Published online Feb 24, 2017. doi: 10.5500/wjt.v7.i1.1
Peer-review started: August 23, 2016
First decision: September 28, 2016
Revised: November 16, 2016
Accepted: December 7, 2016
Article in press: December 9, 2016
Published online: February 24, 2017
Processing time: 190 Days and 16.3 Hours
The evolutionary emergence of an efficient immune system has a fundamental role in our survival against pathogenic attacks. Nevertheless, this same protective mechanism may also establish a negative consequence in the setting of disorders such as autoimmunity and transplant rejection. In light of the latter, although research has long uncovered main concepts of allogeneic recognition, immune rejection is still the main obstacle to long-term graft survival. Therefore, in order to define effective therapies that prolong graft viability, it is essential that we understand the underlying mediators and mechanisms that participate in transplant rejection. This multifaceted process is characterized by diverse cellular and humoral participants with innate and adaptive functions that can determine the type of rejection or promote graft acceptance. Although a number of mediators of graft recognition have been described in traditional immunology, recent studies indicate that defining rigid roles for certain immune cells and factors may be more complicated than originally conceived. Current research has also targeted specific cells and drugs that regulate immune activation and induce tolerance. This review will give a broad view of the most recent understanding of the allogeneic inflammatory/tolerogenic response and current insights into cellular and drug therapies that modulate immune activation that may prove to be useful in the induction of tolerance in the clinical setting.
Core tip: Although the basic mechanisms of transplant allorecognition have been the object of intense study for the last 80 years, graft rejection is still an important obstacle in clinical practice. This review focuses on the principal concepts of transplant immunology and how they apply to the most recent discoveries in the field. It also reviews current treatments used to prolong graft survival and recent approach trends toward tolerance induction in the translational setting.
- Citation: da Silva MB, da Cunha FF, Terra FF, Camara NOS. Old game, new players: Linking classical theories to new trends in transplant immunology. World J Transplant 2017; 7(1): 1-25
- URL: https://www.wjgnet.com/2220-3230/full/v7/i1/1.htm
- DOI: https://dx.doi.org/10.5500/wjt.v7.i1.1
Although the first attempts at organ and tissue transplantation date to many centuries ago, knowledge of the underlying principles that orchestrate the immune response to this surgical procedure only began to be understood in the mid-twentieth century. Initial studies by Medawar and Gibson in the 1940’s showed that allogeneic skin rejection resulted from a response of the recipient to the graft[1,2], and years later, further studies demonstrated the characteristics mediated by cells in this response[3,4]. Since then, great advances have surged as further studies determined the role of different components of the immune system, such as antibodies, antigen-presenting cells (APCs) and T lymphocyte subpopulations, in allograft rejection and tolerance. Nevertheless, rejection is still the main barrier to the success of transplantation, and the development of agents that interfere with the alloimmune response and graft rejection has played a crucial role in the success of organ transplantation. This review will discuss the basic mediators that determine graft rejection and focus on the current immunobiology underlying transplantation research in this area.
Classically, transplantation is classified into four categories according to the origin of material to be grafted: Autologous, syngeneic, allogeneic or xenogeneic. Autologous transplantation occurs when cells, tissues or organs originate from the same individual, or in other words, a patient’s own tissue or organ is transferred. Syngeneic transplantation, in turn, occurs between two syngeneic or genetically identical individuals. A third type, which is the most common in the clinical setting, is allogeneic transplantation, which is performed between individuals of the same species that are genetically different, while xenogeneic transplantation occurs when the donor graft originates from a different species of the recipient.
The immune system has the intrinsic ability to distinguish between self and foreign (non-self) antigens, which allow it to develop a response against foreign organisms in order to destroy them. Specifically, in the context of transplants, this capacity is termed allorecognition and refers to the phenomenon by which the recipient’s immune system recognizes and reacts against donor antigens[5-7]. Thus, the transplantation of tissues or cells between genetically different individuals invariably triggers an immune response that may manifest itself as rejection depending on the magnitude of this response[8-10].
The success of solid organ transplants depends fundamentally on the control of the immune response to foreign molecules that differ among the same species, better known as alloantigens. Currently, a variety of relevant antigens have been described in the context of transplantation, including major histocompatibility complex (MHC) molecules, minor histocompatibility antigens (mHAgs), ABO antigens and endothelial/monocytic cell antigens.
In 1950, Snell[11] and Gorer[7] characterized and determined various antigens responsible for rejection not only in allogeneic tumors but also in healthy allogeneic tissue. Because they were the first antigens discovered regarding the rejection process, these were termed the MHC and are currently known to be the main targets of immune recognition of the surface of donor cells.
This group of genes is common among all vertebrates, and it has an important role in the immune system, mainly in determining the biological identity of individuals. In humans, it is termed human leukocyte antigen (HLA), and it is contained in the short arm of chromosome 6, which is a large chromosomal region with more than 200 coding loci. Based on structural and functional differences as well as on tissue distribution, the HLA products have been divided into three classes (I, II and III), with only classes I and II encoding HLA surface antigens, whereas class III encodes the components C2, C4 and factor B of the complement system[12-14]. These antigens are encoded by different genes inherited from both parents, which are expressed in a codominant fashion[15]. In addition to this, HLA surface antigens are extremely polymorphic[14], which contributes to numerous possible combinations and explains the difficulty in finding close compatibility between individuals. These codominant polymorphic genes influence, among other things, how the immune system responds to the graft recipient. Considering the differential immunogenicity of HLA mismatches observed in epidemiological studies[16], there are some acceptable mismatches, in which the recipient immune system could only weakly react to the donor, enabling longer graft survival. A greater impact of HLA-DR, HLA-A and HLA-B antigens has been observed in renal graft rejection[17], with a much larger effect of DR matching than the others[18,19]. Retrospective analysis of graft survival data also showed that certain HLA mismatch combinations are linked to increased allograft rejection[16,20].
MHC molecules play a critical role in the immune system, which corresponds to the presentation of peptides in a form that allows them to be recognized by T cells. Their highly polymorphic genes encode for cell surface receptors that have a central role in the control of immune recognition of self and non-self as well as subsequent tissue rejection, autoimmunity and immune responses to infectious diseases. Among all genes included in this region, two highly variable groups (MHC class I and class II) with differences in structure and presentation function are central in allorecognition.
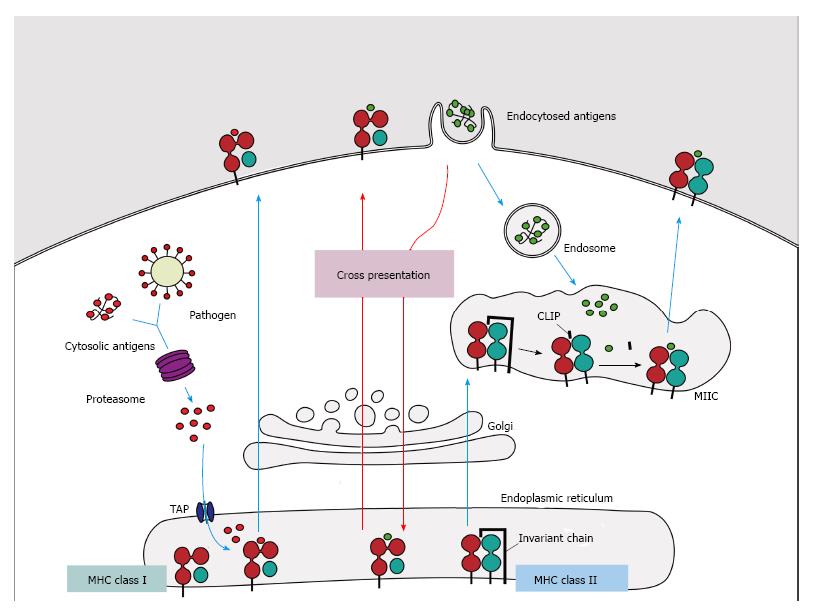
In humans, MHC class I molecules have three loci (HLA-A, HLA-B and HLA-C) and their products result in the classical class I molecules, which are expressed codominantly on all nucleated cells. Structurally, these molecules are formed by a heavy α chain (domains α1, α2 and α3), which is non-covalently associated with a light chain (β2-microglobulin) encoded by a gene located on chromosome 15[12]. These molecules have a groove formed by domains α1 and α2, to which endogenous peptides with length of 8 to 11 amino acids from the cytosol, intracellular parasites or tumors are attached, allowing their presentation on the cell surface of MHC class I-expressing cells, especially to cytotoxic CD8+ T cells[21-23] (Figure 1).
MHC class II molecules, which are encoded by three polymorphic genes (HLA-DR, HLA-DQ and HLA-DP), are constitutively expressed only on APCs, such as macrophages, dendritic cells (DCs), B cells and also thymic epithelial cells, although they may also be induced in other cells such as fibroblasts and endothelial cells under specific stimuli[12]. These molecules consist of a non-covalent association of the α and β polypeptide heterodimer chains, which are encoded by genes of the HLA-D region. Moreover, on class II molecules, the groove region consists of the α1 and β1 domains, and it is slightly larger than in class I molecules, allowing the binding of peptides between 13 and 18 amino acids. These molecules present exogenous peptides (via the endosome) on the surface of APCs[24], especially to helper CD4+ T cells[21-23] (Figure 1).
The MHC is the densest region of the human genome, and it is also one of the most variable, contributing to differences among individuals in immune responsiveness. It is well-known that MHC variants confer susceptibility to many chronic inflammatory and autoimmune conditions, including multiple sclerosis, type I diabetes and Crohn’s disease, as well as infectious diseases such as malaria and HIV[25-27]. Analysis of MHC variants has facilitated the localization of susceptibility loci for autoimmune diseases; however, for most genetic diseases, the specific loci involved remain undefined, and the mechanisms underlying the association of the MHC in autoimmune diseases remains poorly understood.
In 1994, a new group of polymorphic genes located near the HLA-B locus on chromosome 6, termed MHC class I chain-related genes (MIC genes), was described[28]. Only two members of the MIC gene family encode functional proteins, MHC class I chain-related protein A (MICA) and B (MICB), which are highly polymorphic[29]. The expression of these genes are induced by stress, encoding cell-surface glycoproteins that do not associate with β-2 microglobulin and are unable to bind peptides for presentation to T cells[30,31], in contrast to MHC class I molecules. MIC antigens bind to the NKG2D receptor present on NK cells, γδ and CD8 T lymphocytes[29,30], resulting in a cytotoxic response against cells expressing these MIC genes[32]. Moreover, the expression of the MIC gene family in an allograft can generate anti-MIC antibodies, which can lead to cell destruction and progressively to graft failure, as observed in renal allografts[33-35].
Several molecules encoded outside the MHC loci, such as the CD1 family, are structurally and functionally similar to classical MHC molecules and are therefore termed MHC-like molecules. The CD1 family consists of five glycoproteins coding for MHC-like molecules that associate with β2-microglobulin but have a deeper groove that is more hydrophobic than classical MHC molecules; this hydrophobic groove binds to lipid fragments and glycolipid antigens[36,37]. These molecules can present endogenous or exogenous lipid antigens to natural killer T (NKT) cells via the CD1d isoform. NKT cells are essential for cornea allograft survival because they are required for the induction of allospecific T regulatory cells[38]. Furthermore, human CD1d has been identified as a transplantation antigen that mediates a transplantation rejection response in a skin graft mouse model[39].
Acute and hyperacute rejection[40-42] may also occur in the absence of detectable HLA antibodies, suggesting that non-HLA molecules also play roles in rejection. One of these are mHAgs[43], which are peptides presented by MHC class I and II molecules with discrete polymorphisms and considerable allogeneic properties[44]. These antigens were initially characterized to possess a weaker potential to induce rejection in comparison to MHC antigens, although it has been shown that in MHC-compatible transplanted tissues, recognition of mHAgs[43] may also lead to early rejection. This may result from the principle that any polymorphic protein within a species can become a mHAg, thus expanding the possible number of mHAgs between non-identical individuals with compatible MHC. Nevertheless, mHAg-related rejection appears to be restricted to only some immunodominant epitopes[44,45]. Although the molecular basis of this phenomenon is not completely understood[46], these antigens may be encoded by sex chromosomes (the most widely studied are present in the Y chromosome), autosomal chromosomes (with various origins, such as myosin and the BCL2A1 and LBC oncogenes), and ultimately, mitochondrial DNA[47-50]. Additionally, immunity against these antigens is a significant clinical problem, as evidenced by the need for immunosuppression, even in the setting of HLA-identical transplantation, and the incidence of graft-vs-host disease (GVHD) following HLA-identical stem cell transplantation[51].
In addition, there are many other non-HLA antigenic determinants that are expressed on endothelial cells and monocytes that may also be potential targets in allorecognition[33], and non-HLA antibodies reactive with these cells appear to have a deleterious effect in several transplant models[46,52-54]. Moreover, ABO incompatibility arising from differences between the antigens of the ABO system, in turn, has less relevance in graft survival, but may also result in the hyperacute rejection of vascularized grafts such as kidney and heart grafts[55,56].
Antigen presentation is the primary component linking the innate and adaptive immune systems. It does so by permitting lymphocytes to establish effective immune surveillance of their environment through APCs and consequently mounting strong cellular and humoral responses. Nevertheless, this same process, which is essential for the detection of pathogens and potential tumor cells, is also responsible for the recognition of allogeneic antigens in a transplant setting. Thus, the allospecific immune response is mediated mainly by recipient lymphoid cell adaptive responses, which are orchestrated by T and B cells specific for MHC alloantigens expressed by the donor.
To achieve appropriate naïve T cell activation responses, a series of sequential signals are required consisting of: (1) T cell receptor (TCR) recognition; (2) costimulatory molecule signaling; and (3) cytokine activation. Each T lymphocyte has a unique and highly specific TCR on its surface that binds to the peptide-MHC complex on APCs, allowing their recognition as self or non-self. In the context of transplant rejection, this occurs as T cells specific for MHC antigens recognize foreign MHC-peptide complexes, which elicit a highly efficient response. Indeed, it is estimated that the frequency of alloreactive precursor T cells may be up to one thousand times greater than that of common antigens, demonstrating the efficiency of allogeneic immune responses[57]. If a lymphocyte recognizes the complex as non-self, it then becomes activated and begins to proliferate, adopting effector and memory functions that contribute to the response against the graft, which are detailed further in later sections.
B cells also play a major role in adaptive responses by producing antibodies directed against the graft. In this case, antigen presentation occurs when B-cell antigen receptors (BCRs), which consist of cell-surface immunoglobulins, recognize antigens either directly or through MHC presentation. Importantly, in the first setting, direct recognition induces antigen internalization and consequent MHC class II-peptide presentation to T cells, which in turn, along with co-stimulatory activation, drives B cell differentiation into antibody-producing plasma cells and memory B cells[58-60].
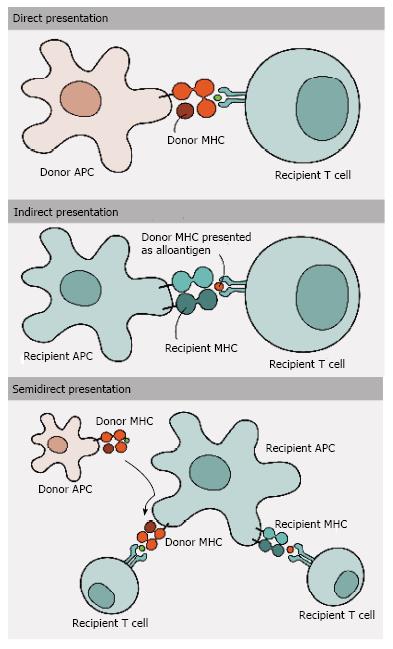
Allogeneic MHC molecules may be presented for recognition by TCRs via four fundamentally different, though not exclusive, pathways and thus may be involved in mediating allograft rejection simultaneously or in different contexts[51] (Figures 1 and 2). With direct presentation, recipient alloreactive T cells are directly activated after the recognition of allogeneic/non-self intact MHC class I and II molecules on the surface of donor APCs[5,61-63]. The presence of APCs in transplanted donor tissue dictates a strong anti-donor response early after engraftment, which decreases over time due the eventual death and removal of these donor APCs[64]. Indirect presentation, on the other hand, involves the capture and processing of allogeneic MHC class I and II donor molecules by recipient APCs[65,66], generating small peptides that are later presented by MHC class II molecules. This presentation results in alloresponses led by CD4+ T cells[67,68] and corresponds to slower responses than those generated via the direct route. The lower frequency of T cells with indirect allospecificity (compared to direct) in the normal repertoire suggests that the direct response dominates the early post-transplant period, while the indirect response develops a role in long-term alloantigen presentation, when donor APCs are already dead[69-71]. Semi-direct presentation, in turn, comprises the interaction between the recipient T cells and APCs, involving the exchange of intact peptide: MHC complexes by direct cell-to-cell contact[72-74] or by the release of small vesicles called exosomes[75,76]. Thus, the recipient APCs are able to present alloantigens directly to recipient T cells, allowing donor MHC and self MHC with donor peptide to be presented on the surface of the same cell. Even so, the precise role of this type of allorecognition in transplant rejection and tolerance remains to be fully elucidated[10].
The fourth type of presentation, cross-presentation, results from the ability of certain APCs to carry peptides that are derived from exogenous antigens on MHC class I molecules, an atypical characteristic, as endogenous antigens are commonly expressed on class I molecules and exogenous are expressed on class II. This type of presentation allows responses to pathogens that do not infect directly or replicate little within the APC[77]; however, this mechanism is not exclusive of infectious diseases, and the efficient priming of CD8+ T cells can occur after allogeneic transplantation as a consequence of cross-presentation of proteins derived from the donor by the recipient DCs[78].
Rejection can be divided into three main types: Hyperacute, acute or chronic, according to the cells and mechanisms involved in tissue damage and the consequent time course of graft loss.
Hyperacute rejection occurs due to the presence of preexisting antibodies towards graft antigens, caused by previous sensitization, which occurs in blood transfusions, organ transplant or even pregnancies. This recognition usually happens as soon as the organ is perfused, and widespread vascular injury associated with thrombosis prevents blood flow, leading to tissue necrosis and consequent graft loss within minutes to hours after the transplant. Nevertheless, this type of rejection is rarely observed in modern medicine due to pre-transplant CDC crossmatch exams that preemptively detect receptor reactivity to donor antigens.
Acute and chronic rejection are more difficult to prevent and less predictable. Acute rejection happens in the first weeks after transplant and is mainly associated with direct antigen presentation pathways, which activate CD4+ T lymphocytes to produce cytokines that amplify inflammation, and CD8+ T lymphocytes, which differentiate into cytotoxic cells upon activation and mediate direct graft cell destruction. These, in turn, also promote monocyte activation at graft sites, which also mediates the balance between tissue damage and repair[79-81].
Moreover, as donor APCs disappear with time, chronic rejection is mainly driven by indirect antigen presentation, where graft antigens are presented by recipient APCs[82,83]. In parallel, various studies also indicate that initial ischemia/reperfusion injury plays an important part in chronic graft rejection, and with time, together these factors ultimately culminate in a particular type of immune activation that causes progressive arterial damage and tissue fibrosis[84,85].
All these types of rejection simply establish a didactic form of characterizing the complex and often concomitant forms of graft rejection. The following portion of the review will approach the main cells mediating the sensitization and effector phases of graft rejection, focusing on the most recent data in literature.
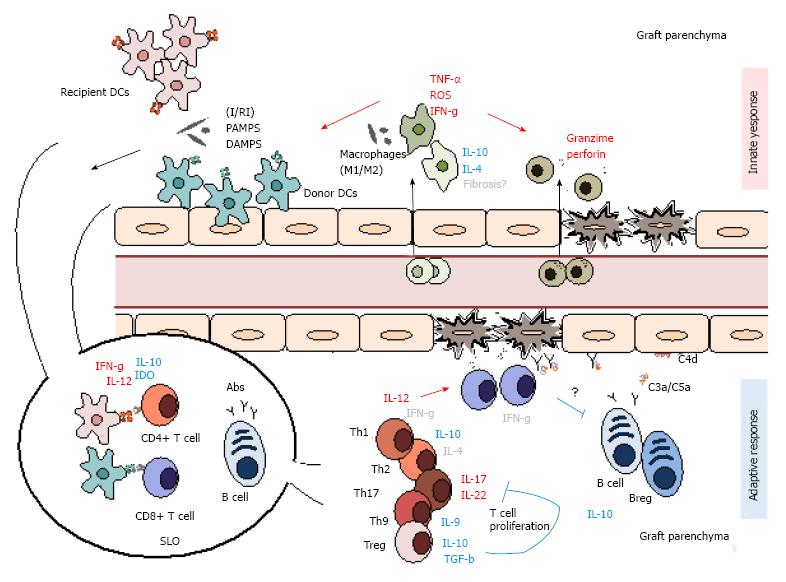
Since the beginning of transplant immunology, scientists have always focused on the adaptive mechanisms responsible for graft rejection and immunological memory, and until recently, little emphasis has been placed on the role of innate cells in allogeneic transplantation. Nonetheless, more recent research has noted that innate immune cells have a crucial role in triggering initial signals in transplant rejection and play an active role in establishing tolerance in transplantation (Figure 3).
The first immunological trigger to unfold during transplantation is almost always of innate origin due to the inevitable physical and ischemia-reperfusion (I/R) injury to solid organs during transplantation in addition to common conditioning regimens, such as chemotherapy, before bone marrow transplantation (BMT). This is particularly important, as it is responsible for the initial activation of innate cells and maturation of APCs to efficiently present antigens to T cells. These signals are expressed as damage-associated molecular patterns (DAMPS), such as heat shock proteins, heparin sulfate and reactive oxygen species (ROS), and activate pattern recognition receptors (PRRs) such as toll-like receptors (TLRs), leading to innate cell activation. These cells, in turn, secrete cytokines and chemokines such as TNF-α and IL-6, which give way to a cascade of events that amplify inflammation and attract further immune cell infiltration. Moreover, some reports have even suggested that innate cells may be able to distinguish allogeneic antigens, putting into question the lasting paradigms that divide the innate and adaptive responses[86,87]. This idea is defended by reports showing differential, memory-like recognition of alloantigens in RAG-/- mice. Some of these reports suggest that NK cells may participate in this phenomenon, showing that these cells develop a stronger IFN-γ response to a secondary stimulus[88]. NK cell-independent recall responses have also been shown in these mice, suggesting that other innate immune cells may also play a bigger role in adaptive immunity than first imagined. Nevertheless, recent research has also suggested that this recognition alone is insufficient to initiate alloimmunity, indicating that effective rejection can take place even in the absence of an innate response[89,90].
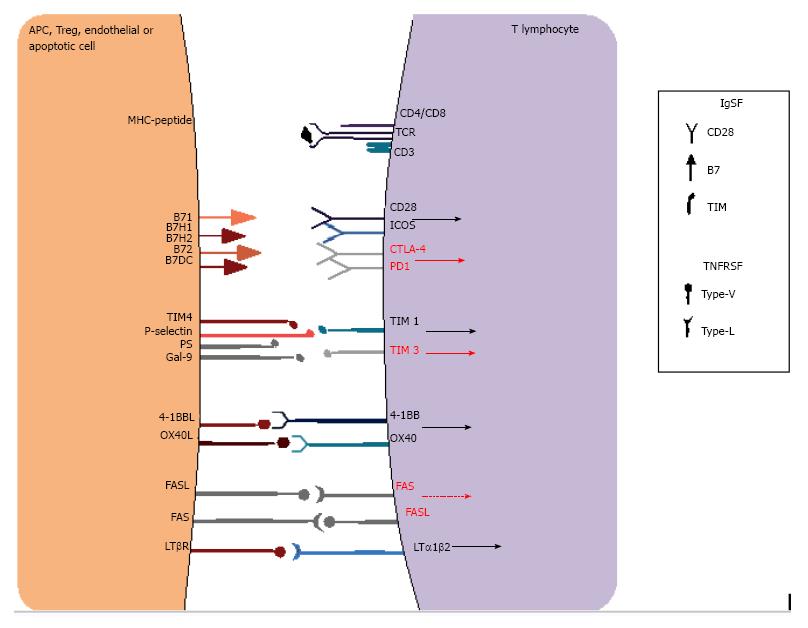
As cited previously, lymphocyte activation depends not only on an appropriate peptide presentation to antigen-specific T lymphocytes but also on the presence of efficient co-stimulatory signals. Therefore, there are two main signals needed for T cell activation: A first signal, involving antigen-specific MHC-peptide complex interaction to TCR molecules present in T cells, and a second signal, which consists of antigen-non-specific co-stimulation receptors on APCs and T cells that in turn drive intracellular activation signals with IL-2 production, T cell differentiation and survival. The basic literature usually describes main APC co-receptors such as B7 (CD80 and CD86), which interact with CD28 on T cells. However, a diverse number of other co-receptors are also known to have positive and negative effects on T cell activation (Figure 4), acting simultaneously at the immune synapse to effect cell activation or inhibition. The majority of known receptors belong to the immunoglobulin superfamily (IgSF) or the tumor necrosis factor receptor superfamily (TNFRSF), including OX40, CD40 and 4-1BB. Without the appropriate stimuli, T cells become anergic or enter apoptosis, and thus, these molecules are important targets for immunosuppression and cancer therapy, which will be detailed further on. Moreover, many different cells, such as DCs, macrophages and even B-lymphocytes, serve as APCs, as they all express both MHC and co-stimulatory molecules. These cells are considered professional APCs, and each have important roles in different contexts of graft allorecognition. It is also important to highlight that non-APCs also regulate lymphocyte activation, as is the case for apoptotic cells that express phosphatidylserine[91-94].
Macrophages are also important mediators of graft rejection, playing a part in antigen presentation and tissue inflammation and damage. These cells have been suggested as predictors of graft failure and are considered by some researchers to be even more reliable predictors than T cell infiltrates[95,96]. Macrophages originate from circulating monocytes, which infiltrate the graft due to multiple chemotactic factors and receptors, such as monocyte chemoattractant protein-1 (MCP-1), macrophage colony-stimulating factor (M-CSF)[97-100], and CX3C chemokine receptor 1 (CX3CR1). Some of these molecules have also been linked to kidney graft infiltration[101,102], differentiating into active mature cells that promote tissue injury. Accordingly, some studies even suggest a central role for CD68 monocytes in allograft dysfunction[103]. Studies assessing the preoperative Campath-1H (Alemtuzumab) treatment of renal recipients demonstrate the effects of monocytes in mediating acute rejection. Because Campath-1H depletes more T lymphocytes that monocytes, this study showed that CD68 monocytes were a dominant population in acute rejection[79,104].
In addition, mature monocytes are especially responsive to I/R injury and are activated soon after DAMP and PAMP stimuli, thereby secreting a range of cytokines that further activate other innate immune cells and also promote lymphocyte activation[105]. Macrophages are also prominent producers of ROS and eicosanoids that induce tissue damage and amplify the inflammatory cascade after tissue engraftment[106]. There are numerous subtypes of macrophages, ranging from inflammatory M1 cells, which produce increased amounts of TNF-α and IFN-γ, to more tolerogenic M2 macrophages, which secrete cytokines such as IL-4 and IL-10 and are associated with wound-healing and regulatory properties[107]. One study has indicated that the transfer of human regulatory macrophages (CD14−/lowHLA-DR+CD80−/lowCD86+CD16−CD64+TLR2− and CD163−/low) induces protection after renal transplant[108]. However, some studies have also associated M2 macrophages with increased allograft fibrosis[109-111]. However, this might depend on the time course of cell activation and the type of macrophage present, as DAMP, PAMP and dead cell clearance also reduces cell stimulation and innate immune activation.
Classically, NK cells are lymphocytes that respond to signals provided by tumor cells or virally infected cells. However, other stress-related signals can activate NK cells[112] through an unbalanced positive signal via membrane receptors. Although these cells share many characteristics with classical lymphoid cells, their activation takes shape through antigen-independent signals and does not produce immunological memory, falling therefore into the category of innate immunity. These cells recognize activating and inhibitory cell surface receptors that indicate cell stress, such as TLRs, class I MHC binding inhibitor receptors (e.g., Ly49), MHC class I-related binding activating receptors (e.g., NKG2D) and Fc receptors (e.g., CD16)[113,114]. In addition, NK cells are also activated by cytokines, such as IL-2, IL-15, IL-12 and IL-18[115]. Moreover, after activation, NK cells go on to perform effector functions such as cytotoxicity (perforin and granzymes) and cytokine production (IFN-γ, TNF-α, IL-22)[116]. Because NK cell class I MHC inhibitory receptors are polymorphic and recognize self-MHC, these cells are readily capable of responding to allogeneic graft cells due to the “missing-self” principle, leading researchers to investigate these cells’ role in graft rejection, especially after BMT. However, literature pertaining to NK cells in allorecognition is contradictory. Some authors demonstrated that these cells are important mediators in GVL (graft vs leukemia) effects, although they may accelerate graft failure due to an attack on donor cells[116,117]. On the other hand, recent research has also indicated that donor cells may evade allorecognition by acquiring host MHC class I molecules through the transfer of surface proteins from receptor cells, therefore inhibiting NK responses[118]. Nonetheless, most articles have shown that NK cells may also facilitate bone marrow engraftment and regulate graft-vs-host disease by suppressing donor and host T cells[119-122].
NKT cells are a heterogeneous population of T cells that express TCRs and NK markers and have properties of both T and NK cells. These cells recognize glycolipid antigens presented by CD1d on APCs instead of MHC molecules. They can be divided into two main subtypes depending on the TCR subchain expressed. Invariant or type I NKT cells express an invariant TCR β-chain (Vα14-Jα18 - mouse or Vα24-Jα18 - human) that is paired with a semi-invariant TCR β-chain (Vb11 - humans or Vβ2, Vβ7 or Vβ8.2 - mice), while type II NKT cells include all other CD1d-dependent T cells[123], with a very small frequency in the peripheral blood. After TCR activation, these cells can modulate the immune system by producing significant amount of Th1, Th2 and Th17 profile cytokines[124-126] and by increasing the expression of co-stimulatory molecules[127].
Recent studies indicate that these cells have tolerogenic effects and are crucial for the induction of peripheral tolerance. NKT cells induced transplantation tolerance towards allogeneic and xenogeneic islet cells transplanted into the liver and towards cardiac allografts[128-130]. The presence of these cells suppresses GvHD and solid organ rejection, which seems to be mediated by the production of IL-4 and IL-10 and by Treg activation[131-133].
DCs are the most prominent APCs involved in antigen presentation, mainly due to their particular ability to capture, process and express peptides via the MHC and their ability to migrate to T cell zones in lymph nodes, expressing high levels of co-stimulatory molecules along with peptides to T lymphocytes. These cells comprise an expressively diverse population that, after differentiating from the common DC precursor (CDP) or monocytes, when activated by danger signals as described above, transition from an immature state (iDCs) with low costimulatory receptor and MHC expression to a mature state (mDC), expressing high levels of costimulatory and MHC molecules.
DCs are classified into various subsets depending on their origin and the way they are activated, with the main types being plasmacytoid DCs (pDCs), conventional or classical DCs (cDCs) and inflammatory monocyte-derived dendritic cells (moDCs). The first population produces significant amounts of Type I and III IFN and diverse chemokines including CXCL1, CXCL3 and others[134,135]. However, they are considered poor APCs and are considered important in the induction of tolerance to grafts, which will be detailed further on.
In contrast, cDCs are efficient APCs that, when mature, produce various cytokines, such as IFN-γ, IL-12 and IL-10, which can direct T-cell activation towards an immunogenic or tolerogenic profile. Research suggests that cDCs are the main APCs responsible for alloantigen presentation during GvHD early after BMT[136]. cDCs are divided into CD8+ or CD8- cells, and there are many different reports on their effects on graft rejection. CD8+ DCs are only expressed in mice (not in humans), but some reports suggest that they have a regulatory role in BMT and solid organ transplantation, where they suppress the activation of other inflammatory DCs by producing indoleamine 2,3-dioxygenase (IDO) and increase Treg numbers and Treg production of IL-10[137-140].
Finally, moDCs possess strong inflammatory properties, differing from cDCs in that they originate from a monocyte precursor and express Gr-1/Ly6C. Although there are almost no in vivo data on the role of this specific population against other cells in graft rejection, some studies indicate that these cells have intense antigen-presenting functions, maybe even more than cDCs[86,87,141,142]. Other studies have also shown that these cells can effectively activate NK cells[143], which are discussed later. Future research shall elucidate the role of these cells in a transplantation setting.
The adaptive immune system has been recognized to have a critical response to organ transplantation. The rejection process is characterized by a highly complex series of cellular and humoral interactions in which T and B lymphocytes as well as DCs exhibit central and essential roles. Nevertheless, the immune response underlying allograft rejection is an ongoing dialogue between the innate and adaptive immune system, whereby innate immune cells modulate and direct the development of adaptive responses through pattern recognition receptor signaling (Figure 3).
To reduce transplant rejection, the biggest challenge faced is overcoming or suppressing adaptive immunity. T cells have a central role in adaptive effector responses due to their cytokine production and cytotoxic functions. After CD4+ T cell activation, the cells differentiate into subtypes, mainly including Th1, Th2, Treg, Th17 and Th9 cells, according to their signature cytokine production. Nevertheless, although these are some of the most studied mediators in transplantation, little consensus exists on their effects on graft rejection, with most of these cell types displaying dual roles in immune activation in transplantation.
In immunology, Th1 cells are considered classic pro-inflammatory actors. These cells are characterized by the expression of the T-bet transcription factor, along with the secretion of IFN-γ, TNF-α/β and IL-2, which in turn stimulate macrophages and lymphocytes towards enhanced effector functions associated with intracellular immunity. Specifically, IL-2 is essential to promoting T cell proliferation, while IFN-γ expression increases CD8+ T cell activation[144]. Many studies correlate IFN-γ expression to kidney graft rejection[145,146]. However, there are also data that showing that IFN-γ may prolong survival by reducing tissue necrosis and local granzyme-perforin secretion[147,148]. This has also been described in GvHD, whereas it prevented early onset of rejection[149], although this effect may depend on conditioning regimens[150]. In addition, IFN-γ expression by Tregs may also be important in reducing GvHD[151].
In contrast to Th1 cells, Th2 cells are traditionally considered immunomodulatory cells associated with extracellular immunity. They express the Gata-3 transcription factor and secrete IL-4, IL-5, IL-10 and IL-13. However, in a transplantation context, some studies demonstrate that Th2 cells have limited immunomodulatory properties[152-154]. Most recent data suggest that Th2 responses may have a negative role in transplant rejection[155]. In addition, some reports also suggest that IL-4 production by Th2 cells may accelerate cardiac and kidney rejection[156,157].
Th17 cells have also an important role in graft rejection. These cells express the transcription factor RORγT and are characterized by IL-17 and IL-22 production. Studies show that the absence of Th17 cells leads to prolonged renal graft survival with reduced IFN-γ and enhanced Treg function[158]. In addition, IL-17/IL-22 levels correlate with acute liver, kidney, islet and lung rejection in addition to GvHD[159-164]. However, the exact role of Th17 cells in transplant rejection may be more complex, as some studies have suggested that Th17 cells are more important for chronic rejection[165].
Finally, there are little data on the role of the recently discovered Th9 cells, which express increased levels of IL-9, in allograft rejection. Two articles suggest that CD4+ T cells that were co-stimulated and polarized with TGF-β and IL-4 in the presence or absence of rapamycin yielded effector cells of the Th9 phenotype that secreted increased IL-9 and expressed a transcription factor profile characteristic of both Th9 and Th2 cells (high GATA-3/low T-bet). Another transcription factor that promotes Th9 is PU.1. Its epigenetic modifications are important for Th9 immunity regulation[166]. These cells may have regulatory functions similar to Th2 cells by reducing IFN-γ alloreactivity and CD4+ and CD8+ T cell engraftment in BMT but also by inhibiting GVHD while increasing GVL[167,168].
CD8+ T cells have an important role in cell-mediated transplant rejection, with distinct cytotoxic effector functions, and were able to be activated even in the absence of CD4+ T cells[169], promoting cellular damage through the secretion of granules containing perforin, granzyme and granulysin. While perforin polymerizes, forming transmembrane pores on target cells, granzymes consist of a class of proteases that cleave substrates in the cytoplasm of target cells, triggering rapid apoptosis. Moreover, granulysin also mediates cell death, inducing ionic unbalance and mitochondria-mediated cell apoptosis in addition to facilitating intracellular bacterial killing[170]. In addition, CD8+ T cells can also express FasL, which binds to Fas receptors on target cells, causing caspase activation and consequently also leading to cell apoptosis. It has also been reported that APO2L/TRAIL constitute an additional pathway of T cell-mediated cytotoxicity[171,172], inducing apoptosis in a FasL- and perforin-independent manner.
In practice, there is no consensus on the specific importance of these cells in the context of allogeneic activation. Although CD8+ T cells may not be essential for some types of allograft rejection[173], others correlate their presence with graft cytotoxicity[174,175]. Recent data have shown that these cells may also inhibit alloantibody production by promoting alloprimed IgG1 (+), resulting in B cell death through FasL- and perforin-mediated apoptosis[176]. Moreover, these cells can also secrete a range of cytokines and are divided into two subclasses, Tc1 or Tc2. Type 1 CD8+ T cells (Tc1) cells mainly secrete IFN-γ, which was recently shown to promote hematopoiesis via increased myeloid differentiation in order to reinforce target cell clearance[177], and on the other hand also reduce IL-4-dependent IgG1 alloantibody production. In parallel, Tc2 cells mainly secrete IL-4 and IL-5 and have been shown to reduce GvHD[178-180].
Memory T cells represent a major challenge in the context of transplantation. Although they have an important role in defense against pathogens, especially in immunocompromised patients, they are also important in transplant rejection. These are very heterogeneous cells, both functionally and phenotypically, expressing different surface markers and residing in lymphoid and non-lymphoid tissues, such as the lung and liver[181,182]. Memory T cells are different from naive T cells because they are long-lasting cells, are antigen-independent persistent, and are capable of self-renewal[183]. Furthermore, they are able to be activated more easily than naïve T cells because they are less dependent on TCR stimulation and on co-stimulatory molecules[184]. These cells can be CD4+ or CD8+ cells, with the CD8+ subtype much more frequent and commonly studied[185]. They are dependent on sensitization, are linked to adaptive immune responses, and are responsible for the recall response[183]. These cells are also derived in an IL-7 dependent manner from effector T cells resistant to apoptosis[186,187]. Memory T cells also have greater and faster responsiveness to antigens than naive T cells because they are derived from effector T cells[188] and are more effective in the immediate response against antigens[189,190].
These cells are also expressly involved in transplant rejection[191-194]. Analysis in patients showed that higher frequencies of memory T cells pre-transplantation are related to higher post-transplantation complications[195,196]. Treatment with immunosuppressive drugs that reduce alloreactive T cells also favors the generation of memory T cells because this generate homeostatic proliferation without antigen stimulation[197], which causes naïve T cells to be converted into effector memory T cells[198,199]. Memory T cells are also involved in heterologous immunity, a process whereby cells activated by pathogens cross-react against alloantigens[200,201].
Memory T cells are also involved in tolerance resistance, mainly because they are highly reactive to donor antigens[191,202,203]. These cells have the ability to break Treg-induced suppression[193,204], constituting a barrier to treatments that aim to induce tolerance in transplantation. To circumvent this, studies have demonstrated that the depletion of memory T cells along with mixed chimerism through BMT after renal transplantation successfully induced a state of delayed tolerance[205].
A recent study has demonstrated that the level of CD38 on CD8+ memory T cells in the peripheral blood can predict the occurrence of GVHD[206]. Thus, the observation of T cell memory and its frequency in recipients may permit the establishment of a relative risk assessment of rejection mediated by these cells, or conversely, the possibility of establishing tolerance and the reduced probability of rejection.
B lymphoid cells are one of the main players in transplant rejection, and along with their antibody-producing properties, they also play an important part in allogeneic responses as APCs and cytokine producers. During B cell ontogeny, these cells go through different maturation stages, starting at the immature B cell stage and roaming to the spleen to complete their maturation. There, the majority of B cells become mature follicular B cells, which circulate between secondary lymphoid organs until they are activated, or marginal zone B cells, which continue in the spleen. Some articles have reported that B cells increase acute GvHD by accentuating T cell activation[207,208]. Chronic GvHD has also been linked to B cell responses via a positive correlation with high levels of autoantibodies[209,210]. Likewise, sex-mismatched BMT has also been associated with H-Y antibodies derived from donor B cells[211]. In addition, B cells also promote T cell activation as a result of antigen presentation and are able to induce graft rejection, even in an antibody-independent manner[212]. However, extensive literature has indicated that B cells may also have important tolerogenic properties in a transplantation setting, mainly via the suppression of T cells and DCs through cytokine production, which will be discussed in detail later in the review.
Antibodies are one of the most important mediators in transplant rejection and play a key role in both acute and chronic rejection. They are produced by transient plasmablasts and long-lived memory plasma B cells resident in secondary lymphoid organs and bone marrow. After transplantation, patients may display pre-existing or de novo donor-specific antibodies (DSAs) that target both HLA and non-HLA molecules. Data suggest that 20% of transplant patients will develop DSA within the first 5 years, and there are substantial data showing that these are responsible for accelerating graft rejection[213,214]. In summary, antigen recognition by antibodies results in the formation of antigen-antibody complexes, which recruit inflammatory cells through Fc receptor recognition and activate the classical pathway of complement activation. This, in turn, leads to the formation of active soluble byproducts that activate inflammatory cells and also leads to the formation of the membrane attack complex (MAC), leading to pore formation and consequent allogeneic cell death. Many studies have demonstrated the important role of complement activation in graft rejection, and many of its byproducts correlate with graft rejection. Both CD3a and C5a have been shown to induce APC and T cell activation, with increased expression of IL-6, costimulatory molecules and MHC II along with reduced FOXP3+ Treg formation[215-217]. In addition, C1q has also been shown to activate DCs, increasing TNF-α production and leading to a Th1 response[218]. Due to the vast formation of byproducts of complement activation, many researchers have also aimed to use these as biomarkers of antibody-mediated rejection. Among these, C4d, which is a product of C4d breakdown and easily localizes to endothelial cells and the basement membrane, has been shown to be of great value[219], although C4d-negative antibody-mediated rejection also exists.
The use of immunosuppressive drugs is essential in cases of solid organ transplantation because it can avoid the immune response against the graft or delay the appearance of de novo baseline disease. Thus, the most frequently used drugs act on pathways that inhibit the proliferation and activation of T cells, the main mechanisms involved in rejection[220]. Commonly, these drugs are used in combination, which can vary according to the patient, the type of transplant and also with the transplant center.
Azathioprine is the oldest immunosuppressive drug to be used in the prevention of rejection, and it was used with the first successful deceased kidney transplantation in 1962[221]. Although currently, it has not been commonly used in transplants, it is still an important treatment for autoimmune and inflammatory diseases[222-224].
Calcineurin inhibitors (CNIs), such as cyclosporin A and tacrolimus, are the most commonly used treatments. Cyclosporin A emerged as an alternative to azathioprine and triggered an important advance in medical transplants[225,226]. Tacrolimus has been the first choice of treatment in most transplant centers in Europe and the United States[223]. These drugs inhibit the calcineurin pathway, avoiding the dephosphorylation of NFAT (nuclear factor of activated T lymphocytes) and its translocation to the nucleus, ultimately blocking the activation of genes involved in T cell activation and, consequently, the propagation of the immune response[226,227]. However, the use of these drugs may induce nephrotoxicity[228,229] and can cause diabetes, dyslipidemia, hypertension, cardiovascular and kidney disease[230,231].
Everolimus and Sirolimus belong to another class of immunosuppressive drugs widely used in kidney transplantation in combination with other drugs. They inhibit mTOR (mammalian target of rapamycin), a kinase protein involved in the activation and proliferation of lymphocytes and tumor growth, among other functions[232], that is also related to the expansion of Treg cells[233,234].
Mycophenolate mofetil has been increasingly used as an initial immunosuppressive drug in recent years[222]. After it is metabolized, it generates mycophenolic acid, which inhibits inosine-5-monophosphate dehydrogenase (IMPDH), an important enzyme involved in purine synthesis. By inhibiting this enzyme, the drug can reduce T and B cell proliferation, in addition to decreasing the recruitment of lymphocytes to sites of inflammation and inducing necrosis in activated lymphocytes[235].
A more recent therapeutic option is Belatacept, a fusion receptor protein that blocks the CD80/CD86-CD28 co-stimulatory pathway, selectively inhibiting T cell activation[236]. Clinical studies have demonstrated that continuous treatment with Belatacept was associated with a consistent improvement in renal function post-transplantation[237-239].
Other treatment alternatives have also been tested. Studies have shown that the use of anti-CD40 can be effective[240] at preventing acute renal transplant rejection[241]. Clinical trials with a JAK3 (Janus kinase) inhibitor, Tofacitinib, in kidney transplantation showed low rates of rejection and a high graft survival, similar to cyclosporin, which was used as a control[242,243]. Phase II studies with Sotrastaurin have also been carried out. This molecule selectively inhibits protein kinase C, blocking T cell activation, although contradictory results regarding its efficacy in preventing rejection have been obtained[244,245].
Moreover, in some, cases, pre-treatment using monoclonal antibodies, such as Alemtuzumab, or polyclonal antibodies, such as anti-thymocyte globulin, can be used as induction therapy at the time of transplantation. This treatment depletes peripheral blood leukocytes, inducing lymphopenia[190], and can stimulate Treg cells[246,247] and regulatory B cells[248], enabling a reduction in the use of other immunosuppressive drugs.
Another class of drugs, proteasome inhibitors, can act directly on T and DC cells. The proteasome is essential for the maintenance and regulation of basic cellular processes, including cell signaling and survival pathways. The inhibition of proteasomal proteolytic activity by proteasome inhibitors suppresses essential immune functions. They can inhibit the activation of nuclear factor (NF)-κB and the transcriptional regulation of pro-inflammatory cytokine release and/or induce the apoptosis of activated immune cells. They can affect T cell activation, function, proliferation, and viability and suppress DC maturation and inhibit DC function. For this reason, they have already been tested in diverse autoimmune diseases, such as systemic lupus erythematosus and rheumatoid arthritis[249-252].
The use of immunosuppressive drugs has been the main option for transplant patients and has provided improvements in graft survival rates. However, many of these drugs present medical complications such as infections, nephrotoxicity, cardiovascular problems and cancer[228,253,254]. Furthermore, the treatment is not able to prevent chronic rejection, and the rates of chronic allograft dysfunction are still very high[255,256]. Additionally, the prolonged use and the high cost of immunosuppressors can lead to non-adherence to treatment[257]. Therefore, alternative therapies are needed, and the induction of tolerance would be an ideal substitute for the use of immunosuppressive drugs[258].
Immunological tolerance is an important mechanism to prevent anti-self immune responses and autoimmune diseases. In central tolerance, which occurs in the fetus thymus before T cell maturation, cells that react against self-antigens are deleted and regulatory T cells are expanded. On the other hand, peripheral tolerance, a secondary process of immunological tolerance, occurs in peripheral lymphoid organs, where there is induction of anergy and deletion of T cells that self-react against antigens that did not exist in the thymus or somehow escaped central deletion[259].
In the context of transplantation, true tolerance is by definition a permanent state of acceptance of alloantigens without the use of immunosuppressive drugs[260], given that in experimental models, animals must retain the ability to reject a third donor organ[261].
The induction of chimeras, or mixed chimerism, is a situation in which donor cells and recipient cells co-exist in the immune system[262], and it is an important technique to induce immunological tolerance. In chimera induction, hematopoietic cells from the donor are transferred to the recipient, and the recipient cells are retained, being only partially replaced by the donor[260]. In parallel, host bone marrow and donor thymus cells cause the central deletion of donor alloreactive T and B cells[260,263], allowing a new concept of what is self.
In experimental models, this method induces donor-specific tolerance and enables prolonged graft acceptance[264]. In humans, Alexander et al[265] demonstrated in a patient who received liver transplantation that the induction of mixed chimerism promoted tolerance and prevented GVHD occurrence. This method has already been used in transplants with good results[266] and is an important way to induce tolerance and prevent rejection or GVHD, allowing the long-term withdrawal of immunosuppressive drugs[267-269].
Currently, the existence of cells capable of regulating the immune response, leading to a more tolerogenic and less inflammatory profile, and restoring the balance of the immune system is well established. In the transplantation context, these cells are responsible for the balance between the survival and rejection of the graft[270]. Regulatory cells of the immune system, such as Tregs[271], tolerogenic DCs[272], and Bregs[273,274], have been detected in recipients that have developed operational tolerance. Therefore, the direct use of these cells, or of elements that stimulate these cells, may be important tools for tolerance induction because they are able to prevent or minimize the use of immunosuppressive drugs and their adverse effects[270,275-278].
Regulatory T cells play an important role in regulating the immune response and are responsible for the balance between the inhibition of autoimmunity (acting in tolerance against self antigens) and preventing tissue damage (acting on innate and adaptive response against non-self antigens)[259]. Two major subtypes of Tregs have been described. Naturally occurring Tregs are generated in the thymus from T-cell precursors expressing CD4, CD25 and the transcription factor Foxp3 and play an important role in maintaining tolerance to self-antigens or other antigens present in the thymus[279,280]. Moreover, induced or adaptive Tregs (iTregs), which are induced in the periphery in various tissues[281,282], express CD4 and Foxp3 and are responsible for the response against antigens not found in the thymus[283]. Thus, both subtypes may be responsible for the recognition of donor alloantigens and for the immune tolerogenic response against them[284].
Treg cells act through different mechanisms that can direct or indirectly inhibit T cell activation and proliferation. These cells can transmit inhibitory signals via cell-cell contact or secrete regulatory cytokines such as TGF-β, IL-10 or IL-35. In addition, they can also limit the availability of trophic factors, such as IL-2, to effector T cells, generate direct toxicity against target cells, or modulate APC functions. Moreover, these cells also act on other immune cells, such as B cells, NK, NKT and mast cells[259,279,283,285].
The induction of operational tolerance to transplantation is strongly associated with Tregs[270,283]. Therefore, the use of these cells has been tested in several ways. The use of these cells as a conditioning therapy before transplantation was able to induce tolerance[286], as was the use of Tregs for the generation of mixed chimerism, where donor Tregs were essential for the suppression of immune response[287,288]. The use of drugs or cytokines that induce Tregs in vivo also improve graft survival[289-291] along with donor alloantigen inoculation pre-transplantation[292], which promotes the expansion and proliferation of Tregs in vivo. Direct inoculation of Tregs or inoculation after ex vivo expansion was also effective in reducing rejection[293-295] and in the prevention of GVHD[296,297]. In humans, clinical trials have also shown that the infusion of Tregs is able to reduce GVHD[298,299]. Importantly, the immunosuppression generated is not global, as the injected Tregs retained the ability to respond to infections[296,298], which was an important advantage in comparison to immunosuppressive drugs.
As described previously, DCs are APCs that participate in T cell activation and are crucial for the activation of the immune response, including the response against alloantigens. When they become mature, they express some co-stimulatory surface markers, such as CD80, CD86, CD40, and MHC II[300]. Immature DCs have decreased expression of MHC II, CD86 and CD40, generating a more tolerogenic profile. Tolerogenic DCs have reduced production of cytokines such as IL-6 and IL-12 and increased IL-10 secretion[301]. Thus, they are capable of inducing clonal deletion, inhibiting memory T cells and inducing or expanding Tregs[277,302].
New therapies based on the transfer of tolerogenic DCs have been tested, especially for autoimmune diseases[278]. Blockade of DC-T cell interactions via co-stimulatory receptors and T cell surface molecules impairs T cell proliferation, preventing an exacerbated immune response[303]. Additionally, immature DCs are also able to promote tolerance in animal models of solid organ and BMT. Treatment with donor immature DCs[304-306] or regulatory DCs[307] in transplantation also prolongs graft survival and the development of GVHD. Moreover, DCs can also be conditioned to become tolerogenic through the use of cytokines, growth factors and drugs[308], and the use of TGF-β[309], and rapamycin[310], for example, were observed to prolong graft survival.
The role of B cells has always been related to the activation of the immune response and transplant rejection, especially through the production of antibodies. However, some B cell subtypes with regulatory functions are also observed to produce regulatory cytokines[311]. Many regulatory B cell subtypes (Bregs) have already been described, including the transitional cell (T1B and T2B), the marginal zone (MZ) B cell, the transitional 2 marginal zone precursor B cell (T2-MZP)[312] and a rarer CD1dhiCD5+ subtype, known as the B10 cell, that has received the most attention[313,314]. MZ B lymphocytes have been shown to produce high levels of IL-10 after the anti-CD40-mediated induction of tolerance[314]. In addition, B10 cells are found mainly in the spleen and also exert their actions exclusively via the production of IL-10, which regulates T-cell activation and inflammatory responses[315]. In an EAE model, Matsushita et al[316] demonstrated that regulatory B cells (B10) exert their function by altering IFN-γ and TNF-α secretion and suppressing T cell proliferation and acting on DCs, downregulating their antigen-presenting ability. Furthermore, another study has also demonstrated that Breg cells play an important role in the induction of Treg cells, maintaining high Treg levels in comparison to Th1 and Th17 cells[317].
B cells are strongly related to operational tolerance. Studies involving transplant patients show an increased percentage of B cells in the blood of tolerant patients compared to patients treated with immunosuppressive drugs or those who have suffered rejection[274,318,319]. B-cell-related genes are also differentially expressed in tolerant patients[273,319]. In addition, when evaluated in vitro, B cells from tolerant patients produced a higher amount of IL10 compared to those from non-tolerant patients[273]. Another study also showed that B cells from patients with chronic rejection do not inhibit autologous T cell proliferation, whereas B cells from healthy patients do[320], confirming the involvement of Breg cells in the tolerance induction process.
Thus, research in recent years has also aimed towards the use of B cells as a cellular therapy to induce tolerance. To this end, Breg cells were shown to induce chimerism and tolerance to donor antigens[321]. Likewise, studies in transplantation models indicated that Breg inoculation is effective towards prolonged graft acceptance[322,323] and the suppression of T cell activation[324], promoting the development of Treg cells, possibly via TGF-β production[325].
Mesenchymal stromal cells have known immunosuppressive properties and are capable of inhibiting T cell function and proliferation, inducing T cell apoptosis and inducing regulatory T cells[326]. The use of MSCs in solid organ transplantation has had important results. MSCs attenuate ischemia-reperfusion injury[327] and prevent graft rejection[328,329]. These cells are able to inhibit the T cell response[330,331] and inhibit the migration of activated T cells into the graft[332,333] in addition to expanding Treg cells[334-336] and tolerogenic DCs[337-339], generating a state of tolerance[326].
Based on evidence in experimental models that MSCs favor the development of tolerance and have demonstrated efficacy and safety, some clinical trials are in development[340]. The infusion of these cells was able to maintain stable graft function via Treg expansion and the reduction of memory T cells[341] and decrease the incidence of acute rejection[342].
Finally, the induction of tolerance is also essential to the fetus, which must tolerate maternal antigens, preventing an immune response against the mother. The immune environment of the developing fetus is specially prepared to generate immune tolerance, especially to non-inherited maternal antigens (NIMAs), protein products derived from polymorphic genes expressed by the mother. Fetal CD4+ T cells have a strong predisposition to differentiate into Tregs after activation by maternal antigens, which actively promotes tolerance to maternal cells residing in fetal tissues[343]. Afterwards, shortly before birth, the fetal cells transition to a more defensive adult-type response, with the ability to combat pathogens[344]. Maternal cells also play an important role in fetal protection during pregnancy. Maternal Treg cells are involved in this process, as they are enriched in the decidua and return to normal levels after birth[345], which does not occur in cases of miscarriage[346,347].
The establishment of microchimerism is the primary factor responsible for the generation of Tregs because fetal cells also have access to the mother. This chimerism occurs both in the maternal tissues and in the fetal tissues, and maternal cells are often found in fetal tissues[343,348], remaining for a long period after birth[349]. Even after development, the ability to generate tolerance to antigens that have been in contact with the fetus is not lost, consisting in a postnatal tolerance. This fact was confirmed in a study by Burlingham et al[350], who showed that patients who received HLA-haploidentical sibling renal transplantation of which the mismatch corresponded to a NIMA had a significant increase in graft survival compared to those in which the mismatch was a non-inherited paternal antigen (NIPA), suggesting a relationship with the exposure to antigens during the fetal period. Other studies using a heart transplantation model also demonstrated that allografts expressing NIMAs were protected from rejection when implanted in offspring mice that had come into contact with the same NIMAs during pregnancy, therefore creating a predisposition to transplantation tolerance in mice as an adult[351], mainly through the induction of NIMA-specific Treg cells[352].
Although the basic mechanisms of transplant allorecognition have been the object of intense study for the last 80 years, graft rejection is still an important obstacle in clinical practice. Allorecognition is an unfortunate disadvantage to the evolution of more effective immunological surveillance and is therefore especially complex to surpass. Nonetheless, current advances have shed light on important mediators that fuel graft rejection, making the search for new therapies possible. In addition, promising discoveries have been made in the search for effective immunosuppressive regimens and, more importantly, the achievement of functional tolerance.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ni Y, Salvadori M S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 2. | Medawar PB. The behaviour and fate of skin autografts and skin homografts in rabbits: A report to the War Wounds Committee of the Medical Research Council. J Anat. 1944;78:176-199. [PubMed] |
| 3. | Mitchison NA. Passive transfer of transplantation immunity. Proc R Soc Lond B Biol Sci. 1954;142:72-87. [PubMed] |
| 4. | Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603-606. [PubMed] |
| 5. | Sherman LA, Chattopadhyay S. The molecular basis of allorecognition. Annu Rev Immunol. 1993;11:385-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 304] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Hernandez-Fuentes MP, Baker RJ, Lechler RI. The alloresponse. Rev Immunogenet. 1999;1:282-296. [PubMed] |
| 7. | Gorer P. The genetic and antigenic basis of tumor transplantation. J Pathol Bacteriol. 1937;44:691-697. |
| 8. | Guild WR, Harrison JH, Merrill JP, Murray J. Successful homotransplantation of the kidney in an identical twin. Trans Am Clin Climatol Assoc. 1955;67:167-173. [PubMed] |
| 9. | Michon L, Hamburger J, Oeconomos N, Delinotte P, Richet G, Vaysse J, Antoine B. [An attempted kidney transplantation in man: medical and biological aspects]. Presse Med. 1953;61:1419-1423. [PubMed] |
| 10. | Afzali B, Lombardi G, Lechler RI. Pathways of major histocompatibility complex allorecognition. Curr Opin Organ Transplant. 2008;13:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Klein J, Sato A. The HLA system. First of two parts. N Engl J Med. 2000;343:702-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 525] [Cited by in RCA: 523] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 13. | King A, Hiby SE, Gardner L, Joseph S, Bowen JM, Verma S, Burrows TD, Loke YW. Recognition of trophoblast HLA class I molecules by decidual NK cell receptors--a review. Placenta. 2000;21 Suppl A:S81-S85. [PubMed] |
| 14. | Bodmer JG, Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Hansen JA, Mach B, Mayr WR. Nomenclature for factors of the HLA system, 1998. Tissue Antigens. 1999;53:407-446. [PubMed] |
| 15. | Chinen J, Buckley RH. Transplantation immunology: solid organ and bone marrow. J Allergy Clin Immunol. 2010;125:S324-S335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 16. | Doxiadis II, Smits JM, Schreuder GM, Persijn GG, van Houwelingen HC, van Rood JJ, Claas FH. Association between specific HLA combinations and probability of kidney allograft loss: the taboo concept. Lancet. 1996;348:850-853. [PubMed] |
| 17. | Opelz G. Correlation of HLA matching with kidney graft survival in patients with or without cyclosporine treatment. Transplantation. 1985;40:240-243. [PubMed] |
| 18. | Gilks WR, Bradley BA, Gore SM, Klouda PT. Substantial benefits of tissue matching in renal transplantation. Transplantation. 1987;43:669-674. [PubMed] |
| 19. | Doxiadis II, de Fijter JW, Mallat MJ, Haasnoot GW, Ringers J, Persijn GG, Claas FH. Simpler and equitable allocation of kidneys from postmortem donors primarily based on full HLA-DR compatibility. Transplantation. 2007;83:1207-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 20. | Reisaeter AV, Leivestad T, Vartdal F, Spurkland A, Fauchald P, Brekke IB, Thorsby E. A strong impact of matching for a limited number of HLA-DR antigens on graft survival and rejection episodes: a single-center study of first cadaveric kidneys to nonsensitized recipients. Transplantation. 1998;66:523-528. [PubMed] |
| 21. | Luz JG, Huang M, Garcia KC, Rudolph MG, Apostolopoulos V, Teyton L, Wilson IA. Structural comparison of allogeneic and syngeneic T cell receptor-peptide-major histocompatibility complex complexes: a buried alloreactive mutation subtly alters peptide presentation substantially increasing V(beta) Interactions. J Exp Med. 2002;195:1175-1186. [PubMed] |
| 22. | Germain RN. MHC-dependent antigen processing and peptide presentation: providing ligands for T lymphocyte activation. Cell. 1994;76:287-299. [PubMed] |
| 23. | York IA, Rock KL. Antigen processing and presentation by the class I major histocompatibility complex. Annu Rev Immunol. 1996;14:369-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 455] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 24. | Chaplin DD. 1. Overview of the human immune response. J Allergy Clin Immunol. 2006;117:S430-S435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 25. | Fellay J, Shianna KV, Ge D, Colombo S, Ledergerber B, Weale M, Zhang K, Gumbs C, Castagna A, Cossarizza A. A whole-genome association study of major determinants for host control of HIV-1. Science. 2007;317:944-947. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1029] [Cited by in RCA: 998] [Article Influence: 55.4] [Reference Citation Analysis (0)] |
| 26. | Fellermann K, Stange DE, Schaeffeler E, Schmalzl H, Wehkamp J, Bevins CL, Reinisch W, Teml A, Schwab M, Lichter P. A chromosome 8 gene-cluster polymorphism with low human beta-defensin 2 gene copy number predisposes to Crohn disease of the colon. Am J Hum Genet. 2006;79:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 386] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 27. | Barcellos LF, Sawcer S, Ramsay PP, Baranzini SE, Thomson G, Briggs F, Cree BC, Begovich AB, Villoslada P, Montalban X. Heterogeneity at the HLA-DRB1 locus and risk for multiple sclerosis. Hum Mol Genet. 2006;15:2813-2824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 220] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 28. | Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1994;91:6259-6263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 596] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Racca AL, Veaute CM, Bailat AS, Gaite L, Arriola M, Hajos SE, Malan Borel IS. Expression of HLA-G and MICA mRNA in renal allograft. Transpl Immunol. 2009;21:10-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Tonnerre P, Gérard N, Chatelais M, Charreau B. MICA gene polymorphism in kidney allografts and possible impact of functionally relevant variants. Transplant Proc. 2010;42:4318-4321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Zwirner N, Fuertes MB, Girart MV, Domaica CI, Rossi LE. Immunobiology of the human MHC class I chain-related gene A (MICA): from transplantation immunology to tumour immune escape. Inmunología. 2006;378-385. |
| 32. | Stastny P. Introduction: MICA/MICB in innate immunity, adaptive immunity, autoimmunity, cancer, and in the immune response to transplants. Hum Immunol. 2006;67:141-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 33. | Sumitran-Holgersson S. Relevance of MICA and other non-HLA antibodies in clinical transplantation. Curr Opin Immunol. 2008;20:607-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Cobbold SP. Rejecting minors--it’s all in the presentation. Transplantation. 2011;91:152-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Mizutani K, Terasaki P, Bignon JD, Hourmant M, Cesbron-Gautier A, Shih RN, Pei R, Lee J, Ozawa M. Association of kidney transplant failure and antibodies against MICA. Hum Immunol. 2006;67:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Martin LH, Calabi F, Milstein C. Isolation of CD1 genes: a family of major histocompatibility complex-related differentiation antigens. Proc Natl Acad Sci USA. 1986;83:9154-9158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 141] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 37. | Calabi F, Milstein C. A novel family of human major histocompatibility complex-related genes not mapping to chromosome 6. Nature. 1986;323:540-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 239] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 38. | Sonoda KH, Taniguchi M, Stein-Streilein J. Long-term survival of corneal allografts is dependent on intact CD1d-reactive NKT cells. J Immunol. 2002;168:2028-2034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 39. | Wang B, Chun T, Rulifson IC, Exley M, Balk SP, Wang CR. Human CD1d functions as a transplantation antigen and a restriction element in mice. J Immunol. 2001;166:3829-3836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 40. | Sumitran-Karuppan S, Tyden G, Reinholt F, Berg U, Moller E. Hyperacute rejections of two consecutive renal allografts and early loss of the third transplant caused by non-HLA antibodies specific for endothelial cells. Transpl Immunol. 1997;5:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 102] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Perrey C, Brenchley PE, Johnson RW, Martin S. An association between antibodies specific for endothelial cells and renal transplant failure. Transpl Immunol. 1998;6:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 42. | Faulk WP, Rose M, Meroni PL, Del Papa N, Torry RJ, Labarrere CA, Busing K, Crisp SJ, Dunn MJ, Nelson DR. Antibodies to endothelial cells identify myocardial damage and predict development of coronary artery disease in patients with transplanted hearts. Hum Immunol. 1999;60:826-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 43. | Barth R, Counce S, Smith P, Snell GD. Strong and weak histocompatibility gene differences in mice and their role in the rejection of homografts of tumors and skin. Ann Surg. 1956;144:198-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 96] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 44. | Simpson E, Scott D, James E, Lombardi G, Cwynarski K, Dazzi F, Millrain M, Dyson PJ. Minor H antigens: genes and peptides. Transpl Immunol. 2002;10:115-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 54] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 45. | Wettstein PJ. Immunodominance in the T-cell response to multiple non-H-2 histocompatibility antigens. II. Observation of a hierarchy among dominant antigens. Immunogenetics. 1986;24:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 46. | Claas FH, Paul LC, van Es LA, van Rood JJ. Antibodies against donor antigens on endothelial cells and monocytes in eluates of rejected kidney allografts. Tissue Antigens. 1980;15:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Goulmy E. Minor histocompatibility antigens: from transplantation problems to therapy of cancer. Hum Immunol. 2006;67:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Goulmy E, Gratama JW, Blokland E, Zwaan FE, van Rood JJ. A minor transplantation antigen detected by MHC-restricted cytotoxic T lymphocytes during graft-versus-host disease. Nature. 1983;302:159-161. [PubMed] |
| 49. | Loveland B, Wang CR, Yonekawa H, Hermel E, Lindahl KF. Maternally transmitted histocompatibility antigen of mice: a hydrophobic peptide of a mitochondrially encoded protein. Cell. 1990;60:971-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 123] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 50. | Bhuyan PK, Young LL, Lindahl KF, Butcher GW. Identification of the rat maternally transmitted minor histocompatibility antigen. J Immunol. 1997;158:3753-3760. [PubMed] |
| 51. | Game DS, Lechler RI. Pathways of allorecognition: implications for transplantation tolerance. Transpl Immunol. 2002;10:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 152] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 52. | Otten HG, van den Bosch JM, van Ginkel WG, van Loon M, van de Graaf EA. Identification of non-HLA target antigens recognized after lung transplantation. J Heart Lung Transplant. 2006;25:1425-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Grandtnerová B, Laca L, Jahnová E, Horváthová M, Baláz V, Hovoricová B, Hudec P, Zarnovicanová M. Hyperacute rejection of living related kidney graft caused by IgG endothelial specific antibodies with a negative monocyte cross-match. Ann Transplant. 2002;7:52-54. [PubMed] |
| 54. | Sumitran-Holgersson S, Ge X, Karrar A, Xu B, Nava S, Broomé U, Nowak G, Ericzon BG. A novel mechanism of liver allograft rejection facilitated by antibodies to liver sinusoidal endothelial cells. Hepatology. 2004;40:1211-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 55. | Mickelson EM, Fefer A, Storb R, Thomas ED. Correlation of the relative response index with marrow graft rejection in patients with aplastic anemia. Transplantation. 1976;22:294-302. [PubMed] |
| 56. | Hume DM, Merrill JP, Miller BF, Thorn GW. Experiences with renal homotransplantation in the human: report of nine cases. J Clin Invest. 1955;34:327-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 414] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 57. | Suchin EJ, Langmuir PB, Palmer E, Sayegh MH, Wells AD, Turka LA. Quantifying the frequency of alloreactive T cells in vivo: new answers to an old question. J Immunol. 2001;166:973-981. [PubMed] |
| 58. | Barroso M, Tucker H, Drake L, Nichol K, Drake JR. Antigen-B Cell Receptor Complexes Associate with Intracellular major histocompatibility complex (MHC) Class II Molecules. J Biol Chem. 2015;290:27101-27112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 59. | Hobeika E, Nielsen PJ, Medgyesi D. Signaling mechanisms regulating B-lymphocyte activation and tolerance. J Mol Med (Berl). 2015;93:143-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 60. | Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537-539. [PubMed] |
| 61. | Warrens AN, Lombardi G, Lechler RI. Presentation and recognition of major and minor histocompatibility antigens. Transpl Immunol. 1994;2:103-107. [PubMed] |
| 62. | Matzinger P, Bevan MJ. Hypothesis: why do so many lymphocytes respond to major histocompatibility antigens? Cell Immunol. 1977;29:1-5. [PubMed] |
| 63. | Kourilsky P, Chaouat G, Rabourdin-Combe C, Claverie JM. Working principles in the immune system implied by the “peptidic self” model. Proc Natl Acad Sci USA. 1987;84:3400-3404. [PubMed] |
| 64. | Afzali B, Lechler RI, Hernandez-Fuentes MP. Allorecognition and the alloresponse: clinical implications. Tissue Antigens. 2007;69:545-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 107] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 65. | Shoskes DA, Wood KJ. Indirect presentation of MHC antigens in transplantation. Immunol Today. 1994;15:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 248] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 66. | Auchincloss H, Lee R, Shea S, Markowitz JS, Grusby MJ, Glimcher LH. The role of “indirect” recognition in initiating rejection of skin grafts from major histocompatibility complex class II-deficient mice. Proc Natl Acad Sci USA. 1993;90:3373-3377. [PubMed] |
| 67. | Taylor AL, Negus SL, Negus M, Bolton EM, Bradley JA, Pettigrew GJ. Pathways of helper CD4 T cell allorecognition in generating alloantibody and CD8 T cell alloimmunity. Transplantation. 2007;83:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 68. | Steele DJ, Laufer TM, Smiley ST, Ando Y, Grusby MJ, Glimcher LH, Auchincloss H. Two levels of help for B cell alloantibody production. J Exp Med. 1996;183:699-703. [PubMed] |
| 69. | Baker RJ, Hernandez-Fuentes MP, Brookes PA, Chaudhry AN, Lechler RI. The role of the allograft in the induction of donor-specific T cell hyporesponsiveness. Transplantation. 2001;72:480-485. [PubMed] |
| 70. | Mason PD, Robinson CM, Lechler RI. Detection of donor-specific hyporesponsiveness following late failure of human renal allografts. Kidney Int. 1996;50:1019-1025. [PubMed] |
| 71. | Hornick PI, Mason PD, Baker RJ, Hernandez-Fuentes M, Frasca L, Lombardi G, Taylor K, Weng L, Rose ML, Yacoub MH. Significant frequencies of T cells with indirect anti-donor specificity in heart graft recipients with chronic rejection. Circulation. 2000;101:2405-2410. [PubMed] |
| 72. | Herrera OB, Golshayan D, Tibbott R, Salcido Ochoa F, James MJ, Marelli-Berg FM, Lechler RI. A novel pathway of alloantigen presentation by dendritic cells. J Immunol. 2004;173:4828-4837. [PubMed] |
| 73. | Game DS, Rogers NJ, Lechler RI. Acquisition of HLA-DR and costimulatory molecules by T cells from allogeneic antigen presenting cells. Am J Transplant. 2005;5:1614-1625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Harshyne LA, Watkins SC, Gambotto A, Barratt-Boyes SM. Dendritic cells acquire antigens from live cells for cross-presentation to CTL. J Immunol. 2001;166:3717-3723. [PubMed] |
| 75. | Morelli AE, Larregina AT, Shufesky WJ, Sullivan ML, Stolz DB, Papworth GD, Zahorchak AF, Logar AJ, Wang Z, Watkins SC. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood. 2004;104:3257-3266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 690] [Cited by in RCA: 812] [Article Influence: 38.7] [Reference Citation Analysis (0)] |
| 76. | Denzer K, van Eijk M, Kleijmeer MJ, Jakobson E, de Groot C, Geuze HJ. Follicular dendritic cells carry MHC class II-expressing microvesicles at their surface. J Immunol. 2000;165:1259-1265. [PubMed] |
| 77. | Rinaldo CR, Piazza P. Virus infection of dendritic cells: portal for host invasion and host defense. Trends Microbiol. 2004;12:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 78. | Dalheimer SL, Richards DM, Mueller DL. Sharing of class I MHC molecules between donor and host promotes the infiltration of allografts by mHAg-reactive CD8 T cells. Am J Transplant. 2005;5:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 79. | Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, Cendales LK, Tadaki DK, Harlan DM, Swanson SJ. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation. 2003;76:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 335] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 80. | Grau V, Garn H, Bette M, Spener F, Steiniger B, Gemsa D, Stehling O. Induction of epidermal fatty acid binding protein in intravascular monocytes of renal allografts. Transplantation. 2003;75:685-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 81. | Gröne HJ, Weber C, Weber KS, Gröne EF, Rabelink T, Klier CM, Wells TN, Proudfood AE, Schlöndorff D, Nelson PJ. Met-RANTES reduces vascular and tubular damage during acute renal transplant rejection: blocking monocyte arrest and recruitment. FASEB J. 1999;13:1371-1383. [PubMed] |
| 82. | Ciubotariu R, Liu Z, Colovai AI, Ho E, Itescu S, Ravalli S, Hardy MA, Cortesini R, Rose EA, Suciu-Foca N. Persistent allopeptide reactivity and epitope spreading in chronic rejection of organ allografts. J Clin Invest. 1998;101:398-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 255] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 83. | Vella JP, Spadafora-Ferreira M, Murphy B, Alexander SI, Harmon W, Carpenter CB, Sayegh MH. Indirect allorecognition of major histocompatibility complex allopeptides in human renal transplant recipients with chronic graft dysfunction. Transplantation. 1997;64:795-800. [PubMed] |
| 84. | Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004;66:523-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 152] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 85. | Menke J, Sollinger D, Schamberger B, Heemann U, Lutz J. The effect of ischemia/reperfusion on the kidney graft. Curr Opin Organ Transplant. 2014;19:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 86. | Oberbarnscheidt MH, Zeng Q, Li Q, Dai H, Williams AL, Shlomchik WD, Rothstein DM, Lakkis FG. Non-self recognition by monocytes initiates allograft rejection. J Clin Invest. 2014;124:3579-3589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 185] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 87. | Zecher D, van Rooijen N, Rothstein DM, Shlomchik WD, Lakkis FG. An innate response to allogeneic nonself mediated by monocytes. J Immunol. 2009;183:7810-7816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 88. | Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA. 2009;106:1915-1919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 505] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 89. | Bingaman AW, Ha J, Waitze SY, Durham MM, Cho HR, Tucker-Burden C, Hendrix R, Cowan SR, Pearson TC, Larsen CP. Vigorous allograft rejection in the absence of danger. J Immunol. 2000;164:3065-3071. [PubMed] |
| 90. | Anderson CC, Carroll JM, Gallucci S, Ridge JP, Cheever AW, Matzinger P. Testing time-, ignorance-, and danger-based models of tolerance. J Immunol. 2001;166:3663-3671. [PubMed] |
| 91. | Rodriguez-Manzanet R, Meyers JH, Balasubramanian S, Slavik J, Kassam N, Dardalhon V, Greenfield EA, Anderson AC, Sobel RA, Hafler DA. TIM-4 expressed on APCs induces T cell expansion and survival. J Immunol. 2008;180:4706-4713. [PubMed] |
| 92. | Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 93. | Pilat N, Sayegh MH, Wekerle T. Costimulatory pathways in transplantation. Semin Immunol. 2011;23:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 94. | Chen L, Flies DB. Molecular mechanisms of T cell co-stimulation and co-inhibition. Nat Rev Immunol. 2013;13:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2216] [Cited by in RCA: 2276] [Article Influence: 189.7] [Reference Citation Analysis (0)] |
| 95. | Tinckam KJ, Djurdjev O, Magil AB. Glomerular monocytes predict worse outcomes after acute renal allograft rejection independent of C4d status. Kidney Int. 2005;68:1866-1874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 96. | Grimm PC, McKenna R, Nickerson P, Russell ME, Gough J, Gospodarek E, Liu B, Jeffery J, Rush DN. Clinical rejection is distinguished from subclinical rejection by increased infiltration by a population of activated macrophages. J Am Soc Nephrol. 1999;10:1582-1589. [PubMed] |
| 97. | Grandaliano G, Gesualdo L, Ranieri E, Monno R, Stallone G, Schena FP. Monocyte chemotactic peptide-1 expression and monocyte infiltration in acute renal transplant rejection. Transplantation. 1997;63:414-420. [PubMed] |
| 98. | Lan HY, Yang N, Brown FG, Isbel NM, Nikolic-Paterson DJ, Mu W, Metz CN, Bacher M, Atkins RC, Bucala R. Macrophage migration inhibitory factor expression in human renal allograft rejection. Transplantation. 1998;66:1465-1471. [PubMed] |
| 99. | Le Meur Y, Jose MD, Mu W, Atkins RC, Chadban SJ. Macrophage colony-stimulating factor expression and macrophage accumulation in renal allograft rejection. Transplantation. 2002;73:1318-1324. [PubMed] |
| 100. | Jose MD, Le Meur Y, Atkins RC, Chadban SJ. Blockade of macrophage colony-stimulating factor reduces macrophage proliferation and accumulation in renal allograft rejection. Am J Transplant. 2003;3:294-300. [PubMed] |
| 101. | Hoffmann U, Bergler T, Segerer S, Rümmele P, Krüger B, Banas MC, Reinhold S, Banas B, Krämer BK. Impact of chemokine receptor CX3CR1 in human renal allograft rejection. Transpl Immunol. 2010;23:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 102. | Kakuta Y, Okumi M, Miyagawa S, Tsutahara K, Abe T, Yazawa K, Matsunami K, Otsuka H, Takahara S, Nonomura N. Blocking of CCR5 and CXCR3 suppresses the infiltration of macrophages in acute renal allograft rejection. Transplantation. 2012;93:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 103. | Girlanda R, Kleiner DE, Duan Z, Ford EA, Wright EC, Mannon RB, Kirk AD. Monocyte infiltration and kidney allograft dysfunction during acute rejection. Am J Transplant. 2008;8:600-607. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 104. | Zhang PL, Malek SK, Prichard JW, Lin F, Yahya TM, Schwartzman MS, Latsha RP, Skaletsky M, Norfolk ER, Brown RE. Monocyte-mediated acute renal rejection after combined treatment with preoperative Campath-1H (alemtuzumab) and postoperative immunosuppression. Ann Clin Lab Sci. 2004;34:209-213. [PubMed] |
| 105. | Laskin DL, Sunil VR, Gardner CR, Laskin JD. Macrophages and tissue injury: agents of defense or destruction? Annu Rev Pharmacol Toxicol. 2011;51:267-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 468] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 106. | West AP, Brodsky IE, Rahner C, Woo DK, Erdjument-Bromage H, Tempst P, Walsh MC, Choi Y, Shadel GS, Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472:476-480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1023] [Cited by in RCA: 1270] [Article Influence: 90.7] [Reference Citation Analysis (0)] |
| 107. | Italiani P, Boraschi D. From Monocytes to M1/M2 Macrophages: Phenotypical vs. Functional Differentiation. Front Immunol. 2014;5:514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1006] [Cited by in RCA: 1519] [Article Influence: 138.1] [Reference Citation Analysis (0)] |
| 108. | Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, Oberg HH, Pascher A, Lützen U, Janssen U. Cutting Edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187:2072-2078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 109. | Pilmore HL, Painter DM, Bishop GA, McCaughan GW, Eris JM. Early up-regulation of macrophages and myofibroblasts: a new marker for development of chronic renal allograft rejection. Transplantation. 2000;69:2658-2662. [PubMed] |
| 110. | Vitalone MJ, O’Connell PJ, Wavamunno M, Fung CL, Chapman JR, Nankivell BJ. Transcriptome changes of chronic tubulointerstitial damage in early kidney transplantation. Transplantation. 2010;89:537-547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 111. | Toki D, Zhang W, Hor KL, Liuwantara D, Alexander SI, Yi Z, Sharma R, Chapman JR, Nankivell BJ, Murphy B. The role of macrophages in the development of human renal allograft fibrosis in the first year after transplantation. Am J Transplant. 2014;14:2126-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 117] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 112. | Long EO, Rajagopalan S. Stress signals activate natural killer cells. J Exp Med. 2002;196:1399-1402. [PubMed] |
| 113. | Lim O, Jung MY, Hwang YK, Shin EC. Present and Future of Allogeneic Natural Killer Cell Therapy. Front Immunol. 2015;6:286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 114. | Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu Rev Immunol. 2013;31:227-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 943] [Article Influence: 78.6] [Reference Citation Analysis (0)] |
| 115. | Alnabhan R, Madrigal A, Saudemont A. Differential activation of cord blood and peripheral blood natural killer cells by cytokines. Cytotherapy. 2015;17:73-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 116. | Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Weisdorf DJ, Miller JS. NK cell education after allogeneic transplantation: dissociation between recovery of cytokine-producing and cytotoxic functions. Blood. 2011;118:2784-2792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 117. | Hamby K, Trexler A, Pearson TC, Larsen CP, Rigby MR, Kean LS. NK cells rapidly reject allogeneic bone marrow in the spleen through a perforin- and Ly49D-dependent, but NKG2D-independent mechanism. Am J Transplant. 2007;7:1884-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 118. | Chow T, Whiteley J, Li M, Rogers IM. The transfer of host MHC class I protein protects donor cells from NK cell and macrophage-mediated rejection during hematopoietic stem cell transplantation and engraftment in mice. Stem Cells. 2013;31:2242-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 119. | Noval Rivas M, Hazzan M, Weatherly K, Gaudray F, Salmon I, Braun MY. NK cell regulation of CD4 T cell-mediated graft-versus-host disease. J Immunol. 2010;184:6790-6798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 120. | Olson JA, Leveson-Gower DB, Gill S, Baker J, Beilhack A, Negrin RS. NK cells mediate reduction of GVHD by inhibiting activated, alloreactive T cells while retaining GVT effects. Blood. 2010;115:4293-4301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 293] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 121. | Hüber CM, Doisne JM, Colucci F. IL-12/15/18-preactivated NK cells suppress GvHD in a mouse model of mismatched hematopoietic cell transplantation. Eur J Immunol. 2015;45:1727-1735. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 122. | Hu B, Bao G, Zhang Y, Lin D, Wu Y, Wu D, Liu H. Donor NK Cells and IL-15 promoted engraftment in nonmyeloablative allogeneic bone marrow transplantation. J Immunol. 2012;189:1661-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 123. | Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: what’s in a name? Nat Rev Immunol. 2004;4:231-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 882] [Cited by in RCA: 954] [Article Influence: 45.4] [Reference Citation Analysis (0)] |
| 124. | Yang YF, Tomura M, Ono S, Hamaoka T, Fujiwara H. Requirement for IFN-gamma in IL-12 production induced by collaboration between v(alpha)14(+) NKT cells and antigen-presenting cells. Int Immunol. 2000;12:1669-1675. [PubMed] |
| 125. | Monteiro M, Graca L. Response to comment on “Induced IL-17-producing invariant NKT cells require activation in presence of TGF-β and IL-1β”. J Immunol. 2013;190:5910-5911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 126. | Di Pietro C, Falcone M. The role of invariant NKT cells in organ-specific autoimmunity. Front Biosci (Landmark Ed). 2014;19:1240-1250. [PubMed] |
| 127. | Singh AK, Gaur P, Das SN. Natural killer T cell anergy, co-stimulatory molecules and immunotherapeutic interventions. Hum Immunol. 2014;75:250-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 128. | Yang SH, Jin JZ, Lee SH, Park H, Kim CH, Lee DS, Kim S, Chung NH, Kim YS. Role of NKT cells in allogeneic islet graft survival. Clin Immunol. 2007;124:258-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 129. | Ikehara Y, Yasunami Y, Kodama S, Maki T, Nakano M, Nakayama T, Taniguchi M, Ikeda S. CD4(+) Valpha14 natural killer T cells are essential for acceptance of rat islet xenografts in mice. J Clin Invest. 2000;105:1761-1767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 115] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 130. | Seino KI, Fukao K, Muramoto K, Yanagisawa K, Takada Y, Kakuta S, Iwakura Y, Van Kaer L, Takeda K, Nakayama T. Requirement for natural killer T (NKT) cells in the induction of allograft tolerance. Proc Natl Acad Sci USA. 2001;98:2577-2581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 131. | Jiang X, Kojo S, Harada M, Ohkohchi N, Taniguchi M, Seino KI. Mechanism of NKT cell-mediated transplant tolerance. Am J Transplant. 2007;7:1482-1490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 132. | Hongo D, Tang X, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 133. | Kim JH, Choi EY, Chung DH. Donor bone marrow type II (non-Valpha14Jalpha18 CD1d-restricted) NKT cells suppress graft-versus-host disease by producing IFN-gamma and IL-4. J Immunol. 2007;179:6579-6587. [PubMed] |
| 134. | Cella M, Jarrossay D, Facchetti F, Alebardi O, Nakajima H, Lanzavecchia A, Colonna M. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5:919-923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1301] [Cited by in RCA: 1295] [Article Influence: 49.8] [Reference Citation Analysis (0)] |
| 135. | Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood. 2006;107:2613-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 179] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 136. | Markey KA, Banovic T, Kuns RD, Olver SD, Don AL, Raffelt NC, Wilson YA, Raggatt LJ, Pettit AR, Bromberg JS. Conventional dendritic cells are the critical donor APC presenting alloantigen after experimental bone marrow transplantation. Blood. 2009;113:5644-5649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 137. | Weber M, Rudolph B, Stein P, Yogev N, Bosmann M, Schild H, Radsak MP. Host-derived CD8 dendritic cells protect against acute graft-versus-host disease after experimental allogeneic bone marrow transplantation. Biol Blood Marrow Transplant. 2014;20:1696-1704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 138. | Toubai T, Malter C, Tawara I, Liu C, Nieves E, Lowler KP, Sun Y, Reddy P. Immunization with host-type CD8{alpha}+ dendritic cells reduces experimental acute GVHD in an IL-10-dependent manner. Blood. 2010;115:724-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 139. | Belladonna ML, Volpi C, Bianchi R, Vacca C, Orabona C, Pallotta MT, Boon L, Gizzi S, Fioretti MC, Grohmann U. Cutting edge: Autocrine TGF-beta sustains default tolerogenesis by IDO-competent dendritic cells. J Immunol. 2008;181:5194-5198. [PubMed] |
| 140. | O'Connell PJ, Li W, Wang Z, Specht SM, Logar AJ, Thomson AW. Immature and mature CD8alpha+ dendritic cells prolong the survival of vascularized heart allografts. J Immunol. 2002;168:143-154. [PubMed] |
| 141. | Cheong C, Matos I, Choi JH, Dandamudi DB, Shrestha E, Longhi MP, Jeffrey KL, Anthony RM, Kluger C, Nchinda G. Microbial stimulation fully differentiates monocytes to DC-SIGN/CD209(+) dendritic cells for immune T cell areas. Cell. 2010;143:416-429. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 477] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 142. | Chow KV, Lew AM, Sutherland RM, Zhan Y. Monocyte-Derived Dendritic Cells Promote Th Polarization, whereas Conventional Dendritic Cells Promote Th Proliferation. J Immunol. 2016;196:624-636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 143. | Münz C, Dao T, Ferlazzo G, de Cos MA, Goodman K, Young JW. Mature myeloid dendritic cell subsets have distinct roles for activation and viability of circulating human natural killer cells. Blood. 2005;105:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 144. | Kolumam GA, Thomas S, Thompson LJ, Sprent J, Murali-Krishna K. Type I interferons act directly on CD8 T cells to allow clonal expansion and memory formation in response to viral infection. J Exp Med. 2005;202:637-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 674] [Cited by in RCA: 766] [Article Influence: 38.3] [Reference Citation Analysis (0)] |
| 145. | Famulski KS, Einecke G, Reeve J, Ramassar V, Allanach K, Mueller T, Hidalgo LG, Zhu LF, Halloran PF. Changes in the transcriptome in allograft rejection: IFN-gamma-induced transcripts in mouse kidney allografts. Am J Transplant. 2006;6:1342-1354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 146. | Nickel P, Presber F, Bold G, Biti D, Schönemann C, Tullius SG, Volk HD, Reinke P. Enzyme-linked immunosorbent spot assay for donor-reactive interferon-gamma-producing cells identifies T-cell presensitization and correlates with graft function at 6 and 12 months in renal-transplant recipients. Transplantation. 2004;78:1640-1646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 147. | Halloran PF, Afrouzian M, Ramassar V, Urmson J, Zhu LF, Helms LM, Solez K, Kneteman NM. Interferon-gamma acts directly on rejecting renal allografts to prevent graft necrosis. Am J Pathol. 2001;158:215-226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 148. | Famulski KS, Sis B, Billesberger L, Halloran PF. Interferon-gamma and donor MHC class I control alternative macrophage activation and activin expression in rejecting kidney allografts: a shift in the Th1-Th2 paradigm. Am J Transplant. 2008;8:547-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 149. | Brok HP, Vossen JM, Heidt PJ. IFN-gamma-mediated prevention of graft-versus-host disease: pharmacodynamic studies and influence on proliferative capacity of chimeric spleen cells. Bone Marrow Transplant. 1998;22:1005-1010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 150. | Welniak LA, Blazar BR, Anver MR, Wiltrout RH, Murphy WJ. Opposing roles of interferon-gamma on CD4+ T cell-mediated graft-versus-host disease: effects of conditioning. Biol Blood Marrow Transplant. 2000;6:604-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 151. | Koenecke C, Lee CW, Thamm K, Föhse L, Schafferus M, Mittrücker HW, Floess S, Huehn J, Ganser A, Förster R. IFN-γ production by allogeneic Foxp3+ regulatory T cells is essential for preventing experimental graft-versus-host disease. J Immunol. 2012;189:2890-2896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 152. | Bushell A, Niimi M, Morris PJ, Wood KJ. Evidence for immune regulation in the induction of transplantation tolerance: a conditional but limited role for IL-4. J Immunol. 1999;162:1359-1366. [PubMed] |
| 153. | Mariotti J, Foley J, Ryan K, Buxhoeveden N, Kapoor V, Amarnath S, Fowler DH. Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an interleukin-4/STAT6 pathway. Blood. 2008;112:4765-4775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 154. | Onodera K, Hancock WW, Graser E, Lehmann M, Sayegh MH, Strom TB, Volk HD, Kupiec-Weglinski JW. Type 2 helper T cell-type cytokines and the development of “infectious” tolerance in rat cardiac allograft recipients. J Immunol. 1997;158:1572-1581. [PubMed] |
| 155. | Sadeghi M, Daniel V, Weimer R, Wiesel M, Hergesell O, Opelz G. Pre-transplant Th1 and post-transplant Th2 cytokine patterns are associated with early acute rejection in renal transplant recipients. Clin Transplant. 2003;17:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 156. | Sabet-Baktach M, Eggenhofer E, Rovira J, Renner P, Lantow M, Farkas SA, Malaisé M, Edtinger K, Shaotang Z, Koehl GE. Double deficiency for RORγt and T-bet drives Th2-mediated allograft rejection in mice. J Immunol. 2013;191:4440-4446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 157. | Karczewski J, Karczewski M, Glyda M, Wiktorowicz K. Role of TH1/TH2 cytokines in kidney allograft rejection. Transplant Proc. 2008;40:3390-3392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 158. | Kwan T, Chadban SJ, Ma J, Bao S, Alexander SI, Wu H. IL-17 deficiency attenuates allograft injury and prolongs survival in a murine model of fully MHC-mismatched renal allograft transplantation. Am J Transplant. 2015;15:1555-1567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 159. | Snell GI, Levvey BJ, Zheng L, Bailey M, Orsida B, Williams TJ, Kotsimbos TC. Interleukin-17 and airway inflammation: a longitudinal airway biopsy study after lung transplantation. J Heart Lung Transplant. 2007;26:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 160. | Tsaur I, Gasser M, Aviles B, Lutz J, Lutz L, Grimm M, Lange V, Lopau K, Heemann U, Germer CT. Donor antigen-specific regulatory T-cell function affects outcome in kidney transplant recipients. Kidney Int. 2011;79:1005-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 161. | Sugimoto K, Itoh T, Takita M, Shimoda M, Chujo D, SoRelle JA, Naziruddin B, Levy MF, Shimada M, Matsumoto S. Improving allogeneic islet transplantation by suppressing Th17 and enhancing Treg with histone deacetylase inhibitors. Transpl Int. 2014;27:408-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 162. | Vanaudenaerde BM, De Vleeschauwer SI, Vos R, Meyts I, Bullens DM, Reynders V, Wuyts WA, Van Raemdonck DE, Dupont LJ, Verleden GM. The role of the IL23/IL17 axis in bronchiolitis obliterans syndrome after lung transplantation. Am J Transplant. 2008;8:1911-1920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 163. | Malard F, Bossard C, Brissot E, Chevallier P, Guillaume T, Delaunay J, Mosnier JF, Moreau P, Grégoire M, Gaugler B. Increased Th17/Treg ratio in chronic liver GVHD. Bone Marrow Transplant. 2014;49:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 164. | Bommiasamy H. IL-22 Is Critical To The Pathogenesis Of Cutaneous Gvhd Mediated By Th17 Cells. Blood. 2013;122:4482. |
| 165. | Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124:374-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 296] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 166. | Ramming A, Druzd D, Leipe J, Schulze-Koops H, Skapenko A. Maturation-related histone modifications in the PU.1 promoter regulate Th9-cell development. Blood. 2012;119:4665-4674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 167. | Mangus CW, Massey PR, Fowler DH, Amarnath S. Rapamycin resistant murine th9 cells have a stable in vivo phenotype and inhibit graft-versus-host reactivity. PLoS One. 2013;8:e72305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 168. | Ramadan A, Zhang J, Griesenauer B, Kapur R, Hanenberg H, Sun J, Kaplan M, Paczesny S. IL-33/ST2 activation of IL-9-secreting T cells alters the balance of fatal immunity and tumor immunity (TRAN1P.926). J Immunol. 2015;194:140.148-140.148. |
| 169. | Halamay KE, Kirkman RL, Sun L, Yamada A, Fragoso RC, Shimizu K, Mitchell RN, McKay DB. CD8 T cells are sufficient to mediate allorecognition and allograft rejection. Cell Immunol. 2002;216:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 170. | Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melián A, Bogdan C. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 783] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 171. | Thomas WD, Hersey P. TNF-related apoptosis-inducing ligand (TRAIL) induces apoptosis in Fas ligand-resistant melanoma cells and mediates CD4 T cell killing of target cells. J Immunol. 1998;161:2195-2200. [PubMed] |
| 172. | Kayagaki N, Yamaguchi N, Nakayama M, Kawasaki A, Akiba H, Okumura K, Yagita H. Involvement of TNF-related apoptosis-inducing ligand in human CD4+ T cell-mediated cytotoxicity. J Immunol. 1999;162:2639-2647. [PubMed] |
| 173. | Krams SM, Hayashi M, Fox CK, Villanueva JC, Whitmer KJ, Burns W, Esquivel CO, Martinez OM. CD8+ cells are not necessary for allograft rejection or the induction of apoptosis in an experimental model of small intestinal transplantation. J Immunol. 1998;160:3673-3680. [PubMed] |
| 174. | Lowry RP, Forbes RD, Blackburn JH, Marghesco DM. Immune mechanisms in organ allograft rejection. V. Pivotal role of the cytotoxic-suppressor T cell subset in the rejection of heart grafts bearing isolated class I disparities in the inbred rat. Transplantation. 1985;40:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 175. | Haspot F, Li HW, Lucas CL, Fehr T, Beyaz S, Sykes M. Allospecific rejection of MHC class I-deficient bone marrow by CD8 T cells. Am J Transplant. 2014;14:49-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 176. | Zimmerer JM, Pham TA, Wright CL, Tobin KJ, Sanghavi PB, Elzein SM, Sanders VM, Bumgardner GL. Alloprimed CD8(+) T cells regulate alloantibody and eliminate alloprimed B cells through perforin- and FasL-dependent mechanisms. Am J Transplant. 2014;14:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 177. | Schürch CM, Riether C, Ochsenbein AF. Cytotoxic CD8+ T cells stimulate hematopoietic progenitors by promoting cytokine release from bone marrow mesenchymal stromal cells. Cell Stem Cell. 2014;14:460-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 178. | Fowler DH, Gress RE. Th2 and Tc2 cells in the regulation of GVHD, GVL, and graft rejection: considerations for the allogeneic transplantation therapy of leukemia and lymphoma. Leuk Lymphoma. 2000;38:221-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 97] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 179. | Erdmann AA, Jung U, Foley JE, Toda Y, Fowler DH. Co-stimulated/Tc2 cells abrogate murine marrow graft rejection. Biol Blood Marrow Transplant. 2004;10:604-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 180. | Jung U, Foley JE, Erdmann AA, Eckhaus MA, Fowler DH. CD3/CD28-costimulated T1 and T2 subsets: differential in vivo allosensitization generates distinct GVT and GVHD effects. Blood. 2003;102:3439-3446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 181. | Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413-2417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1486] [Cited by in RCA: 1522] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 182. | Obhrai JS, Oberbarnscheidt MH, Hand TW, Diggs L, Chalasani G, Lakkis FG. Effector T cell differentiation and memory T cell maintenance outside secondary lymphoid organs. J Immunol. 2006;176:4051-4058. [PubMed] |
| 183. | Chang JT, Wherry EJ, Goldrath AW. Molecular regulation of effector and memory T cell differentiation. Nat Immunol. 2014;15:1104-1115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 460] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 184. | London CA, Lodge MP, Abbas AK. Functional responses and costimulator dependence of memory CD4+ T cells. J Immunol. 2000;164:265-272. [PubMed] |
| 185. | Harrington LE, Janowski KM, Oliver JR, Zajac AJ, Weaver CT. Memory CD4 T cells emerge from effector T-cell progenitors. Nature. 2008;452:356-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 204] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 186. | Li J, Huston G, Swain SL. IL-7 promotes the transition of CD4 effectors to persistent memory cells. J Exp Med. 2003;198:1807-1815. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 256] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 187. | Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol. 2003;4:1191-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1385] [Cited by in RCA: 1518] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 188. | Valujskikh A, Li XC. Frontiers in nephrology: T cell memory as a barrier to transplant tolerance. J Am Soc Nephrol. 2007;18:2252-2261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 189. | Chalasani G, Dai Z, Konieczny BT, Baddoura FK, Lakkis FG. Recall and propagation of allospecific memory T cells independent of secondary lymphoid organs. Proc Natl Acad Sci USA. 2002;99:6175-6180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 190. | Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 389] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 191. | Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176:1394-1401. [PubMed] |
| 192. | Minamimura K, Sato K, Yagita H, Tanaka T, Arii S, Maki T. Strategies to induce marked prolongation of secondary skin allograft survival in alloantigen-primed mice. Am J Transplant. 2008;8:761-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 193. | Yang J, Brook MO, Carvalho-Gaspar M, Zhang J, Ramon HE, Sayegh MH, Wood KJ, Turka LA, Jones ND. Allograft rejection mediated by memory T cells is resistant to regulation. Proc Natl Acad Sci USA. 2007;104:19954-19959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 194. | Li XC, Kloc M, Ghobrial RM. Memory T cells in transplantation - progress and challenges. Curr Opin Organ Transplant. 2013;18:387-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 195. | Heeger PS, Greenspan NS, Kuhlenschmidt S, Dejelo C, Hricik DE, Schulak JA, Tary-Lehmann M. Pretransplant frequency of donor-specific, IFN-gamma-producing lymphocytes is a manifestation of immunologic memory and correlates with the risk of posttransplant rejection episodes. J Immunol. 1999;163:2267-2275. [PubMed] |
| 196. | Nadazdin O, Boskovic S, Murakami T, Tocco G, Smith RN, Colvin RB, Sachs DH, Allan J, Madsen JC, Kawai T. Host alloreactive memory T cells influence tolerance to kidney allografts in nonhuman primates. Sci Transl Med. 2011;3:86ra51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 197. | Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Huang X, Markmann JF, Kassaee A, Rosengard BR, Hancock WW. Homeostatic proliferation is a barrier to transplantation tolerance. Nat Med. 2004;10:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 335] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 198. | Goldrath AW, Bogatzki LY, Bevan MJ. Naive T cells transiently acquire a memory-like phenotype during homeostasis-driven proliferation. J Exp Med. 2000;192:557-564. [PubMed] |
| 199. | Sener A, Tang AL, Farber DL. Memory T-cell predominance following T-cell depletional therapy derives from homeostatic expansion of naive T cells. Am J Transplant. 2009;9:2615-2623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 200. | Taylor DK, Neujahr D, Turka LA. Heterologous immunity and homeostatic proliferation as barriers to tolerance. Curr Opin Immunol. 2004;16:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 201. | Selin LK, Brehm MA. Frontiers in nephrology: heterologous immunity, T cell cross-reactivity, and alloreactivity. J Am Soc Nephrol. 2007;18:2268-2277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 202. | Koyama I, Nadazdin O, Boskovic S, Ochiai T, Smith RN, Sykes M, Sogawa H, Murakami T, Strom TB, Colvin RB. Depletion of CD8 memory T cells for induction of tolerance of a previously transplanted kidney allograft. Am J Transplant. 2007;7:1055-1061. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 101] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 203. | Krummey SM, Ford ML. Heterogeneity within T Cell Memory: Implications for Transplant Tolerance. Front Immunol. 2012;3:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 204. | Afzali B, Mitchell PJ, Scottà C, Canavan J, Edozie FC, Fazekasova H, Lord GM, John S, Barber LD, Hernandez-Fuentes MP. Relative resistance of human CD4(+) memory T cells to suppression by CD4(+) CD25(+) regulatory T cells. Am J Transplant. 2011;11:1734-1742. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 205. | Yamada Y, Boskovic S, Aoyama A, Murakami T, Putheti P, Smith RN, Ochiai T, Nadazdin O, Koyama I, Boenisch O. Overcoming memory T-cell responses for induction of delayed tolerance in nonhuman primates. Am J Transplant. 2012;12:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 206. | Khandelwal P, Lane A, Chaturvedi V, Owsley E, Davies SM, Marmer D, Filipovich AH, Jordan MB, Marsh RA. Peripheral Blood CD38 Bright CD8+ Effector Memory T Cells Predict Acute Graft-versus-Host Disease. Biol Blood Marrow Transplant. 2015;21:1215-1222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 207. | Schultz KR, Paquet J, Bader S, HayGlass KT. Requirement for B cells in T cell priming to minor histocompatibility antigens and development of graft-versus-host disease. Bone Marrow Transplant. 1995;16:289-295. [PubMed] |
| 208. | Iori AP, Torelli GF, De Propris MS, Milano F, Pupella S, Gozzer M, Mancini F, Milani ML, Intoppa S, Cerretti R. B-cell concentration in the apheretic product predicts acute graft-versus-host disease and treatment-related mortality of allogeneic peripheral blood stem cell transplantation. Transplantation. 2008;85:386-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 209. | Patriarca F, Skert C, Sperotto A, Zaja F, Falleti E, Mestroni R, Kikic F, Calistri E, Filì C, Geromin A. The development of autoantibodies after allogeneic stem cell transplantation is related with chronic graft-vs-host disease and immune recovery. Exp Hematol. 2006;34:389-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 105] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 210. | Svegliati S, Olivieri A, Campelli N, Luchetti M, Poloni A, Trappolini S, Moroncini G, Bacigalupo A, Leoni P, Avvedimento EV. Stimulatory autoantibodies to PDGF receptor in patients with extensive chronic graft-versus-host disease. Blood. 2007;110:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 167] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 211. | Miklos DB, Kim HT, Miller KH, Guo L, Zorn E, Lee SJ, Hochberg EP, Wu CJ, Alyea EP, Cutler C. Antibody responses to H-Y minor histocompatibility antigens correlate with chronic graft-versus-host disease and disease remission. Blood. 2005;105:2973-2978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 307] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 212. | Zeng Q, Ng YH, Singh T, Jiang K, Sheriff KA, Ippolito R, Zahalka S, Li Q, Randhawa P, Hoffman RA. B cells mediate chronic allograft rejection independently of antibody production. J Clin Invest. 2014;124:1052-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 213. | Lefaucheur C, Loupy A, Hill GS, Andrade J, Nochy D, Antoine C, Gautreau C, Charron D, Glotz D, Suberbielle-Boissel C. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol. 2010;21:1398-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 641] [Cited by in RCA: 651] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 214. | Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, Catrou PG, Bolin P, Kendrick WT, Kendrick SA. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95:410-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 314] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 215. | Peng Q, Li K, Wang N, Li Q, Asgari E, Lu B, Woodruff TM, Sacks SH, Zhou W. Dendritic cell function in allostimulation is modulated by C5aR signaling. J Immunol. 2009;183:6058-6068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 216. | Strainic MG, Shevach EM, An F, Lin F, Medof ME. Absence of signaling into CD4+ cells via C3aR and C5aR enables autoinductive TGF-β1 signaling and induction of Foxp3+ regulatory T cells. Nat Immunol. 2013;14:162-171. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 215] [Cited by in RCA: 259] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 217. | Kwan WH, van der Touw W, Paz-Artal E, Li MO, Heeger PS. Signaling through C5a receptor and C3a receptor diminishes function of murine natural regulatory T cells. J Exp Med. 2013;210:257-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 179] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 218. | Csomor E, Bajtay Z, Sándor N, Kristóf K, Arlaud GJ, Thiel S, Erdei A. Complement protein C1q induces maturation of human dendritic cells. Mol Immunol. 2007;44:3389-3397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 219. | Mauiyyedi S, Pelle PD, Saidman S, Collins AB, Pascual M, Tolkoff-Rubin NE, Williams WW, Cosimi AA, Schneeberger EE, Colvin RB. Chronic humoral rejection: identification of antibody-mediated chronic renal allograft rejection by C4d deposits in peritubular capillaries. J Am Soc Nephrol. 2001;12:574-582. [PubMed] |
| 220. | Halloran PF. Immunosuppressive drugs for kidney transplantation. N Engl J Med. 2004;351:2715-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1087] [Cited by in RCA: 1043] [Article Influence: 49.7] [Reference Citation Analysis (0)] |
| 221. | Merrill JP, Murray JE, Takacs FJ, Hager EB, Wilson RE, Dammin GJ. Successful transplantation of kidney from a human cadaver. JAMA. 1963;185:347-353. [PubMed] |
| 222. | Organ Procurement and Transplantation Network and Scientific Registry of Transplant Recipients 2010 data report. Am J Transplant. 2012;12 Suppl 1:1-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 223. | van Gelder T, van Schaik RH, Hesselink DA. Pharmacogenetics and immunosuppressive drugs in solid organ transplantation. Nat Rev Nephrol. 2014;10:725-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 224. | Konidari A, Matary WE. Use of thiopurines in inflammatory bowel disease: Safety issues. World J Gastrointest Pharmacol Ther. 2014;5:63-76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 36] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 225. | Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med. 2000;342:605-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1431] [Cited by in RCA: 1432] [Article Influence: 57.3] [Reference Citation Analysis (0)] |
| 226. | Tedesco D, Haragsim L. Cyclosporine: a review. J Transplant. 2012;2012:230386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 227. | Rao A, Luo C, Hogan PG. Transcription factors of the NFAT family: regulation and function. Annu Rev Immunol. 1997;15:707-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1991] [Cited by in RCA: 2038] [Article Influence: 72.8] [Reference Citation Analysis (0)] |
| 228. | Zununi Vahed S, Ardalan M, Samadi N, Omidi Y. Pharmacogenetics and drug-induced nephrotoxicity in renal transplant recipients. Bioimpacts. 2015;5:45-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 229. | Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RD. Calcineurin inhibitor nephrotoxicity: longitudinal assessment by protocol histology. Transplantation. 2004;78:557-565. [PubMed] |
| 230. | Ojo AO, Held PJ, Port FK, Wolfe RA, Leichtman AB, Young EW, Arndorfer J, Christensen L, Merion RM. Chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:931-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1703] [Cited by in RCA: 1637] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 231. | Bloom RD, Reese PP. Chronic kidney disease after nonrenal solid-organ transplantation. J Am Soc Nephrol. 2007;18:3031-3041. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 232. | Harris TE, Lawrence JC. TOR signaling. Sci STKE. 2003;2003:re15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 191] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 233. | Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338-8347. [PubMed] |
| 234. | Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, Hou JZ, Negrin RS. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 320] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 235. | Staatz CE, Tett SE. Pharmacology and toxicology of mycophenolate in organ transplant recipients: an update. Arch Toxicol. 2014;88:1351-1389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 150] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 236. | Satyananda V, Shapiro R. Belatacept in kidney transplantation. Curr Opin Organ Transplant. 2014;19:573-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 237. | Rostaing L, Vincenti F, Grinyó J, Rice KM, Bresnahan B, Steinberg S, Gang S, Gaite LE, Moal MC, Mondragón-Ramirez GA. Long-term belatacept exposure maintains efficacy and safety at 5 years: results from the long-term extension of the BENEFIT study. Am J Transplant. 2013;13:2875-2883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 238. | Kirk AD, Guasch A, Xu H, Cheeseman J, Mead SI, Ghali A, Mehta AK, Wu D, Gebel H, Bray R. Renal transplantation using belatacept without maintenance steroids or calcineurin inhibitors. Am J Transplant. 2014;14:1142-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 239. | Masson P, Henderson L, Chapman JR, Craig JC, Webster AC. Belatacept for kidney transplant recipients. Cochrane Database Syst Rev. 2014;11:CD010699. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 240. | Okimura K, Maeta K, Kobayashi N, Goto M, Kano N, Ishihara T, Ishikawa T, Tsumura H, Ueno A, Miyao Y. Characterization of ASKP1240, a fully human antibody targeting human CD40 with potent immunosuppressive effects. Am J Transplant. 2014;14:1290-1299. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 241. | Watanabe M, Yamashita K, Suzuki T, Kamachi H, Kuraya D, Koshizuka Y, Ogura M, Yoshida T, Aoyagi T, Fukumori D. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant. 2013;13:1976-1988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 242. | Vincenti F, Silva HT, Busque S, O’Connell PJ, Russ G, Budde K, Yoshida A, Tortorici MA, Lamba M, Lawendy N. Evaluation of the effect of tofacitinib exposure on outcomes in kidney transplant patients. Am J Transplant. 2015;15:1644-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 243. | Vincenti F, Tedesco Silva H, Busque S, O’Connell P, Friedewald J, Cibrik D, Budde K, Yoshida A, Cohney S, Weimar W. Randomized phase 2b trial of tofacitinib (CP-690,550) in de novo kidney transplant patients: efficacy, renal function and safety at 1 year. Am J Transplant. 2012;12:2446-2456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 244. | Russ GR, Tedesco-Silva H, Kuypers DR, Cohney S, Langer RM, Witzke O, Eris J, Sommerer C, von Zur-Mühlen B, Woodle ES. Efficacy of sotrastaurin plus tacrolimus after de novo kidney transplantation: randomized, phase II trial results. Am J Transplant. 2013;13:1746-1756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 245. | Pascher A, De Simone P, Pratschke J, Salamé E, Pirenne J, Isoneimi H, Bijarnia M, Krishnan I, Klupp J. Protein kinase C inhibitor sotrastaurin in de novo liver transplant recipients: a randomized phase II trial. Am J Transplant. 2015;15:1283-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 246. | Lopez M, Clarkson MR, Albin M, Sayegh MH, Najafian N. A novel mechanism of action for anti-thymocyte globulin: induction of CD4+CD25+Foxp3+ regulatory T cells. J Am Soc Nephrol. 2006;17:2844-2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 292] [Cited by in RCA: 294] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 247. | Bloom DD, Chang Z, Fechner JH, Dar W, Polster SP, Pascual J, Turka LA, Knechtle SJ. CD4+ CD25+ FOXP3+ regulatory T cells increase de novo in kidney transplant patients after immunodepletion with Campath-1H. Am J Transplant. 2008;8:793-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 248. | Heidt S, Hester J, Shankar S, Friend PJ, Wood KJ. B cell repopulation after alemtuzumab induction-transient increase in transitional B cells and long-term dominance of naïve B cells. Am J Transplant. 2012;12:1784-1792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 249. | Al-Homsi AS, Feng Y, Duffner U, Al Malki MM, Goodyke A, Cole K, Muilenburg M, Abdel-Mageed A. Bortezomib for the prevention and treatment of graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Exp Hematol. 2016;44:771-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 250. | Al-Homsi AS, Lai Z, Roy TS, Kouttab N. Effect of novel proteasome and immunoproteasome inhibitors on dendritic cell maturation, function, and expression of IκB and NFκB. Transpl Immunol. 2013;29:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 251. | Berges C, Haberstock H, Fuchs D, Miltz M, Sadeghi M, Opelz G, Daniel V, Naujokat C. Proteasome inhibition suppresses essential immune functions of human CD4+ T cells. Immunology. 2008;124:234-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 252. | Verbrugge SE, Scheper RJ, Lems WF, de Gruijl TD, Jansen G. Proteasome inhibitors as experimental therapeutics of autoimmune diseases. Arthritis Res Ther. 2015;17:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 94] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 253. | Sarno G, Muscogiuri G, De Rosa P. New-onset diabetes after kidney transplantation: prevalence, risk factors, and management. Transplantation. 2012;93:1189-1195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 254. | Bottomley MJ, Harden PN. Update on the long-term complications of renal transplantation. Br Med Bull. 2013;106:117-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 255. | Jevnikar AM, Mannon RB. Late kidney allograft loss: what we know about it, and what we can do about it. Clin J Am Soc Nephrol. 2008;3 Suppl 2:S56-S67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 256. | Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4:378-383. [PubMed] |
| 257. | Evans RW, Applegate WH, Briscoe DM, Cohen DJ, Rorick CC, Murphy BT, Madsen JC. Cost-related immunosuppressive medication nonadherence among kidney transplant recipients. Clin J Am Soc Nephrol. 2010;5:2323-2328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 258. | Saidi RF, Hejazii Kenari SK. Clinical transplantation and tolerance: are we there yet? Int J Organ Transplant Med. 2014;5:137-145. [PubMed] |
| 259. | Xing Y, Hogquist KA. T-cell tolerance: central and peripheral. Cold Spring Harb Perspect Biol. 2012;4:pii: a006957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 332] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 260. | Girlanda R, Kirk AD. Frontiers in nephrology: immune tolerance to allografts in humans. J Am Soc Nephrol. 2007;18:2242-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 261. | Alex Bishop G, Bertolino PD, Bowen DG, McCaughan GW. Tolerance in liver transplantation. Best Pract Res Clin Gastroenterol. 2012;26:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 262. | Fehr T, Sykes M. Clinical experience with mixed chimerism to induce transplantation tolerance. Transpl Int. 2008;21:1118-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 56] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 263. | Sachs DH, Sykes M, Kawai T, Cosimi AB. Immuno-intervention for the induction of transplantation tolerance through mixed chimerism. Semin Immunol. 2011;23:165-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 264. | Kawai T, Sogawa H, Boskovic S, Abrahamian G, Smith RN, Wee SL, Andrews D, Nadazdin O, Koyama I, Sykes M. CD154 blockade for induction of mixed chimerism and prolonged renal allograft survival in nonhuman primates. Am J Transplant. 2004;4:1391-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 168] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 265. | Alexander SI, Smith N, Hu M, Verran D, Shun A, Dorney S, Smith A, Webster B, Shaw PJ, Lammi A. Chimerism and tolerance in a recipient of a deceased-donor liver transplant. N Engl J Med. 2008;358:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 266. | Kawai T, Cosimi AB, Sachs DH. Preclinical and clinical studies on the induction of renal allograft tolerance through transient mixed chimerism. Curr Opin Organ Transplant. 2011;16:366-371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 267. | Scandling JD, Busque S, Dejbakhsh-Jones S, Benike C, Millan MT, Shizuru JA, Hoppe RT, Lowsky R, Engleman EG, Strober S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N Engl J Med. 2008;358:362-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 395] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 268. | Kawai T, Cosimi AB, Spitzer TR, Tolkoff-Rubin N, Suthanthiran M, Saidman SL, Shaffer J, Preffer FI, Ding R, Sharma V. HLA-mismatched renal transplantation without maintenance immunosuppression. N Engl J Med. 2008;358:353-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 890] [Cited by in RCA: 831] [Article Influence: 48.9] [Reference Citation Analysis (0)] |
| 269. | Leventhal J, Abecassis M, Miller J, Gallon L, Ravindra K, Tollerud DJ, King B, Elliott MJ, Herzig G, Herzig R. Chimerism and tolerance without GVHD or engraftment syndrome in HLA-mismatched combined kidney and hematopoietic stem cell transplantation. Sci Transl Med. 2012;4:124ra28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 343] [Cited by in RCA: 326] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 270. | Wood KJ, Bushell A, Hester J. Regulatory immune cells in transplantation. Nat Rev Immunol. 2012;12:417-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 335] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 271. | Martínez-Llordella M, Puig-Pey I, Orlando G, Ramoni M, Tisone G, Rimola A, Lerut J, Latinne D, Margarit C, Bilbao I. Multiparameter immune profiling of operational tolerance in liver transplantation. Am J Transplant. 2007;7:309-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 272. | Mazariegos GV, Zahorchak AF, Reyes J, Ostrowski L, Flynn B, Zeevi A, Thomson AW. Dendritic cell subset ratio in peripheral blood correlates with successful withdrawal of immunosuppression in liver transplant patients. Am J Transplant. 2003;3:689-696. [PubMed] |
| 273. | Newell KA, Asare A, Kirk AD, Gisler TD, Bourcier K, Suthanthiran M, Burlingham WJ, Marks WH, Sanz I, Lechler RI. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest. 2010;120:1836-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 563] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 274. | Shabir S, Girdlestone J, Briggs D, Kaul B, Smith H, Daga S, Chand S, Jham S, Navarrete C, Harper L. Transitional B lymphocytes are associated with protection from kidney allograft rejection: a prospective study. Am J Transplant. 2015;15:1384-1391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 92] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 275. | Singer BD, King LS, D’Alessio FR. Regulatory T cells as immunotherapy. Front Immunol. 2014;5:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 276. | Stolp J, Turka LA, Wood KJ. B cells with immune-regulating function in transplantation. Nat Rev Nephrol. 2014;10:389-397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 277. | Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011;23:252-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 122] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 278. | Gordon JR, Ma Y, Churchman L, Gordon SA, Dawicki W. Regulatory dendritic cells for immunotherapy in immunologic diseases. Front Immunol. 2014;5:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 133] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 279. | Benoist C, Mathis D. Treg cells, life history, and diversity. Cold Spring Harb Perspect Biol. 2012;4:a007021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 280. | Geiger TL, Tauro S. Nature and nurture in Foxp3(+) regulatory T cell development, stability, and function. Hum Immunol. 2012;73:232-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 281. | Feuerer M, Hill JA, Kretschmer K, von Boehmer H, Mathis D, Benoist C. Genomic definition of multiple ex vivo regulatory T cell subphenotypes. Proc Natl Acad Sci USA. 2010;107:5919-5924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 177] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 282. | Verhagen J, Wegner A, Wraith DC. Extra-thymically induced T regulatory cell subsets: the optimal target for antigen-specific immunotherapy. Immunology. 2015;145:171-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 283. | Issa F, Chandrasekharan D, Wood KJ. Regulatory T cells as modulators of chronic allograft dysfunction. Curr Opin Immunol. 2011;23:648-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 284. | Francis RS, Feng G, Tha-In T, Lyons IS, Wood KJ, Bushell A. Induction of transplantation tolerance converts potential effector T cells into graft-protective regulatory T cells. Eur J Immunol. 2011;41:726-738. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 285. | Wing JB, Sakaguchi S. Multiple treg suppressive modules and their adaptability. Front Immunol. 2012;3:178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 286. | Joffre O, Santolaria T, Calise D, Al Saati T, Hudrisier D, Romagnoli P, van Meerwijk JP. Prevention of acute and chronic allograft rejection with CD4+CD25+Foxp3+ regulatory T lymphocytes. Nat Med. 2008;14:88-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 439] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 287. | Pilat N, Klaus C, Gattringer M, Jaeckel E, Wrba F, Golshayan D, Baranyi U, Wekerle T. Therapeutic efficacy of polyclonal tregs does not require rapamycin in a low-dose irradiation bone marrow transplantation model. Transplantation. 2011;92:280-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 288. | Joffre O, Gorsse N, Romagnoli P, Hudrisier D, van Meerwijk JP. Induction of antigen-specific tolerance to bone marrow allografts with CD4+CD25+ T lymphocytes. Blood. 2004;103:4216-4221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 142] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 289. | Shin HJ, Baker J, Leveson-Gower DB, Smith AT, Sega EI, Negrin RS. Rapamycin and IL-2 reduce lethal acute graft-versus-host disease associated with increased expansion of donor type CD4+CD25+Foxp3+ regulatory T cells. Blood. 2011;118:2342-2350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 290. | Hester J, Schiopu A, Nadig SN, Wood KJ. Low-dose rapamycin treatment increases the ability of human regulatory T cells to inhibit transplant arteriosclerosis in vivo. Am J Transplant. 2012;12:2008-2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 291. | Moore C, Tejon G, Fuentes C, Hidalgo Y, Bono MR, Maldonado P, Fernandez R, Wood KJ, Fierro JA, Rosemblatt M. Alloreactive regulatory T cells generated with retinoic acid prevent skin allograft rejection. Eur J Immunol. 2015;45:452-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 292. | Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080-1086. [PubMed] |
| 293. | Nadig SN, Wieckiewicz J, Wu DC, Warnecke G, Zhang W, Luo S, Schiopu A, Taggart DP, Wood KJ. In vivo prevention of transplant arteriosclerosis by ex vivo-expanded human regulatory T cells. Nat Med. 2010;16:809-813. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 249] [Cited by in RCA: 248] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 294. | Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 295. | Issa F, Hester J, Goto R, Nadig SN, Goodacre TE, Wood K. Ex vivo-expanded human regulatory T cells prevent the rejection of skin allografts in a humanized mouse model. Transplantation. 2010;90:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 168] [Cited by in RCA: 166] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 296. | Nguyen VH, Shashidhar S, Chang DS, Ho L, Kambham N, Bachmann M, Brown JM, Negrin RS. The impact of regulatory T cells on T-cell immunity following hematopoietic cell transplantation. Blood. 2008;111:945-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 139] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 297. | Ermann J, Hoffmann P, Edinger M, Dutt S, Blankenberg FG, Higgins JP, Negrin RS, Fathman CG, Strober S. Only the CD62L+ subpopulation of CD4+CD25+ regulatory T cells protects from lethal acute GVHD. Blood. 2005;105:2220-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 319] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 298. | Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 833] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 299. | Brunstein CG, Miller JS, Cao Q, McKenna DH, Hippen KL, Curtsinger J, Defor T, Levine BL, June CH, Rubinstein P. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood. 2011;117:1061-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 829] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 300. | Raïch-Regué D, Glancy M, Thomson AW. Regulatory dendritic cell therapy: from rodents to clinical application. Immunol Lett. 2014;161:216-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 301. | Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2370] [Cited by in RCA: 2338] [Article Influence: 106.3] [Reference Citation Analysis (0)] |
| 302. | Divito SJ, Wang Z, Shufesky WJ, Liu Q, Tkacheva OA, Montecalvo A, Erdos G, Larregina AT, Morelli AE. Endogenous dendritic cells mediate the effects of intravenously injected therapeutic immunosuppressive dendritic cells in transplantation. Blood. 2010;116:2694-2705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 303. | Mackern-Oberti JP, Vega F, Llanos C, Bueno SM, Kalergis AM. Targeting dendritic cell function during systemic autoimmunity to restore tolerance. Int J Mol Sci. 2014;15:16381-16417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 304. | Lutz MB, Suri RM, Niimi M, Ogilvie AL, Kukutsch NA, Rössner S, Schuler G, Austyn JM. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 are maturation resistant and prolong allograft survival in vivo. Eur J Immunol. 2000;30:1813-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 305. | Pêche H, Trinité B, Martinet B, Cuturi MC. Prolongation of heart allograft survival by immature dendritic cells generated from recipient type bone marrow progenitors. Am J Transplant. 2005;5:255-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 108] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 306. | Bériou G, Pêche H, Guillonneau C, Merieau E, Cuturi MC. Donor-specific allograft tolerance by administration of recipient-derived immature dendritic cells and suboptimal immunosuppression. Transplantation. 2005;79:969-972. [PubMed] |
| 307. | Sato K, Yamashita N, Yamashita N, Baba M, Matsuyama T. Regulatory dendritic cells protect mice from murine acute graft-versus-host disease and leukemia relapse. Immunity. 2003;18:367-379. [PubMed] |
| 308. | Hu J, Wan Y. Tolerogenic dendritic cells and their potential applications. Immunology. 2011;132:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 309. | Thomas DC, Wong FS, Zaccone P, Green EA, Wållberg M. Protection of islet grafts through transforming growth factor-β-induced tolerogenic dendritic cells. Diabetes. 2013;62:3132-3142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 310. | Turnquist HR, Raimondi G, Zahorchak AF, Fischer RT, Wang Z, Thomson AW. Rapamycin-conditioned dendritic cells are poor stimulators of allogeneic CD4+ T cells, but enrich for antigen-specific Foxp3+ T regulatory cells and promote organ transplant tolerance. J Immunol. 2007;178:7018-7031. [PubMed] |
| 311. | Chesneau M, Michel L, Degauque N, Brouard S. Regulatory B cells and tolerance in transplantation: from animal models to human. Front Immunol. 2013;4:497. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 312. | Evans JG, Chavez-Rueda KA, Eddaoudi A, Meyer-Bahlburg A, Rawlings DJ, Ehrenstein MR, Mauri C. Novel suppressive function of transitional 2 B cells in experimental arthritis. J Immunol. 2007;178:7868-7878. [PubMed] |
| 313. | Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1012] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 314. | Lal G, Nakayama Y, Sethi A, Singh AK, Burrell BE, Kulkarni N, Brinkman CC, Iwami D, Zhang T, Bromberg JS. Interleukin-10 From Marginal Zone Precursor B-Cell Subset Is Required for Costimulatory Blockade-Induced Transplantation Tolerance. Transplantation. 2015;99:1817-1828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 315. | Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 342] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 316. | Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory T cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240-2252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 313] [Cited by in RCA: 300] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 317. | Carter NA, Vasconcellos R, Rosser EC, Tulone C, Muñoz-Suano A, Kamanaka M, Ehrenstein MR, Flavell RA, Mauri C. Mice lacking endogenous IL-10-producing regulatory B cells develop exacerbated disease and present with an increased frequency of Th1/Th17 but a decrease in regulatory T cells. J Immunol. 2011;186:5569-5579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 378] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 318. | Pallier A, Hillion S, Danger R, Giral M, Racapé M, Degauque N, Dugast E, Ashton-Chess J, Pettré S, Lozano JJ. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int. 2010;78:503-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 218] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 319. | Sagoo P, Perucha E, Sawitzki B, Tomiuk S, Stephens DA, Miqueu P, Chapman S, Craciun L, Sergeant R, Brouard S. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest. 2010;120:1848-1861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 436] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 320. | Nouël A, Ségalen I, Jamin C, Doucet L, Caillard S, Renaudineau Y, Pers JO, Le Meur Y, Hillion S. B cells display an abnormal distribution and an impaired suppressive function in patients with chronic antibody-mediated rejection. Kidney Int. 2014;85:590-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 321. | Fehr T, Haspot F, Mollov J, Chittenden M, Hogan T, Sykes M. Alloreactive CD8 T cell tolerance requires recipient B cells, dendritic cells, and MHC class II. J Immunol. 2008;181:165-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 322. | Yan Y, van der Putten K, Bowen DG, Painter DM, Kohar J, Sharland AF, McCaughan GW, Bishop GA. Postoperative administration of donor B cells induces rat kidney allograft acceptance: lack of association with Th2 cytokine expression in long-term accepted grafts. Transplantation. 2002;73:1123-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 323. | Zhao G, Moore DJ, Lee KM, Kim JI, Duff PE, O’Connor MR, Hirohashi T, Lei J, Yang M, Markmann JF. An unexpected counter-regulatory role of IL-10 in B-lymphocyte-mediated transplantation tolerance. Am J Transplant. 2010;10:796-801. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 324. | Moreau A, Blair PA, Chai JG, Ratnasothy K, Stolarczyk E, Alhabbab R, Rackham CL, Jones PM, Smyth L, Elgueta R. Transitional-2 B cells acquire regulatory function during tolerance induction and contribute to allograft survival. Eur J Immunol. 2015;45:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 325. | Lee KM, Stott RT, Zhao G, SooHoo J, Xiong W, Lian MM, Fitzgerald L, Shi S, Akrawi E, Lei J. TGF-β-producing regulatory B cells induce regulatory T cells and promote transplantation tolerance. Eur J Immunol. 2014;44:1728-1736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 326. | Wang Y, Chen X, Cao W, Shi Y. Plasticity of mesenchymal stem cells in immunomodulation: pathological and therapeutic implications. Nat Immunol. 2014;15:1009-1016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 1058] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 327. | Liu H, Liu S, Li Y, Wang X, Xue W, Ge G, Luo X. The role of SDF-1-CXCR4/CXCR7 axis in the therapeutic effects of hypoxia-preconditioned mesenchymal stem cells for renal ischemia/reperfusion injury. PLoS One. 2012;7:e34608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 210] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 328. | Casiraghi F, Azzollini N, Cassis P, Imberti B, Morigi M, Cugini D, Cavinato RA, Todeschini M, Solini S, Sonzogni A. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933-3946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 321] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 329. | Ge W, Jiang J, Arp J, Liu W, Garcia B, Wang H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation. 2010;90:1312-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 272] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 330. | Casiraghi F, Azzollini N, Todeschini M, Cavinato RA, Cassis P, Solini S, Rota C, Morigi M, Introna M, Maranta R. Localization of mesenchymal stromal cells dictates their immune or proinflammatory effects in kidney transplantation. Am J Transplant. 2012;12:2373-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 331. | Coulson-Thomas VJ, Gesteira TF, Hascall V, Kao W. Umbilical cord mesenchymal stem cells suppress host rejection: the role of the glycocalyx. J Biol Chem. 2014;289:23465-23481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 332. | Eggenhofer E, Steinmann JF, Renner P, Slowik P, Piso P, Geissler EK, Schlitt HJ, Dahlke MH, Popp FC. Mesenchymal stem cells together with mycophenolate mofetil inhibit antigen presenting cell and T cell infiltration into allogeneic heart grafts. Transpl Immunol. 2011;24:157-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 333. | Hara Y, Stolk M, Ringe J, Dehne T, Ladhoff J, Kotsch K, Reutzel-Selke A, Reinke P, Volk HD, Seifert M. In vivo effect of bone marrow-derived mesenchymal stem cells in a rat kidney transplantation model with prolonged cold ischemia. Transpl Int. 2011;24:1112-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 334. | Kim YH, Wee YM, Choi MY, Lim DG, Kim SC, Han DJ. Interleukin (IL)-10 induced by CD11b(+) cells and IL-10-activated regulatory T cells play a role in immune modulation of mesenchymal stem cells in rat islet allografts. Mol Med. 2011;17:697-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 335. | Xu DM, Yu XF, Zhang D, Zhang MX, Zhou JF, Tan PH, Ding YC. Mesenchymal stem cells differentially mediate regulatory T cells and conventional effector T cells to protect fully allogeneic islet grafts in mice. Diabetologia. 2012;55:1091-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 336. | Mougiakakos D, Jitschin R, Johansson CC, Okita R, Kiessling R, Le Blanc K. The impact of inflammatory licensing on heme oxygenase-1-mediated induction of regulatory T cells by human mesenchymal stem cells. Blood. 2011;117:4826-4835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 163] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 337. | Li H, Guo Z, Jiang X, Zhu H, Li X, Mao N. Mesenchymal stem cells alter migratory property of T and dendritic cells to delay the development of murine lethal acute graft-versus-host disease. Stem Cells. 2008;26:2531-2541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 88] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 338. | Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113:6576-6583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 501] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 339. | Zhang B, Liu R, Shi D, Liu X, Chen Y, Dou X, Zhu X, Lu C, Liang W, Liao L. Mesenchymal stem cells induce mature dendritic cells into a novel Jagged-2-dependent regulatory dendritic cell population. Blood. 2009;113:46-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 230] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 340. | Casiraghi F, Remuzzi G, Perico N. Mesenchymal stromal cells to promote kidney transplantation tolerance. Curr Opin Organ Transplant. 2014;19:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 341. | Perico N, Casiraghi F, Introna M, Gotti E, Todeschini M, Cavinato RA, Capelli C, Rambaldi A, Cassis P, Rizzo P. Autologous mesenchymal stromal cells and kidney transplantation: a pilot study of safety and clinical feasibility. Clin J Am Soc Nephrol. 2011;6:412-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 232] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 342. | Tan J, Wu W, Xu X, Liao L, Zheng F, Messinger S, Sun X, Chen J, Yang S, Cai J. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. JAMA. 2012;307:1169-1177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 423] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 343. | Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562-1565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 688] [Cited by in RCA: 638] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 344. | Burt TD. Fetal regulatory T cells and peripheral immune tolerance in utero: implications for development and disease. Am J Reprod Immunol. 2013;69:346-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 345. | Somerset DA, Zheng Y, Kilby MD, Sansom DM, Drayson MT. Normal human pregnancy is associated with an elevation in the immune suppressive CD25+ CD4+ regulatory T-cell subset. Immunology. 2004;112:38-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 582] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 346. | Sasaki Y, Sakai M, Miyazaki S, Higuma S, Shiozaki A, Saito S. Decidual and peripheral blood CD4+CD25+ regulatory T cells in early pregnancy subjects and spontaneous abortion cases. Mol Hum Reprod. 2004;10:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 537] [Cited by in RCA: 566] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 347. | Winger EE, Reed JL. Low circulating CD4(+) CD25(+) Foxp3(+) T regulatory cell levels predict miscarriage risk in newly pregnant women with a history of failure. Am J Reprod Immunol. 2011;66:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 348. | Gammill HS, Stephenson MD, Aydelotte TM, Nelson JL. Microchimerism in recurrent miscarriage. Cell Mol Immunol. 2014;11:589-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 349. | Maloney S, Smith A, Furst DE, Myerson D, Rupert K, Evans PC, Nelson JL. Microchimerism of maternal origin persists into adult life. J Clin Invest. 1999;104:41-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 335] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 350. | Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657-1664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 222] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 351. | Andrassy J, Kusaka S, Jankowska-Gan E, Torrealba JR, Haynes LD, Marthaler BR, Tam RC, Illigens BM, Anosova N, Benichou G. Tolerance to noninherited maternal MHC antigens in mice. J Immunol. 2003;171:5554-5561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 352. | Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749-6761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |