Published online Jun 28, 2018. doi: 10.5500/wjt.v8.i3.75
Peer-review started: January 23, 2018
First decision: February 9, 2018
Revised: February 20, 2018
Accepted: April 1, 2018
Article in press: April 1, 2018
Published online: June 28, 2018
Processing time: 154 Days and 11.8 Hours
To investigate the relationship between post-liver transplantation (LT) glycemic control and LT outcomes.
A qualitative systematic review on relevant prospective interventions designed to control glucose levels including insulin protocols. Studies investigating an association between glycemic control and post-LT outcomes such as mortality, graft rejection, and infection rate were reviewed. PubMed, EMBASE, and other databases were searched through October 2016.
Three thousands, six hundreds and ninety-two patients from 14 studies were included. Higher mortality rate was seen when blood glucose (BG) ≥ 150 mg/dL (P = 0.05). BG ≥ 150 mg/dL also led to higher rates of infection. Higher rates of graft rejection were seen at BG > 200 mg/dL (P < 0.001). Mean BG ≥ 200 mg/dL was associated with more infections (P = 0.002). Nurse-initiated protocols and early screening strategies have shown a reduction in negative post-LT outcomes.
Hyperglycemia in the perioperative period is associated with poor post-LT outcomes. Only a few prospective studies have designed interventions aimed at managing post-LT hyperglycemia, post-transplant diabetes mellitus (PTDM) and their impact on post-LT outcomes.
Core tip: Despite the importance of post-liver transplantation (LT) glycemic control, there are no evidence-based guidelines on how to manage hyperglycemia in the post-LT period. The aim of this qualitative systematic review is to determine potential associations between glucose levels post-LT and outcomes such as mortality, graft rejection, infection rate, and other related post-LT outcomes. In addition, we analyzed methods for targeting glycemic control including specific therapeutic regimens or insulin protocols utilized in LT recipients.
- Citation: Paka P, Lieber SR, Lee RA, Desai CS, Dupuis RE, Barritt AS. Perioperative glucose management and outcomes in liver transplant recipients: A qualitative systematic review. World J Transplant 2018; 8(3): 75-83
- URL: https://www.wjgnet.com/2220-3230/full/v8/i3/75.htm
- DOI: https://dx.doi.org/10.5500/wjt.v8.i3.75
Hyperglycemia after liver transplantation (LT) is a common phenomenon associated with increased risk of allograft rejection[1,2]. Poor glycemic control is also implicated in other post-LT complications including infection[3-5], acute kidney injury[6], new onset diabetes after transplantation (NODAT)[7,8], and malignancy, in addition to complications related to the metabolic syndrome including increased cardiovascular risk[9]. Despite the importance of post-LT glycemic control, there are no evidence-based guidelines on how to manage hyperglycemia in the post-LT period. Moreover, it is unclear what degree of glycemic control is associated with graft failure and complications such as infections. Similarly, predictors for poor glycemic control and NODAT in LT recipients have not been identified, apart from donor graft steatosis[9], post-LT immunosuppression[10-12], steroid use, and hepatitis C virus (HCV) infection[13]. These gaps in our existing knowledge necessitate a review of the literature on glycemic control and perioperative outcomes in LT recipients.
Given the increasing prevalence of non-alcoholic fatty liver disease (NAFLD) and metabolic syndrome, many patients will arrive at transplant with some degree of insulin resistance. Post-LT hyperglycemic management will be essential to improving patient care and outcomes. The incidence of NODAT ranges from 20% to 44% among LT recipients, with rates varying depending on methodology used[8,9,11,14]. The aim of this qualitative systematic review is to analyze methods for targeting glycemic control including specific therapeutic regimens or insulin protocols utilized in LT recipients, and to determine associations between glycemic control and post-LT outcomes such as mortality, graft rejection, or infection rate. To achieve this goal, we reviewed prospective interventions targeting glucose control, as well as retrospective studies that examined the association between glucose control and relevant perioperative transplant outcomes.
Our qualitative systematic review included a priori search criteria of journal articles and conference abstracts among adult (age ≥ 18 years) human orthotopic or living donor LT recipients. Studies were limited to the English language and had to include at least one relevant outcome of interest such as patient survival, graft rejection, infection rate, acute kidney injury, and graft survival. Given the focus on perioperative glucose control, study outcomes were limited to glucose control during the first year post-LT.
A health sciences librarian with clinical input from our study team designed the apriori search strategy. PubMed (https://http://www.ncbi.nlm.nih.gov/pubmed), EMBASE (https://http://www.elsevier.com/solutions/embase-biomedical-research), SCOPUS (https://http://www.scopus.com), Clinical trials.gov (https://clinicaltrials.gov), and WHO ICTRP (http://www.who.int/ictrp/en) using the search terms outlined in Supplementary Table 1. Searches were performed on October 18, 2016 and updated in December 2017. All studies prior to this date were included.
| Ref. | Country | Year | n | Group 1 | Group 2 | Study outcome(s) | Comments |
| Ammori et al[5] | United States | 2007 | 184 | Strict glucose control (BG < 150 mg/dL) | Poor Glucose control (BG ≥ 150 mg/dL) | Mortality Infection rate | |
| Chung et al[25] | South Korea | 2014 | 211 | BG decline during the Neohepatic Phase (Yes) | BG decline during the Neohepatic Phase (No) | Mortality, length of ICU stay, early allograft dysfunction, MELD Score recovery | Outcomes were assessed relative to the drop in hyperglycemia after the neohepatic phase |
| Gelley et al[21] | Hungary | 2011 | 310 | De novo diabetes | Control | HepC recurrence and association with NODAT | |
| Hartog et al[23] | United Kingdom | 2014 | 430 | DBD | DCD | NODAT | |
| Keegan et al[17] | United States | 2010 | 161 (158 were available for analysis) | Pre-protocol | Protocol | Mortality Morbidity Graft function | |
| Linder et al[18] | United States | 2016 | 114 | PTDM | Non-PTDM | PTDM | BPAR, allograft failure, death, CMV infection are additional endpoints |
| Park et al[4] | United States/Taiwan | 2009 | 680 | SSI (Yes) | SSI (No) | SSI | |
| Trail et al[20] | United States | 1996 | 497 | PTDM | Case-control | PTDM morbidity | PTDM leading to infections and graft rejection |
| Wallia et al[1] | United States | 2010 | 144 | BG > 200 mg/dL | BG < 200 mg/dL | Graft rejection, infection, and re-hospitalization | Graft survival and prolonged ventilation |
| Wallia et al[19] | United States | 2011 | 73 | Glucose management service | Non-Glucose Management Service | Graft rejection, infection, and re-hospitalization | Graft survival and prolonged ventilation |
| Yoo et al[6] | South Korea | 2016 | 304 | Normoglycemia (BG: 80-200 mg/dL) | Mild hyperglycemia (BG: 200-250 mg/dL) | AKI | Group 3: Moderate hyperglycemia (250-300 mg/dL) Group 4: Severe hyperglycemia (> 300 mg/dL) |
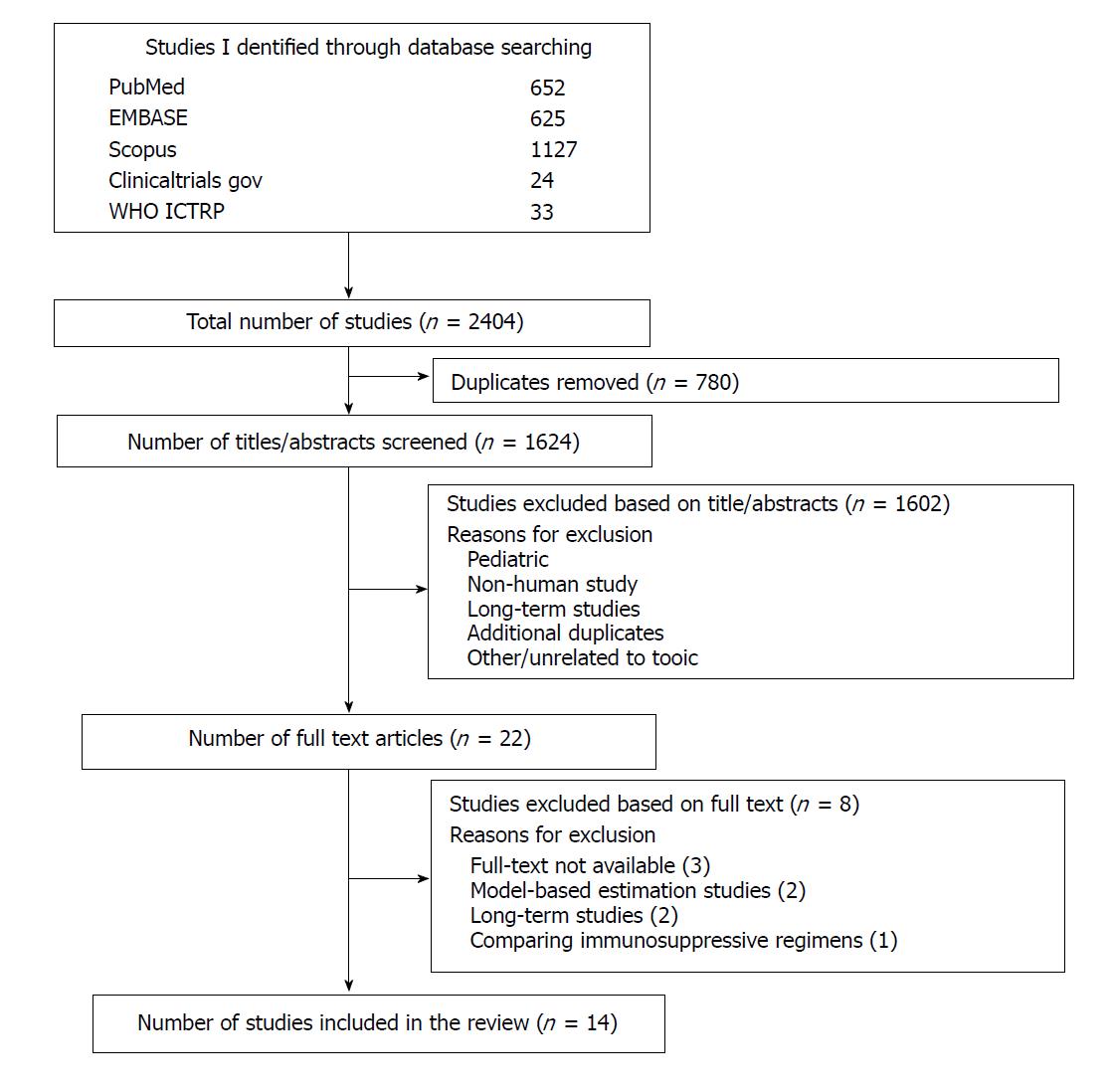
Using the various databases outlined above, our search yielded a total of 1624 results after removing duplicate results. Four reviewers (PP, SRL, RAL, and ASB) independently screened the titles and abstracts of 406 search results each for potential eligibility and a consensus was reached to include a total of 14 studies in the final analysis (Figure 1). Although the search strategy was designed to exclude patients receiving other transplants from this review, some of these studies included patients that received combined liver-kidney transplantation. These patients were included since the results were reported in a composite manner (i.e., data for liver transplantation alone patients vs combined liver-kidney transplantation patients were not reported separately). Overall, the number of liver-kidney transplantation patients was relatively small, and the results were predominantly driven by LT recipients alone.
Four reviewers independently assessed the risk of bias in each study. Selected studies were reviewed based on representativeness of study population, comparability of cohorts, adequate assessment of outcomes, sufficient length of follow-up, adequacy of follow-up, and source of study funding. The prospective randomized study was assessed using the Cochrane risk of bias tool and the Newcastle-Ottawa Scale (NOS) was used for the cohort/case-control studies[15,16].
This qualitative systematic review includes results from 14 full text articles. Of the 1624 records identified electronically, 780 were duplicates and 109 were eligible after abstract review. Of the 109, there were 22 articles that were reviewed and retrieved in full-text form. Of these, 8 were excluded and data from 14 full text articles (11 retrospective studies, 2 prospective studies and 1 cross-sectional study) were found to be eligible and included in this review (Figure 1).
The characteristics of the included studies are shown in Tables 1 and 2. A total of 3692 patients (3077 patients were retrospectively studied; 615 patients were prospectively studied) from 14 studies were included. The studies spanned 20 years from 1996 to 2016 with most occurring in the past decade and included transplants performed in the United States, United Kingdom, Taiwan, Spain, South Korea, and Hungary.
| Ref. | Country | Year | n | Group 1 | Group 2 | Outcome | Comment |
| Alvarez-Sotomayor et al[24] | Spain | 2016 | 344 | Diabetes before transplantation | No diabetes before transplantation | PTDM | Cross-sectional study |
| Villanueva et al[22] | United States | 2005 | 107 | Rosiglitazone | - | PTDM | |
| Welsh et al[28] | United States | 2016 | 164 | Intensive glycemic control | Moderate glycemic control | Hypoglycemia | Insulin requirements |
Supplementary Table 2 shows the risk of bias assessment of all the included trials. Of the 14 studies that were included, 11 were retrospective in nature and carry a potential to be inherently biased. NOS was used to assess risk of bias for the cohort/case-control studies and a modified version of the NOS was used for the single cross-sectional study. The single randomized prospective study, for the most part was deemed to have minimal bias utilizing the Cochrane risk of bias tool[15,16].
Clinical outcomes for each trial are summarized in Table 3. Major outcomes of interest in relation to blood glucose (BG) level include mortality, graft rejection, infection rate, acute kidney injury (AKI), graft survival, post-transplant diabetes mellitus (PTDM), and NODAT.
| Outcome of interest | Important findings | Data sources |
| Mortality | Mean BG ≥ 150 mg/dL increases mortality Nurse initiated insulin protocol did not impact mortality PTDM influenced glucose levels but did not change mortality | Ammori et al[5] (retrospective study) Keegan et al[17] (retrospective study Linder et al[18] (retrospective study) |
| Graft rejection | Mean BG > 200 mg/dL increases risk of rejection Although, mean BG were lower with the use of GMS, it did not lead to lower rate of rejection Conflicting evidence exists relating to the development of PTDM and its relation to rejection | Wallia et al[1] (retrospective study) Wallia et al[19] (retrospective study) Linder et al[18] and Trail et al[20] (retrospective studies) |
| Infection rate | BG ≥ 150 mg/dL is associated with higher infection rate BG ≥ 200 mg/dL increases risk of SSIs Use of GMS led to lower rate of infection Higher BG levels post-LT also led to increased incidence of HCV recurrence No association between BG levels and post-LT CMV infection Development of PTDM did not lead to higher infection rate | Ammori et al[5] (retrospective study) Park et al[27] (retrospective study) Wallia et al[1] (retrospective study) Gelley et al[21] (retrospective study) Linder et al[18] (retrospective study) Trail et al[20] (retrospective study) |
| Post-transplant diabetes mellitus/new onset diabetes mellitus | Rosiglitazone ± sulfonylurea is a potential option for the management of PTDM Post-LT hyperglycemia is associated with the development of PTDM Insulin use was significantly higher in PTDM patients with inadequate BG | Villanueva et al[22] (prospective study) Linder et al[18] (retrospective study) Alvarez-Sotomayor et al[24] (retrospective study) |
| Acute kidney injury and graft survival | High glucose variability is associated with post-LT acute kidney injury No association between post-LT BG levels and graft survival | Yoo et al[6] (retrospective study) Wallia et al[1] and Trail et al[20] (retrospective studies) |
Three studies evaluated the relation between glycemic control and mortality in orthotopic liver transplantation (OLT) patients. Ammori et al[5] found a statistically significant association between glycemic control and mortality. The retrospective review compared patients with strict glycemic control (mean blood glucose < 150 mg/dL) vs those with poor control (mean blood glucose ≥ 150 mg/dL). A total of 184 patients were analyzed (n = 60 for strict control, n = 124 for poorly controlled). The strict control group had a mean glucose of 135 mg/dL while the poorly controlled group had a mean glucose level of 184 mg/dL. Baseline donor and recipient characteristics for both groups were similar with the exception of recipient age (47 ± 2 years vs 53 ± 1 year; strict vs poor control, respectively). The Kaplan Meier survival analysis showed a significantly improved one-year survival rate in the strict glucose control group (91.2%) as compared to that in the poorly controlled group (78.1%). The one-year mortality rate was found to be 8.8% and 21.9% (P = 0.05) for patients in the strict controlled group and poorly glucose control group respectively.
Keegan et al[17] also evaluated the impact of perioperative glycemic control in OLT patients. This retrospective analysis studied the impact of the initiation of a nurse-initiated protocol for glycemic management (protocol group) vs glycemic management prior to the initiation of the protocol (pre-protocol group). Prior to the implementation of the protocol, a variety of insulin infusion protocols and ad hoc sliding scales were used at the discretion of the physician for glycemic control. Under the protocol, a nurse would initiate a continuous intravenous (IV) insulin infusion within 48 h post-OLT if a patient’s BG was greater than 130 mg/dL. The insulin infusion would be titrated as necessary (based on hourly readings) to reach a target BG goal of 80-130 mg/dL. The primary purpose of this quality improvement study was to identify the percentage of all measurements that were in the hypoglycemic (BG < 60 mg/dL) or severely hyperglycemia (BG > 250 mg/dL) range. These measurements were compared between pre-protocol and protocol groups. A total of 158 patients were available for analysis (n = 84 in the pre-protocol group; n = 77 in protocol group). Severe hyperglycemia was observed in 90 of the 581 measurements (15.5%) in the pre-protocol group and 15 of the 539 (2.8%) in the protocol group (OR for protocol group 0.16; CI: 0.09-0.28). Statistical significance, however, was not seen in one-year mortality between the protocol group and the pre-protocol group. Four out of 75 patients (5.3%) died in the protocol group, compared with 5 out of 83 patients (6.0%) in the pre-protocol group (OR for death in the protocol group, 0.89; 95%CI: 0.23-3.42; P = 0.86).
Linder et al[18] evaluated the insulin burden between liver transplant patients that developed PTDM vs patients that did not. BG levels between these two groups were reported as well as mortality rates. A total of 114 patients were retrospectively analyzed and while postoperative BG levels were similar in the ICU setting between the two groups, a statistically significant difference in floor (non-ICU) average BG levels (mg/dL) was seen between patients that developed PTDM and those that did not (184.7 ± 31.5 and 169.3 ± 31.4 respectively, P = 0.013). Statistically significant differences in one-month average BG levels were also seen-176.0 ± 31.1 for the PTDM group and 160.6 ± 28.0 for the non-PTDM group (P = 0.007). However, there was no significant difference in one-year mortality in the PTDM and non-PTDM groups.
Four studies examined the association between glycemic levels and graft rejection in liver transplant recipients. In a retrospective analysis conducted by Wallia et al[1] (n = 144), there was a statistically significant association between glucose level and graft rejection. Higher rates of rejection were seen in patients with a mean BG level > 200 mg/dL compared to those that had a mean BG level < 200 mg/dL (76.7% and 35.1% respectively; P < 0.001). A retrospective subgroup analysis by Wallia et al[19] (n = 73) studied the effect of a glucose management service (GMS) on blood glucose levels and its impact on clinical outcomes including graft rejection. The GMS consisted of a group of nurse practitioners supervised by an endocrinologist responsible for managing BG. The BG levels were managed by the primary transplant team in the non-GMS group. The mean inpatient BG level during the peri-transplant period was 189.0 ± 45.0 in the non-GMS group and 157.9 ± 32.3 in the GMS group (statistical significance data not provided). Although, patients in the non-GMS group had higher BG levels, hyperglycemia did not lead to higher rates of graft rejection (45% in the non-GMS group vs 29% in the GMS group, P = 0.156).
In the previously described retrospective analysis by Linder et al[18], biopsy-proven acute rejection (BPAR) was also studied as an outcome and there was a statistically higher incidence in PTDM vs non-PTDM patients (41.7% vs 24.2% respectively, P = 0.048). Similarly, a retrospective study by Trail et al[20] (n = 497), studied morbidity, including graft rejection, in DM patients after LT compared with matched control patients. Mean fasting blood glucose for patients with PTDM was 122.3 ± 5.0 mg/dL compared to 101.9 ± 3.9 mg/dL for the matched control patients (P < 0.01). Despite the statistically significant difference in glycemic levels between the PTDM group and matched control group, the number of rejection episodes was similar between the two groups, i.e., rates of rejection were not significantly different between groups.
Six retrospective studies evaluated the association between glucose levels and infection. Park et al[4] studied the association between intraoperative hyperglycemia and surgical site infection (SSI) postoperatively in a retrospective study (n = 680). Of the 680 patients, 76 (11.2%) experienced SSI after LT. Severe hyperglycemia (defined as mean BG ≥ 200 mg/dL) was seen in 37.8% of the 76 patients with SSIs compared to only 21.9% of the 604 non-SSI patients (P = 0.002) suggesting an association between the occurrence of SSIs and mean BG levels ≥ 200 mg/dL. Similarly, In the study by Ammori et al[5], infectious complications when assessed 30 d post-LT were significantly associated with worse glucose control-among the strict glucose control group (mean BG < 150 mg/dL), there were 60 (30%) post-LT infections, compared to 124 (48%) infections in the poor glucose control group (mean BG ≥ 150 mg/dL) (P = 0.02). The retrospective subgroup analysis by Wallia et al[19] found that the patients in the non-GMS group with higher BG levels exhibited higher rate of infection compared to the patients in the GMS group at one-year post-LT follow up (79% vs 51% respectively, P = 0.015). Gelley at al[21] found that higher early postoperative fasting plasma glucose led to higher incidence of HCV recurrence (diagnosed with histology criteria of the Knodell score), although no data was shown with regards to BG levels.
In contrast to the above studies, Linder et al[18] showed no association between glycemic level and post-LT CMV infection (patients with PTDM had higher BG levels compared to non-PTDM patients). Similarly, Trail et al[20] also showed no significant difference in infectious rates between patients with PTDM and those without PTDM. This study also evaluated the severity of infection as well as the type of infection and no differences were seen between the two groups.
Villanueva and Baldwin evaluated the use of Rosiglitazone (ROSI) therapy for patients with PTDM. DM was diagnosed according to the American Diabetes Association (ADA) criteria (symptoms of hyperglycemia with post-prandial BG ≥ 200 mg/dL, or fasting BG ≥ 126 mg/dL on two separate occasions). The study followed 40 patients that developed PTDM that were initially stabilized by twice-daily NPH and regular insulin. These patients were subsequently started on ROSI 4 mg/d with the treatment goal to discontinue insulin while maintaining a target goal of HBA1c ≤ 6.5%. Thirty of the patients that were initially treated with insulin were able to discontinue insulin within 3-4 mo. Three patients required chronic insulin therapy despite ROSI ± a sulfonylurea, and were considered insulin dependent. ROSI monotherapy was sufficient in 12 patients (30%), whereas 25 patients (62.5%) required ROSI + sulfonylurea to maintain insulin independence and normoglycemia. ROSI was continued at 4 mg/d in 25 patients while 15 patients required an increase to 8 mg/d. PTDM patients treated with ROSI maintained a mean HBA1C of 5.6% ± 0.8 (target BG levels were < 100 mg/dL for fasting glucose and < 140 mg/dL for post prandial glucose). A commonly seen side effect among patients treated with ROSI was edema (13%). These data suggest ROSI ± sulfonylurea may be a potential intervention that can reduce insulin burden in patients with PTDM[22].
Linder et al[18] also showed that patients who developed PTDM had significantly higher BG levels (1-mo average BG) suggesting post-LT hyperglycemia could play a role in the development of PTDM. Multivariate analysis for predictors of PTDM showed the use of Basiliximab was a negative independent predictor [AOR 0.182 (0.040-0.836), P = 0.03] and rejection was a positive independent predictor [AOR 3.237 (1.214-8.633), P = 0.019] for the development of PTDM. Hartog et al[23] demonstrated that pulse high-dose steroids was an independent predictor of NODAT [OR 3.1 (1.7-5.6), P = 0.001]. In addition, this study also demonstrated donor graft type was associated with early occurrence of NODAT (within 15 d post-LT). Multivariate analysis showed donation after cardiac death (DCD) graft type was associated with significantly early occurrence of NODAT compared to donation after brain death (DBD) graft type [OR 6.5 (2.3-18.4), P = 0.001].
In addition to the previously mentioned outcomes, PTDM has also been associated with higher insulin use in post-LT patients. A cross-sectional study by Alvarez-Sotomayor et al[24] evaluated 344 patients of whom 141 patients experienced PTDM (157 total but 16 patients did not have HbA1c readings prior to enrollment). Patients with PTDM who had adequate glycemic control (defined as HbA1c < 7%), were significantly less dependent on insulin (39.4%) compared to patients with inadequate glycemic control (80.8%) (OR 6.6, 95%CI: 1.8-24.6, P < 0.001). Finally, Chung et al[25] found male sex, emergency surgery, surgical time (≤ 9 h), and serum lactate (> 5 mmol/L) to be independent predictors for refractive hyperglycemia (RH), however, most post-LT outcomes were not significant in relation to RH.
Other outcomes of interest including AKI, graft survival, and complications related to hospitalization were not studied extensively. Three studies evaluated graft survival and no statistically significant association was seen between post-LT glycemic control and graft survival[1,19,20]. Similarly, no association was seen between BG levels and re-hospitalizations[1,19]. A study by Yoo et al[6] demonstrated no association between hyperglycemia and AKI in LT recipients; however, patients with greater glucose variability, as defined by the SD of blood glucose levels, more commonly presented with AKI (P = 0.019). Using SD as a surrogate marker for glucose variability, patients were divided into quartiles according to the SD of intraoperative and postoperative (initial 48 h of ICU admission) blood glucose levels. Patients with the lowest SD were assigned to the first quartile, ranging to those with the highest SD who were assigned to the fourth quartile. Glucose variability was significantly associated with AKI among patients in the third quartile (23.3% of patients with no AKI vs 30.3% with AKI, OR 2.47, CI: 1.22-5.00, P = 0.012) and fourth quartile (22.1% with no AKI and 31.1% with AKI, OR 2.16, CI: 1.05-4.42, P = 0.035).
This qualitative systematic review of 14 studies examined post-LT glucose control, interventions designed to target glucose control, and associations with post-LT outcomes including infection rate, PTDM, AKI, graft survival and mortality. Ultimately, this review concludes that perioperative hyperglycemia leads to unfavorable post-LT outcomes; however, the degree to which it plays a role may depend on the specific outcome in question. There is strong evidence to support an association between perioperative hyperglycemia and post-LT outcomes such as high infection rate and graft rejection[1,4,5,18-20,26]. A review by Park et al[27] that focused specifically on intraoperative hyperglycemia found a similar association between hyperglycemia and infection rate. In contrast, the strength of the evidence that exists to support an association between perioperative hyperglycemia and outcomes such as mortality and graft survival is not as well founded[1,5,17-20]. High glucose variability may also be a factor with the development of certain complications such as AKI[6]. In addition, donor graft type (DCD vs DBD) may also play a role in the early occurrence of NODAT (within 15 d post-LT)[23].
What was difficult to discern from these studies was the target BG level associated with poor post-LT outcomes. The studies in this review used different target BG levels to evaluate different outcomes, thus making it difficult to associate the degree of glycemic control with certain outcomes and also limiting comparisons that could be made between studies. The studies also varied in their definition of PTDM, the timing of glucose monitoring (immediate post-operative to days post-LT), and the medications used to manage hyperglycemia (ranging from insulin infusion to oral meds). The variability in the studies is what limits the comparisons that can be made and is the reason we can only perform a qualitative review of the literature. Additionally, most of the studies were retrospective observational studies and were not designed to study the specific association between hyperglycemia and post-LT outcomes. Finally, there were some studies that included a small number of combined liver-kidney transplant recipients and the results were reported in a composite manner, thereby making it difficult to detect LT-specific associations between glucose control and post-LT outcomes.
In this review, all of the relevant literature regarding glucose control and post-LT outcomes was compiled systematically using an apriori search strategy of the major medical literature databases. The data were compiled in a qualitative, descriptive manner due to the heterogeneity among research strategies and outcomes that exist in published literature
The conclusions from this review have robust implications for clinical practice. It is imperative to monitor glucose control pre- and post-LT. Along with hyperglycemia, it is also important to consider complications associated with strict glycemic control such as hypoglycemia and high insulin burden when deciding specific BG levels to target. Welsh et al[28] demonstrated the impact of hypoglycemia (defined as glucose ≤ 70 mg/dL) pertaining to intensive and moderate glycemic control in post-LT patients. There were a higher number of hypoglycemic patients in the intensive group and these hypoglycemic patients had significantly higher peak insulin drip rates, higher peak insulin glargine, and more importantly had significantly longer hospital stay. Therefore, it is crucial to target perioperative BG levels within a range that would limit complications associated with both hyper- and hypoglycemia. A reasonable target, based on our findings would be a range between 120 mg/dL to 150 mg/dL, given that BG ≥ 150 mg/dL were associated with negative post-LT outcomes. In addition, interventions through nurse-initiated glucose management protocols to achieve specific target BG levels, early screening to identify patients at high-risk for PTDM, and use of oral agents for management of PTDM seem to be a promising approaches to minimize post-LT outcomes[17,22,24]. Although not discussed extensively in this review, optimizing immunosuppression regimens may also play an important role as noted by the potential association between basiliximab and pulse steroids with PTDM[18,23]. Song et al[29] conducted a retrospective study in China and demonstrated that lower exposure of tacrolimus (measured by mean tacrolimus concentration at 6 mo) was associated with less risk of developing NODAT and its related complications. This suggested that not only optimizing the regimen important but also the dosing of immunosuppressive drugs utilized in the regimen need to be optimized. Such recommendations would be strengthened by prospective randomized data and thus highlights the need for further study in this area.
The need for close monitoring of glucose levels post-LT will become even more important in the future. More patients with insulin resistance will come to transplant in the coming years. NAFLD is the fastest growing indication for transplant and will become the leading indication over the next decade[30,31]. The change in disease etiology may also be accompanied by donor grafts from older patients with DM and obesity that may be more susceptible to poor outcomes from hyperglycemic stressors[32]. As NAFLD increases prevalence, the transplant community will see more NAFLD among both living donors as well. A reliable assessment of hepatic steatosis is of paramount importance for living donor selection as significant steatosis can impact the postoperative outcomes of recipients and safety of the donor[33]. Because of these challenges, the focus could be on developing and establishing a standardized protocol for the monitoring of blood glucose levels. The frequency of test like hemoglobin A1c, glucose tolerance test, and use of tools such as continuous glucose monitoring should be further explored.
Prospective clinical studies need to further examine the impact of perioperative glycemic control in LT recipients with specific attention to the outcomes listed above. An ideal target range for BG levels needs to be determined and specifically investigated in terms of reducing negative outcomes associated with both hypo- and hyperglycemia, as well as adverse events related to post-LT complications due to impaired glucose control.
There are no standard guidelines to properly manage hyperglycemia in the perioperative period of liver transplantation.
Understanding the importance of blood glucose level and proper strategies to manage post-liver transplantation hyperglycemia could help reduce adverse outcomes
The primary objective was to identify an ideal blood glucose level to achieve in the perioperative period for patients undergoing liver transplantation. In addition, exploring treatment regimens to achieve the target blood glucose can help identify better strategies for the management of these patients in the future.
This is a qualitative systematic review that utilized key search terms to find studies on PubMed and other common databases. The search terms were in relation to liver transplantation and blood glucose level management in the perioperative period.
A total of 14 studies fit the criteria to properly study the objectives. The findings from this qualitative review suggests that blood glucose levels greater than or equal to 150 mg/dL in the perioperative period generally leads to negative post-liver transplantation outcomes. Specifically, there was an increased risk of infections, graft rejection, PTDM, and mortality. Graft survival was not impacted by hyperglycemia and there was an increased risk of acute kidney injury with high glucose variability in the perioperative period.
The findings from the compiled studies in this review suggest a blood glucose level between 120 mg/dL and 150 mg/dL could potentially be an ideal target to manage hyperglycemia post-liver transplantation. In addition, early screening, use of oral agents, and utilizing resources such as a glucose management service could be potential strategies to limit adverse outcomes post-transplantation.
Future studies can validate the findings from this review through a prospective study while implementing some of the strategies discussed in this review to minimize post-liver transplantation outcomes.
The authors would like to thank Rachael Posey of The UNC Health Sciences Library for her assistance in designing the search strategy.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Sanal MG, Tarantino G S- Editor: Wang XJ L- Editor: A E- Editor: Tan WW
| 1. | Wallia A, Parikh ND, Molitch ME, Mahler E, Tian L, Huang JJ, Levitsky J. Posttransplant hyperglycemia is associated with increased risk of liver allograft rejection. Transplantation. 2010;89:222-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Marvin MR, Morton V. Glycemic control and organ transplantation. J Diabetes Sci Technol. 2009;3:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Nieto-Rodriguez JA, Kusne S, Mañez R, Irish W, Linden P, Magnone M, Wing EJ, Fung JJ, Starzl TE. Factors associated with the development of candidemia and candidemia-related death among liver transplant recipients. Ann Surg. 1996;223:70-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Park C, Hsu C, Neelakanta G, Nourmand H, Braunfeld M, Wray C, Steadman RH, Hu KQ, Cheng RT, Xia VW. Severe intraoperative hyperglycemia is independently associated with surgical site infection after liver transplantation. Transplantation. 2009;87:1031-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 5. | Ammori JB, Sigakis M, Englesbe MJ, O’Reilly M, Pelletier SJ. Effect of intraoperative hyperglycemia during liver transplantation. J Surg Res. 2007;140:227-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Yoo S, Lee HJ, Lee H, Ryu HG. Association Between Perioperative Hyperglycemia or Glucose Variability and Postoperative Acute Kidney Injury After Liver Transplantation: A Retrospective Observational Study. Anesth Analg. 2017;124:35-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Galindo RJ, Wallia A. Hyperglycemia and Diabetes Mellitus Following Organ Transplantation. Curr Diab Rep. 2016;16:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 8. | Wallia A, Illuri V, Molitch ME. Diabetes Care After Transplant: Definitions, Risk Factors, and Clinical Management. Med Clin North Am. 2016;100:535-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Lv C, Zhang Y, Chen X, Huang X, Xue M, Sun Q, Wang T, Liang J, He S, Gao J. New-onset diabetes after liver transplantation and its impact on complications and patient survival. J Diabetes. 2015;7:881-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 10. | Lane JT, Dagogo-Jack S. Approach to the patient with new-onset diabetes after transplant (NODAT). J Clin Endocrinol Metab. 2011;96:3289-3297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Gosmanov AR, Dagogo-Jack S. Predicting, managing and preventing new-onset diabetes after transplantation. Minerva Endocrinol. 2012;37:233-246. [PubMed] |
| 12. | Driscoll CJ. Risk factors for posttransplant diabetes mellitus: a review of the literature. Prog Transplant. 2007;17:295-300; quiz 301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Räkel A, Karelis AD. New-onset diabetes after transplantation: risk factors and clinical impact. Diabetes Metab. 2011;37:1-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 14. | First MR, Dhadda S, Croy R, Holman J, Fitzsimmons WE. New-onset diabetes after transplantation (NODAT): an evaluation of definitions in clinical trials. Transplantation. 2013;96:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 15. | Higgins JPT, Green S (eds). Cochrane Handbook for System-atic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration. 2011; Available from: http://methods.cochrane.org/bias/. |
| 16. | Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2013; Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 17. | Keegan MT, Vrchota JM, Haala PM, Timm JV. Safety and effectiveness of intensive insulin protocol use in post-operative liver transplant recipients. Transplant Proc. 2010;42:2617-2624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Linder KE, Baker WL, Rochon C, May ST, Sheiner PA, Martin ST. Evaluation of Posttransplantation Diabetes Mellitus After Liver Transplantation: Assessment of Insulin Administration as a Risk Factor. Ann Pharmacother. 2016;50:369-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Wallia A, Parikh ND, O’Shea-Mahler E, Schmidt K, DeSantis AJ, Tian L, Levitsky J, Molitch ME. Glycemic control by a glucose management service and infection rates after liver transplantation. Endocr Pract. 2011;17:546-551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Trail KC, McCashland TM, Larsen JL, Heffron TG, Stratta RJ, Langnas AN, Fox IJ, Zetterman RK, Donovan JP, Sorrell MF. Morbidity in patients with posttransplant diabetes mellitus following orthotopic liver transplantation. Liver Transpl Surg. 1996;2:276-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 21. | Gelley F, Zadori G, Firneisz G, Wagner L, Fehervári I, Gerlei Z, Fazakas J, Papai S, Lengyel G, Sarvary E. Relationship between hepatitis C virus recurrence and de novo diabetes after liver transplantation: the Hungarian experience. Transplant Proc. 2011;43:1281-1282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Villanueva G, Baldwin D. Rosiglitazone therapy of posttransplant diabetes mellitus. Transplantation. 2005;80:1402-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 23. | Hartog H, May CJ, Corbett C, Phillips A, Tomlinson JW, Mergental H, Isaac J, Bramhall S, Mirza DF, Muiesan P. Early occurrence of new-onset diabetes after transplantation is related to type of liver graft and warm ischaemic injury. Liver Int. 2015;35:1739-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Alvarez-Sotomayor D, Satorres C, Rodríguez-Medina B, Herrero I, de la Mata M, Serrano T, Rodríguez-Perálvarez M, D’Avola D, Lorente S, Rubín A. Controlling Diabetes After Liver Transplantation: Room for Improvement. Transplantation. 2016;100:e66-e73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Chung HS, Lee S, Kwon SJ, Park CS. Perioperative predictors for refractory hyperglycemia during the neohepatic phase of liver transplantation. Transplant Proc. 2014;46:3474-3480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Morbitzer KA, Taber DJ, Pilch NA, Meadows HB, Fleming JN, Bratton CF, McGillicuddy JW, Baliga PK, Chavin KD. The impact of diabetes mellitus and glycemic control on clinical outcomes following liver transplant for hepatitis C. Clin Transplant. 2014;28:862-868. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Park CS. Predictive roles of intraoperative blood glucose for post-transplant outcomes in liver transplantation. World J Gastroenterol. 2015;21:6835-6841. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 28. | Welsh N, Derby T, Gupta S, Fulkerson C, Oakes DJ, Schmidt K, Parikh ND, Norvell JP, Levitsky J, Rademaker A. Inpatient hypoglycemic events in a comparative effectiveness trial for glycemic control in a high-risk population. Endocr Pract. 2016;22:1040-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 29. | Song JL, Gao W, Zhong Y, Yan LN, Yang JY, Wen TF, Li B, Wang WT, Wu H, Xu MQ. Minimizing tacrolimus decreases the risk of new-onset diabetes mellitus after liver transplantation. World J Gastroenterol. 2016;22:2133-2141. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 22] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 30. | Yi Z, Mayorga ME, Orman ES, Wheeler SB, Hayashi PH, Barritt AS Th. Trends in Characteristics of Patients Listed for Liver Transplantation Will Lead to Higher Rates of Waitlist Removal Due to Clinical Deterioration. Transplantation. 2017;101:2368-2374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Pais R, Barritt AS 4th, Calmus Y, Scatton O, Runge T, Lebray P, Poynard T, Ratziu V, Conti F. NAFLD and liver transplantation: Current burden and expected challenges. J Hepatol. 2016;65:1245-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 330] [Article Influence: 36.7] [Reference Citation Analysis (1)] |
| 32. | Orman ES, Mayorga ME, Wheeler SB, Townsley RM, Toro-Diaz HH, Hayashi PH, Barritt AS 4th. Declining liver graft quality threatens the future of liver transplantation in the United States. Liver Transpl. 2015;21:1040-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 104] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 33. | Kim JM, Ha SY, Joh JW, Sinn DH, Jeong WK, Choi GS, Gwak GY, Kwon CH, Kim YK, Paik YH. Predicting Hepatic Steatosis in Living Liver Donors via Noninvasive Methods. Medicine (Baltimore). 2016;95:e2718. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |