2 patients who received apixaban 2.5 mg twice daily. There were no significant changes in serum tacrolimus level three days after the initiation of NOACs among patients treated with tacrolimus (pre- and post-NOACs tacrolimus levels were 7.2516 and 7.8867 ng/mL, P = 0.55, respectively). Also, after one-year of treatment with NOACs there were no significant changes in the pre- and post-NOACs serum creatinine level (P = 0.772) and estimated glomerular filtration rates (P = 0.232).
No thromboembolic events or significant changes in renal profile were observed in our cohort of kidney transplant recipients who were treated with NOACs for at least a year. However, a few bleeding events were observed. This calls for further well-planned randomized controlled trials to assess the efficacy and safety of NOACs among renal transplant recipients.
Core tip: No consensus is available in the literature about whether novel oral anticoagulants are effective and safe for renal transplant recipients. This is one of the first attempts to investigate the profile, safety and effectiveness of novel oral anticoagulants for adult renal transplant recipients. We investigated the role of novel oral anticoagulants in terms of its effect on thromboembolism, bleeding, creatinine clearance and immunosuppressive agents.
- Citation: Bukhari MA, Al-Theaby A, Tawhari M, Al-Shaggag A, Pyrke R, Gangji A, Treleaven D, Ribic C. Efficacy and safety of non-vitamin K antagonist oral anticoagulants post-kidney transplantation. World J Transplant 2019; 9(6): 134-144
- URL: https://www.wjgnet.com/2220-3230/full/v9/i6/134.htm
- DOI: https://dx.doi.org/10.5500/wjt.v9.i6.134
Non-Vitamin K antagonists also known as novel oral anticoagulants (NOACs) were developed as alternatives to vitamin K antagonists, primarily warfarin, as they do not require routine monitoring and have limited drug-drug and drug-food interactions[1]. NOACs are gaining popularity over the past few years as stroke-preventing agents for people with atrial fibrillation (AF)[1]. NOACs have also been recommended for the treatment of systemic embolic events in patients with nonvalvular AF and for the treatment of venous thromboembolism (VTE)[1-3]. They are recommended by the Canadian Cardiovascular Society guidelines for the management of AF with a class I recommendation[4]. Four NOACs, (dabigatran, rivaroxaban, apixaban, and edoxaban) have received approval from the United States Food and Drug Administration for the prevention of AF.
Kidney transplantation is considered the treatment of choice for patients with end-stage renal disease (ESRD) and has been shown to improve quality of life and survival rate for most patients compared to those maintained on dialysis[5,6]. AF occurs in over 7% of kidney transplant recipients in the first 3 years after transplantation and is associated with reduced graft and patient survival[7]. NOACs represent a valuable anticoagulation therapy for kidney transplant recipients, which are at higher risk of bleeding and thrombotic complications. However, NOACs use in renal transplant patients is not yet recommended as they are excreted via kidney and there are concerns it may interact with immunosuppressive therapy[5,7]. Indeed, as substrates of CYP3A4, apixaban and rivaroxaban, and p-glycoprotein, dabigatran; NOACs were suggested to interact with calcineurin inhibitors (CNIs) in a small retrospective study[8]. In heart and lung transplant recipients, a recent study showed that NOACs were effective and safe but associated with high rate of drug interactions that require dose reduction (by 45%)[9].
Given the fact that NOACs don’t require frequent monitoring and due to their low interactions and lower risk of spontaneous bleeding, these agents carry a great advantage over warfarin[1]. However, the efficacy and safety of these agents in kidney transplantation are not well studied yet. In this study, we aimed to assess the safety of NOACs administration in patients after kidney transplantation, and to provide recommendations and guidelines on therapeutic strategies in these patients.
This was a retrospective study carried out among adult patients who were actively on the following NOACs (apixaban, rivaroxaban or dabigatran) in our renal transplantation program from December 2015 to December 2016. The patients were identified primarily through the electronic medical record system (patient data linkage). We also included renal transplant recipients whose anticoagulation therapy with NOACs were stopped or changed but had at least one-year record of use of NOACs corresponding with our study period (i.e., up to one year of use be December 1, 2016).
Only records of adult patients (age ≥ 18 years) were included. Data of pediatric renal transplant recipients, adult patients with medication adherence issues, and those who stopped NOACs >12 mo prior to the study, were excluded from the analysis. The electronic records of the patients were retrieved from the electronic medical record system (Patient link). The data of patients with incomplete information were available in the electronic medical record system were extracted from the patients’ paper charts. Data on the clinical and laboratory profile of the patients were extracted.
The study was approved by Hamilton Integrated Research Ethics Board (HiREB). Also, because this was a retrospective study of anonymized/deidentified electronic records, HiREB waived request for informed consent from patients. Data were analyzed with SPSS 22.0 (IBM Corp., NY, United States). Continuous variables were expressed as means ± standard deviations and categorical variables were expressed as percentages. Chi-square tests were used for categorical variables and unpaired t-tests and one-way analyses of variance were used to compare continuous variables. P values < 0.05 were considered significant. The statistical methods of this study were reviewed by Dr. Mamta Gupta PhD (Public Health and Epidemiology/MPH Epidemiology and Biostatistics) from the Department of Epidemiology and Biostatistics, Alchemist Research and Data Analysis, Chandigarh, 160 036, India.
Our cohort included a total of 47 patients; only 42 patients were retained for further analysis after excluding 5 patients due to incomplete data. The clinical characteristics of patients are presented in Table 1. Most patients were males 25 (59.5%) and the vast majority 28 (66.7%) were older than 60 years old with 11 (26.2%) being ≥ 75 years old. The mean age in our cohort was 64.7 ± 13.88 years. The mean duration since renal transplantation of the patients was 8.8 ± 7.4 years (range 1 to 30 years). The average estimated glomerular filtration rate (eGFR) was 62.90 ± 18.98 mL/min/1.73 m2. No significant difference in eGFR among age groups was noticed. A total of 38 patients were white (90.5%); only 2 were Asian, 1 Indian and 1 Hispanic. The Most common causes of ESRD in our cohort were hypertension and polycystic kidney disease, occurring in 8 patients (19.0%) each, followed by 7 patients with diabetic nephropathy and chronic glomerulonephritis (16.7%) (Table 2).
| Age | No. patients | Age (yr, mean ± SD) | No. males | Weight | n | Estimated glomerular filtration rate |
| ≤ 30 | 1 | 30 | 0 | 52 | 0 | 54 |
| 31-45 | 5 | 40.4 ± 5.86 | 2 | 96.20 ± 31.06 | 2 | 56.00 ± 18.67 |
| 46-60 | 8 | 56.4 ± 2.51 | 6 | 98.88 ± 29.79 | 3 | 65.13 ± 21.94 |
| > 60 | 28 | 72.0 ± 6.71 | 17 | 78.25 ± 14.77 | 14 | 63.82 ± 18.88 |
| Total | 42 | 64.7 ± 13.88 | 25 | 83.69 ± 22.32 | 19 | 62.90 ± 18.98 |
| Variable | Age group (yr) | Total | |
| < 75 | ≥ 75 | ||
| Primary cause of ESRD | |||
| Diabetic nephropathy | 6 (19.4) | 1 (9.1) | 7 (16.7) |
| Hypertension | 6 (19.4) | 2 (18.2) | 8 (19.0) |
| Glomerulonephritis | 4 (12.9) | 3 (27.3) | 7 (16.7) |
| Polycystic kidney disease | 6 (19.4) | 2 (18.2) | 8 (19.0) |
| Chronic Interstitial nephritis | 3 (9.7) | 1 (9.1) | 4 (9.5) |
| Reflux/Congenital | 3 (9.7) | 2 (18.2) | 3 (7.1) |
| Other | 3 (9.7) | 2 (18.2) | 5 (11.9) |
| NOACs | |||
| Dabigatran 150 mg bid | 1 (3.2) | 1 (3.2) | 2 (4.8) |
| Dabigatran-Low Dose | 1 (3.2) | 0 (0.0) | 1 (2.4) |
| Apixaban 5 mg bid | 11 (35.5) | 1 (9.1) | 12 (28.6) |
| Apixaban-Low Dose | 10 (32.3) | 7 (63.6) | 17 (40.5) |
| Rivaroxaban 20 mg/d | 5 (16.1) | 0 (0.0) | 5 (11.9) |
| Rivaroxaban Low Dose | 3 (9.7) | 2 (18.2) | 5 (11.9) |
| Cause of NOAC initiation | |||
| VTE | 8 (25.8) | 2 (18.2) | 10 (23.8) |
| AF | 17 (54.8) | 8 (72.7) | 25 (59.5) |
| Other | 2 (6.5) | 0 (0) | 2 (4.8) |
| VTE and AF | 4 (12.9) | 1 (9.1) | 5 (11.9) |
| Calcineurin inhibitors | |||
| Advagraf | 22 (71.0) | 5 (45.5) | 27 (64.3) |
| Prograf | 3 (9.7) | 1 (9.1) | 4 (9.5) |
| Cyclosporin | 1 (3.2) | 4 (36.4) | 5 (11.9) |
| Sirolimus | 3 (9.7) | 1 (9.1) | 4 (9.5) |
| None | 2 (4.8) | 0 (0) | 2 (4.8) |
| Clopidogrel | |||
| Yes | 4 (12.9) | 1 (9.1) | 5 (11.9) |
| No | 27 (87.1) | 10 (90.9) | 37 (88.1) |
A total 29 patients (69%) were treated with apixaban, 10 patients (23.8%) with rivaroxaban and 3 patients (7.14%) with dabigatran (Table 2). Among those that were on apixaban, 58.6% were on low dose of 2.5 mg bid and 41.3% were on full dose of 5 mg bid. Similarly, of the 10 patients on rivaroxaban, 5 were on a full daily dose of 20 mg and 5 were on reduced daily dose of 15 mg. In our cohort, 25 patients (59.5%) were on NOACs due to AF, 10 patients (23.8%) due to VTE and 5 patients (11.9%) due to both AF and VTE. Most patients were on tacrolimus-based anti-rejection (immunosuppressive) therapy (31; 76.8%) and 5 patients (11.9%) were on a cyclosporine-based regimen, and only 4 patients (9.6%) were on sirolimus-based regimen. In addition, all the 42 patients (100%) received oral prednisolone and mycophenolate mofetil. Table 3 shows the profile of the immunosuppressive agents received according the type of NOAC agent. NOACs were used without a con-comitant antiplatelets therapy in 37 of the patients (88.1%).
| Case 1 | Case 2 | Case 3 | |
| Age | 77 | 73 | 87 |
| Gender | Male | Female | Female |
| NOACs on use | Rivaroxaban | Apixaban | Apixaban |
| NOACs dose | 15mg daily | 2.5mg bid | 2.5mg bid |
| Type of bleeding | Major | Non-major | Non-major |
| Site of bleeding | Intra-ocular | Bleeding per rectum | Bleeding per rectum |
| Time to bleed | > 1 yr post starting | > 1 yr post starting | > 1 yr post starting |
| Base line Cr/eGFR | 93/72.6 | 67/79.5 | 122/38.44 |
| Cr/eGFR at bleeding | 144/38.6 | 58/93.9 | 147/31.0 |
| CNI in use | Cyclosporin | Tacrolimus | Cyclosporin |
| CNI level at bleeding time | C0: 91 | 5.8 (within target) | C0: 116 |
| Antiplatelet used | None | None | None |
| note | Rivaroxaban was on hold at the time of bleeding. Bled post cataract surgery. | ||
Overall, we observed 3 bleeding events (7.1%) in our cohort consisting of 1 patient treated with rivaroxaban 15 mg daily and 2 patients who received apixaban 2.5 mg twice daily (Table 4). One of these was a major bleeding event which occurred while rivaroxaban was on hold for over a month in preparation for a cataract surgery. The patient had a background of severe retinopathy and had intraocular bleeding one day after the surgery. This bleeding event was assumed to be unrelated to the medication, and rivaroxaban was resumed a few months later. This patient didn’t experience any further bleeding events after rivaroxaban resumption. The other two bleeding events were bleeding per-rectum events that occurred in two ladies on low-dose apixaban. There were no significant reduction in the patients creatinine, eGFR or CNI levels at the time of the events. The bleeding events in both cases were minor, didn’t cause hemodynamic instability, and didn’t require surgical intervention or complete cessation of NOACs.
| NOAC | Calcineurin inhibitor used, n (%) | Total | ||||
| Advograf | Pyograf | Cycosporin | Sirolimus | None | ||
| Dabigatran 150 mg bid | 2 (7.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 2 (4.8) |
| Dabigatran-low dose | 1 (3.7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.4) |
| Apixaban 5 mg bid | 8 (29.6) | 1 (25.0) | 0 (0) | 2 (50.0) | 1 (50.0) | 12 (28.6) |
| Apixaban-low dose | 10 (37.0) | 2 (50.0) | 3 (60.0) | 2 (50.0) | 0 (0) | 17 (40.5) |
| Rivaroxaban 20 mg/d | 4 (14.8) | 1 (25.0) | 0 (0) | 0 (0) | 0 (0) | 5 (11.9) |
| Rivaroxaban low dose | 2 (7.4) | 0 (0) | 2 (40.0) | 0 (0) | 1 (50.0) | 5 (11.9) |
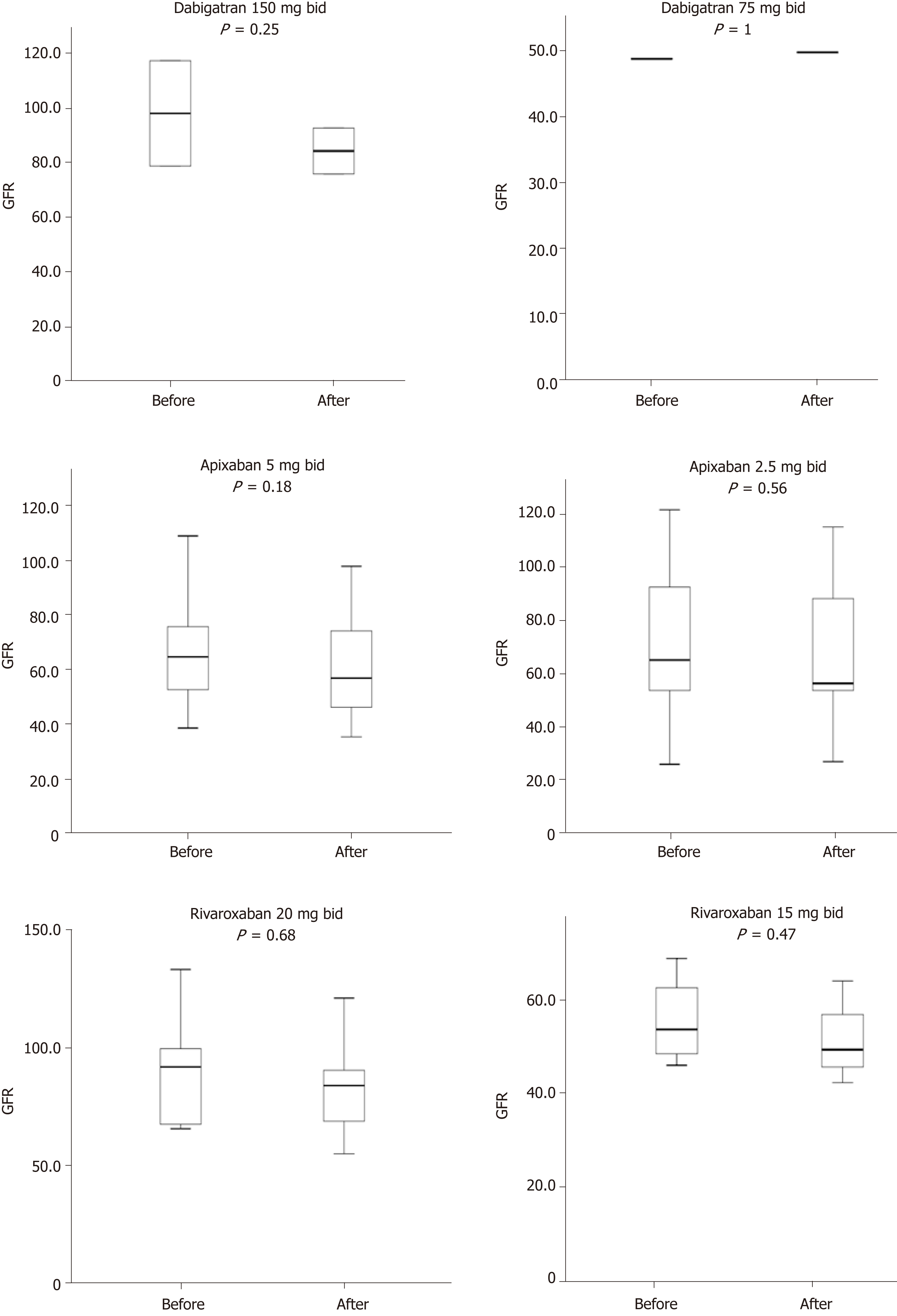
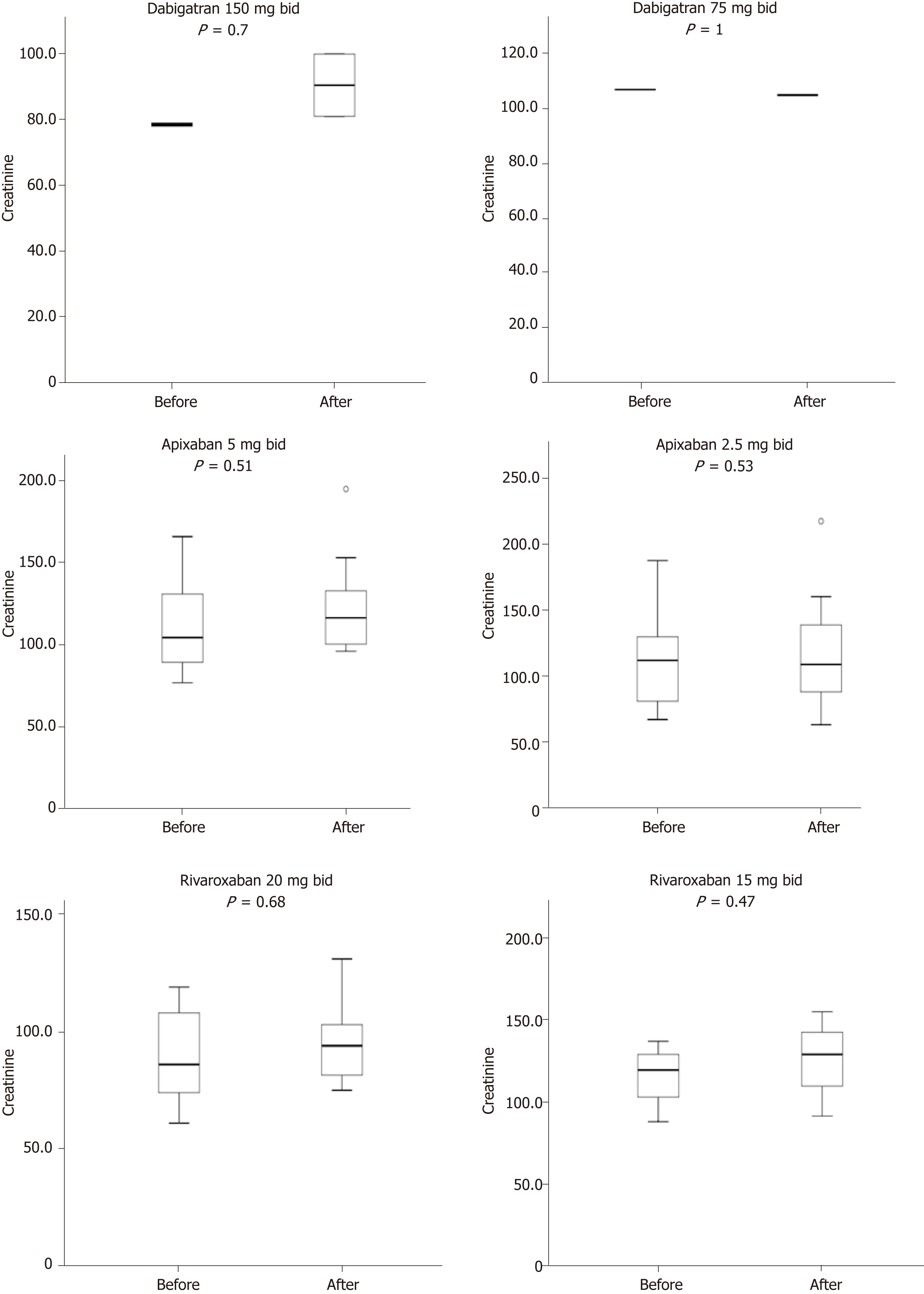
On the other hand, no thromboembolic events (0%) were observed. In addition, no significant change in serum tacrolimus level was observed three days after the initiation of NOACs among patients treated with tacrolimus (pre- and post-NOACs serum tacrolimus level was 7.25 and 7.89 ng/mL, P = 0.55). Similarly, after one year of treatment with NOACs there was no significant change in the pre- and post-NOACs serum creatinine level with mean levels of 107.6 μmol/L and 113.11 μmol/L (P = 0.772) respectively, (median 107.5 vs 108.5 μmol/L, respectively). This is summarized in Figure 1. Besides, as shown in Figure 2, pre- and post-NOACs eGFR levels after one-year of treatment with NOACs did not significantly change with respective mean levels of 72.2 mL/min/1.73 m2 and 65.9 mL/min/1.73 m2 (P = 0.232; median: 68.2 vs 60.4 mL/min/1.73 m2, respectively).
Dabigatran was the first NOAC agent released into the European market for VTE prophylaxis post joint replacement surgeries in 2008[1]. It was the first NOAC agent to get Food and Drug Administration approval for AF in 2010, and VTE in 2014. International recommendations suggested the need to change NOACs name from novel oral anticoagulation drugs to non-vitamin K antagonist agents keeping the same acronym; NOACs[10].
To our knowledge, this is the first study that addresses the efficacy and safety of NOACs in kidney transplantation recipients. Our results show that NOACs treatment has no effect on kidney function. Indeed, none of the NOACs used in our study induced changes in creatinine or eGFR levels after treatment. A previous study on lungs and heart transplantation suggested that NOACs can interact with CNIs[9]. Moreover, Wannhoff et al[11] suggested that cyclosporine has a higher rate of drug interaction with rivaroxaban in another liver transplantation study. On the other hand, Vanhove et al[12] reported similar, but clinically insignificant (< 20% change), interaction that didn’t warrant CNI dose adjustments in transplant recipients.
In our study, we didn’t report any thromboembolic event in any of the patients after CNI initiation. This might suggest NOACs are as effective in kidney trans-plantation population as the general population. Also, we had a few bleeding events with low doses (2.5 mg twice daily) of apixaban and a moderate dose (15 mg daily) of rivaroxaban, which may suggest a good safety profile. However, there is a need to further assess the mechanisms of bleeding in patients exposed to NOACs. Although our study indicates that NOACs may be safe and effective for the prevention and treatment of thromboembolic events in renal transplant recipients, there is a need to highlight some of its important advantages and disadvantages compared to other vitamin K antagonists. Its major advantages include absence of food interactions, few strong drug interactions, predictable pharmacokinetic and pharmacodynamic properties, a rapid onset and offset of action, a short half-life, and the absence of the need for laboratory monitoring[13].
However, pharmacokinetic and pharmacodynamic studies show that NOACs elimination is dependent on renal clearance to varying extents; but compared with vitamin K antagonists, the efficacy and safety of the NOACs is preserved in patients with moderate renal impairment[14,15]. There is a need to administer NOACs with caution in individuals with severe kidney or hepatic damage particularly the elderly. This is because up to 25%, 33% and 80% of apixaban, rivaroxaban and dabigatran, respectively are eliminated through the kidneys as an active drug[13-15]. In severe renal or hepatic damage, the elimination of the drug may be affected requiring adjustments in the dosing of the NOAC agent.
Our analysis only included renal transplant recipients with an eGFR of > 54 mL/min/1.73 m2. Therefore, dosage adaptation of the NOACs should ideally not be necessary. However, considering the very limited or no prior experience in the use of NOACs in kidney transplant recipients (with/without renal impairment), doses of NOACs were administered to the patients in this study using the Health Canada dosing algorithm for each of the NOACs according to renal function and clinical status of the patients[14,16]. Thus, the effectiveness of NOACs observed in our data can only be interpreted in the context of kidney transplant recipients with sufficiently preserved renal function. Several clinical trials such as the EINSTEIN, ARISTOTLE, and RE-LY trials have previously demonstrated the safety and efficacy of these NOACs in individuals with varying levels of renal impairment[17-19].
In the present study, 3 of the subjects received dabigatran with tacrolimus-based CNIs. Previous studies have called for caution in the use of NOACs and immuno-suppressive agents due to the potential for drug-drug interactions[8,20,21]. A study suggested that dabigatran should not be administered to patients receiving CNIs because CNIs are known substrates of both CYP 450 3A4 and P-gp, and can lead to increased exposure to dabigatran[8,20]. Because of the limited evidence of NOACs usage with CNIs in the setting of solid organ transplantation, this clinical recommendation was made based on an underpowered analysis of nine heart transplant recipients immunosuppressed with CNIs and treated with dabigatran for AF, VTE, or atrial thrombus[8]. In the study, patients who received tacrolimus with dabigatran were more likely to require a decrease in tacrolimus dose during therapy and numerically had more major bleeding events[8]. However, observations from the RE-LY trial indicate that concomitant use of dabigatran with P-gp inhibitors (like amiodarone or verapamil) increased dabigatran exposure but was not associated with significant differences in the event rate or bleeding[22,23]. A recent review indicates that in patients receiving dabigatran etexilate for the treatment and prevention of VTE, there is no need for dose adjustments and no contraindication to its co-administration with P-gp inhibitors so long as the patients have a creatinine clearance greater than 50 mL/min[24]. All the patients in our study had creatinine clearance greater than 50 mL/min and none of those who received dabigatran had a bleeding event. Recent expert opinion conclude that provided adequate attention is given to renal function, the co-administration of NOACs and CNIs in solid organ transplantation is safe and effective[24].
This study has some limitations. First, this was a retrospective observational study, therefore any reported association does not imply causation. Second, all the patients in this study had sufficiently preserved renal function (creatinine clearance > 50 mL/min), therefore we cannot report on the safety or efficacy of the NOACs in kidney transplant recipients with substantial renal impairment. Third, more than half of the patients received low doses of the NOAC agent. Therefore, our finding may not reflect the outcomes in renal transplant recipients treated with higher doses of NOAC agent.
In conclusion, our study suggests that NOACs may be safe and effective for the prevention and treatment of thromboembolic events in renal transplant recipients with limited complications. Further studies need to be conducted to assess the effectiveness and safety profile of NOACs compared to other vitamin K antagonists (e.g., warfarin) in kidney transplant population.
Novel oral anticoagulants are increasingly being used in recent times for preventing stroke in individuals with atrial fibrillation and for the management of systemic embolic events and venous thromboembolism. With the increased risk of atrial fibrillation and thrombotic events observed in kidney transplant recipients, whether novel oral anticoagulants have clinical significance in this group of patients remains unclear.
Novel oral anticoagulants are being used as an oral anticoagulation agent for the prevention of embolic events in individuals with atrial fibrillation and for the treatment of venous thromboembolism. They also have the advantage of not requiring frequent monitoring and having a lower adverse effects profile. There are concerns regarding the clinical use of novel oral anticoagulants in renal transplant recipients because of its renal excretion and the likelihood of its interaction with immunosuppressive agents. Although, novel oral anticoagulants have successfully been used for anticoagulation in heart-lung transplant recipients, its use for this role in kidney transplant recipients is unknown.
We performed this retrospective study to assess the efficacy and safety of novel oral anticoagulants administration in patients after kidney transplantation, and to provide recommendations and guidelines on therapeutic strategies in these patients.
This was a retrospective study carried out among adult patients who were actively on the following novel oral anticoagulants (apixaban, rivaroxaban or dabigatran) in our renal transplantation program from December 2015 to December 2016. The outcomes of interest include the profile of the patients, thromboembolic and bleeding events, and kidney dysfunction.
The authors observed 3 (7.1%) bleeding events in the cohort. Also, no (0%) thromboembolic events were observed. In addition, no significant changes in pre- and post- novel oral anticoagulants tacrolimus level, creatinine level, and estimated glomerular filtration rates were observed.
Novel oral anticoagulants appear to be as effective in the renal transplantation population as in the general population. Also, we had a few bleeding events and no changes in renal function after the initiation of novel oral anticoagulants which suggests a good safety profile.
This study demonstrated that novel oral anticoagulants are safe and effective in renal transplant recipients. There is a need for further clinical studies to assess the mechanisms of bleeding in patients exposed to novel oral anticoagulants. Randomised controlled trials are needed to compare the effectiveness and safety of novel oral anticoagulants compared to other vitamin K antagonists (e.g., warfarin) in kidney transplant population.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Hanna R, Kita K, Staufer K S-Editor: Yan JP L-Editor: A E-Editor: Xing YX
| 1. | Amin A. Choosing Non-Vitamin K Antagonist Oral Anticoagulants: Practical Considerations We Need to Know. Ochsner J. 2016;16:531-541. [PubMed] |
| 2. | Patel P, Pandya J, Goldberg M. NOACs vs. Warfarin for Stroke Prevention in Nonvalvular Atrial Fibrillation. Cureus. 2017;9:e1395. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 3. | Bromley A, Plitt A. A Review of the Role of Non-Vitamin K Oral Anticoagulants in the Acute and Long-Term Treatment of Venous Thromboembolism. Cardiol Ther. 2018;7:1-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Verma A, Cairns JA, Mitchell LB, Macle L, Stiell IG, Gladstone D, McMurtry MS, Connolly S, Cox JL, Dorian P, Ivers N, Leblanc K, Nattel S, Healey JS; CCS Atrial Fibrillation Guidelines Committee. 2014 focused update of the Canadian Cardiovascular Society Guidelines for the management of atrial fibrillation. Can J Cardiol. 2014;30:1114-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 326] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 5. | Suthanthiran M, Strom TB. Renal transplantation. N Engl J Med. 1994;331:365-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 346] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 6. | Kapoor A, Kwan KG, Whelan JP. Commercial renal transplantation: A risky venture? A single Canadian centre experience. Can Urol Assoc J. 2011;5:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 7. | Malyszko J, Lopatowska P, Mlodawska E, Musialowska D, Malyszko JS, Tomaszuk-Kazberuk A. Atrial fibrillation in kidney transplant recipients: is there a place for the novel drugs? Nephrol Dial Transplant. 2018;33:1304-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Shuster JE, Larue SJ, Vader JM. Dabigatran may have more significant drug interactions with calcineurin inhibitors than oral anti-Xa inhibitors (Abstracts S417). J Heart Lung Transplant. 2016;S417. |
| 9. | Lichvar AB, Moore CA, Ensor CR, McDyer JF, Teuteberg JJ, Shullo MA. Evaluation of Direct Oral Anticoagulation Therapy in Heart and Lung Transplant Recipients. Prog Transplant. 2016;26:263-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Husted S, de Caterina R, Andreotti F, Arnesen H, Bachmann F, Huber K, Jespersen J, Kristensen SD, Lip GY, Morais J, Rasmussen LH, Siegbahn A, Storey RF, Weitz JI; ESC Working Group on Thrombosis Task Force on Anticoagulants in Heart Disease. Non-vitamin K antagonist oral anticoagulants (NOACs): No longer new or novel. Thromb Haemost. 2014;111:781-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Wannhoff A, Weiss KH, Schemmer P, Stremmel W, Gotthardt DN. Increased levels of rivaroxaban in patients after liver transplantation treated with cyclosporine A. Transplantation. 2014;98:e12-e13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Vanhove T, Spriet I, Annaert P, Maertens J, Van Cleemput J, Vos R, Kuypers D. Effect of the Direct Oral Anticoagulants Rivaroxaban and Apixaban on the Disposition of Calcineurin Inhibitors in Transplant Recipients. Ther Drug Monit. 2017;39:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 13. | Mekaj YH, Mekaj AY, Duci SB, Miftari EI. New oral anticoagulants: their advantages and disadvantages compared with vitamin K antagonists in the prevention and treatment of patients with thromboembolic events. Ther Clin Risk Manag. 2015;11:967-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 291] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 14. | Turpie AGG, Purdham D, Ciaccia A. Nonvitamin K antagonist oral anticoagulant use in patients with renal impairment. Ther Adv Cardiovasc Dis. 2017;11:243-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Mikuni M, Fujii S, Yaoeda H. [Stereophotography of the ocular fundus. 2. Observation method]. Ganka. 1968;10:311-318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Andrade JG, Macle L, Nattel S, Verma A, Cairns J. Contemporary Atrial Fibrillation Management: A Comparison of the Current AHA/ACC/HRS, CCS, and ESC Guidelines. Can J Cardiol. 2017;33:965-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 17. | EINSTEIN Investigators. Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2261] [Article Influence: 150.7] [Reference Citation Analysis (0)] |
| 18. | Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, Keltai M, Lanas F, Lopes RD, Lopez-Sendon J, Granger CB, Wallentin L. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. 2012;33:2821-2830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 410] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 19. | Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, Ezekowitz MD, Reilly PA, Siegbahn A, Yusuf S, Wallentin L. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial analysis. Circulation. 2014;129:961-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 20. | Lee RA, Gabardi S. Current trends in immunosuppressive therapies for renal transplant recipients. Am J Health Syst Pharm. 2012;69:1961-1975. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Salerno DM, Tsapepas D, Papachristos A, Chang JH, Martin S, Hardy MA, McKeen J. Direct oral anticoagulant considerations in solid organ transplantation: A review. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, Yusuf S, Wallentin L, Haertter S, Staab A. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9:2168-2175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 245] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 23. | Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, Brueckmann M, Pogue J, Alings M, Amerena JV, Avezum A, Baumgartner I, Budaj AJ, Chen JH, Dans AL, Darius H, Di Pasquale G, Ferreira J, Flaker GC, Flather MD, Franzosi MG, Golitsyn SP, Halon DA, Heidbuchel H, Hohnloser SH, Huber K, Jansky P, Kamensky G, Keltai M, Kim SS, Lau CP, Le Heuzey JY, Lewis BS, Liu L, Nanas J, Omar R, Pais P, Pedersen KE, Piegas LS, Raev D, Smith PJ, Talajic M, Tan RS, Tanomsup S, Toivonen L, Vinereanu D, Xavier D, Zhu J, Wang SQ, Duffy CO, Themeles E, Yusuf S. The Long-Term Multicenter Observational Study of Dabigatran Treatment in Patients With Atrial Fibrillation (RELY-ABLE) Study. Circulation. 2013;128:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 24. | Lam E, Bashir B, Chaballa M, Kraft WK. Drug interactions between direct-acting oral anticoagulants and calcineurin inhibitors during solid organ transplantation: considerations for therapy. Expert Rev Clin Pharmacol. 2019;12:781-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |