Published online Dec 24, 2017. doi: 10.5500/wjt.v7.i6.359
Peer-review started: July 20, 2017
First decision: September 5, 2017
Revised: November 30, 2017
Accepted: December 5, 2017
Article in press: December 6, 2017
Published online: December 24, 2017
Processing time: 160 Days and 16.1 Hours
Biliary mucoceles after deceased donor liver transplantation are a rarity, and mucoceles mimicking a gallbladder from the recipient remnant cystic duct have not been described until this case. We describe a 48-year-old male who presented with right upper quadrant pain and was found to have a recipient cystic duct mucocele 3 mo after receiving a deceased donor liver transplant. We describe the clinical presentation, laboratory and imaging findings (including the appearance of a gallbladder), multidisciplinary approach and surgical resolution of this mucocele originating from the recipient cystic duct, and a review of the literature.
Core tip: Biliary mucoceles after deceased donor liver transplantation are a rarity, and mucoceles mimicking a gallbladder from the recipient remnant cystic duct have not been described until this case. We describe a 48-year-old male who presented with right upper quadrant pain and was found to have a recipient cystic duct mucocele 3 mo after receiving a deceased donor liver transplant. We describe the clinical presentation, laboratory and imaging findings (including the appearance of a gallbladder), multidisciplinary approach and surgical resolution of this mucocele originating from the recipient cystic duct, and a review of the literature.
- Citation: Chaly T, Campsen J, O’Hara R, Hardman R, Gallegos-Orozco JF, Thiesset H, Kim RD. Mucocele mimicking a gallbladder in a transplanted liver: A case report and review of the literature. World J Transplant 2017; 7(6): 359-363
- URL: https://www.wjgnet.com/2220-3230/full/v7/i6/359.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i6.359
Orthotopic deceased donor liver transplantation has become the standard of care for patients with end stage liver disease secondary to hepatitis C virus (HCV) and hepatocellular cancer (HCC). The standard biliary anastomosis is a duct-to-duct anastomosis performed in either a running or interrupted fashion using absorbable suture. In addition, a donor cholecystectomy is routinely performed after reperfusion and in instances of a long recipient cystic duct, this duct is either opened or over sewn. We present a case of a 48-year-old male who underwent an uncomplicated deceased donor liver transplant and was found to have a mucocele which mimicked a gallbladder 3 mo post-operatively. A biliary mucocele is a complication after deceased donor liver transplantation that has not been well described in the literature. Furthermore, mucoceles from the recipient vs donor remnant cystic duct have not yet been described. Such a biliary abnormality can be difficult for the surgeon to both diagnose and treat. It can be extremely difficult to make the diagnosis of a recipient duct vs a donor duct mucocele preoperatively even with excellent imaging.
A 48-year-old man with end stage liver disease secondary to HCV and HCC received a liver transplant from a deceased donor, and per routine, a graft cholecystectomy was performed and confirmed by pathology. The donor cystic duct was ligated with a 2-0 silk tie, and the recipient cystic duct was over sewn with a 4-0 proline suture. The patient’s post-operative course was unremarkable, and the patient was subsequently discharged on post-operative day 8. The patient did well in the weeks after the transplant with his liver functions tests (LFTs) indicating excellent graft function.
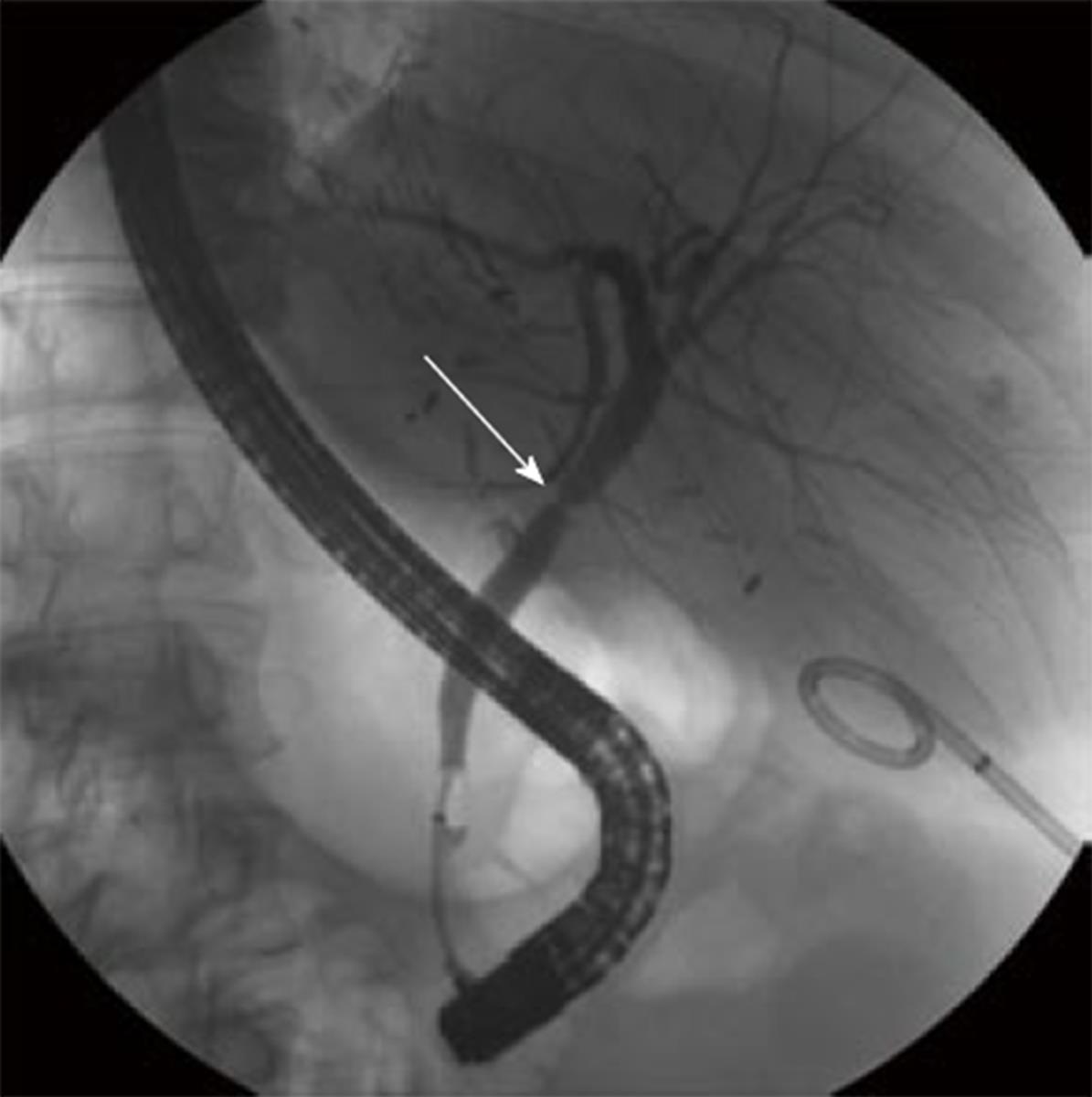
Three months after the transplant, the patient complained of right upper quadrant pain. He was found to have abnormal LFTs, specifically an elevated gamma-glutamyltransferase (GGT), alkaline phosphatase (alk phos), and total bilirubin (T.bili). A liver biopsy showed no signs of rejection or recurrent HCV. A magnetic resonance cholangiopancreatography (MRCP) showed an anastomotic biliary stricture. An endoscopic retrograde cholangiopancreatography (ERCP) demonstrated an anastomotic stricture, a covered stent was placed, and the biliary duct then displayed free flowing contrast through the biliary system (Figure 1). The alk phos, GGT and T.bili normalized.
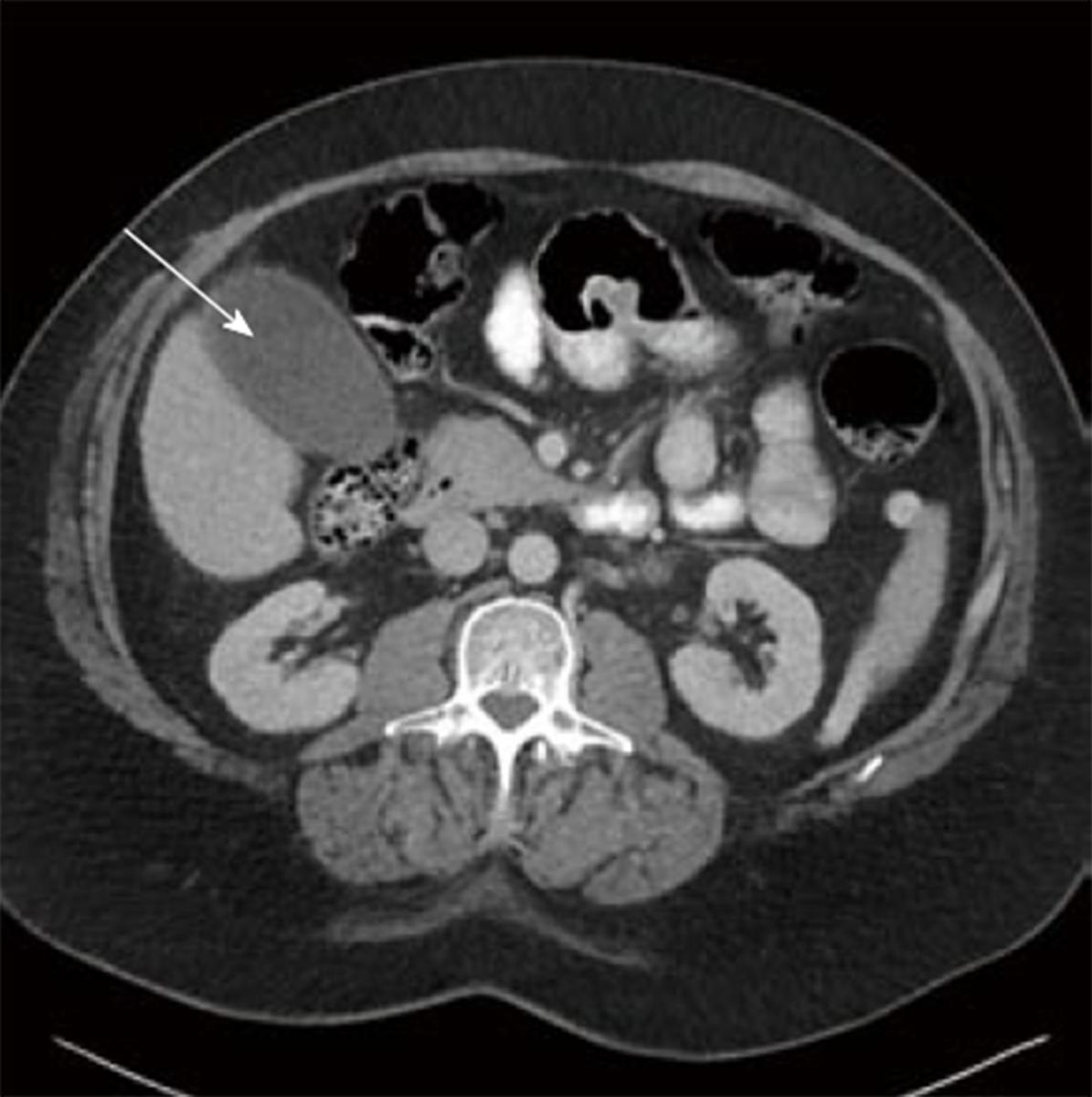
In the 4 wk following the procedures, the patient complained of persistent right upper quadrant pain with associated nausea, vomiting, and intermittent elevated temperatures. A CT scan showed what appeared to be a fluid collection mimicking a gallbladder (Figure 2). The suspected collection was presumed to be either a hematoma or biloma. Given the patients clinical symptoms of right upper quadrant pain, nausea, and vomiting, it was decided to place a drain in the collection via interventional radiology.
Following drain placement, bile tinged fluid was extracted, and a biloma was presumed. The patient was asymptomatic following drain placement, and his liver graft function was normal. The patient was seen for a follow up visit in clinic, during which the drain exhibited signs of minimal output and was subsequently removed.
Three weeks following drain removal the patient had a recurrence of his symptoms. His right upper quadrant pain was now increased in caliber, which forced in-patient admission. The patient clinically appeared to have cholangitis. On repeat imaging, the collection had now re-appeared inferior to the liver. A drain was once again placed and had evidence of purulence and bile tinged fluid. In addition to the interventional radiology procedure, the patient had an ERCP for stent removal, which showed resolution of his previous biliary stricture.
A diagnosis of cystic duct remnant mucocele was made given its appearance and recurrence, and ablation with sclerosing agents (ETOH) was attempted by interventional radiology on three separate occasions but was of little benefit. The patient continued to have recurrent symptoms with drain removal and a re-appearance of the mucocele.
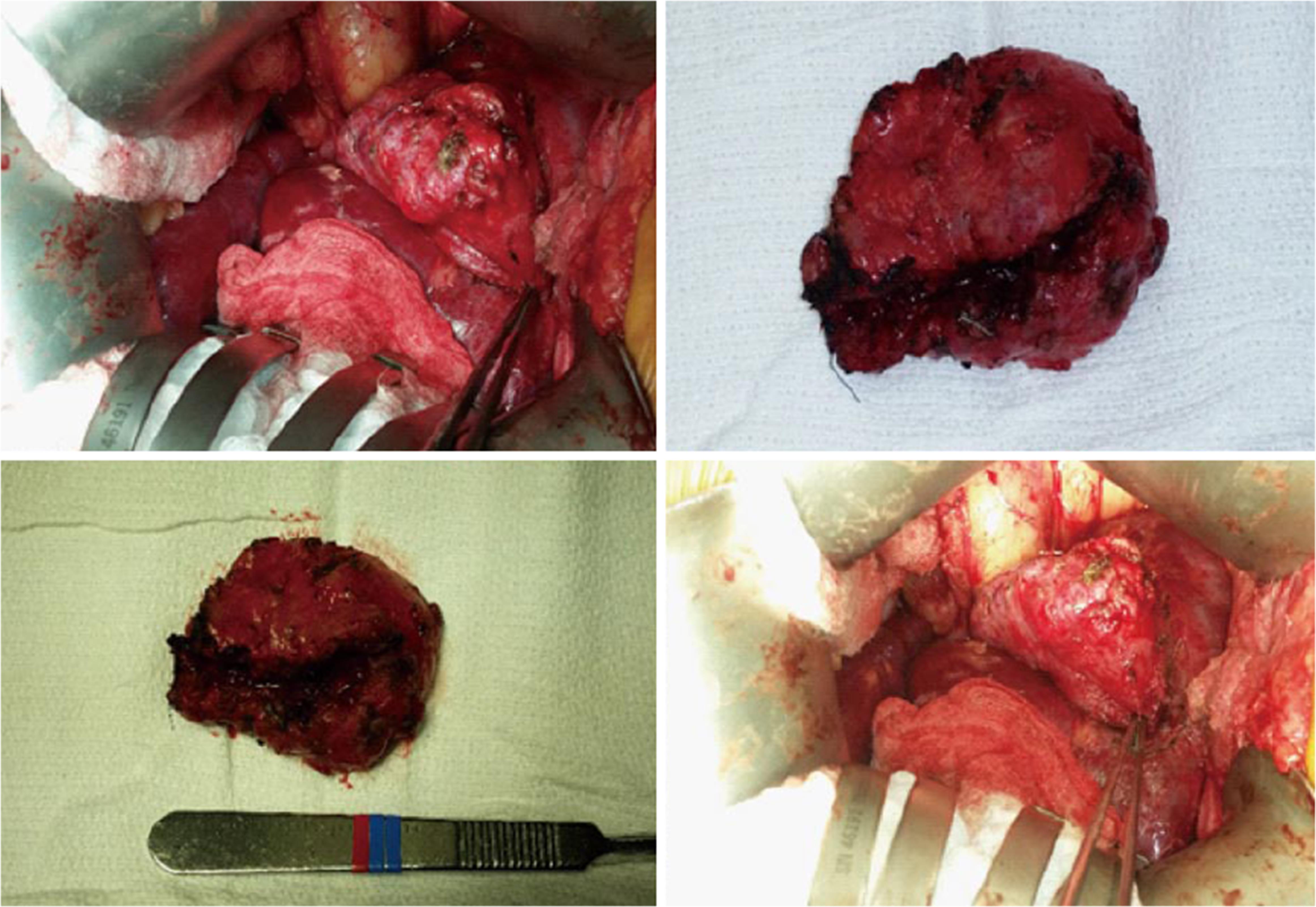
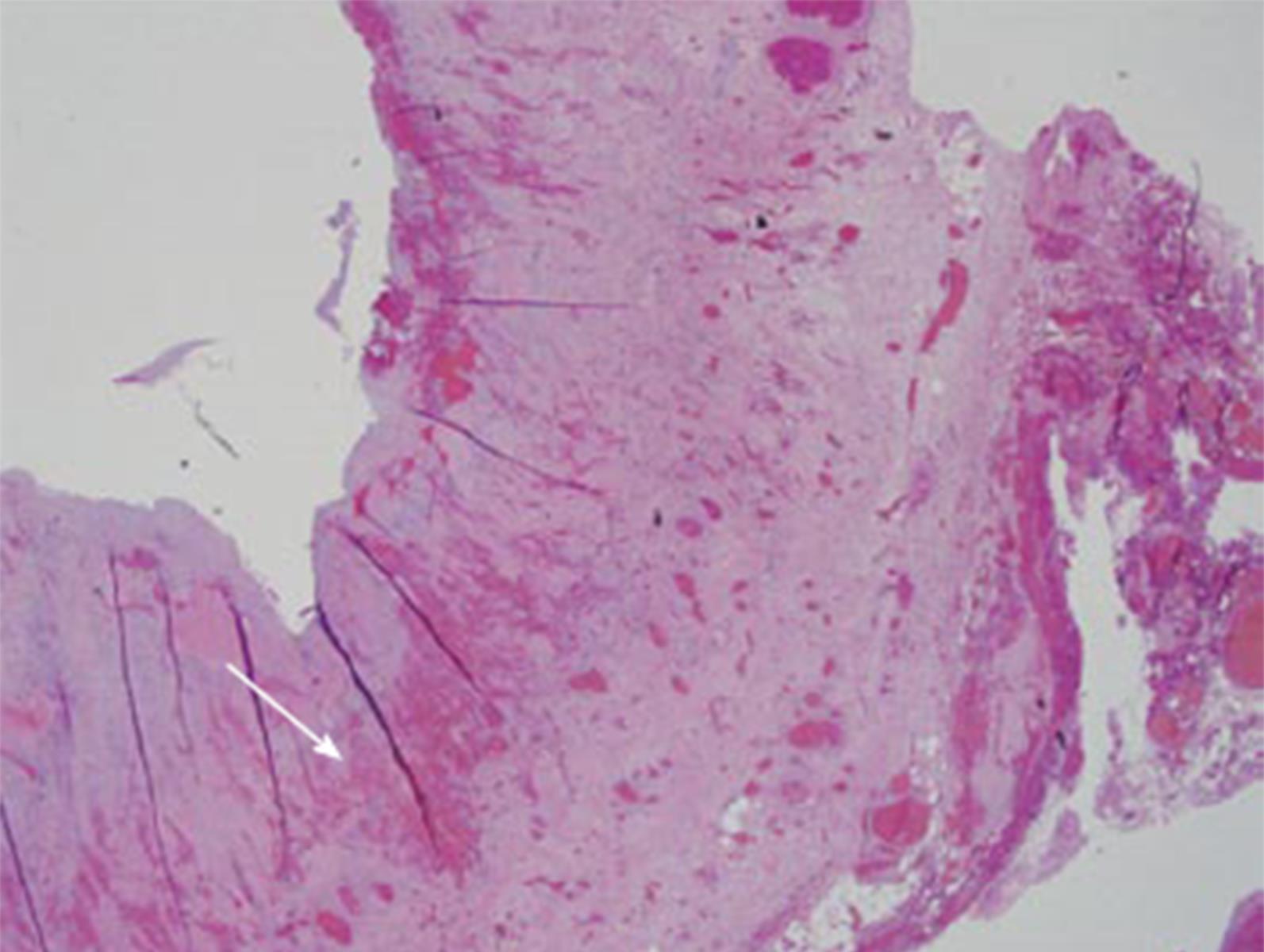
After repeated attempts at minimally invasive techniques, 12 mo after transplant, the patient underwent an exploratory laparotomy. Abdominal exploration revealed a mucocele originating from the remnant of the recipient’s cystic duct (Figure 3). This was subsequently surgically excised, and the defect was closed with proline suture. The patient did well after the operation with complete resolution of his symptoms. Furthermore, the patient’s liver graft function remained excellent, with normalization of his alk phos, GGT and T.bili. The final pathology report revealed a cystic duct mucocele with signs of cholangitis and scarring. The mucosal wall had biliary mucosa (Figure 4).
Orthotopic liver transplant is an accepted therapy for patients with end stage liver disease. Technical complications remain a significant cause of morbidity and mortality with biliary complications accounting for approximately 13% to 19% of the morbidities. Anastomotic biliary strictures and bile leaks account for most of these[1,2].
It is well known that the junction of the cystic and common hepatic ducts can be variable. In approximately 20% of cases, the cystic duct descends for a considerable distance, either along the right side of, or posterior to, the common duct before joining the common duct[2-4].
The presence of a recipient cystic duct mucocele after deceased donor liver transplant is a very rare anatomical complication. The reported cases in the literature are few, and they all describe instances in which the donor cystic duct remained long to avoid damage to the donor common bile duct with resultant cystic duct mucocele. Chaterjee et al[5] describe a case in which there was high ligation of the donor cystic duct and incorporation of the cystic duct into the anastomosis to facilitate drainage but resulted in a donor duct mucocele nonetheless. Caputo et al[2] describe 13 patients in a 13-year history of 283 liver transplants, who developed donor duct mucoceles without encountering a recipient duct mucocele. Most of the reported cases demonstrate a mucocele derived from the donor anatomy as opposed to the recipient anatomy as described here[6-8]. In our case, it is believed that the recipient cystic duct was not adequately oversewn or ligated at the time of the initial liver transplantation. It is also possible that the ERCP stent had inappropriately obstructed the recipient cystic duct causing this mucocele; however, once removed, the mucocele persisted, making this unlikely.
The diagnosis of a mucocele, whether recipient or donor in origin, can be difficult but a rigorous diagnostic evaluation can aid in the diagnosis. With elevated liver enzymes after liver transplantation one must first exclude a vascular insult such as hepatic artery thrombosis or portal vein thrombosis, and an ultrasound with doppler of the liver transplant is necessary. In addition, an ultrasound may provide information on the biliary tree and whether there is intrahepatic biliary dilation indicating a possible biliary stricture. With a normal ultrasound examination, a liver biopsy is an appropriate next step to rule out rejection. With vascular compromise and rejection ruled out, the pathway may proceed in a variety of ways. A cholescintigraphy (HIDA) scan will provide the clinician with information in regard to the uptake and excretion of bile through the intrahepatic and extrahepatic biliary system. In rare instances, it may also reveal a possible mucocele. With a normal HIDA scan, a CT scan of the abdomen will give a better understanding of the hilar anatomy and assist in diagnosing any fluid collections, bilomas, or potential mucoceles. In addition, an MRCP can also aid in this diagnosis and exclude any biliary strictures. Given the condensed area of the hepatic hilum, it is difficult to distinguish the actual origin of the mucocele. If the mucocele were small and/or compressing the common duct, similar to Mirizzi’s syndrome, this would point to the diagnosis of a donor duct mucocele. If there was no compression on the common duct and scans gave the impression of a remnant gall bladder, this may point to a recipient duct origin. From a clinical standpoint, however, the constellation of symptoms between a recipient duct and donor duct mucocele are very similar. Therefore, while imaging criteria may suggest a certain origin, the final diagnosis may not be achieved until operative intervention.
Treatment of cystic duct mucoceles, whether donor or recipient in nature, can be achieved by either interventional radiology (IR) or surgery. The IR approach is safe, effective, and can be achieved by percutaneous drain placement and ethanol ablation. Surgically, if the mucocele is donor duct in origin, excision of the mucocele followed by roux-en-y hepaticojejunostomy has been described as an accepted treatment method[2,5]. In the instance of a recipient duct mucocele, one would expect, depending on the recipient variation of cystic duct anatomy, to have a well-drained cystic duct either into the recipient common duct or directly into the duodenum. Regardless of the anatomy, our patient developed a mucocele from the recipient remnant cystic duct, and only required simple excision.
When a transected cystic duct is encountered during preparation for liver transplant, it should be excised completely, even if it is close to the common duct. If this is not possible, the cystic duct should be excised as much as possible, rather than creating a blind mucosa-lined sac with the potential of enlarging and creating obstruction. However, this should be done under the discretion of the surgeon, as injury to the right hepatic artery, the common bile duct and its branches, and the biliary tract blood supply are possibilities. If excision of the cystic duct is thought too dangerous, its distal end can be incorporated in the anastomotic suture line to allow drainage of the cystic duct stump. However, wherever the valves of Heister are left to drain a blind biliary pouch that produces mucous, the risk of mucocele remains.
The diagnosis of cystic duct mucocele in the appropriate setting is usually made by radiologic modalities. Demonstration of fluid collection at the porta hepatis is a nonspecific finding. However, a combination of a well-defined, round, fluid collection adjacent to the common hepatic duct would confirm evidence. In some cases, the mucocele appears as a gallbladder and immediately calls into question whether a graft cholecystectomy was performed.
A recipient cystic duct mucocele is a rarity after deceased donor liver transplantation. It is a recognized complication to observe a donor duct mucocele that may compress the common bile duct. However, we describe for the first time a patient with a more ambiguous clinical picture found to have a recipient duct mucocele, showing the importance of the consideration of this complication in the post-transplant patient.
A 48-year-old male who presented with right upper quadrant pain and was found to have a recipient cystic duct mucocele 3 mo after receiving a deceased donor liver transplant.
A diagnosis of cystic duct remnant mucocele was made given its appearance and recurrence, and ablation with sclerosing agents (ETOH) was attempted by interventional radiology on three separate occasions but was of little benefit.
Biloma, anastomotic bile leak, pancreatic cyst, pancreatic pseudo cysts, and cystic duct mucocele after transplantation.
Liver function test revealed elevated canallicullar enzymes, complete blood count showed elevated white blood cells, and blood cultures were unremarkable.
Abdominal X-ray (nonspecific), abdominal CT (mass or fluid collection at porta hepatis), abdominal MRI (mass or fluid collection at porta hepatis), MRCP (showed filling of the fluid collection ), and ERCP (showed filling of the fluid collection).
Resection and histologic analysis of the fluid filled mucocele revealed chronic cholangitis with prominent fibrosis, granulation tissue formation through mucosa, muscularis, and adventitia.
Surgical resection of the mass, and perioperative antibiotics.
See the reference list: Ref. [2,4,7].
Mucocele: A swelling like a sac that is due to distension of a hollow organ or cavity with mucus; MRCP: Magnetic resonance cholangiopancreatography: A special type of magnetic resonance imaging (MRI) exam that produces detailed images of the hepatobiliary and pancreatic systems, including the liver, gallbladder, bile ducts, pancreas and pancreatic duct; ERCP: Endoscopic retrograde cholangiopancreatography: A technique that combines the use of endoscopy and fluoroscopy to diagnose and treat certain problems of the biliary or pancreatic ductal systems; Gamma-glutamyltransferase: A transferase (a type of enzyme) that catalyzes the transfer of gamma-glutamyl functional groups from molecules such as glutathione to an acceptor that may be an amino acid, a peptide or water (forming glutamate); Mirizzi’s Syndrome: A rare complication in which a gallstone becomes impacted in the cystic duct or neck of the gallbladder causing compression of the common bile duct or common hepatic duct, resulting in obstruction and jaundice; Hepaticojejunostomy: Biliary-enteric anastomosis is usually to smaller ducts, which can be multiple if the injury or stricture is above the bifurcation of the right and left ducts.
Cystic duct mucoceles after transplantation require a high index of suspicion requiring h and p, lab tests (liver function test, complete blood count, blood cultures), and imaging studies (abdominal X-ray, abdominal CT, abdominal MRI, ERCP) with resolution through surgical resection.
Manuscript source: Unsolicited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Coelho JCU, Kai K, Zhang CW S- Editor: Kong JX L- Editor: A E- Editor: Yan JL
| 1. | Caputo M, Spagnol A, Minguzzi MT, Papa S, Calculli L, Soro A, Grazi GL, Morganti M, Gavelli G. Incidence of dilatation of residual cystic duct in liver transplantation. Our experience with 152 cases. Radiol Med. 1995;89:647-650. [PubMed] |
| 2. | Caputo M, Piolanti M, Riccioli LA, Pazienza L, Fabbro E, Gruppioni F, Grazi G, Gavelli G. Nonobstructive residual mucocele of the cystic duct. Reassessment of complications in our 13 years’ experience with liver transplantation. Radiol Med. 2000;100:354-356. [PubMed] |
| 3. | Liang TB, Zhao ZC, Jia CK, Zheng SS. Mucocele formation of cystic bile duct remnant after orthotopic liver transplantation. Chin Med J (Engl). 2007;120:254-256. [PubMed] |
| 4. | Koneru B, Zajko AB, Sher L, Marsh JW, Tzakis AG, Iwatsuki S, Starzl TE. Obstructing mucocele of the cystic duct after transplantation of the liver. Surg Gynecol Obstet. 1989;168:394-396. [PubMed] |
| 5. | Chatterjee S, Das D, Hudson M, Bassendine MF, Scott J, Oppong KE, Sen G, French JJ. Mucocele of the cystic duct remnant after orthotopic liver transplant: a problem revisited. Exp Clin Transplant. 2011;9:214-216. [PubMed] |
| 6. | Piardi T, Panaro F, Gheza F, Wolf P. ‘Cystic’ lesion in the porta hepatis after liver transplantation. Liver Int. 2011;31:859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 7. | Abcarian PW, Emond JC, Ring EJ. Cystic duct remnant mucocele in a liver transplant recipient. J Vasc Interv Radiol. 1994;5:127-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Zajko AB, Bennett MJ, Campbell WL, Koneru B. Mucocele of the cystic duct remnant in eight liver transplant recipients: findings at cholangiography, CT, and US. Radiology. 1990;177:691-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |