Published online Sep 24, 2016. doi: 10.5500/wjt.v6.i3.542
Peer-review started: April 29, 2016
First decision: June 17, 2016
Revised: July 6, 2016
Accepted: July 29, 2016
Article in press: August 1, 2016
Published online: September 24, 2016
Processing time: 147 Days and 17.8 Hours
Hemodynamic monitoring has long formed the cornerstone of heart failure (HF) and pulmonary hypertension diagnosis and management. We review the long history of invasive hemodynamic monitors initially using pulmonary artery (PA) pressure catheters in the hospital setting, to evaluating the utility of a number of implantable devices that can allow for ambulatory determination of intracardiac pressures. Although the use of indwelling PA catheters has fallen out of favor in a number of settings, implantable devices have afforded clinicians an opportunity for objective determination of a patient’s volume status and pulmonary pressures. Some devices, such as the CardioMEMS and thoracic impedance monitors present as part of implantable cardiac defibrillators, are supported by a body of evidence which show the potential to reduce HF related morbidity and have received regulatory approval, whereas other devices have failed to show benefit and, in some cases, harm. Clearly these devices can convey a considerable amount of information and clinicians should start to familiarize themselves with their use and expect further development and refinement in the future.
Core tip: Hemodynamic monitoring forms the cornerstone of heart failure (HF) and pulmonary hypertension diagnosis and management. We review invasive hemodynamic monitors including a number of implantable devices that can allow for ambulatory determination of a variety of intracardiac pressures. These implantable devices have afforded clinicians an opportunity for objective determination of a patient’s volume status and pulmonary pressures. Devices such as the CardioMEMS and thoracic impedance monitors are supported by a body of evidence that show the potential to reduce HF related morbidity. Clinicians should start to familiarize themselves with their use and expect further development and refinement in the future.
- Citation: Davey R, Raina A. Hemodynamic monitoring in heart failure and pulmonary hypertension: From analog tracings to the digital age. World J Transplant 2016; 6(3): 542-547
- URL: https://www.wjgnet.com/2220-3230/full/v6/i3/542.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i3.542
Heart failure (HF) is an increasingly prevalent disease affecting, by some estimates, over 23 million people worldwide[1]. It is a clinical syndrome characterized by the inability of the heart to adequately provide effective net forward blood flow, either due to left ventricular systolic dysfunction (heart failure with reduced ejection fraction or HFrEF), as a result of left ventricular diastolic dysfunction and/or valvular disease (heart failure with preserved ejection fraction or HFpEF), or due to right sided HF related to pulmonary arterial hypertension (PAH) or primary right ventricular (RV) dysfunction. This may result in both acute and chronic volume overloaded states, poor end organ perfusion and significant morbidity and mortality. In the United States, HF is the most common cause for hospitalizations in those over age 65 with over 1 million admissions per year[2]. Despite improvements in contemporary medical therapy for HFrEF, long term morbidity and mortality remain unacceptable and 30-d rehospitalization rates remain roughly 23%[3]. For HFpEF patients, there is still no disease modifying therapy which has been shown to improve survival in randomized clinical trials[4].
PAH is a far rarer condition affecting perhaps 52 out of one million in the population at any given time[5]. However, it is a progressive and insidious disease characterized by remodeling of the pulmonary arterial tree, associated with endothelial dysfunction, vascular smooth muscle hypertrophy, and vasoconstriction[6]. The gradual rise in RV afterload leads to compensatory RV hypertrophy and dilatation, but if left untreated, culminates in RV dysfunction, fall in cardiac output and clinical symptoms[7].
The right heart catheter (RHC) has long been considered the gold standard for the diagnosis of PAH and also for monitoring disease progression. It has also been shown to be effective in determining the etiology of patients in shock, and hemodynamic parameters obtained from RHC have prognostic utility in HF patients. Moreover, in selected patients with advanced HF, there may be a role for hemodynamically tailored HF therapy with use of an indwelling Swan-Ganz catheter in the intensive care unit, though this approach has not been associated with superior survival[8,9].
Standard RHC does, however, have significant limitations and over the past two decades, a number of newer implantable hemodynamic monitors (IHMs) have been developed for use in HF patients. The increasing adoption of IHM in HF and PAH patients may afford new opportunities for improving clinical outcomes in these disease states and thus forms the subject of this review.
The RHC was first developed by Forssmann et al[10] in 1929 after experimenting on himself to find a way to both measure intra-cardiac pressures and deliver therapies[10]. After further pulmonary artery (PA) catheter development and refinement by Drs. Swan and Ganz in 1970, it gained widespread use in the management of advanced HF and shock despite relatively limited evidence regarding its efficacy in reducing deleterious clinical outcomes.
Though it is an invasive procedure, RHC has since become recognized as safe with a relatively low rate of complications, especially when performed in referral centers[11]. However RHC has a variety of limitations, many of which are inherent to the RHC procedure and its associated technology.
In general, at most centers RHC is performed in the supine position at rest, and the catheter does not lend itself well to either ambulatory or frequent measurements outside of an inpatient setting. Indeed, even in-hospital readings must be taken in a meticulous fashion to avoid the issues inherent to the procedure such as respiratory variation in pressures and inappropriate pressure transducer placement and zeroing.
In an effort to limit variation and standardize measurements from a PA catheter, many centers take readings at end-expiration and with the patient supine which, while allowing for reproducibility, is likely not an entirely accurate physiologic assessment of the patient’s hemodynamics during their day to day activities[12].
The Swan-Ganz catheter, in part due to its perceived safety, was widely adopted in a number of clinical scenarios and as a result, a number of significant associated adverse events were reported[8]. Therefore, the ESCAPE trial was undertaken to assess the value of PA catheterization in HF patients. Published in 2005, ESCAPE showed that the routine use of PA catheterization for patients admitted with HF was not associated with a significantly decreased length of stay, due in part to an increased infection risk; however, its applicability to disease states such as overt cardiogenic shock has not been shown[9] and such patients were, in large part, excluded from ESCAPE.
Although the indwelling PA catheter has fallen out of favor with clinicians for uncomplicated HF, the overall goal of accurate and reproducible hemodynamic monitoring to assess volume status, filling pressures and cardiac output remains very valuable in preventing adverse events in this group, including hospital readmissions. As a part of the Affordable Care Act in the United States, the Centers for Medicare and Medicaid Services has identified HF as a disease state warranting readmission measures and the assessment of penalties are to begin in 2016 for readmission rates deemed to be in excess of the national average[13].
With a view to managing volume in the ambulatory setting, a number of different IHMs have been developed. Perhaps the most frequently used at present are those devices that measure thoracic impedance via the RV lead on an implanted cardiac defibrillation or cardiac resynchronization device. Specifically, these devices attempt to gauge the degree of pulmonary congestion by measuring the resistance to flow of a current passed across the lung. Since tissue will conduct current more readily with increasing amounts of fluid, impedance will drop as a patient’s volume status expands. In clinical practice, this is usually reported by the device using an algorithm that indexes these values and can signal the clinician of an abnormal trend upon device interrogation. The FAST study showed that decrements in thoracic impedance were more closely correlated with negative HF endpoints than standard home weight monitoring[14] but these readings have proved difficult for clinicians to incorporate in clinical practice[15].
Other IHM devices have targeted intravascular pressures directly with a view to increasing sensitivity and applicability to clinical practice. The first of these devices used a diaphragm-tipped pressure catheter that would be passively placed in a vascular structure. In the case of the Medtronic Chronicle device, a generator was implanted subcutaneously and attached to a lead with its electrode tip placed subcutaneously in the RV by passive fixation. This allowed for remote measurement of RV systolic and diastolic pressure, imputed PA diastolic pressure, heart rate, activity, RV dp/dt, and core body temperature[16]. The HeartPOD was a device from St Jude Medical deployed via a femoral venous approach and then crossing the intra-atrial septum to sit directly in the left atrium. An antenna coil could then be subcutaneously implanted in the femoral region or reflected back into a superior venous position[17].
COMPASS-HF[18] was a single-blinded prospective study designed to use the Chronicle device in patients with HFpEF and HFrEF and tailor medical therapy based either on standard assessments alone (control arm) or with the use of the device data. They randomized a total of 274 patients and although the primary endpoint of HF-related events, including hospitalizations and urgent clinic visits, decreased by 21% it failed to reach statistical significance. The device was not granted FDA approval and so did not reach market.
The initial study HeartPOD study[19] showed promise but the follow-up study LAPTOP-HF was terminated early for safety reasons due to procedural complications related to the required trans-septal puncture.
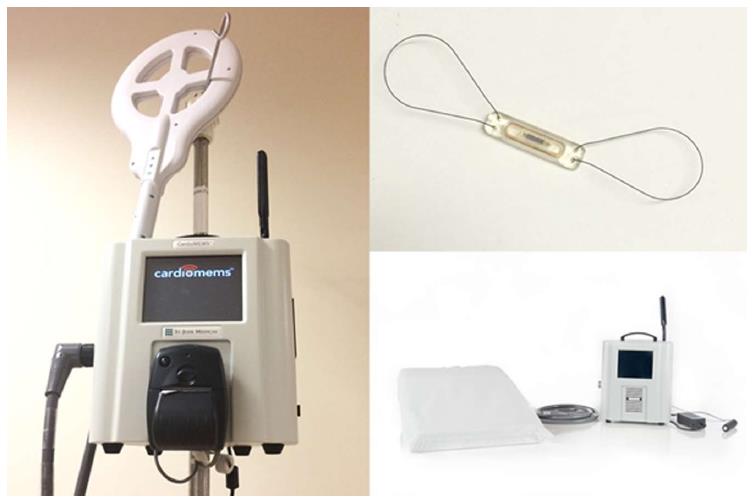
More recently, the CardioMEMS device from St Jude was studied in the CHAMPION trial[20]. This was a multi-center, single-blind, prospective trial which enrolled 550 patients total to both arms and, similarly to previous studies, both HFpEF and HFrEF were included. As with previous studies, medical therapies in the treatment arm were guided by the use of the PA pressures provided by the device. Patients were followed for a mean of 15 mo. The primary endpoint was HF related hospitalizations and this was significantly reduced by 37% with minimal device-related adverse events (1.4%) and 100% device reliability. Follow-up data showing open-access to the PA pressures reported by the device led to a 48% readmission rate reduction in the former control group and, in patients who had repeat RHC, the mean difference in the mean PA pressure between the device and direct invasive measurement was 1 mmHg[21].
Unlike the aforementioned devices, the CardioMEMS device is a percutaneously delivered pressure sensor that is placed in a PA branch and interrogated via a wireless detection system which can then be remotely reviewed by clinicians in close to real-time via upload to a website (Figures 1 and 2). This has the benefit to the clinician of understanding a patient’s ambulatory right-sided pressures and, by extension, volume status in a format similar to RHC. In addition, this device did not have a percutaneous lead or generator that was prone to failure or infection and could last for the life of the patient. These factors and the success of the CHAMPION trial led to the approval of the device by the FDA in order to reduce hospitalizations in HF patients.
There may also be a role for IHMs in the risk stratification of patients requiring advanced HF therapies including left ventricular assist device (LVAD) implantation and transplantation. Data from the CHAMPION trial showed that treatment group progressed faster to LVAD therapy (167 d vs 266 d), had faster declines in PA pressures and ultimately, a quicker bridge to cardiac transplantation (177 d vs 370 d)[22]. Furthermore, the CardioMEMS device may provide a way to monitor exercise responsiveness in patients with LVAD implants[23] and could provide a novel way to measure PA pressure in those with total artificial hearts (TAH) whose PA pressures were not previously measureable due to the inherent limitations of the TAH implant.
Although neither the Medtronic Chronicle nor the CardioMEMS device were expressly designed for the management of PAH, ongoing knowledge of a patient’s PA pressures in this disease might be extremely valuable, especially if an estimate of cardiac output could be derived from the sensor to calculate total pulmonary resistance. With regard to therapeutic interventions in PAH, the device could allow for guided up and down titration of therapy and thus prevent the sequelae of RV failure or high cardiac output states via direct measurement of these parameters. It could also give some insight into those patients with medication compliance issues. There have been several case series that have been conducted to investigate the role of these monitors in guiding PH therapies.
The Chronicle device was studied in 5 patients with PAH who were prescribed iloprost - a prostacyclin analog - via an inhaled, aerosolized delivery. The device clearly demonstrated a drop in RV systolic pressures in the immediate post-inhalation period and importantly showed that the duration of drug effect was much shorter than was expected[24]. The authors postulated that patients who were at rest during the delivery of the drug may even have a more pronounced pressure lowering and indeed, further study with iloprost and IHMs showed that in fact, with exercise, there was a significantly blunted pressure lowering effect[25]. The Chronicle device also identified 13 out of 15 PAH patients who had a greater than 30 m decrease in 6 min walk distance on the basis of improvement in pressure measurements[26].
The CardioMEMS device is currently being studied in PAH as part of an NHLBI funded pilot study in PAH. This is a single center study investigating long-term pressure measurements and titration of therapy based on device interpretations. Early data has shown that instead of titrating to a pre-specified, protocolled dose of parenteral prostacyclin, IHM-guided therapy has allowed for early recognition of optimal dosing. As compared with standard therapy, this has allowed for enhanced cost savings due to lower drug dosing (in one case, approximately United States $ 29000 was saved), minimization of prostacyclin-related side effects, and decreased risk associated with repeat RHC izations[27].
Ambulatory hemodynamic monitoring in HF and PAH is clearly still developing but the use of these devices is being gradually expanded outside the traditional role of fluid management in HFrEF. As we gain more experience with the current generation of devices such as the CardioMEMS IHM in clinical practice, device design will continue to evolve and already a variety of even more sophisticated sensors are under development.
As the CardioMEMs sensor continues to be evaluated in the management of PAH, IHMs hold the promise of a more precise and accurate titration of medical therapies and may also allow for determining which patients are at higher risk of adverse events, thus allowing for earlier and more aggressive interventions.
Clinicians should eagerly await and critically scrutinize data from forthcoming studies looking at expanding roles for these devices.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Albacker T, Puddu PE S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011;8:30-41. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1550] [Cited by in RCA: 1418] [Article Influence: 101.3] [Reference Citation Analysis (0)] |
| 2. | Desai AS, Stevenson LW. Rehospitalization for heart failure: predict or prevent? Circulation. 2012;126:501-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 461] [Article Influence: 35.5] [Reference Citation Analysis (0)] |
| 3. | Ross JS, Chen J, Lin Z, Bueno H, Curtis JP, Keenan PS, Normand SL, Schreiner G, Spertus JA, Vidán MT. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 332] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 4. | Li J, Becher PM, Blankenberg S, Westermann D. Current treatment of heart failure with preserved ejection fraction: should we add life to the remaining years or add years to the remaining life? Cardiol Res Pract. 2013;2013:130724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Peacock AJ, Murphy NF, McMurray JJ, Caballero L, Stewart S. An epidemiological study of pulmonary arterial hypertension. Eur Respir J. 2007;30:104-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 511] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 6. | Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23-42, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 255] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 7. | Fuster V, Steele PM, Edwards WD, Gersh BJ, McGoon MD, Frye RL. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation. 1984;70:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 713] [Cited by in RCA: 607] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 8. | Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Binanay C, Califf RM, Hasselblad V, O’Connor CM, Shah MR, Sopko G, Stevenson LW, Francis GS, Leier CV, Miller LW. Evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness: the ESCAPE trial. JAMA. 2005;294:1625-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1058] [Cited by in RCA: 1062] [Article Influence: 53.1] [Reference Citation Analysis (0)] |
| 10. | Forssmann W. Die Sondierung des Rechten Herzens. Klinische Wochenschrift. 1929;8:2085-2087. [RCA] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 259] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Hoeper MM, Lee SH, Voswinckel R, Palazzini M, Jais X, Marinelli A, Barst RJ, Ghofrani HA, Jing ZC, Opitz C. Complications of right heart catheterization procedures in patients with pulmonary hypertension in experienced centers. J Am Coll Cardiol. 2006;48:2546-2552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 377] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 12. | Kovacs G, Avian A, Pienn M, Naeije R, Olschewski H. Reading pulmonary vascular pressure tracings. How to handle the problems of zero leveling and respiratory swings. Am J Respir Crit Care Med. 2014;190:252-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 122] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 13. | Readmissions Reduction Program (HRRP) - Centers for Medicare and Medicaid Services Baltimore: Department of Health & Human Services, 2016. [accessed 2016 Apr 13]. Available from: https: //http://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. |
| 14. | Abraham WT, Compton S, Haas G, Foreman B, Canby RC, Fishel R, McRae S, Toledo GB, Sarkar S, Hettrick DA. Intrathoracic impedance vs daily weight monitoring for predicting worsening heart failure events: results of the Fluid Accumulation Status Trial (FAST). Congest Heart Fail. 2011;17:51-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 157] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 15. | Tang WH, Tong W. Measuring impedance in congestive heart failure: current options and clinical applications. Am Heart J. 2009;157:402-411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Steinhaus D, Reynolds DW, Gadler F, Kay GN, Hess MF, Bennett T. Implant experience with an implantable hemodynamic monitor for the management of symptomatic heart failure. Pacing Clin Electrophysiol. 2005;28:747-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Pretorius V, Birgersdotter-Green U, Heywood JT, Hafelfinger W, Gutfinger DE, Eigler NL, Love CJ, Abraham WT. An implantable left atrial pressure sensor lead designed for percutaneous extraction using standard techniques. Pacing Clin Electrophysiol. 2013;36:570-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Bourge RC, Abraham WT, Adamson PB, Aaron MF, Aranda JM, Magalski A, Zile MR, Smith AL, Smart FW, O’Shaughnessy MA. Randomized controlled trial of an implantable continuous hemodynamic monitor in patients with advanced heart failure: the COMPASS-HF study. J Am Coll Cardiol. 2008;51:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 402] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 19. | Ritzema J, Troughton R, Melton I, Crozier I, Doughty R, Krum H, Walton A, Adamson P, Kar S, Shah PK. Physician-directed patient self-management of left atrial pressure in advanced chronic heart failure. Circulation. 2010;121:1086-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 236] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 20. | Abraham WT, Adamson PB, Bourge RC, Aaron MF, Costanzo MR, Stevenson LW, Strickland W, Neelagaru S, Raval N, Krueger S. Wireless pulmonary artery haemodynamic monitoring in chronic heart failure: a randomised controlled trial. Lancet. 2011;377:658-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1118] [Cited by in RCA: 1225] [Article Influence: 87.5] [Reference Citation Analysis (0)] |
| 21. | Abraham WT, Stevenson LW, Bourge RC, Lindenfeld JA, Bauman JG, Adamson PB. Sustained efficacy of pulmonary artery pressure to guide adjustment of chronic heart failure therapy: complete follow-up results from the CHAMPION randomised trial. Lancet. 2016;387:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 463] [Article Influence: 51.4] [Reference Citation Analysis (0)] |
| 22. | Feldman D, Naka Y, Cabuay B, Takayama H, Bauman J, Cowart P, Corcoran K, Levy W, Moazami N. 241 A Wireless Hemodynamic Pressure Sensor before and after Ventricular Assist Device Placement: A Sub-Study of the CHAMPION Trial. J Heart Lung Transpl. 2011;30:S86. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Agarwal R, Kamath MY, Correa-Jacque P, Benza RL, editors . 1262 - Use of Cardiomems to Detect Abnormal Exercise Response in a Patient with a Left Ventricular Assist Device. ISHLT: Washington, D.C 2016; . |
| 24. | Fruhwald FM, Kjellström B, Perthold W, Watzinger N, Maier R, Grandjean PA, Klein W. Continuous hemodynamic monitoring in pulmonary hypertensive patients treated with inhaled iloprost. Chest. 2003;124:351-359. [PubMed] |
| 25. | Wonisch M, Fruhwald FM, Maier R, Watzinger N, Hödl R, Kraxner W, Perthold W, Klein WW. Continuous haemodynamic monitoring during exercise in patients with pulmonary hypertension. Int J Cardiol. 2005;101:415-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Kjellström B, Frantz RP, Benza RL, Bennett T, Bourge RC, McGoon MD. Hemodynamic ranges during daily activities and exercise testing in patients with pulmonary arterial hypertension. J Card Fail. 2014;20:485-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Benza RL, Doyle M, Correa-Jaque P, Cham M, White J, Raina A, Biederman R. A Study to Explore the Feasibility and Safety of Using CardioMEMs HF System in PAH Patients. D26 Assessment of the Right Ventricle in Health and Disease: American Thoracic Society 2015; A5529-A. |