Published online Aug 9, 2018. doi: 10.5500/wjt.v8.i4.110
Peer-review started: March 8, 2018
First decision: April 4, 2018
Revised: April 9, 2018
Accepted: May 11, 2018
Article in press: May 13, 2018
Published online: August 9, 2018
Processing time: 154 Days and 12.8 Hours
To compare survival of kidney transplants from deceased extended criteria donors (ECD) according to: (1) donor graft histological score; and (2) allocation of high score grafts either to single (SKT) or dual (DKT) transplant.
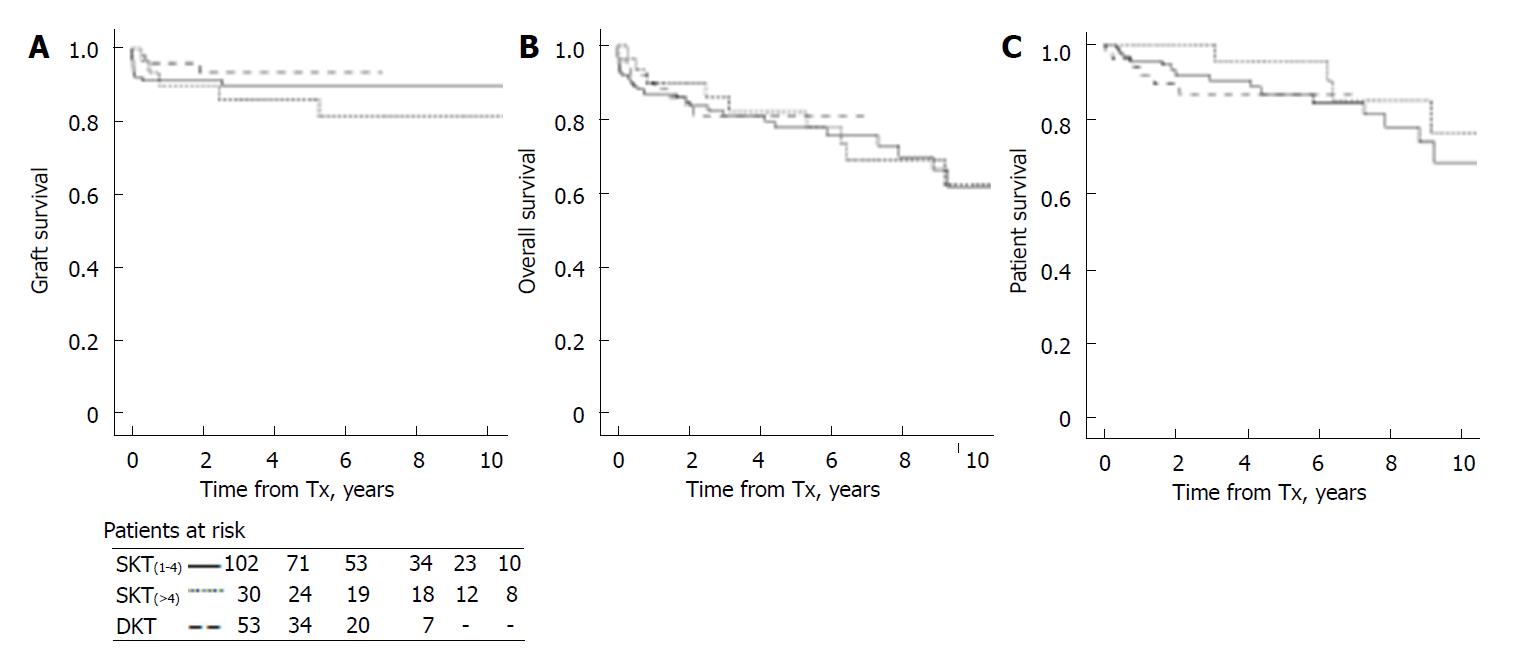
Renal biopsy was performed as part of either a newly adopted DKT protocol, or of surveillance protocol in the past. A total 185 ECD graft recipients were categorized according to pre-implantation graft biopsy into 3 groups: SKT with graft score 1 to 4 [SKT(1-4), n = 102]; SKT with donor graft score 5 to 8 [SKT(> 4), n = 30]; DKT with donor graft score 5 to 7 (DKT, n = 53). Graft and patient survival were analyzed by Kaplan-Meier curves and compared by log-rank test. Mean number of functioning graft years by transplant reference, and mean number of dialysis-free life years by donor reference in recipients were also calculated at 1, 3 and 6 years from transplantation.
There were no statistically significant differences in graft and patient survival between SKT(1-4) and SKT(> 4), and between SKT(> 4) and DKT. Recipient renal function (plasma creatinine and creatinine clearance) at 1 years did not differ in SKT(1-4) and SKT(> 4) (plasma creatinine 1.71 ± 0.69 and 1.69 ± 0.63 mg/dL; creatinine clearance 49.6 + 18.5 and 52.6 + 18.8 mL/min, respectively); DKT showed statistically lower plasma creatinine (1.46 ± 0.57, P < 0.04) but not different creatinine clearance (55.4 + 20.4). Due to older donor age in the DKT group, comparisons were repeated in transplants from donors older than 70 years, and equal graft and patient survival in SKT and DKT were confirmed. Total mean number of functioning graft years by transplant reference at 1, 3 and 6 post-transplant years were equal between the groups, but mean number of dialysis-free life years by donor reference were significantly higher in SKT (mean difference compared to DKT at 6 years: 292 [IQR 260-318] years/100 donors in SKT(1-4) and 292.5 [(IQR 247.8-331.6) in SKT(> 4)].
In transplants from clinically suitable ECD donors, graft survival was similar irrespective of pre-implantation biopsy score and of allocation to SKT or DKT. These results suggest use of caution in the use of histology as the only decision criteria for ECD organ allocation.
Core tip: Pre-implantation biopsy of grafts from elderly donors is under appraisal as a means to direct the acceptance/discard decision of organs for transplantation and the best allocation to single rather than dual transplant. Presented data shows that in recipients of grafts from older donors, rated suitable to donate according to clinical data and preserved renal function, graft and patient survival did not differ in the two categories of transplants with graft histological score in the lower (1-4) or higher (5-8) range of a scale in use. Additionally, allocation of higher score grafts to single or dual transplant did not result in different survival in time, but observed total number of dialysis free life years in recipients up to 6 years was lower for the dual kidney transplant (DKT) allocation. We suggest that older donors rated suitable to donation by clinical decision and preserved renal function may be allocated to single kidney transplant without biopsy; if biopsy is performed, higher scores than those in actual use should be considered for allocation to DKT.
- Citation: Colussi G, Casati C, Colombo VG, Camozzi MLP, Salerno FR. Renal transplants from older deceased donors: Is pre-implantation biopsy useful? A monocentric observational clinical study. World J Transplant 2018; 8(4): 110-121
- URL: https://www.wjgnet.com/2220-3230/full/v8/i4/110.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i4.110
Organ shortage is widely held the most urgent problem in the field of kidney transplantation[1]. In order to increase the donor pool and the chance of transplantation to patients on wait list most transplant programs are increasingly accepting suboptimal, so called “extended criteria”, donors (ECD)[2,3]. Despite worse performance than transplants from young donors in terms of delayed graft function (DGF), primary non function (PNF), short and long term renal function and overall graft survival[2-4], transplants from ECD may offer a survival advantage in comparison with not-being transplanted and remaining on wait list, at least for specific patient categories[5-7]. In quantitative terms, several reports indicate that graft survival from adequately selected ECD may not be much lower as compared to grafts from “standard” donors[8-10]. In our series, a retrospective analysis of death-censored graft-survival of transplants from clinically suitable, i.e., with preserved renal function and anatomy, donors older than 60 years was only 8.2% lower than that from younger than 60-year donors after 10 years (84.0% vs 92.2%). Thus, elderly donors may be a precious source of transplantable organs.
In some countries (among which Italy), dual (DKT) rather than single kidney transplantation (SKT) from ECD has gained popularity as a means of limiting elderly organ discard[11-15]; a simplistic rational is that quantity of functioning nephrons in one kidney from elderly donors may be insufficient to sustain adequate function in recipients, while double such a quantity may provide adequate compensation. Moreover organ senescence and age-related pathology might also benefit from doubling tissue mass. A critical issue is how to measure and quantitate these variables; common assumption is that histology, and its translation into quantitative scores, may allow a more objective evaluation of organ quality than clinical data (renal function, anatomy, comorbidities). Several reports have shown similar survival of high histological score organs (assumed to represent poor-quality grafts) used as DKT as compared to SKT with low histological score grafts (again assumed to represent better-quality organs)[11-13]; these results have been credited to support the validity of biopsy-based organ allocation. On the other hand, other reports have shown equal survival of grafts from elder donors (all allocated to SKT) independently of pre-implantation histological score, i.e., low (1 to 3)[8,16,17] vs high [4 to 6(8) or 4 to 5[16,17]] score. Score 4 constitutes the limit for differential allocation of ECD grafts to SKT rather than DKT in the biopsy-based protocol in use in our transplant area. We and others[18] have reported that DKT recipients who lost one graft due to surgical complications were able to maintain adequate organ function, despite bad histological score of the surviving graft. Thus, it would appear that current biopsy protocol for allocation of ECD grafts to SKT or DKT may foster unbalanced allocation to DKT of grafts suitable for SKT, somehow reducing transplant benefits from available donors. In the present analysis, we have taken advantage of donor kidney pre-implantation biopsies performed in the past, i.e., before adopting current biopsy-based DKT program, as a component of post-transplant surveillance protocol; we have reviewed all available biopsies from ECD and scored them according to current criteria within the DKT program. Several grafts, allocated to SKT, happened retrospectively to show > 4 histological score, a value which would actually indicate allocation to DKT. The aim of the study was to retrospectively compare the outcome of SKT from ECD categorized according to histological score, i.e., up to 4, or higher than 4; in addition, outcome of SKT from grafts with low or high histological score was also compared to outcome of DKT from grafts with high histological score according to current protocol. Graft survival in time and measured renal function at one year in recipients were main outcomes; in addition, dialysis-free life years in recipients at 1, 3 and 6 years within each transplant category were also evaluated using the restricted mean survival time methodology[19-21].
All renal transplants from older than 60-year donors performed in our Centre from 1 Jan 2000 to 30 Oct 2017 were analyzed, provided that a pre-implantation biopsy was available. Up to 30 Nov 2010 only SKT were performed; irrespective of age and comorbidities, donor suitability was based on clinical data which included normal lower pre-donation plasma creatinine, eGFR (Cockroft-Gault formula) higher than 60 mL/min per 1.73 m2, proteinuria absent or “trace”, and anatomy permissive (echography and/or surgical inspection). Pre-implantation biopsy was not required, and was only performed for cause, e.g., in case of pre-donation acute renal failure or more than trivial proteinuria, to ascertain any specific pathology, or as part of a post-transplant surveillance protocol, in which case histological data were analyzed only time after transplantation.
After 1 Dec 2010 our Centre joined to a biopsy-based DKT program designed and coordinated by our inter-regional regulatory agency, NITp[11], where it is publicly registered[22], and which is shared by all transplant Centers of the area. Within this program older than 60-year donors are allocated to SKT or DKT according to clinical and histological criteria: Donors older than 70 years, or aged 60-70 years with any of arterial hypertension treated with ≥ 2 drugs, drug-treated type 2diabetes mellitus, death due to cerebrovascular event (with exclusion of trauma and aneurism rupture as cause of brain death), proteinuria higher than 0.5 g/L, eGFR (Cockcroft-Gault) less than 60 mL/min per 1.73 m2 undergo pre-implantation biopsy, and are allocated to SKT if histological score is ≤ 4, to DKT if mean score is 5-7, and discarded if mean score is > 7 (Table 1); these donors are collectively defined “high-risk” ECD. When only one of partner kidneys had a score > 4, it was at discretion of the transplant Centre to perform DKT or SKT with the lower score graft. Donors in the 60-70 year-range, without any of the above comorbidities, collectively defined “low-risk” ECD, are allocated to SKT without biopsy.
| Glomerular global sclerosis | 0 = no glomeruli globally sclerosed |
| 1 = less than 20% | |
| 2 = 20%-50% | |
| 3 = > 50% | |
| Arteries/arterioles wall thickness1 | 0 = normal appearance |
| 1 = less than lumen diameter | |
| 2 = equal/slightly higher than lumen diameter | |
| 3 = higher than lumen diameter/severe lumen reduction | |
| Tubular atrophy | 0 = absent |
| 1 = less than 20% tubuli affected | |
| 2 = 20%-50% | |
| 3 = > 50% | |
| Interstitial fibrosis | 0 = absent |
| 1 = less than 20% parenchymal tissue substituted | |
| 2 = 20%-50% tissue | |
| 3 = > 50% tissue |
Application to the program is additive to that for standard donors and requires signature of a specific informed consent; in our Centre we also require recipient’s age older than 62 years. Consent includes either DKT or SKT from the same donor categorized as “high-risk” ECD. All donors were brain-dead; transplants from living, cardiac-death, ABO- or HLA-incompatible donors, as well as simultaneous kidney and any other organ transplants were not included. Both first and non-first transplants were included. A pre-transplant negative T and B-lymphocyte CDC was a pre-requisite for transplantation and forbidden donor antigens, according to actual or historical HLA antibodies in recipient, were carefully avoided by the allocation agency; allocation algorithm in use in our inter-regional area searches for best HLA match first, then for immunization status, listing time and age match in all transplant categories except in DKT protocol, where HLA match is not considered.
Informed consent was obtained from all the patients applying for renal transplantation in our Centre at the time of listing and at the time of transplantation, and additionally for applying to the DKT program. Consent for anonymous use of clinical data was included in the consent form. This study has been conducted according to principles of the declaration of Helsinki and complies with the declaration of Istanbul. As a standard of care, anonymous study no approval by ethic committee was needed.
We analyzed and compared 3 groups of transplants: Group 1, SKT from older than 60-year donors with pre-implantation graft biopsy score, either before or within the DKT protocol, ≤ 4 (SKT(1-4)); group 2, SKT with graft pre-implantation biopsy score, either before or within the DKT protocol, ≥ 5 (SKT(> 4)); we included within these 2 SKT categories also 6 DKT recipients who had early removal of one graft for surgical complications with score in remaining graft ≥ 5 (5 patients) or < 5 (1 patient); group 3, DKT with graft pre-implantation biopsy score 4 to 7 according to the DKT protocol (DKT). As already said, only in the DKT protocol histological score was known before transplant and used for differential graft allocation, while in the pre-DKT period it was only a retrospective information.
For every donor-recipient pair, in each group, we collected and analyzed clinical data of interest, age, sex, HLA mismatches (loci A, B, DRB1), type and length of dialysis in recipients, plasma creatinine and eGFR in donor and plasma creatinine and creatinine clearance (24 h urine) at 3 mo and 1 years post-transplant in recipients, and biopsy-proven rejection of any type in the first 18 mo after transplantation in recipients. Outcomes of interest were death-censored graft survival (i.e., freedom from dialysis or re-transplantation), overall graft survival (i.e., graft loss or patient death with functioning graft, whichever came first, corresponding to patients alive with functioning graft), patient survival (i.e., death with functioning graft) and renal function in recipients at 3 and 12 mo from transplantation; we also evaluated: Early graft losses (EGL, i.e., no dialysis-freedom, or need of permanent dialysis, within 3 mo after transplantation), DGF (need of dialysis for any cause in the first week after transplantation), mean years of functioning graft at 1, 3 and 6 years from transplantation with reference to initial transplants and total dialysis-free life years at the same times with reference to donors.
Data base update was closed on 31 Jan 2018, allowing for at least 3 mo uncensored follow up in all patients; since only in few cases total follow-up was longer than 6 years in the DKT group, and longer of 10 years in both the SKT groups, follow up was censored at 6 years in DKT and 10 years in SKT(1-4) and SKT(> 4).
Biopsies within the DKT program were either wedge or core biopsies, according to harvesting Centre practice, while our historical biopsies were all core needle. Score was evaluated on paraffin-embedded, hematoxylin-eosin stained slides; in the DKT program score was calculated by any of participating Centre pathologists and communicated to NITp; all our pre-DKT surveillance biopsies were viewed and scored by collaborative work of a pathologist (Camozzi MLP) and two nephropathologists (Colombo VG and Casati C). A minimum of at least 10 glomeruli were required for a biopsy to be representative.
Immunosuppression protocols at our Centre did not change in all observation period (Jan 2000 to Oct 2017), and included in most patients rATG induction (3.5 mg/kg in 7 d, 7 mg/kg if ≥ 2nd transplant), cyclosporine-A starting pre-transplantation as a 10 mg/kg oral load, mycophenolate mofetil/mycophenolic acid starting on p.o. day 1 (1g or 720 mg bid) and corticosteroids (methylprednisolone 500 mg at reperfusion, rapidly tapered down to 8 mg/d on p.o. day 11 and 4 mg/d after 3 mo). In a minority of patients, tacrolimus, everolimus, belatacept or sirolimus were used (Table 2). Post-transplant heparin anticoagulation was started in 2011 only in DKT, after that a higher than usual graft vein thrombosis was observed in this type of transplant, as described also by others[23].
| Transplant category1 | SKT(1-4) | SKT(> 4) | DKT |
| n | 102 | 30 | 53 |
| Donors, M/F | 54/48 | 16/14 | 29/24 |
| Donor age, yr (mean, SD) | 68.9 ± 5.7 | 66.9 ± 6.7 | 75.3 ± 5.0b |
| Score of transplanted graft, median (IQR) | 3 (3-4)d | 5 (5-6)a | 5 (4-5) |
| Donor comorbidities | |||
| Donor age > 70 yr, n (%) | 47 (46) | 8 (27) | 47 (89)b |
| Arterial hypertension | 40 (39) | 21 (70) | 34 (64) |
| Diabetes | 9 (9) | 6 (20) | 7 (13) |
| Cerebrovascular cause of death | 33 (32) | 17 (57) | 29 (55) |
| KDPI1 | 89.4 ± 8.0 | 89.9 ± 9.2 | 96.9 ± 3.4b |
| KDRI1 | 1.65 ± 0.27 | 1.7 ± 0.33 | 2.02 ± 0.31b |
| Recipients, M/F | 68/34 | 20/10 | 37/16 |
| Recipient age (mean ± SD, yr) | 61.0 ± 7.2 | 60.2 ± 6.0 | 67.3 ± 4.6b |
| Years on dialysis, median (IQR) | 3.5 (0.1-13.5) | 3.4 (0.8-9.5) | 2.1 (0.3-8.5)b |
| Dialysis mode, n (%) | |||
| Hemodialysis | 84 (82) | 25 (83) | 40 (75) |
| Peritoneal dialysis | 16 (16) | 5 (17) | 12 (23) |
| Pre-emptive | 2 (2) | 0 | 1 (2) |
| Renal disease n (%)1 | |||
| GN/systemic | 35 (34) | 10 (34) | 19 (36) |
| ADPKD | 24 (23) | 4 (13) | 6 (11) |
| Vascular/hypertension | 10 (10) | 3 (10) | 3 (6) |
| Diabetes | 11 (11) | 4 (13) | 7 (13) |
| Other | 14 (14) | 6 (20) | 12 (23) |
| Unknown | 8 (8) | 3 (10) | 6 (11) |
| 1st-2nd-3rd Tx | 95-6-1 | 28-2-0 | 51-2-0 |
| HLA-MM (median, IQR) | 4 (3-5) | 4 (3-5) | 4 (4-5) |
| CITa (mean ± SD, h) | 15.0 ± 3.6 | 15.9 ± 4.2 | 16.1 ± 3.1 |
Descriptive statistics are given as numbers, percentages and mean ± SD or median (1st and 3rd interquartile range, IQR) according to data distribution; inter-category differences were checked by ANOVA, followed by Scheffé post-hoc test; Fisher’s exact test was used for comparison of frequencies; Pearson’s coefficient was used for correlation analysis between pairs of data. Survival analysis was estimated as event free cumulative survival using the Kaplan–Meier method and compared using the log-rank Mantel-Cox test.
We estimated the mean number of years the allografts were functioning before loss for any cause (failure or death with functioning graft) by the restricted mean survival analysis[19-21]; it is computed as the total area under the survival curve at specific times (we repeated the procedure at 1, 3 and 6 post-transplant years), and indicates the mean time (years) the grafts remained functional at any defined time. Conceptually, this evaluation indicates mean dialysis-free life years for every transplanted patient at any defined time. From this value we extrapolated total dialysis-free life years for every 100 donors at any time in each of the 3 groups of transplants; for this calculation each donor was made equal to 1.6 SKT, according to data of our regional agency on utilization of overall retrieved grafts[24], very close to the 1.67 figure for ECD of another transplant program[8], and to 1 DKT. SPSS Statistics software v.21 was used for all analyses. Two-tailed P values < 0.05 were considered significant.
In the DKT protocol (after Dec 2010) there were 196 older than 60-year donors, of which 131 qualified for biopsy showing score 4 or less in 66 (allocated to SKT) and score 5 or higher (up to 7) in 65, of which 59 were allocated to DKT and 6 to SKT, with score 5 in 5 and 6 in 1; we accepted these 6 grafts as SKT to avoid discard, since the corresponding partner grafts, with lower than 5 scores, had already been allocated to SKT or was anatomically unsuitable. Six of the 59 DKT, with early removal of one graft for surgical complications, have been included, according to score in remaining graft, in the SKT(> 4) (5 cases: score 6 in 4 cases and score 5 in 1) or SKT(1-4) (1 case, score 3) categories.
In the pre-DKT period, pre-implantation biopsy was available in 72 older than 60-year donors; in 18 cases available tissue was insufficient for adequate scoring, 35 grafts showed score 4 or less, and 19 score 5 or higher (range 5-8). Thus, our analysis concerns 102 SKT(1-4), 30 SKT(> 4), and 53 DKT. Summary data of baseline donor and recipient characteristics in the 3 transplant categories are given in Table 2 and main post-transplant events of interest in Table 3. Donor and recipient age was higher, and time on dialysis prior to transplant shorter, in the DKT category, while donor and recipient sex distribution was equal. Also KDPI and KDRI were higher in the DKT category, mostly as a consequence of older age (see below). Donors older than 70 years were 102, of which 47 were allocated to DKT and 55 to SKT. Histological score was lower by selection in SKT(1-4) than SKT(> 4) and DKT, and was also higher in SKT(> 4) than in DKT. Median and total follow-up was shorter in DKT, due to contribution to follow-up from the pre-DKT years only in the 2 SKT categories. All other donor and recipient characteristics, including donor comorbidities, recipient dialysis mode, renal disease, HLA mismatches, number of transplants, immunosuppression, graft cold ischemia time, were not different between categories. There were no major differences in events of interest along follow up between categories, apart higher incidence of DGF, i.e., need of dialysis in the first week after transplantation, in SKT(> 4). Early graft losses were 9 (7.1%) in all 126 original SKT, i.e., excluding 6 original DKT included here in the SKT groups, of which 4 (3.2%) where associated to graft vascular thrombosis and 5 (4.0%) where “unexplained” PNF; in DKT there were 2 of 59 surgical (thrombosis and hemorrhage) early losses (3.4%), but overall vascular graft thrombosis occurred in 8 of 118 grafts (6.8%) (P < 0.10 vs SKT).
| Transplant category1 | SKT(1-4) | SKT(> 4) | DKT |
| n | 102 | 30 | 53 |
| Initial immunosuppression, n (%) | |||
| rATG | 95 (93) | 25 (83) | 53 (100) |
| Basilix imab | 8 (8) | 3 (10) | 0 |
| Cyclosporin | 91 (89) | 25 (83) | 50 (94) |
| Tacrolimus | 9 (9) | 2 (7) | 3 (6) |
| Mycophenolate | 91 (89) | 27 (90) | 50 (94) |
| Everolimus | 9 (9) | 4 (13) | 3 (6) |
| Sirolimus | 1 (1) | 1 (3) | 0 |
| Belatacept | 2 (2) | 1 (3) | 0 |
| Steroids | 84 (82) | 28 (93) | 50 (94) |
| Tx duration2, yr (median, IQR) | 4.1 (1.6-7.4) | 7.0 (2.6-9.9) | 2.7 (1.4-4.8)a |
| Total follow-up, pt-years | 467.8 | 180.5 | 161.7 |
| DGF3, % | 42.1 | 56.6c | 24.5 |
| EGL3, n (%) | |||
| All | 8 (7.8) | 1 (3.3) | 2 (3.8) |
| PNF3 | 4 (3.9) | 1 (3.3) | 0 |
| Surgical | 4 (3.9) | 0 | 2 (3.8) |
| BPAR3, n (%) | 10 (9.8) | 3 (10.0) | 3 (5.6) |
| Graft failure4, n (n/100 pt-yr) | 10 (2.1) | 6 (3.3) | 3 (1.8) |
| Pt-death, n (n/100 pt-yr) | 16 (3.4) | 4 (2.0) | 6 (3.5) |
Donor histological score did not show any significant correlation with donor age (r = 0.11, P > 0.10), donor plasma creatinine (r = 0.05) and eGFR (r = -0.01), recipient creatinine clearance at 3 mo and 1 years after transplantation (r = -0.05 and 0.05, respectively; all P > 0.25), and donor KDPI and KDRI indices (r = 0.05 and 0.10, respectively, P > 0.10). Both KDPI and KDRI were strongly correlated with donor age (r = 0.70 and 0.78, respectively, P < 0.0001), and donor eGFR (r = -0.31 and -0.36, P < 0.001).
There were no statistically significant differences in graft, patient and overall survival in recipients of SKT(1-4)vs SKT(> 4) (P = 0.41, 0.78 and 0.31 for graft, overall and patients survival), and between DKT and both SKT(1-4) and SKT(> 4) (respectively P = 0.40 and 0.23 for graft, 0.71 and 0.85 for patient and graft, and 0.81 and 0.36 for patient survival) (Figure 1).
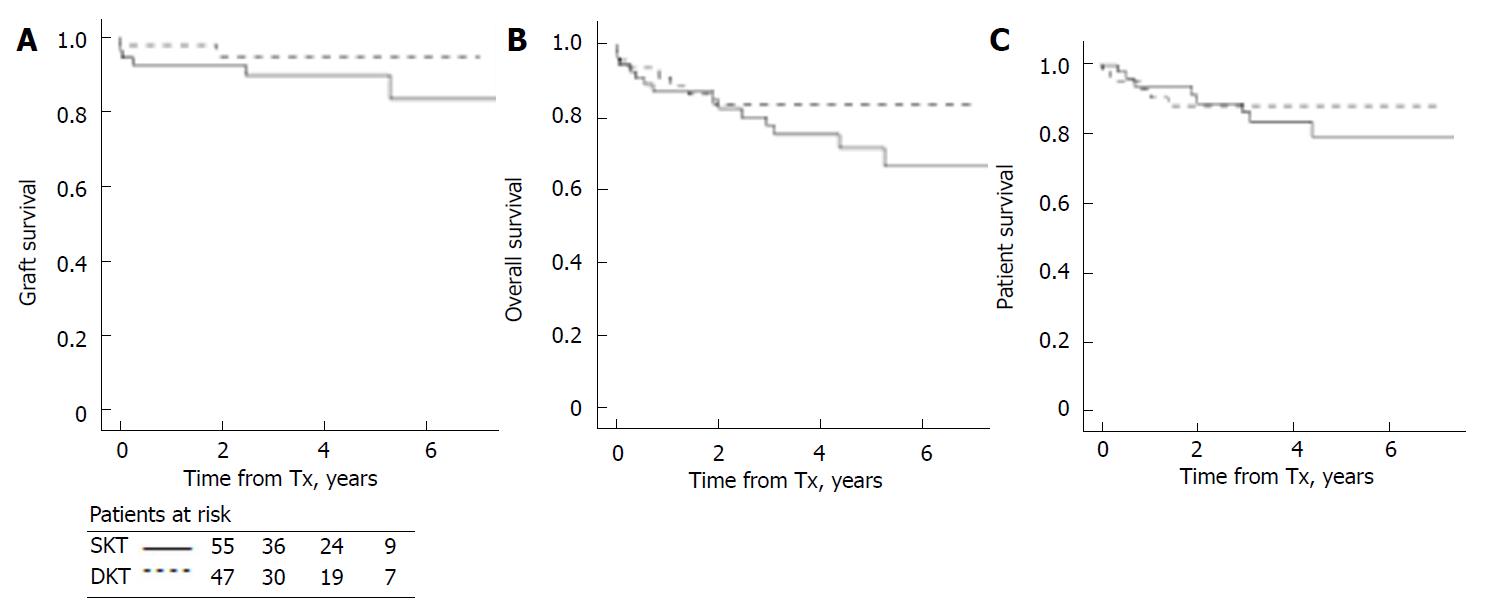
To account for differences in donor age, we repeated survival analysis in recipients of older than 70-year donors, i.e., in the highest age risk range according to definitions in the DKT protocol in use: there were 47 older than 70 years donors with organs allocated to DKT and 55 to SKT (47 in SKT(1-4) and 8 in the SKT(> 4) categories); since survival data were equal for SKT(1-4) and SKT(> 4), we pooled together all SKT. Donor age was 76.4 + 4.0 in the DKT group, and 74.2 + 3.6 in the SKT group (P < 0.004). Recipient age was 67.3 + 4.8 in DKT and 63.2 + 6.2 in SKT (P < 0.001). Histological score was 5 (IQR 4-6) in DKT and 4 (IQR 3-4) in SKT (P < 0.01). For homogeneity, follow-up was closed at 6 years in both groups. Again, there were no statistically significant differences in graft (P = 0.24), patient (P = 0.64) and patient and graft survival (P = 0.28) (Figure 2).
Renal function in donors and recipients of each transplant category is shown in Table 4. Donor plasma creatinine and eGFR did not statistically differ between transplant categories.
| Transplant category1 | SKT(1-4) | SKT(> 4) | DKT |
| D-Pcr, mg/dL | 0.88 ± 0.31 | 0.92 ± 0.28 | 0.81 ± 0.23 |
| D-eGFR | 83.9 ± 27.7 | 87.1 ± 26.0 | 79.1 ± 21.8 |
| n | 102 | 30 | 53 |
| R-Pcr 3 mo | 1.92 ± 0.98 | 2.12 ± 1.12 | 1.56 ± 0.75a |
| R-CCr 3 mo | 45.0 ± 19.3 | 43.4 ± 21.8 | 49.6 ± 19.6 |
| n | 94 | 28 | 49 |
| R-Pcr 12 mo | 1.71 ± 0.69 | 1.69 ± 0.63 | 1.46 ± 0.57c |
| R-CCr 12 mo | 49.6 ± 18.5 | 52.6 ± 18.8 | 55.4 ± 20.4 |
| n | 83 | 25 | 45 |
At 3 and 12 post-transplant months, recipients alive with a non-failed graft showed similar levels of plasma creatinine and measured creatinine clearance in SKT(1-4) and SKT(> 4), while in DKT plasma creatinine was lower than in SKT at both times, with statistical significant difference at 3 mo vs both SKT(1-4) and SKT(> 4) and only versus SKT(1-4) at 12 mo. Differences in creatinine clearance did not reach statistical significance (Table 4).
Table 5 shows that the mean number of functioning graft years by transplant reference at 1, 3 and 6 years from transplantation was equal for all 3 transplant categories; for clarity, calculations were referred to 100 transplants. Extrapolation of total dialysis free life years by donor reference at the same time showed significant differences by allocation (i.e., SKT or DKT), with statistically higher figures for both SKT categories at any time. In this extrapolation, we have conservatively chosen an utilization factor of 1.6, rather than 2, SKT for each donor according to published statistics[8,24]. Thus, our data are a minimal realistic estimation of benefits of SKT vs DKT, accounting for observed differences in overall survival.
| 1 yr | 3 yr | 6 yr | ||
| RNFGY (× 100 Tx) | SKT(1-4) | 93.3 (86.9-99.7) | 261.0 (253.3-268.7) | 499.6 (490.5-508.7) |
| SKT(> 4) | 93.8 (85.2-102.4) | 279.5 (266.9-292.1) | 499.9 (482.5-517.2) | |
| DKT | 97.7 (91.4-104.1) | 275.0 (264.1-285.8) | 507.3 (496.0-524.3) | |
| TDFLY (× 100 donors) | SKT(1-4) | 149.3 (139.1-159.6) | 417.6 (405.4-429.9) | 799.3 (784.7-813.9) |
| SKT(> 4) | 150.1 (136.2-163.9) | 447.2 (427.1-467.4) | 799.8 (772.1-827.6) | |
| DKT | 97.7 (91.4-104.1) | 275.0 (264.1-285.8) | 507.3 (496.0-524.3) | |
| Vs DKT, difference | ||||
| SKT(1-4) | 51.6 (35.0-68.2) | 142.7 (119.5-165.8) | 292.0 (260.4-317.9) | |
| SKT(> 4) | 52.3 (32.2-72.5) | 172.3 (141.2-203.3) | 292.5 (247.8-331.6) |
Our data shows that graft and overall survival in recipients of renal transplants from elderly donors, allocated to SKT, is not statistically different according to histological score of transplanted grafts, i.e., score 4 or lower as compared to score 5 or higher (up to 8); additionally, they also show that survival of grafts of score 5 or higher does not differ by organ allocation to SKT rather than DKT, at least within the available follow-up of 6 years. Also measured GFR at one year from transplantation in not-failed grafts (a generic predictor of survival expectation) does not differ between SKT with differential score grafts, and is marginally better in DKT than in SKT. Thus, our data would indicate that, for organs rated suitable for transplantation clinically, histological information has uncertain usefulness to predict outcome; additionally, current score scale for allocation to DKT appears to hold little discrimination power between grafts which could or could not perform adequately as SKT.
While the biopsy protocol for allocation of elderly donors to SKT or DKT according to score actually in use in our transplant area, which operates on a 19 million population area, dictates allocation of organs with score higher than 4 to DKT, we were able to find out from SKT performed in the past recipients who happened to receive higher than score 4 grafts, as disclosed by surveillance biopsies which were a posteriori scored using criteria of the protocol in use. To these recipients, we added 5 DKT recipients who retained a single high-score graft due to early loss of the corresponding partner graft for vascular complications and 6 other high score grafts in the DKT era whose paired graft had been allocated to SKT in other Centers or was unsuitable for transplantation. The SKT(1-4) recipients were part of both the recent DKT protocol and past transplant activity with available surveillance biopsy. SKT(1-4) and SKT(> 4) groups were well matched concerning donor and recipient characteristics, and differed only in donor graft score by intended, afterward selection; thus, observational data in these 2 groups offer unbiased, clinically relevant, information. DKT category instead showed older age in donors and accordingly in recipients. To overcome this bias, we repeated outcome survival analysis considering only transplants from donors in the most extreme age range, i.e., older than 70 years and up to 88 in DKT and 85 in SKT. In this analysis, due to observed equal graft and patient survival between SKT with different score ranges, we only compared DKT to SKT allocation. Again, there were no statistically significant differences in graft and patient survival between DKT and SKT recipients. Unfortunately, also in this sub-analysis the two populations were not homogeneous, since mean donor and recipient ages were 2 and 4 years older, respectively, and histological score higher in DKT; we think that these small differences have little impact on interpretation of results, even though we recognize that we cannot evade the general assumption that equal outcome with worst graft histology may sustain the validity of DKT allocation by score.
“High risk donors” as defined in our regional DKT protocol (older than 70 years, or 60-69-year-old with comorbidities) are 10 years ahead of canonical ECD definition (older than 60-year or 50-59-year-old with comorbidities)[3,25]. The overwhelming majority of our ECD (84%) were “high risk” according to the above definition. Despite this donor connotation, our medium (in DKT) and long-term data (in SKT(1-4) and SKT(> 4)) shows not inferior graft and patient survival in recipients of these donor grafts than that commonly described for ECD in general[9,10], and confirms the potential wealth of older donor organs. Survival figures did not change, too, by restricting survival analysis to donors older than 70 years, indicating that also very old donors may be safe, if renal function is permissive. Others have described similar survival in recipients of grafts from donors older than 75 years as compared to grafts from younger ECD[10], or in recipients of grafts from ECD donors which differed by decades in the range from 60 to 80 years[12].
Our data that histology appears a poor predictor of transplant outcome confirms other published reports: Hofer et al[8] showed similar medium term (8 years) survival of grafts with score 0-3 as compared to score 4-6, with worst survival only for grafts with extremely high score (i.e., 7-12). These latter were only 8 out of 106 ECD (7.5%), and 4 out of 305 SCD (1.3%); it is uncertain if so severe histology entailed any degree of impaired function, which might have indicated for cause biopsy. Carta et al[17] report equal short term (3 years) graft and patient survival in SKT recipients of score 4-5 as compared to score 0-3 grafts. Foss et al[26] allocated to SKT by clinical criteria 54 grafts from older than 75-year donors and retrospectively could not find any relationship between 5 years graft survival and pre-implantation score (ranging 0 to 8), with equal 1-year plasma creatinine levels in recipients of score 0-4 as compared to score 5-8 grafts.
No single component of histological score has been shown to be consistently associated to post-transplant outcome[8,17,27]; definition of a score limit for graft allocation or for acceptance/discard has so far entailed some empiricism. The original DKT protocol in NITp area contemplated a score above 3 for organ allocation to DKT[28,29], and has been changed to score 4 as a result of favorable outcome of SKT with score 4 grafts[14,30]. Our and others’[8,17,26] data suggests that even higher score grafts, from clinically suitable donors, may perform well as SKT. So, further appraisal from clinical series comparing outcome of grafts with equal histology but differentially allocated to SKT or DKT appears at least desirable. Ideally, such a comparison of outcome should be implemented with the new concept of population-average dialysis-free life years by donors, which may somehow temper the interpretation of the more direct and usual concept of time survival by recipients (see below).
As reported[8,16,17,26] also in our hands histological score, despite being credited as a senescence index, had no relationship with donor age, nor did it correlate with renal function in donors and recipients. It was shown to correlate mostly with hypertension and vascular disease in donors[8,16], a finding consistent with the marginally lower incidence (just below statistical significance) of hypertension in our SKT(1-4) donors in comparison with SKT(> 4) and DKT. It was even not correlated with donor KDPI and KDRI, as shown by equal values of these indices in either SKT category. Higher KDPI/KDRI in DKT were almost the exclusive effect of older donor age, as indicated by the very strong correlation of these indices with donor age, much stronger than that with donor eGFR. Thus, our data adds evidence that current tools to predict organ quality, i.e., histology, KDPI and even pump perfusion[31] have little reliability in predicting individual graft outcome and are no better than clinical evaluation.
Lack of correlation between histological score and graft outcome we have shown has to be commented within the frame of donors with well-preserved renal function; while there is no doubt that donor grafts with severe pathology are poor candidates for transplantation, it is disputable that such grafts associate with well-preserved renal function. In healthy kidney live donors it was shown that while number of glomeruli falls with age, single nephron GFR does not change up to 70 years, so that total GFR proportionally falls[32]; thus preserved GFR may select donors with a lesser degree of age-related nephron loss. Our results indicate that reliance only in histology for organ allocation may not always be well founded, and that even though function does not predict histology it remains a reliable predictor of graft outcome. In ECD with well-preserved renal function, as the majority of ECD in the present series, biopsy should better be avoided. Causes of discordance between histology and outcome have already been commented, and may reside in any of recognized biases of histology, including its randomness, differences in technique and process, and pathologist expertise among others[8,26,27].
We underscore that donors (of any category) who present with impaired renal function, either long standing, acute or uncertain, are a different context. Biopsy in these donors is of definite help in defining specific underlying pathologies (i.e., acute vs chronic, reversible vs irreversible lesions); while grafts with acute, reversible pathologies (more commonly acute tubular necrosis) perform well as SKT[33], grafts with chronic lesions require integration of both clinical and histological information to guide mainly in the decision between DKT vs discard. We think that donors with pre-existing marginal renal function and anatomy should be the main candidates to histological evaluation, with the aim to ascertain that at least 50% of renal mass is viable. We acknowledge that such an achievement may not be easy; within the frame of current score scale, we suggest that a level of at least up to 2 for any individual score should be allowed, summing up to a total of 8 as acceptable score for DKT.
DKT was proposed as a means to reduce discard rate of grafts from marginal donors (defined on the basis of vascular disease and/or older age)[34]; organs from these donors have been often perceived to offer inadequate function if used as SKT. Indeed, survival in time of these organs allocated to SKT is lower in comparison with grafts from younger, or standard, donors[9,10]. In one study early graft loss from any cause was 10.1% (4.2% from unexplained PNF) in recipients of ECD grafts against 4.1 (all causes) and 1.5 (PNF), respectively, in standard donor grafts[35]; these and other’s[36] figures in ECD transplants are not far from ours in all SKT (7.1% early loss for any cause, with 4.0% PNF). As for survival in time, the population-average relative risk of graft failure (including patient death) at 10 years from transplantation was 1.7 times higher in recipients of an ECD graft as compared to a standard donor graft[8,10]. Translated into quantitative numbers, after 10-year follow-up the mean time to graft failure was only 8 mo shorter for recipients of an ECD graft as compared to standard donor graft[10]. Thus, despite inherent detriments as compared to younger donor grafts, absolute benefits of ECD organs at a population level are not trivial, and foster in many European transplant communities a call to a wider use rather than to discard of these organs[10,20]. In this perspective DKT, even assuming that it effectively reduces early and long-time losses, may not allow an equally efficient use of available organs as SKT. It is claimed that, due to bad histology, these organs could not perform adequately if allocated to SKT. We have shown instead that SKT of grafts with bad histological score is associated with similar graft and patient survival in recipients as compared to DKT.
We have tried to quantitate benefits from ECD transplants according to allocation to SKT or DKT; from the observed survival curves, we calculated mean number of functioning graft years at specific time points in recipients by transplant reference and mean dialysis-free life years by donor reference. Dialysis-free life years may be viewed as a good indicator of transplant benefits, as far as it includes both quality of life related to transplantation and social cost savings. Dialysis-free life years were greater in SKT than DKT at any time of our analysis, and the difference increased rather than lessen in time. This data would favor SKT over DKT from the same donors; moreover, since our follow up was not long, longer-reaching series are needed to confirm maintenance in time of these benefits. Better renal function at 1 years justifies a longer survival expectation in time for DKT; on the other hand, it has to be appreciated that in the long-term immunological mechanisms are a prevalent cause of graft loss[37], and may become the main determinant of graft survival. Thus, any long-term scenario remains simple speculation unless longer term observational data is available.
Despite a rather small number of cases, this study allows an unbiased comparison of clinical outcome of renal transplants categorized by graft histology and allocation. Donor and recipient characteristics, immunosuppression and clinical management were homogeneous between groups, except for donors’ and recipients’ older age in the DKT group. In addition to canonical survival analysis by Kaplan Meier methodology, this study has evaluated novel outcome data in use in clinical transplantation based on the restricted mean survival time methodology, allowing to infer on quantitative dialysis-free life years made possible by differential allocation.
Main limit of the study is the rather short follow up of our DKT population, which advocates for a longer time analysis. Older age in donor and recipients of DKT may also constitute a bias in comparison to SKT categories, however reanalysis of results in older than 70-year donors, with very small mean donor and recipients age difference, confirmed the results in the whole series.
In conclusion, our data shows that grafts older than 60 years of age from deceased donors, allocated to SKT on the basis of clinical suitability, perform equally well in recipients irrespective of categorization according to histological score, up to 4 or greater than 4, and that high-score grafts perform equally well in recipients irrespective of allocation to SKT or DKT. With respect to observed survival figures at 1, 3 and 6 years, overall dialysis-free life years per any donor number were greater for SKT than DKT allocation of equally scored grafts. For clinically suitable organs, histology appears unable to predict and improve the population-average graft survival. Thus, indications for DKT allocation of ECD grafts should perhaps be revised, with DKT being limited to use mainly for organs clinically unsuitable for SKT due to inadequate function and/or imaging/anatomy. In this context, new criteria have to be sought to guide decision not on allocation, but rather on acceptance vs discard.
In renal transplantation a hot topic is the best use of older donor grafts: these organs are associated with an higher risk of early and late graft failure, yet this donor category has become the most prevalent one in western countries. Pre-implantation biopsy of grafts from elderly donors is commonly used to guide in the acceptance/discard of organs, and/or in their allocation to single or dual kidney transplant.
There is no universal agreement in the literature on usefulness of biopsy to predict post-transplant graft outcome; additionally, a main concern with dual kidney allocation is a reduction of transplants made possible by available donors.
The main objectives of our study were to retrospectively compare outcome data of transplants with older donor grafts categorized according to pre-implantation histology into a low-score or high-score category; additionally, high-score grafts were compared by allocation to either dual kidney or single kidney transplant category.
All renal-only transplants in our Center from 1 Jan 2000 to 30 Oct 2017 from donors older than 60 years and with available pre-implantation graft biopsy were retrospectively evaluated. Before Dec 2010 grafts were allocated only to single kidney transplant, irrespective of histology; after that date we adopted a biopsy-based protocol (DKT protocol), which dictated allocation to single kidney transplant of grafts with low histological score (1 to 4), and to dual kidney transplant of grafts with high histological score (4 to 7).
A total of 185 patients with pre-implantation biopsy were available, 102 with low histological score (4 or less), 83 with high histological score (5 to 8), of which 30 were allocated to single kidney transplant (score 5 to 8) and 53 to dual kidney transplant (score 5 to 7). Donors allocated to single kidney transplant did not differ between the low score and high score categories as concerns age, sex distribution, renal function, comorbidities, KDPI and KDRI indices, while they were older and with higher KDPI/KDRI indices in the dual kidney transplant category. Up to 10 years after transplant, we did not observe any differences in graft, patient and overall survival between recipients of a single kidney transplant with either low or high histological score, or between recipients of high histological score grafts allocated either to single or dual kidney transplant. These results were confirmed in a sub-analysis based only on the oldest donors (older than 70 years). We also calculated the total number of dialysis free life years in recipients of either a single or dual kidney transplant by available donors, showing a significantly higher value for recipients of a single kidney transplant up to the available follow-up of 6 years.
Our study shows that the histological score in use in our transplant area does not predict post-transplant outcome in recipients of a single kidney transplant; additionally, allocation of grafts with similar histological score to single or dual kidney transplant is associated with equal survival up to the available follow-up of 6 years. We propose that renal biopsy is not indicated in older donors with preserved renal function and anatomy, and that organ allocation to single kidney transplant allows the best use of these donors. We propose that pre-implantation biopsy be limited to donors of any age with abnormal renal function, to ascertain type and reversibility of underlying pathology; dual kidney transplant allocation should be considered for bad function grafts with chronic histological pathology, provided that at least 50% viable tissue be reasonably ascertained.
Main lesson of our study is that histological score scale in current clinical use does not allow to discriminate between organs which could or could not function adequately as single kidney transplant. This implies the risk of underutilization of available donors. A prospective randomization of equal score grafts to single or dual kidney transplant, and a longer follow-up are strongly desirable to ascertain any advantages or inconveniences of dual vs single kidney allocation.
The Authors wish to thank Michele Nichelatti, PhD, specialist in Health Statistics, for helpful suggestions and expert supervision of all statistics of this article, and Maruska Nizzi for expert language editing of the article.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, B
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kute VB, Stavroulopoulos A, Veroux M S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Fehr T, Immer F. Marginal organ allocation: old and new REALity. Transpl Int. 2017;30:1212-1214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Rosengard BR, Feng S, Alfrey EJ, Zaroff JG, Emond JC, Henry ML, Garrity ER, Roberts JP, Wynn JJ, Metzger RA. Report of the Crystal City meeting to maximize the use of organs recovered from the cadaver donor. Am J Transplant. 2002;2:701-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 247] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Metzger RA, Delmonico FL, Feng S, Port FK, Wynn JJ, Merion RM. Expanded criteria donors for kidney transplantation. Am J Transplant. 2003;3 Suppl 4:114-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 515] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 4. | van Ittersum FJ, Hemke AC, Dekker FW, Hilbrands LB, Christiaans MH, Roodnat JI, Hoitsma AJ, van Diepen M. Increased risk of graft failure and mortality in Dutch recipients receiving an expanded criteria donor kidney transplant. Transpl Int. 2017;30:14-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Ojo AO, Hanson JA, Meier-Kriesche H, Okechukwu CN, Wolfe RA, Leichtman AB, Agodoa LY, Kaplan B, Port FK. Survival in recipients of marginal cadaveric donor kidneys compared with other recipients and wait-listed transplant candidates. J Am Soc Nephrol. 2001;12:589-597. [PubMed] |
| 6. | Merion RM, Ashby VB, Wolfe RA, Distant DA, Hulbert-Shearon TE, Metzger RA, Ojo AO, Port FK. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 556] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 7. | Rose C, Schaeffner E, Frei U, Gill J, Gill JS. A Lifetime of Allograft Function with Kidneys from Older Donors. J Am Soc Nephrol. 2015;26:2483-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Hofer J, Regele H, Böhmig GA, Gutjahr G, Kikić Z, Mühlbacher F, Kletzmayr J. Pre-implant biopsy predicts outcome of single-kidney transplantation independent of clinical donor variables. Transplantation. 2014;97:426-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 9. | Querard AH, Foucher Y, Combescure C, Dantan E, Larmet D, Lorent M, Pouteau LM, Giral M, Gillaizeau F. Comparison of survival outcomes between Expanded Criteria Donor and Standard Criteria Donor kidney transplant recipients: a systematic review and meta-analysis. Transpl Int. 2016;29:403-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Querard AH, Le Borgne F, Dion A, Giral M, Mourad G, Garrigue V, Rostaing L, Kamar N, Loupy A, Legendre C. Propensity score-based comparison of the graft failure risk between kidney transplant recipients of standard and expanded criteria donor grafts: Toward increasing the pool of marginal donors. Am J Transplant. 2018;18:1151-1157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 11. | Pierobon ES, Sandrini S, De Fazio N, Rossini G, Fontana I, Boschiero L, Gropuzzo M, Gotti E, Donati D, Minetti E. Optimizing utilization of kidneys from deceased donors over 60 yr: five-year outcomes after implementation of a combined clinical and histological allocation algorithm. Transpl Int. 2013;26:833-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Messina M, Diena D, Dellepiane S, Guzzo G, Lo Sardo L, Fop F, Segoloni GP, Amoroso A, Magistroni P, Biancone L. Long-Term Outcomes and Discard Rate of Kidneys by Decade of Extended Criteria Donor Age. Clin J Am Soc Nephrol. 2017;12:323-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Ruggenenti P, Silvestre C, Boschiero L, Rota G, Furian L, Perna A, Rossini G, Remuzzi G, Rigotti P. Long-term outcome of renal transplantation from octogenarian donors: A multicenter controlled study. Am J Transplant. 2017;17:3159-3171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 14. | Fernández-Lorente L, Riera L, Bestard O, Carrera M, Gomà M, Porta N, Torras J, Melilli E, Gil-Vernet S, Grinyó JM. Long-term results of biopsy-guided selection and allocation of kidneys from older donors in older recipients. Am J Transplant. 2012;12:2781-2788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, Palanisamy A, Reeves-Daniel A, Doares W, Kaczmorski S, Gautreaux MD. Dual kidney transplants from adult marginal donors successfully expand the limited deceased donor organ pool. Clin Transplant. 2016;30:380-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Navarro MD, López-Andréu M, Rodríguez-Benot A, Ortega-Salas R, Morales ML, López-Rubio F, García PA. Significance of preimplantation analysis of kidney biopsies from expanded criteria donors in long-term outcome. Transplantation. 2011;91:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Carta P, Zanazzi M, Caroti L, Buti E, Mjeshtri A, Di Maria L, Raspollini MR, Minetti EE. Impact of the pre-transplant histological score on 3-year graft outcomes of kidneys from marginal donors: a single-centre study. Nephrol Dial Transplant. 2013;28:2637-2644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 18. | Cruzado JM, Fernandez L, Riera L, Bestard O, Carrera M, Torras J, Gil Vernet S, Melilli E, Ngango L, Grinyó JM. Revisiting double kidney transplantation: two kidneys provide better graft survival than one. Transplant Proc. 2011;43:2165-2167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Royston P, Parmar MK. Restricted mean survival time: an alternative to the hazard ratio for the design and analysis of randomized trials with a time-to-event outcome. BMC Med Res Methodol. 2013;13:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 391] [Cited by in RCA: 664] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 20. | Pippias M, Jager KJ, Caskey F, Casula A, Erlandsson H, Finne P, Heaf J, Heinze G, Hoitsma A, Kramar R. Kidney transplant outcomes from older deceased donors: a paired kidney analysis by the European Renal Association-European Dialysis and Transplant Association Registry. Transpl Int. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Lim WH, Chang S, Chadban S, Campbell S, Dent H, Russ GR, McDonald SP. Donor-recipient age matching improves years of graft function in deceased-donor kidney transplantation. Nephrol Dial Transplant. 2010;25:3082-3089. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Nord Italia Transplant Program, Regione Lombardia. Potocollo per l’utilizzo dei reni da donator anziano. [accessed 26 Dec 2017]. Available from: http://www.policlinico.mi.it/AMM/nitp/area_operatore/protocolli/02/141230Rene_ProtocolloDonatoriAnziani.pdf. |
| 23. | Cocco A, Shahrestani S, Cocco N, Hameed A, Yuen L, Ryan B, Hawthorne W, Lam V, Pleass H. Dual kidney transplant techniques: A systematic review. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 24. | Centro trasfusionale e di immunologia dei trapianti, Ospedale Maggiore di Milano, Policlinico IRCCS. Reports di attività. [accessed 30 Sep 2017]. Available from: http://cm.argonet.it/websites/policmi/staging/home_ctit.nsf/wAssets /IDCW-8HTBUL/$file/Report%20NITp%20maggio%202011.pdf. |
| 25. | Port FK, Bragg-Gresham JL, Metzger RA, Dykstra DM, Gillespie BW, Young EW, Delmonico FL, Wynn JJ, Merion RM, Wolfe RA. Donor characteristics associated with reduced graft survival: an approach to expanding the pool of kidney donors. Transplantation. 2002;74:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 635] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 26. | Foss A, Heldal K, Scott H, Foss S, Leivestad T, Jørgensen PF, Scholz T, Midtvedt K. Kidneys from deceased donors more than 75 yr perform acceptably after transplantation. Transplantation. 2009;87:1437-1441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Wang CJ, Wetmore JB, Crary GS, Kasiske BL. The Donor Kidney Biopsy and Its Implications in Predicting Graft Outcomes: A Systematic Review. Am J Transplant. 2015;15:1903-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 121] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Remuzzi G, Cravedi P, Perna A, Dimitrov BD, Turturro M, Locatelli G, Rigotti P, Baldan N, Beatini M, Valente U. Long-term outcome of renal transplantation from older donors. N Engl J Med. 2006;354:343-352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 382] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 29. | Ekser B, Furian L, Broggiato A, Silvestre C, Pierobon ES, Baldan N, Rigotti P. Technical aspects of unilateral dual kidney transplantation from expanded criteria donors: experience of 100 patients. Am J Transplant. 2010;10:2000-2007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 30. | Losappio V, Stallone G, Infante B, Schena A, Rossini M, Maiorano A, Fiorentino M, Ditonno P, Lucarelli G, Battaglia M. A single-center cohort study to define the role of pretransplant biopsy score in the long-term outcome of kidney transplantation. Transplantation. 2014;97:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Doshi MD, Reese PP, Hall IE, Schröppel B, Ficek J, Formica RN, Weng FL, Hasz RD, Thiessen-Philbrook H, Parikh CR. Utility of Applying Quality Assessment Tools for Kidneys With KDPI ≥80. Transplantation. 2017;101:1125-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 32. | Denic A, Mathew J, Lerman LO, Lieske JC, Larson JJ, Alexander MP, Poggio E, Glassock RJ, Rule AD. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N Engl J Med. 2017;376:2349-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 247] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 33. | Heilman RL, Smith ML, Kurian SM, Huskey J, Batra RK, Chakkera HA, Katariya NN, Khamash H, Moss A, Salomon DR. Transplanting Kidneys from Deceased Donors With Severe Acute Kidney Injury. Am J Transplant. 2015;15:2143-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 34. | Johnson LB, Kuo PC, Dafoe DC, Drachenberg CB, Schweitzer EJ, Alfrey EJ, Ridge LA, Salvatierra P, Papadimitriou JC, Mergner WJ. The use of bilateral adult renal allografts - a method to optimize function from donor kidneys with suboptimal nephron mass. Transplantation. 1996;61:1261-1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Hamed MO, Chen Y, Pasea L, Watson CJ, Torpey N, Bradley JA, Pettigrew G, Saeb-Parsy K. Early graft loss after kidney transplantation: risk factors and consequences. Am J Transplant. 2015;15:1632-1643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 36. | Helanterä I, Räihä J, Finne P, Lempinen M. Early failure of kidney transplants in the current era-a national cohort study. Transpl Int. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 37. | Einecke G, Sis B, Reeve J, Mengel M, Campbell PM, Hidalgo LG, Kaplan B, Halloran PF. Antibody-mediated microcirculation injury is the major cause of late kidney transplant failure. Am J Transplant. 2009;9:2520-2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 558] [Cited by in RCA: 560] [Article Influence: 35.0] [Reference Citation Analysis (0)] |