Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.43
Peer-review started: July 12, 2016
First decision: September 9, 2016
Revised: October 9, 2016
Accepted: November 27, 2016
Article in press: November 29, 2016
Published online: February 24, 2017
Processing time: 231 Days and 2.1 Hours
To emphasize the effectiveness and versatility of prosthesis, and good tolerance by patients with incisional hernia (IH).
From December 2001 to February 2016, 270 liver transplantations were performed at San Camillo Hospital. IH occurred in 78 patients (28.8%). IH usually appeared early within the first year post-orthotopic liver transplantation. In the first era, fascial defect was repaired by primary closure for defects smaller than 2.5 cm or with synthetic mesh for greater defects. Recently, we started using biological mesh (Permacol™, Covidien). We present a series of five transplanted patients submitted to surgery for abdominal wall defect correction repaired with biological mesh (Permacol™, Covidien).
In our cases, the use of biological prosthesis (Permacol™, Covidien) have proven to be effective and versatile in repairing hernia defects of different kinds; patients did not suffer infections of the prosthesis and no recurrence was observed. Furthermore, the prosthesis remains intact even in the years after surgery.
The cases that we presented show that the use of biological mesh (Permacol™, Covidien) in transplanted patients may be safe and effective, being careful in the management of perioperative immunosuppression and renal and graft function, although the cost of the product itself has been the main limiting factor and there is need for prospective studies for further evaluations.
Core tip: Incisional hernia (IH) following abdominal organ transplantation have a high rate, and even more in immunosuppressed patients. Several factors have been described to be associated with IH in transplant patients. Herein, we present our preliminary experience with porcine dermal collagen mesh.
- Citation: Vennarecci G, Mascianà G, De Werra E, Sandri GBL, Ferraro D, Burocchi M, Tortorelli G, Guglielmo N, Ettorre GM. Effectiveness and versatility of biological prosthesis in transplanted patients. World J Transplant 2017; 7(1): 43-48
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/43.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.43
Incisional hernia (IH) following abdominal organ transplantation have a high rate. Every year thousands of transplant procedures are performed worldwide. Equally, the number of IH in this population is growing every year. This post-operative complication rate is estimated for kidney transplant, liver transplant and pancreas transplant as ranging from 1.6% to 18%[1,2], from 1.7% to 32.4%[3,4] and 13% to 34.8%[5,6] respectively.
Different causes have been proposed to increase IH risk. Among them are: Pre-transplant malnutrition, presence of abundant ascites for liver candidates, type of incision and type of wall closure, co-morbidities such as diabetes and obesity, multiple surgeries, and male sex. Compromised wound healing process is major in patients with an immunosuppressive regimen; nonetheless, this therapy increases the infections rate. The European Hernia Society recommend to use a porcine dermal collagen (PDC) mesh in these cases. In spite of this, no proven benefit vs synthetic mesh (SM) has been described.
Recent studies have shown that biological prostheses have a greater ability to integrate into tissues, resist bacterial colonization, reduce cytotoxic or allergic reactions, and provide similar functional results, compared with SM[7,8]. This article shows the experience of our surgical division in the use of PDC mesh (Permacol™, Covidien) in transplanted patients, emphasizing their effectiveness and versatility, and good tolerance by the patients.
From December 2001 to February 2016, 270 liver transplantations were performed at San Camillo Hospital. The transplant procedures were performed with the piggy-back technique without venous-venous bypass. Surgical access was obtained by a bilateral subcostal laparotomy with a cranial midline extension or a J-shaped (Makuuchi) laparotomy. Closure of the abdomen was performed with a slowly absorbable two-layer running sling suture. All patients received a triple immunosuppressive therapy with steroid, tacrolimus and mycophenolate. Everolimus has been used since 2010 in patients with renal dysfunction and/or associated hepatocellular carcinoma (HCC). IH occurred in 78 patients (28.8%). IH usually appeared early within the first year post-orthotopic liver transplantation (OLT). The elective surgical repair of the abdominal defect was delayed until the patient recovered good general condition. On average, repair was performed at a median of 29 mo (range: 22-45 mo) after OLT. IH was diagnosed by physical examination. In the first era, the fascial defect was repaired by primary closure for defects smaller than 2.5 cm or with SM for greater defects. Whenever possible, the sublay technique with implantation of the mesh between the closed posterior fascia and the muscle in the majority of patients was used. Otherwise, a dual-mesh prosthesis was implanted intraperitoneally. Recently, we started using PDC mesh (Permacol™, Covidien). The patient’s management included everolimus withdrawal before surgery, early nasogastric tube removal to facilitate oral feeding, administration of immunosuppressive therapy, peri-operative antibiotic administration, monitoring “graft function”, monitoring patient for local or chest infections, and e.v. fluid administration to avoid dehydration and renal dysfunction. In our practice, we applied a third-generation cephalosporin until the tube-drain removal.
Herein, we present a case series of OLT patients submitted to surgery for abdominal wall defect correction repaired with PDC mesh (Permacol™, Covidien), including: 1 case of subcostal/epigastric IH; 1 case of paraombelical IH; 1 case of reconstruction of the diaphragm in a patient with HCC recurrence infiltrating the diaphragm; 1 case of large-for-size liver graft mismatch; and 1 case of epigastric IH in a heart transplant (HT) patient (Table 1).
| Case No. | Age/sex | Type of transplant | Immunosuppressive therapy | Hernia size, cm | Time from transplantation to repair | Recurrence | Follow-up duration |
| 1 | 52/male | Liver | Tacrolimus + Everolimus | 10 × 8 | 8 mo | None | 2 yr |
| 2 | 58/male | Heart | Steroids + Tacrolimus | 10 × 10 | 5 yr | None | 3 yr |
| 3 | 55/male | Liver | Steroids + Tacrolimus + Everolimus | 8 × 8 | 6 mo | None | 5 yr |
| 4 | 58/female | Liver | Steroids + Tacrolimus + Everolimus | 20 × 15 | 3 d | None | 3 mo |
| 5 | 70/male | Liver | Tacrolimus | 6 × 7 | 4 yr | None | 6 mo |
A 52-year-old male was admitted to the hospital with a giant IH in the epigastrium region 4 years after OLT. A PDC (10 cm × 15 cm) mesh (Permacol™, Covidien) was positioned without tension to the edges of the fascia defect, and fixed with 2-0 interrupted polypropylene sutures. We used a Jackson-Pratt drain (Cardinal Health™) above the mesh construct. The skin was closed with interrupted sutures. Prophylactic antibiotics were given until post-operative d (POD) 5. The patient continued immunosuppressive therapy without any changes. The drain was removed and the patient was discharged on POD 5 without complications. No hernia recurrence was observed at 2-year follow-up after surgery.
A 58-year-old male was admitted with a subxiphoid-epigastric IH 5 years after a HT. The surgical access was a sternotomy with a subxiphoid extension. The abdominal IH occurred within 1 year from HT. The patient was on an immunosuppressive regimen with steroids, once-daily tacrolimus and everolimus. Everolimus was stopped 2 mo before surgery. Physical examination showed that the defect was about 20 cm in diameter. The operative procedure started with incision xypho-supraumbilical. The hernia sac was prepared and isolated by adhesions with cutaneous scar to the back-end of the rectus abdominis without opening the sac. The dissection was continued with the preparation of the rear end of the rectum to the lateral margin; the fascia was sutured on midline obtaining the reduction of the hernia sac in subfascial position. Permacol™ mesh (molded with diameter 15 cm × 13 cm) was implanted using the sublay technique and sutured with 0 interrupted polypropylene sutures. We placed 1 drain in the subfascial over the prosthesis and then sutured the front fascia of the rectus abdominis. Everolimus was restarted 2 wk after surgery. The drain was removed and the patient was discharged on POD 5 without complications. No hernia recurrence was observed at 3-year follow-up after surgery (Figure 1).
A 55-year-old male received a liver transplant 6 years earlier for autoimmune-related liver cirrhosis. At the time of the transplant procedure, the patient’s giant umbilical hernia (10 cm × 8 cm) was not repaired. The hernia sac was opened carefully, and no adhesions were found. The PDC mesh (Permacol™, Covidien) was fixed with not-absorbable sutures at the muscle-aponeurotic plane, bridging the defect without primary fascial apposition. A drain was placed in the subcutaneous plain. The subcutaneous tissue and skin were closed with interrupted sutures. Antibiotics were given until POD 6. The patient continued immunosuppressive therapy without any changes, including steroids at 7.5 mg daily. The drain was removed and the patient was discharged on POD 6 without complications. At 5 years after the surgery no hernia recurrence was observed.
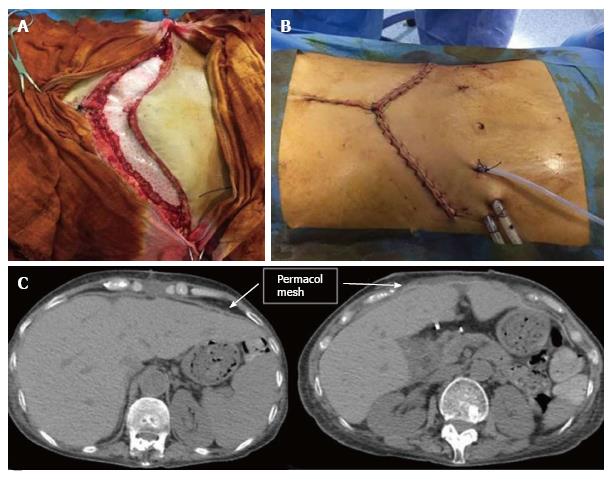
A 58-year-old female received a liver transplant in November 2015 for a primary biliary cirrhosis. The surgical access was a bilateral subcostal laparotomy with a cranial midline extension. Due to large-for-size liver graft mismatch, with a graft-to-recipient-weight-ratio of 3.3%, and presence of bowel edema, abdominal wall closure was not possible at the end of procedure. In order to prevent the onset of a compartment syndrome, a temporary wound closure with Bogota Bag was performed. After 3 d, a PDC mesh (Permacol™, Covidien) was molded (28 cm × 18 cm) and sutured at the muscle-aponeurotic plane with 0 interrupted polypropylene sutures (Figure 2A). We placed 1 drain in the subcutaneous plain and the skin was closed with continuous sutures above the mesh (Figure 2B). Post-operative course was characterized by respiratory distress (classified as Dindo-Clavien Grade II) resolved at POD 3. The patient was discharged on POD 5 and followed as out-patient. Three mo after the liver transplant, a CT scan showed the complete integrity of the biological prosthesis, and the patient had an excellent functional result (Figure 2C) and a normally perfusioned graft.
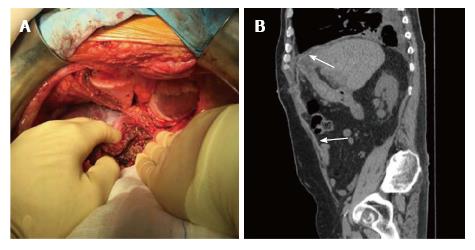
Four years after OLT for HCC, a 70-year-old male was admitted to the hospital with a recurrence of HCC infiltrating the peritoneum pericardium and diaphragm. Abdominal exploration showed a neoplasm of left lobe liver graft with infiltration of the diaphragm which extended to the pleura and pericardium. The operative procedure included a left lobectomy of the graft with resection of the diaphragm “en bloc” with the adjacent portion of right pleura and pericardium. The resection created a wide pleura-pericardial wall defect (Figure 3A). The wall defect was sheltered by apposition of a PDC mesh (Permacol™, Covidien) sutured to the diaphragm with 2-0 continuous polypropylene sutures. At the end of procedure, the subcostal wall defect was repaired by apposition of the same prosthesis used before. Everolimus therapy was discontinued 7 d before IH repair until POD 7. A mild pleural effusion (Figure 3B) was observed as post-operative complication.
The rate of IH after OLT is estimated to range from 1.7% to 32.4%[9,10]. In OLT patients several risk factors have been defined, including male sex, elevated body mass index, wound infection, hematoma, ascites, repeat interventions, immunosuppressive drugs, low platelets count, abdominal wall closure technique, diabetes mellitus and smoking history[11,12]. Different techniques are available to repair the IH, including open techniques with primary fascia closure and open or laparoscopic repair with synthetic or biological mesh[13]. Although permanent mesh prostheses are considered the best treatment for minimizing IH recurrence, they have been associated with a high risk of complications due to their non-absorbable characteristics, such as erosion into the abdominal viscera, protrusion, extrusion, adhesion, infection and bowel fistulae, that can lead to more complex and costly surgery[14].
Biological mesh was introduced as an alternative to SM in the 1990s[15]. The bioprosthetic materials are taken from several different species (bovine, porcine and equine) and from different organs (pericardium, skin and bowel submucosal)[14]. Biological mesh prostheses allow neo-vascularization and regeneration due to infiltration of native fibroblasts and they are incorporated into the surrounding tissue. During incorporation, they generate active neofascia to withstand the mechanical forces of the abdominal wall[16]. Recent studies have shown that biological prosthesis have a greater ability to integrate into tissues being colonized by host cells and blood vessels, resist bacterial colonization minimizing the risk of infection, reduce cytotoxic or allergic reactions, and provide similar functional results, compared with synthetic prosthesis. Porcine dermis is the closest to human dermis and it is not cytotoxic, hemolytic, pyrogenic or allergenic, and it does not elicit a foreign body response[17]. It is soft and flexible, and it has bilateral smooth surfaces with high tensile strength[17]. It is sold in sheets, allowing it to be cut to shape, and provides the largest grafts available (maximum size, 28 cm × 40 cm)[16,17]. In animal studies, a porcine dermal collagen implant produced a substantially weaker inflammatory response and less extensive, less dense adhesions[17,18].
To date, no prospective studies have been performed for which surgical technique in abdominal closure in IH is best, neither in indications about use of PDC mesh (Permacol™, Covidien). Some retrospective studies have shown that the use of a biological prosthesis may improve clinical outcome[19]. Schaffellner et al[20] reported an experience of 3 cases of ventral IH after OLT, and they did not observe wound healing disorders or signs of post-operative infections.
Our experience is limited to the use of PDC mesh (Permacol™, Covidien) in patients who underwent liver transplant and HT. In our series, biological mesh has been also used to bridge fascial defects, defined as placement of the PDC between edges of the rectus sheath where primary closure was not feasible; although, the data reported in the literature are not in favor of the use of biological prostheses in bridge repairing[21,22]. Of the 2 cases examined, the first (case 5) had a follow-up that was too short to consider a recurrence of IH, and the other (case 2) showed a good outcome, with no hernia recurrence at 3-year follow-up after surgery.
A grading system to stratify patients according to their risk factors for adverse surgical site occurrences has been proposed by the Ventral Hernia Working Group (VHWG)[23]. In this grading system, the immunosuppressed transplanted patients are classified as grade 2, which suggests that a PDC mesh may improve the outcome[23].
An Italian study described the biological meshes as useful and found a lower rate of infection and recurrence in transplanted patients[24]. Nonetheless, the use of banked fascia lata allografts seemed to provide a biocompatible, safe and effective alternative to other biological meshes[15].
Biological prosthesis is related with decreased number of infections, recurrence and mesh removal, compared to SM. The cases that we have presented show that the use of PDC mesh (Permacol™, Covidien) in transplanted patients may be safe and effective, being careful of the management of perioperative immunosuppression and renal and graft function; although, the cost of the product itself has been the main limiting factor and there is a need for randomized controlled trials for further evaluations. Our experience with PDC has been successful for several reasons. The prostheses have proven to be effective and versatile in repairing hernia defects of different kinds; moreover, in our series, patients did not suffer infections of the prosthesis and no recurrence was observed, even in cases in which they were used to bridge fascial defects. Furthermore, the prosthesis has remained intact even in the years after surgery.
Incisional hernia (IH) is a common complication after organ transplantation. Considering the immunosuppressed status, transplanted patients may have an increased risk of post-operative morbidity.
In this study, the use of biological mesh (Permacol™, Covidien) in transplanted patients, emphasizes its effectiveness and versatility, and good tolerance by the immunosuppressed patients.
To date, no prospective studies have been performed for surgical technique in abdominal closure in IH, neither regarding indications about use of porcine dermal collagen mesh.
IH following abdominal organ transplantation has a high rate and is related to the immunosuppressive status of the patient. Each year, thousands of new transplantations are performed and in the same way the number of IH has increased in these patients.
A porcine dermal collagen mesh prosthesis has a greater ability to integrate into tissues, resist bacterial colonization, reduce cytotoxic or allergic reactions, and provide similar functional results.
It is a well-written paper.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Darecchio A, Demirag A, Feretis M, Galun D, Salvadori M S- Editor: Qiu S L- Editor: Filipodia E- Editor: Lu YJ
| 1. | Mazzucchi E, Nahas WC, Antonopoulos I, Ianhez LE, Arap S. Incisional hernia and its repair with polypropylene mesh in renal transplant recipients. J Urol. 2001;166:816-819. [PubMed] |
| 2. | Knight RJ, Villa M, Laskey R, Benavides C, Schoenberg L, Welsh M, Kerman RH, Podder H, Van Buren CT, Katz SM. Risk factors for impaired wound healing in sirolimus-treated renal transplant recipients. Clin Transplant. 2007;21:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 3. | Piazzese E, Montalti R, Beltempo P, Bertelli R, Puviani L, Pacilè V, Nardo B, Cavallari A. Incidence, predisposing factors, and results of surgical treatment of incisional hernia after orthotopic liver transplantation. Transplant Proc. 2004;36:3097-3098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Gastaca M, Valdivieso A, Ruiz P, de Urbina JO. Reducing the incidence of incisional hernia after liver transplantation. Transpl Int. 2010;23:559-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Piros L, Máthé Z, Földes K, Langer RM. Incisional hernia after simultaneous pancreas kidney tranplantation: a single-center experience from Budapest. Transplant Proc. 2011;43:1303-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Hanish SI, Petersen RP, Collins BH, Tuttle-Newhall J, Marroquin CE, Kuo PC, Butterly DW, Smith SR, Desai DM. Obesity predicts increased overall complications following pancreas transplantation. Transplant Proc. 2005;37:3564-3566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Abdelfatah MM, Rostambeigi N, Podgaetz E, Sarr MG. Long-term outcomes (& gt; 5-year follow-up) with porcine acellular dermal matrix (Permacol) in incisional hernias at risk for infection. Hernia. 2015;19:135-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Beale EW, Hoxworth RE, Livingston EH, Trussler AP. The role of biologic mesh in abdominal wall reconstruction: a systematic review of the current literature. Am J Surg. 2012;204:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Vennarecci G, Guglielmo N, Pelle F, Felli E, Ettorre GM. The use of Permacol™ surgical implant for subxiphoid incisional hernia repair in cardiac transplant patients. Int J Surg. 2015;21:68-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Smith CT, Katz MG, Foley D, Welch B, Leverson GE, Funk LM, Greenberg JA. Incidence and risk factors of incisional hernia formation following abdominal organ transplantation. Surg Endosc. 2015;29:398-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 11. | Chang EI, Galvez MG, Padilla BE, Freise CE, Foster RD, Hoffman WY. Ten-year retrospective analysis of incisional herniorrhaphy following renal transplantation. Arch Surg. 2011;146:21-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 12. | Lo Monte AI, Damiano G, Maione C, Gioviale MC, Lombardo C, Buscemi G, Romano M. Use of intraperitoneal ePTFE Gore dual-mesh plus in a giant incisional hernia after kidney transplantation: a case report. Transplant Proc. 2009;41:1398-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Smart NJ, Marshall M, Daniels IR. Biological meshes: a review of their use in abdominal wall hernia repairs. Surgeon. 2012;10:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Bellows CF, Smith A, Malsbury J, Helton WS. Repair of incisional hernias with biological prosthesis: a systematic review of current evidence. Am J Surg. 2013;205:85-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Tiengo C, Giatsidis G, Azzena B. Fascia lata allografts as biological mesh in abdominal wall repair: preliminary outcomes from a retrospective case series. Plast Reconstr Surg. 2013;132:631e-639e. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 16. | Slater NJ, van der Kolk M, Hendriks T, van Goor H, Bleichrodt RP. Biologic grafts for ventral hernia repair: a systematic review. Am J Surg. 2013;205:220-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 17. | Pentlow A, Smart NJ, Richards SK, Inward CD, Morgan JD. The use of porcine dermal collagen implants in assisting abdominal wall closure of pediatric renal transplant recipients with donor size discrepancy. Pediatr Transplant. 2008;12:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Kaleya RN. Evaluation of implant/host tissue interactions following intraperitoneal implantation of porcine dermal collagen prosthesis in the rat. Hernia. 2005;9:269-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Brewer MB, Rada EM, Milburn ML, Goldberg NH, Singh DP, Cooper M, Silverman RP. Human acellular dermal matrix for ventral hernia repair reduces morbidity in transplant patients. Hernia. 2011;15:141-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Schaffellner S, Sereinigg M, Wagner D, Jakoby E, Kniepeiss D, Stiegler P, Haybäck J, Müller H. Ventral incisional hernia (VIH) repair after liver transplantation (OLT) with a biological mesh: experience in 3 cases. Z Gastroenterol. 2016;54:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Iacco A, Adeyemo A, Riggs T, Janczyk R. Single institutional experience using biological mesh for abdominal wall reconstruction. Am J Surg. 2014;208:480-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Patel KM, Nahabedian MY, Albino F, Bhanot P. The use of porcine acellular dermal matrix in a bridge technique for complex abdominal wall reconstruction: an outcome analysis. Am J Surg. 2013;205:209-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Breuing K, Butler CE, Ferzoco S, Franz M, Hultman CS, Kilbridge JF, Rosen M, Silverman RP, Vargo D. Incisional ventral hernias: review of the literature and recommendations regarding the grading and technique of repair. Surgery. 2010;148:544-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 702] [Cited by in RCA: 729] [Article Influence: 48.6] [Reference Citation Analysis (0)] |