INTRODUCTION
The intestine is often deemed one of the most difficult organs to be transplanted because of its unique structure and enhanced immune response[1-3]. Over the past several decades, intestinal transplantation (ITx) has achieved remarkable advancement not only in volume of transplants but also in outcomes, owing to progress in various aspects of organ preservation, surgical technique, immunosuppression, and postoperative management[4-7]. Despite improvements in short-term outcome, long-term survival of both patient and graft after ITx has been well behind other solid-organ transplants, with 10-year survival rates under 50%[5,8]. Allograft dysfunction and/or loss due to acute and chronic rejection continue to be major barriers to the success of intestinal allografts[6]. Therefore, it is essential to further delineate mechanisms for graft failure and to develop treatment strategies that will provide long-term intestinal graft function.
Traditionally, intestinal allograft rejection has mainly been regarded as a T-cell-mediated process, whereas the humoral immunity has received less attention in the evaluation of intestinal rejection. A potential role for antibodies in graft rejection has long been suspected because antibodies to human leukocyte antigens (HLA) are often detected in patients with rejection[9-11]. To date, HLA antibodies are well recognized as causes for hyperacute rejection, acute antibody-mediated rejection (ABMR) and chronic ABMR following kidney or heart transplantation[12-14]. Isolated reports suggest that HLA antibodies also affect lung, liver, or pancreas transplants[15-17]. Much of the evidence indicates that an early diagnosis and aggressive treatment of acute ABMR are critical for improving graft and patient outcomes in kidney or heart transplantation[18,19]. In recent years, several groups demonstrate that, as with other solid-organ transplantation, HLA antibodies appear to be a significant risk factor for the development of acute and chronic rejection after ITx and worsen the overall prognosis for both patient and graft[20-22]. ABMR has increasingly emerged as a potential form of graft dysfunction after ITx. The strategies to decrease or eliminate preformed HLA antibodies, early recognition and appropriate management of newly-formed (de novo) antibodies may further improve outcomes in intestinal allograft recipients.
This review summarizes what is currently known regarding antibody-mediated injury to the intestine and potential solutions to this problem and to emphasize the areas that require further study.
DONOR-SPECIFIC ANTIBODIES AND PRETRANSPLANT SENSITIZATION
Alloantibodies directed against donor HLA, called donor-specific antibodies (DSAs), may be present at the time of transplantation (preformed DSA) or develop de novo following organ grafting. These donor HLA antigens are commonly expressed on endothelial cells, epithelial cells, or other organ specific targets. Over the past several decades, analyzing transplant recipients for DSAs has become an important part of immune monitoring before and after transplantation[23]. The earliest method developed in the 1960s was complement-dependent cytotoxicity (CDC) cross-matching of the recipient’s serum with the donor’s lymphocytes in the presence of complement. This simple test substantially reduces the occurrence of hyperacute rejection, but its sensitivity and specificity (due to non-HLA antibodies) are very low. Flow cytometry cross-matching developed in the 1970s is based on the detection of serum antibodies binding to donor lymphocytes, and it is more sensitive than CDC cross-matching. Current solid-phase immunoassays such as Luminex single-antigen beads provide important advantages in sensitivity and specificity over cell-based assays and are widely used in most transplant centers around the world[24].
Compared with other solid-organ transplants, sensitization is relatively higher in intestinal allograft recipients, most likely due to previous multiple operations, blood transfusions, recurrent line infections, or pregnancies. High panel reactive antibody (PRA) levels are observed in 18%-30% of intestinal transplant candidates on the waiting list, compared to the sensitization rate of 10%-15% in kidney and heart transplant candidates[22,25,26]. Indeed, in our experience the incidence of sensitization was as high as 30%, implying that intestine recipients are an immunologically high-risk population[21].
HYPERACUTE REJECTION
As with other solid-organ transplants, an intestinal allograft placed into a highly sensitized recipient may be subject to very rapid loss because of hyperacute rejection. This severe form of acute rejection was originally described for clinical kidney allografts transplanted into recipients with circulating antibody against the donor[27]. The kidney graft rapidly develops a beefy red or blue appearance and immediately fails[28]. The pathogenesis involves the binding of preformed DSA to HLA on endothelial cells and the subsequent activation of the classical complement cascade leading to the formation of the membrane attack complex and endothelial damage. Because of its strong clinical relevance, cross-matching of the recipient’s serum and the donor’s lymphocytes prior to transplantation became a standard protocol of kidney transplant programs throughout the world.
The kidney and heart are most susceptible to hyperacute rejection, and the liver is relatively resistant[29,30]. To date, hyperacute rejection has not been sufficiently studied in ITx[31]. Hyperacute rejection, although rare, can occur in intestinal allograft recipients who are highly sensitized with the presence of DSAs. This aggressive form of rejection occurs almost exclusively in the pre-sensitized patient with a very high titer of preformed HLA antibodies and is the result of a severe antibody-mediated response to the vasculature endothelium, characterized histologically by vascular injury, thrombosis, and ischemia. In a case report of hyperacute rejection, Ruiz et al[32] described an isolated intestinal allograft recipient with the presence of a positive cross-match and multiple preformed DSAs. The intestinal allograft became dusky immediately following graft reperfusion and the recipient showed hypoxia, hypotension, and acidosis. Subsequent mucosal biopsy specimens exhibited severe vascular congestion with thrombi, hemorrhage, and leukocyte infiltration. Immunofluorescence revealed the deposits of IgG, IgM, C4d, and C3 on the endothelium, suggesting that antibodies can directly injury the intestinal allograft. In this isolated case, the intestinal graft was successfully saved after a combination of intensified tacrolimus, alemtuzumab, rituximab, and plasmapheresis.
ACUTE ABMR
In the earlier series, Bond et al[9] reported outcomes of 23 cross-matching positive grafts in 124 recipients (18%) and illustrated that a positive cross-match was associated with increased frequency of acute rejection after ITx, especially with an isolated intestine. They showed 43.5% (10 out of 23 positive cross-matching) allografts failed at a follow-up of two years. The simultaneous liver allograft as part of a composite visceral transplant appeared to improve the negative effect of the preformed antibodies and positive cross-matching. Later, Ruiz et al[33] in Miami and Wu et al[10] in Pittsburgh respectively described the vascular changes of intestinal allograft recipients in the setting of a positive cross-match. In the recipients with a higher PRA and a positive cross-match, the pathology showed significant vascular congestion and submucosal hemorrhage with deposition of C4d, IgG, and IgM. They found a lower graft survival in the recipients with the early significant vascular lesions[33]. Based on these early results and lessons learned from the other solid-organ transplantation, a positive CDC cross-match has been considered relatively prohibitive for an isolated intestine transplant in most intestinal transplant programs.
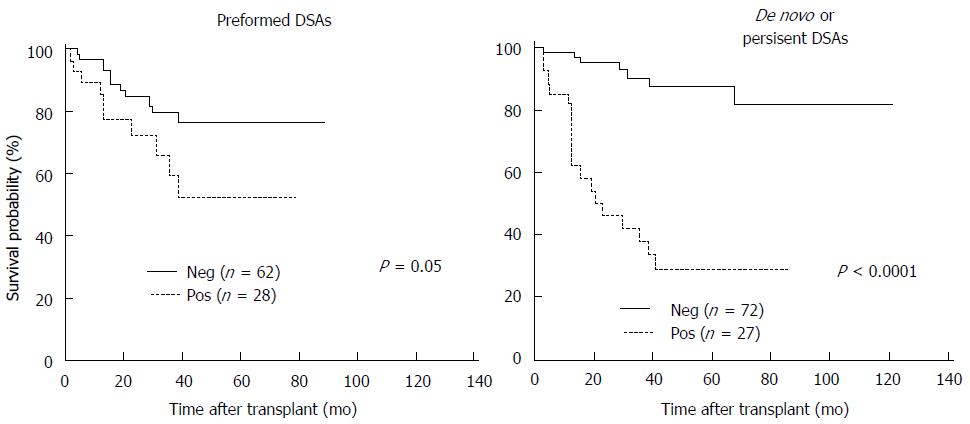
A decade later, Wu et al[34] evaluated an adverse impact of HLA antibodies on intestinal allograft outcome. This study initially retrospectively analyzed a total of 117 recipients who received a primary liver-exclusive intestine allograft during the period between 2000 and 2009. The results further confirmed that a positive cross-match with preformed DSA significantly increased rate and severity of acute rejection after transplant and the formation of de novo DSA after ITx was associated with the worst clinical outcome (Figure 1). Tsai et al[20] prospectively examined the impact of pre- and post-transplant DSA on intestinal allograft rejection. Thirteen recipients were subsequently followed up for DSA levels by a sensitive Luminex assay pre- and posttransplant. They found that the presence of DSA was closely related to an increasing number of rejection episodes and severe acute rejection grading. A combination of rituximab, plasmapheresis, IVIg, or bortezomib therapies to eliminate DSA was associated with clinical improvement of acute rejection. The authors suggest that frequent intestinal graft biopsies combined with serial measurement of DSAs are valuable for evaluation of cellular and humoral immunity of acute rejection.
Figure 1 The Kaplan-Meier graft survival for the presence of performed donor-specific antibodies before transplant and newly formed (de novo) donor-specific antibodies after transplant.
Patients with preformed donor-specific antibodies (DSA) had significantly lower graft survival than those without preformed DSA. The graft survival was markedly worse in patients with de novo DSA or persistent DSA.
Our group further analyzed 194 primary intestinal/multivisceral allograft recipients in which one-third had a positive CDC cross-match prior to surgery[21]. In 156 recipients, 49 (31%) had preformed DSA before ITx; 19 (39%) had persistent DSA after ITx; and 19 (18%) developed de novo DSA. The authors again showed preformed DSA significantly increased frequency and severity of acute rejection. Overall cumulative risk of acute rejection was significantly higher in a positive cross-match compared to a negative cross-match. The recipients with higher levels of DSAs, as measured by a single antigen Luminex assay, developed an increased incidence of steroid-resistant rejection which responded poorly to OKT3 treatment, and 1-year graft survival in DSA-positive recipients was significantly inferior to that of DSA-negative recipients. Twenty-one (11%) of recipients were diagnosed with acute ABMR, and most ABMR cases occurred in the first three months after transplant. The incidence of acute ABMR was substantially elevated in recipients with performed, persistent DSA and de novo DSA and 11 (52%) of acute ABMR cases led to allograft failure.
It is important to note that intestinal transplant recipients can mount humoral immune response after transplantation even in the setting of a negative cross-math. Gerlach et al[35] reported thirteen patients undergoing intestinal/multivisceral transplants with non-donor-specific HLA antibodies before ITx and found that the development of de novo DSAs after ITx was associated with severe graft dysfunction. They observed that only three recipients had non-donor-specific HLA antibodies before transplantation; 15 (50%) cases developed de novo DSA during the first 6 mo; and only two recipients developed DSA 10 years after transplantation. In their small series, all patients with de novo DSAs showed simultaneous acute cellular rejection at the time of DSA occurrence. Luckily, nine of the 10 patients diagnosed with acute ABMR were successfully treated with a combination of plasmapheresis and intravenous immunoglobulin (IVIg). In case of persistence of DSA and/or treatment-refractory rejection, additional rituximab and/or bortezomib were beneficial.
DIAGNOSIS OF ACUTE ABMR
Up to date, diagnostic criteria for acute ABMR after ITx have not been established and there is no consensus on the characteristic clinicopathologic features. However, several reports addressing a unique form of allograft rejection that is consistent with the definition of acute ABMR which was defined by The National Conference to Assess Antibody-Mediated Rejection in Solid Organ Transplantation in kidney and heart transplantation[36,37].
Wu et al[10] initially described a characteristic clinical and pathologic syndrome during the early postoperative course in intestine recipients with a positive cross-math. They observed that the strongly positive cross-match recipients exhibited serious mucosal damage instantly after graft reperfusion, including mucosal congestion, bluish discoloration, and focal hemorrhage in the allograft. Pathology showed severe capillary congestion, neutrophilic infiltration, hemorrhage, epithelial injury, and thrombi within the lamina propria microvasculature, but without evidence of histologic neutrophilic or necrotizing arteritis, and the immunofluorescent findings were unremarkable. In contrast, the recipients with a weakly positive crossmatch, as well as the cross-match negative recipients, had none of these characteristic clinical, endoscopic, or microscopic findings.
C4d is a footprint of antibody-triggered classical complement activation and its deposition has become pivotal to the diagnosis of acute ABMR in kidney and heart transplants[37,38]. However, there is no generally acceptable consensus on the use of C4d staining in diagnosing acute ABMR after ITx. Earlier studies showed that C4d deposition had no difference in biopsies between acute rejection and no rejection and claimed that C4d had no clinical relevance as diagnosing humoral rejection in intestinal allografts[39,40]. Unfortunately these earlier studies neither correlated C4d with the levels of HLA antibodies nor examined these antibodies by a relatively sensitive methodology. Ruiz et al[33] demonstrated that post-transplant vascular lesions in intestinal allografts at earlier time periods were associated with higher levels of pre-transplant PRA or a positive CDC cross-match. In intestinal recipients with the vascular changes, C4d staining can be seen in the small vasculatures. Of the patients with no significant vascular alterations, C4d deposition was negative or trace. Our team evaluated the utility of C4d in intestinal biopsies at the time of suspected acute ABMR and showed a diffuse C4d staining was mainly observed in recipients with a positive DSA, while focal or minimal C4d staining was observed in intestinal biopsies with no evidence of rejection[21]. Similar to other solid-organ transplants, our results emphasize clinical significance of a diffuse C4d deposition in intestinal allografts, suggesting that C4d together with higher titers of DSA, is a very useful marker to detect acute ABMR after ITx.
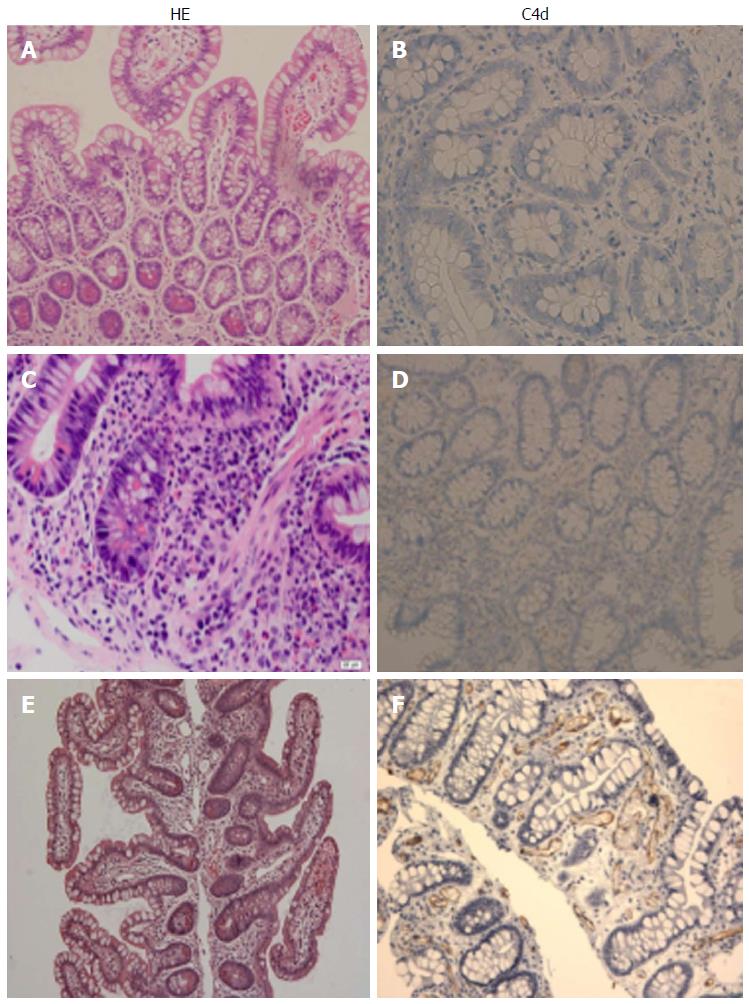
Based on the established diagnostic criteria for kidney transplant, including the presence of circulating DSAs, acute tissue injury, C4d deposition and clinical allograft dysfunction, we performed a retrospective single-center analysis to investigate the incidence, risk factors and clinical outcomes of acute ABMR after ITx (unpublished data). Acute ABMR was diagnosed in 18 (10.3%) out of 175 primary intestinal/multivisceral transplants with a median occurrence of 10 d (range, 4-162) after ITx. All eighteen patients were sensitized to HLA class I and/or II antigens with the presence of performed DSAs. A cross-match was positive in 14 (77.8%) recipients. Twelve of 18 patients (66.7%) developed de novo DSA after ITx. Pathological features of acute ABMR include C4d deposition, prominent hemorrhage and congestion with scattered fibrin thrombin in the lamina propria (Figure 2). Despite initial improvement after treatment, eleven (61.1%) lost graft due to rejection. Of those, nine (50%) received enterectomies and four (22.2%) underwent retransplantation after acute ABMR. At a median follow-up of 32.3 mo (range, 13.3-76.4 mo), eight (44.4%) recipients died. We conclude that acute ABMR can be a fulminant form of intestinal rejection, especially in a liver-free transplant and survivors are at an increased risk of developing refractory rejection. Our studies suggest that no morphologic findings are specific for acute ABMR in intestinal allografts, and the diagnosis is best made using serologic, clinical, and histologic data together.
Figure 2 Histopatholgy of the intestinal allografts.
A and B: No rejection: Normal mucosal architecture of small bowel biopsy after transplant. No staining for C4d is seen in the capillaries of the lamina propria; C and D: Acute cellular rejection (ACR): There is mononuclear infiltration, crypt epithelial injury, and apoptotic bodies in the lamina propria. A weak staining for C4d is sometimes present in a patient with ACR; E and F: Acute antibody-mediated rejection: There is prominent hemorrhage and congestion with scattered fibrin thrombin in the lamina propria. Widespread and bright staining for C4d is present in the capillaries of the lamina propria.
PREVENTION AND TREATMENT OF ACUTE ABMR
Due to rarity of ITx, no standard protocols are currently available for prevention and treatment of acute ABMR. Therapeutic strategies are predominantly based on case reports, small series, and renal transplant data.
The avoidance of a known HLA DSA target at the time of transplant remains a primary preventive strategy. With the development of solid-phase assays, the ability to detect and minimize DSA prior to transplantation is possible. Luminex single-antigen assay of DSA has led to the application of the virtual cross-match, in which known recipient HLA antibodies are compared to donor HLA prior to transplantation. At the time of a donor organ offer, the titer, MFI, and total number of DSA can be evaluated for the virtual cross-match. Hawksworth et al[25] evaluated the virtual cross-matching for organ allocation and immunological risk reduction in sensitized isolated intestinal transplants. In their study, higher DSA titers (more than 1:16) were considered a contraindication for an isolated intestinal transplant. They observed that clinical outcomes were comparable between sensitized (PRA > 20%) and control (PRA < 20%) recipients in terms of 1-year freedom from rejection, 1-year patient survival, and 1-year graft survival. The authors conclude that a virtual cross-matching strategy to optimize organ allocation is valuable in sensitized patients to successfully undergo isolated ITx with good short-term outcomes. However, this strategy may affect the sensitized potential recipient’s access to ITx.
The use of preoperative desensitization strategies to decrease DSA titers with plasmapheresis, ATG, IVIg, and mycophenolate has been described with good tolerability and reduction of early rejection episodes and equivalent posttransplant outcomes to unsensitized patients[41]. The Indiana group reported their experience with combined rabbit ATG and rituximab as induction therapy, a positive cross-match was not related to an increased risk of acute rejection and graft failure. They suggested that combined use of anti-IL2 receptor antibody may be beneficial in the liver-free intestinal transplant. The authors conclude that with anti-thymocyte globulin plus rituximab induction, a positive cross-match had reasonable outcomes after intestinal/multivisceral transplantation. Garcia-Roca et al[42] recently presented two living donor intestinal candidates with a positive cross-match that was successfully converted to a negative cross-match using desensitization protocol prior to transplantation. The first case had 67% for PRA HLA class I and 100% for class II and had DSA with a positive flow cytometry cross-match with a potential donor. The second case was sensitized with 80% for PRA class I and 26% for class II; no DSAs were identified. In this case, the standard cytotoxic cross-match was negative, but the flow cytometry cross-match was positive for B cell. Both cases were successfully desensitized with steroids, thymoglobulin, multiple plasmapheresis, followed by IVIg, achieving a complete negative cross-match at the time of transplant. ITx was successfully performed in both cases after desensitization protocol. Humoral rejection did not occur during the initial 6 and 9 mo follow-up.
It has been well-known that combined liver and ITx can be performed against a positive cross-math, suggesting that the liver graft protects the subsequent intestinal transplant from the harmful antibodies. Testa et al[43] described a highly sensitized case in which a cross-match remained positive after multiple plasmapheresis. With a liver transplant, the cross-match quickly became negative allowing subsequent bowel grafting in one week. We described our single-center experience in retransplanted recipients and compared cases who underwent liver-free retransplants with those who underwent liver-inclusive retransplants[44]. The graft survival rates at 1, 3 and 5 years in liver-free retransplants were markedly worse than those in liver-inclusive retransplants. The majority of liver-free retransplants underwent enterectomy due to either severe acute cellular rejection or chronic rejection. Six recipients died due to rejection-related complications. Compared to liver-free retransplants, the frequency and grading of acute rejection were markedly decreased in liver-inclusive retransplants. We did not see cases with chronic rejection during the study period and two patients died due to graft-vs host disease and infection in this group, respectively. We conclude that a liver-inclusive retransplant offers a better long-term clinical outcome, suggesting that the liver-intestine combined transplantation should be considered when retransplantation is unavoidable.
The treatment of comfirmed acute ABMR has routinely included a combination of corticosteroids, IVIg, plasmapheresis, ATG, and rituximab. Bortezomib, a proteasome inhibitor, has been reported to reduce or eliminate DSA after transplantation[45]. Gerlach et al[46] described ten intestinal recipients with a diagnosis of acute ABMR. After combined therapies including bortezomib, 9 cases were successfully treated with a good graft function. DSAs were completely cleared in 8 patients, and detectable in only one. Eculizumab, a humanized monoclonal antibody against complement C5, has successfully been used to treat acute ABMR in renal transplant. Recently, Fan et al[47] described a case in which eculizumab was administered to reverse acute ABMR in a desensitization-resistant intestinal retransplant patient. His primary intestinal allograft failed due to ABMR eight years after ITx. Two donors were used in his initial allograft (one for the intestinal graft and another for the abdominal wall graft). He underwent a second intestinal graft which had to be resected a month later due to uncontrolled severe acute ABMR. The patient became highly immunized due to three HLA unmatched different organs, as reflected by 100% PRA and serum high titers of DSAs. He received the third liver-inclusive multivisceral transplant and developed severe acute ABMR on day 3 post-transplantation. Acute ABMR was successfully salvaged with antibody-targeted desensitization regimens. Although PRA levels remained higher, the titers of DSAs significantly decreased below the cut-off level of 3000 MFI (mean fluorescent intensity) within a month after the third transplant. The favorable outcomes in this extremely difficult case may be attributed to the use of Eculizumab and the immunoprotective effect of the liver graft.
CHRONIC REJECTION
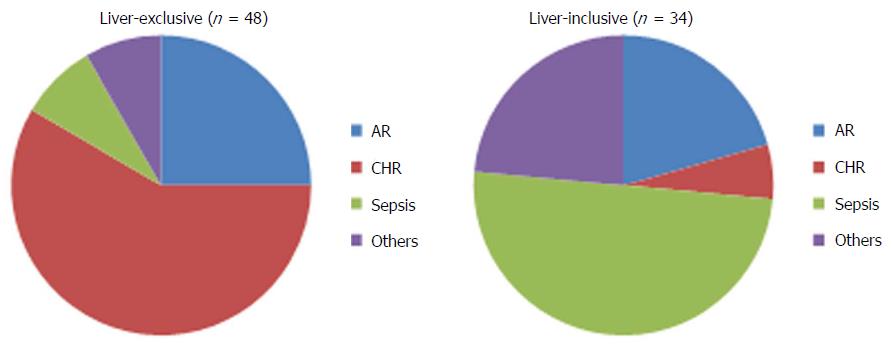
Chronic rejection or enteropathy is a significant barrier to long-term graft and patient survival of intestinal allograft. The incidence of chronic rejection ranges between 15%-20% after ITx[6,48]. Pathologically, it is characterized by concentric vasculopathy, luminal occlusion, leukocyte infiltration, and a marked fibrotic change[49]. These histologic findings are the end results of a complex, multi-stage process of repeated immune- and non-immune-mediated cellular injury and inflammation. Repetitive insults exhaust the recipient’s natural repair mechanisms leading to fibrotic replacement and intestinal failure[50]. An isolated small bowel transplant appears to render the graft more susceptible to chronic rejection compared to a liver-inclusive transplant[6,44,51] (Figure 3).
Figure 3 The causes of graft loss in the liver-exclusive and liver-inclusive intestinal transplants.
In liver-exclusive transplants, chronic rejection was the leading cause of graft loss. In liver-inclusive transplants, however, infection was the major cause of graft loss. AR: Acute rejection; CHR: Chronic rejection.
The causes of chronic rejection resulting from graft tissue injury are multifactorial and both immune- and non-immune-mediated factors can contribute to graft injury. Emerging evidence suggests that immune-mediated injuries to the graft are the fundamental cause of chronic rejection[3,52]. Several studies have identified severe acute rejection, recurrent episodes of rejection, the cumulative burden of acute rejection, and late-onset acute rejection as risk factors for chronic rejection[6,21]. Recently, the role of humoral alloimmunity has also appeared to be closely related to chronic rejection[21,53]. The major target of humoral immunity appears to be the graft endothelium, which can be activated and injured by HLA antibodies. However, the mechanism by which humoral alloimmunity leading to chronic rejection is not well understood, and whether the presence of antibody is an initiating event or merely a response to tissue damage remains to be defined.
A large observational study investigating the potential effect of HLA antibodies on the intestinal chronic rejection came from our group[21]. We retrospectively analyzed 194 consecutive intestine transplants which showed the incidence of chronic rejection at 36 cases (19%) with an average of 21 ± 10 mo (range 2-88 mo) follow-up. Cumulative risk of chronic rejection was slightly higher in recipients with a positive cross-match vs a negative cross-match. Cumulative probability of chronic rejection was markedly elevated in recipients in the setting of the presence of preformed DSAs before ITx together with persistent DSAs after ITx. The formation of de novo DSAs was closely related severe chronic rejection and subsequent graft loss. The graft survival was markedly decreased in the DSA-positive patients and the graft loss due to chronic rejection was irreversible in one-third patients. The liver-inclusive transplant was associated with better clearance of preformed DSAs, lower rates of de novo DSA formation, and therefore reduced rates of chronic rejection. The results illustrate a strong relationship between DSAs and an increased risk of chronic rejection and allograft failure.
CONCLUSION
Increasing and compelling evidence indicates that antibody-mediated graft injury is closely related to poor outcomes in ITx. The presence of preformed DSAs should alert the clinician of the increased risk of ABMR. The avoidance of a known DSA target at the time of transplant remains a major preventive strategy and may improve unsatisfactory outcomes in intestine recipients. The development of de novo DSA after ITx usually portends a poor prognosis with an increased risk of uncontrolled acute rejection, chronic rejection, and allograft loss. The better understanding of mechanisms of antibody-mediated graft injury, establishment of the diagnostic criteria, and optimal management of DSAs are needed to improve clinical outcomes of ITx.
ACKNOWLEDGMENTS
The author would like to thank the surgical team and the nursing staff at Xijing Hospital of Digestive Diseases, the Fourth Military Medical University, for their excellent patient care. The authors thank Mr. Yinglun Wu for help with English grammar.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: China
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Cid J, Gurkan A S- Editor: Ji FF L- Editor: A E- Editor: Wu HL