Published online Oct 22, 2018. doi: 10.5500/wjt.v8.i6.203
Peer-review started: July 19, 2018
First decision: July 29, 2018
Revised: August 9, 2018
Accepted: August 27, 2018
Article in press: August 27, 2018
Published online: October 22, 2018
Processing time: 93 Days and 3.7 Hours
For decades, kidney diseases related to inappropriate complement activity, such as atypical hemolytic uremic syndrome and C3 glomerulopathy (a subtype of membranoproliferative glomerulonephritis), have mostly been complicated by worsened prognoses and rapid progression to end-stage renal failure. Alternative complement pathway dysregulation, whether congenital or acquired, is well-recognized as the main driver of the disease process in these patients. The list of triggers include: surgery, infection, immunologic factors, pregnancy and medications. The advent of complement activation blockade, however, revolutionized the clinical course and outcome of these diseases, rendering transplantation a viable option for patients who were previously considered as non-transplantable cases. Several less-costly therapeutic lines and likely better efficacy and safety profiles are currently underway. In view of the challenging nature of diagnosing these diseases and the long-term cost implications, a multidisciplinary approach including the nephrologist, renal pathologist and the genetic laboratory is required to help improve overall care of these patients and draw the optimum therapeutic plan.
Core tip: The recent progress in our understanding of the pathophysiology of complement-mediated diseases is gaining considerable popularity. Complement dysregulation due to inherited or acquired factors is currently the culprit mechanism. Several constitutional abnormalities usually trigger the process of recurrence, with a subsequent high rate of graft loss. The development of the terminal complement inhibitor “eculizumab” is a breakthrough in controlling abnormal complement activation. While diagnosing complement abnormalities is one challenge, treatment cost with this new agent is another major hurdle in any health care system. New lines of promising therapies are currently in the pipeline.
- Citation: Abbas F, El Kossi M, Kim JJ, Shaheen IS, Sharma A, Halawa A. Complement-mediated renal diseases after kidney transplantation - current diagnostic and therapeutic options in de novo and recurrent diseases. World J Transplantation 2018; 8(6): 203-219
- URL: https://www.wjgnet.com/2220-3230/full/v8/i6/203.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i6.203
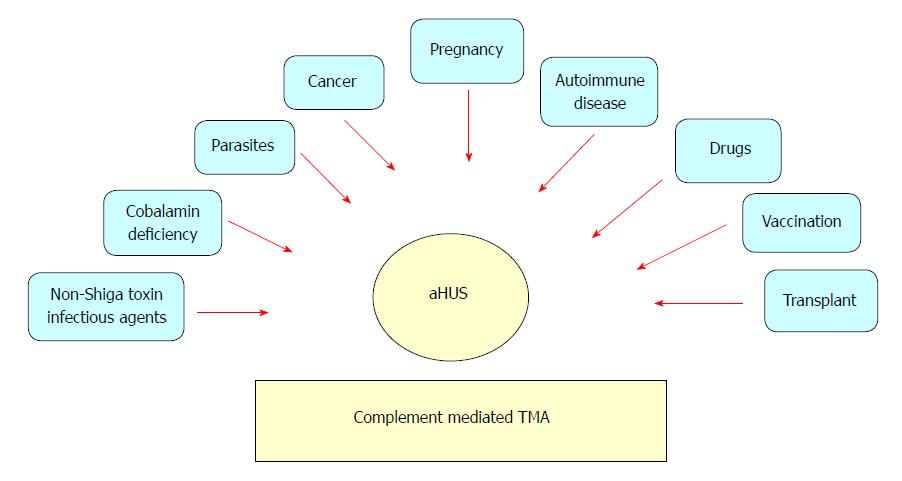
The complement components can be seen in biopsies of almost all types of glomerulonephritis, which can be broadly divided into two main groups: (1) “complement over-activation” includes IgA nephropathy (IgAN) and immune complex membranoproliferative glomerulonephritis (MPGN); and (2) “complement dysregulation” that encompasses atypical hemolytic uremic syndrome (aHUS) and C3 glomerulopathy (C3G)[1]. While complement activation is triggered by immune complex formation in the former group, genetic mutations are the driver of complement over-activation in the latter one. This explains why the disease process in the former class is potentially modifiable by immunosuppression in the post-transplantation period, which is not the case in the latter class. Our understanding of the biogenetic causes of C3G and aHUS/thrombotic microangiopathy (TMA) has been expanding. The mechanisms of these diseases not only affect their clinical history, but also affect the recurrence rate[2]. The role of complement in C3G evolution is now well-recognized[3]. Recent progress in understanding the pathophysiology of MPGN led to newer classifications of MPGN into immune complex-mediated and complement-mediated subtypes. The hallmark of complement-mediated MPGN is the deposition of C3 and other complement products in glomerular tissues[4]. This is caused by dysregulation and loss of control of the AP complement pathway[5]. The AP is tightly regulated under physiological conditions. It can be disrupted through either inherited (mutations/polymorphisms) or acquired (autoantibodies) interferences to the regulating components. Histological staining using immunofluorescence (IF) is currently the best determinant technique, and C3G is defined by dominant C3 with dispersed, reduced or absent immunoglobulin (Ig). Based on electron microscopy (EM) examination, C3G subdivides into complement three glomerulonephritis (C3GN) and dense deposit disease (DDD). In C3GN, discrete deposits can be seen in the mesangium and capillary walls (subendothelial and subepithelial regions). On the contrary, DDD deposits are large in size, extremely dense (osmiophilic) and intramembranous, which leads to a characteristic thickening of the glomerular basement membrane (GBM)[5]. The term aHUS is applied to a heterogenous group of diseases (Figure 1) that share TMA manifestations with an associated decline in renal function (classically, no IF staining of C3 or any other complement components). In aHUS, complement abnormalities (either genetic mutations or acquired autoantibodies) are well-recognized mechanisms with a clearly associated complement-mediated TMA[1]. In this article, we will discuss various types of complement-mediated renal diseases after kidney transplantation and their current therapeutic options.
In view of the lack of prospective controlled trials concerned with complement-mediated diseases post-kidney transplant, we tried to shed the light in this review on the most recent expert opinions, with regard to the best tools of management for these devastating diseases.
DDD and C3GN share some salient features that include proteinuria, hematuria and increased serum creatinine concentration[6,7]. Recurrence of C3G is typically encountered one to two years after transplant[7]. C3G comprises a spectrum of diseases that result from aberrant control of complement activation, deposition and dysregulation, leading to C3 glomerular deposition with characteristic electron-dense deposits (EDD) in EM (Table 1).
| Morphological features of C3G | |
| Light microscopy | Active lesions |
| Mesangial expansion with or without hypercellularity | |
| Endocapillary hypercellularity including monocytes and/or neutrophils | |
| Capillary wall thickening with double contours (combination of capillary wall thickening + mesangial increase is referred to as a membranoproliferative pattern) | |
| Fibrinoid necrosis | |
| Cellular/fibrocellular crescents | |
| Chronic lesions | |
| Segmental or global glomerulosclerosis | |
| Fibrous crescents | |
| IF microscopy | Typically dominant C3 staining |
| Electron microscopy | DDD: Dense osmiophilic mesangial and intramembranous electron dense deposits. |
| C3GN: Amorphous mesangial with or without capillary wall deposits including subendothelial, intramembranous and subepithelial EDD | |
| Subepithelial “humps” may be seen in both DDD and C3GN |
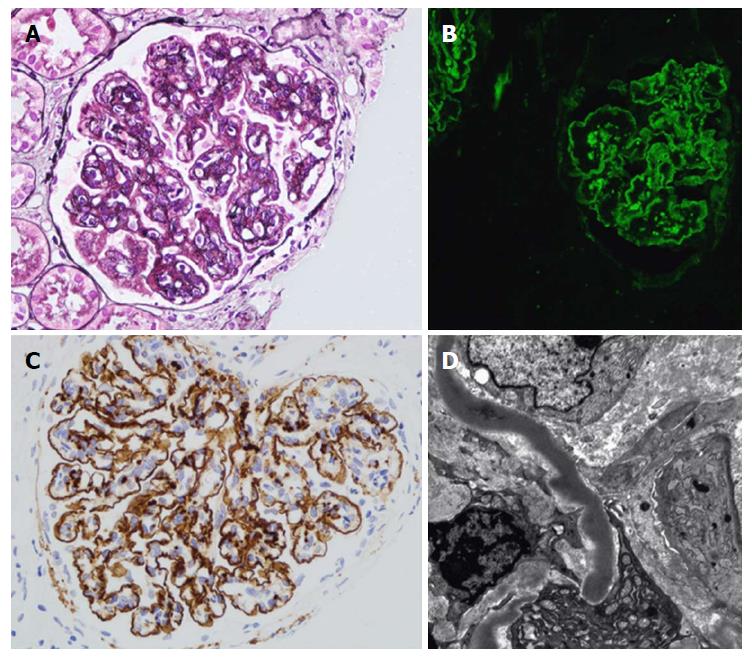
Renal biopsy is crucial for C3G diagnosis. LM is not helpful, due to its extremely diverse appearance. IF is the mainstay for diagnosis. A unique criterion in IF studies is the presence of dominant C3 staining, which is twice as intense as any other immunoreactant (IgG, IgM, IgA, and C1q)[8]. Ninety percent of DDD patients, but fewer C3GN patients, can be diagnosed through applying this criterion[8]. Repeated biopsy may be required to confirm the diagnosis. As C3G may present in acute infection, C3 can be observed with post-infectious GN. Humps are no longer pathognomonic criteria of post-infectious GN, however they can also be encountered in C3G. However, the presence of double contours in the GBM raises the possibility of C3G diagnosis. To differentiate DDD from C3GN, EM studies should be accomplished, as it has pivotal clinical implications. Moreover, staining for IgG as well as light chains on pronase-digested paraffin should be applied for all cases of C3GN on standard IF, particularly in adults (Figure 2 and Table 1)[9,10].
TMA is mostly presented 3-6 mo post-transplant, but it can occur at any time after renal transplantation[13]. Presentation of TMA is not universal, ranging from the renal-limited form up to a complete systemic picture with its classic triad of thrombocytopenia, microangiopathic hemolytic anemia (MAHA) and decline in renal function[14]. MAHA is defined as increased LDH, decline in HB and haptoglobin, and appearance of schistocytes in peripheral blood smears. On the other hand, localized (renal-limited) TMA usually presents later in the post-transplant course. In the acute stage, evidence of endothelial injury with platelet aggregation (thrombosis), fibrinoid necrosis, as well as glomerular ischemia can be seen. On the other hand, chronic lesions show duplication and multilayering of the GBM, with clustering of the matrix layers and vessel wall cells leading to the characteristic onion skin shape appearance (Table 2)[15]. As TMA is not always present with full-blown systemic pictures, genetic studies to unmask the underlying complement defect are ultimately mandated, particularly if no other clear cause has been associated (e.g., AMR-associated TMA). AMR can give a TMA-like picture, as it is an antibody interaction with the endothelium. This is also a fundamental maneuver to differentiate de novo from recurrent disease (positive genetic testing), with consequent clinical therapeutic implications[16].
| Active lesions | Chronic lesions |
| Glomeruli: Thrombi - Endothelial swelling or denudation - Fragmented RBCs - Subendothelial flocculent material. EM: Mesangiolysis - Microaneurysms | Glomeruli: LM: Double contours of peripheral capillary walls, with variable mesangial interposition - EM: New subendothelial basement membrane - Widening of the subendothelial zone |
| Arterioles: Thrombi - Endothelial swelling or denudation - Intramural fibrin - Fragmented red blood cells - Intimal swelling - Myocyte necrosis | Arterioles: Hyaline deposits |
| Arteries: Thrombi - Myxoid intimal swelling - Intramural fibrin - Fragmented red blood cells | Arteries: Fibrous intimal thickening with concentric lamination (onion skin) |
Twenty percent of aHUS patients express extrarenal manifestations. Their relation to complement activation and TMA evolution is unclear. Drusen is rarely seen in TMA[17]. Drusen formation, which represents an accumulation of lipids and complement-rich proteins between Bruch’s membrane and the retinal pigmentary epithelium, is commonly reported in age-related macular degeneration but present at a much earlier age with C3G[18]. In C3G, retinal drusen and acquired partial lipodystrophy have been commonly reported. The latter is most commonly encountered with C3 nephritic factors. Factor D, an essential agent for C3 convertase formation, is highly concentrated in adipocytes that undergo C3 nephritic factor-induced complement-dependent lysis[19] (Table 3).
| aHUS | DDD/C3GN |
| Digital gangrene, skin | Retinal drusen |
| Cerebral artery thrombosis/stenosis | Acquired partial lipodystrophy |
| Extracerebral artery stenosis | |
| Cardiac involvement/myocardial infarction | |
| Ocular involvement | |
| Neurologic involvement | |
| Pancreatic, gastrointestinal involvement | |
| Pulmonary involvement | |
| Intestinal involvement |
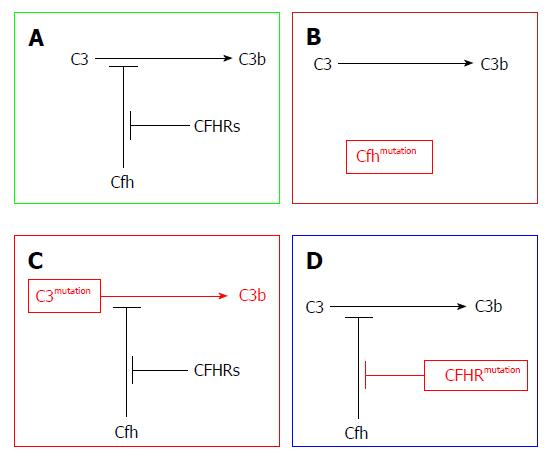
The new classification of MPGN encompasses two subtypes: the immune complex-mediated GN (ICGN) and complement-mediated GN (CGN), recently named (C3G). The former is characterized by both Ig as well as complement component deposition in kidney tissues as recognized by IF studies. The latter is characterized by dominant complement deposition with smaller amounts of Ig deposition. Further subdivision of C3G into C3GN and DDD can be attained through EM studies[20]. Both subtypes are triggered through dysregulation of any part of the AP. For example, patients may develop the C3 convertase-stabilizing factor called C3NeF, which leads to uncontrolled complement activation. Loss-of-function mutations in complement regulatory proteins (CFH or CFI)[20-23] or gain-of-function mutations in C3 leads to CFH resistance, which has been postulated as an underlying mechanism (Figure 3).
Pathophysiology of AP activation in DDD and C3GN is nearly the same. In both disorders, disturbance of the fluid phase is triggered as a result of aberrant gene mutations or the presence of autoantibodies. However, the presence of C3 nephritic factor (C3NeF) is by far the most commonly acquired complement defect. C3NeF has the ability to block CFH-mediated decay by stabilizing C3 convertase[5,24]. By binding to C3 convertase, C3NeF has the ability to trigger it approximately ten times[25,26]. C3 convertase can also block the action of CFH, CR1, as well as decay-accelerating factor (DAF).
C3NeF is prevalent in 50%-80% C3G patients[27]. Other autoantibodies have also been found (e.g., autoantibodies against factor B[28], CFH[29,30] and C3 convertase)[28]. In C3G, CFH mutations have been frequently reported. Different forms of mutations can be presented as defective or completely absent protein H. These mutations can be seen in homozygous or heterozygous forms[31,32]. C3NeF can also be encountered, which denotes the clustering of different risk factor varieties. More recently, genetic mutations involving the CFHR gene have been reported in the C3G cohort of patients[33]. CFHR group genetic mutations[34], deletions[35], duplications[36], as well as hybrid genes[37] have also been observed in C3G patients, either in an isolated manner or in a familial cohort. Malik and his associates[38] reported that members of one family can develop C3G as an result of aberrant copies of CFHR3 and CFHR1 loci. The presence of familial C3G underscores the genetic basis of several C3G varieties and their relation to AP dysregulation.
To summarize, complement dysregulation is the specific etiology of C3G, which could be genetic or acquired. While genetic causes encompasse complement gene mutations, acquired causes include the C3NeFs, which have the ability to impede normal complement regulation[1]. Moreover, genetic varieties constitute the pathophysiologic basis of C3G and aHUS evolution (Table 4). Recently, a robust correlation between CFH-related proteins and a variety of complement-mediated diseases have been documented. Functional parameters (e.g., complement regulators and CFH competitors) have recently attained significant popularity[39].
| Genetic defect | Phenotypical expression |
| Duplication in CFHR5 gene | C3 glomerulopathy (CFHR5 nephropathy) |
| Duplication in CFHR1 gene | C3 glomerulopathy |
| Hybrid CFHR3/CFHR1 | C3 glomerulopathy |
| Hybrid CFHR2/CFHR5 | C3 glomerulopathy |
| Hybrid CFH/CFHR1 | aHUS |
| Hybrid CFH/CFHR3 | aHUS |
Both TMA and C3G have a common underlying causation: AP dysregulation. However, the question that arises is “which factors influence the evolution of one disease rather than the other?”[40]. The prevalence of the fluid phase complement activation dysregulation in animal models suggests that C3G is the responsible factor. On the other hand, complement activation involving capillary walls can result in TMA evolution[41]. Furthermore, absolute CFH deficiency is in favor of an activation of the fluid phase complement with subsequent C3G evolution, while the lack of an aberrant CFH binding region is in favor of TMA evolution[41]. It has also been postulated that CFH and CFH/CFHR mutations induce aHUS to inhibit CFH-binding to many cell surfaces, while C3G-associated mutations in CFHRs cannot inhibit CFH binding to endothelial cell surfaces[42]. The prevalence of familial C3G mutations serves as a robust indicator of the genetic base of C3G recurrence[1].
Despite the well-known DDD variants of C3, its pathogenesis has only recently been recognized. The five-year graft survival rate was only 50% in one retrospective study of 75 children[6]. In adults, a majority of the recipients developed recurrence in post-transplant periods, with 25% of them losing their allografts[43]. In another broader cohort that included eighty adults and children with C3G, Medjeral-Thomas et al[44], reported histological recurrence in all six DDD recipients. Graft loss had resulted in 50% of his cases. For recipients who developed DDD recurrence, the ten-year graft survival rate has been reported to be up to 57.5% in an UNOS review[45]. Risk factors for DDD recurrent disease and graft loss are not well-recognized. However, the histological recurrence rate was reported to be more than 70%[46,47]. Recurrence may present spontaneously in post-transplant periods, though it may take several years to manifest[47]. This discrepancy raises some questions, such as the impact of the longevity of follow-ups, the need for tissue diagnosis, and the real rate of DDD recurrence.
