Published online Dec 24, 2016. doi: 10.5500/wjt.v6.i4.729
Peer-review started: July 12, 2016
First decision: September 12, 2016
Revised: October 5, 2016
Accepted: November 21, 2016
Article in press: November 23, 2016
Published online: December 24, 2016
Processing time: 161 Days and 10.4 Hours
To examine the risk of late-onset post-transplant lymphoproliferative disorder (PTLD) in the presence of persisting high Epstein-Barr virus (EBV) in EBV naïve pediatric heart transplant (HT) recipients.
A retrospective review of the medical records of the 145 pediatric HT recipients who had serial EBV viral load monitoring at our center was performed. We defined EBV naive patients whose EBV serology either IgM or IgG in the blood were negative at the time of HT and excluded passive transmission from mother to child in subjects less than 6 mo of age.
PTLD was diagnosed in 8 out of 145 patients (5.5%); 6/91 (6.5%) in those who were EBV seropositive and 2/54 (3.7%) in the EBV naïve group at the time of HT (P = 0.71). We found 32/145 (22%) patients with persistently high EBV load during continuing follow-up; 20/91 (22%) in EBV seropositive group vs 12/54 (22%) in EBV naïve group (P = 0.97). There was no significant association between pre-HT serostatus and EBV load after transplant (P > 0.05). In the EBV seropositive group, PTLD was diagnosed in 15% (3/20) of patients with high EBV vs 4.2% (3/71) of patients with low or undetectable EBV load (P = 0.14) whereas in EBV naïve patients 8.3% (1/12) of those with high EBV load and 2.3% (1/42) with low or undetectable EBV load (P = 0.41). There was a highly significant association between occurrence of PTLD in those with high EBV load and duration of follow up (4.3 ± 3.9 years) after HT by Cochran-Armitage test for the entire cohort (P = 0.005). At least one episode of acute rejection occurred in 72% (23/32) of patients with high EBV vs 36% (41/113) patients with low or undetectable EBV after HT (P < 0.05).
There is an association between persistently high EBV load during post-HT follow up and the occurrence of late-onset PTLD in pediatric HT recipients irrespective of serostatus at the time of transplant. The occurrence of allograft rejection increased in patients with high EBV load presumably due to reduction in immunosuppression.
Core tip: Post-transplant lymphoproliferative disorder (PTLD) after heart transplantation is a severe complication where there is still limited information is available. There are many publications on estimations of PTLD frequency in different settings and types of patient, as well as the factors associated with its appearance and prognosis. But, most studies do not take into account the length of follow-up which may be misleading given that patients are exposed to the risk of immunosuppression over a long period of follow-up. This study is unique that, it is a single center study span over a period of 18 years in which maintenance immunosuppression therapy and management of rejection episodes remained same throughout. Although, a single center study result cannot be generalized, however it adds to the existing literature for risk stratification of these patients based on whole blood Epstein-Barr virus (EBV) polymerase chain reaction (PCR) after accounting for the time since transplant and patients¡¯ pre-transplant EBV serostatus. This paper also highlights the risk of acute rejection after reduction or alteration in immunosuppression in patients with high EBV load by PCR without any effect on the occurrence of PTLD.
- Citation: Das B, Morrow R, Huang R, Fixler D. Persistent Epstein-Barr viral load in Epstein-Barr viral naïve pediatric heart transplant recipients: Risk of late-onset post-transplant lymphoproliferative disease. World J Transplant 2016; 6(4): 729-735
- URL: https://www.wjgnet.com/2220-3230/full/v6/i4/729.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i4.729
Post-transplant lymphoproliferative disorder (PTLD) is the most common malignancy occurring in 3.5%-9% of pediatric HT recipients[1-4]. It is characterized by uncontrolled proliferation of lymphoid lineage cells, the vast majority of which are B-cell lymphomas, in a context of posttransplant immunosuppression. In some situations, reducing the immunosuppression can reverse the proliferation, thus differentiating it somewhat from truly irreversible malignancies. Most but not all PTLD cases have a strong relationship with Epstein-Barr virus (EBV).
The development of PTLD is influenced by a variety of factors including the type, intensity, and cumulative amount of immunosuppression, and the EBV status of the donor and recipient. Children are at greatest risk for the development of PTLD since they are often seronegative at transplant and acquire a primary EBV infection post-heart transplant (HT) in the setting of immunosuppression. Nevertheless, the factors that account for whether or not a particular child develops EBV-associated PTLD are undetermined. Diagnosis and effective treatment of PTLD is hampered by our inability to determine which children are at risk of developing EBV-associated PTLD.
The onset of PTLD is usually preceded by an elevated EBV load in the peripheral blood which is highly sensitive but not a specific marker for development of PTLD in renal transplant recipients[5,6]. Routine long-term post-HT EBV monitoring identifies a group of children who carry persistent viral loads for months to years after solid organ transplants[5-8]. Patients with a persistently elevated level of circulating EBV may have an increased risk of PTLD[2,9]. Previously, a single center study reported that a high EBV load did not predict PTLD in early post-heart and heart-lung transplant period[10]. However, another single center study suggested that exposure to EBV and higher intensity immunosuppression was associated with increased risk of PTLD in pediatric HT recipients[11]. A more recent study has shown that early onset PTLD in solid organ transplant recipients appears mainly as an EBV-driven disease especially favored by insufficient immunosurveillance[12,13]. These contradictory findings leave the long-term clinical significance of chronic high EBV load unknown. We hypothesized that patients with persistently high EBV viral load at any time after their transplant in the setting of immunosuppression may be at increased risk for development of PTLD.
The objective of the study was to examine the risk of late-onset PTLD (> 1 year post-transplant) in the presence of persisting high EBV and to determine whether patients’ serostatus at the time of HT changed the risk.
All pediatric HT patients transplanted between 1995 and 2013 who had known EBV serology at the time of transplant were included in this retrospective descriptive study. For this study, we defined EBV naive patients whose EBV serology either IgM or IgG in the blood were negative at the time of HT and excluded passive transmission from mother to child in subjects less than 6 mo of age. Data collection included demographics, clinical data, pre-HT EBV serological status, serial post-HT EBV load, diagnosis of PTLD and acute rejection episodes during post-HT follow-up. The presence of EBV virus in whole blood was measured by quantitative polymerase chain reaction (PCR) using a cut-off of 1000 copies/mL according to our institution protocol.
Viral (EBV) load testing was done in whole blood using PCR every 2 wk for 3 mo, every month for 3 mo, every 3 to 6 mo for a duration of 1 year after HT, and thereafter annually. Additional EBV PCR levels were drawn if EBV PCR was rising, with any increased immunosuppression for treatment of allograft rejection, or if clinically indicated by symptoms such as protracted fever, gastrointestinal symptoms, unexplained elevated liver enzymes, lymphadenopathy, tonsillar hypertrophy, obstructive sleep apnea, unexplained anemia, pancytopenia, atypical lymphocytes or eosinophilia, persistent headache or focal neurological symptoms.
Our protocol for follow-up of patients based upon EBV PCR positivity included: (1) EBV PCR < 1000 copies/mL: No change in immunosuppression, routine follow-up as per above protocol; (2) EBV PCR 1000-9999 copies/mL: No change in immunosuppression, repeat EBV PCR every 2 wk; and (3) EBV PCR ≥ 10000 copies/mL (> 2 consecutive tests and remains positive for > 12 mo): We reduced immunosuppression with a goal for tacrolimus trough level 3-5 ng/mL, cyclosporine trough level 50-75 ng/mL, and decreased mycophenolate mofetil/azathioprine dose to half of the initial dose and closely monitored for any signs of acute rejections. For this study analysis, we divided all patients into 3 groups based on EBV viral load as a continuous value anytime during post-TX follow-up: group I: Negative EBV or EBV PCR < 1000 copies/mL; group II: EBV PCR 1000-9999 copies/mL; and group III: EBV PCR ≥ 10000 copies/mL (persistently positive for > one year). During follow-up, patients who had transiently increased EBV PCR in excess of 10000 but did not persist for a year were included in group II.
PTLD was defined according to the 2008 World health Organization (WHO) classification system, but early lesions such as lymphoid hyperplasia with scattered positive in situ hybridization using EBV encoded RNA detected by the Epstein-Barr early region (EBER) immunostaining assay[14] was excluded for this study as PTLD. All biopsy proved polymorphic, monomorphic and classical Hodgkin lymphoma-type PTLD patients were evaluated by our oncology service and treatment was guided as per oncology protocol.
Acute Allograft rejection was defined as ISHLT grade 2R or higher or an episode of clinically significant decline in cardiac function treated with steroid bolus or anti-T cell therapies. Endomyocardial biopsy was performed per our institutional protocol for all patients and frequency of biopsy was not modified based on high EBV load or reduction of immunosuppression. However, patients whose immunosuppression was decreased as a result of high EBV load were monitored closely clinically and by echocardiogram for any graft dysfunction.
All patients received basiliximab (simulect) and methyl prednisone for induction at the time of transplant as per our institution protocol since 2001. Between 1995-2000, our induction therapy was only methyl prednisone. Maintenance immunosuppression includes triple therapy of tacrolium/cyclosporine, mycophenolate mofetil (MMF), and steroids. Steroids were withdrawn after one year routinely unless there are more than one rejection episode within first year after transplant. This study was approved by our institutional IRB.
Descriptive analyses of the continuous and categorical data were performed using mean, standard deviation, median, quartiles, frequency and proportion as appropriate. Fisher’s exact test and χ2 tests were used to test binary variables between two groups. Cochran-Armitage test and logistic regression were used to test the association between post-HT EBV load, duration of follow-up and incidence of PTLD. The statistical analyses were performed with SAS 9.3.
A total of 145 patients were followed from 1995 to 2013 for mean 4.3 ± 3.9 years (interquartile range 1.5 to 6.0 years) post-HT. Mean age at HT was 6.6 ± 6.3 years, median age 4.8 years with interquartile range 0.69 to 12.0 years. EBV was first detected at a median of 1 year (range 0.1 to 16 years) post-HT. Patients were then sub-grouped based on age at transplant into 0-6 mo, 6 mo to 1 year, 1-7 years and 7-20 years vs EBV load as shown in Table 1. The proportions of high EBV load are 38.8%, 27.4%, 18.4% and 15.4% in age group 0-6 mo, 6 mo to 1 year, 1-7 years and 7-20 years, respectively. Cochran-Armitage test with square root transformation to age and the logistic regression showed that patients’ age at HT was negatively associated with high EBV load (P = 0.03), which means patients with younger age had high risk for high EBV during follow up. One year old was chosen as the threshold for younger patients. χ2 test showed that patients 1 year old or younger were more likely to have high EBV during follow up than patients older than 1 year old (P = 0.01). The relative risk for developing high EBV load in patients having transplant at 1 year old or younger is 2.16 (95%CI: 1.19-3.92) over patients having transplant at older age irrespective of their pre-HT EBV serological status.
| Group I (EBV PCR negative or < 1000) (n = 66) | Group II (EBV PCR 1000-9999) (n = 47) | Group III (EBV PCR≥10000) (n = 32) | |
| EBV Naïve at HT | 29/66 (44%) | 13/47 (28%) | 12/32 (37%) |
| Age at transplant | |||
| 0 up to < 6 mo | 9 | 10 | 12 |
| 6 mo < 1 yr | 3 | 5 | 3 |
| 1 yr up to < 7 yr | 28 | 8 | 8 |
| ≥ 7 yr up to 20 yr | 26 | 24 | 9 |
| Post-HT Follow-up (yr) | 4.5 ± 3.2 | 4.8 ± 4.2 | 4.6 ± 5.3 |
| No of PTLD | 1 | 3 | 4 |
| No of total Rejections | 40 | 42 | 48 |
| Number of patients with ≥ 1 episodes of rejections | 19 | 22 | 23 |
The clinical characteristics of individual PTLD patient are described in Table 2. All patients were treated by reducing immunosuppression; five patients received rituximab, two patients received chemotherapy and one patient received chemotherapy plus radiation therapy. Three patients underwent tumor resection and all patients survived the treatment of PTLD. One patient died two years after treatment of PTLD due to non-cardiac cause.
| Patient (gender) | Year of HT | HT to PTLD (yr) | EBV serologyat HT | EBV loadat PTLD | Organ involved in PTLD | CD20 positivity | EBER status of PTLD | Histological diagnosis | Treatment |
| 1 (M) | 1995 | 16 | Positive | < 10000 | Retroperitoneal lymph node | Neg | Neg | Hodgkin Lymphoma | Chemotherapy |
| 2 (F) | 1996 | 14 | Positive | < 10000 | Cervical Lymph node | Neg | Neg | Diffuse large B-cell lymphoma | Chemotherapy |
| 3 (M) | 1999 | 12 | Positive | > 10000 | Retroperitoneal lymph node | Pos | Neg | Polymorphic PTLD | Rituximab |
| 4 (M) | 2001 | 3 | Positive | > 10000 | Pharynx | Neg | Pos | Intermediate between Hodgkin and large cell lymphoma | Chemotherapy plus Radiation |
| 5 (F) | 2009 | 3 | Negative | > 10000 | Cervical Lymph node | Pos | Pos | Polymorphic PTLD | Rituximab |
| 6 (F) | 2000 | 14 | Positive | < 1000 | Brain-Temporal Lobe | Pos | Pos | Polymorphic PTLD | Rituximab |
| 7 (M) | 2000 | 3 | Negative | < 10000 | Small intestine | Pos | Pos | Polymorphic PTLD | Rituximab |
| 8 (F) | 2005 | 6 | Positive | > 10000 | Retroperitoneal lymph node | Pos | Pos | Large B-cell Lymphoma | Rituximab |
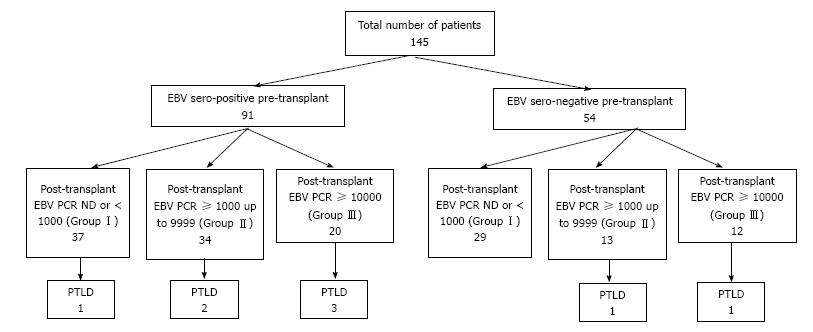
Figure 1 describes the distribution of patients’ EBV serological status at the time of HT, EBV viral load by PCR post-HT, and number of patients who developed PTLD for the entire cohort. Out of 145 patients, 54 (37%) were EBV seronegative and 91 (63%) were EBV seropositive at the time of transplant and 22% from each group developed persistently high EBV viral load during follow-up after HT (P = 0.97). There were 6 cases (6.4%) of PTLD in EBV seropositive group vs 2 cases (3.9%) in EBV naive group (P = 0.71). In the EBV seropositive group, PTLD was diagnosed in 15 % (3/20) of patients with persistently high EBV vs 4.2% (3/71) of patients with low or undetectable EBV load (P = 0.14) whereas in EBV naïve patients PTLD was diagnosed in 8.3% (1/12) who had persistently high EBV load and 2.3% (1/42) with low or undetectable EBV load (P = 0.41). There was no significant association between pre-HT serostatus and post-HT EBV viral load after transplant (P > 0.05).
For the entire cohort of 145 patients, we found 65/145 (44.8%) had negative or EBV < 1000 copies/mL (Group I), 48/145 (33%) had EBV load between 1000-9999 copies/mL (Group II), and 32/145 (22%) patients had EBV load ≥ 10000 copies/mL (Group III) during follow-up irrespective of initial serological status at the time of transplant. PTLD was diagnosed in 8 out of 145 patients (5.5%) at a median of 4.4 years (mean 7.5 ± 6.5 years, interquartile range 2.7 to 13.7 years) after heart HT. PTLD was diagnosed in 12.5% (4/32) of patients with persistently high EBV vs 3.5% (4/113) of patients with low or undetectable EBV load (P = 0.07 by Fisher’s exact test). High viral load could predict PTLD with sensitivity 50% (95%CI: 15.7%-84.30%), specificity 79.66% (95%CI: 71.8%-85.97%), positive likelihood ratio 2.45 (95%CI: 1.14-5.27), negative likelihood ratio 0.63 (95%CI: 0.31-1.26) and positive predictive value 12.5% (95%CI: 3.51%-2.88%). There is a significant association between persistently high EBV load during a sum of follow up over 11 ± 7 years after HT and the occurrence of PTLD by Cochran-Armitage test (P = 0.005).
There was at least one episode of acute rejection (Grade 2R) in 23 patients with high EBV load after reduction of their immunosuppression (Table 1). On the other hand, 41 patients with low or negative EBV load who had no change in their immunosuppression had at least one episode of rejection. Thus, a larger proportion of patients 72% (23/32) with persistently high EBV load had acute rejections vs 36% (41/113) patients with low or negative EBV load (P < 0.05). Furthermore, there was an increase in frequency of total rejection episodes in patients with persistently high EBV load by 150% (48/32) vs 72.5% (82/113) in patients with low or negative EBV load (P < 0.05).
The incidence of PTLD in our study at 5.5% is similar with other series reported[1]. The occurrence of PTLD is dependent on the transplanted organ type and patient-specific risk factors. The strongest risk factor for PTLD is the development of primary EBV infection after transplantation[2,12,15,16]. Schubert et al[2] have reported 8.2% incidence of PTLD in pediatric HT recipients and the EBV association was 83% as a risk factor for development of PTLD. EBV monitoring in peripheral blood using PCR has been reported to have variable sensitivity and lack of specificity as an indicator of risk for developing PTLD[5,10,17-19]. Among pediatric HT recipients studied by Bingler et al[20] those with high EBV load were more likely to develop late-onset PTLD, occurring as long as 8.4 years after HT. In this study, we showed that patients who underwent HT at younger age (Table 1) are at higher risk for development of high EBV load over time (P = 0.05) irrespective their serological status at the time of HT. This observation is of clinical importance because many potential risk factors for development of PTLD such as persistent EBV viremia and overall immunosuppression are a function of duration of follow-up and may not be observed in early post-HT period. The occurrence of PTLD is highest in younger patients; age may not be an independent risk factor but may depend upon the likelihood of the recipient being exposed to long-term immunosuppression.
One of the limitations of the current study is the fact that we had incomplete data on the donor EBV status. Therefore, we could not determine whether high EBV load was the result of primary infection derived from community exposure or related to donor transmission. Asymptomatic high EBV load also predicts other adverse outcomes, such as graft dysfunction or acute rejection[20,21]. Jabs et al[21] showed that EBV viremia occurring immediately after renal transplant was associated with subsequent rejection episodes, and they speculate that T cell responses to viral infection might cross-react with the graft. In another study, Smith et al[22] have showed that subclinical cytomegalovirus and EBV viremia occurring in the early post-transplant period was associated with higher incidence of allograft injury. The authors did not find evidence of significant viral replication in the renal allograft at 2 years after transplant, suggesting that graft dysfunction is not related to chronic infection[22]. We found a higher rate of rejection episodes (mostly grade 2R) in pediatric HT recipients with persistently high EBV load compared to those patients with low or negative EBV PCR. The mechanisms of rejection are not clear from this study but may include viral cytopathic effects, increased expression of alloantigen, adhesion molecule expression by endothelial cells, or indirect inflammatory effects due to cytokine release, or a combination of multiple mechanisms leading to allograft injury.
In our practice, we do reduce maintenance immunosuppression in patients who have persistently raised EBV PCR ≥ 10000 copies/mL of whole blood. A link between EBV load and level of immunosuppression in adult HT patients was noted[23]. We hypothesize that reduction of immunosuppression has probably contributed for higher allograft rejection episodes in patients with high EBV load. Therefore, we recommend close monitoring for allograft rejection must be done after reduction of immunosuppressive therapy.
In this study, we have used methyl prednisone as induction therapy from 1995 through 2000 and basiliximab and methyl-prednisone as induction therapy from 2001 through 2013. Our standard maintenance immunosuppression (tacrolimus/cyclosporine, MMF or azathioprine and steroids) and consistent decrease in immunosuppression strategy in response to a high EBV support to the notion that it is overall immunosuppression exposure during the life time of the patient which compromises anti-tumor and anti-viral immunosurveillance capacity and thus facilitate development of PTLD. We have not used sirolimus or evorlimus routinely and we cannot comment regarding the effect of proliferation signal inhibitors on EBV PCR or PTLD from this study.
This study must be viewed in light of some limitations. It was a single-center retrospective study and thus findings may not be generalizable. Some patients were transferred to another center and also transitioned to an adult HT program, thus complete follow-up data for a small portion of patients were not available. However, this is a well-studied patient population in which maintenance immunosuppression therapy and management of rejection episodes remained same throughout and we followed our standardized institutional protocol strictly.
In conclusion, there is an association between persistently high EBV load and the occurrence of late-onset PTLD in pediatric HT recipients especially considering cumulative incidences at different lengths of follow-up. Patients ≤ 1 year of age at the time of HT are more likely to have persistently high EBV PCR during follow up than patients > 1 year of age at the time of transplant irrespective of their EBV serological status. Reduction of immunosuppression in the face of persistently high EBV load did not change the proportions of patient who had late-onset PTLD but did increase the risk of allograft rejection significantly. Based on our findings, there is a need for research to better determine other factors that might be predictive of PTLD. Currently, there is a multi-center study sponsored by National Institute of Allergy and Infectious Diseases examining the role of viral (EBV) and immunological biomarker associated with development of PTLD after transplantation[24]. This study will provide further insight to identify surrogate markers that can predict development of PTLD.
Post-transplant lymphoproliferative disorder (PTLD) is a significant complication after heart transplantation. Most but not all PTLD cases have a strong relationship with Epstein-Barr virus (EBV). This condition straddles the disciplines of transplantation, immunology, oncology, and virology. PTLD presents significant problems for the clinician because it is difficult to predict and has high morbidity and mortality rates. In addition, it has the potential for graft loss due to disease itself or the need to reduce immunosuppression, which increases the risk of graft rejection.
The goal of this study is to review a single center experience of late-onset PTLD in the presence of persisting high EBV in pediatric heart transplant recipients.
This study showed that there is an association between persistently high EBV load during post-transplant follow up and the occurrence of late-onset PTLD in pediatric heart transplant recipients. The occurrence of PTLD is highest in younger patients; age may not be an independent risk factor but may depend upon the likelihood of the recipient being exposed to long-term immunosuppression. The incidence of allograft rejection increased in patients with high EBV load presumably due to reduction in immunosuppression.
Late-onset PTLD is less likely to be associated with patients’ EBV serostatus at the time of transplant. Also, late-onset PTLD may be more likely extra-nodal and heterogeneous.
Detection of persistently high EBV viral DNA by polymerase chain reaction (PCR) from the peripheral blood is associated with late-onset PTLD in pediatric heart transplant recipients.
The research is methodological well performed, clearly written, and the data is honestly presented.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Burgler S, Kim ST, Taheri S S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ
| 1. | Webber SA, Naftel DC, Fricker FJ, Olesnevich P, Blume ED, Addonizio L, Kirklin JK, Canter CE. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. 2006;367:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 174] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 2. | Schubert S, Abdul-Khaliq H, Lehmkuhl HB, Yegitbasi M, Reinke P, Kebelmann-Betzig C, Hauptmann K, Gross-Wieltsch U, Hetzer R, Berger F. Diagnosis and treatment of post-transplantation lymphoproliferative disorder in pediatric heart transplant patients. Pediatr Transplant. 2009;13:54-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 56] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Katz BZ, Pahl E, Crawford SE, Kostyk MC, Rodgers S, Seshadri R, Proytcheva M, Pophal S. Case-control study of risk factors for the development of post-transplant lymphoproliferative disease in a pediatric heart transplant cohort. Pediatr Transplant. 2007;11:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 42] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Mendoza F, Kunitake H, Laks H, Odim J. Post-transplant lymphoproliferative disorder following pediatric heart transplantation. Pediatr Transplant. 2006;10:60-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Höcker B, Fickenscher H, Delecluse HJ, Böhm S, Küsters U, Schnitzler P, Pohl M, John U, Kemper MJ, Fehrenbach H. Epidemiology and morbidity of Epstein-Barr virus infection in pediatric renal transplant recipients: a multicenter, prospective study. Clin Infect Dis. 2013;56:84-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Tanaka E, Sato T, Ishihara M, Tsutsumi Y, Hisano M, Chikamoto H, Akioka Y, Dohno S, Maeda A, Hattori M. Asymptomatic high Epstein-Barr viral load carriage in pediatric renal transplant recipients. Pediatr Transplant. 2011;15:306-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Rowe DT, Webber S, Schauer EM, Reyes J, Green M. Epstein-Barr virus load monitoring: its role in the prevention and management of post-transplant lymphoproliferative disease. Transpl Infect Dis. 2001;3:79-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Lau AH, Soltys K, Sindhi RK, Bond G, Mazariegos GV, Green M. Chronic high Epstein-Barr viral load carriage in pediatric small bowel transplant recipients. Pediatr Transplant. 2010;14:549-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | D’Antiga L, Del Rizzo M, Mengoli C, Cillo U, Guariso G, Zancan L. Sustained Epstein-Barr virus detection in paediatric liver transplantation. Insights into the occurrence of late PTLD. Liver Transpl. 2007;13:343-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 10. | Benden C, Aurora P, Burch M, Cubitt D, Lloyd C, Whitmore P, Neligan SL, Elliott MJ. Monitoring of Epstein-Barr viral load in pediatric heart and lung transplant recipients by real-time polymerase chain reaction. J Heart Lung Transplant. 2005;24:2103-2108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Manlhiot C, Pollock-Barziv SM, Holmes C, Weitzman S, Allen U, Clarizia NA, Ngan BY, McCrindle BW, Dipchand AI. Post-transplant lymphoproliferative disorder in pediatric heart transplant recipients. J Heart Lung Transplant. 2010;29:648-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Schober T, Framke T, Kreipe H, Schulz TF, Großhennig A, Hussein K, Baumann U, Pape L, Schubert S, Wingen AM. Characteristics of early and late PTLD development in pediatric solid organ transplant recipients. Transplantation. 2013;95:240-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 90] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Rogers BB, Sommerauer J, Quan A, Timmons CF, Dawson DB, Scheuermann RH, Krisher K, Atkins C. Epstein-Barr virus polymerase chain reaction and serology in pediatric post-transplant lymphoproliferative disorder: three-year experience. Pediatr Dev Pathol. 1998;1:480-486. [PubMed] |
| 14. | Niedobitek G, Herbst H. In situ detection of Epstein-Barr virus and phenotype determination of EBV-infected cells. Methods Mol Biol. 2006;326:115-137. [PubMed] [DOI] [Full Text] |
| 15. | Allen UD, Farkas G, Hébert D, Weitzman S, Stephens D, Petric M, Tellier R, Ngan B, Fecteau A, West L. Risk factors for post-transplant lymphoproliferative disorder in pediatric patients: a case-control study. Pediatr Transplant. 2005;9:450-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Savoie A, Perpête C, Carpentier L, Joncas J, Alfieri C. Direct correlation between the load of Epstein-Barr virus-infected lymphocytes in the peripheral blood of pediatric transplant patients and risk of lymphoproliferative disease. Blood. 1994;83:2715-2722. [PubMed] |
| 17. | Kimura H, Ito Y, Suzuki R, Nishiyama Y. Measuring Epstein-Barr virus (EBV) load: the significance and application for each EBV-associated disease. Rev Med Virol. 2008;18:305-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 115] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Gulley ML, Tang W. Using Epstein-Barr viral load assays to diagnose, monitor, and prevent posttransplant lymphoproliferative disorder. Clin Microbiol Rev. 2010;23:350-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 165] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 19. | Stevens SJ, Verschuuren EA, Verkuujlen SA, Van Den Brule AJ, Meijer CJ, Middeldorp JM. Role of Epstein-Barr virus DNA load monitoring in prevention and early detection of post-transplant lymphoproliferative disease. Leuk Lymphoma. 2002;43:831-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 76] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 20. | Bingler MA, Feingold B, Miller SA, Quivers E, Michaels MG, Green M, Wadowsky RM, Rowe DT, Webber SA. Chronic high Epstein-Barr viral load state and risk for late-onset posttransplant lymphoproliferative disease/lymphoma in children. Am J Transplant. 2008;8:442-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Jabs WJ, Maurmann S, Wagner HJ, Müller-Steinhardt M, Steinhoff J, Fricke L. Time course and frequency of Epstein-Barr virus reactivation after kidney transplantation: linkage to renal allograft rejection. J Infect Dis. 2004;190:1600-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, McDonald RA. Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol. 2010;21:1579-1586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Doesch AO, Konstandin M, Celik S, Kristen A, Frankenstein L, Sack FU, Schnabel P, Schnitzler P, Katus HA, Dengler TJ. Epstein-Barr virus load in whole blood is associated with immunosuppression, but not with post-transplant lymphoproliferative disease in stable adult heart transplant patients. Transpl Int. 2008;21:963-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | National Institute of Allergy and Infectious Diseases (NIAID). Biomarkers for Post-Transplant Lymphoproliferative Disorders in Children. n: ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). [accessed 2016 Jul 1] 2016; Available from: http//clinicaltrials.gov/ct2/show/NCT02182986. |