Published online Dec 24, 2016. doi: 10.5500/wjt.v6.i4.712
Peer-review started: April 27, 2016
First decision: June 16, 2016
Revised: October 20, 2016
Accepted: November 1, 2016
Article in press: November 3, 2016
Published online: December 24, 2016
Processing time: 231 Days and 22.5 Hours
To evaluate and compare the outcomes of kidney transplant (KT) from deceased donors among standard criteria, acute kidney injury (AKI) and expanded criteria donors (ECDs).
This retrospective study included 111 deceased donor kidney transplant recipients (DDKT). Deceased donors were classified as standard criteria donor (SCD), AKI donor and ECD. AKI was diagnosed and classified based on change of serum Cr by acute kidney injury network (AKIN) criteria. Primary outcome was one-year estimated glomerular filtration rate (eGFR) calculated from Cr by CKD-EPI. Multivariate regression analysis was done by adjusting factors such as type of DDKT, %Panel-reactive antibodies, cold ischemic time, the presence of delayed graft function and the use of induction therapy. Significant factors that can affect the primary outcomes were then identified.
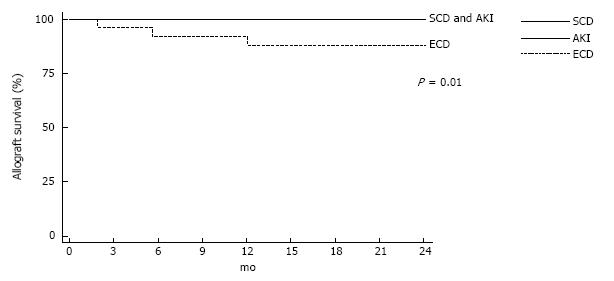
ECD group had a significantly lower eGFR at one year (33.9 ± 17.3 mL/min) when compared with AKI group (56.6 ± 23.9) and SCD group (63.6 ± 19.9) (P < 0.001). For AKI group, one-year eGFR was also indifferent among AKIN stage 1, 2 or 3. Patients with AKIN stage 3 had progressive increase of eGFR from 49.6 ± 27.2 at discharge to 61.9 ± 29.0 mL/min at one year. From Kaplan-Meier analysis, AKI donor showed better two-year graft survival than ECD (100% vs 88.5%, P = 0.006). Interestingly, AKI group had a stable eGFR at one and two year. The two-year eGFR of AKI group was not significantly different from SCD group (56.6 ± 24.5 mL/min vs 58.6 ± 23.2 mL/min, P = 0.65).
Kidney transplantations from deceased donors with variable stage of acute kidney injuries were associated with favorable two-year allograft function. The outcomes were comparable with KT from SCD. This information supports the option that deceased donors with AKI are an important source of organ for kidney transplantation even in the presence of stage 3 AKI.
Core tip: Many concerns about problems from using kidneys donated from donors who had acute kidney injury (AKI) before organ procurement lead to underutilization of such kidneys. Several kidneys have unnecessary been discarded in recent year. Here, we describe the comparable allograft and patient outcomes between using kidney from standard criteria donor and donor with AKI. Kidney transplantations from deceased donors with variable stages of acute kidney injuries were associated with favorable allograft function. This information supports the option that deceased donors with AKI are an important source of organ for kidney transplantation and can remedy the problem of organ shortage.
- Citation: Wiwattanathum P, Ingsathit A, Kantachuvesiri S, Arpornsujaritkun N, Tirapanich W, Sumethkul V. Stabilization of estimated glomerular filtration rate in kidney transplantation from deceased donors with acute kidney injuries. World J Transplant 2016; 6(4): 712-718
- URL: https://www.wjgnet.com/2220-3230/full/v6/i4/712.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i4.712
An increasing tendency to perform kidney transplant (KT) from deceased donors other than standard criteria donor (SCD) is the result of disparity between the number of patient being in the waiting list for transplantation and utilized donor pool[1]. Types of non-ideal deceased donor include donors with acute kidney injury (AKI) and expanded criteria donor (ECD) are being used for expanding donor pool[2]. However, there are concerns about worse allograft outcomes when using kidneys form AKI donors. Therefore, a significant number of kidneys from AKI donors with high terminal serum creatinine level have been discarded. Hence, the plan to solve problem of organ shortage cannot be accomplished.
Increased incidence of delayed graft function (DGF)[3,4] is a significant disadvantage of using kidneys from AKI donors. This can lead to increased hospital stay and cost of treatment or even worse allograft function[5] when compare with KT from SCD. In addition, it is uncertain whether KT from AKI donor is associated with increased risk of acute rejection or allograft loss when compare with KT from using kidney from standard deceased donor[3,4]. Since AKI can occur from different causes and have different severities, the outcomes of KT from donors with AKI may be varied. Theoretically, KT from donors with mild degree of AKI may have favorable outcomes than KT from severe AKI. However, it is not universally agreed to use kidneys from donors with AKI. There are studies reporting association of discarding kidney in the presence of AKI of deceased donor[6]. We conducted a study aimed to determine outcomes of kidney transplantation from deceased donors with variable degrees of acute kidney injuries.
A retrospective cohort of 243 KT recipients from our single center hospital during 1st January 2012 to 31st December 2013 was reviewed. Inclusion criteria were (1) deceased donor kidney transplant (DDKT) recipient; (2) Age ≥ 15 years old; (3) Negative lymphocytotoxic cross match result at the time of transplantation; and (4) Panel-reactive antibodies (PRA) luminex < 20%. Exclusion criteria were: (1) recipients who had combined solid organ transplantation; and (2) donor whose terminal serum creatinine increased ≥ 0.3 mg/dL but not ≥ 1.5-fold from baseline. From these inclusion and exclusion criteria (excluded 115 cases due to living related kidney transplantation, 8 cases due to age < 15 years, 1 case due to combined solid organ transplant and 8 cases due to terminal serum creatinine increased ≥ 0.3 mg/dL but not ≥ 1.5-fold from baseline), total 111 KT recipients who received DDKT were enrolled in the study. This study was approved by the study center Institutional Review Board/Ethics Committee.
Baseline transplantation data and the clinical outcomes at two-year were collected from all patients then compared outcomes by statistical analysis. Study populations were stratified into 3 groups according to the donor status: (1) Standard criteria deceased donor (SCD); (2) Deceased donor with AKI donor; and (3) Expanded criteria deceased donor (ECD). AKI donor was recognized by a rising of serum creatinine more than 0.3 mg/dL and defined by AKI Network criteria (AKIN criteria[7]) based on baseline to terminal serum creatinine (Cr) as follows: Stage 1, increase in Cr ≥ 1.5 to < 2-fold increase; stage 2, 2 to < 3 fold increase and stage 3, ≥ 3-fold increase. However, we did not included AKI donors who have terminal serum creatinine less than 1.5 fold from baseline to ensure that degree of AKI was significant enough to have impacts on transplantation outcomes. ECD was defined by any donor over the age of 60, or a donor over the age of 50 with two of the following: A history of high blood pressure, a creatinine greater than or equal to 1.5 mg/dL, or death resulting from a stroke. All other donors were classified as SCD.
Primary outcome was estimated glomerular filtration rate (eGFR) at one year as calculated from Cr by CKD-EPI equation. Secondary outcomes were eGFR at discharge and two year, rate of DGF (defined as requirement of dialysis within 7 d after transplantation), two-year allograft and patient survival.
Continuous variables were described as mean values (SD) and median values (range) for data with normal distribution and non-normal distribution respectively. Categorical variables were described as frequency and percentage. Student t test (or Mann-Whitney U test) was used to compare the difference between groups for continuous data. A χ2 test (or Fisher’s exact test) was used to compare the difference between groups for categorical data. Multivariate regression analysis was used to determine independently significant factors (type of DDKT, %PRA, cold ischemic time, the presence of DGF and the use of induction therapy) that may affect one-year eGFR. Allograft survival and patient survival were presented by Kaplan Meier analysis. All analyses were performed using Stata statistical software, version 13.0 (Stata Corp., Collage Station, TX). P < 0.5 was considered significant. The statistical review of the study was performed by a biomedical statistician.
A total of 119 DDKT recipients were enrolled. Eight recipients receiving kidney from AKI donors whose terminal serum creatinine increased ≥ 0.3 mg/dL but not ≥ 1.5-fold from baseline and were excluded. One hundred and eleven patients were included in the analysis. There were 32 recipients in SCD group, 51 in AKI group and 28 in ECD group. Recipient and donor characteristics are shown in Table 1. All recipient baseline characteristics were similar among 3 groups. Donor age was older in ECD group than the other groups. Most donors were male and the proportion was highest in AKI group. Basiliximab (Simulect®) was commonly used for induction in both SCD (34.4%) and AKI group (47.1%). Antithymocyte globulin (ATG) was frequently used in ECD group (39.9%). However, the different in prescribing induction therapy was not statistically significant (P = 0.19). Maintenance immunosuppressive regiments were shown in Table 1. The combination of cyclosporine and everolimus was more commonly used in AKI and ECD donor when compared with standard criteria deceased donor (P = 0.05).
| DDKT (n = 111) | SCD (32) | AKI (51) | ECD (28) | aP-value | bP-value |
| Recipients | |||||
| Age year (mean ± SD) | 42.7 ± 13.8 | 43.9 ± 12.0 | 43.1 ± 12.3 | 0.68 | 0.67 |
| Male n (%) | 19 (59.4) | 35 (68.6) | 16 (57.1) | 0.48 | 0.55 |
| Pre KT dialysis | 1 | 1 | |||
| Hemodialysis n (%) | 26 (81.3) | 42 (82.4) | 23 (82.1) | ||
| Peritoneal dialysis n (%) | 6 (18.8) | 9 (17.7) | 5 (17.9) | ||
| Comorbid n (%) | |||||
| DM | 2 (6.25) | 8 (15.7) | 3 (10.7) | 0.3 | 0.48 |
| HT | 30 (93.8) | 49 (96.1) | 25 (89.3) | 0.67 | 0.41 |
| CAD | 1 (3.1) | 1 (1.9) | 1 (3.6) | 1 | 1 |
| Cause of ESRD n (%) | 0.91 | 0.73 | |||
| Unknown (no biopsy) | 23 (23.2) | 33 (31.8) | 18 (18.9) | ||
| Diabetic nephropathy | 1 (0.9) | 1 (1.3) | 1 (0.8) | ||
| IgA nephropathy | 1 (1.9) | 2 (2.6) | 3 (1.5) | ||
| Chronic glomerulonephritis | 2 (0.9) | 1 (1.3) | 0 (0.8) | ||
| Blood group n (%) | 0.14 | 0.38 | |||
| A | 4 (12.5) | 13 (25.5) | 7 (25.0) | ||
| B | 13 (40.6) | 13 (25.5) | 7 (25.0) | ||
| AB | 4 (12.5) | 2 (3.9) | 3 (10.7) | ||
| O | 11 (34.4) | 23 (45.1) | 11 (39.3) | ||
| PRA - % median (range) | 0 (0.85) | 0 (0.0) | 0 (0.0) | 0.03 | 0.04 |
| Second KT n (%) | 2 (6.25) | 3 (5.88) | 1 (3.57) | 1 | 1 |
| Total HLA mismatch - (mean ± SD) | 2.5 (1.2) | 2.3 (1.1) | 2.1 (1.1) | 0.52 | 0.76 |
| Donors | |||||
| Age, year (mean ± SD) | 33.9 ± 14.8 | 41.0 ± 12.0 | 61.2 ± 7.0 | 0.02 | < 0.001 |
| Male n (%) | 24 (75.0) | 44 (86.3) | 17 (60.7) | 0.25 | 0.04 |
| Terminal serum creatinine (mg/dL) - median (range) | 0.91 (0.73, 1.13) | 2.22 (1.65, 3.20) | 1.28 (0.99, 2.70) | < 0.001 | |
| Cold ischemic time, minute (mean ± SD) | 1099 ± 291 | 1129 ± 294 | 1261 ± 242 | 0.65 | 0.5 |
| Immunosuppressive drugs | |||||
| Induction n (%) | 0.11 | 0.19 | |||
| No | 16 (50.0) | 16 (31.4) | 9 (32.1) | ||
| ATG | 5 (15.6) | 11 (21.6) | 11 (39.3) | ||
| Simulect | 11 (34.4) | 24 (47.1) | 8 (28.6) | ||
| Maintenance n (%) | 0.05 | 0.005 | |||
| Tacrolimus/mycophenolate/prednisolone | 16 (50.0) | 27 (52.9) | 13 (46.4) | ||
| Cyclosporin A/mycophenolate/prednisolone | 15 (46.8) | 16 (31.4) | 5 (17.9) | ||
| Cyclosporin A/everolimus/prednisolone | 0 | 7 (13.7) | 8 (28.6) | ||
| Everolimus/mycophenolate/prednisolone | 1 (3.1) | 0 | 0 |
eGFR at discharge was 64.1 ± 22.1, 52.5 ± 22.9 and 35.5 ± 17.9 mL/min for SCD, AKI and ECD group. eGFR at one year was 63.6 ± 19.9, 56.6 ± 23.9 and 33.9 ± 17.3 mL/min for SCD, AKI and ECD group. eGFR at two year was 58.6 ± 23.2, 56.6 ± 24.5 and 29.9 ± 19.2 mL/min in SCD, AKI and ECD group respectively (Table 2). Two-year eGFR was significant lower in ECD group (P < 0.001) when compared with the other groups but was not different between SCD group and AKI group (P = 0.65). For AKI group, two-year eGFR was also indifferent among degree of AKI as classified by AKIN stage 1, 2 or 3 (Table 3). Two-year eGFR for AKI group with AKIN stage 1, 2 and 3 was 53.4 ± 24.3, 54.0 ± 21.4 and 64.0 ± 29.4 mL/min (P = 0.79). While two-year eGFR in both SCD and ECD groups decreased over time after transplantation, two-year eGFR in AKI group had tendency to improve over time after transplantation especially in AKIN stage 3 (Table 3). In AKIN stage 3 group, two-year eGFR progressively improved form 49.6 ± 27.2 mL/min after transplant to 64.0 ± 29.4 mL/min. However, this change was not statistically different (P = 0.12). Univariate regression analysis showed that the use of ECD and presence of DGF were significantly associated with decreased of eGFR at one year by univariate model. However, multivariate regression analysis showed that use of ECD is the only factor that was associated with declining one-year eGFR (Table 4).
| Outcomes | SCD (32) | AKI (51) | ECD (28) | aP-value | bP-value |
| Cr at discharge - mg/dL (mean ± SD) | 1.35 ± 0.51 | 1.70 ± 0.84 | 2.41 ± 1.00 | 0.04 | < 0.001 |
| Cr at 1 yr - mg/dL (mean ± SD) | 1.35 ± 0.50 | 1.59 ± 0.75 | 2.64 ± 1.38 | 0.14 | < 0.001 |
| Cr at 2 yr - mg/dL (mean ± SD) | 1.52 ± 0.63 | 1.68 ± 1.06 | 3.29 ± 2.12 | 0.47 | < 0.001 |
| eGFR at discharge - mL/min (mean ± SD) | 64.1 ± 22.1 | 52.5 ± 22.9 | 35.5 ± 17.9 | 0.03 | < 0.001 |
| eGFR at 1 yr - mL/min (mean ± SD) | 63.6 ± 19.9 | 56.6 ± 23.9 | 33.9 ± 17.3 | 0.19 | < 0.001 |
| eGFR at 2 yr - mL/min (mean ± SD) | 58.6 ± 23.2 | 56.6 ± 24.5 | 29.9 ± 19.2 | 0.65 | < 0.001 |
| DGF n (%) | 10 (31.2) | 29 (56.9) | 21 (77.8) | 0.03 | 0.001 |
| Length of stay - d (mean ± SD) | 24.4 ± 8.3 | 31.1 ± 14.7 | 37.9 ± 15.3 | 0.02 | 0.002 |
| Nephrectomy n (%) | 0 | 2 (3.9) | 2 (7.4) | 0.52 | 0.27 |
| Acute rejection | 5 (15.7) | 10 (19.6) | 6 (27.4) | 0.70 | 0.8 |
| ACR | 2 (6.3) | 6 (11.8) | 2 (7.1) | ||
| ABMR | 1 (3.1) | 3 (5.9) | 3 (10.7) | ||
| ACR + ABMR | 2 (6.3) | 1 (1.9) | 1 (3.6) | ||
| Graft loss n (%) | 0 | 0 | 3 (11.5) | NS | 0.01 |
| Death n (%) | 2 (6.3) | 3 (5.1) | 3 (10.7) | 0.63 | 0.57 |
| CMV n (%) | 7 (5.2) | 6 (8.3) | 5 (4.5) | 0.23 | 0.46 |
| BK virus nephropathy n (%) | 1 (3.1) | 3 (5.69) | 0 | 1 | 0.69 |
| eGFR - mean ± SD | SCD (n)(32) | AKIN stage (n) | P-value | ||
| 1 (18) | 2 (21) | 3 (12) | |||
| eGFR at discharge - mL/min | 64.1 ± 22.1 | 49.8 ± 20.7 | 57.1 ± 23.7 | 49.6 ± 27.2 | 0.87, 0.07 |
| eGFR at 1 yr - mL/min | 63.6 ± 19.9 | 52.9 ± 21.2 | 57.1 ± 21.5 | 61.9 ± 29.0 | 0.47, 0.92 |
| eGFR at 2 yr - mL/min | 58.6 ± 23.2 | 53.4 ± 24.3 | 54.0 ± 21.4 | 64.0 ± 29.4 | 0.79, 0.54 |
| Factors | Univariate | Multivariate | ||||
| B-coefficient | P-value | 95%CI | B-coefficient | P-value | 95%CI | |
| Type of donor | ||||||
| SCD | Reference | NA | Reference | NA | ||
| AKI | -6.73 | 0.17 | -16.41, 2.94 | -3.7 | 0.49 | -14.52, 7.13 |
| ECD | -29.76 | < 0.001 | -41.67, -17.85 | -25.43 | < 0.001 | -38.80, -12.05 |
| PRA > 20% | 3.49 | 0.64 | -7.37, 14.34 | 3.62 | 0.53 | -7.82, 15.05 |
| DGF | 12.3 | 0.008 | 3.22, 21.39 | 6.17 | 0.18 | -2.91, 15.25 |
| HLA mismatch ≥ 3 | -2.99 | 0.53 | -12.45, 6.46 | -7.12 | 0.11 | -15.77, 1.53 |
| CIT > 24 h | -14.72 | 0.03 | -27.99, -1.45 | -9.54 | 0.14 | -22.19, 3.12 |
| Received Induction | 4.49 | 0.36 | -5.28, 14.25 | 1.8 | 0.72 | -8.09, 11.70 |
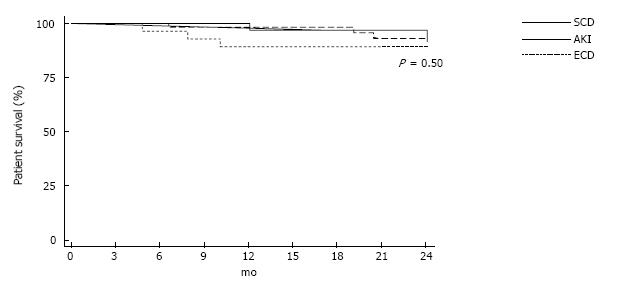
Rate of DGF was lowest in SCD group and highest in ECD group. DGF occurred 31.2%, 56.9% and 77.8% for each group (P = 0.001). Rate of acute rejection was not differed among the three groups (Table 2). Two-year allograft survival was 100%, 100% and 88.5% for each group (Figure 1, P = 0.01). Two-year patient survival rate was similar among three groups (Figure 2). Cardiovascular death was responsible for cause of death in 1, 3 and 1 recipient in SCD, AKI and ECD group respectively. Infection related death was responsible for cause of death in 1 recipient both from SCD and ECD group. Rate of CMV and BK virus infection were not difference among 3 groups (Table 2).
Our findings suggest that transplantation from deceased donors with AKI have comparable outcome when compared with SCD. The outcomes include both eGFR and two year patient survival. In addition, eGFR of AKI group did not decline after two year follow up. In contrast, eGFR in ECD group significantly declined after two year. This finding supports the view that kidneys with AKI may have recovery after a period of time.
In native kidney, after injury subsides, kidney can repair itself and restore normal or sub-normal function over time depends on severity and duration of injury[8]. Our finding suggests that these processes also occur in transplanted kidney. As shown in AKI group, one-year eGFR had progressive increase from baseline and stable at two-year follow up in all three groups. However, there are difficulties to predict the ability of each kidney allograft regarding the ability to recovery from acute kidney injuries. A calculation of “Kidney Donor Profile Index”[9,10] has been proposed to predict the risk of graft loss after deceased donor kidney transplantation. The involved donors’ parameters include age, height, weight, ethnicity, history of hypertension, history of diabetes, causes of death, serum creatinine, HCV status and donation after circulatory death status. However, the calculations of KDPI use a single value of serum creatinine and may or may not be indicative the presence of AKI in the donors. Evidences from some studies showed worse allograft function from AKI donor. These suggested that not all kidneys from AKI donor were suitable for transplantation. Researches providing such information are necessary and useful for making decision on which kidney should be used or discarded.
In the recent years, kidneys form AKI donor were underutilization. As shown in some studies that there are high discard rate of deceased donor with high serum creatinine. About 20%-30% of kidneys from AKI donors were discarded and sometime more than 40 percent were discarded when terminal serum creatinine > 2.0 mg/dL[3,6,11]. In contrast, our study has shown that KT from deceased donors with AKI is associated with comparable clinical outcomes with standard criteria deceased donors. Thus, our results show that kidneys from AKI donor are important source for organ transplantation and should not be discarded.
The limitation of our study is that there may be selection bias regarding the quality of kidneys when compare with other studies[3,6]. Pre-implantation biopsy and organ perfusion machine are not routinely used in this study for the organ procurement process. These can lead to more kidneys being used when organ retrieval process was satisfied as judged by the clinician.
In summary, kidney transplantations from deceased donors with variable stages of acute kidney injuries were associated with favorable two-year allograft function and survival. The outcomes were comparable with KT from those of standard criteria deceased donors. This information supports the option that deceased donors with AKI are an important source of organ for kidney transplantation even in the presence of stage 3 AKI. However, not all kidneys from AKI donor may be used for transplantation. Further studies are required to determine and clarify the optimal use of kidneys with AKI and the precise parameters that can identify suitable kidneys form AKI donor suitable for proceeding to transplantation.
Organ shortage is a common problem worldwide. Kidney transplantations from non-ideal deceased donors are a potential option to minimize this problem. Acute kidney injury (AKI) donor and expanded criteria donor (ECD) are important sources of deceased donors. However, there are several challenging issues about the outcomes of using kidney form AKI donors or ECD. This can lead to the discard of using deceased donors with high terminal serum creatinine (Cr). The “old to old” concept has been proposed to be the model of allocating kidneys from ECD. However, there is no consensus guideline regarding the use of kidneys from AKI donors. The authors therefore evaluate the outcomes of kidney transplant from deceased donors with several stages of AKI and compare with that of standard criteria donors (SCDs) and ECDs.
Results from some studies showed worse allograft function when transplantation from AKI donor. These suggest that not all kidneys from AKI donor were suitable for transplantation. Researches providing such information are necessary and useful for decision whether which kidney should be used or discarded.
Many kidneys from AKI donors were discarded because of concerning about poor allograft outcomes. This study showed that kidney transplantation from deceased donors with variable stage of acute kidney injuries was associated with equivalent allograft function and survival when compare with SCD.
Kidneys from AKI donors are important sources of organ for transplantation that can mitigate the problem of organ shortage.
KT: Kidney transplant; SCD: Standard criteria donor; AKI: Acute kidney injury; ECD: Expanded criteria donor; DM: Diabetes mellitus; HT: Hypertension; CAD: Cardiovascular disease; ESRD: End stage renal disease; PRA: Panel reactive antibody; HLA: Human leukocyte antigen; Cr: Creatinine; eGFR: Estimated glomerular filtration rate; ATG: Antithymocyte globulin; DGF: Delayed graft function; ACR: Acute cellular rejection; ABMR: Antibody mediated rejection; CMV: Cytomegalo virus.
The article is well written and relevant.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Thailand
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Hammes M, Sinha R, Watanabe T, Zhang ZH S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Schnitzler MA, Stewart DE, Cherikh WS, Wainright JL, Snyder JJ. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14 Suppl 1:11-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 278] [Article Influence: 25.3] [Reference Citation Analysis (0)] |
| 2. | Abouna GM. Organ shortage crisis: problems and possible solutions. Transplant Proc. 2008;40:34-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 243] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 3. | Heilman RL, Smith ML, Kurian SM, Huskey J, Batra RK, Chakkera HA, Katariya NN, Khamash H, Moss A, Salomon DR. Transplanting Kidneys from Deceased Donors With Severe Acute Kidney Injury. Am J Transplant. 2015;15:2143-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 116] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Yu CC, Ho HC, Yu TM, Ou YC, Shu KH, Cheng CL, Su CK, Chen WM, Wang SS, Chen CS. Kidneys from standard-criteria donors with different severities of terminal acute kidney injury. Transplant Proc. 2014;46:3335-3338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 5. | Kolonko A, Chudek J, Pawlik A, Wilk J, Jałowiecki P, Więcek A. Acute kidney injury before organ procurement is associated with worse long-term kidney graft outcome. Transplant Proc. 2011;43:2871-2874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Hall IE, Schröppel B, Doshi MD, Ficek J, Weng FL, Hasz RD, Thiessen-Philbrook H, Reese PP, Parikh CR. Associations of deceased donor kidney injury with kidney discard and function after transplantation. Am J Transplant. 2015;15:1623-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 7. | Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4846] [Cited by in RCA: 4989] [Article Influence: 277.2] [Reference Citation Analysis (0)] |
| 8. | Bidani A, Churchill P. Hemodynamically mediated acute renal failure. N Engl J Med. 1986;314:1515-1516. [PubMed] |
| 9. | Gandolfini I, Buzio C, Zanelli P, Palmisano A, Cremaschi E, Vaglio A, Piotti G, Melfa L, La Manna G, Feliciangeli G. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes. Am J Transplant. 2014;14:2515-2525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Rao PS, Schaubel DE, Guidinger MK, Andreoni KA, Wolfe RA, Merion RM, Port FK, Sung RS. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 686] [Cited by in RCA: 786] [Article Influence: 49.1] [Reference Citation Analysis (1)] |
| 11. | Kayler LK, Garzon P, Magliocca J, Fujita S, Kim RD, Hemming AW, Howard R, Schold JD. Outcomes and utilization of kidneys from deceased donors with acute kidney injury. Am J Transplant. 2009;9:367-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 6.6] [Reference Citation Analysis (0)] |