Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.70
Peer-review started: June 3, 2016
First decision: July 5, 2016
Revised: December 2, 2016
Accepted: December 27, 2016
Article in press: December 29, 2016
Published online: February 24, 2017
Processing time: 267 Days and 4.7 Hours
To systematically review reports on deceased-donor-lobar lung transplantation (ddLLTx) and uniformly describe size matching using the donor-to-recipient predicted-total lung-capacity (pTLC) ratio.
We set out to systematically review reports on ddLLTx and uniformly describe size matching using the donor-to-recipient pTLC ratio and to summarize reported one-year survival data of ddLLTx and conventional-LTx. We searched in PubMed, CINAHL via EBSCO, Cochrane Database of Systematic Reviews via Wiley (CDSR), Database of Abstracts of Reviews of Effects via Wiley (DARE), Cochrane Central Register of Controlled Trials via Wiley (CENTRAL), Scopus (which includes EMBASE abstracts), and Web of Science for original reports on ddLLTx.
Nine observational cohort studies reporting on 301 ddLLTx met our inclusion criteria for systematic review of size matching, and eight for describing one-year-survival. The ddLLTx-group was often characterized by high acuity; however there was heterogeneity in transplant indications and pre-operative characteristics between studies. Data to calculate the pTLC ratio was available for 242 ddLLTx (80%). The mean pTLCratio before lobar resection was 1.25 ± 0.3 and the transplanted pTLCratio after lobar resection was 0.76 ± 0.2. One-year survival in the ddLLTx-group ranged from 50%-100%, compared to 72%-88% in the conventional-LTx group. In the largest study ddLLTx (n = 138) was associated with a lower one-year-survival compared to conventional-LTx (n = 539) (65.1% vs 84.1%, P < 0.001).
Further investigations of optimal donor-to-recipient size matching parameters for ddLLTx could improve outcomes of this important surgical option.
Core tip: Deceased-donor-lobar lung transplantation (ddLLTx) is an important and so far underutilized surgical option for lung transplant candidates with small chest cavities. It is only performed at a few specialized centers and frequently performed in high urgency cases. Outcome is acuity-driven and is expected to improve as more elective cases are done. The size matching decision for ddLLTx is complex and based on varying parameters. Systematically using the predicted Total Lung Capacity ratio as the size matching tool could help to identify sizing thresholds to maximize the risk/benefit balance for ddLLTx.
- Citation: Eberlein M, Reed RM, Chahla M, Bolukbas S, Blevins A, Van Raemdonck D, Stanzi A, Inci I, Marasco S, Shigemura N, Aigner C, Deuse T. Lobar lung transplantation from deceased donors: A systematic review. World J Transplant 2017; 7(1): 70-80
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/70.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.70
Lung transplantation (LTx) is an established therapy for appropriately selected patients suffering from end-stage lung disease. Since the implementation of the Lung Allocation Scoring (LAS) system, characteristics of candidates on the wait list have changed to include a sicker group of patients with a greater proportion of restrictive lung diseases (LAS diagnoses group D)[1,2]. As a consequence, wait-list mortality rates are again rising despite higher wait-list transplant rates compared to the pre-LAS era[3]. Potential LTx-recipients with short stature and small thoracic cavities have longer waiting times on the LTx list, as donor lungs considered to be size-appropriate are particularly limited[3,4]. This often affects patients with cystic fibrosis and pulmonary fibrosis[4]. In both groups, LTx can become an urgent issue when significant disease exacerbations occur, and in this setting in particular patients are at high risk for wait list mortality. Higher acuity at the time of LTx is in turn associated with decreased survival[5].
Three operative solutions exist to increase the utilization of available deceased donors for patients with small chest cavities[6-8]. These include: (1) deceased lobar lung transplant (ddLLTx)[6,8]; (2) split lung transplant (a form of ddLLTx, where the left lung allograft is divided and then each resulting lobe is implanted into the two hemithoraces)[9]; and (3) peripheral atypical resection. ddLLTx was first described by Bisson et al[8] in 1994. Subsequently, several single center reports on ddLLTx have been published[6,7,9-16].
The best size-matching parameter remains debatable. Chest X-ray parameters, calculation of the ratio between donor and recipient heights, calculation of the ratio of predicted total lung capacity (pTLC) between donor and recipient (pTLCratio) and estimation based on visual inspection in the operating room are commonly used strategies[17]. Amongst these the pTLCratio has the largest evidence base to support its use[17-30].
Therefore, we set out to systematically review reports on ddLLTx with the aim to describe the size matching between donor and recipient uniformly using the pTL-Cratio[31-33]. Specifically we intended to compare the pTLCratio that would have occurred using the entire donor lungs (pTLCratioFull) to the pTLCratio that was transplanted via the lobar transplantation (pTLCratioLobar). The second objective was to perform a systematic review and meta-analysis of one-year survival after ddLLTx.
A health sciences librarian ran extensive literature searches in PubMed, CINAHL via Ebsco, Cochrane Database of Systematic Reviews via Wiley (CDSR), Database of Abstracts of Reviews of Effects via Wiley (DARE), Cochrane Central Register of Controlled Trials via Wiley (CENTRAL), Scopus (which includes EMBASE abstracts), and Web of Science. No filters for date, language, or any other parameter were used. The PubMed strategy described below was modified as needed for use in other electronic databases. Full search strategies are available upon request.
The search strategy was for PubMed: (((((“Lung Transplantation”[Mesh] OR lung transplant*[Text Word] OR lung graft*[text word])) OR ((“Tissue and Organ Procurement”[Mesh] OR “Tissue Donors”[Mesh] OR “Organ Transplantation”[Mesh] OR organ procurement*[text word] OR tissue procurement*[text word] OR tissue donor*[text word] OR organ donor*[text word] OR organ transplant*[text word]) AND (Lung[Mesh] OR Lung[text word] OR Lungs[text word])))) AND ((lobar[text word] OR lobe*[text word]))) AND ((“Cadaver”[Mesh] OR Cadaver*[text word] OR Dead[text word] OR Nonliving[text word] OR Non-living[text word])).
For an identified study to be included in the systematic review it had to: (1) involve human participants; (2) have full text available in English; and (3) report on recipients of ddLLTx. For an identified study to be included in the meta-analysis it had to meet the following additional criteria: one year survival data is available for: (1) a conventional lung transplant cohort (either in same study or from a contemporary publication from the same center); and (2) a ddLLTx cohort. When overlapping data, i.e., several publications from same center, study selection favored most recent data. The corresponding authors of the studies selected for inclusion in the systematic analysis were contacted to seek unpublished updated center data.
The methodological quality of the selected studies was evaluated using criteria from the United States Preventative Services Task Force.
Data extracted included author name, year of publication, location of center, number of patients in ddLLTx cohort, number of patients in conventional-LTx cohort, study-years, indication for transplantation and acuity at time of transplant. Outcome data extracted included rate of primary graft dysfunction (PGD), ICU and hospital length of stay (LOS), FEV1(%-predicted) at 6 mo and peak FEV1, survival at 1 year and 5 years.
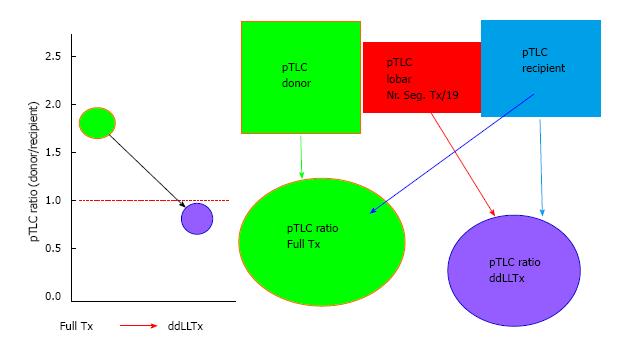
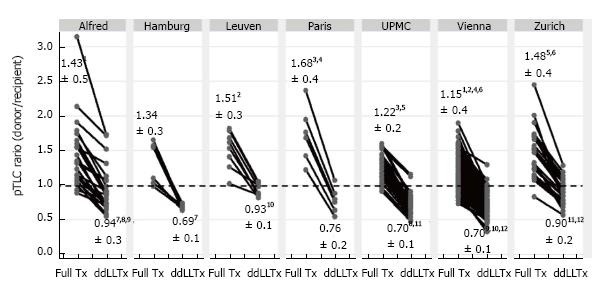
The parameter(s) used for the size matching were extracted for each study. For all studies that did not report recipient pTLC (pTLCrecipient), full donor pTLC (pTLCdonorFull) and donor pTLC after lobar resection (pTLCdonorLobar) the study authors were contacted and asked to provide: recipient age, height and sex (to calculate pTLCrecipient[18]); donor age, height and sex (to calculate pTLCdonorFull[18]) and information on donor lobes transplanted [to calculate pTLCdonorLobar = (pTLCdonorFull)× (number donor lung segments transplanted/19)] for each donor and recipient pair. From this the pTLCratio that would have occurred using the entire donor lungs was calculated as pTLCratioFull = pTLCdonorFull/pTLCrecipient. The pTLC ratio that was actually transplanted via the lobar transplantation was calculated as pTLCratioLobar = pTLCdonorLobar/pTLCrecipient, Figure 1.
The primary outcome of interest was one-year-survival. Secondary outcomes were occurrence of PGD, ICU and hospital LOS, FEV1 (6 mo and peak) and 5-year survival.
We expressed pTLCratioFull and pTLCratioLobar as means ± standard deviation for the entire cohort and stratified by transplant indication and transplant center. We assessed for differences in mean pTLCratioFull and pTLCratioLobar between transplant indications and centers by one-way ANOVA analysis of variance, with bonferroni adjustment for multiple comparisons. We extracted dichotomous data for one-year-survival form all studies reporting number of patients with events and total participants. We performed a meta-analysis and pooled the one-year-mortality data to calculate relative risks (risk ratios, RRs) with 95% confidence interval (CI). We used the statistic of I2 to test for the heterogeneity, with I2 < 25%, 25%-75% and > 75% to represent low, moderate and high degree of inconsistency, respectively. In analyses, if the heterogeneity was low then we used a fixed-effect model, or else applied the random-effect model. We performed a sensitivity analysis, in which a study was removed at a time while the rest was analyzed, to evaluate whether the results could have markedly been affected by that single study. We used Egger’s linear regression test to find a potential publication bias. All analyses were performed with Stata (Version10.0, Stata Corporation, College Station, TX, United States). A 2-tailed P value of less than 0.05 was considered statistically significant.
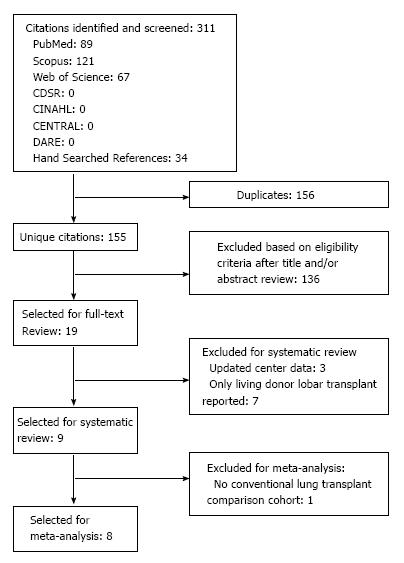
Our search identified 155 unique citations. Of these, 32 abstracts and 18 full-text publications were assessed (Figure 2). Nine studies fulfilled our inclusion criteria for final review[6,7,10-16] (Table 1). Reviewer agreement on selection of abstracts was 100% (K = 1.0) and on inclusion of articles for the final review it was 100% (K = 1.0).
| Author | Year | Country | Center | Time | Nr | Indication/diagnosis | Acuity | ||||
| CF | IPF | IPAH | COPD | Other | |||||||
| Couetil | 1997 | France | Paris | 1993-1994 | 7 | 3 | 1 | 2 | 1 | - | Not reported |
| Espinosa | 2010 | Spain | Reina Sofia | 2003-2009 | 6 | - | - | - | - | - | 2 ICU, |
| 2 Hosp, | |||||||||||
| 2 Outpatient | |||||||||||
| Deuse | 2011 | Germany | Hamburg | 2009-2012 | 71 | 2 | 5 | - | - | - | 1 ECMO |
| Marasco | 2012 | Australia | Alfred | 1990-2012 | 271 | 6 | 5 | - | 4 | 12 | Not reported |
| Inci | 2012 | Swiss | Zurich | 2000-2012 | 23 | 10 | 8 | - | 3 | 2 | 3 ECMO, 1 MV, |
| Shigemura | 2013 | United States | UPMC | 2010-2012 | 351 | 4 | 17 | - | - | 14 | 7 ECMO, 9 MV, LAS 72-94 |
| Mitilian | 2013 | France | Foch | 1988-2012 | 50 | 35 | 7 | - | 3 | 5 | 2 ECMO |
| Aigner | 2014 | Austria | Vienna | 2001-2012 | 1381 | 48 | 46 | 8 | 16 | 20 | 27 MV, 18 ECMO |
| Stanzi | 2014 | Belgium | Leuven | 2005-2012 | 8 | 8 | - | - | - | - | All outpatients |
All nine reports were single center retrospective cohort studies. Seven reports originated in Europe[6,7,10,12,14-16], one in Australia[11], and one in North America[13]. The study period ranged from 1988-2012. Four centers reported on fewer than 10 recipients of ddLLTx, two had 20-35 ddLLTx recipients, and two reported 50 or more ddLLTx cases.
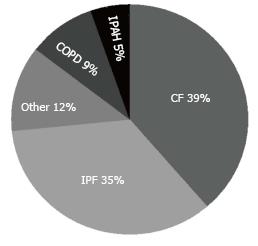
In the nine studies including 301 ddLLTx, the indications were available in eight studies (295 ddLLTx) and were predominantly cystic fibrosis (39%) and interstitial lung diseases (35%) (Figure 3). Six of the nine studies qualified the acuity of ddLLTx and these were often characterized by high acuity (Table 1).
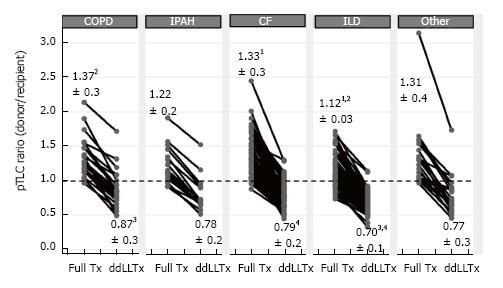
The size matching parameter used was the pTLCratio in five of nine studies, often in combination with visual inspection of fully inflated allograft and recipient chest cavity size in the operating room. Donor and recipient height and CXR characteristics were used in 2 studies (Table 2). Two studies reported pTLCdonorFull, pTLCdonorLobar and pLTCrecipient[6,11]. Data to calculate these parameters were provided for five additional studies[7,12,13,15,16] and pTLCdonorFull, pTLCdonorLobar and pLTCrecipient was then available for 242 of 301 donor-recipient pairs of ddLLTx (Figure 1). The mean pTLCdonorFull was 6.42 ± 1.0 L and after lobar resections was reduced to pTLCdonorLobar 3.83 ± 0.8 L. The mean pLTCrecipient was 5.27 ± 1.0 L. The mean pTLCratioFull was 1.25 ± 0.3 and was reduced to a mean pTLCratioLobar 0.76 ± 0.2. Stratified by transplant indication, the interstitial lung diseases group had the lowest mean pTLCratioFull (1.12 ± 0.03), which was significantly lower than COPD (1.37 ± 0.3) and CF (1.33 ± 0.3) (Figure 4). After lobar resections the transplanted mean pTLCratioLobar was also the lowest in interstitial lung diseases group (0.70 ± 0.1) and significantly lower than COPD (0.87 ± 0.3) and CF (0.79 ± 0.2) (Figure 4). Stratified by transplant centers the pTLCratioFull ranged from 1.15 ± 0.4 to 1.68 ± 0.4 (Figure 5). The transplanted pTLCratioLobar ranged between transplant centers from 0.69 ± 0.1 to 0.94 ± 0.3 (Figure 5).
| Center | Size matching parameter | pTLC donor (full) | pTLC donor (lobar) | pTLC recipient | pTLCratio (full) | pTLCratio (lobar) |
| Paris | pTLCratio | 6.91 ± 0.7 | 3.11 ± 0.3 | 4.28 ± 1.1 | 1.69 ± 0.4 | 0.76 ± 0.5 |
| Reina Sofia | Not reported | Not provided | Not provided | Not provided | Not provided | Not provided |
| Hamburg1 | pTLCratio | 6.96 ± 1.2 | 3.64 ± 0.7 | 5.27 ± 1.0 | 1.35 ± 0.3 | 0.69 ± 0.1 |
| Alfred1 | pTLCratio, CXR | 6.82 ± 1.2 | 4.81 ± 1.1 | 5.12 ± 1.4 | 1.44 ± 0.5 | 0.94 ± 0.3 |
| Zurich1 | Visual inspection, height | 7.21 ± 0.8 | 4.45 ± 0.7 | 5.04 ± 0.9 | 1.48 ± 0.4 | 0.90 ± 0.2 |
| UPMC1 | Height, CXR, visual inspection | 6.28 ± 0.7 | 3.76 ± 0.7 | 5.22 ± 0.8 | 1.22 ± 0.9 | 0.73 ± 0.5 |
| Foch | pTLCratio, visual inspection | Not provided | Not provided | Not provided | 1.65 | Not provided |
| Vienna1 | pTLCratio, visual inspection | 6.19 ± 1.1 | 3.80 ± 0.9 | 5.45 ± 1.0 | 1.15 ± 0.2 | 0.70 ± 0.1 |
| Leuven1 | Visual inspection, height | 6.70 ± 1.2 | 4.11 ± 0.3 | 4.42 ± 0.4 | 1.52 ± 0.4 | 0.93 ± 0.3 |
Nine studies (301 patients) provided data on one-year survival after ddLLTx (Table 3). One-year survival in the ddLLTx groups ranged from 50%-100%. We identified survival information for a conventional-LTx comparison group within the same institution for eight studies. One-year survival was 72%-88% in the conventional-LTx groups, which was not statistically different within each individual study, with the exception of the largest study, where ddLLTx was associated with a higher risk of mortality (65.1% vs 84.1% one-year survival, P < 0.001)[15].
| Center | ComparisonGroup with CLTx (number, diagnosis) | PGD (grade)PostOP-ECMO | ICU LOS (d) | Hospital LOS(d) | Survival 1 year | Survival 5 years |
| Paris | No | Not reported | Not reported | Not reported | ddLLTx: 86% | Not reported |
| Reina Sofia | Yes (149 - mixed)1 | Not reported | Not reported | Not reported | ddLLTx: 50%, CLTx: 72%1 | Not reported |
| Hamburg | Yes (28 - mixed)4 | Not reported | Not reported | Not reported | ddLLTx: 85%, CLTx: 72%4 | Not reported |
| Alfred | Yes (329 - mixed) | ddLLTx: 56% ≥ PGD (2) | LLT: 12; CLTx: 4 | ddLLTx: 30 CLTx: 21 | ddLLTx: 81%, CLTx: 84% (P = 0.115) | ddLLTx: 52%5, CLTx: 37.5%5 (P = 0.115) |
| Zurich | Yes (219 - mixed) | ddLLTx: 13% PGD (not spec.) | Not reported | Not reported | ddLLTx: 82%; CLTx: 88% (P = 0.56) | ddLLTx: 64%; CLTx: 69% (P = 0.56) |
| UPMC | Yes (691 - mixed)2, Yes (65 - high LAS)3 | ddLLTx: 36% ECMO | Not reported | Not reported | ddLLTx: 76%; CLTx: 83%1; (high LAS): 72%2 | Not reported |
| Foch | Yes (445 - mixed) | ddLLTx: 54% ≥ PGD (1) 20% ECMO | ddLLTx: 17 | ddLLTx: 43 | ddLLTx: 60%, CLTx: 78% (NS) | ddLLTx: 46%, CLTx: 51% (NS) |
| Vienna | Yes (778 - mixed) | ddLLTx: 44% ≥ PGD1 32% ECMO | ddLLTx: 17; CLTx: 6 | ddLLTx: 33.5 CLTx: 22 | ddLLTx: 65.1; CLTx: 84.8% (P < 0.001) | ddLLTx: 54.9% CLTx: 69.9% (P < 0.001) |
| Leuven | Yes (66 - all CF) | ddLLTx: 25% PGD (3) at 48 h vs CLTx: 9% | ddLLTx: 12 CLTx: 5 | ddLLTx: 37 CLTx: 24 | ddLLTx: 100%; CLTx: 88.4% (NS) | Not reported |
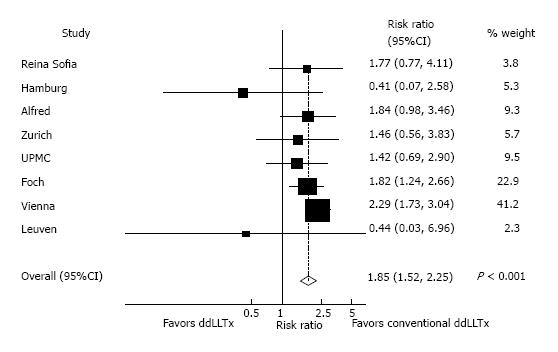
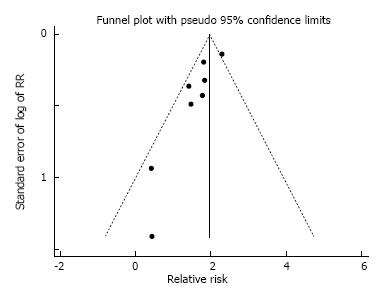
In pooled analysis of unadjusted data from eight studies, ddLLTx-recipients (n = 284) had a relative risk of one-year mortality of 1.85 (95%CI: 1.52-2.25, P < 0.001) compared with conventional-LTx-recipients (n = 2777) (Figure 6). There was low heterogeneity as indicated by an I2 of 0% (P = 0.47). In an analysis for possible publication bias by performing a linear regression of the standard normal deviate against precision (Egger test) showed that the intercepts did significantly deviate from zero (P = 0.007, for one-year-survival), indicating the presence of publication bias. Visual inspection of the funnel plot showed asymmetry (Figure 7). This also indicated the presence of publication bias, limiting the interpretation of the meta-analysis.
Five studies described the occurrence of primary graft dysfunction (PGD) and described rates ranging between 13%-56% in ddLLTx (Table 3). One study reported ddLLTx PGD rates compared to conventional-LTx. At 48 h, PGD grade 3 rates were 25% in ddLLTx (n = 8), compared to 9% in the conventional-LTx (n = 66) group[16]; this difference, however, was not statistically significant in that study. Three studies reported on postoperative ECMO needs, which ranged from 20%-36% in the ddLLTx groups[13-15]. Four studies reported on ICU LOS. This ranged from 12 to 27 d in ddLLTx, compared to 4-6 d in conventional-LTx, Table 3. Five studies reported on FEV1 in the post-ddLLTx period, Table 4. At 3-6 mo following ddLLTx FEV1 (%-predicted) ranged from 52.6%-75.3%. Peak FEV1 (%-predicted) following ddLLTX ranged from 67.3%-85.2%. Only one study compared FEV1 (%-predicted) between ddLLTx (n = 8) and conventional-LTx (n = 66) cohorts[16]. In that study, at 3 mo ddLLTx FEV1 (%-predicted) was 64.5%, compared to 76% (P-value non-significant) in conventional-LTx and peak FEV1 (%-predicted) was 80.5% and 99% (P-value non-significant) for the respective cohorts[16]. Two studies reported on the correlation between FEV1(%-predicted) and the transplanted pTLCratio (= pTLCratioLobar) following ddLLTx and both studies found a significant correlation between the size of the transplanted lungs and FEV1(%predicted), Table 4. Four studies reported on 5 year survival following ddLLTx and this ranged from 37.5%-54.9%, compared to 51%-69.9% in the conventional-LTx groups, Table 3[11,12,14,15]. Five-year-survival was not statistically different within each individual study, with the exception of the largest study, where ddLLTx was associated with a higher risk of mortality (54.9% vs 69.9% five-year survival, P < 0.001)[15].
| Center (Nr of ddLLTx) | Comparison group (Nr) | FEV1 (%) 3-6 mo | Peak FEV1 (%) | Correlation to pTLCratio |
| Paris (7) | No | 6 mo: 62% | 81% | Not reported |
| Reina Sofia (6) | No | Not reported | Not reported | Not reported |
| Hamburg (3) | No | Not reported | Not reported | Not reported |
| Alfred (23) | No | 6 mo: 52.6% | Not reported | Yes FEV1(%) at 3 mo correlates with pTLCratioLobar (r = 0.549, P = 0.028) |
| Zurich (23) | No | 6 mo: 75.3% | 76.80% | Yes FEV1(%) at 3 mo correlates with pTLCratioLobar (r = 0.485, P = 0.04) |
| UPMC (25) | No | Not reported | 85.20% | Not reported |
| Foch (50) | No | 6 mo: 61.1% | 67.30% | |
| Vienna | No | Not reported | Not reported | Not reported |
| Leuven (6) | Yes CLTx (66) | 3 mo: ddLLTx: 64.5% CLTx: 76% | ddLLTx: 80.5 CLTx: 99% | Not reported |
The technique of deceased donor lobar lung transplantation (ddLLTx) is an important surgical option for LTx-candidates with small chest cavities and adds to our armamentarium of LTx techniques. The lung is a special organ that allows parenchyma resections to reduce its size without necessarily compromising the functionality of the remaining tissue. Amongst other solid organs, this remarkable feature is only shared by the liver, not by the heart or the kidneys and split liver transplants have already been established as a reliable tool to increase the donor pool for children[34]. After all, the anatomical organization of the graft and the number of individual lobes transplanted should be less of a concern than the total amount of lung parenchyma provided for the recipient.
Lobectomies are straightforward procedures, but are still rarely performed in the context of LTx. However lobectomies add to the surgical complexity of the LTx operation and may thus prolong the operative time. More importantly, when performed on the back-table, cooling may be impaired and the graft is exposed to warm ischemic time. These disadvantages need to be weighed against the advantages of significantly increasing the potential donor pool and reducing waiting times and waiting list mortality in LTx-candidates with small chest cavities[3]. Because prolonged waiting times often correlate with patient deconditioning, timely transplantation may also reduce the procedural risk for some patients. Differences in surgical strategies among centers include the preferred choice of lobes transplanted. Isolated lower and upper lobe transplants carry the fundamental advantage of not creating a bronchial stump as does bi-lobar transplantation of right upper + middle or upper + lower lobes. Although there is a considerable size mismatch between the recipient main bronchus and a lobar graft bronchus, careful adjustment during surgery allows tension-free alignment in most of the cases. Airway complications have been described and in one study, anastomotic stenoses were reported to occur more frequently in ddLLTx than in full-size transplantation[7,10,11,14,16,35]. However, most airway complications were bronchial stenoses that were amenable for bronchoscopic treatment[14,35].
The size matching parameter utilized to make the decision to perform a ddLLTx varied between studies and some degree of surgeon-specific assessment based on visual inspection was repeatedly reported. However, among objective parameters, the pTLCratio was most frequently reported and offers the possibility to compare practices and results among centers. To our knowledge, this is the first study that uniformly analyzes size matching for ddLLTx based on the pTLCratio.
Although all 9 centers reporting ddLLTx for down-sizing have somewhat different patient populations and surgical philosophies, there were remarkable similarities. The mean recipient’s pTLCs were mostly reported at around 5 L, only in two reports (Paris and Leuven) the mean recipient pTLCs were in the 4-4.5 L range, reflecting a higher proportion of pediatric recipients. Although the decision to perform a ddLLTx was based on different sizing considerations, the down-sizing performed as reflected by the pTLCratioLobar was similar among centers and averaged at 0.76 ± 0.2. The general preference towards undersizing in the setting of fibrotic lung diseases[17,36] was also evident in this systematic review, where the interstitial lung diseases group had the lowest mean pTLCratioFull (1.12 ± 0.03) and after lobar resections the transplanted mean pTLCratioLobar was also the lowest in interstitial lung diseases group (0.70 ± 0.1) (Figure 4).
In previous studies the pTLCratio was found to be an independent predictor of survival after LTx[21,22,25-28,37]. In an analysis of the SRTR database in the post-LAS era, the pTLCratio showed an independent and nonlinear association with one-year-survival after LTx, irrespective of LTx indication[27]. There was a declining risk of death with higher pTLCratio from 0.5 to about 1.3, where an inflection occurred with rising risk at pTLCratios > 1.3[27]. Furthermore, in an ancillary study to the Lung-Transplant-Outcomes-Group, oversized allografts were associated with a decreased risk of PGD grade 3 after bilateral-LTx[36]. This association was most apparent in recipients with risk factors for PGD[38]. There are concerns that in the intra-operative and early post-LTx period, hemodynamic compromise can occur in the setting of a profoundly oversized allograft secondary to a compartment-syndrome-like picture occurring after chest closure. Also, persistent atelectasis may hamper overall oxygenation and increase the risk for pulmonary infections. However in a single center study oversized allografts (mean pTLCratio 1.18 ± 0.14, range 1.01-1.63), when compared with undersized allografts (mean pTLCratio 0.89 ± 0.09, range 0.63-1.00), were not associated with an increase in post-LTx complications. On the contrary, oversized allografts were associated with a shorter hospital LOS after LTx and lower resource utilization[20]. These previous data linking the pTLCratio to important post-LTx outcomes could suggest that for severely oversized pTLCratioFull (in excess of > 1.4) a ddLLTx could be an important surgical option however should be performed only in special circumstances in cases with lower pTLCratioFull.
The principal finding was that the ddLLTx-group appeared to have a higher risk for one-year mortality than the conventional-LTx-group. In the meta-analysis the ddLLTx and conventional-LTx-groups were unmatched and the outcomes were unadjusted for confounders. Furthermore, the Egger test and visual inspection of the funnel plot for the 1 year survival meta-analysis indicated the presence of publication bias. In terms of publication bias, an underreporting of unsuccessful ddLLTx cases is or appears more likely than an underreporting of superior outcomes of ddLLTx compared to conventional LTx. Because of the above issues, the results of the meta-analysis need to be interpreted with caution. The majority of the included single center studies showed no statistically significant survival difference, although most studies suggested a trend towards higher one-year mortality in the ddLLTx-group. The largest single center study, however, showed a significantly higher risk for one-year mortality in the ddLLTx-group. Importantly, there are significant clinical differences between the ddLLTx and conventional-LTx-groups, which are not adjusted for in the pooled analysis. Because ddLLTx is more frequently used in very urgent cases to realize timely LTx, it is likely that the one-year-survival differences between ddLLTx and conventional-LTx groups are due to the high acuity of the ddLLTx-group. In the Vienna experience, for example, patients receiving ddLLTx were significantly more urgent and more frequently on mechanical ventilation or ECMO support pre-LTx[15]. The Pittsburgh experience also supports the notion of an acuity-driven mortality risk associated with ddLLTx. Only very urgent patients with LAS > 70 were considered as candidates for ddLLTx. This very high acuity ddLLTx group achieved a 76% one-year survival (n = 35)[13], which was similar to that of the high-LAS-cohort (LAS > 50) receiving full-sized lung transplants (72% one-year survival, n = 108)[39]. Resource utilization following ddLLTx seems to reflect the pre-transplant high acuity of the recipients. In three studies reporting on postoperative ECMO needs, this ranged from 20-36% in the ddLLTx groups[13-15]. Four studies reported on ICU LOS and this ranged from 12 to 27 d in ddLLTx, compared to 4-6 d in conventional-LTx (Table 3). It thus remains to be seen if elective ddLLTx in routine LTx-candidates achieves outcomes comparable to those of elective full-sized LTx. This is supported by the experience of the Leuven group, where a cohort of eight stable outpatient LTx-candidates with cystic fibrosis had a 100% one-year survival after ddLLTx[16]. Other centers also reported favorable results with ddLLTx in elective, non-urgent cases[40].
Our study has several limitations. All of the included reports were retrospective observational cohort studies. Although this study systematically analyzed size matching using the pTLCratio, data for its calculation was not available for all patients of the ddLLTx-cohort. Physiologically there a notable difference between a CF patient with short stature and a normal sized IPF patient with the exceptionally small chest cavity from the fibrotic lung disease. For this systematic review only aggregate data on outcomes was available and these two groups could not be analyzed separately. However the pTLC of the recipient would adequately reflect the “normal” chest cavity size of these two very different populations. Whereas using the actually measured total lung capacity or visual inspection of the chest cavities on imaging or in the operating room largely reflects the disease specific effects of the underlying lung diseases on the chest cavity size. However, such alterations in chest cavity size have been shown to be quickly reversible. Assessing chest cavity size via opto-electronic-plethysmography post-LTx demonstrated that, irrespective of LTx-indication, the chest volume and the response to exercise was not different from normal controls[41]. In this systematic review 2 studies reported on donor and recipient pTLC and both studies used regression equation based on sex and height to derive pTLC[6,11]. Whereas for the calculations of donor and recipient pTLC done as part of this systematic review from data provided by the authors of five of the included studies[7,12,13,15,16] were based on age, sex and height[18]. While the latter approach accounts for the main determinants of lung size, the race effect on lung size remains unaccounted for with both approaches. The best regression equation to calculate pTLC is not defined, but computed tomography (CT) and CT-volumetry is increasingly used to derive comprehensive and refined regression equations for pTLC[42]. There were wide variations in rates of PGD, likely in part due to variation in definitions, surveillance methods, and reporting. Despite between-institution variability, each individual institution reportedly treated ddLLTx and conventional-LTx cohorts similarly. The majority of the included reports originated in Europe[6,7,10,12,14-16] with only one originating from Australia[11] and one in North America[13]. The organ allocation mechanisms vary by region. Furthermore there were differences in the patient populations and surgical philosophies, which limit the interpretation of aggregate data. The optimal strategy for size matching decisions and thresholds to perform a ddLLTx, especially for recipient with restrictive lung disease, remains to be defined. Important open questions include: (1) Is there a threshold where the risk of implanting an oversized full allograft exceeds the risks of a ddLLTx and ddLLTx should be recommended? (2) When ddLLTx leads to a very undersized lobar allograft based on the pTLCratioLobar, is there a threshold where the risks of PGD and poor outcomes start to rise substantially? and (3) Would the risk of PGD and the overall outcome of reasonably matched ddLLTx compare to those of full-size allografts if performed routinely in elective cases?
In conclusion, ddLLTx is an important and so far underutilized surgical option for lung transplant candidates with small pTLC. It is only performed at a few specialized centers and frequently performed in high urgency cases. Outcome is acuity-driven and is expected to improve as more elective cases are done. Systematically using the pTLCratio as the size matching tool could help to identify sizing thresholds to maximize the risk/benefit balance for ddLLTx.
Alison Beer participated in developing the literature search strategy and in registering the meta-analysis at PROSPERO International prospective register of systematic reviews (PROSPERO 2014:CRD42014004308). Robert M Reed is funded in part by the Flight Attendant Medical Research Institute (FAMRI).
Lung transplantation (LTx) is an established therapy for appropriately selected patients suffering from end-stage lung disease. Potential LTx-recipients with short stature and small thoracic cavities have longer waiting times on the LTx list, as donor lungs considered to be size-appropriate are particularly limited. Deceased-donor-lobar lung transplantation (ddLLTx) is an important and so far underutilized surgical option for lung transplant candidates with small chest cavities. The size matching decision for ddLLTx is complex and based on varying parameters.
The best donor-to-recipient size-matching parameter in LTx remains controversial. Chest X-ray parameters, calculation of the ratio between donor and recipient heights, calculation of the ratio of predicted total lung capacity (pTLC) between donor and recipient (pTLCratio) and estimation based on visual inspection in the operating room are commonly used strategies. Amongst these the pTLCratio has the largest evidence base to support its use. Systematically using the pTLCratio as the size matching tool could help to identify sizing thresholds to maximize the risk/benefit balance for ddLLTx.
In this systematic review the authors’ analyzed all reports on ddLLTx and uniformly described size matching using the donor-to-recipient predicted-total lung-capacity (pTLC) ratio and summarized reported one-year survival data of ddLLTx and conventional-LTx. Nine observational cohort studies reporting on 301 ddLLTx met the inclusion criteria for systematic review of size matching, and eight for describing one-year-survival. The ddLLTx-group was often characterized by high acuity; however there was heterogeneity in transplant indications and pre-operative characteristics between studies. Data to calculate the pTLCratio was available for 242 ddLLTx (80%). The mean pTLCratio before lobar resection was 1.25 ± 0.3 and the transplanted pTLCratio after lobar resection was 0.76 ± 0.2. One-year survival in the ddLLTx-group ranged from 50%-100%, compared to 72%-88% in the conventional-LTx group. In the largest study ddLLTx (n = 138) was associated with a lower one-year-survival compared to conventional-LTx (n = 539) (65.1% vs 84.1%, P < 0.001).
ddLLTx is an important and so far underutilized surgical option for lung transplant candidates with small pTLC. It is only performed at a few specialized centers and frequently performed in high urgency cases. Outcome is acuity-driven and is expected to improve as more elective cases are done. Systematically using the pTLCratio as the size matching tool could help to identify sizing thresholds to maximize the risk/benefit balance for ddLLTx.
The technique of deceased donor lobar lung transplantation (ddLLTx) is an important surgical option for LTx-candidates with small chest cavities. The lung is a special organ that allows parenchyma resections to reduce its size without necessarily compromising the functionality of the remaining tissue. Amongst other solid organs, this remarkable feature is only shared by the liver, not by the heart or the kidneys and split liver transplants have already been established as a reliable tool to increase the donor pool for children.
The authors have prepared an excellent review of the literature concerning the lobar transplantation (LTx). That technique is one of the new possibility for improving the number of LTx and to save a larger number of patients in very poor respiratory condition. The work is absolutely important and deserves a priority publication.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Belliato M, Nosotti M, Salvadori M S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
| 1. | Eberlein M, Garrity ER, Orens JB. Lung allocation in the United States. Clin Chest Med. 2011;32:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 2. | Maxwell BG, Mooney JJ, Lee PH, Levitt JE, Chhatwani L, Nicolls MR, Zamora MR, Valentine V, Weill D, Dhillon GS. Increased resource use in lung transplant admissions in the lung allocation score era. Am J Respir Crit Care Med. 2015;191:302-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Aigner C, Winkler G, Jaksch P, Ankersmit J, Marta G, Taghavi S, Wisser W, Klepetko W. Size-reduced lung transplantation: an advanced operative strategy to alleviate donor organ shortage. Transplant Proc. 2004;36:2801-2805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Keeshan BC, Rossano JW, Beck N, Hammond R, Kreindler J, Spray TL, Fuller S, Goldfarb S. Lung transplant waitlist mortality: height as a predictor of poor outcomes. Pediatr Transplant. 2015;19:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 5. | Merlo CA, Weiss ES, Orens JB, Borja MC, Diener-West M, Conte JV, Shah AS. Impact of U.S. Lung Allocation Score on survival after lung transplantation. J Heart Lung Transplant. 2009;28:769-775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Couetil JP, Tolan MJ, Loulmet DF, Guinvarch A, Chevalier PG, Achkar A, Birmbaum P, Carpentier AF. Pulmonary bipartitioning and lobar transplantation: a new approach to donor organ shortage. J Thorac Cardiovasc Surg. 1997;113:529-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Deuse T, Sill B, von Samson P, Yildirim Y, Kugler C, Oldigs M, Klose H, Meierling S, Rabe KF, Reichenspurner H. Surgical technique of lower lobe lung transplantation. Ann Thorac Surg. 2011;92:e39-e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Bisson A, Bonnette P, el Kadi NB, Leroy M, Colchen A. Bilateral pulmonary lobe transplantation: left lower and right middle and lower lobes. Ann Thorac Surg. 1994;57:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Inci I, Benden C, Kestenholz P, Hillinger S, Schneiter D, Ganter M, Bechir M, Grünenfelder J, Weder W. Simultaneous bilateral lobar lung transplantation: one donor serves two recipients. Ann Thorac Surg. 2013;96:e69-e71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Espinosa D, Algar FJ, Moreno P, Illana J, Alvarez A, Cerezo F, Baamonde C, Santos F, Vaquero JM, Redel J. Experience of the Reina Sofia hospital in lobar lung transplantation. Transplant Proc. 2010;42:3214-3216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Marasco SF, Than S, Keating D, Westall G, Whitford H, Snell G, Gooi J, Williams T, Pick A, Zimmet A. Cadaveric lobar lung transplantation: technical aspects. Ann Thorac Surg. 2012;93:1836-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Inci I, Schuurmans MM, Kestenholz P, Schneiter D, Hillinger S, Opitz I, Boehler A, Weder W. Long-term outcomes of bilateral lobar lung transplantation. Eur J Cardiothorac Surg. 2013;43:1220-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Shigemura N, D’Cunha J, Bhama JK, Shiose A, Abou El Ela A, Hackmann A, Zaldonis D, Toyoda Y, Pilewski JM, Luketich JD. Lobar lung transplantation: a relevant surgical option in the current era of lung allocation score. Ann Thorac Surg. 2013;96:451-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | Mitilian D, Sage E, Puyo P, Bonnette P, Parquin F, Stern M, Fischler M, Chapelier A. Techniques and results of lobar lung transplantations. Eur J Cardiothorac Surg. 2014;45:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Slama A, Ghanim B, Klikovits T, Scheed A, Hoda MA, Hoetzenecker K, Jaksch P, Matilla J, Taghavi S, Klepetko W. Lobar lung transplantation--is it comparable with standard lung transplantation? Transpl Int. 2014;27:909-916. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Stanzi A, Decaluwe H, Coosemans W, De Leyn P, Nafteux P, Van Veer H, Dupont L, Verleden GM, Van Raemdonck D. Lobar lung transplantation from deceased donors: a valid option for small-sized patients with cystic fibrosis. Transplant Proc. 2014;46:3154-3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Barnard JB, Davies O, Curry P, Catarino P, Dunning J, Jenkins D, Sudarshan C, Nair S, Tsui S, Parmar J. Size matching in lung transplantation: an evidence-based review. J Heart Lung Transplant. 2013;32:849-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 18. | Mason DP, Batizy LH, Wu J, Nowicki ER, Murthy SC, McNeill AM, Budev MM, Mehta AC, Pettersson GB, Blackstone EH. Matching donor to recipient in lung transplantation: How much does size matter? J Thorac Cardiovasc Surg. 2009;137:1234-40.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Eberlein M, Permutt S, Brown RH, Brooker A, Chahla MF, Bolukbas S, Nathan SD, Pearse DB, Orens JB, Brower RG. Supranormal expiratory airflow after bilateral lung transplantation is associated with improved survival. Am J Respir Crit Care Med. 2011;183:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Eberlein M, Arnaoutakis GJ, Yarmus L, Feller-Kopman D, Dezube R, Chahla MF, Bolukbas S, Reed RM, Klesney-Tait J, Parekh KR. The effect of lung size mismatch on complications and resource utilization after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 21. | Eberlein M, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Shlobin OA, Shelhamer JH, Reed RM, Pearse DB, Orens JB. Lung size mismatch in bilateral lung transplantation is associated with allograft function and bronchiolitis obliterans syndrome. Chest. 2012;141:451-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 22. | Eberlein M, Reed RM, Permutt S, Chahla MF, Bolukbas S, Nathan SD, Iacono A, Pearse DB, Fessler HE, Shah AS. Parameters of donor-recipient size mismatch and survival after bilateral lung transplantation. J Heart Lung Transplant. 2012;31:1207-1213.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Dezube R, Arnaoutakis GJ, Reed RM, Bolukbas S, Shah AS, Orens JB, Brower RG, Eberlein M. The effect of lung-size mismatch on mechanical ventilation tidal volumes after bilateral lung transplantation. Interact Cardiovasc Thorac Surg. 2013;16:275-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 24. | Eberlein M, Bolukbas S, Pena T, Reed RM. eComment. Lung size mismatch and graft dysfunction immediately after reperfusion. Interact Cardiovasc Thorac Surg. 2016;22:320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Eberlein M, Diehl E, Bolukbas S, Merlo CA, Reed RM. An oversized allograft is associated with improved survival after lung transplantation for idiopathic pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32:1172-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Eberlein M, Reed RM, Bolukbas S, Parekh KR, Arnaoutakis GJ, Orens JB, Brower RG, Shah AS, Hunsicker L, Merlo CA. Lung size mismatch and survival after single and bilateral lung transplantation. Ann Thorac Surg. 2013;96:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Eberlein M, Reed RM, Maidaa M, Bolukbas S, Arnaoutakis GJ, Orens JB, Brower RG, Merlo CA, Hunsicker LG. Donor-recipient size matching and survival after lung transplantation. A cohort study. Ann Am Thorac Soc. 2013;10:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 28. | Reed RM, Eberlein M. Sizing strategies in heart and lung transplantation: you cannot manage what you do not measure. Future Cardiol. 2014;10:303-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Loizzi D, Aigner C, Jaksch P, Scheed A, Mora B, Sollitto F, Klepetko W. A scale for decision making between whole lung transplantation or lobar transplantation. Eur J Cardiothorac Surg. 2010;37:1122-1125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 30. | Force SD. Invited Commentary. Ann Thorac Surg. 2015;100:2057-2058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 31. | Eberlein M, Bolukbas S, Reed RM. Bilateral lobar lung transplantation and size mismatch by pTLC-ratio. Eur J Cardiothorac Surg. 2013;44:394-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Reed RM, Eberlein M. Sizing considerations in lobar lung transplantation. Transpl Int. 2014;27:e132-e133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 33. | Eberlein M, Reed RM. Donor to recipient sizing in thoracic organ transplantation. World J Transplant. 2016;6:155-164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 34. | Hong JC, Yersiz H, Busuttil RW. Where are we today in split liver transplantation? Curr Opin Organ Transplant. 2011;16:269-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Weder W, Inci I, Korom S, Kestenholz PB, Hillinger S, Eich C, Irani S, Lardinois D. Airway complications after lung transplantation: risk factors, prevention and outcome. Eur J Cardiothorac Surg. 2009;35:293-298; discussion 298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 36. | Taher H, Reed RM, Eberlein M. Characterization of Donor to Recipient Size Matching in Lung Transplantation. Austin J Pulm Respir Med. 2014;1:1014. |
| 37. | Christie JD, Bellamy S, Ware LB, Lederer D, Hadjiliadis D, Lee J, Robinson N, Localio AR, Wille K, Lama V. Construct validity of the definition of primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1231-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 132] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 38. | Eberlein M, Reed RM, Bolukbas S, Diamond JM, Wille KM, Orens JB, Brower RG, Christie JD. Lung size mismatch and primary graft dysfunction after bilateral lung transplantation. J Heart Lung Transplant. 2015;34:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 39. | Horai T, Shigemura N, Gries C, Pilewski J, Bhama JK, Bermudez CA, Zaldonis D, Toyoda Y. Lung transplantation for patients with high lung allocation score: single-center experience. Ann Thorac Surg. 2012;93:1592-1597; discussion 1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Sill B, Oelschner C, Oldigs M, Deuse T. Elective Lobar Lung Transplantation - A Single Center Experience. Thorac cardiovasc Surg. 2015;63:ePP42. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 41. | Wilkens H, Weingard B, Lo Mauro A, Schena E, Pedotti A, Sybrecht GW, Aliverti A. Breathing pattern and chest wall volumes during exercise in patients with cystic fibrosis, pulmonary fibrosis and COPD before and after lung transplantation. Thorax. 2010;65:808-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 42. | Konheim JA, Kon ZN, Pasrija C, Luo Q, Sanchez PG, Garcia JP, Griffith BP, Jeudy J. Predictive equations for lung volumes from computed tomography for size matching in pulmonary transplantation. J Thorac Cardiovasc Surg. 2016;151:1163-1169.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |