Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.34
Peer-review started: August 10, 2016
First decision: September 12, 2016
Revised: October 27, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: February 24, 2017
Processing time: 198 Days and 12.6 Hours
To analyse the risk factors and outcomes of delayed graft function (DGF) in patients receiving a steroid sparing protocol.
Four hundred and twenty-seven recipients of deceased donor kidney transplants were studied of which 135 (31.6%) experienced DGF. All patients received monoclonal antibody induction with a tacrolimus based, steroid sparing immunosuppression protocol.
Five year patient survival was 87.2% and 94.9% in the DGF and primary graft function (PGF) group respectively, P = 0.047. Allograft survival was 77.9% and 90.2% in the DGF and PGF group respectively, P < 0.001. Overall rejection free survival was no different between the DGF and PGF groups with a 1 and 5 year rejection free survival in the DGF group of 77.7% and 67.8% compared with 81.3% and 75.3% in the PGF group, P = 0.19. Patients with DGF who received IL2 receptor antibody induction were at significantly higher risk of rejection in the early post-transplant period than the group with DGF who received alemtuzumab induction. On multivariate analysis, risk factors for DGF were male recipients, recipients of black ethnicity, circulatory death donation, preformed DSA, increasing cold ischaemic time, older donor age and dialysis vintage.
Alemtuzumab induction may be of benefit in preventing early rejection episodes associated with DGF. Prospective trials are required to determine optimal immunotherapy protocols for patients at high risk of DGF.
Core tip: Alemtuzumab induction may help mitigate the early rejection risk associated with delayed graft function following renal transplantation. This may help with the management of recipients of transplants at high risk of delayed graft function, as it may lessen the need for repeated histological sampling.
- Citation: Willicombe M, Rizzello A, Goodall D, Papalois V, McLean AG, Taube D. Risk factors and outcomes of delayed graft function in renal transplant recipients receiving a steroid sparing immunosuppression protocol. World J Transplant 2017; 7(1): 34-42
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/34.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.34
Delayed graft function (DGF) is associated with adverse allograft and patient outcomes[1-6]. The incidence of DGF has increased over the recent years in concordance with the expanding use of marginal donors[4,7]. Risk factors for DGF are well established and include both recipient and donor characteristics mediated through immunological and non-immunological mechanisms[1,4,6,8,9]. Strategies to reduce the incidence of DGF are imperative in order to improve transplant outcomes and minimise cost. Hypothermic machine perfusion has been shown to reduce the risk and severity of DGF but whether this will translate into beneficial long term outcomes is not known[10-15]. There are also numerous trials currently in progress which are focusing on immunomodulation of the transplant prior to engraftment in order to reduce the ischaemic reperfusion injury, which is thought to be the pathological mechanism behind DGF and its sequelae[16,17]. Such agents include complement (e.g., Mirococept® and Eculizumab®) and chemokine (e.g., Reparixin®) inhibitors[16,17].
Rejection has been shown to be associated with DGF with a reported incidence as high as 40%-50%[2-4,6,18,19]. Therefore, the type of immunosuppression protocol used may impact on the natural corollary of DGF and there is evidence to show that DGF outcomes may be improved with the use of lymphocyte depleting antibody induction[4,19-23]. Neither ATG nor alemtuzumab have been shown to reduce the risk of DGF, however it has been demonstrated that their use is associated with a decrease in the incidence of rejection episodes[4,19-24]. Conversely, even in the absence of DGF steroid avoidance protocols have been shown to be associated with a higher number of rejection episodes despite good medium term allograft survival[25-29]. The additional risk posed by using steroid sparing regimens to the incidence of rejection and outcomes in DGF has not been formally trialled.
The aim of this study is to describe the risk factors and outcomes of DGF in a large cohort of ethnically diverse, deceased donor recipients treated with monoclonal antibody induction and a steroid sparing immunosuppression protocol.
We retrospectively analysed 427 patients who received a deceased donor transplant at Imperial College Kidney and Transplant centre between 2005 and 2012. We excluded all patients who had lost their graft within 24 h due to technical reasons, recipients of living donor kidneys and simultaneous kidney-pancreas grafts. We included both deceased donor following circulatory death (DCD) and deceased donor following brain death (DBD) donors. All patients were CDC (T and B cell) and T cell flow cytometry cross match negative at the time of transplantation; patients with preformed donor specific antibodies detected by luminex methods only were included. Patient demographics are shown in Table 1.
| Factor | DGF n = 135 (%) | PGF n = 292 (%) | P value | |
| Recipient age | Years (mean) | 51.43 ± 12.19 | 47.45 ± 13.93 | 0.0046 |
| Donor age | Years (mean) | 51.56 ± 13.05 | 47.00 ± 15.99 | 0.0041 |
| Recipient gender | Male | 105 (77.8) | 178 (61.0) | 0.0009 |
| Female | 30 (22.2) | 114 (39.0) | ||
| Donor gender | Male | 69 (51.1) | 123 (42.1) | 0.12 |
| Female | 66 (48.9) | 167 (57.2) | ||
| Ethnicity | Black | 35 (25.9) | 41 (14.0) | 0.004 |
| Non-black | 100 (74.1) | 251 (86.0) | ||
| Time on RRT | Years (mean) | 6.37 ± 5.44 | 5.00 ± 5.07 | 0.012 |
| Regrafts | 1st | 114 (84.4) | 261 (89.4) | 0.2 |
| > 2nd | 21 (15.6) | 31 (10.6) | ||
| Donation type | DCD | 45 (33.3) | 32 (11.0) | < 0.00001 |
| DBD | 90 (66.6) | 259 (88.7) | ||
| CIT | Hours (mean) | 24.70 ± 7.82 | 21.29 ± 7.58 | 0.000023 |
| HLA mismatch | Mean | 3.47 ± 1.30 | 3.19 ± 1.58 | 0.079 |
| Preformed DSA | DSA+ | 17 (12.6) | 18 (6.2) | 0.039 |
| DSA- | 118 (87.4) | 274 (93.8) | ||
| Induction | Alemtuzumab | 113 (83.7) | 292 (84.9) | 0.86 |
| IL2RA | 22 (16.3) | 44 (15.1) | ||
| Recipient Diabetes | Yes | 35 (25.9) | 46 (15.8) | 0.02 |
| No | 100 (74.1) | 246 (84.2) | ||
All patients received monoclonal antibody induction with either anti-CD52 antibody [alemtuzumab (Mabcampath, Genzyme, United Kingdom)] or an anti-CD25 antibody [daclizumab (Zenpax®, Roche Inc, NJ) or basiliximab (Simulect®, Novartis Pharma Corp, NJ)]. All patients receive alemtuzumab induction unless they have a relative contraindication, which includes a past history of malignancy, hepatitis or previous significant immunosuppressive burden, when they receive an anti-CD25 antibody. Historically, patients enrolled into a clinical trial may also have received an anti-CD25 antibody at induction[29]. Maintenance immunosuppression included a steroid sparing, tacrolimus based regimen of tacrolimus monotherapy in the alemtuzumab induced patients and tacrolimus with the addition of mycophenolate mofetil in the anti-CD25 induced patients. All patients received a steroid sparing protocol of 500 mg methylprednisolone at the time of transplantation followed by one week of oral corticosteroids, which consists of 3 d of 30 mg prednisolone twice a day followed by 4 d of 30 mg once daily. Rejection episodes were diagnosed by biopsy and classified using the Banff 07 Classification of Renal Allograft Pathology[30]. Donor specific antibodies were detected using LABScreen® single antigen beads.
DGF was defined as the need for dialysis in the first week post-transplant.
All analyses were performed using Medcalc version 10.4.3. Comparisons of means and frequencies of normally distributed variables were calculated using t-tests and χ2/Fisher’s exact tests. Kaplan-Meier survival analysis was used to calculate time of event from index biopsy and statistical significance was determined by log rank testing. Cox proportional regression plots were used for multivariable analyses, variables with a significance level of P < 0.1 on univariant analysis were included in the multivariable analysis using a stepwise method selection. A P value of < 0.05 was deemed statistically significant.
The 135/427 (31.6%) of recipients of a deceased donor renal allograft experienced DGF. Patient and allograft outcomes were compared between the DGF and PGF (primary graft function) group, with a mean follow up was 42.62 ± 19.96 mo.
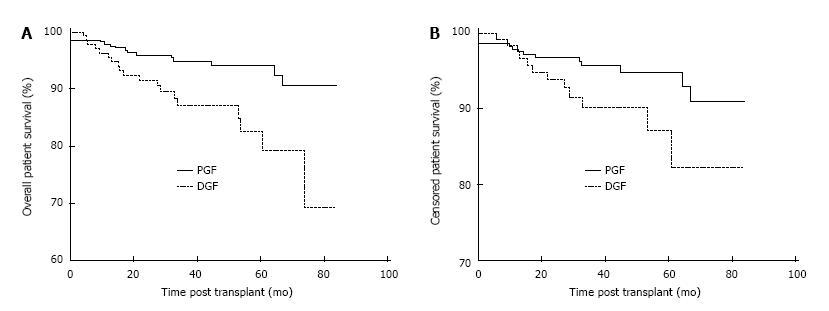
Patient survival was negatively impacted by the development of DGF post-transplant. Overall patient survival at 1, 3 and 5 years post-transplant was 96.3%, 87.2% and 82.5% in the DGF group and 97.9%, 95.0% and 94.2% in the PGF group, P < 0.01 as shown in Figure 1A. Censoring at the time of allograft failure, 1, 3 and 5 year patient survival was 98.4%, 90.2% and 87.2% in the DGF group and 97.9%, 95.7% and 94.9%, P = 0.047 in the PFG as shown in Figure 1B. The causes of death in the 11/135 (8.1%) DGF patients who died with a functioning graft were cardiovascular 4/11 (36.4%), sepsis 4/11 (36.4%), malignancy 1/11 (9.1%), autoimmune disease 1/11 (9.1%) and unknown 1/11 (9.1%).
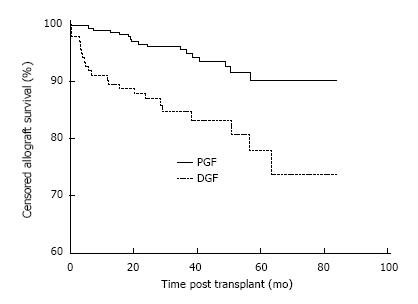
Allograft survival was also inferior in the DGF group. Censored allograft survival in the DGF group was 90.3%, 84.7% and 77.9% at 1, 3 and 5 years compared with 99.0%, 95.5% and 90.2% in the PGF group, P < 0.001 as shown in Figure 2. The causes of allograft failure in the 23/135 (17.0%) of patients with DGF were late technical losses in 4/23 (17.4%) (2 renal vein thrombosis, 2 ureteric complications), rejection in 6/23 (26.1%), BK nephropathy in 1/23 (4.3%), progressive scarring in 6/23 (26.1%) and multifactorial aetiologies in 6/23 (26.1%).
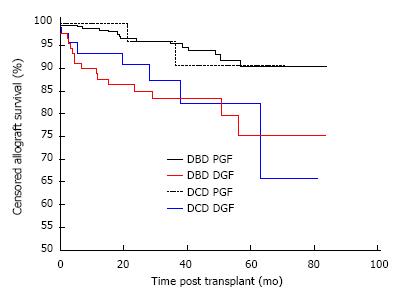
The development of DGF but not donor type impacted on allograft survival. Overall allograft survival in recipients of DBD and DCD kidneys with PGF was 90.3% and 90.7% respectively, which was significantly higher than recipients of DBD and DCD kidneys with DGF, which was 75.3% and 65.8% respectively, P = 0.0016 as shown in Figure 3. Comparing outcome by donor type, there was no difference in survival between DBD and DCD kidneys with PGF, P = 0.84 or with independently, DBD and DCD kidneys with DGF, P = 0.73.
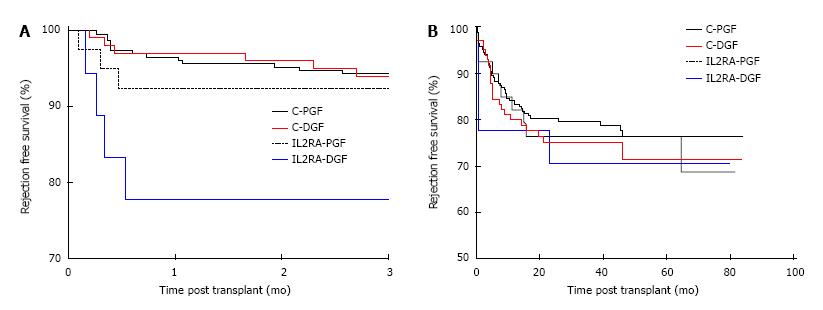
Patients with preformed DSA were at increased risk of rejection when compared with patients with no DSA, with a one year rejection free survival of 58.9% and 82.1% in the DSA+ and DSA-groups respectively, P < 0.001. Preformed DSA were also more frequent in the DGF group, with 17/135 (12.59%) and 18/292 (6.16%) patients having preformed DSA in the DGF and PGF groups respectively, P = 0.03. Censoring for DSA positive patients, the overall rejection free survival was no different between the DGF and PGF groups. The 1, 3 and 5 year rejection free survival in the DGF group was 77.7%, 72.2% and 67.8% compared with 81.3%, 77.7% and 75.3% in the PGF group, P = 0.19. However, comparing early rejection episodes by induction agent used and the occurrence of DGF, patients receiving an IL2RA who had DGF (IL2-DGF) were at significantly higher risk of rejection than the alemtuzumab-DGF (C-DGF) group in the first 3 mo post-transplant as shown in Figure 4A. The 3 mo rejection free survival was 93.0%, 92.9%, 92.5% and 77.8% in the C-PGF, C-DGF, IL2RA-PGF and IL2RA-DGF groups respectively, P = 0.03. However, this effect was not maintained and the overall rejection free survival was no different, with a rejection free survival of 76.4%, 71.5%, 76.5% and 70.7% in the C-PGF, C-DGF, IL2RA-PGF and IL2RA-DGF groups respectively, P = 0.75 as shown in Figure 4B. Induction agent had no subsequent impact on graft loss and patients with DGF had inferior allograft survival to those with PGF in the alemtuzumab and IL2RA groups, P = 0.0014. Allograft survival in the C-DGF group compared with the IL2-DGF group was 73.6% and 76.6%, P = 0.78 and 89.7% and 89.7% in the C-PGF and IL2-PGF groups respectively, P = 0.58.
De novo DSA free survival was lower in the DGF group in the first month only, with a DSA free survival of 89.8% and 95.3% in the DGF and PGF groups respectively, P = 0.04. At 3, 12, 36 and 60 mo the DSA free survival was 88.1%, 83.0%, 77.3% and 77.3% in the DGF group and 92.3%, 86.8%, 81.6% and 78.5% in the PGF group, P = 0.16, 0.29, 0.26 and 0.38 respectively.
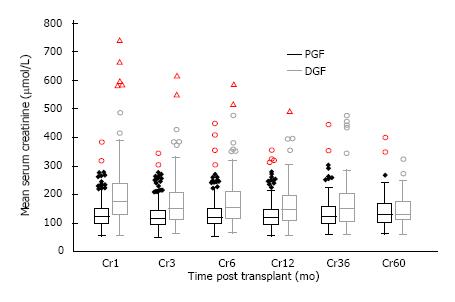
Allograft function of patients who remained dialysis independent was inferior in the DGF groups in the short to medium term as shown in Figure 5. Mean serum creatinine was 203.4 ± 120.0, 172.3 ± 86.6, 161.9 ± 74.9, 167.2 ± 86.1 and 149.6 ± 59.4 μmol/L at 1, 6, 12, 36, 60 mo post-transplant in the DGF group compared with 132.4 ± 48.6, 133.8 ± 56.7, 127.8 ± 43.6, 138.4 ± 47.7 and 143.0 ± 65.2 μmol/L in the PGF group; giving a P value of < 0.01 at 1 to 12 mo, a P value of 0.015 at 36 mo and 0.70 at 60 mo.
new onset diabetes after transplant (NODAT) free survival at 1, 3 and 5 years in the DGF group was 91.0%, 87.7% and 80.3% which was no different from the PGF group, which had a 1, 3 and 5 year NODAT free survival of 92.6%, 87.0% and 82.4%, P = 0.88.
The baseline demographics for the patients who developed DGF are shown in Table 2. On univariate analysis we found that both older donor and recipient age was associated with risk of DGF. The mean age of the recipient in the DGF and PGF groups was 51.4 ± 12.2 and 47.5 ± 13.9 respectively, P < 0.01; whilst the mean donor age was 51.6 ± 13.1 and 47.0 ± 16 respectively, P < 0.01. Male recipients were at higher risk of DGF, with 105/135 (77.8%) of the DGF group being male compared with 178/292 (61.0%) of the PGF group, P < 0.001. Donor gender did not influence DGF. Black recipients were more likely to experience DGF when compared with recipients of other ethnicities, with 35/135 (25.9%) of the DGF and 41/292 (14.0%) of the PGF group being of Black ethnic origin P = 0.004. Patients with DGF had spent longer on dialysis therapy pre-transplantation, with a mean dialysis vintage of 6.37 ± 5.44 and 5.00 ± 5.07 years in the DGF compared with PGF groups, P = 0.012. Recipients with DGF were more likely to be diabetic, with 35/135 (25.9%) of patients with DGF having diabetes compared with 46/292 (15.8% of the PGF group, P = 0.02). There were a significantly higher proportion of DCD donors in the DGF group, with 45/135 (33.3%) of the DGF patients receiving a DCD graft compared with 32/292 (11.0%) of the PGF group, P < 0.001. There was also a significant difference in the mean cold ischaemic time (CIT) between the groups, with a CIT of 24.70 ± 7.82 and 21.29 ± 7.58 h in the DGF and PGF groups respectively, P < 0.001.
| Variable | OR | 95%CI | P value |
| Black Ethnicity | 2.27 | 1.28-4.00 | 0.0047 |
| Female gender | 0.43 | 0.25-0.73 | 0.0017 |
| DCD donor | 4.09 | 2.33-7.20 | < 0.0001 |
| Preformed DSA | 2.36 | 1.07-5.18 | 0.0326 |
| CIT | 1.05 | 1.02-1.08 | 0.0009 |
| Donor age | 1.02 | 1.01-1.04 | 0.0049 |
| Time on dialysis | 1.07 | 1.02-1.11 | 0.0023 |
Statistically significant variables by univariate analysis were placed into a multivariable model. These included donor and recipient age, recipient being of male gender and black ethnicity, diabetic recipients, the presence of preformed DSA, DCD donors, CIT and dialysis vintage. Independent categorical risk factors for DGF were found to be black ethnicity [OR = 2.27 (1.3-4.0), P = 0.005], receiving a DCD graft [OR = 4.1 (2.3-7.2), P < 0.001], the presence of preformed DSA [OR = 2.36 (1.1-5.2), P = 0.03, with female gender being protective [OR = 0.43 (0.25-0.7), P = 0.002]. Continuous variables associated with DGF were CIT [HR = 1.05 (1.0-1.1) P < 0.001], with a CIT of > 20 h being most predictive of DGF; donor age [OR = 1.02 (1.01-1.04), P = 0.005] with a donor age of > 36 years being most predictive and time on dialysis [OR = 1.07 (1.02-1.11), P = 0.002], with risk increasing after 3.1 years. Recipient age and diabetes were not retained in the model.
In this descriptive study of the outcomes of DGF in a large series of ethnically diverse, deceased donor recipients receiving a steroid sparing immunosuppression protocol, we found that DGF is associated with inferior allograft and patient survival. This is in accordance with published DGF studies incorporating the use of corticosteroids[1-6]. Rejection was not increased in patients who experienced DGF compared with the PGF group, however we found that the rejection patterns differed depending upon the type of induction antibody used. Patients receiving IL2RA induction who had DGF were more likely to have rejection in the first 3 mo compared with those patients who received alemtuzumab induction. Risk factors associated with the development of DGF in our cohort were consistent with other studies and included donor age, recipients of a DCD organ, CIT, recipient gender and ethnicity, length of time on dialysis and the presence of preformed DSA[8,9]. This highlights CIT as a modifiable risk factor for DGF and efforts to reduce CIT are crucial in order to prevent DGF.
According to registry data, the incidence of DGF has increased over the past 2 decades, with an incidence of 21.3% reported in the United States in 2011[7]. Single centre series, depending on their patient population have reported an incidence of up to 45%[1]. The incidence of 27.4% we found in our deceased donor recipients despite steroid sparing is within this reported range. Inferior allograft outcomes are widely reported following DGF with increased risk of graft failure, rejection and poor function[1,2,4-6]. Less studies have analysed patient survival following DGF. Although there are individual series in which patient survival has been shown to be reduced, a meta-analysis did not demonstrate a significant association between DGF and death[1-3,23]. However, Narayanan et al[3] found that DGF following live donation was associated with death with a functioning graft.
To date no immunosuppression protocol has been shown to influence the development of DGF. However, it is recognised that immunosuppression and more precisely, the type of induction agent used can impact on the subsequent outcomes of DGF[4,20-23]. It has been shown that DGF is associated with increased risk of rejection[1,4,6,31]. However, this risk may be dependent upon the immunosuppression protocol as several studies have shown that the use of lymphocyte depleting induction agents, either ATG or alemtuzumab may reduce the risk of rejection in patients with DGF[4,20-23]. The effectiveness of ATG in preventing rejection in DGF may be dose dependent, which has not been reported post alemtuzumab[4,21,23,32]. Regarding further comparisons between ATG and alemtuzumab, in a prospective RCT in which the effectiveness of alemtuzumab vs ATG induction was examined in high risk patients with early steroid withdrawal, the incidence of early biopsy proven acute rejection (BPAR) was less in the patients who received alemtuzumab[26]. Despite, the overall incidence of DGF in that particular study being low due to the exclusion of marginal donors, the results might favour alemtuzumab over ATG to prevent early DGF associated rejection[26]. Several other studies have shown that alemtuzumab may mitigate the rejection risk of DGF[20,33,34]. Knechtle et al[20] in a retrospective study comparing alemtuzumab, thymoglobulin and anti-CD25 antibody induction, showed that alemtuzumab reduced the incidence of rejection in patients with DGF and improved allograft survival however the patients in this study were receiving maintenance corticosteroids. Tyson et al[31] in a RCT comparing ATG and alemtuzumab induction, had a similar proportion of marginal donors and DGF between the two arms and showed the incidence of BPAR to be less in the alemtuzumab arm. It should be noted that although alemtuzumab is associated with reduced early BPAR, alemtuzumab has been shown to be associated with a higher incidence of late BPAR resulting in equivocal rejection rates between ATG and alemtuzumab overall[26,35]. However, the use of alemtuzumab may be useful in the management of patients at high risk of DGF given the low early rejection risk, which may reduce the need for frequent biopsies.
Steroid sparing protocols have been shown to be associated with an increased rejection rate, although there is no adverse impact on allograft survival[25]. Conversely, corticosteroids use post-transplant is associated with NODAT, hypertension, hypercholesterolaemia and patient death secondary to cardiovascular and infectious complications[25-29]. The patient demographic at risk of DGF, which include older males and ethnic minorities are already more likely to have many of these complications and therefore steroids might confound the problem. Diabetes is a relatively new risk factor to be reportedly associated with DGF and peri-operative hyperglycaemia has been shown to exacerbate the ischaemic reperfusion injury in both animal and human models[36,37]. Steroid avoidance or early withdrawal might therefore help with diabetic control in the crucial recovery period.
Irish et al[8,9] formulated a predictive model of DGF by performing a multivariable logistic regression analysis of 24337 deceased donor transplant recipients in the United States. Given the relationship between DGF and allograft loss, their model predicts not only patients with increased risk of DGF but also those at risk of subsequent graft failure[8,9]. They found that the most significant risk factors for DGF to be CIT, donor creatinine, recipient body mass index, donor age and recipients of DCD organs[8,9]. They did not address the risk of low level preformed DSA, however they did find that the contribution to the overall risk according to the level of peak panel reactive antibodies (%) and previous transplantation diminished between two consecutive eras of immunosuppression[8,9]. Minimising CIT is an important variable in the lowering risk of DGF and improving outcomes and we accept that our mean CIT is higher than the average reported[38,39]. One study indicated a CIT of > 18 h was strongly associated with DGF and allograft failure[39]. Although cold storage slows the ischaemic damage, even in hypothermic conditions prolonged ischaemic times result in a more severe ischaemic reperfusion injury[17,40]. The superiority of hypothermic machine perfusion over static cold storage in preventing DGF is still an area of controversy and the long term benefit is not known[10-13]. The mechanisms through which machine perfusion is thought to minimise ischaemic injury include maintaining the patency of the vascular bed, providing nutrients and low level oxygen along with the ability to remove metabolic toxins[41]. In practice, machine perfusion is not universally available, therefore the most important modifiable factor in reducing DGF remains minimising CIT[38,40,42].
In conclusion, DGF is associated with inferior allograft and patient outcomes in patients receiving monoclonal antibody induction and a steroid sparing protocol. There is an increased risk of early rejection in patients with DGF receiving IL2RA compared with alemtuzumab induction, which implies that type of immunosuppression is important in the management of patients at risk of DGF. With an increase in the use of marginal donors, prospective studies into optimal immunotherapy protocols for these high risk patients are needed. Donor and recipient characteristics also contribute to the risk of DGF and CIT remains an important modifiable risk factor.
Delayed graft function (DGF) post renal transplantation is associated with adverse patient and allograft outcomes. The incidence of DGF has been increasing with the use of extended criterion donors, and strategies to reduce DGF are required in order to improve outcomes. Risk factors for DGF are well established and include both recipient and donor characteristics mediated through immunological and non-immunological mechanisms.
Significant research has been carried out to establish methods of optimising extended criterion allografts pre-implantation in order to provide the best outcomes. Most of these methods either involve hypothermic machine perfusion or immunomodulation of the transplant prior to engraftment in order to reduce ischaemic reperfusion injury. This study highlights the importance of immunosuppression post-transplant as a means to reduce any further injury to these allografts secondary to alloimmune responses.
In this study the authors directly compare the outcomes of DGF in patients receiving a steroid sparing immunosuppressive protocol but who either receive an IL2 receptor antibody or alemtuzumab induction.
This study shows how the type of induction immunosuppression may help in managing patients at high risk of DGF. By reducing the risk of early rejection in these patients, it may help with long term outcomes by preventing a secondary injury due to alloimune responses. If risk of rejection is low, it may also reduce the need for frequent histological examination.
Delayed graft function can be described in many ways, but the authors use one of the most common definitions, which is the need for dialysis in the first 7 d post renal transplant.
The paper is very exciting and instructive article.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United Kingdom
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Salvadori M, Sert I, Vlachopanos G, Xu H S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Yarlagadda SG, Coca SG, Formica RN, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24:1039-1047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 501] [Cited by in RCA: 567] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 2. | Grosso G, Corona D, Mistretta A, Zerbo D, Sinagra N, Giaquinta A, Cimino S, Ekser B, Giuffrida G, Leonardi A. Delayed graft function and long-term outcome in kidney transplantation. Transplant Proc. 2012;44:1879-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 3. | Narayanan R, Cardella CJ, Cattran DC, Cole EH, Tinckam KJ, Schiff J, Kim SJ. Delayed graft function and the risk of death with graft function in living donor kidney transplant recipients. Am J Kidney Dis. 2010;56:961-970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Siedlecki A, Irish W, Brennan DC. Delayed graft function in the kidney transplant. Am J Transplant. 2011;11:2279-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 587] [Article Influence: 41.9] [Reference Citation Analysis (0)] |
| 5. | Hirt-Minkowski P, Amico P, Hönger G, Praehauser C, Steiger J, Koller MT, Gürke L, Mayr M, Schaub S. Delayed graft function is not associated with an increased incidence of renal allograft rejection. Clin Transplant. 2012;26:E624-E633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 6. | Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63:968-974. [PubMed] |
| 7. | OPTN SRTR Annual Data Report 2011. 2013-06-13. Available from: http//srtr.transplant.hrsa.gov. |
| 8. | Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10:2279-2286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 9. | Irish WD, McCollum DA, Tesi RJ, Owen AB, Brennan DC, Bailly JE, Schnitzler MA. Nomogram for predicting the likelihood of delayed graft function in adult cadaveric renal transplant recipients. J Am Soc Nephrol. 2003;14:2967-2974. [PubMed] |
| 10. | Watson CJ, Wells AC, Roberts RJ, Akoh JA, Friend PJ, Akyol M, Calder FR, Allen JE, Jones MN, Collett D. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10:1991-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 155] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 11. | Lam VW, Laurence JM, Richardson AJ, Pleass HC, Allen RD. Hypothermic machine perfusion in deceased donor kidney transplantation: a systematic review. J Surg Res. 2013;180:176-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, van Kasterop-Kutz M, van der Heide JJ, Squifflet JP, van Heurn E. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360:7-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 781] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 13. | Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, Rahmel A, Squifflet JP, van Heurn E, Monbaliu D. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252:756-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 231] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 14. | Deng R, Gu G, Wang D, Tai Q, Wu L, Ju W, Zhu X, Guo Z, He X. Machine perfusion versus cold storage of kidneys derived from donation after cardiac death: a meta-analysis. PLoS One. 2013;8:e56368. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 15. | O’Callaghan JM, Morgan RD, Knight SR, Morris PJ. Systematic review and meta-analysis of hypothermic machine perfusion versus static cold storage of kidney allografts on transplant outcomes. Br J Surg. 2013;100:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 16. | Available from: http// www.isrctn.com/ISRCTN49958194. |
| 17. | Dompé Farmaceutici S. p.A. Reparixin in prevention of delayed graft dysfunction after kidney transplantation. In: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). Available from: http//www.clinicaltrials.gov/ct2/show/NCT00248040. |
| 18. | Morgan RD, O’Callaghan JM, Knight SR, Morris PJ. Alemtuzumab induction therapy in kidney transplantation: a systematic review and meta-analysis. Transplantation. 2012;93:1179-1188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 19. | Kosieradzki M, Rowiński W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40:3279-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 217] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 20. | Knechtle SJ, Fernandez LA, Pirsch JD, Becker BN, Chin LT, Becker YT, Odorico JS, D’alessandro AM, Sollinger HW. Campath-1H in renal transplantation: The University of Wisconsin experience. Surgery. 2004;136:754-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 105] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 21. | Noël C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, Charpentier B, Touchard G, Berthoux F, Merville P. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20:1385-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355:1967-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 573] [Cited by in RCA: 550] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 23. | Patel SJ, Duhart BT, Krauss AG, Moore LW, Egidi MF, Amiri HS, Gaber LW, Gaber AO. Risk factors and consequences of delayed graft function in deceased donor renal transplant patients receiving antithymocyte globulin induction. Transplantation. 2008;86:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Mourad G, Morelon E, Noël C, Glotz D, Lebranchu Y. The role of Thymoglobulin induction in kidney transplantation: an update. Clin Transplant. 2012;26:E450-E464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 25. | Woodle ES, Alloway RR, Hanaway MJ, Buell JF, Thomas M, Roy-Chaudhury P, Trofe J. Early corticosteroid withdrawal under modern immunosuppression in renal transplantation: multivariate analysis of risk factors for acute rejection. Transplant Proc. 2005;37:798-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Hanaway MJ, Woodle ES, Mulgaonkar S, Peddi VR, Kaufman DB, First MR, Croy R, Holman J. Alemtuzumab induction in renal transplantation. N Engl J Med. 2011;364:1909-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 27. | Opelz G, Döhler B. Association between steroid dosage and death with a functioning graft after kidney transplantation. Am J Transplant. 2013;13:2096-2105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 28. | Borrows R, Chan K, Loucaidou M, Lawrence C, Van Tromp J, Cairns T, Griffith M, Hakim N, McLean A, Palmer A. Five years of steroid sparing in renal transplantation with tacrolimus and mycophenolate mofetil. Transplantation. 2006;81:125-128. [PubMed] |
| 29. | Chan K, Taube D, Roufosse C, Cook T, Brookes P, Goodall D, Galliford J, Cairns T, Dorling A, Duncan N. Kidney transplantation with minimized maintenance: alemtuzumab induction with tacrolimus monotherapy--an open label, randomized trial. Transplantation. 2011;92:774-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, Halloran PF, Baldwin W, Banfi G, Collins AB. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753-760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1514] [Cited by in RCA: 1498] [Article Influence: 88.1] [Reference Citation Analysis (0)] |
| 31. | Tyson M, Castle E, Andrews P, Heilman R, Mekeel K, Moss A, Mulligan D, Reddy K. Early graft function after laparoscopically procured living donor kidney transplantation. J Urol. 2010;184:1434-1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Liu Y, Zhou P, Han M, Xue CB, Hu XP, Li C. Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: a meta-analysis. Transplant Proc. 2010;42:1667-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Farney AC, Doares W, Rogers J, Singh R, Hartmann E, Hart L, Ashcraft E, Reeves-Daniels A, Gautreaux M, Iskandar SS. A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation. 2009;88:810-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, Mattiazzi A, Cordovilla T, Roth D, Kupin W. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80:457-465. [PubMed] |
| 35. | Ciancio G, Burke GW, Gaynor JJ, Roth D, Kupin W, Rosen A, Cordovilla T, Tueros L, Herrada E, Miller J. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow-up. Clin Transplant. 2008;22:200-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 75] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Parekh J, Bostrom A, Feng S. Diabetes mellitus: a risk factor for delayed graft function after deceased donor kidney transplantation. Am J Transplant. 2010;10:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 37. | Parekh J, Roll GR, Feng S, Niemann CU, Hirose R. Peri-operative hyperglycemia is associated with delayed graft function in deceased donor renal transplantation. Clin Transplant. 2013;27:E424-E430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Giblin L, O’Kelly P, Little D, Hickey D, Donohue J, Walshe JJ, Spencer S, Conlon PJ. A comparison of long-term graft survival rates between the first and second donor kidney transplanted--the effect of a longer cold ischaemic time for the second kidney. Am J Transplant. 2005;5:1071-1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Opelz G, Döhler B. Multicenter analysis of kidney preservation. Transplantation. 2007;83:247-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 40. | van der Vliet JA, Warlé MC, Cheung CL, Teerenstra S, Hoitsma AJ. Influence of prolonged cold ischemia in renal transplantation. Clin Transplant. 2011;25:E612-E616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 41. | Taylor MJ, Baicu SC. Current state of hypothermic machine perfusion preservation of organs: The clinical perspective. Cryobiology. 2010;60:S20-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 98] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 42. | Kienzl-Wagner K, Schneiderbauer S, Bösmüller C, Schneeberger S, Pratschke J, Ollinger R. Nighttime procedures are not associated with adverse outcomes in kidney transplantation. Transpl Int. 2013;26:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |