Published online Dec 24, 2016. doi: 10.5500/wjt.v6.i4.751
Peer-review started: July 21, 2016
First decision: September 5, 2016
Revised: November 4, 2016
Accepted: November 27, 2016
Article in press: November 29, 2016
Published online: December 24, 2016
Processing time: 148 Days and 12.5 Hours
To evaluate cardiac magnetic resonance imaging (CMR) as a non-invasive tool to detect acute cellular rejection (ACR) in children after heart transplant (HT).
Thirty pediatric HT recipients underwent CMR at the time of surveillance endomyocardial biopsy (EMB) and results were compared to 14 non-transplant controls. Biventricular volumes, ejection fractions (EFs), T2-weighted signal intensities, native T1 times, extracellular volumes (ECVs) and presence of late gadolinium enhancement (LGE) were compared between patients and controls and between patients with International Society of Heart and Lung Transplantation (ISHLT) grade ≥ 2R rejection and those with grade 0/1R. Heart rate (HR) and brain natriuretic peptide (BNP) were assessed as potential biomarkers.
Significant ACR (ISHLT grade ≥ 2R) was an infrequent event in our population (5/30, 17%). Ventricular volumes, EFs, LGE prevalence, ECVs, native T1 times, T2 signal intensity ratios, HR and BNP were not associated with the presence of ≥ 2R ACR.
In this pilot study CMR did not reliably identify ACR-related changes in pediatric HT patients.
Core tip: After heart transplantation the diagnosis of significant acute cellular rejection (ACR) changes management. It is associated with adverse outcome. Endomyocardial biopsy is the gold standard for the detection of ACR but has important limitations. This prospective trial examined the use of cardiac magnetic resonance imaging (CMR) for the diagnosis of ACR in pediatric heart transplant recipients. Significant rejection was a rare event in our cohort and was not associated with changes in CMR parameters in this pilot study.
- Citation: Greenway SC, Dallaire F, Kantor PF, Dipchand AI, Chaturvedi RR, Warade M, Riesenkampff E, Yoo SJ, Grosse-Wortmann L. Magnetic resonance imaging of the transplanted pediatric heart as a potential predictor of rejection. World J Transplant 2016; 6(4): 751-758
- URL: https://www.wjgnet.com/2220-3230/full/v6/i4/751.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i4.751
Acute cellular rejection (ACR) is an immune-mediated process leading to allograft damage and decreased graft survival and is a serious and potentially lethal complication after heart transplant (HT). The gold standard for the detection of rejection is an endomyocardial biopsy (EMB). However, EMB is an invasive procedure, exposes the patient to ionizing radiation and carries a small but important risk of serious complications[1-4].
Cardiac magnetic resonance imaging (CMR) has been proposed as a non-invasive method for the detection of rejection in adults after HT. However, CMR measurements used in adults for the detection of rejection or myocardial inflammation, including T2-weighted imaging[5,6], native T1 times and extracellular volume fractions (ECVs) derived from T1 mapping[7], myocardial thickness, ventricular volumes and ejection fraction (EF)[8,9] have not been systematically evaluated in pediatric HT recipients with EMB-proven ACR.
In this pilot study we sought to assess the utility of parameters of ventricular function and myocardial tissue characterization for the non-invasive detection of ACR in children and adolescents after HT.
This single center, prospective, cross-sectional study was approved by the institutional research ethics board and included pediatric (age < 18 years) HT patients who were scheduled for a clinically-indicated EMB between April 2010 and March 2011. All consecutive and eligible patients without contraindications to contrast-enhanced CMR during the study period were invited to participate. In patients who underwent more than one CMR/EMB procedure during the study period only the first set of investigations was analyzed for this study. Following written informed consent, CMR was performed immediately prior to cardiac catheterization and EMB. Control subjects were asymptomatic relatives of patients diagnosed with arrhythmogenic right ventricular cardiomyopathy (ARVC) who had normal echocardiograms, electrocardiograms (ECGs), signal-averaged ECGs as well as CMR scans and who were negative for ARVC-associated mutations if testing had been performed. Control subjects did not receive gadolinium as part of their CMR study. Heart rate (HR) was obtained from the average HR during the short axis CMR cine acquisition for ventricular volumetry.
Standardized immunosuppression post-transplantation for all patients included the use of thymoglobulin for induction (1-5 doses depending on risk factors), tacrolimus and mycophenolate mofetil. Perioperative steroids were discontinued 6 mo post-HT until 2007 and thereafter were discontinued at 5 d post-HT. Routine surveillance for rejection included serial echocardiograms, ECGs and cardiac catheterization with decreasing frequency over time post-transplantation.
At the authors’ institution right ventricular EMBs are obtained at 1, 6 and 12 mo and then annually up to 5 years post-HT; thereafter only if there is clinical or echocardiographic suspicion for rejection. During the EMB five or six tissue samples were obtained from the right ventricular surface of the interventricular septum, stained with hematoxylin and eosin and evaluated using light microscopy. Samples were graded by a hospital pathologist who was blinded to the CMR and biochemistry results (below) and reported according to the International Society of Heart and Lung Transplantation (ISHLT) Standardized Cardiac Biopsy Grading Criteria[10]. Congruent with clinical practice grades 2R and 3R were classified as significant ACR and grades 0R and 1R as non-significant ACR. Tissue samples were also evaluated for the presence of antibody-mediated rejection (AMR) by C4d immunohistochemical staining.
CMR was performed using a 1.5 Tesla scanner (Magnetom Avanto, Siemens AG Healthcare Sector, Erlangen, Germany) and a phased-array multi-channel surface receiver coil.
A stack of multiphase short axis slices was acquired using the steady state free precession technique for left and right ventricular volumes, as described previously[11,12]. Ventricular volumes were extracted from the cine short axis stack in end-diastole and end-systole in the routine clinical fashion using commercially available software (QMass, version 7.2, Medis, Leiden, The Netherlands). Ventricular volumes were reported as indexed to recipient body surface area. EFs for both ventricles were calculated using end-diastolic and end-systolic volumes. The presence of late gadolinium enhancement (LGE) was determined qualitatively on standard long-axis (4-chamber, 2-chamber and 3-chamber) and short-axis slices using phase-sensitive inversion-recovery acquisitions > 10 min after the administration of 0.2 mmol/kg gadopentetate dimeglumine (Magnevist®, Bayer, Leverkusen, Germany).
We previously described our T1 mapping approach for these patients in detail[13]. In short, a modified Look-Locker inversion recovery sequence (MOLLI) with inversion pulses of 100 msec and 150 msec, respectively, as well as 3 and 5 single-shot images after these inversion pulses was used to measure native and post-contrast longitudinal relaxation T1 times of myocardium and blood. Images were acquired in diastole at a single mid-ventricular short axis slice orientation before and > 10 min after administration of contrast (same injection as described above for LGE). Breathholds were used in cooperative patients and all other patients were scanned during free breathing. Longitudinal relaxation times (T1 times) were measured using commercially available software (CVI42, Circle Cardiovascular Imaging, Calgary, AB, Canada). Contours were drawn in the interventricular septum, the left ventricular (LV) free wall and in a region encompassing the entire LV myocardium. T1 times in the blood pool were measured in the LV cavity. The ECV was calculated using pre- and post-gadolinium T1 times of blood and myocardium as well as the patient’s hematocrit, obtained at the time of the scan[14].
An ECG-gated turbo spin-echo readout sequence without fat saturation pulse preceded by a double inversion recovery dark-blood preparation and the following parameters was obtained in a single midventricular short axis slice[15]: Inplane spatial resolution 1.6 mm, slice thickness 6-10 mm, TE 59 ms. Imaging was performed in diastole during every other or every third heartbeat, depending on the HR, to achieve a TR of at least 1000 ms. The scanner’s body coil was used for a homogeneous signal reception within the field of view. Myocardial signal intensity was measured around the circumference of the short axis slice and normalized to that of skeletal muscle using a dedicated module within the CVI42 software[16].
A blood sample was drawn upon insertion of the peripheral intravenous cannula needed for the CMR and analyzed for brain natriuretic peptide (BNP) levels (Modular Analytics, Roche Diagnostics, Laval, QC, Canada).
CMR data from transplant patients were stratified according to the presence (grade ≥ 2R) or absence (grade 0R or 1R) of significant ACR. Most variables were not normally distributed and results are thus presented as medians, 10th and 90th percentiles. Medians between groups were compared using a non-parametric Wilcoxon two-sample test or the Kruskal-Wallis test where appropriate. A P-value < 0.05 was considered statistically significant. All analyses were performed using SAS for Windows 9.4 (SAS Institute Inc., Cary, NC, United States). Statistical review of the study was performed by a biomedical statistician (FD).
The CMR studies from 14 non-transplant pediatric controls and 30 pediatric HT recipients were included in this study. The EMBs from 25 HT patients (83%) showed no significant ACR (ISHLT grades 0R or 1R) while 5 (17%) demonstrated significant rejection (ISHLT 2R). No patient had ISHLT grade 3R ACR. None of our HT patients were identified as having AMR. There were no statistically significant differences between the transplant groups with < 2R and ≥ 2R ISHLT rejection with respect to age at CMR or for time since transplant (Table 1). Patients with grade 2R rejection were younger than the controls and “no rejection” groups at the time of CMR but this difference was not statistically significant. HR and BNP were significantly increased in both groups of HT patients compared to controls but there were no statistically significant differences between the “no rejection” and “rejection” HT groups.
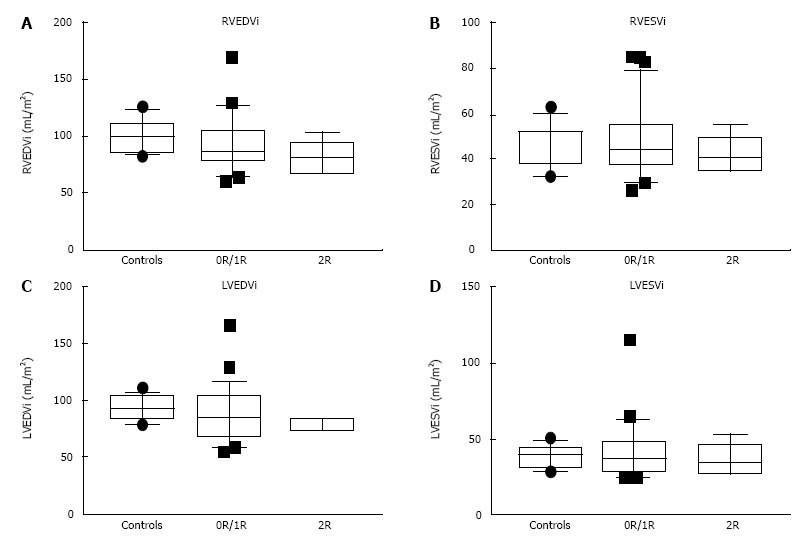
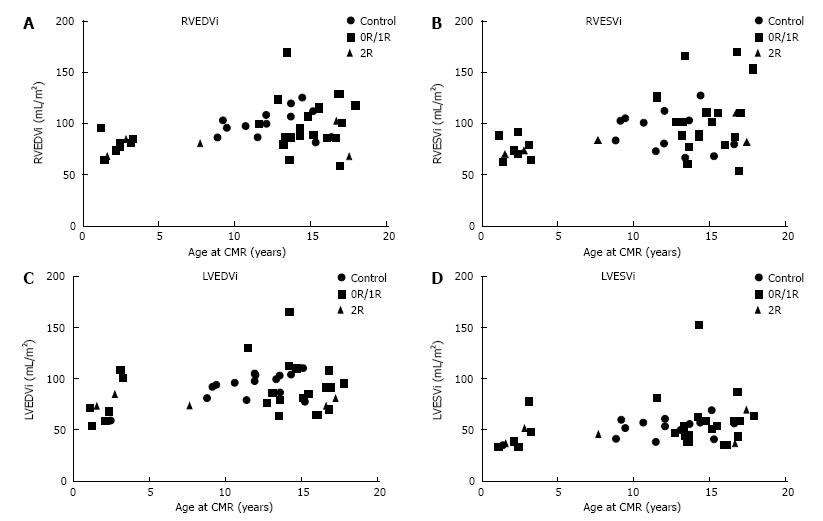
Biventricular end-diastolic volumes were decreased in the rejection group compared to the controls but not in the group without rejection (Figure 1). LVEF was decreased and LV mass increased only in the “no rejection” group compared to controls (Table 2). However, no significant differences between HT patients with and without clinically important rejection were observed with regards to ventricular volumes, ejection fractions, LV mass or LV mass/volume ratio. The absence of a significant change in ventricular volumes with rejection may be confounded by the increase in ventricular size with age (Figure 2). There was no significant association between BNP and CMR parameters.
| Controls | No rejection (0R/1R) | Rejection (2R) | |
| Number | 14 | 25 | 5 |
| RVEDVi (mL/m2) | 98.5 (85.7, 120.4) | 86.6 (64.1, 124.4) | 80.6a (68.1, 102.7) |
| RVESVi (mL/m2) | 50.5 (33.9, 56.1) | 44.1 (30.7, 77) | 41 (34.9, 55.4) |
| RVEF (%) | 53.4 (48, 60) | 50 (41, 57) | 47.4 (40, 56) |
| LVEDVi (mL/m2) | 93 (79.2, 104) | 85.2 (58.9, 112) | 74.1c (73.7, 85) |
| LVESVi (mL/m2) | 40.3 (29.8, 45) | 37.5 (24.6, 60.9) | 33.8 (27.2, 52) |
| LVEF (%) | 58.8 (53.2, 63) | 54e (46, 64) | 56 (36, 63) |
| LV mass (g/m2) | 53.5 (45.8, 61) | 61.5h (50, 84.6) | 66.1 (48, 80) |
The MOLLI sequence for T1 mapping became available to us after study enrollment had begun and therefore this data was available only in a subgroup of patients (Table 3). With regards to patient demographics there were no significant differences between this patient subset and the entire cohort. There were no significant differences in native T1 times and ECV fraction between patients with < 2R and ≥ 2R ISHLT rejection. LGE was not observed in any of the HT patients. Native T1 times and ECV were not quantified and LGE imaging was not obtained in controls who did not receive contrast.
| No rejection (0R/1R) | Rejection (2R) | |
| Number | 18 | 4 |
| Female (%) | 8 (44) | 1 (25) |
| Days post-transplant | 485 (13, 1818) | 142 (12, 800) |
| Age at CMR (yr) | 13.2 (1.4, 16.9) | 5.3 (1.6, 16.8) |
| Native T1 (ms) | ||
| IVS | 1008 (963, 1067) | 976 (967, 1026) |
| LV free wall | 988 (903, 1018) | 978 (924, 1016) |
| Entire LV | 991 (930, 1031) | 978 (944, 1020) |
| Hematocrit | 0.37 (0.26, 0.44) | 0.35 (0.29, 0.38) |
| ECV | ||
| IVS | 0.3 (0.26, 0.34) | 0.29 (0.26, 0.33) |
| LV free wall | 0.27 (0.24, 0.34) | 0.28 (0.25, 0.31) |
| Entire LV | 0.29 (0.26, 0.33) | 0.29 (0.27, 0.32) |
The global ratios of myocardial:skeletal muscle T2 signal intensities on a mid-ventricular short axis slice were similar between groups and did not differ between controls (median 1.37, 10th percentile 1.29, 90th percentile 1.67) and transplant patients with no rejection (median 1.3, 10th percentile 1.02, 90th percentile 1.6) or with rejection (median 1.3, 10th percentile 1.12, 90th percentile 1.47). There were no significant differences between transplant patients with < 2R and ≥ 2R ACR rejection.
Despite a growing body of evidence in adult HT patients and important information from animal experiments the role of CMR for the detection of ACR in children has not been explored[17-19]. CMR tissue characterization overcomes important limitations of EMB such as the potential of containing scar from a previous EMB in the histological sample and the fact that specimens are collected from the RV surface of the interventricular septum and may not be representative of the remainder of the myocardium[17]. The current study compared descriptors of myocardial edema, expansion of the myocardial extracellular space, presence of patchy myocardial scarring as well as ventricular size and function between controls and HT recipients as well as between HT patients with < 2R and ≥ 2R ISHLT rejection. However, in contrast to the experience in adult HT populations for several of these parameters, we were unable to demonstrate an association of any of them with ACR in pediatric HT recipients[17,19].
None of the 30 patients in our study displayed patchy myocardial scarring as evidenced by LGE. This finding is in contrast to studies in adult HT recipients which found myocardial scarring on LGE imaging in a sizeable proportion (although this was not correlated with rejection)[19,20]. The reason for this discrepancy remains unclear, but may be related to the younger age of the donor hearts used for pediatric HT[21,22]. While LGE reflects patchy myocardial scarring of a certain size native T1 and ECV are regarded as measures of expansion of the extracellular matrix. Both are elevated in states of increased myocardial fibrosis or edema. Acute rejection is characterized histologically by inflammation of the myocardium while chronic or repeated episodes of rejection have been associated with fibrotic remodeling[7,23,24]. Native T1 and ECV have been explored as markers of ACR in a pilot study in adults after HT but an association with rejection has yet to be demonstrated[8]. In the current study, albeit in a limited number of patients, ECV and native T1 times did not distinguish between < 2R and ≥ 2R ISHLT rejection. T2-weighted imaging is an established approach to detect tissue edema in inflammatory conditions and in the heart it is used as a marker for myocardial edema in myocarditis[6]. Studies that employed T2 signal intensity for the non-invasive detection of rejection have yielded mixed results in adult HT patients[5,9,25]. Our early results did not reveal increased signal intensity on T2-weighted imaging in patients with ACR. T2 mapping is another approach to myocardial edema which has yielded promising results in adult ACR[19,26-28], but this technique was not available to us at the time of the study. When discussing the lack of agreement between CMR markers and histological indicators of ACR, important shortcomings of EMB as the gold standard for the detection of ACR must be considered. Marie et al[26] found T2 mapping CMR to be “positive” for significant rejection several weeks before a follow-up EMB confirmed it suggesting a lack of sensitivity for EMB.
Ventricular size, LV myocardial mass, and function did not distinguish between patients with < 2R and ≥ 2R ISHLT rejection in our study. An increase in indexed right ventricular end-diastolic volume has emerged as a potential predictor of rejection in adults[19], but the trend in our patients was in the opposite direction for both right and left ventricular end-diastolic volumes. The use of ventricular volumes as a biological marker is potentially problematic for two reasons: Firstly, there is often a size mismatch between the donor and the recipient which can be up to two-fold in children. This mismatch is fairly random and quite possibly obscures any association between ventricular size and the presence of rejection. Secondly, indexing to body surface area, although standard practice, is a crude strategy for normalizing ventricular volumes in children. Z-scores are more reliable in ensuring comparability across a spectrum of ages, body sizes and genders, but universally accepted Z-scores for CMR volumes are missing.
Another potential sign of inflammation is myocardial swelling as evidenced by increased LV “mass”. Studies in adults have shown an increase in LV wall thickness during episodes of rejection[28,29]. However, an increase in LV mass in HT patients also occurs unrelated to rejection due to myocardial hypertrophy either as an adverse effect of medications[30], myocardial TNF-α expression[31] or hypertension. In our study there was no significant difference between HT patients with and without ≥ 2R ACR with regards to LV mass.
With regards to non-CMR parameters, higher HRs were noted in the HT recipients as compared to controls due to denervation during the transplant operation. However, in our small cohort HR did not differ significantly between patients with and without significant rejection. An elevated BNP has also been proposed as a marker for rejection in pediatric cardiac transplant patients[32] and, although elevated in the transplant patients, there was no significant difference between the transplant rejection groups.
The most important limitation of this pilot study is the small number of patients with ≥ 2R rejection which may have obscured associations of EMB with CMR parameters. The number of patients with available T1 mapping data, in particular, was very small. The small numbers may have also augmented the effects of potential confounders, for example donor:recipient size mismatch in HT patients, and thereby affected the comparability of ventricular volumes. The relatively low prevalence of ACR in the current era is related to improved immunosuppression regimes and, consistent with contemporary outcomes[33], none of the patients in our study had severe grade 3R rejection. The incidence of moderate (grade 2R) ACR (17%) was similar to the 13%-23% found by others[8,19,20]. T1 relaxometry and T2-weighted imaging were based exclusively on measurements in a single mid-ventricular short axis slice. Many experts now recommend a wider representation of all regions of the LV in tissue characterization. Since many of the measures we assessed are associated with intramyocardial edema, which is rare in 2R rejection, it is perhaps unsurprising that the studied CMR parameters were unchanged. It is possible that, rather than detecting acute rejection, CMR may have a greater role in identifying long-term changes in the myocardium perhaps associated with cardiac allograft vasculopathy.
Studies in adults have produced mixed results with regards to the use of CMR as a screening tool for rejection and our pilot study did not identify CMR parameters altered by the presence of 2R rejection. However, myocardial tissue characterization by CMR is undergoing continuous refinement. Given the conceptual association between ACR and myocardial inflammation and the multiple disadvantages of EMB, CMR should continue to be evaluated for its ability to non-invasively detect rejection. Larger trials producing sizable cohorts of patients with clinically-significant rejection episodes and including T2 relaxometry are recommended.
Cardiac magnetic resonance imaging (CMR) has been proposed as a non-invasive method for the detection of rejection in adults after heart transplant (HT). However, CMR measurements used in adults for the detection of rejection or myocardial inflammation have not been systematically evaluated in pediatric HT recipients with biopsy-proven acute cellular rejection (ACR). In this pilot study, the authors sought to assess the utility of parameters of ventricular function and myocardial tissue characterization for the non-invasive detection of ACR in children and adolescents after HT.
CMR tissue characterization overcomes important limitations of endomyocardial biopsy (EMB) such as the potential of containing scar from a previous EMB in the histological sample and the fact that specimens are collected from the RV surface of the interventricular septum and may not be representative of the remainder of the myocardium.
CMR has shown potential utility in adult heart transplant recipients. However, in this pilot study CMR did not reliably identify ACR-related changes in pediatric heart transplant patients.
Given the multiple disadvantages of EMB, CMR should continue to be evaluated for its ability to non-invasively detect rejection. Larger trials producing sizable cohorts of patients with clinically-significant rejection episodes and including T2 imaging are recommended.
EMB: Invasive procedure used to sample the endomyocardium of the right ventricle to diagnose rejection; ACR: Damage created by T-cell mediated immune response directed by the recipient against the transplanted organ; T1- and T2-weighted imaging: MRI sequences that are used to differentiate tissues based mainly on their composition of fat and water.
The authors have produced an interesting study evaluating the use of CMR scanning as a means to diagnose acute cellular rejection in paediatric HT recipients.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Canada
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bramhall S, Sijens PE S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Saraiva F, Matos V, Gonçalves L, Antunes M, Providência LA. Complications of endomyocardial biopsy in heart transplant patients: a retrospective study of 2117 consecutive procedures. Transplant Proc. 2011;43:1908-1912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 2. | Daly KP, Marshall AC, Vincent JA, Zuckerman WA, Hoffman TM, Canter CE, Blume ED, Bergersen L. Endomyocardial biopsy and selective coronary angiography are low-risk procedures in pediatric heart transplant recipients: results of a multicenter experience. J Heart Lung Transplant. 2012;31:398-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 3. | Fiorelli AI, Coelho GH, Aiello VD, Benvenuti LA, Palazzo JF, Santos Júnior VP, Canizares B, Dias RR, Stolf NA. Tricuspid valve injury after heart transplantation due to endomyocardial biopsy: an analysis of 3550 biopsies. Transplant Proc. 2012;44:2479-2482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 4. | Strecker T, Rösch J, Weyand M, Agaimy A. Endomyocardial biopsy for monitoring heart transplant patients: 11-years-experience at a german heart center. Int J Clin Exp Pathol. 2013;6:55-65. [PubMed] |
| 5. | Smart FW, Young JB, Weilbaecher D, Kleiman NS, Wendt RE, Johnston DL. Magnetic resonance imaging for assessment of tissue rejection after heterotopic heart transplantation. J Heart Lung Transplant. 1993;12:403-410. [PubMed] |
| 6. | Friedrich MG, Sechtem U, Schulz-Menger J, Holmvang G, Alakija P, Cooper LT, White JA, Abdel-Aty H, Gutberlet M, Prasad S. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. 2009;53:1475-1487. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1951] [Cited by in RCA: 1744] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 7. | Radunski UK, Lund GK, Stehning C, Schnackenburg B, Bohnen S, Adam G, Blankenberg S, Muellerleile K. CMR in patients with severe myocarditis: diagnostic value of quantitative tissue markers including extracellular volume imaging. JACC Cardiovasc Imaging. 2014;7:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 162] [Article Influence: 14.7] [Reference Citation Analysis (1)] |
| 8. | Miller CA, Naish JH, Shaw SM, Yonan N, Williams SG, Clark D, Bishop PW, Ainslie MP, Borg A, Coutts G. Multiparametric cardiovascular magnetic resonance surveillance of acute cardiac allograft rejection and characterisation of transplantation-associated myocardial injury: a pilot study. J Cardiovasc Magn Reson. 2014;16:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Almenar L, Igual B, Martínez-Dolz L, Arnau MA, Osa A, Rueda J, Palencia M. Utility of cardiac magnetic resonance imaging for the diagnosis of heart transplant rejection. Transplant Proc. 2003;35:1962-1964. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1265] [Cited by in RCA: 1380] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 11. | Grosse-Wortmann L, Roche SL, Yoo SJ, Seed M, Kantor P. Early changes in right ventricular function and their clinical consequences in childhood and adolescent dilated cardiomyopathy. Cardiol Young. 2010;20:418-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Lee W, Yoo SJ, Roche SL, Kantor P, van Arsdell G, Park EA, Redington A, Grosse-Wortmann L. Determinants and functional impact of restrictive physiology after repair of tetralogy of Fallot: new insights from magnetic resonance imaging. Int J Cardiol. 2013;167:1347-1353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Riesenkampff E, Messroghli DR, Redington AN, Grosse-Wortmann L. Myocardial T1 mapping in pediatric and congenital heart disease. Circ Cardiovasc Imaging. 2015;8:e002504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Flett AS, Hayward MP, Ashworth MT, Hansen MS, Taylor AM, Elliott PM, McGregor C, Moon JC. Equilibrium contrast cardiovascular magnetic resonance for the measurement of diffuse myocardial fibrosis: preliminary validation in humans. Circulation. 2010;122:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 741] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 15. | Simonetti OP, Finn JP, White RD, Laub G, Henry DA. “Black blood” T2-weighted inversion-recovery MR imaging of the heart. Radiology. 1996;199:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 449] [Cited by in RCA: 417] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 16. | Eitel I, Friedrich MG. T2-weighted cardiovascular magnetic resonance in acute cardiac disease. J Cardiovasc Magn Reson. 2011;13:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 166] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 17. | Butler CR, Thompson R, Haykowsky M, Toma M, Paterson I. Cardiovascular magnetic resonance in the diagnosis of acute heart transplant rejection: a review. J Cardiovasc Magn Reson. 2009;11:7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Lu W, Zheng J, Pan XD, Zhang MD, Zhu TY, Li B, Sun LZ. Diagnostic performance of cardiac magnetic resonance for the detection of acute cardiac allograft rejection: a systematic review and meta-analysis. J Thorac Dis. 2015;7:252-263. [PubMed] |
| 19. | Butler CR, Savu A, Bakal JA, Toma M, Thompson R, Chow K, Wang H, Kim DH, Mengel M, Haykowsky M. Correlation of cardiovascular magnetic resonance imaging findings and endomyocardial biopsy results in patients undergoing screening for heart transplant rejection. J Heart Lung Transplant. 2015;34:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 20. | Krieghoff C, Barten MJ, Hildebrand L, Grothoff M, Lehmkuhl L, Lücke C, Andres C, Nitzsche S, Riese F, Strüber M. Assessment of sub-clinical acute cellular rejection after heart transplantation: comparison of cardiac magnetic resonance imaging and endomyocardial biopsy. Eur Radiol. 2014;24:2360-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Boucek MM, Mathis CM, Boucek RJ, Hodgkin DD, Kanakriyeh MS, McCormack J, Gundry SR, Bailey LL. Prospective evaluation of echocardiography for primary rejection surveillance after infant heart transplantation: comparison with endomyocardial biopsy. J Heart Lung Transplant. 1994;13:66-73. [PubMed] |
| 22. | Webber SA, Naftel DC, Parker J, Mulla N, Balfour I, Kirklin JK, Morrow R. Late rejection episodes more than 1 year after pediatric heart transplantation: risk factors and outcomes. J Heart Lung Transplant. 2003;22:869-875. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Kellman P, Wilson JR, Xue H, Bandettini WP, Shanbhag SM, Druey KM, Ugander M, Arai AE. Extracellular volume fraction mapping in the myocardium, part 2: initial clinical experience. J Cardiovasc Magn Reson. 2012;14:64. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 205] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 24. | Riesenkampff E, Chen CK, Kantor PF, Greenway S, Chaturvedi RR, Yoo SJ, Greiser A, Dipchand AI, Grosse-Wortmann L. Diffuse Myocardial Fibrosis in Children After Heart Transplantations: A Magnetic Resonance T1 Mapping Study. Transplantation. 2015;99:2656-2662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 25. | Aherne T, Tscholakoff D, Finkbeiner W, Sechtem U, Derugin N, Yee E, Higgins CB. Magnetic resonance imaging of cardiac transplants: the evaluation of rejection of cardiac allografts with and without immunosuppression. Circulation. 1986;74:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 26. | Marie PY, Angioï M, Carteaux JP, Escanye JM, Mattei S, Tzvetanov K, Claudon O, Hassan N, Danchin N, Karcher G. Detection and prediction of acute heart transplant rejection with the myocardial T2 determination provided by a black-blood magnetic resonance imaging sequence. J Am Coll Cardiol. 2001;37:825-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 120] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 27. | Lund G, Morin RL, Olivari MT, Ring WS. Serial myocardial T2 relaxation time measurements in normal subjects and heart transplant recipients. J Heart Transplant. 1988;7:274-279. [PubMed] |
| 28. | Wisenberg G, Pflugfelder PW, Kostuk WJ, McKenzie FN, Prato FS. Diagnostic applicability of magnetic resonance imaging in assessing human cardiac allograft rejection. Am J Cardiol. 1987;60:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 29. | Revel D, Chapelon C, Mathieu D, Cochet P, Ninet J, Chuzel M, Champsaur G, Dureau G, Amiel M, Helenon O. Magnetic resonance imaging of human orthotopic heart transplantation: correlation with endomyocardial biopsy. J Heart Transplant. 1989;8:139-146. [PubMed] |
| 30. | Mano A, Nakatani T, Yahata Y, Kato T, Hashimoto S, Wada K, Ishibashi-Ueda H. Reversible myocardial hypertrophy induced by tacrolimus in a pediatric heart transplant recipient: case report. Transplant Proc. 2009;41:3831-3834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Stetson SJ, Perez-Verdia A, Mazur W, Farmer JA, Koerner MM, Weilbaecher DG, Entman ML, Quiñones MA, Noon GP, Torre-Amione G. Cardiac hypertrophy after transplantation is associated with persistent expression of tumor necrosis factor-alpha. Circulation. 2001;104:676-681. [PubMed] |
| 32. | Knecht KR, Alexander ML, Swearingen CJ, Frazier EA. NTproBNP as a marker of rejection in pediatric heart transplant recipients. Pediatr Transplant. 2012;16:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Thrush PT, Hoffman TM. Pediatric heart transplantation-indications and outcomes in the current era. J Thorac Dis. 2014;6:1080-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |