Published online Sep 24, 2016. doi: 10.5500/wjt.v6.i3.532
Peer-review started: March 31, 2016
First decision: May 17, 2016
Revised: May 31, 2016
Accepted: July 11, 2016
Article in press: July 13, 2016
Published online: September 24, 2016
Processing time: 176 Days and 20.6 Hours
The successful of transplantation is determined by the shared human leukocyte antigens (HLAs) and ABO blood group antigens between donor and recipient. In recent years, killer cell receptor [i.e., killer cell immunoglobulin-like receptor (KIR)] and major histocompatibility complex (MHC) class I chain-related gene molecule (i.e., MICA) were also reported as important determinants of transplant compatibility. At present, several different genotyping techniques (e.g., sequence specific primer and sequence based typing) can be used to characterize blood group, HLA, MICA and KIR and loci. These molecular techniques have several advantages because they do not depend on the availability of anti-sera, cellular expression and have greater specificity and accuracy compared with the antibody-antigen based typing. Nonetheless, these molecular techniques have limited capability to capture increasing number of markers which have been demonstrated to determine donor and recipient compatibility. It is now possible to genotype multiple markers and to the extent of a complete sequencing of the human genome using next generation sequencer (NGS). This high throughput genotyping platform has been tested for HLA, and it is expected that NGS will be used to simultaneously genotype a large number of clinically relevant transplantation genes in near future. This is not far from reality due to the bioinformatics support given by the immunogenetics community and the rigorous improvement in NGS methodology. In addition, new developments in immune tolerance based therapy, donor recruitment strategies and bioengineering are expected to provide significant advances in the field of transplantation medicine.
Core tip: Transplantation is a systematic medical procedure for patients with organ failure and haematological disorders. Immunologically compatible donor and recipient are determined by several genetic markers which include matching for ABO blood group, human leukocyte antigen, MICA and killer cell immunoglobulin-like receptors. The elucidation of genes code for these markers of tissue identity reviewed here and significant advancement in the field of transplant immunology are expected to have a positive impact on transplantation medicine. These include both the waitlisted and transplanted patients.
- Citation: Edinur HA, Manaf SM, Che Mat NF. Genetic barriers in transplantation medicine. World J Transplant 2016; 6(3): 532-541
- URL: https://www.wjgnet.com/2220-3230/full/v6/i3/532.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i3.532
Transplantation is a systematic medical procedure for patients with organ failure and haematological disorders[1,2]. Transplantation can be classified into four categories: Autograft, isograft, allograft and xenograft based on the origins and the recipients of the grafts (cells, tissues or organs). In autograft transplantation (also known as autologous transplantation), a graft is taken and transplanted from different parts of the same individual. The processes of transferring grafts between genetically identical and non-identical individuals of the same species are known as isograft and allograft transplantation, respectively. In contrast, xenograft refers to the transplantation of grafts between two different species such as from baboon to human. Implantation of human cancer cells in mice for tumour study is also assumed to be xenograft transplantation[3,4].
The current practice of allograft transplantation is to have as many match for ABO and human leukocyte antigen (HLA) loci as possible between the donor and recipient. However, this is not the case for isograft and autograft as the transplanted graft originated from the genetically identical resources. Incompatibility between donor and recipient will cause rejection since the graft will be considered as non-self by the recipient’s immune surveillance and the rate of graft rejection will vary depending on time courses, types of tissue or organ grafted and the immune responses involved.
In general, there are three types of graft rejections, i.e., hyperacute, acute and chronic rejection[4]. These types of rejections are categorized based on the speed that the rejection occurs. For hyperacute rejection, this process may occur within minutes or hours, and is usually not longer than 24 h. Sometimes, hyperacute rejection may occur immediately during the surgery process. This type of rejection is due to preformed alloantibodies against the mismatched ABO and HLA antigens between patient and donor. The alloantibodies may exist due to previous transplantation or transfusion, pregnancy or infections[5]. This pre-existing antibody can activate the complement system and cause injury to the endothelial cells which will then lead to platelet adhesion and thrombosis. Therefore, the graft will never be vascularised and the organ must be removed immediately. The hyperacute rejection may be managed with systematic antibody screening and cross matching between donor and recipient[6].
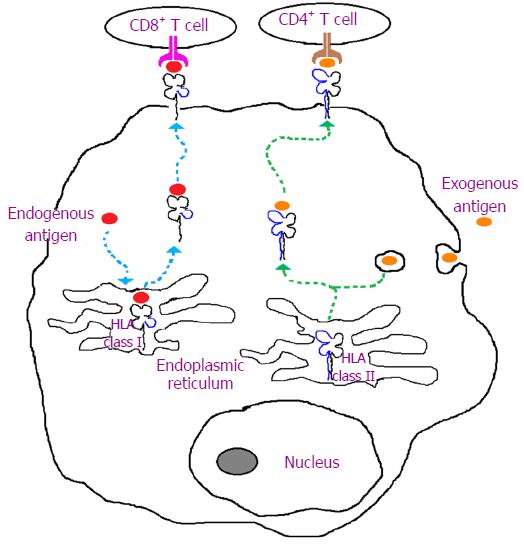
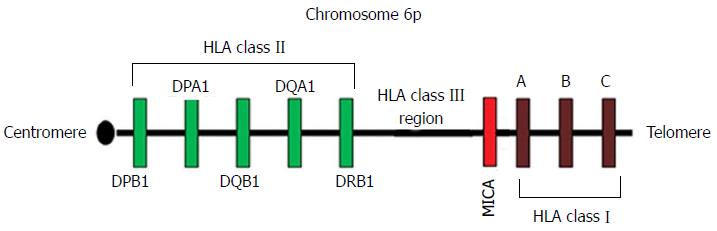
The most common type of graft rejection is acute rejection. The onset of rejection varies from weeks to months and is largely attributed to HLA incompatibility. This type of rejection involves both cellular- and humoral-mediated immunity. However, the cellular-mediated immune responses are more significant through either direct recognition of non-self HLA molecules on the surface of the graft or indirect antigenic peptide presentation by self HLA molecules to T cells[7-9] (Figure 1). The CD4+ T cells will also secrete several types of cytokines such as interleukin-4 (IL-4) and IL-2. These cytokines will then lead to several mechanisms including inflammation, recruitment of other inflammatory cells and may also induce T and B cell proliferations[9]. The major histocompatibility complex (MHC) class I chain-related gene A (MICA) molecules are also important markers of tissue identity and have been implicated in transplant immunology[10,11]. The stress-induced MICA has previously known as PERB11.1 glycoproteins and are coded for by the gene located on the classical class I subregion of MHC[12] (Figure 2) and incompatibility between the donor and recipient for the MICA antigen will trigger cytotoxic activity of lymphocytes (CD8+ and γδ T cells) and natural killer (NK) cells[11,13-15] (see the following sub-sections). The role of MICA in graft rejection and donor specific antibodies to MICA antigens have been reported by several others[11,16-18].
The third type of rejection is chronic rejection which takes place months to years following transplantation procedure. It induces chronic damage via the production of cytokines and alloantibodies which activate the classical pathway of complement system[19,20]. However, the actual mechanism of this rejection is not very well understood. It is usually characterized by fibrosis and arteriosclerosis, due to extensive proliferation of smooth muscle cells. Repairing process of damaged tissues and macrophages activation in chronic rejection can lead to fibrosis formation[21-23].
The transplanted allograft can also trigger immune reactions [i.e., graft vs host disease (GVHD)] against mismatched antigens possessed by the recipients. The GVHD is predominantly occurs in bone marrow transplantation which involves alloreactivity of donor’s lymphocytes against the incompatible tissues of the immune-suppressed host [8]. However, improved outcomes were observed in haplo-identical (i.e., a single HLA haplotype-mismatched) stem cell transplantation[24-26]. In this context, donor’s NK cells will recognize leukaemia cells as non-self and initiate alloreactivity (i.e., graft vs leukaemia effect) against the cancerous cells after haplo-identical stem cell transplantation[27-29]. The inhibitory and alloreactivity of NK cells are determined by HLA molecules which acting as ligands (Table 1) for their immunoglobulin-like receptors [i.e., killer cell immunoglobulin-like receptors (KIRs)][29,30] (see the following sub-sections). Thus, this receptor-ligand incompatible might lead to either NK alloreactivity against transplanted graft or GVHD. Our understanding of this immune surveillance has provided the basis for the adoptive infusion of NK cells as part of immunological based modality in transplantation and ultimately reduce the potential toxicity effects of other immunosuppression agents[29,31,32] (see later).
| KIR | Alleles | Protein variants | HLA ligands |
| 2DL1 | 43 | 24 | C2 |
| 2DL2 | 28 | 11 | C1, C2 |
| 2DL3 | 34 | 17 | C1, C2 |
| 2DL4 | 46 | 22 | G |
| 2DL5 | 41 | 17 | Unknown |
| 2DS1 | 15 | 7 | C2 |
| 2DS2 | 22 | 8 | Unknown |
| 2DS3 | 14 | 5 | Unknown |
| 2DS4 | 30 | 13 | A*11, some C |
| 2DS5 | 16 | 11 | Unknown |
| 3DL1 | 73 | 58 | Bw4 |
| 3DS1 | 16 | 12 | Unknown |
| 3DL2 | 84 | 61 | A*03,-11 |
| 3DL3 | 107 | 55 | Unknown |
| 3DP1 | 22 | 0 | 0 |
| 2DP1 | 23 | 0 | 0 |
The immunosuppressive therapy is used to increase the survival rate of the graft, especially during acute rejection. However, this therapy cannot be used for chronic rejection since it is difficult to manage. This therapy does not only involve drugs but also antibodies[33,34]. Examples of the drugs that have been used in immunosuppressive therapy are like mycophenolate mofetil, cyclosporine, tacrolimus and sirolimus[35-38]. Each of these drugs has their own mechanism of action which will result in immune cells suppression. For example, mycophenolate mofetil is administered to block proliferation of lymphocytes by inhibiting the key enzyme that is important for purine synthesis and DNA replication[36] while cyclosporine is given to inhibit transcription factor for T-cell activation[39,40]. For antibodies, a number of monoclonal and polyclonal antibodies have been given to the patients in preventing graft rejection. Most of these antibodies are specific for T cells or T cell sub-populations and they are very effective for blocking T cells activation and binding[41,42].
However, most of the immunosuppressive agents can cause various side effects to the recipient on their long term use. Besides that, the immunosuppression effects of the agents are not specific only on the graft, but also attack the overall body systems including the lymphocyte maturation. Hence, this will put the recipient at a high risk of getting other infections, cancer, cardiovascular diseases and metabolic bone diseases[33,43-45]. Additionally, the recipient will have a chance of getting transplant rejection once they stop taking these immunosuppression agents. As an alternative, researchers are working on finding a new therapy that maintains the health of the graft without compromising the immune system. This new method involves inducing immune tolerance and mainly focus on T cell depletion in thymus (i.e., central tolerance) and suppression of mature T cells in lymph nodes (i.e., peripheral tolerance)[20,46].
The key element in tolerance induction is specificity, which means the recipient immune system is not completely paralyzed. For example, the traditional antithymocyte globulin (TGA) was used as immunosuppressive agent drugs to prevent an acute rejection in organ transplantation[47-49]. As an alternative, this treatment is replaced with another antibody known as anti-IL-2Rα receptor antibodies. This type of antibody is widely used to replace TGA as it does not cause chronic expression of cytokines and improves the development of immune tolerance[50-53]. Besides anti-IL-2Rα, the combination of costimulatory molecule blockage with inhibitory of signal activation also appear to be effective in inducing tolerance in a few animal studies. Interaction between T cell receptor and costimulatory signals such as CD28 is required for T cell activation. Thus, blockage of the CD28 and its ligands (i.e., B7 family molecules) resulted in transplantation tolerance[46,54,55] and induction of anergic state in T cells activation[56]. In addition, another molecule that binds to ligand for T cell activation (e.g., CD152 or also known CTLA-4) also has a potential in inducing tolerance. For example, treatment with CTLA-4 immunoglobulin (Ig) during bone marrow transplantation in murine models was able to induce long-term survival rate of allograft[57]. Similarly, Ig treatment of other ligand for T cell receptor (e.g., PD-1) and costimulatory molecule (e.g., CD40) have also been shown to limit T cell proliferation and activation[58-60]. Acute rejection in non-human primates is also preventable by anti-CD40L treatment with or without CTLA-Ig[61,62].
Besides using inhibitory molecules, Treg (CD4+CD25+) and NK cells can also be used to suppress CD4+ and CD8+ T cell proliferation[63-67] and reduced rejection and GVHD[68-74]. Other than post-transplant, infusion of Treg cells before a transplant procedure is found to promote immune reconstitution and improve immunity to opportunistic infection, hence, preventing GVHD[75]. By increasing NK cells by total lymphoid irradiation, the immune tolerance is induced after organ and HSC transplantation[76]. A study suggests that the interaction of NK cells and Treg cells can promote immune tolerance. IL-4, which is secreted by NK cells, induces the expression of negative costimulatory molecules on the Treg cells[77]. The purification of NK cells in allogeneic transplantation may be achieved by depleting CD3+ cells followed by CD56+ cell enrichment[78]. Donors are also reported safe in completed clinical trials of NK cells infusion[79-81]. Stimulated NK cells with IFN-γ, IL-2 and anti-CD3 show MHC-independent cytotoxicity effect and NK cells infusion is proven safe to use after autologous HSCT[82]. The strategies of using immune cell infusion therapy have significantly increased the level of immune tolerance against allogeneic graft. New discoveries on Treg and NK cells administration posit that they appear to be effective in inducing transplant tolerance and rapid immune reconstitution. This may help to induce a better protection of infection or cancer relapse and consequently reducing GVHD incidence.
Immunologically compatible donor and recipient are determined by several genetic markers which include matching for ABO blood groups, HLA, MICA and KIRs (see preceding sections). These antigens are encoded by highly polymorphic and independent loci in our genome and are distributed differently between individuals and populations. Incompatibility between the donor and recipient for these antigens will lead to either allograft lost or GVHD. In the following sub-sections, we discuss the molecular bases for the genes encoded for the determinants of transplant compatibility.
The ABO is important blood group in transfusion and transplantation and consists of three antigens; A, B and O. These red cell antigens are determined by the ABO allelic variants (A, B and O alleles) on the long arm of chromosome 9. The co-dominant A and B alleles differ by four nucleotide substitutions (C526G, G703A, C796A and G803C) while the ∆261G deletion differentiates between the recessive O and A alleles[83-85]. The α1,3-N-acetylgalactosaminyltransferase encoded by A allele and α1,3-D-galactosyltransferase encoded by B alleles then convert H antigens, the products of H gene located on human chromosome 19 to either A or B antigens, respectively[86]. In contrast, there is no enzymatic activity on H antigen for those bearing the O allele due to the ∆261G deletion on the background of O allele. Thus, the A, B, O and AB phenotypes are determined by the three ABO allelic variants; A, B and O alleles.
The HLA class I molecules consist of a non-polymorphic β2-microglobulin and a highly polymorphic α-chain glycoprotein encoded by the genes within MHC on the chromosome 6[87-89]. There are three types of HLA class I molecules (A, C and B) with their specificities depend on the polymorphic α-chain encoded by HLA-A, -B and -C genes in the classical class I sub-region of MHC[90]. In contrast, both α- and β-chains of class II HLA molecules (DP, DQ and DR) are encoded by genes in the classical class II sub-region of MHC[12] (Figure 2). The HLA class I and II gene clusters within MHC are separated by the class III sub-region which codes for complement components and not part of endogenous and exogenous peptide presentation to CD8+ and CD4+ cells, respectively[91-93] (Figure 1).
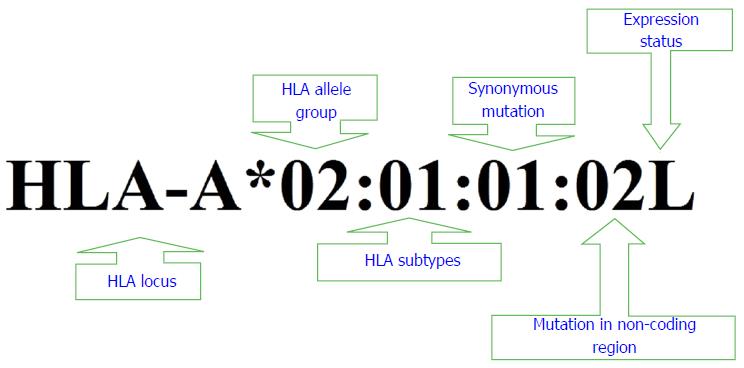
The World Health Organization has developed an alphanumeric nomenclature to name HLA antigens, genes and alleles (Figure 3). This systematic alphanumeric nomenclature begins with letters representing specific HLA gene and followed by an asterisk and two sets of digits specific for HLA allele group and glycoprotein. Two additional sets of digits are then used to specify synonymous nucleotide changes and mutation outside the non-coding region, respectively. Suffixes (e.g., L: low cell surface expression, N: Null, C: Allele is expressed in cytoplasm but not on the cell surface and A: Aberrant expression) may be added to the end of this string of numbering system to indicate expression status of particular HLA alleles[12,94].
The MICA molecules are stress induced antigens encoded by a gene within MHC region (Figure 2) and are expressed by a wide range of cells including monocytes, keratinocytes and fibroblasts[14,87,95-97]. Unlike HLA class I molecule, MICA is not linked to β2-microglobulin and NK cells and CD8+ T (αβ and γδ) cells reactivity are stimulated through interaction of MICA and its ligand, the NKG2D receptor[13-15,98]. Variants of MICA gene are largely due to single nucleotide polymorphism and repeated units of alanine (i.e., 4 to 10 Ala residues) in exons 2, 3 and 4 and exon 5, respectively[99-102] (see González-Galarza et al[100] for the list of populations characterized for MICA). The diversity within MICA gene reflect its role in immunity and as a marker of tissue identity[96,97].
The NK cells recognize healthy and unhealthy cells through either their lectin-like or immunoglobulin-like receptors encoded by NK and leukocyte receptor complexes located on human chromosome 12 and 19, respectively[103,104]. The leukocyte receptor complex also code for KIRs, one of the highly polymorphic transmembrane glycoprotein receptors expressed by NK cells[105,106]. Currently there are 16 KIR genes and more than 570 genotypes (combinations of haplotype A and B KIR genes - Table 2) and 600 alleles were documented in public databases[99,100].
| KIR gene | KIR haplotype | |||||
| A | A | A | B | B | B | |
| 1KIR3DL1 | *015 | *086 | *005 | *007 | *086 | X |
| 1KIR2DL1 | *003 | *003 | *003 | *010 | *004 | X |
| 1KIR2DL3 | *001 | *001 | *001 | X | X | X |
| 1KIR2DS4 | *001 | *001 | *010 | *003 | *001 | X |
| 2KIR2DL2 | X | X | X | *003 | *001 | *001 |
| 2KIR2DL5 | X | X | X | *B002 | *B002 | A*001 |
| 2KIR3DS1 | X | X | X | X | X | *013 |
| 2KIR2DS1 | X | X | X | X | X | *002 |
| 2KIR2DS2 | X | X | X | *001 | *001 | X |
| 2KIR2DS3 | X | X | X | *001 | *003 | X |
| 2KIR2DS5 | X | X | X | X | X | *001 |
| 3KIR2DL4 | *001 | *028 | *011 | *006 | *028 | *005 |
| 3KIR3DL2 | *002 | *002 | *010 | *002 | *002 | *007 |
| 3KIR3DL3 | *013 | *002 | *009 | *014 | *013 | *003 |
| 1KIR2DP1 | *009 | *001 | *001 | *004 | *007 | *007 |
| 3KIR3DP1 | *001 | *001 | *003 | *001 | *003 | *003 |
Each KIR is classified according to the number of their extracellular immunoglobulin (two and three domains and assigned as 2D and 3D, respectively) and the length of cytoplasmic (short and long and assigned as S and L, respectively) domains, respectively[107]. The KIRs with short and long cytoplasmic domains are activating and inhibitory receptors and transduce their signals through DAP-12 and tyrosine-based motifs, respectively. The only exception is for KIR2DL4 which transmits both, inhibitory and stimulatory signals[99]. The highly diverse and complex of KIRs were also reported for their ligands, the HLA class I molecules (Table 1) and both have significant influences in transplantation and pathogenesis of various diseases[108].
Typing of ABO and HLA, antibody screening and cross matching are three important procedures in determining the compatibility between donors and recipients. These procedures have been largely conducted using serological approaches (e.g., complement dependent cytotoxicity test, ELISA, Luminex and flow cytometric assays; see Howell et al[8] for details). Alloantibodies against the transplanted organs/cells are usually developed in highly transfused patients or due to previous transplantation and pregnancy. These are the three main events where individuals might be exposed to non-self antigens including the clinically important transplant antigens such as ABO antigens, HLA and MICA. Thus, antibody screening and cross matching are crucial to avoid allograft lost. Nowadays, molecular typing techniques such as those using sequence specific oligonucleotide primer, and Sanger sequencing have largely been used for genotyping of ABO, HLA and MICA and KIR genes. These molecular techniques have several advantages as they are not dependent on the availability of anti-sera, cellular expression and have greater specificity and accuracy as compared with the antibody-antigen based typing (recently reviewed by Howell et al[8], Dunn[109] and Edinur et al[110]).
Advances in the field of molecular biology and genetics have contributed immense benefits to the medical field including in transplantation medicine. A number of molecular techniques have been developed following the elucidation of molecular bases of the genes encoding for transplant determinants. Currently, several different genotyping platforms can be used to screen blood group, HLA, MICA, and KIR loci (see Howell et al[8], Dunn[109], Edinur et al[110] and Finning et al[111]). It is now possible to genotype multiple markers and to the extent of complete sequencing of human genome using the next generation sequencer (NGS). This high throughput genotyping platform has been tested for HLA (e.g., see Bentley et al[112], Holcomb et al[113], Wang et al[114] and Skibola et al[115]) and it is expected that NGS will be used to simultaneously genotype large number of clinically relevant transplantation genes in near future. This is not far from reality due to bioinformatics support given by the immunogenetics community and the rigorous improvement in NGS methodology (see Robinson et al[94] and Grada et al[116]). In addition, new developments in immune tolerance based therapy, donor recruitment strategies and bioengineering (tissue engineering and regenerative medicine) will provide significant advances in the field of transplantation medicine. This paper provides only brief discussions of these new developments, while others[20,46,110,117,118] have conducted systematic reviews of them.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: Malaysia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
P- Reviewer: Boucek CD, Kin T, Lee WC, Peng SM S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Heidary Rouchi A, Mahdavi-Mazdeh M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int J Organ Transplant Med. 2015;6:93-98. [PubMed] |
| 2. | Park B, Yoo KH, Kim C. Hematopoietic stem cell expansion and generation: the ways to make a breakthrough. Blood Res. 2015;50:194-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 54] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 3. | Kindt TJ, Osborne BA, Goldsby RA. Kuby Immunology. New York: W. H. Freeman & Company 2007; 425-443. |
| 4. | Chong AS, Alegre ML. The impact of infection and tissue damage in solid-organ transplantation. Nat Rev Immunol. 2012;12:459-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 5. | Wood K, Shankar S, Mittal S. Concepts and challenges in organ transplantation: rejection, immunosuppressant and tolerance. In: Clinical immunology: principles and practice. 4th ed. Elsevier California 2013; 989-995. |
| 6. | Puttarajappa C, Shapiro R, Tan HP. Antibody-mediated rejection in kidney transplantation: a review. J Transplant. 2012;2012:193724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 7. | Hennecke J, Wiley DC. T cell receptor-MHC interactions up close. Cell. 2001;104:1-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 8. | Howell WM, Carter V, Clark B. The HLA system: immunobiology, HLA typing, antibody screening and crossmatching techniques. J Clin Pathol. 2010;63:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Plant N, Wood P. Transplantation, ABO incompatibility and immunology. Anaesth Intens Care Med. 2009;10:227-230. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 10. | Suárez-Alvarez B, López-Vázquez A, Gonzalez MZ, Fdez-Morera JL, Díaz-Molina B, Blanco-Gelaz MA, Pascual D, Martínez-Borra J, Muro M, Alvarez-López MR. The relationship of anti-MICA antibodies and MICA expression with heart allograft rejection. Am J Transplant. 2007;7:1842-1848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Tonnerre P, Gérard N, Chatelais M, Poli C, Allard S, Cury S, Bressollette C, Cesbron-Gautier A, Charreau B. MICA variant promotes allosensitization after kidney transplantation. J Am Soc Nephrol. 2013;24:954-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Marsh SG, Albert ED, Bodmer WF, Bontrop RE, Dupont B, Erlich HA, Fernández-Viña M, Geraghty DE, Holdsworth R, Hurley CK. Nomenclature for factors of the HLA system, 2010. Tissue Antigens. 2010;75:291-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3449] [Cited by in RCA: 3346] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 13. | Muro M, Lopez-Hernandez R, Llorente S, Bolarin JM, Martinez P, Boix F, Salgado G, Bosch A, Martinez H, Eguia J. MICA Molecules in Disease and Transplantation, a Double-Edged Sword? Curr Immunol Rev. 2012;8:307-325. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of NK cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 1999;285:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2208] [Cited by in RCA: 2301] [Article Influence: 88.5] [Reference Citation Analysis (0)] |
| 15. | Zwirner NW, Dole K, Stastny P. Differential surface expression of MICA by endothelial cells, fibroblasts, keratinocytes, and monocytes. Hum Immunol. 1999;60:323-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 16. | Gautier AC, Devys A, Cheneau ML, Simon PH, Martin C, Allard S, Hourmant M, Bignon JD. MICA compatibility and immunization in third kidney transplantations. Transplant Proc. 2009;41:663-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Zhang Q, Cecka JM, Gjertson DW, Ge P, Rose ML, Patel JK, Ardehali A, Kobashigawa JA, Fishbein MC, Reed EF. HLA and MICA: targets of antibody-mediated rejection in heart transplantation. Transplantation. 2011;91:1153-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Zou Y, Stastny P, Süsal C, Döhler B, Opelz G. Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med. 2007;357:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 323] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 19. | Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando). 2009;23:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Ruiz P, Maldonado P, Hidalgo Y, Gleisner A, Sauma D, Silva C, Saez JJ, Nuñez S, Rosemblatt M, Bono MR. Transplant tolerance: new insights and strategies for long-term allograft acceptance. Clin Dev Immunol. 2013;2013:210506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Demetris AJ, Murase N, Lee RG, Randhawa P, Zeevi A, Pham S, Duquesnoy R, Fung JJ, Starzl TE. Chronic rejection. A general overview of histopathology and pathophysiology with emphasis on liver, heart and intestinal allografts. Ann Transplant. 1997;2:27-44. [PubMed] |
| 22. | Bhatti AB, Usman M. Chronic Renal Transplant Rejection and Possible Anti-Proliferative Drug Targets. Cureus. 2015;7:e376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Khan MA, Nicolls MR. Complement-mediated microvascular injury leads to chronic rejection. Adv Exp Med Biol. 2013;735:233-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 24. | Wu S, Zeng YJ, Zhang C, Deng TX, Xu YQ, Zhang X. The role of the killer cell immunoglobulin-like receptor (KIR) “missing self” model in unrelated donor HSCT: a meta-analysis. Transplant Proc. 2015;47:558-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2010;16:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 26. | Sobecks RM, Wang T, Askar M, Gallagher MM, Haagenson M, Spellman S, Fernandez-Vina M, Malmberg KJ, Müller C, Battiwalla M. Impact of KIR and HLA Genotypes on Outcomes after Reduced-Intensity Conditioning Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant. 2015;21:1589-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 27. | Aversa F. Haploidentical haematopoietic stem cell transplantation for acute leukaemia in adults: experience in Europe and the United States. Bone Marrow Transplant. 2008;41:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 28. | Kolb HJ, Schmid C, Barrett AJ, Schendel DJ. Graft-versus-leukemia reactions in allogeneic chimeras. Blood. 2004;103:767-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 307] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 29. | Davis CT, Rizzieri D. Immunotherapeutic applications of NK cells. Pharmaceuticals (Basel). 2015;8:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 30. | Rajalingam R, Gebel HM. KIR-HLA mismatching in human renal allograft transplantation: emergence of a new concept. Am J Transplant. 2011;11:1771-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Bleakley M, Riddell SR. Molecules and mechanisms of the graft-versus-leukaemia effect. Nat Rev Cancer. 2004;4:371-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 287] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 32. | Locatelli F, Moretta F, Brescia L, Merli P. Natural killer cells in the treatment of high-risk acute leukaemia. Semin Immunol. 2014;26:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 78] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 33. | Watson CJ, Dark JH. Organ transplantation: historical perspective and current practice. Br J Anaesth. 2012;108 Suppl 1:i29-i42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 149] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 34. | Page AJ, Ford ML, Kirk AD. Memory T-cell-specific therapeutics in organ transplantation. Curr Opin Organ Transplant. 2009;14:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 35. | Anil Kumar MS, Irfan Saeed M, Ranganna K, Malat G, Sustento-Reodica N, Kumar AM, Meyers WC. Comparison of four different immunosuppression protocols without long-term steroid therapy in kidney recipients monitored by surveillance biopsy: five-year outcomes. Transpl Immunol. 2008;20:32-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 36. | Lindenfeld J, Miller GG, Shakar SF, Zolty R, Lowes BD, Wolfel EE, Mestroni L, Page RL, Kobashigawa J. Drug therapy in the heart transplant recipient: part II: immunosuppressive drugs. Circulation. 2004;110:3858-3865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 37. | Jain A, Sharma R, Ryan C, Tsoulfas G, Orloff M, Abt P, Kashyap R, Batzold P, Sauberman L, Safadjou S. Potential immunological advantage of intravenous mycophenolate mofetil with tacrolimus and steroids in primary deceased donor liver transplantation and live donor liver transplantation without antibody induction. Liver Transpl. 2008;14:202-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Lo A, Stratta RJ, Alloway RR, Egidi MF, Shokouh-Amiri MH, Grewal HP, Gaber LW, Gaber AO. Initial clinical experience with interleukin-2 receptor antagonist induction in combination with tacrolimus, mycophenolate mofetil and steroids in simultaneous kidney-pancreas transplantation. Transpl Int. 2001;14:396-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 39. | Wiseman AC. Immunosuppressive Medications. Clin J Am Soc Nephrol. 2016;11:332-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 159] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 40. | Hernández GL, Volpert OV, Iñiguez MA, Lorenzo E, Martínez-Martínez S, Grau R, Fresno M, Redondo JM. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2. J Exp Med. 2001;193:607-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 329] [Cited by in RCA: 330] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 41. | Kirk AD, Hale DA, Mannon RB, Kleiner DE, Hoffmann SC, Kampen RL, Cendales LK, Tadaki DK, Harlan DM, Swanson SJ. Results from a human renal allograft tolerance trial evaluating the humanized CD52-specific monoclonal antibody alemtuzumab (CAMPATH-1H). Transplantation. 2003;76:120-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 335] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 42. | Pearl JP, Parris J, Hale DA, Hoffmann SC, Bernstein WB, McCoy KL, Swanson SJ, Mannon RB, Roederer M, Kirk AD. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant. 2005;5:465-474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 389] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 43. | Woodward RS, Schnitzler MA, Baty J, Lowell JA, Lopez-Rocafort L, Haider S, Woodworth TG, Brennan DC. Incidence and cost of new onset diabetes mellitus among U.S. wait-listed and transplanted renal allograft recipients. Am J Transplant. 2003;3:590-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 297] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 44. | Wei L, MacDonald TM, Walker BR. Taking glucocorticoids by prescription is associated with subsequent cardiovascular disease. Ann Intern Med. 2004;141:764-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 502] [Cited by in RCA: 533] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 45. | Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 763] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 46. | Alpdogan O, van den Brink MR. Immune tolerance and transplantation. Semin Oncol. 2012;39:629-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 47. | Colak T, Sevmiş S, Karakayali H, Moray G, Haberal M. One center’s experience with antithymocyte globulin treatment for acute rejection in renal transplantation. Transplant Proc. 2008;40:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 48. | Deeks ED, Keating GM. Rabbit antithymocyte globulin (thymoglobulin): a review of its use in the prevention and treatment of acute renal allograft rejection. Drugs. 2009;69:1483-1512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 49. | Saull HE, Enderby CY, Gonwa TA, Wadei HM. Comparison of alemtuzumab vs. antithymocyte globulin induction therapy in primary non-sensitized renal transplant patients treated with rapid steroid withdrawal. Clin Transplant. 2015;29:573-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 50. | Pham K, Kraft K, Thielke J, Oberholzer J, Sankary H, Testa G, Benedetti E. Limited-dose Daclizumab versus Basiliximab: a comparison of cost and efficacy in preventing acute rejection. Transplant Proc. 2005;37:899-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 51. | Vincenti F. Potential of daclizumab in solid organ transplantation. BioDrugs. 1999;11:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 52. | Swiatecka-Urban A. Anti-interleukin-2 receptor antibodies for the prevention of rejection in pediatric renal transplant patients: current status. Paediatr Drugs. 2003;5:699-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 53. | Peng W, Liu G, Xie W, Huang H, Wu J, Shou Z, Chen J. Interleukin-2 receptor antagonist compared with antithymocyte globulin induction therapy in kidney transplantation from donors after cardiac death. Int J Clin Pract Suppl. 2015;23-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 54. | Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T cells. J Immunol. 1991;147:2461-2466. [PubMed] |
| 55. | Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 621] [Cited by in RCA: 599] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 56. | Bour-Jordan H, Esensten JH, Martinez-Llordella M, Penaranda C, Stumpf M, Bluestone JA. Intrinsic and extrinsic control of peripheral T-cell tolerance by costimulatory molecules of the CD28/ B7 family. Immunol Rev. 2011;241:180-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 309] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 57. | Pearson TC, Alexander DZ, Hendrix R, Elwood ET, Linsley PS, Winn KJ, Larsen CP. CTLA4-Ig plus bone marrow induces long-term allograft survival and donor specific unresponsiveness in the murine model. Evidence for hematopoietic chimerism. Transplantation. 1996;61:997-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 92] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Habicht A, Kewalaramani R, Vu MD, Demirci G, Blazar BR, Sayegh MH, Li XC. Striking dichotomy of PD-L1 and PD-L2 pathways in regulating alloreactive CD4(+) and CD8(+) T cells in vivo. Am J Transplant. 2007;7:2683-2692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 41] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 59. | Haspot F, Fehr T, Gibbons C, Zhao G, Hogan T, Honjo T, Freeman GJ, Sykes M. Peripheral deletional tolerance of alloreactive CD8 but not CD4 T cells is dependent on the PD-1/PD-L1 pathway. Blood. 2008;112:2149-2155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 61] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173:945-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 775] [Cited by in RCA: 889] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 61. | Kirk AD, Harlan DM, Armstrong NN, Davis TA, Dong Y, Gray GS, Hong X, Thomas D, Fechner JH, Knechtle SJ. CTLA4-Ig and anti-CD40 ligand prevent renal allograft rejection in primates. Proc Natl Acad Sci USA. 1997;94:8789-8794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 726] [Article Influence: 25.9] [Reference Citation Analysis (0)] |
| 62. | Kirk AD, Burkly LC, Batty DS, Baumgartner RE, Berning JD, Buchanan K, Fechner JH, Germond RL, Kampen RL, Patterson NB. Treatment with humanized monoclonal antibody against CD154 prevents acute renal allograft rejection in nonhuman primates. Nat Med. 1999;5:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 671] [Cited by in RCA: 628] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 63. | Shevach EM. CD4+ CD25+ suppressor T cells: more questions than answers. Nat Rev Immunol. 2002;2:389-400. [PubMed] |
| 64. | Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4(+)CD25(-)Foxp3(-) Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. J Exp Med. 2007;204:285-297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 452] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 65. | Collison LW, Workman CJ, Kuo TT, Boyd K, Wang Y, Vignali KM, Cross R, Sehy D, Blumberg RS, Vignali DA. The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature. 2007;450:566-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1324] [Cited by in RCA: 1515] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 66. | Nakamura K, Kitani A, Strober W. Cell contact-dependent immunosuppression by CD4(+)CD25(+) regulatory T cells is mediated by cell surface-bound transforming growth factor beta. J Exp Med. 2001;194:629-644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1190] [Cited by in RCA: 1198] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 67. | Rubtsov YP, Rasmussen JP, Chi EY, Fontenot J, Castelli L, Ye X, Treuting P, Siewe L, Roers A, Henderson WR. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 1252] [Article Influence: 73.6] [Reference Citation Analysis (0)] |
| 68. | Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood. 2002;99:3493-3499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 841] [Article Influence: 36.6] [Reference Citation Analysis (0)] |
| 69. | Hoffmann P, Ermann J, Edinger M, Fathman CG, Strober S. Donor-type CD4(+)CD25(+) regulatory T cells suppress lethal acute graft-versus-host disease after allogeneic bone marrow transplantation. J Exp Med. 2002;196:389-399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 867] [Cited by in RCA: 869] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 70. | Graca L, Thompson S, Lin CY, Adams E, Cobbold SP, Waldmann H. Both CD4(+)CD25(+) and CD4(+)CD25(-) regulatory cells mediate dominant transplantation tolerance. J Immunol. 2002;168:5558-5565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 71. | Cobbold SP, Castejon R, Adams E, Zelenika D, Graca L, Humm S, Waldmann H. Induction of foxP3+ regulatory T cells in the periphery of T cell receptor transgenic mice tolerized to transplants. J Immunol. 2004;172:6003-6010. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 317] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 72. | Feng G, Wood KJ, Bushell A. Interferon-gamma conditioning ex vivo generates CD25+CD62L+Foxp3+ regulatory T cells that prevent allograft rejection: potential avenues for cellular therapy. Transplantation. 2008;86:578-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 73. | Kingsley CI, Karim M, Bushell AR, Wood KJ. CD25+CD4+ regulatory T cells prevent graft rejection: CTLA-4- and IL-10-dependent immunoregulation of alloresponses. J Immunol. 2002;168:1080-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 588] [Cited by in RCA: 590] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 74. | Waldmann H, Graca L, Cobbold S, Adams E, Tone M, Tone Y. Regulatory T cells and organ transplantation. Semin Immunol. 2004;16:119-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 75. | Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 833] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 76. | Higuchi M, Zeng D, Shizuru J, Gworek J, Dejbakhsh-Jones S, Taniguchi M, Strober S. Immune tolerance to combined organ and bone marrow transplants after fractionated lymphoid irradiation involves regulatory NK T cells and clonal deletion. J Immunol. 2002;169:5564-5570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 75] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 77. | Hongo D, Tang X, Dutt S, Nador RG, Strober S. Interactions between NKT cells and Tregs are required for tolerance to combined bone marrow and organ transplants. Blood. 2012;119:1581-1589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 78. | Skeate R, Singh C, Cooley S, Geller M, Northouse J, Welbig J, Slungaard A, Miller J, McKenna D. Hemolytic anemia due to passenger lymphocyte syndrome in solid malignancy patients treated with allogeneic natural killer cell products. Transfusion. 2013;53:419-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 79. | Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051-3057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1210] [Cited by in RCA: 1423] [Article Influence: 71.2] [Reference Citation Analysis (0)] |
| 80. | Geller MA, Cooley S, Judson PL, Ghebre R, Carson LF, Argenta PA, Jonson AL, Panoskaltsis-Mortari A, Curtsinger J, McKenna D. A phase II study of allogeneic natural killer cell therapy to treat patients with recurrent ovarian and breast cancer. Cytotherapy. 2011;13:98-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 337] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 81. | Rubnitz JE, Inaba H, Ribeiro RC, Pounds S, Rooney B, Bell T, Pui CH, Leung W. NKAML: a pilot study to determine the safety and feasibility of haploidentical natural killer cell transplantation in childhood acute myeloid leukemia. J Clin Oncol. 2010;28:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 500] [Article Influence: 33.3] [Reference Citation Analysis (0)] |
| 82. | Leemhuis T, Wells S, Scheffold C, Edinger M, Negrin RS. A phase I trial of autologous cytokine-induced killer cells for the treatment of relapsed Hodgkin disease and non-Hodgkin lymphoma. Biol Blood Marrow Transplant. 2005;11:181-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 171] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 83. | Daniels G. The molecular genetics of blood group polymorphism. Hum Genet. 2009;126:729-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 84. | Veldhuisen B, van der Schoot CE, de Haas M. Blood group genotyping: from patient to high-throughput donor screening. Vox Sang. 2009;97:198-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 68] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 85. | Ogasawara K, Bannai M, Saitou N, Yabe R, Nakata K, Takenaka M, Fujisawa K, Uchikawa M, Ishikawa Y, Juji T. Extensive polymorphism of ABO blood group gene: three major lineages of the alleles for the common ABO phenotypes. Hum Genet. 1996;97:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 86. | Yamamoto F, Marken J, Tsuji T, White T, Clausen H, Hakomori S. Cloning and characterization of DNA complementary to human UDP-GalNAc: Fuc alpha 1----2Gal alpha 1----3GalNAc transferase (histo-blood group A transferase) mRNA. J Biol Chem. 1990;265:1146-1151. [PubMed] |
| 87. | Horton R, Wilming L, Rand V, Lovering RC, Bruford EA, Khodiyar VK, Lush MJ, Povey S, Talbot CC, Wright MW. Gene map of the extended human MHC. Nat Rev Genet. 2004;5:889-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 780] [Cited by in RCA: 829] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 88. | Schwartz BD. The human major histocompatibility human leukocyte antigen (HLA) complex. Basic and clinical immunology. California: Appleton & Lange 1991; 45-60. |
| 89. | Goodfellow PN, Jones EA, Van Heyningen V, Solomon E, Bobrow M, Miggiano V, Bodmer WF. The beta2-microglobulin gene is on chromosome 15 and not in the HL-A region. Nature. 1975;254:267-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 197] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Brodsky FM. Antigen presentation & the major histocompatibility complex. Medical Immunology. Stanford: Appleton & Lange 1997; 83-94. |
| 91. | Abbas AK, Lichtman AH, Pober JS. Cellular and molecular immunology. Philadelphia, Pennsylvania, USA: W.B. Saunders Company 2000; 63-101. |
| 92. | Sargent CA, Dunham I, Campbell RD. Identification of multiple HTF-island associated genes in the human major histocompatibility complex class III region. EMBO J. 1989;8:2305-2312. [PubMed] |
| 93. | Shiina T, Hosomichi K, Inoko H, Kulski JK. The HLA genomic loci map: expression, interaction, diversity and disease. J Hum Genet. 2009;54:15-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 522] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 94. | Robinson J, Halliwell JA, Hayhurst JD, Flicek P, Parham P, Marsh SG. The IPD and IMGT/HLA database: allele variant databases. Nucleic Acids Res. 2015;43:D423-D431. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1479] [Cited by in RCA: 1499] [Article Influence: 136.3] [Reference Citation Analysis (0)] |
| 95. | Groh V, Bahram S, Bauer S, Herman A, Beauchamp M, Spies T. Cell stress-regulated human major histocompatibility complex class I gene expressed in gastrointestinal epithelium. Proc Natl Acad Sci USA. 1996;93:12445-12450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 784] [Cited by in RCA: 795] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 96. | Stephens HA. MICA and MICB genes: can the enigma of their polymorphism be resolved? Trends Immunol. 2001;22:378-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 154] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 97. | Zou Y, Stastny P. Role of MICA in the immune response to transplants. Tissue Antigens. 2010;76:171-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 98. | Bahram S, Bresnahan M, Geraghty DE, Spies T. A second lineage of mammalian major histocompatibility complex class I genes. Proc Natl Acad Sci USA. 1994;91:6259-6263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 585] [Cited by in RCA: 596] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 99. | Robinson J, Mistry K, McWilliam H, Lopez R, Marsh SG. IPD--the Immuno Polymorphism Database. Nucleic Acids Res. 2010;38:D863-D869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 144] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 100. | González-Galarza FF, Takeshita LY, Santos EJ, Kempson F, Maia MH, da Silva AL, Teles e Silva AL, Ghattaoraya GS, Alfirevic A, Jones AR. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res. 2015;43:D784-D788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 619] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 101. | Pérez-Rodríguez M, Argüello JR, Fischer G, Corell A, Cox ST, Robinson J, Hossain E, McWhinnie A, Travers PJ, Marsh SG. Further polymorphism of the MICA gene. Eur J Immunogenet. 2002;29:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 102. | Robinson J, Pérez-Rodríguez M, Waller MJ, Cuillerier B, Bahram S, Yao Z, Albert ED, Madrigal JA, Marsh SG. MICA sequences 2000. Immunogenetics. 2001;53:150-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 103. | Carrington M, Martin MP. The impact of variation at the KIR gene cluster on human disease. Curr Top Microbiol Immunol. 2006;298:225-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 104. | Parham P, Norman PJ, Abi-Rached L, Guethlein LA. Human-specific evolution of killer cell immunoglobulin-like receptor recognition of major histocompatibility complex class I molecules. Philos Trans R Soc Lond B Biol Sci. 2012;367:800-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 105. | Yokoyama WM, Plougastel BF. Immune functions encoded by the natural killer gene complex. Nat Rev Immunol. 2003;3:304-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 437] [Cited by in RCA: 437] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 106. | Martin AM, Kulski JK, Witt C, Pontarotti P, Christiansen FT. Leukocyte Ig-like receptor complex (LRC) in mice and men. Trends Immunol. 2002;23:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 107. | Middleton D, Curran M, Maxwell L. Natural killer cells and their receptors. Transpl Immunol. 2002;10:147-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 108. | Single RM, Martin MP, Gao X, Meyer D, Yeager M, Kidd JR, Kidd KK, Carrington M. Global diversity and evidence for coevolution of KIR and HLA. Nat Genet. 2007;39:1114-1119. [PubMed] |
| 109. | Dunn PP. Human leucocyte antigen typing: techniques and technology, a critical appraisal. Int J Immunogenet. 2011;38:463-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 110. | Edinur HA, Chambers GK, Dunn PP. Recent Developments in Transplantation and Transfusion Medicine. Ann Transplant. 2015;20:424-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 111. | Finning K, Bhandari R, Sellers F, Revelli N, Villa MA, Muñiz-Díaz E, Nogués N. Evaluation of red blood cell and platelet antigen genotyping platforms (ID CORE XT/ID HPA XT) in routine clinical practice. Blood Transfus. 2015;1-8. [RCA] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 112. | Bentley G, Higuchi R, Hoglund B, Goodridge D, Sayer D, Trachtenberg EA, Erlich HA. High-resolution, high-throughput HLA genotyping by next-generation sequencing. Tissue Antigens. 2009;74:393-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 113. | Holcomb CL, Höglund B, Anderson MW, Blake LA, Böhme I, Egholm M, Ferriola D, Gabriel C, Gelber SE, Goodridge D. A multi-site study using high-resolution HLA genotyping by next generation sequencing. Tissue Antigens. 2011;77:206-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 114. | Wang C, Krishnakumar S, Wilhelmy J, Babrzadeh F, Stepanyan L, Su LF, Levinson D, Fernandez-Viña MA, Davis RW, Davis MM. High-throughput, high-fidelity HLA genotyping with deep sequencing. Proc Natl Acad Sci USA. 2012;109:8676-8681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 148] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 115. | Skibola CF, Akers NK, Conde L, Ladner M, Hawbecker SK, Cohen F, Ribas F, Erlich HA, Goodridge D, Trachtenberg EA. Multi-locus HLA class I and II allele and haplotype associations with follicular lymphoma. Tissue Antigens. 2012;79:279-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 116. | Grada A, Weinbrecht K. Next-generation sequencing: methodology and application. J Invest Dermatol. 2013;133:e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 152] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 117. | Moon KH, Ko IK, Yoo JJ, Atala A. Kidney diseases and tissue engineering. Methods. 2016;99:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 118. | Yamanaka S, Yokoo T. Current Bioengineering Methods for Whole Kidney Regeneration. Stem Cells Int. 2015;2015:724047. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |