Published online Aug 26, 2019. doi: 10.5500/wjt.v9.i4.81
Peer-review started: February 22, 2019
First decision: April 16, 2019
Revised: April 23, 2019
Accepted: July 29, 2019
Article in press: July 30, 2019
Published online: August 26, 2019
Processing time: 180 Days and 10.9 Hours
Kidney transplantation is the treatment of choice for management of end-stage renal disease. However, in diabetic patients, the underlying metabolic disturbance will persist and even may get worse after isolated kidney transplantation. Pancreatic transplantation in humans was first introduced in 1966. The initial outcome was disappointing. However, this was changed after the improvement of surgical techniques together with better patient selection and the availability of potent and better-tolerated immune-suppression like cyclosporine and induction antibodies. Combined kidney and pancreas transplantation will not only solve the problem of organ failure, but it will also stabilise or even reverse the metabolic complications of diabetes. Combined kidney and pancreas transplantation have the best long term outcome in diabetic cases with renal failure. Nevertheless, at the cost of an initial increase in morbidity and risk of mortality. Other transplantation options include pancreas after kidney transplantation and islet cell transplantation. We aim by this work to explore various options which can be offered to a diabetic patient with advanced chronic kidney disease. Our work will provide a simplified, yet up-to-date information regarding the different management options for those diabetic chronic kidney failure patients.
Core tip: Kidney transplantation is the treatment of choice for end-stage renal disease. Combined kidney-pancreas transplantation provides the patients with the highest long term survival. There are different surgical approaches for combined kidney-pancreas transplantation with recognised advantages and limitations of each technique. Islet cell transplantation is a minimally invasive treatment option but carries a risk of sensitisation to a wide range of human leukocyte antigen antigens.
- Citation: Aref A, Zayan T, Pararajasingam R, Sharma A, Halawa A. Pancreatic transplantation: Brief review of the current evidence. World J Transplant 2019; 9(4): 81-93
- URL: https://www.wjgnet.com/2220-3230/full/v9/i4/81.htm
- DOI: https://dx.doi.org/10.5500/wjt.v9.i4.81
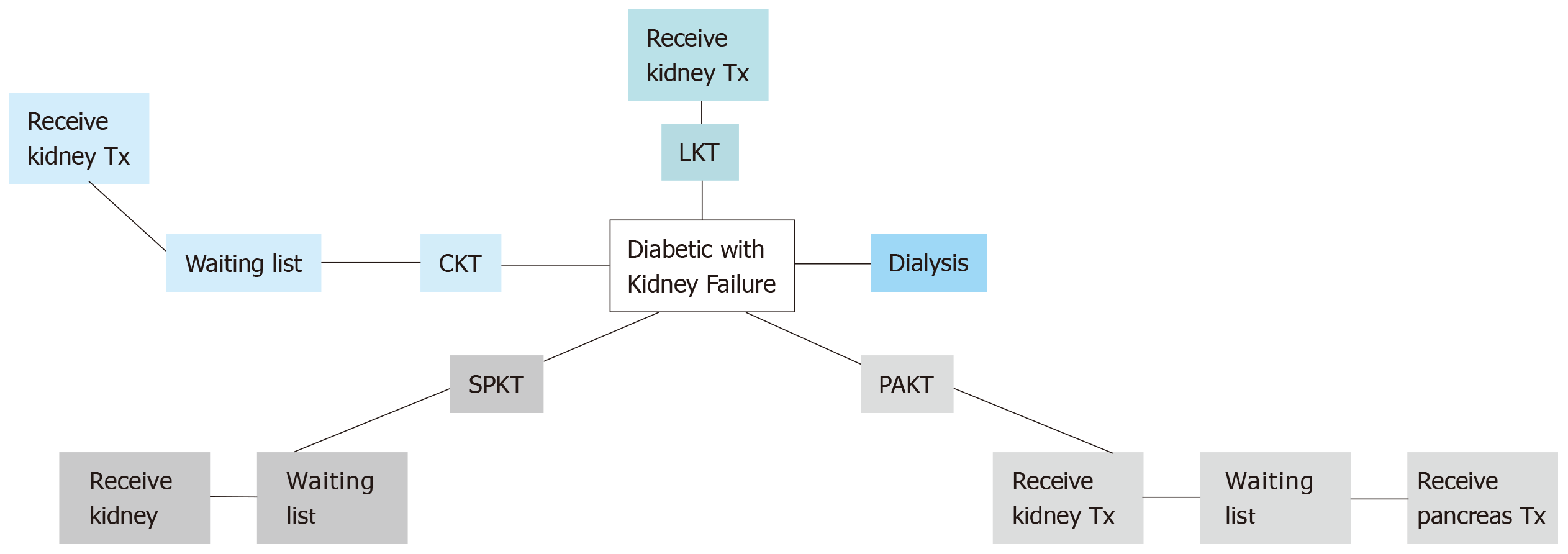
Diabetes mellitus (DM) is the leading cause of chronic kidney disease (CKD) and end-stage renal disease (ESRD) worldwide[1]. Successful pancreas transplantation provides optimisation of glucose metabolism for diabetic patients[2]. The current management options for diabetic patients with advanced CKD are summarised in Figure 1.
Pancreas transplant alone (PTA) is another option for managing diabetic patients with normal renal function. Moreover, transplantation of the islets of Langerhans is a promising alternative to whole pancreas transplantation which can provide adequate glycemic control without exposing the recipient to major surgical interventions[2].
Diabetes is classified into two main subtypes, type 1 and type 2, based on the American Diabetes Association classification system. The discrimination between the two types of DM may be difficult in many cases[3]. Table 1 summaries the main characteristics of type 1 and type 2 DM in children and adolescents[4].
| Type 1 diabetes | Type 2 diabetes | |
| Prevalence | Common, increasing | Increasing |
| Age at presentation | Throughout childhood | Puberty |
| Onset | Typically, acute severe | Insidious to severe |
| Ketosis at onset | Common | 5% to 10%1 |
| Affected relative | 5% to 10% | 75% to 90% |
| Female: male | 1:1 | Approximately 2:1 |
| Inheritance | Polygenic | Polygenic |
| HLA-DR3/4 | Strong association | No association |
| Ethnicity | Most common in non- Hispanic white | All2 |
| Insulin secretion | Decreased/absent | Variable |
| Insulin sensitivity | Normal when controlled | Decreased |
| Insulin dependence | Permanent | Variable |
| Obese or overweight | 20% to 25% overweight3 | > 80% obese |
| Acanthosis nigricans | 12%4 | 50% to 90%4 |
| Pancreatic antibodies | Yes5 | No6 |
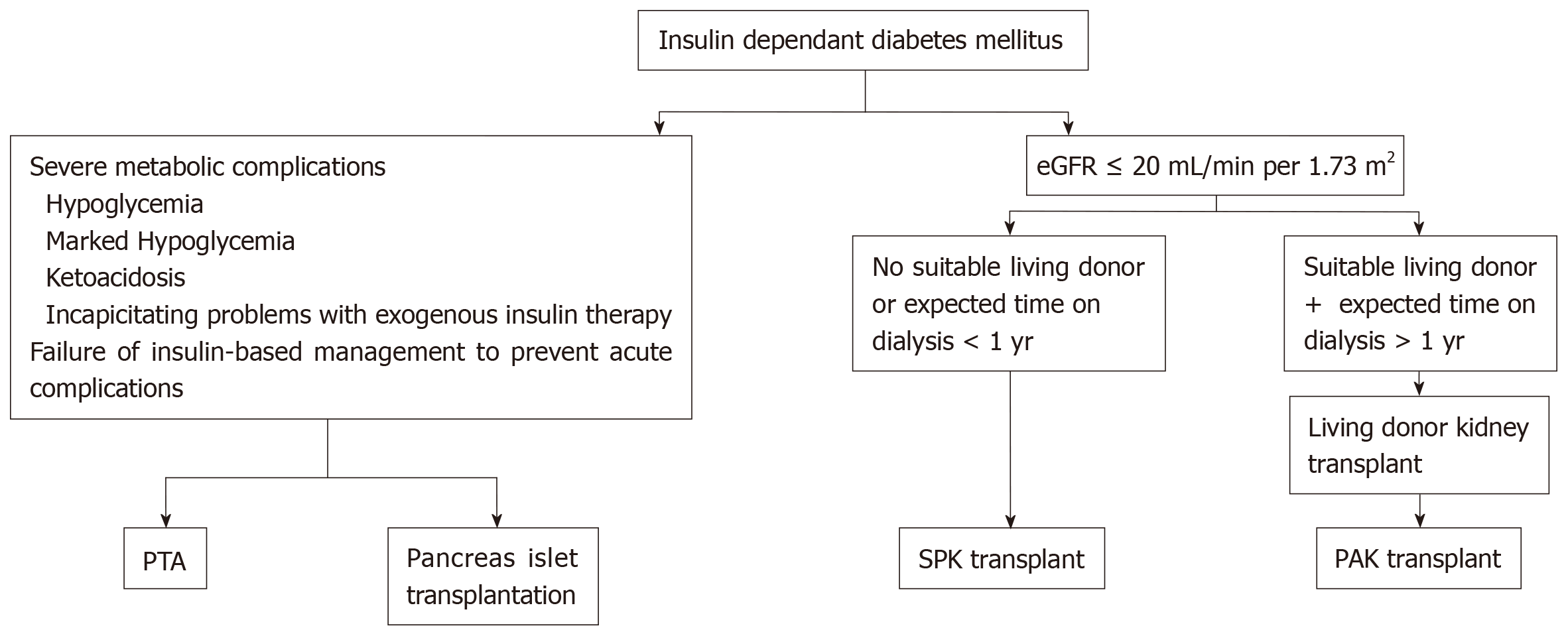
Pancreas transplantation is offered primarily to type 1 diabetic CKD patients, an approach that was supported by the fact of absence of endogenous insulin and normal insulin sensitivity. However, some cases with insulin-dependent type 2 diabetes in the United States have been accepted on simultaneous pancreas-kidney (SPK) waiting list if their body mass index (BMI) is less than 30 kg/m2, requiring insulin, but < 1.5 U/kg per day. About 6% only of SPK waiting list cases are type 2 DM[2]. The plan for transplan-tation modality is simplified in Figure 2.
In advanced CKD, Preemptive kidney transplantation from a living donor will offer the patient the highest patient survival rate at five years reaching up to 91% (compared to 84% for non-extended-criteria donor transplant, and 70% for extended-criteria donor transplants)[5]. Nevertheless, this management option will not usually solve diabetes-related medical condition (as DM control may be impaired if steroids were used post kidney transplantation either as maintenance therapy or for treatment of rejection episodes)[1,2].
In the United Kingdom, the national five-year patient survival is 88% for SPK recipients, and 78% for pancreas only transplant recipients. Pancreas allograft survival rate at five years is 75% for SPK recipients and 45% pancreas-only transplants[6].
A retrospective analysis of long-term survival of 18549 patients with type 1 DM in the United States has demonstrated that the patient survival at eight years for SPK recipients was similar to living-donor kidney recipients (about 72%) while the survival for cadaveric kidney recipients was only 55%[7]. SPK is associated with significantly elevated early mortality risk most probably secondary to the surgical procedure itself, and the related complications, which result in prolonged and recurrent hospitalisation is the first few months post-transplantation[1,7]. On the other hand, the long-term outcome for SPK is better than any other transplantation option in diabetic patients[1,7]. Recent data regarding kidney allograft survival with various types of kidney pancreas transplantation are summarized in Table 2[8].
| Type of the allograft | 1 yr | 5 yr | 10 yr |
| SPK | 3.1% | 16.5% | 37.7% |
| PAK (deceased donor) | 3.3% | 21.2% | 51.2% |
| PAK (living donor) | 3.0% | 13.7% | 37.0% |
The kidney outcomes for pancreas after kidney (PAK) were from the time of pancreas transplant, which may explain the lower survival rates compared to those of SPK recipients[8]. The maintenance of a functioning pancreas allograft was associated with the favourable long-term outcome with SPK most probably secondary to stabili-sation or even improvement of most of the DM associated systemic complications as illustrated in Table 3.
| Ref. | Patient cohorts | Outcomes of interest | Time after transplant (yr) | Results |
| Cardiovascular disease | ||||
| Fiorina et al[9], 2000 | SPK (n = 42) vs KTA (n = 26) vs type 1 diabetes (n = 20) | Left ventricular systolic and diastolic function assessed by radionuclideventriculography | 4 yr | Left ventricular ejection fraction was higher in SPK recipients than in KTA recipients [75.7 (SD 1.8%) vs 65.3% (2.8%); P = 0.02] and type 1 diabetes controls (75.7 (1.8%) vs 61.2 (3.7%); P = 0.004). |
| Biesenbach et al[10], 2005 | SPK (n = 12) vs KTA (n = 10) | Composite endpoint of myocardial infarction, stroke, and amputation | 10 yr | Lower incidence of myocardial infarction (16% vs 50%), stroke (16% vs 40%), and amputations (16% vs 30%) in SPK vs KTA recipients (P < 0.05 for composite endpoint of all three events) |
| Diabetic nephropathy | ||||
| Fioretto et al[11], 1998 | PTA: Pre-transplant vspost-transplant (n = 8) | Native kidney biopsy:structural morphology | 10 yr | Improvement in glomerular basement membrane thickening, tubular basement membrane thickening, and mesangial expansion after transplantation compared with before |
| before and after transplant | ||||
| Boggi et al[12], 2011 | PTA: Pre-transplant vs post-transplant (n = 71) | Proteinuria and estimatedGFR (eGFR) | Up to 4 yr | Overall, proteinuria decreased from 1.36 (SD 2.72) g/d pre-transplant to 0.29 (0.51) g/d post-transplant (P < 0.01) eGFR decreased by about 20% from 94 (39) mL/min per 1.73m2 to 75 (22) mL/min per 1.73 m2 (P < 0.01) |
| Diabetic neuropathy | ||||
| Havrdova et al[13], 2016 | SPK: Pre-transplant vspost-transplant (n = 12) | Epidermal nerve fiberdensity on skin biopsy, autonomic function tests, and nerve conduction studies | Up to 8 yr | No improvement in epidermal nerve fiber density or functional deficits on autonomic function tests |
| Boggi et al[12], 2011 | PTA: Pre-transplant vspost-transplant (n = 71) | Clinical neurologicexamination (vibration threshold), nerve conduction studies, and autonomic function tests (lying-to-standing test) | Up to 4 yr | Significant improvement in mean vibration thresholds, nerve conduction studies, and autonomic function tests after PTA compared with before |
| Diabetic retinopathy | ||||
| Boggi et al[12], 2011 | PTA: Pre-transplant vs post-transplant (n = 71) | Visual acuity scores andfundoscopic examination | Up to 4 yr | Before transplantation, 7.5% of patients had no retinopathy and remained lesion-free at 4 yr. Of the 29.5% with non-proliferative retinopathy, 75% improved and 25% remained unchanged. In the remainder with proliferative retinopathy, lesions remained stable in 82% and progressed in 18% |
| Giannarelli et al[14], 2006 | PTA (n = 33) vs type 1 diabetes (n = 35) | Visual acuity scores, fundoscopic examination, and angiography in selected cases | Up to 30 mo | Before transplant, 9% of patients with PTA and 6% of those with type1 diabetes had no retinopathy, 24% and 29% had non-proliferative retinopathy, and 67% and 66% had proliferative retinopathy. Overall, the percentage of patients with improved or stabilized retinopathy was significantly higher in the PTA group (P < 0.01) |
| Koznarova et al[15], 2000 | SPK (n = 43) Vs KTA (n = 45) | Visual acuity scores and fundoscopic examination | 3 yr | In the SPK group, fundoscopic findings at the end of follow-up had improved, stabilized, or deteriorated in 21.3%, 61.7%, and 17.0%, respectively. In the KTA group these figures were 6.1 %, 48.8%, and 45.1% (P < 0.001) |
Data collected from 20,854 pancreas transplant recipients between 1996 and 2012 by the United Network for Organ Sharing (UNOS) was analysed for patient and graft survival[16]. The best graft survival outcome was observed in recipient ranged between 40-49 years old. Additionally, the study documented an inverse relationship between recipient age and patient survival, with reduced patient survival in those who are older than 50 years[16].
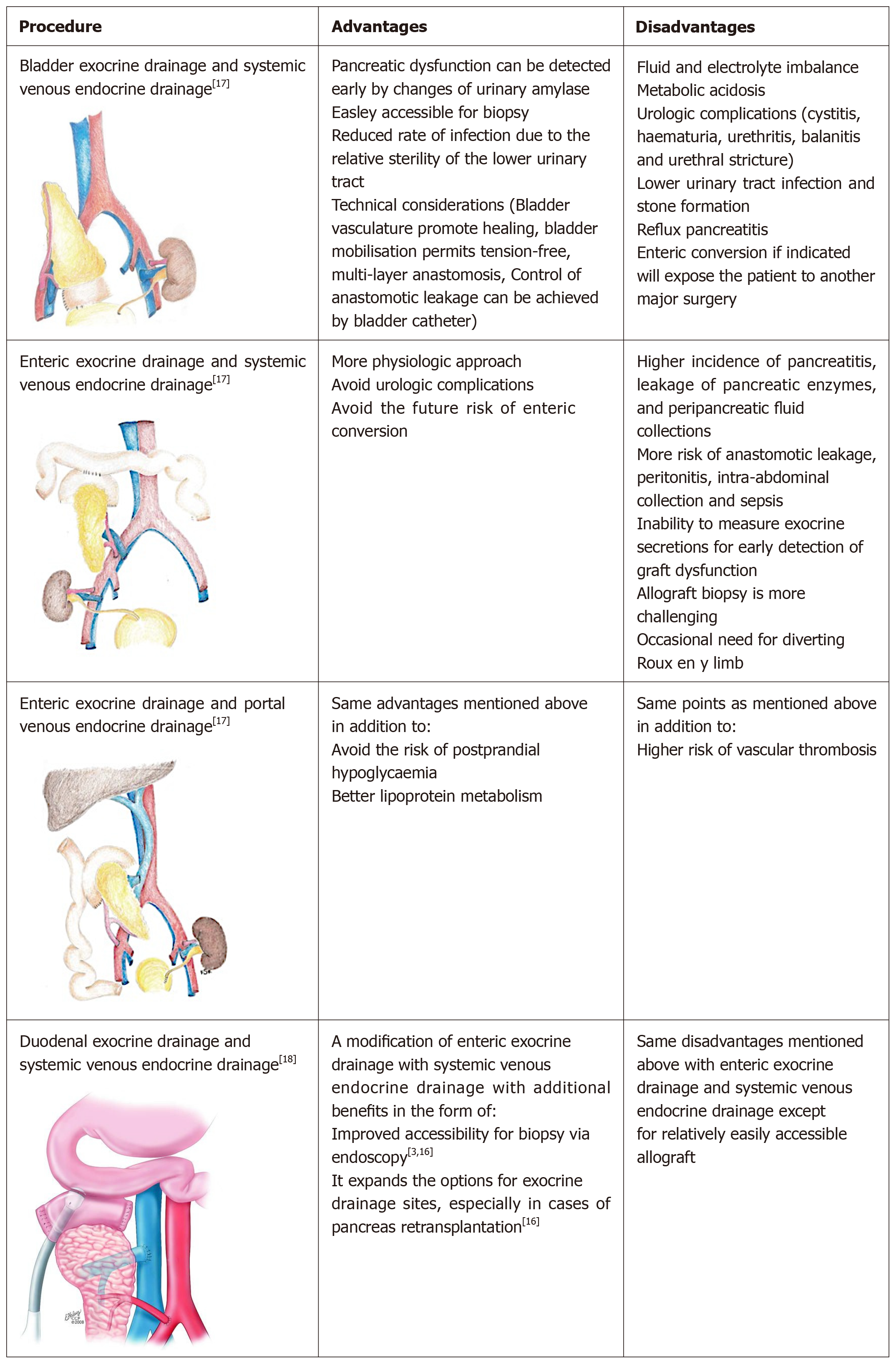
In SPK operation, the kidney is usually transplanted into the left iliac fossa by the traditional approach using the iliac vessels for vascular anastomosis[2]. There are several options for pancreatic implantation reflecting the fact that there is no standard optimal technique, each surgical option has its advantages as well as disadvantages. One of the challenges is the exocrine and endocrine drainage of the pancreatic allograft[2]. The various pancreatic implantations techniques are simplified in Figure 3[2,3,17,18], while the possible complications of pancreatic transplantation were summerized in Table 4[19].
| Complications | |
| Early complications | |
| Allograft parenchymal complications | Acute pancreatitis |
| Necrotizing pancreatitis | |
| Fistulous tracts | |
| Infection and abscesses | |
| Entric complications | Anastomosis leakage at duodeno-enterostomy |
| Ileus Colonic infection. | |
| Vascular complications | Venous or arterial graft thrombosis |
| Acute bleeding | |
| Late complications | |
| Allograft parenchymal complications | Rejection |
| Pseudocyst formation | |
| Post-transplant lymphoproliferative disease | |
| Enteric complications | Small bowel obstruction |
| Colonic infection | |
| Vascular complications | Arterial or venous pseudoaneurysms |
The patient evaluation should follow the local protocol for transplant candidate. This includes detailed medical, surgical, and psychosocial history; a meticulous physical examination; and laboratory evaluation. However, the pretransplant workup should be very strict to identify any possible undiagnosed condition related to DM that will negatively affect the outcome. Particular attention should be given for assessing cardiovascular status and the presence of peripheral vascular disease[20].
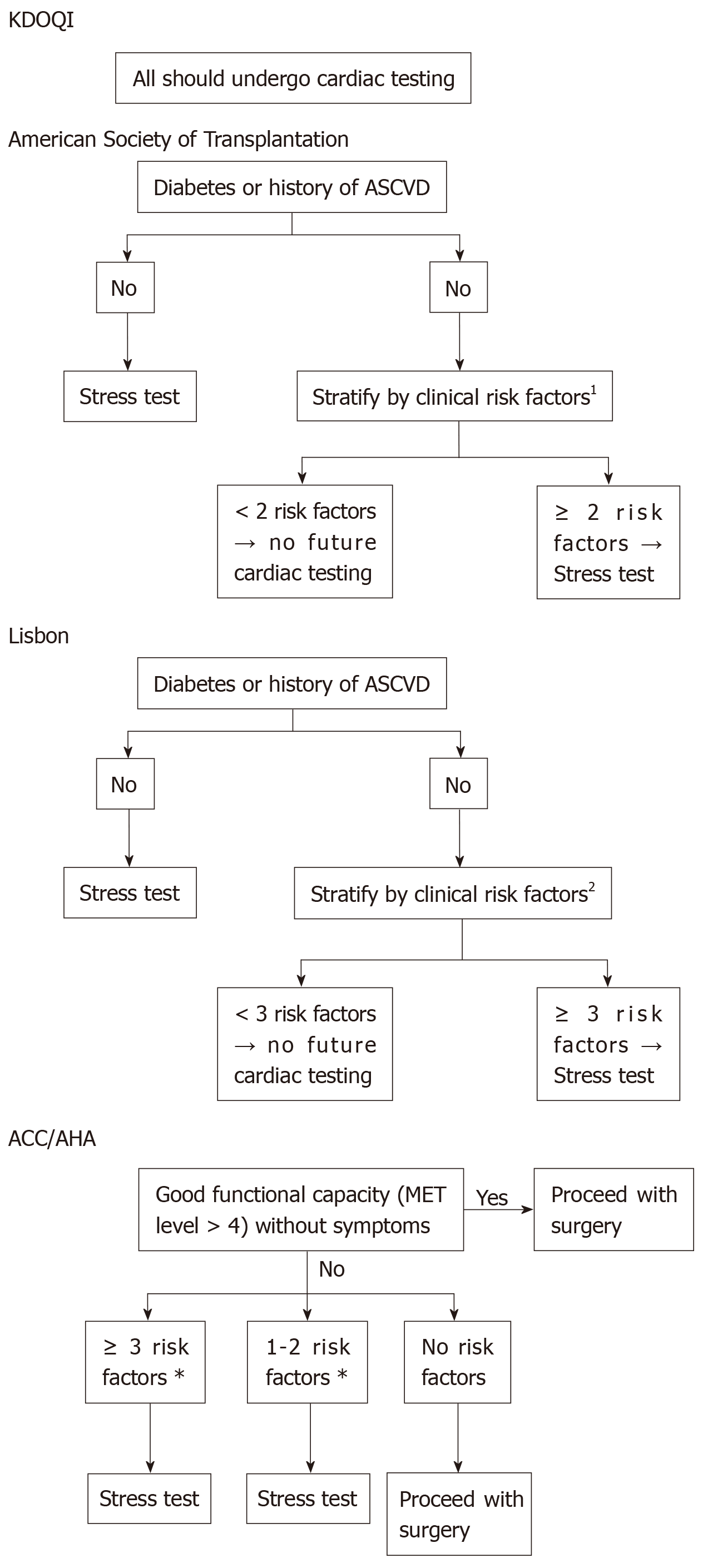
Pancreatic transplantation is associated with an increased risk of mortality in the early post-operative period, and the most frequent cause of death is of cardiovascular event[20]. There is no universally standardised cardiovascular screening protocol for asymptomatic CKD patients[21]. Some of the internationally published protocols are illustrated in Figure 4[21].
The initial cardiac assessment could be suggested by myocardial perfusion imaging (MPI) together with exercise-based (+/- dobutamine) stress test, and results should be interpreted by an expert cardiologist[21]. Myocardial perfusion studies provide valuable information regarding functional capacity, the extent of myocardial viability, and the extent of stress-induced ischemia as well as the degree of stress defect reversibility[21]. Some studies demonstrated an increased risk of cardiovascular events among patients who fail to complete exercise stress test regardless of the presence of negative test results[21].
The decisions regarding coronary catheterisation and revascularisation should be considered based on cardiologist recommendations. Patients with significant coronary pathology that is not amenable to revascularisation are not candidates for pancreatic transplantation[20].
Following successful transplantation BTS recommends reviewing the recipients in clinic twice to three times per week for the first month, weekly visits for the next two months, monthly for another three months, then every 2-3 months later on[22]. The clinic visit should include a detailed history of any new symptoms, careful medical examination and appropriate laboratory investigations (including immune-suppressant drug levels if possible). The patient care should involve a multidis-ciplinary team including a pharmacist, social worker, dietician, and psychologist[22].
Meticulous pancreatic donor and recipient selection criteria together with the modern immune suppression protocols have steadily decreased the incidence of pancreatic rejection to range between 10% to 20% in the first year post-transplant[2]. The majority of the early complications of the transplantation can be attributed to surgical and technical failures rather than an immunological injury. Complications include anastomotic leak, vascular thrombosis of the graft, graft pancreatitis, and infection[2,23].
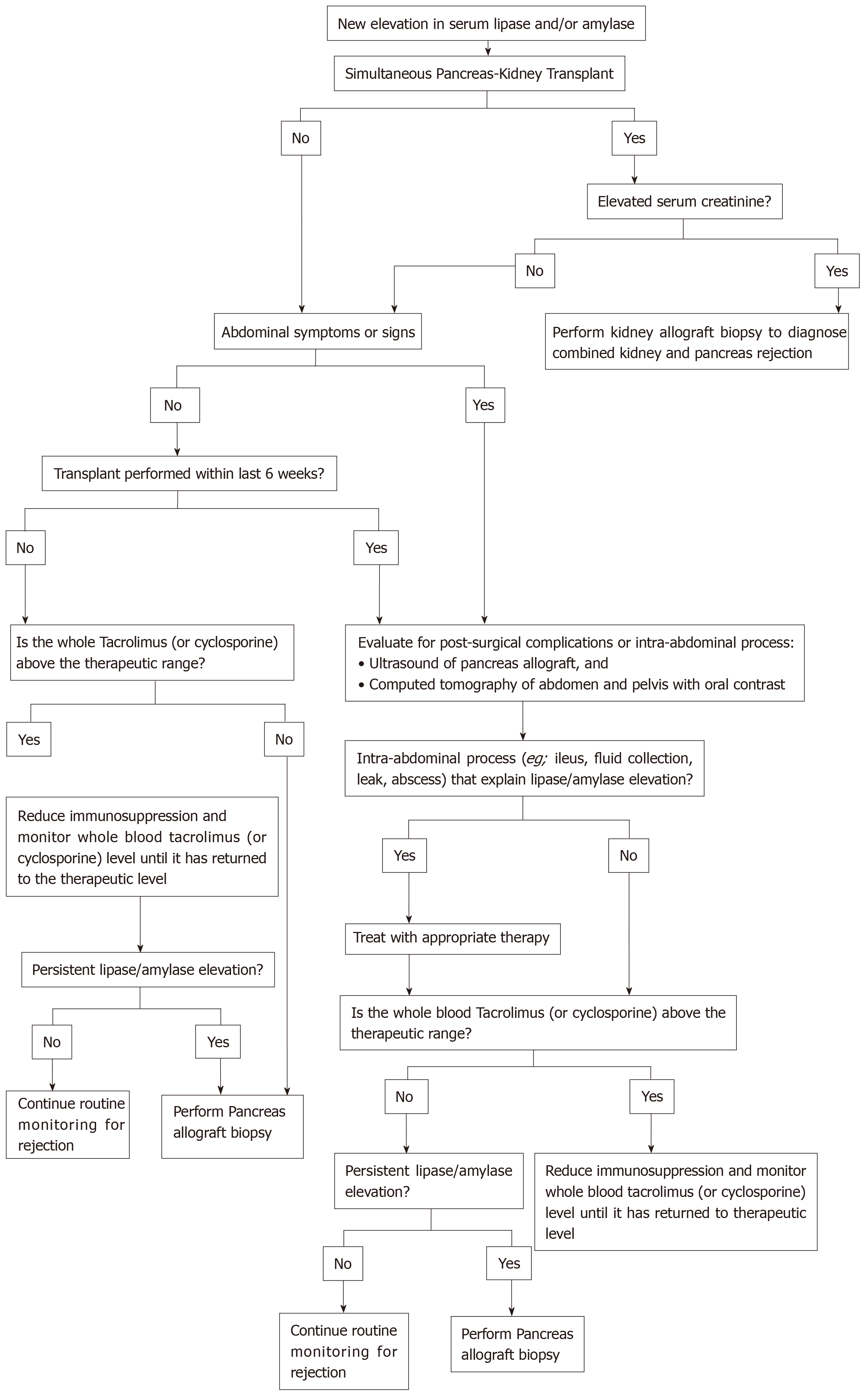
Most cases of pancreas allograft rejection are asymptomatic, so we should keep a high index of suspicion to detect allograft rejection early enough to allow early initiation of the proper therapy. The islet cells are spared in the initial phase of rejection, and hyperglycaemia is a late finding[2]. We should start our workup once allograft dysfunction is suspected (e.g., elevated serum amylase and/or lipase)[2,24]. A recom-mended approach for evaluation of pancreatic allograft dysfunction is illustrated in Figure 5[24].
Maintenance immunosuppressive therapy for pancreatic transplantation is similar for that used for kidney transplantation. Most centres use a combination of a calcineurin inhibitor (predominantly tacrolimus), an antimetabolite (mycophenolate mofetil or mycophenolate sodium), and low-dose corticosteroids[2,20]. Induction therapy with lymphocyte-depleting agents (e.g., antithymocyte globulin and alemtuzumab) allows early steroid withdrawal and steroid free regimens which are adopted by some centres[2].
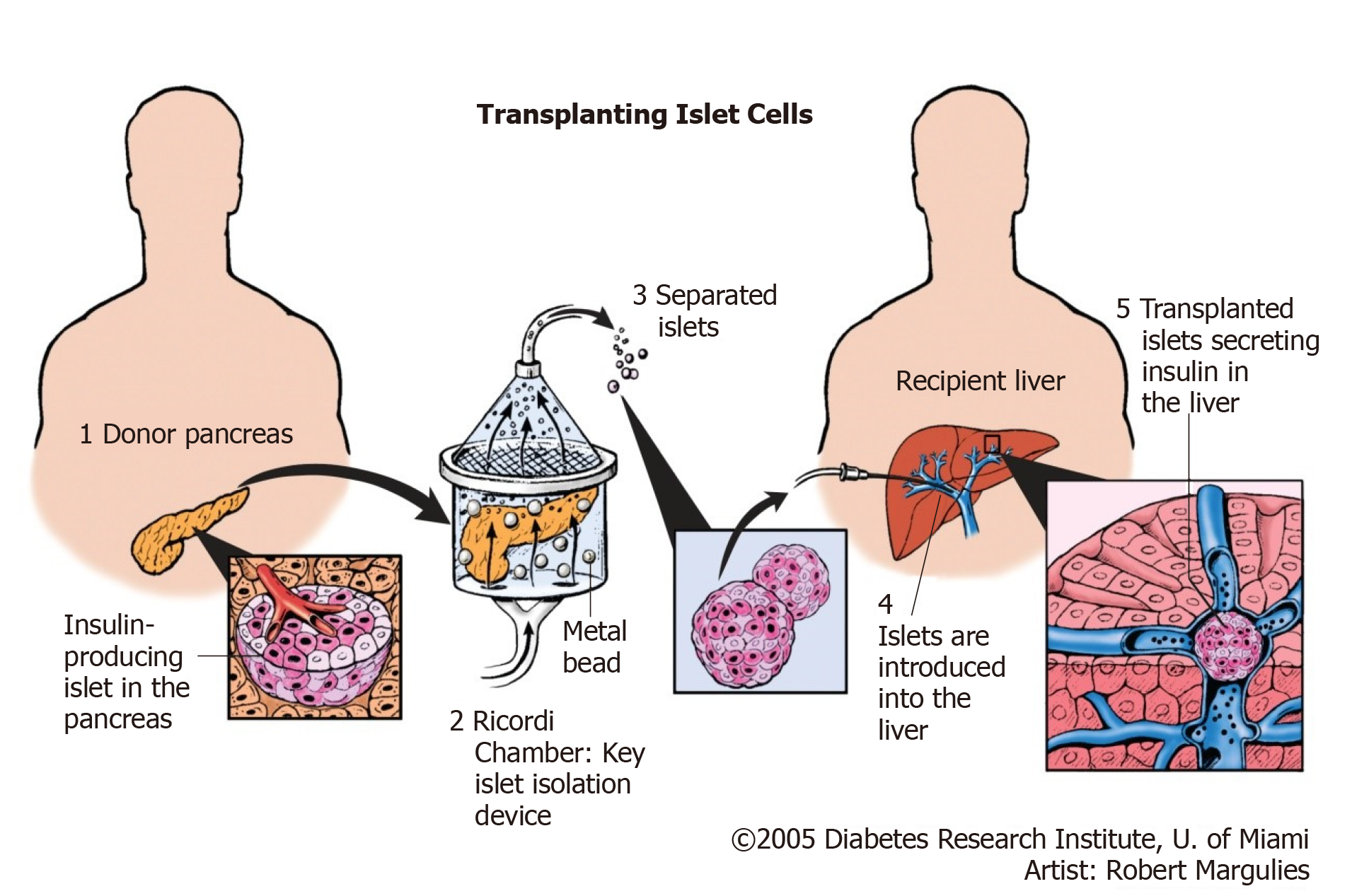
Islet transplantation is an evolving and promising therapeutic option for management of type 1 DM. Successful isolation of islet cells from the whole pancreas is followed by infusion of the cells to the portal vein of the recipient via a percutaneous catheter as illustrated in Figure 6[25].
Keeping in mind that the major mass of the pancreas if formed of exocrine gland with only scattered clusters of endocrine cells, separation of islet cells from exocrine part will not only allow transplantation via minimally invasive technique (infusion of islets isolated from cadaveric pancreas via the portal vein), but it will also avoid vascular and allograft duodenal anastomoses, hence avoiding an essential source of surgical complications[2].
On the other hand, this therapeutic option is facing significant challenges that include: Achieving insulin independence necessitates transplantation of an adequate islet mass, which requires isolation from multiple donors (typically 2 to 4 donors), thus islet cell recipients are exposed to numerous human leukocyte antigen (HLA) mismatches which may jeopardize the possibility of future transplantation due to sensitization and formation of donor-specific antibodies[2,23]; The patient would require lifelong immune suppression even if received islet cell transplantation alone[2,23]; Despite the satisfactory short-term outcome of this technique (about 80% of the cases remained insulin independent after two years), the long-term outcome is still disappointing[2]; In the case of advanced CKD in addition to DM, Islet cell transplan-tation alone is not a valid option in the management of such medical condition.
There is no individual management plan for diabetic patients with advanced CKD; instead, we have different management options that depend on the patient co-morbidities as well as personal preferences. Nevertheless, each option has its limitations and possible complications. The best management plan for diabetic patient approaching ESRD is SPK which will offer the best long-term survival, in addition to the better quality of life and regression of most of DM complications. However, this approach is associated with early increased risk of morbidity and mortality. PTA and islet cell transplantation are possible options for managing diabetic patients. However, they are not suitable alone for patients with concomitant advanced CKD. The pretransplant workup for SPK is more stringent compared to kidney transplan-tation alone to minimise the risk of early postoperative morbidity and mortality and to achieve long-term patient and graft survival. Islet cell transplantation carries the risk of sensitisation against a group of HLA antigens, which makes the patients less likely to get a compatible kidney allograft in the future. PAK is not recommended above the age of 50 as it is negatively affecting the survival of patients older than 50 years. Additionally, it may result in loss of kidney allograft as a complication of this major intervention.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Koukoulaki M, Parajuli S S-Editor: Ji FF L-Editor: Filipodia E-Editor: Qi LL
| 1. | Steddon S, Chesser A, Cunningham J, Ashman N. Oxford Handbook of Nephrology and Hypertension. Second edition, Oxford University Press. 2014;. [DOI] [Full Text] |
| 2. | Danovitch GM. Handbook of Kidney Transplantation. Sixth Edition, Wolters Kluwer. 2017;606. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 3. | Srinivas TR, Shoskes DA. Kidney and Pancreas Transplantation: A Practical Guide. Available from: https://www.springer.com/us/book/9781607616412. |
| 4. | Misra M, Levitsky LL. Epidemiology, presentation, and diagnosis of type 1 diabetes mellitus in children and adolescents. 2018; Available from: https://www.uptodate.com/contents/epidemiology-presentation-and-diagnosis-of-type-1-diabetes-mellitus-in-children-and-adolescents. |
| 5. | Vella J. Patient survival after renal transplantation. 2018; Available from: https://www.uptodate.com/contents/patient-survival-after-renal-transplantation#!. |
| 6. | Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017;357:j1321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 7. | Reddy KS, Stablein D, Taranto S, Stratta RJ, Johnston TD, Waid TH, McKeown JW, Lucas BA, Ranjan D. Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis. 2003;41:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Kandaswamy R, Stock PG, Gustafson SK, Skeans MA, Curry MA, Prentice MA, Fox A, Israni AK, Snyder JJ, Kasiske BL. OPTN/SRTR 2016 Annual Data Report: Pancreas. Am J Transplant. 2018;18 Suppl 1:114-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 9. | Fiorina P, La Rocca E, Astorri E, Lucignani G, Rossetti C, Fazio F, Giudici D, di Carlo V, Cristallo M, Pozza G, Secchi A. Reversal of left ventricular diastolic dysfunction after kidney-pancreas transplantation in type 1 diabetic uremic patients. Diabetes Care. 2000;23:1804-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 52] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Biesenbach G, Königsrainer A, Gross C, Margreiter R. Progression of macrovascular diseases is reduced in type 1 diabetic patients after more than 5 years successful combined pancreas-kidney transplantation in comparison to kidney transplantation alone. Transpl Int. 2005;18:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 11. | Fioretto P, Steffes MW, Sutherland DE, Goetz FC, Mauer M. Reversal of lesions of diabetic nephropathy after pancreas transplantation. N Engl J Med. 1998;339:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 839] [Cited by in RCA: 746] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 12. | Boggi U, Vistoli F, Amorese G, Giannarelli R, Coppelli A, Mariotti R, Rondinini L, Barsotti M, Piaggesi A, Tedeschi A, Signori S, De Lio N, Occhipinti M, Mangione E, Cantarovich D, Del Prato S, Mosca F, Marchetti P. Results of pancreas transplantation alone with special attention to native kidney function and proteinuria in type 1 diabetes patients. Rev Diabet Stud. 2011;8:259-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Havrdova T, Boucek P, Saudek F, Voska L, Lodererova A, Üçeyler N, Vondrova H, Skibova J, Lipar K, Sommer C. Severe Epidermal Nerve Fiber Loss in Diabetic Neuropathy Is Not Reversed by Long-Term Normoglycemia After Simultaneous Pancreas and Kidney Transplantation. Am J Transplant. 2016;16:2196-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 14. | Giannarelli R, Coppelli A, Sartini MS, Del Chiaro M, Vistoli F, Rizzo G, Barsotti M, Del Prato S, Mosca F, Boggi U, Marchetti P. Pancreas transplant alone has beneficial effects on retinopathy in type 1 diabetic patients. Diabetologia. 2006;49:2977-2982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Koznarová R, Saudek F, Sosna T, Adamec M, Jedináková T, Boucek P, Bartos V, Lánská V. Beneficial effect of pancreas and kidney transplantation on advanced diabetic retinopathy. Cell Transplant. 2000;9:903-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 58] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Siskind E, Maloney C, Akerman M, Alex A, Ashburn S, Barlow M, Siskind T, Bhaskaran M, Ali N, Basu A, Molmenti E, Ortiz J. An analysis of pancreas transplantation outcomes based on age groupings--an update of the UNOS database. Clin Transplant. 2014;28:990-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | El-Hennawy H, Stratta RJ, Smith F. Exocrine drainage in vascularized pancreas transplantation in the new millennium. World J Transplant. 2016;6:255-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Wee AC, Krishnamurthi V. Pancreas Transplantation: Surgical Techniques. In: Srinivas T, Shoskes D (eds). Kidney and Pancreas Transplantation. Current Clinical Urology. Humana Press, Totowa, NJ 2011; . [DOI] [Full Text] |
| 19. | França M, Certo M, Martins L, Varzim P, Teixeira M, Henriques AC, Ribeiro AM, Alves FC. Imaging of pancreas transplantation and its complications. Insights Imaging. 2010;1:329-338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 20. | Alhamad T, Stratta RJ. Pancreas-kidney transplantation in diabetes mellitus: Patient selection and pretransplant evaluation. 2018; Available from: https://www.uptodate.com/contents/pancreas-kidney-transplantation-in-diabetes-mellitus-patient-selection-and-pretransplant-evaluation#!. |
| 21. | Halawa A, Aref A, Sharma A, Zayan T. Evaluation of the Cardiovascular Prior to Transplantation; An Endless Debate. Urol Nephrol Open Access J. 2017;4:00126. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | British Transplantation Society. Active BTS Guidelines Standards. 2018; Available from: https://bts.org.uk/guidelines-standards/. |
| 23. | Robertson RP. Pancreas and islet transplantation in diabetes mellitus. 2018; Available from: https://www.uptodate.com/contents/pancreas-and-islet-transplantation-in-diabetes-mellitus. |
| 24. | Alhamad T, Kukla A, Stratta RJ. Pancreas allograft rejection. 2018; Available from: https://www.uptodate.com/contents/pancreas-allograft-rejection#!. |
| 25. | Diabetes Research Institute, University of Miami. Transplanting islet cells. 2018; Available from: https://www.diabetesresearch.org/images-video. |