There is no documented relation between mode of presentation, C3 serum levels, or C3NeF levels and C3GN recurrence[48]. The only trustworthy risk factor correlated with C3 recurrence is the presence of heavy proteinuria, with two thirds of C3 patients showing vulnerability to recurrence and a high incidence of graft loss[5,7,27]. All the available data about recurrence are based on case series, with the largest by Zand et al[7] that failed to reveal robust evidence of recurrence risk. This observation is partially explained by the heterogeneity of complement defects implicated in C3GN evolution. Early reports postulated HLA-B8 DR3 and living related donation as possible risk factors for recurrence[49]. However, the more recent reports suggested the following: (1) history of graft loss owing to recurrence[50]; (2) aggressive histopathological alterations in native kidney biopsy; and (3) hybrid CFHR3 1 gene-related C3GN. Wong et al[51] have recently reported a high rate of C3G recurrence (five patients received a total of eight kidney transplants). Four (50%) renal allografts had disease recurrence, of which three had biopsy-proven recurrence, with time to recurrence ranging from as early as 2 wk following living-related donor transplantation, to 93 and 101 mo for the two remaining allografts, respectively[51].
The declining appearance of proteinuria, hematuria or eGFR is a strong indicator of C3G recurrence. Final diagnosis is usually made through LM, IF, and EM studies of kidney biopsy. After histopathological examination, a thorough evaluation of any genetic mutation in the AP should be accomplished, especially if these studies were not previously fulfilled with the native kidney disease.
A robust work-up of analytic studies including genetic, biochemical and pathological evaluation should be instituted, including the following: (1) complement components and complement regulatory protein levels; (2) peripheral WBC MCP levels; (3) screening for antibodies to CFH and C3NeFs; and (4) mutation screening of CFH, CFI, CFB, C3, and MCP. Furthermore, recombination in the CFHR region should be tested[52].
In both DDD and C3GN, recurrent disease is usually associated with allograft loss[6,44,53]. The one-year allograft survival was reported to be 94%, with 69% at five years, and 28% at ten years. Three predictive criteria for progression to ESRD were recognized: (1) crescentic GN; (2) severe arteriolar sclerosis by LM; and (3) decline of renal function at the time of first biopsy[44].
Compared to recurrent TMA, the prognosis of de novo TMA is quite poor. Fifty percent of patients may lose their graft within a couple of years after diagnosis[54,55]. Many reports were in favor of this attitude[54-56]. Before the era of eculizumab (EZ), Schwimmer et al[54] reported that 54% of systemic TMA can develop dialysis requiring AKI, and about 38% lost their allograft. However, no one patient with localized TMA has complicated with TMA-related allograft loss or a need for dialysis. Nevertheless, both systemic and localized forms may experience unfavorable long-term graft survival[54,57].
The therapeutic approach for de novo C3G therapy is similar to that of recurrent disease. Very minimal information is available regarding de novo C3G[58].
In light of the paucity of data from controlled studies, some experts have suggested an approach that depends on disease severity (i.e., mild, moderate and severe) based on the degree of proteinuria and the magnitude of allograft dysfunction (Table 5): (1) conservative measures, as with other glomerulotides, including RAS blockade and lipid-lowering agents; (2) glucocorticoids, MMF, rituximab and PE have been used with variable success[59,60]. In selected patients, MMF has been reported to be effective in C3GN controls in a retrospective study[12,61]; and (3) EZ was firstly reported by Bomback et al[62], in treating six patients with C3G (three with DDD and three with C3GN) in an open-labelled trial. EZ dose is guided by previous experience in aHUS and used for one year. Improved kidney function was observed in two patients; one patient showed partially improved proteinuria, while another patient showed better histological and laboratory findings[62]. Notably, elevated serum membrane attack complex (MAC) levels were associated with clinical improvement[63]. Duration of therapy is not yet defined. The beneficial effects of EZ in DDD recurrence[46] and C3GN recurrence[64] have been shown in case reports[65]. However, histopathological evidence of disease progression has been observed in subsequent biopsies. This highlights the fact that there is no standard accepted biomarker for disease monitoring, which can be used to assess the patient’s response to treatment and predict better renal function.
| All patients | Moderate disease | Severe disease |
| Lipid control | Urine protein > 500 mg/24 h despite supportive therapy, or | Urine protein > 2000 mg/24 h despite immunosuppression and supportive therapy or |
| Optimal BP control (< 90% in children and ≤ 120/80 mm Hg in adults) | Moderate inflammation on renal biopsy or | Severe inflammation represented by marked endo- or extracapillary proliferation with/without crescent formation despite immunosuppression and supportive therapy or |
| Optimal nutrition for both normal growth in children and healthy weight in adults | Recent increase in serum creatinine suggesting risk for progressive disease | Increased S. Cr suggesting risk for progressive disease at onset despite immunosuppression and supportive therapy |
| Recommendation | Recommendations | |
| Prednisone | Methylprednisolone pulse-dosing as well as other anti-cellular immune suppressants have had limited success in rapidly progressive disease | |
| Mycophenolate mofetil | Data are insufficient to recommend eculizumab as a first-line agent for the treatment of rapidly progressive disease |
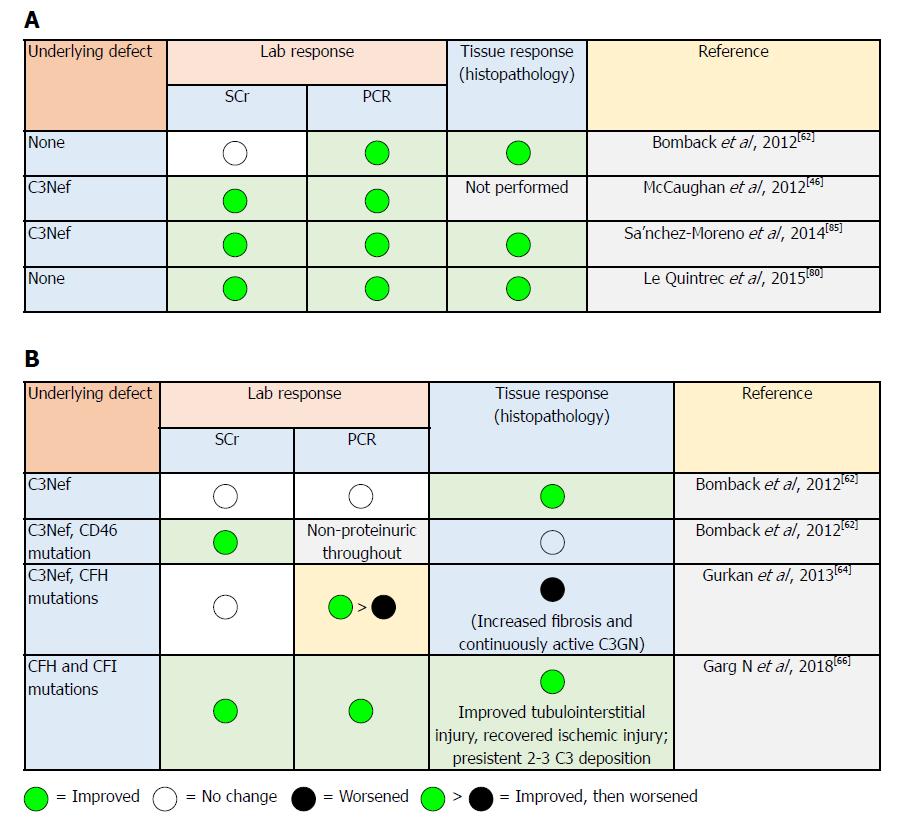
In 2018, Garg et al[66] described the spectrum of C3 pathophysiology and its clinical implications. The observed variability of the degrees of upstream (site of C3 convertase) and downstream (site of C5 convertase) complement dysregulation may result in variable phenotypic differences[67,68]. Consequently, the nature of this spectrum will be reflected clinically on disease progress in two ways: firstly, the variability in response to EZ therapy (Figure 4)[66]. In C3G, if the dominant process focused on activation of C5 convertase (resulting in increased soluble C5b-9 levels), EZ will be of therapeutic benefit. On the other hand, patients with the dominant process focused on dysregulation at the level of C3 convertase (increased C3 split product levels), the impact of EZ therapy will be less impressive, and the process of uncontrolled complement dysregulation will persist with consequent ongoing renal injury. Secondly, future application of “soluble C5b-9” as well as “C3 degradation product” measurements will be feasible in monitoring EZ therapy (and other newly introduced C3 convertase inhibitors agents) and, thereby, will help in predicting its response[66]: (1) compstatin is a C3 inhibitory peptide that can block C3 and its convertase interaction, so that all of the three complement pathways are activated; (2) CP40 is a compstatin analog with a selective C3 inhibitor property. CP40 can prevent in vitro complement-mediated hemolysis induced by C3GN patient sera. Moreover, it can abort dysregulated AP activation induced by autoantibodies and genetic mutations[63]. Since C3d is the major complement fragment deposited in C3GN and DDD, CP40 represents a promising therapeutic agent. CP40 has been evaluated in paroxysmal nocturnal hemoglobinuria and hemodialysis-induced inflammation[69,70]. If CP40 is able to offer a disease-specific targeted therapy, this agent may represent a breakthrough in C3G control; (3) other novel therapeutics: antibody-based agents targeting complement function by blocking particular components of C3 convertase to hamper its formation and/or function (e.g., anti-C3b monoclonal antibodies reported by Paixao-Cavalcante et al[71], anti-FB antibodies as described by Subias[72], and anti-properdin antibodies as professed by Pauly et al[73] targeting complement blockade are all under thorough evaluation[74]). Soluble complement receptor1 (CR1): a robust regulator of complement activity in vitro, soluble CR1 can prevent dysregulation of the AP C3 convertase. The safety and efficacy of the soluble CR1 in normalizing complement activity in pediatric patients with ESRD have been reported. With its ability to breakdown active C3b, soluble CR1 infusion can induce clinical improvement in C3GN as well as in the serum levels of MAC in patients with DDD recurrence[37].
Methods of achieving C3GN control are summarized in Table 5[34,75-86]. Until enough data from randomized control trials become available, the guidelines related to complement blockade therapy of C3GN should be based on those applied in aHUS (Table 6)[12].
| CH50 (total complement activity) | AH50 (alternative pathway hemolytic activity) | Eculizumab trough | Alternative assays |
| Measures the combined activity of all of the complement pathways | Measures combined activity of alternative and terminal complement pathways | May be a free or bound level | The following assays are under investigation |
| Tests the functional capability of serum complement components to lyse 50 % of sheep erythrocytes in a reaction mixture | Tests functional capability of alternate or terminal pathway complement components to lyse 50% of rabbit erythrocytes in a Mg2+-EGTA buffer | ELISA: using C5-coated plates, patient sera, and an anti-human IgG detection system | Free C5 |
| Low in congenital complement deficiency (C1-8) or during complement blockade | Will be low in congenital C3, FI, FB, properdin, FH, and FD deficiencies or during terminal complement blockade | Not affected by complement deficiencies | In vitro human microvascular endothelial cell test |
| Normal range: Assay dependent | Normal range is assay-dependent. | Recommended trough level during complement blockade: 50-100 μg/mL | SC5b-9 (also referred to as sMAC and TCC) remain detectable in aHUS remission, so not recommended as a monitoring tool |
| Recommended goal during therapeutic complement blockade: < 10% of normal | Recommended goal during complement blockade: < 10% of normal |
Minimal data is available concerning renal transplantation for C3G. The available recommendations (Table 7) are currently based on expert opinion. Recurrence post-transplant is common, with about half of the patients with C3G at risk of losing their grafts[12].
| Timing | Donor selection | Risk reduction |
| Avoid transplantation during acute period of renal loss | No specific recommendation can be made on donor choice. When considering living donors, high risk of recurrence should be weighed against presumed risk of waiting on cadaveric donor list | C3G histological recurrence is as high as 90%[7,87] |
| Avoid transplantation during acute inflammation | Limited data suggest: rapid progression to ESRD in native kidneys increases recurrence risk[87] | |
| No data supporting whether specific complement abnormalities (e.g., high titer C3Nef, low C3 or high soluble C5b-9) predict increased risk for relapse | There are no known strategies to reduce recurrence risk of C3G | |
| Clinical recurrence should drive decision to treat[7] | ||
| In absence of clinical trials, use of anti-complement therapy is based solely on a small open-label trial and positive case reports[62] (the impact of publication bias is unknown) | ||
| C3G associated with monoclonal gammopathy has a high rate of recurrence[7] |
For cases of TMA secondary to medication, switching of the culprit drug to another agent (mTOR or CNI) is associated with a better response[88-90]. The first line of therapy of de novo TMA should encompass withdrawal of the offending drug, an essential step that is usually associated with correction of the hematological profile[57].
Plasmapheresis (PE) and intravenous immunoglobulins (IVIG) (particularly with AMR-associated TMA): fresh-frozen plasma (FFP) is advised as a reposition fluid, which must be type-specific, ordered in advance and thawed before use, despite the high risk of reactions; however, it replaces all plasma constituents and is appropriate for patients with TMA[91]. Before the era of EZ, the following supportive explanations have been provided: (1) proven efficacy in TTP[92]; (2) a graft salvage rate of more than 80%, as reported by Karthikeyan et al[13]. He addressed two possible benefits for this type of therapy: clearance of the platelet aggregation factors (e.g., thromboxane A2) and replenishment of the deficient agents (e.g., PGI2-stimulating factor)[13]; (3) with frequent possibility of the presence of underlying complement dysregulation, commencing PE therapy will also be beneficial in two ways: clearance of the aberrant complement components, and replacement with normally functioning complement proteins[93]; (4) clearance of the anti-HLA antibodies in AMR-associated aHUS improved patient outcome[55,94]; (5) PE/IVIG therapy was successfully associated with a 100% response rate in five solid organ transplants complicated by a systemic form of TMA. There was no evidence of relapse after cessation of the culprit drug (e.g., tacrolimus) in a recent report[57].
Belatacept, a fusion protein composed of the Fc fragment of human IgG1 linked to the extracellular domain of cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4), selectively inhibits T cell activation through a co-stimulatory blockade[95].
EZ, an anti-C5 agent that blocks lytic C5b-9 MAC generation, not only revolutionized aHUS therapy but was also effective in preventing its recurrence[96]. The role of complement activation in TMA evolution has been recognized in a majority of de novo TMA patients. Chua et al[97], for example, reported deposition of C4d in all biopsies of post-transplant TMA. Efficacy of this agent has also been documented in the management of resistant cases of medication-associated de novo TMA, including those with unidentified genetic mutations[98-103]. Moreover, efficacy of EZ has been also shown in some cases of resistant AMR-associated TMA[103-111]. However, Loupy et al[112] reported a similar graft survival (95.8% vs 89.7% at two years post-transplant, respectively) and estimated GFR (52.6 mL/min vs 46.7 mL/min) in comparing PE-treated recipients with the EZ-treated group. Considering the high cost of this drug, utilization of this agent is better confined to PE-dependent patients, AMR-associated TMA and to cases with refractory hemolysis.
Minimal work-up of genetic studies should include: CFH, CFI, CFHR, CFB, MCP and C3[113]. All cases with suspected TMA should be screened for all complement components and its related proteins. Cases with isolated membrane cofactor protein (MCP) mutations (not combined with other gene defects) may be safe for kidney donation. Cases with documented TMA and with a lack of definitive genetic defects may proceed with kidney transplantation under the umbrella of intensive PE therapy[114]. Polygenic patterns of TMA should be dealt with cautiously in case of living donation[115].
Avoid trigger factors that stimulate complement activity (e.g., ischemia-reperfusion injury, viral infection and culprit medications)[52]. Immunosuppressive regimens devoid of medications related to TMA evolution[116] are advised. PE therapy alone is not sufficient for TMA cure and prevention, with the following explanations postulated: (1) PE alone frequently failed to prevent TMA recurrence[117]; (2) TMA regression cannot be preserved after cessation of therapy; and (3) recipients treated with PE showed an evidence of “subclinical” disease[118], which declares that PE has no influence on complement activity. Prophylactic use of rituximab proved to be beneficial as an anti-CFH-antibody[119], and this effect can be augmented with the addition of PE therapy[120,121]. The anti-C5 monoclonal antibody EZ has been reported to be successful in preventing TMA recurrence in recipients with CFH, CFH/CFHR1 hybrid gene mutations as well as in C3 gene mutations[122-125].
Eighty percent of kidney transplantation recipients with TMA proved to be associated with genetic mutations[126]. Based on the fact that a TMA episode is suspected with trigger factor (e.g., surgery), a robust suggestion is to protect the patient with complement blockade, if not already instituted[127]. Unfortunately, this suggestion lacks appropriate evidence[12].
Given a clear role of complement blockade in the management of TMA, two regimens have been suggested: (1) minimal dosage to achieve complement blockade; and (2) a dose withdrawal scheme (Table 8)[84]. EZ monitoring, however, is mandated for better response (Table 6)[128-131].
| Minimal dose | Discontinuation |
| Desire to continue dosing with the minimal dose required to achieve a pre-identified level of complement blockade1 | Desire to discontinue complement blockade |
| Dose reduction or interval extension | No consensus exists regarding tapering of dose |
| Goal CH50 < 10% (recommended) | |
| Goal AH50 < 10% (recommended) | |
| Goal eculizumab trough >100 μg/mL |
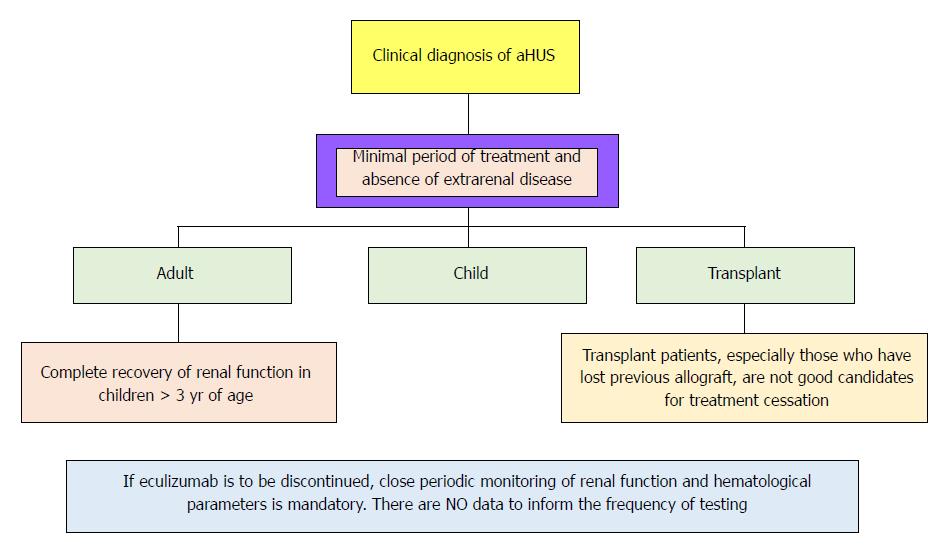
There is not enough data supporting life-long therapy. However, sustaining EZ seems to be reasonable in certain situations. Figure 5 represents a small guide, meanwhile early biomarkers of disease recurrence and complement activation became available.
The lacunae in satisfactory data still present as proper dosage, dose intervals, and duration of therapy[132], as well as the impact of this type of therapy on transplant spectrum[133].
In 2013, Verhave et al[118] reported the feasibility of successful kidney transplantation without EZ therapy in four patients with high-risk aHUS. Patients received living donor kidneys with a therapeutic regimen consisting of: Basiliximab for induction, tacrolimus in low dosage, prednisone, and MMF for maintenance immunosuppression. A statin has also been added. Further precautions include: lowering BP as much as tolerable and minimizing the cold ischemic time. For the next 16-21 mo, no recurrence or rejection events have been reported[118]. The following conclusion has been addressed: successful kidney transplantation in recurrent aHUS patients can be achieved with an EZ-free regimen through: (1) decreasing cold ischemic time; (2) minimizing the risk of rejection; and (3) preserving endothelial integrity[118].
Timing of transplant: six months after commencing, dialysis should elapse before proceeding in transplant, as renal recovery can be observed several months after initiation of EZ therapy[137,138]. Two prerequisites should be fulfilled before commencing renal transplantation: (1) resolution of the extrarenal manifestations of TMA; and (2) recovery of TMA hematological parameters. The magnitude of recurrence risk may be used to evaluate the recipient’s need for complement blockade (Table 9)[1].
| Gene mutation | Location | Functional Impact | Mutation frequency in aHUS (%) | Recurrence after transplantation (%) |
| CFH | Plasma | Loss | 20-30 | 75-90 |
| CFI | Plasma | Loss | 2-12 | 45-80 |
| CFB | Plasma | Gain | 1-2 | 100 |
| C3 | Plasma | Gain | 5-10 | 40-70 |
| MCP | Membrane | Loss | 10-15 | 15-20 |
| THBD | Membrane | Loss | 5 | One case |
| Homozygous CFHR1 del (3%-8%) | Circulating | Undetermined | 14-23 (> 90% with anti-CHF AB) | NA |
The role of complement cascade in the evolution of kidney diseases either in the native kidney or post-transplant is well recognized. The prognosis of aHUS and, in some cases, C3G is greatly improved after commencing complement blockade. These agents are not only curative, but also successful in preventing post-transplant disease recurrence. Owing to the inherited nature of most of these diseases, the maintenance of this therapy is recommended despite cost burden. Consequently, the need for regimens allowing safe withdrawal of these agents is urgently required. However, newer therapies (e.g., new monoclonal antibodies, recombinant proteins, and small interfering RNA (siRNA) agents) hold promise for the near future[139,140].
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gonzalez F, Jamil AK, Nacif LS S- Editor: Ji FF L- Editor: Filipodia E- Editor: Huang Y
| 1. | Salvadori M, Bertoni E. Complement related kidney diseases: Recurrence after transplantation. World J Transplant. 2016;6:632-645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 21] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 2. | Nester CM, Barbour T, de Cordoba SR, Dragon-Durey MA, Fremeaux-Bacchi V, Goodship TH, Kavanagh D, Noris M, Pickering M, Sanchez-Corral P. Atypical aHUS: State of the art. Mol Immunol. 2015;67:31-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 208] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 3. | Zipfel PF, Skerka C, Chen Q, Wiech T, Goodship T, Johnson S, Fremeaux-Bacchi V, Nester C, de Córdoba SR, Noris M. The role of complement in C3 glomerulopathy. Mol Immunol. 2015;67:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 4. | Sethi S, Fervenza F C. Understanding MPGN in the Native and Transplanted Kidney. Kidney Week. 2017; Available from: http://www.kidneynews.org/kidney-news/special-sections/glomerular-disease/understanding-mpgn-in-the-native-and-transplanted-kidney. |
| 5. | Sethi S, Fervenza FC, Zhang Y, Zand L, Vrana JA, Nasr SH, Theis JD, Dogan A, Smith RJ. C3 glomerulonephritis: clinicopathological findings, complement abnormalities, glomerular proteomic profile, treatment, and follow-up. Kidney Int. 2012;82:465-473. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 220] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 6. | Braun MC, Stablein DM, Hamiwka LA, Bell L, Bartosh SM, Strife CF. Recurrence of membranoproliferative glomerulonephritis type II in renal allografts: The North American Pediatric Renal Transplant Cooperative Study experience. J Am Soc Nephrol. 2005;16:2225-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 71] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Zand L, Lorenz EC, Cosio FG, Fervenza FC, Nasr SH, Gandhi MJ, Smith RJ, Sethi S. Clinical findings, pathology, and outcomes of C3GN after kidney transplantation. J Am Soc Nephrol. 2014;25:1110-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 8. | Hou J, Markowitz GS, Bomback AS, Appel GB, Herlitz LC, Barry Stokes M, D’Agati VD. Toward a working definition of C3 glomerulopathy by immunofluorescence. Kidney Int. 2014;85:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 144] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 9. | Larsen CP, Ambuzs JM, Bonsib SM, Boils CL, Cossey LN, Messias NC, Silva FG, Wang YH, Gokden N, Walker PD. Membranous-like glomerulopathy with masked IgG kappa deposits. Kidney Int. 2014;86:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Larsen CP, Messias NC, Walker PD, Fidler ME, Cornell LD, Hernandez LH, Alexander MP, Sethi S, Nasr SH. Membranoproliferative glomerulonephritis with masked monotypic immunoglobulin deposits. Kidney Int. 2015;88:867-873. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 11. | Barbour TD, Pickering MC, Terence Cook H. Dense deposit disease and C3 glomerulopathy. Semin Nephrol. 2013;33:493-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Goodship TH, Cook HT, Fakhouri F, Fervenza FC, Frémeaux-Bacchi V, Kavanagh D, Nester CM, Noris M, Pickering MC, Rodríguez de Córdoba S. Atypical hemolytic uremic syndrome and C3 glomerulopathy: conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int. 2017;91:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 478] [Article Influence: 53.1] [Reference Citation Analysis (1)] |
| 13. | Karthikeyan V, Parasuraman R, Shah V, Vera E, Venkat KK. Outcome of plasma exchange therapy in thrombotic microangiopathy after renal transplantation. Am J Transplant. 2003;3:1289-1294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Nadasdy T. Thrombotic microangiopathy in renal allografts: the diagnostic challenge. Curr Opin Organ Transplant. 2014;19:283-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Bouatou Y, Bacchi VF, Villard J, Moll S, Martin PY, Hadaya K. Atypical Hemolytic Uremic Syndrome Recurrence after Renal Transplantation: C3-Glomerulonephritis as an Initial Presentation. Transplant Direct. 2015;1:e9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Matar D, Naqvi F, Racusen LC, Carter-Monroe N, Montgomery RA, Alachkar N. Atypical hemolytic uremic syndrome recurrence after kidney transplantation. Transplantation. 2014;98:1205-1212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | Recalde S, Tortajada A, Subias M, Anter J, Blasco M, Maranta R, Coco R, Pinto S, Noris M, García-Layana A. Molecular Basis of Factor H R1210C Association with Ocular and Renal Diseases. J Am Soc Nephrol. 2016;27:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Fritsche LG, Chen W, Schu M, Yaspan BL, Yu Y, Thorleifsson G, Zack DJ, Arakawa S, Cipriani V, Ripke S. Seven new loci associated with age-related macular degeneration. Nat Genet. 2013;45:433-439, 439e1-439e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 513] [Cited by in RCA: 597] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 19. | Mathieson PW, Würzner R, Oliveria DB, Lachmann PJ, Peters DK. Complement-mediated adipocyte lysis by nephritic factor sera. J Exp Med. 1993;177:1827-1831. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 69] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Sethi S, Fervenza FC, Zhang Y, Nasr SH, Leung N, Vrana J, Cramer C, Nester CM, Smith RJ. Proliferative glomerulonephritis secondary to dysfunction of the alternative pathway of complement. Clin J Am Soc Nephrol. 2011;6:1009-1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 115] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Sethi S, Fervenza FC. Membranoproliferative glomerulonephritis: pathogenetic heterogeneity and proposal for a new classification. Semin Nephrol. 2011;31:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 135] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 22. | Rincón B, Bernis C, Garcia A, Traver JA. Mesangiocapillary glomerulonephritis associated with hydatid disease. Nephrol Dial Transplant. 1993;8:783-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Goules A, Masouridi S, Tzioufas AG, Ioannidis JP, Skopouli FN, Moutsopoulos HM. Clinically significant and biopsy-documented renal involvement in primary Sjögren syndrome. Medicine (Baltimore). 2000;79:241-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 121] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 24. | Servais A, Noël LH, Roumenina LT, Le Quintrec M, Ngo S, Dragon-Durey MA, Macher MA, Zuber J, Karras A, Provot F. Acquired and genetic complement abnormalities play a critical role in dense deposit disease and other C3 glomerulopathies. Kidney Int. 2012;82:454-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 401] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 25. | Dragon-Durey MA, Blanc C, Marinozzi MC, van Schaarenburg RA, Trouw LA. Autoantibodies against complement components and functional consequences. Mol Immunol. 2013;56:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 26. | Smith RJ, Harris CL, Pickering MC. Dense deposit disease. Mol Immunol. 2011;48:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 27. | Thomas S, Ranganathan D, Francis L, Madhan K, John GT. Current concepts in C3 glomerulopathy. Indian J Nephrol. 2014;24:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 28. | Chen Q, Müller D, Rudolph B, Hartmann A, Kuwertz-Bröking E, Wu K, Kirschfink M, Skerka C, Zipfel PF. Combined C3b and factor B autoantibodies and MPGN type II. N Engl J Med. 2011;365:2340-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 29. | Goodship TH, Pappworth IY, Toth T, Denton M, Houlberg K, McCormick F, Warland D, Moore I, Hunze EM, Staniforth SJ. Factor H autoantibodies in membranoproliferative glomerulonephritis. Mol Immunol. 2012;52:200-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 30. | Lorcy N, Rioux-Leclercq N, Lombard ML, Le Pogamp P, Vigneau C. Three kidneys, two diseases, one antibody? Nephrol Dial Transplant. 2011;26:3811-3813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 31. | Dragon-Durey MA, Frémeaux-Bacchi V, Loirat C, Blouin J, Niaudet P, Deschenes G, Coppo P, Herman Fridman W, Weiss L. Heterozygous and homozygous factor h deficiencies associated with hemolytic uremic syndrome or membranoproliferative glomerulonephritis: report and genetic analysis of 16 cases. J Am Soc Nephrol. 2004;15:787-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 264] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 32. | Servais A, Noël LH, Dragon-Durey MA, Gübler MC, Rémy P, Buob D, Cordonnier C, Makdassi R, Jaber W, Boulanger E. Heterogeneous pattern of renal disease associated with homozygous factor H deficiency. Hum Pathol. 2011;42:1305-1311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 33. | Skerka C, Chen Q, Fremeaux-Bacchi V, Roumenina LT. Complement factor H related proteins (CFHRs). Mol Immunol. 2013;56:170-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 191] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 34. | Besbas N, Gulhan B, Gucer S, Korkmaz E, Ozaltin F. A novel CFHR5 mutation associated with C3 glomerulonephritis in a Turkish girl. J Nephrol. 2014;27:457-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 35. | Chen Q, Manzke M, Hartmann A, Büttner M, Amann K, Pauly D, Wiesener M, Skerka C, Zipfel PF. Complement Factor H-Related 5-Hybrid Proteins Anchor Properdin and Activate Complement at Self-Surfaces. J Am Soc Nephrol. 2016;27:1413-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 36. | Gale DP, de Jorge EG, Cook HT, Martinez-Barricarte R, Hadjisavvas A, McLean AG, Pusey CD, Pierides A, Kyriacou K, Athanasiou Y. Identification of a mutation in complement factor H-related protein 5 in patients of Cypriot origin with glomerulonephritis. Lancet. 2010;376:794-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 255] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 37. | Zhang Y, Nester CM, Holanda DG, Marsh HC, Hammond RA, Thomas LJ, Meyer NC, Hunsicker LG, Sethi S, Smith RJ. Soluble CR1 therapy improves complement regulation in C3 glomerulopathy. J Am Soc Nephrol. 2013;24:1820-1829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Malik TH, Lavin PJ, Goicoechea de Jorge E, Vernon KA, Rose KL, Patel MP, de Leeuw M, Neary JJ, Conlon PJ, Winn MP. A hybrid CFHR3-1 gene causes familial C3 glomerulopathy. J Am Soc Nephrol. 2012;23:1155-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 39. | Tortajada A, Garcìa SP, Gastoldi S, Fernandez JG, Martin Merinero H, Arjona E, Noris M, Rodriguez de Cordoba S. Prevalent FHR1 mutant protein generated by gene conversion reveals crucial role of factor H polymorhisms in atypical hemolytic uremic syndrome. Immunobiology. 2016;221:1199. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 40. | Thurman JM. Complement in kidney disease: core curriculum 2015. Am J Kidney Dis. 2015;65:156-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 41. | Goicoechea de Jorge E, Pickering MC. Atypical hemolytic uremic syndrome: telling the difference between H and Y. Kidney Int. 2010;78:721-723. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Noris M, Remuzzi G. Glomerular Diseases Dependent on Complement Activation, Including Atypical Hemolytic Uremic Syndrome, Membranoproliferative Glomerulonephritis, and C3 Glomerulopathy: Core Curriculum 2015. Am J Kidney Dis. 2015;66:359-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 43. | Cochat P, Fargue S, Mestrallet G, Jungraithmayr T, Koch-Nogueira P, Ranchin B, Zimmerhackl LB. Disease recurrence in paediatric renal transplantation. Pediatr Nephrol. 2009;24:2097-2108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 44. | Medjeral-Thomas NR, O’Shaughnessy MM, O’Regan JA, Traynor C, Flanagan M, Wong L, Teoh CW, Awan A, Waldron M, Cairns T. C3 glomerulopathy: clinicopathologic features and predictors of outcome. Clin J Am Soc Nephrol. 2014;9:46-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 169] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 45. | Angelo JR, Bell CS, Braun MC. Allograft failure in kidney transplant recipients with membranoproliferative glomerulonephritis. Am J Kidney Dis. 2011;57:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 46. | McCaughan JA, O’Rourke DM, Courtney AE. Recurrent dense deposit disease after renal transplantation: an emerging role for complementary therapies. Am J Transplant. 2012;12:1046-1051. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Andresdottir MB, Assmann KJ, Hoitsma AJ, Koene RA, Wetzels JF. Renal transplantation in patients with dense deposit disease: morphological characteristics of recurrent disease and clinical outcome. Nephrol Dial Transplant. 1999;14:1723-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 48. | Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363-2372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 49. | Andresdottir MB, Assmann KJ, Hoitsma AJ, Koene RA, Wetzels JF. Recurrence of type I membranoproliferative glomerulonephritis after renal transplantation: analysis of the incidence, risk factors, and impact on graft survival. Transplantation. 1997;63:1628-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Little MA, Dupont P, Campbell E, Dorman A, Walshe JJ. Severity of primary MPGN, rather than MPGN type, determines renal survival and post-transplantation recurrence risk. Kidney Int. 2006;69:504-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 51. | Wong L, Moran S, Lavin PJ, Dorman AM, Conlon PJ. Kidney transplant outcomes in familial C3 glomerulopathy. Clin Kidney J. 2016;9:403-407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 52. | Zuber J, Le Quintrec M, Morris H, Frémeaux-Bacchi V, Loirat C, Legendre C. Targeted strategies in the prevention and management of atypical HUS recurrence after kidney transplantation. Transplant Rev (Orlando). 2013;27:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 53. | Pickering MC, D’Agati VD, Nester CM, Smith RJ, Haas M, Appel GB, Alpers CE, Bajema IM, Bedrosian C, Braun M. C3 glomerulopathy: consensus report. Kidney Int. 2013;84:1079-1089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 54. | Schwimmer J, Nadasdy TA, Spitalnik PF, Kaplan KL, Zand MS. De novo thrombotic microangiopathy in renal transplant recipients: a comparison of hemolytic uremic syndrome with localized renal thrombotic microangiopathy. Am J Kidney Dis. 2003;41:471-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 55. | Satoskar AA, Pelletier R, Adams P, Nadasdy GM, Brodsky S, Pesavento T, Henry M, Nadasdy T. De novo thrombotic microangiopathy in renal allograft biopsies-role of antibody-mediated rejection. Am J Transplant. 2010;10:1804-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 86] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Fortin MC, Raymond MA, Madore F, Fugère JA, Pâquet M, St-Louis G, Hébert MJ. Increased risk of thrombotic microangiopathy in patients receiving a cyclosporin-sirolimus combination. Am J Transplant. 2004;4:946-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Garg N, Rennke HG, Pavlakis M, Zandi-Nejad K. De novo thrombotic microangiopathy after kidney transplantation. Transplant Rev (Orlando). 2018;32:58-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 62] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 58. | Java A, Gaut JP, Brennan DC. De novo membranoproliferative glomerulonephritis III in a renal transplant patient: case report and review of the literature. Transpl Int. 2012;25:e58-e61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 59. | Habbig S, Mihatsch MJ, Heinen S, Beck B, Emmel M, Skerka C, Kirschfink M, Hoppe B, Zipfel PF, Licht C. C3 deposition glomerulopathy due to a functional factor H defect. Kidney Int. 2009;75:1230-1234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 60. | Nester CM, Smith RJ. Treatment options for C3 glomerulopathy. Curr Opin Nephrol Hypertens. 2013;22:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 61. | Rabasco C, Cavero T, Román E, Rojas-Rivera J, Olea T, Espinosa M, Cabello V, Fernández-Juarez G, González F, Ávila A, Baltar JM, Díaz M, Alegre R, Elías S, Antón M, Frutos MA, Pobes A, Blasco M, Martín F, Bernis C, Macías M, Barroso S, de Lorenzo A, Ariceta G, López-Mendoza M, Rivas B, López-Revuelta K, Campistol JM, Mendizábal S, de Córdoba SR, Praga M; Spanish Group for the Study of Glomerular Diseases (GLOSEN). Effectiveness of mycophenolate mofetil in C3 glomerulonephritis. Kidney Int. 2015;88:1153-1160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 107] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 62. | Bomback AS, Smith RJ, Barile GR, Zhang Y, Heher EC, Herlitz L, Stokes MB, Markowitz GS, D’Agati VD, Canetta PA. Eculizumab for dense deposit disease and C3 glomerulonephritis. Clin J Am Soc Nephrol. 2012;7:748-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 263] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 63. | Sanghera P, Ghanta M, Ozay F, Ariyamuthu VK, Tanriover B. Kidney Diseases Associated With Alternative Complement Pathway Dysregulation and Potential Treatment Options. Am J Med Sci. 2017;354:533-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | Gurkan S, Fyfe B, Weiss L, Xiao X, Zhang Y, Smith RJ. Eculizumab and recurrent C3 glomerulonephritis. Pediatr Nephrol. 2013;28:1975-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 65. | Herlitz LC, Bomback AS, Markowitz GS, Stokes MB, Smith RN, Colvin RB, Appel GB, D’Agati VD. Pathology after eculizumab in dense deposit disease and C3 GN. J Am Soc Nephrol. 2012;23:1229-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 66. | Garg N, Zhang Y, Nicholson-Weller A, Khankin EV, Borsa NG, Meyer NC, McDermott S, Stillman IE, Rennke HG, Smith RJ. C3 glomerulonephritis secondary to mutations in factors H and I: rapid recurrence in deceased donor kidney transplant effectively treated with eculizumab. Nephrol Dial Transplant. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 67. | Sethi S, Nester CM, Smith RJ. Membranoproliferative glomerulonephritis and C3 glomerulopathy: resolving the confusion. Kidney Int. 2012;81:434-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 68. | Rose KL, Paixao-Cavalcante D, Fish J, Manderson AP, Malik TH, Bygrave AE, Lin T, Sacks SH, Walport MJ, Cook HT. Factor I is required for the development of membranoproliferative glomerulonephritis in factor H-deficient mice. J Clin Invest. 2008;118:608-618. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 67] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 69. | Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094-2101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 157] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 70. | Reis ES, DeAngelis RA, Chen H, Resuello RR, Ricklin D, Lambris JD. Therapeutic C3 inhibitor Cp40 abrogates complement activation induced by modern hemodialysis filters. Immunobiology. 2015;220:476-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 71. | Paixão-Cavalcante D, Torreira E, Lindorfer MA, Rodriguez de Cordoba S, Morgan BP, Taylor RP, Llorca O, Harris CL. A humanized antibody that regulates the alternative pathway convertase: potential for therapy of renal disease associated with nephritic factors. J Immunol. 2014;192:4844-4851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 72. | Subías M, Tortajada A, Gastoldi S, Galbusera M, López-Perrote A, Lopez Lde J, González-Fernández FA, Villegas-Martínez A, Dominguez M, Llorca O. A novel antibody against human factor B that blocks formation of the C3bB proconvertase and inhibits complement activation in disease models. J Immunol. 2014;193:5567-5575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Pauly D, Nagel BM, Reinders J, Killian T, Wulf M, Ackermann S, Ehrenstein B, Zipfel PF, Skerka C, Weber BH. A novel antibody against human properdin inhibits the alternative complement system and specifically detects properdin from blood samples. PLoS One. 2014;9:e96371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Zhang Y, Shao D, Ricklin D, Hilkin BM, Nester CM, Lambris JD, Smith RJ. Compstatin analog Cp40 inhibits complement dysregulation in vitro in C3 glomerulopathy. Immunobiology. 2015;220:993-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 75. | Bonucchi D, Leonelli M, Damiano F, Granito M, Ghiandai G, De Amicis S, Americo C, Ligabue G, Albertazzi V, Cappelli G. [Post-transplant recurrence of glomerulonephritis: a complex clinical case]. G Ital Nefrol. 2010;27 Suppl 52:S82-S84. [PubMed] |
| 76. | Daina E, Noris M, Remuzzi G. Eculizumab in a patient with dense-deposit disease. N Engl J Med. 2012;366:1161-1163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 77. | Garnier AS, Augusto JF, Pellier I, Subra JF, Sayegh J. Successful long-term outcome of kidney transplantation in a patient with X-linked thrombocytopenia: 9-year follow-up. Transplantation. 2014;98:e57-e58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 78. | Inman M, Prater G, Fatima H, Wallace E. Eculizumab-induced reversal of dialysis-dependent kidney failure from C3 glomerulonephritis. Clin Kidney J. 2015;8:445-448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 79. | Kerns E, Rozansky D, Troxell ML. Evolution of immunoglobulin deposition in C3-dominant membranoproliferative glomerulopathy. Pediatr Nephrol. 2013;28:2227-2231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 80. | Le Quintrec M, Lionet A, Kandel C, Bourdon F, Gnemmi V, Colombat M, Goujon JM, Frémeaux-Bacchi V, Fakhouri F. Eculizumab for treatment of rapidly progressive C3 glomerulopathy. Am J Kidney Dis. 2015;65:484-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 81. | Oosterveld MJ, Garrelfs MR, Hoppe B, Florquin S, Roelofs JJ, van den Heuvel LP, Amann K, Davin JC, Bouts AH, Schriemer PJ. Eculizumab in Pediatric Dense Deposit Disease. Clin J Am Soc Nephrol. 2015;10:1773-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 82. | Ozkaya O, Nalcacioglu H, Tekcan D, Genc G, Meydan BC, Ozdemir BH, Baysal MK, Keceligil HT. Eculizumab therapy in a patient with dense-deposit disease associated with partial lipodystropy. Pediatr Nephrol. 2014;29:1283-1287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 83. | Radhakrishnan S, Lunn A, Kirschfink M, Thorner P, Hebert D, Langlois V, Pluthero F, Licht C. Eculizumab and refractory membranoproliferative glomerulonephritis. N Engl J Med. 2012;366:1165-1166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 84. | Rousset-Rouvière C, Cailliez M, Garaix F, Bruno D, Laurent D, Tsimaratos M. Rituximab fails where eculizumab restores renal function in C3nef-related DDD. Pediatr Nephrol. 2014;29:1107-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 85. | Sánchez-Moreno A, De la Cerda F, Cabrera R, Fijo J, López-Trascasa M, Bedoya R, Rodríguez de Córdoba S, Ybot-González P. Eculizumab in dense-deposit disease after renal transplantation. Pediatr Nephrol. 2014;29:2055-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 86. | Vivarelli M, Pasini A, Emma F. Eculizumab for the treatment of dense-deposit disease. N Engl J Med. 2012;366:1163-1165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 114] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 87. | Lu DF, Moon M, Lanning LD, McCarthy AM, Smith RJ. Clinical features and outcomes of 98 children and adults with dense deposit disease. Pediatr Nephrol. 2012;27:773-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 88. | Czubkowski P, Pawłowska J, Jankowska I, Teisseyre M, Kamińska D, Markiewicz M, Ryżko J. Successful sirolimus rescue in tacrolimus-induced thrombotic microangiopathy after living-related liver transplantation. Pediatr Transplant. 2012;16:E261-E264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 89. | Franco A, Hernandez D, Capdevilla L, Errasti P, Gonzalez M, Ruiz JC, Sanchez J; HUS-Sirolimus Spanish Study Group. De novo hemolytic-uremic syndrome/thrombotic microangiopathy in renal transplant patients receiving calcineurin inhibitors: role of sirolimus. Transplant Proc. 2003;35:1764-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 90. | Epperla N, Hemauer K, Hamadani M, Friedman KD, Kreuziger LB. Impact of treatment and outcomes for patients with posttransplant drug-associated thrombotic microangiopathy. Transfusion. 2017;57:2775-2781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 91. | McLeod BC. Therapeutic apheresis: use of human serum albumin, fresh frozen plasma and cryosupernatant plasma in therapeutic plasma exchange. Best Pract Res Clin Haematol. 2006;19:157-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Bell WR, Braine HG, Ness PM, Kickler TS. Improved survival in thrombotic thrombocytopenic purpura-hemolytic uremic syndrome. Clinical experience in 108 patients. N Engl J Med. 1991;325:398-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 558] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 93. | Le Quintrec M, Lionet A, Kamar N, Karras A, Barbier S, Buchler M, Fakhouri F, Provost F, Fridman WH, Thervet E. Complement mutation-associated de novo thrombotic microangiopathy following kidney transplantation. Am J Transplant. 2008;8:1694-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 147] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 94. | Djamali A, Kaufman DB, Ellis TM, Zhong W, Matas A, Samaniego M. Diagnosis and management of antibody-mediated rejection: current status and novel approaches. Am J Transplant. 2014;14:255-271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 286] [Cited by in RCA: 286] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 95. | Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. 2014;CD010699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 96. | Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169-2181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1034] [Cited by in RCA: 1110] [Article Influence: 92.5] [Reference Citation Analysis (0)] |
| 97. | Chua JS, Baelde HJ, Zandbergen M, Wilhelmus S, van Es LA, de Fijter JW, Bruijn JA, Bajema IM, Cohen D. Complement Factor C4d Is a Common Denominator in Thrombotic Microangiopathy. J Am Soc Nephrol. 2015;26:2239-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 95] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 98. | Shochet L, Kanellis J, Simpson I, Ta J, Mulley W. De novo thrombotic microangiopathy following simultaneous pancreas and kidney transplantation managed with eculizumab. Nephrology (Carlton). 2017;22 Suppl 1:23-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 99. | Dedhia P, Govil A, Mogilishetty G, Alloway RR, Woodle ES, Abu Jawdeh BG. Eculizumab and Belatacept for De Novo Atypical Hemolytic Uremic Syndrome Associated With CFHR3-CFHR1 Deletion in a Kidney Transplant Recipient: A Case Report. Transplant Proc. 2017;49:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 100. | Ikeda T, Okumi M, Unagami K, Kanzawa T, Sawada A, Kawanishi K, Omoto K, Ishida H, Tanabe K. Two cases of kidney transplantation-associated thrombotic microangiopathy successfully treated with eculizumab. Nephrology (Carlton). 2016;21 Suppl 1:35-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 101. | Safa K, Logan MS, Batal I, Gabardi S, Rennke HG, Abdi R. Eculizumab for drug-induced de novo posttransplantation thrombotic microangiopathy: A case report. Clin Nephrol. 2015;83:125-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 102. | Loirat C, Babu S, Furman R, Sheerin N, Cohen D, Gaber O. Eculizumab Efficacy and Safety in Patients With Atypical Hemolytic Uremic Syndrome (aHUS) Resistant to Plasma Exchange/Infusion. Poster presented at the 16th Congress of European Hematology Association (EHA);. 2011;Abstract 0979. |
| 103. | Loirat C, Muus P, Legendre C, Douglas K, Hourmant M, Delmas Y. A Phase II Study of Eculizumab in Patients with Atypical Hemolytic Uremic Syndrome Receiving Chronic Plasma Exchange/Infusion. Poster presented at the 16th Congress of European Hematology Association (EHA);. 2011;Abstract 0979. |
| 104. | Stegall MD, Diwan T, Raghavaiah S, Cornell LD, Burns J, Dean PG, Cosio FG, Gandhi MJ, Kremers W, Gloor JM. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant. 2011;11:2405-2413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 442] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 105. | González-Roncero F, Suñer M, Bernal G, Cabello V, Toro M, Pereira P, Angel Gentil M. Eculizumab treatment of acute antibody-mediated rejection in renal transplantation: case reports. Transplant Proc. 2012;44:2690-2694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 106. | Chehade H, Rotman S, Matter M, Girardin E, Aubert V, Pascual M. Eculizumab to treat antibody-mediated rejection in a 7-year-old kidney transplant recipient. Pediatrics. 2015;135:e551-e555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 107. | Locke JE, Magro CM, Singer AL, Segev DL, Haas M, Hillel AT, King KE, Kraus E, Lees LM, Melancon JK. The use of antibody to complement protein C5 for salvage treatment of severe antibody-mediated rejection. Am J Transplant. 2009;9:231-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 237] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 108. | Lonze BE, Dagher NN, Simpkins CE, Locke JE, Singer AL, Segev DL, Zachary AA, Montgomery RA. Eculizumab, bortezomib and kidney paired donation facilitate transplantation of a highly sensitized patient without vascular access. Am J Transplant. 2010;10:2154-2160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 109. | Stewart ZA, Collins TE, Schlueter AJ, Raife TI, Holanda DG, Nair R, Reed AI, Thomas CP. Case report: Eculizumab rescue of severe accelerated antibody-mediated rejection after ABO-incompatible kidney transplant. Transplant Proc. 2012;44:3033-3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 110. | Tran D, Boucher A, Collette S, Payette A, Royal V, Senécal L. Eculizumab for the Treatment of Severe Antibody-Mediated Rejection: A Case Report and Review of the Literature. Case Rep Transplant. 2016;2016:9874261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Orandi BJ, Zachary AA, Dagher NN, Bagnasco SM, Garonzik-Wang JM, Van Arendonk KJ, Gupta N, Lonze BE, Alachkar N, Kraus ES. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation. 2014;98:857-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 112. | Loupy A, Viglietti D, Mengel M. Complement inhibition in HLA-incompatible kidney transplants: persisting antibody-mediated injury despite marked decrease of clinical ABMR. Am J Transplant. 2015;15:1139-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 113. | Vernon KA, Gale DP, de Jorge EG, McLean AG, Galliford J, Pierides A, Maxwell PH, Taube D, Pickering MC, Cook HT. Recurrence of complement factor H-related protein 5 nephropathy in a renal transplant. Am J Transplant. 2011;11:152-155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 114. | Loirat C, Fremeaux-Bacchi V. Hemolytic uremic syndrome recurrence after renal transplantation. Pediatr Transplant. 2008;12:619-629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 115. | Zuber J, Le Quintrec M, Sberro-Soussan R, Loirat C, Frémeaux-Bacchi V, Legendre C. New insights into postrenal transplant hemolytic uremic syndrome. Nat Rev Nephrol. 2011;7:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 135] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 116. | Noris M, Remuzzi G. Overview of complement activation and regulation. Semin Nephrol. 2013;33:479-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 571] [Article Influence: 51.9] [Reference Citation Analysis (0)] |
| 117. | Zuber J, Le Quintrec M, Krid S, Bertoye C, Gueutin V, Lahoche A, Heyne N, Ardissino G, Chatelet V, Noël LH. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant. 2012;12:3337-3354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 118. | Verhave JC, Westra D, van Hamersvelt HW, van Helden M, van de Kar NC, Wetzels JF. Living kidney transplantation in adult patients with atypical haemolytic uraemic syndrome. Neth J Med. 2013;71:342-347. [PubMed] |
| 119. | Goicoechea de Jorge E, Caesar JJ, Malik TH, Patel M, Colledge M, Johnson S, Hakobyan S, Morgan BP, Harris CL, Pickering MC. Dimerization of complement factor H-related proteins modulates complement activation in vivo. Proc Natl Acad Sci USA. 2013;110:4685-4690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 120. | Kwon T, Dragon-Durey MA, Macher MA, Baudouin V, Maisin A, Peuchmaur M, Fremeaux-Bacchi V, Loirat C. Successful pre-transplant management of a patient with anti-factor H autoantibodies-associated haemolytic uraemic syndrome. Nephrol Dial Transplant. 2008;23:2088-2090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 121. | Waters AM, Pappworth I, Marchbank K, Bockenhauer D, Tullus K, Pickering MC, Strain L, Sebire N, Shroff R, Marks SD. Successful renal transplantation in factor H autoantibody associated HUS with CFHR1 and 3 deficiency and CFH variant G2850T. Am J Transplant. 2010;10:168-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 122. | Zimmerhackl LB, Hofer J, Cortina G, Mark W, Würzner R, Jungraithmayr TC, Khursigara G, Kliche KO, Radauer W. Prophylactic eculizumab after renal transplantation in atypical hemolytic-uremic syndrome. N Engl J Med. 2010;362:1746-1748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 123. | Román-Ortiz E, Mendizabal Oteiza S, Pinto S, López-Trascasa M, Sánchez-Corral P, Rodríguez de Cordoba S. Eculizumab long-term therapy for pediatric renal transplant in aHUS with CFH/CFHR1 hybrid gene. Pediatr Nephrol. 2014;29:149-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 124. | Nester C, Stewart Z, Myers D, Jetton J, Nair R, Reed A, Thomas C, Smith R, Brophy P. Pre-emptive eculizumab and plasmapheresis for renal transplant in atypical hemolytic uremic syndrome. Clin J Am Soc Nephrol. 2011;6:1488-1494. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 125. | Krid S, Roumenina LT, Beury D, Charbit M, Boyer O, Frémeaux-Bacchi V, Niaudet P. Renal transplantation under prophylactic eculizumab in atypical hemolytic uremic syndrome with CFH/CFHR1 hybrid protein. Am J Transplant. 2012;12:1938-1944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 126. | Pérez-Caballero D, González-Rubio C, Gallardo ME, Vera M, López-Trascasa M, Rodríguez de Córdoba S, Sánchez-Corral P. Clustering of missense mutations in the C-terminal region of factor H in atypical hemolytic uremic syndrome. Am J Hum Genet. 2001;68:478-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 225] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 127. | Licht C, Heinen S, Józsi M, Löschmann I, Saunders RE, Perkins SJ, Waldherr R, Skerka C, Kirschfink M, Hoppe B. Deletion of Lys224 in regulatory domain 4 of Factor H reveals a novel pathomechanism for dense deposit disease (MPGN II). Kidney Int. 2006;70:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 158] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 128. | West CD, Witte DP, McAdams AJ. Composition of nephritic factor-generated glomerular deposits in membranoproliferative glomerulonephritis type 2. Am J Kidney Dis. 2001;37:1120-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 129. | Dragon-Durey MA, Sethi SK, Bagga A, Blanc C, Blouin J, Ranchin B, André JL, Takagi N, Cheong HI, Hari P. Clinical features of anti-factor H autoantibody-associated hemolytic uremic syndrome. J Am Soc Nephrol. 2010;21:2180-2187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 222] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 130. | Khandelwal P, Gupta A, Sinha A, Saini S, Hari P, Dragon Durey MA, Bagga A. Effect of plasma exchange and immunosuppressive medications on antibody titers and outcome in anti-complement factor H antibody-associated hemolytic uremic syndrome. Pediatr Nephrol. 2015;30:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 131. | Noris M, Galbusera M, Gastoldi S, Macor P, Banterla F, Bresin E, Tripodo C, Bettoni S, Donadelli R, Valoti E. Dynamics of complement activation in aHUS and how to monitor eculizumab therapy. Blood. 2014;124:1715-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 261] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 132. | Tanimoto T, Oshima Y, Kami M. Eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;369:1378-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 133. | Zuber J, Fakhouri F, Roumenina LT, Loirat C, Frémeaux-Bacchi V; French Study Group for aHUS/C3G. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 364] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 134. | Sheerin NS, Kavanagh D, Goodship TH, Johnson S. A national specialized service in England for atypical haemolytic uraemic syndrome-the first year’s experience. QJM. 2016;109:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 135. | Wetzels JF, van de Kar NC. Discontinuation of eculizumab maintenance treatment for atypical hemolytic uremic syndrome. Am J Kidney Dis. 2015;65:342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 136. | Ardissino G, Possenti I, Tel F, Testa S, Salardi S, Ladisa V. Discontinuation of eculizumab treatment in atypical hemolytic uremic syndrome: an update. Am J Kidney Dis. 2015;66:172-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 137. | Povey H, Vundru R, Junglee N, Jibani M. Renal recovery with eculizumab in atypical hemolytic uremic syndrome following prolonged dialysis. Clin Nephrol. 2014;82:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 138. | Licht C, Greenbaum LA, Muus P, Babu S, Bedrosian CL, Cohen DJ, Delmas Y, Douglas K, Furman RR, Gaber OA. Efficacy and safety of eculizumab in atypical hemolytic uremic syndrome from 2-year extensions of phase 2 studies. Kidney Int. 2015;87:1061-1073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 268] [Cited by in RCA: 307] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 139. | Thurman JM, Le Quintrec M. Targeting the complement cascade: novel treatments coming down the pike. Kidney Int. 2016;90:746-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 140. | Le KN, Gibiansky L, van Lookeren Campagne M, Good J, Davancaze T, Loyet KM, Morimoto A, Strauss EC, Jin JY. Population Pharmacokinetics and Pharmacodynamics of Lampalizumab Administered Intravitreally to Patients With Geographic Atrophy. CPT Pharmacometrics Syst Pharmacol. 2015;4:595-604. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |