Published online Sep 24, 2016. doi: 10.5500/wjt.v6.i3.451
Peer-review started: April 26, 2016
First decision: June 16, 2016
Revised: June 25, 2016
Accepted: July 14, 2016
Article in press: July 18, 2016
Published online: September 24, 2016
Processing time: 150 Days and 18.2 Hours
Organ transplantation saves thousands of lives every year but the shortage of donors is a major limiting factor to increase transplantation rates. To allow more patients to be transplanted before they die on the wait-list an increase in the number of donors is necessary. Patients with devastating irreversible brain injury, if medically suitable, are potential deceased donors and strategies are needed to successfully convert them into actual donors. Multiple steps in the process of deceased organ donation can be targeted to increase the number of organs suitable for transplant. In this review, after describing this process, we discuss current challenges and potential strategies to expand the pool of deceased donors.
Core tip: An increase in the number of donors is necessary to allow more patients to be transplanted before they die on the wait-list. Multiple steps in the process of deceased organ donation can be targeted to increase the number of organs suitable for transplant.
- Citation: Girlanda R. Deceased organ donation for transplantation: Challenges and opportunities. World J Transplant 2016; 6(3): 451-459
- URL: https://www.wjgnet.com/2220-3230/full/v6/i3/451.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i3.451
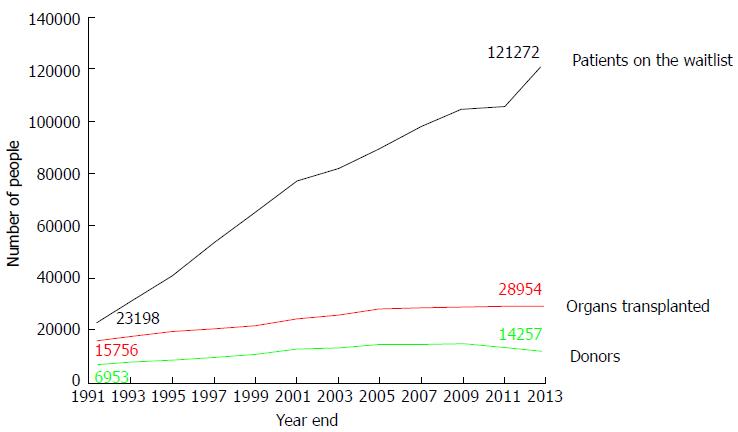
Several obstacles have been overcome over the last few decades to make organ transplantation an effective life-saving treatment for many patients. Among them, the refinement of surgical techniques and the availability of effective immunosuppressive regimens against rejection have played a major role. However, only the availability of donated organs from deceased persons (DD) has made it possible for organ transplantation to become an established, worldwide treatment for patients with organ failure. Without the “gift of life” from deceased donors, it is difficult to imagine how so many lives could have been saved. Currently, the shortage of organs is a major obstacle to making organ transplantation more accessible to a larger number of candidates. Only 30973 transplants from 15064 donors have been performed in the United States in the year 2015, while more than 121000 candidates were waiting for a transplant[1]. Furthermore, the gap between the number of patients on the wait list and the limited number of available organs continues to widen. As a consequence, more than 6000 patients die every year while waiting for a transplant. In the ideal situation of an unlimited organ supply, virtually no patient would die on the wait list. Instead, due to the persistent scarcity of organs, a candidate for transplant has a 10%-30% chance of dying, depending on the organ, while on the wait list to receive an organ.
The common parameter adopted in different countries to measure the activity of organ donation has been traditionally the number of donors/million population. Although this metric is prone to the flaws of regional variations in health status, it is still used worldwide[2]. In this review, because our observations are limited to the United States, we will refer instead to the total number of donors/year.
The shortage of organs has been recognized worldwide as a major limiting factor to organ transplantation. The World Health Organization and several international agencies have addressed organ shortage at different levels[3-7]. Over the past decade, several initiatives have been put into place in the United States to address the shortage of organs. Among them, The Organ Donation Breakthrough Collaborative, funded by the Division of Transplantation in the Health Resources and Services Administration of the Department of Health and Human Services, was launched in September 2003 with the intent of increasing the number of organs available for transplant. The goal of this initiative was to achieve a donor conversion rate (i.e., from eligible to actual donor, see below) of 75% or higher across the country. Since its inception, more than 180 hospitals have met or exceeded this goal. Another goal proposed in this initiative was to increase the number of organs transplanted per donor. Subsequently, the Institute of Medicine (IoM) published the document “Organ Donation: Opportunities for Action”[8]. This report emphasized that the current system of organ donation could be greatly improved and offered a number of specific recommendations to help increase the supply of transplantable organs. Given the wide variation in consent rate, ranging between 30% and 70%, across Organ Procurement Organizations (OPO), the IoM recommended the identification of best practices and their dissemination among institutions in the organ-procurement and transplantation system. In addition, the IoM report suggested to devote research efforts to identify new ways to improve the system and increase donation rates. Importantly, among them, it was recommended to integrate organ donation in the process of end-of-life care, recognizing that patients and their families should be offered the opportunity to donate as part of the standard care at the end of life. Still, after those and other efforts, over the last decade the donation rate from deceased donors has remained stagnant in the United States (Figure 1).
The vast majority (80%-90%) of organs from DD are procured after declaration of death by neurologic criteria (or “brain death”, BD). Brain death is determined after irreversible cessation of brain stem activity documented by bedside neurologic tests (reflexes, Table 1).
| Corneal reflex |
| Cough reflex |
| Facial motor response to painful stimuli |
| Gag reflex |
| Oculocephalic reflex (“Doll’s eyes”) |
| Oculovestibular reflex (caloric response) |
| Pupillary response to light |
The oxygenation of a comatose person who suffered a devastating irreversible brain injury fulfilling the criteria for brain death is maintained by mechanical ventilation, while cardio-circulatory activity and organ perfusion is supported, if needed, by inotropic medications.
Unlike BD donors, a proportion of DD, currently 16% of the organs procured nationally, are recovered after declaration of death by circulatory criteria [donation after cardiac death (DCD)][9]. In this scenario, patients who have suffered severe brain injury but do not fulfill the criteria for brain death, may still be organ donors if the patient, by advance directive, or the patient’s family decides to withdraw life support. In these circumstances, after consent for organ donation has been obtained, the patient is brought to the operating room where ventilation is disconnected and life-sustaining medications are withdrawn. After the cessation of cardio-circulatory activity for 2-5 min, depending on the local protocol, the patient is pronounced dead by a member of the primary team. After declaration of death the organ procurement team arrives to the operating room and begins organ recovery. The different dynamics involved in BD and DCD pathways and their implications on organ allocation and function are beyond the scope of this review. For historical purposes, it is interesting to note that at the beginning of organ transplantation in the 1960s all organs were procured from DCD donors, since the concept and legislation of brain death had not been developed. Only in 1968, an ad hoc committee at Harvard Medical School defined brain death as the state of irreversible coma with unresponsiveness and lack of receptivity, absence of movement and breathing and absence of brain-stem reflexes[10]. Since then, the vast majority of DD have been BD. Only over the past decade there has been an increase in the proportion of DCD from 7% in 2005 to the current 16% of all deceased donors, with wide regional variation ranging between 7%-30%. The recent increase in the proportion of DCD donors has paired with only a small increase in the total number of DD. This has raised the legitimate concern whether the BD pool is curtailed as a result of more DCD donors being pursued. Specifically, the question is raised whether some of the DCD donors could/would have progressed to BD had life support been continued for a sufficient time to allow BD to occur. In a multicenter report from 27 European countries participating in a survey on organ donation, including 10 countries with established DCD programs, the number of both DBD and DCD overall increased during the interval 2000-2009. However, DBD decreased of about 20% in three countries with a predominant DCD activity, implying that DCD might have negatively impacted on DBD activity[11]. Ideally, in order to increase the overall donation rate, the expansion of the DCD pathway should have an additive rather than detrimental effect on DBD, so that, in aggregate, more potential donors become actual donors compared to the DBD pathway alone. Indeed, a recent study from the New England Organ Bank, one of the top ranking OPOs in the United States by percentage of DCD (> 30%), reports a 5-year experience with 331 DCD donors without a concomitant reduction of DBD, suggesting that a DCD program may actually expand the donor pool rather than curtailing it. The results of this study also show that overall more potential donors had been identified that would have not been realized without the DCD program[12]. Regardless, DCD alone and/or in combination with current DBD practices are unlikely to bridge the gap between current organ availability and need. In addition to DCD, other strategies to optimize the current limited organ pool are needed, including the use of less-than-ideal organs (“marginal organs”) and split techniques (in case of the liver). While these strategies partially mitigate the donor shortage, still do not resolve the problem of organ shortage and call for additional initiatives. Among them, a considerable attention has been given lately in several countries to the pool of potential donors.
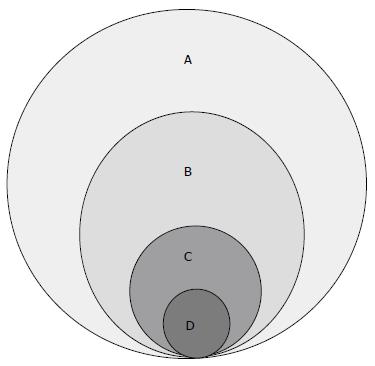
Multiple recent studies from different countries, including the United States, have documented the potential for increasing the number of deceased donors. The Iberoamerican Network/Council on Donation and Transplantation has reported a 52% increase in deceased donation in less than 10 years in Central and South America[3], indirectly demonstrating that the pool of potential donors was previously incompletely exploited. According to a report from Spain, 2.3% of hospital deaths and 12.4% of deaths in the intensive care unit could yield potential donors, making the number of actual donors up to 21% higher if all potentials were to be identified and followed[13]. The Spanish donation system, among the top performing worldwide, has been widely recognized as a valid model in both BD and DCD pathways and includes an internal hospital chart review of patients who died in ICU performed by transplant coordinators followed by an external periodic audit. Although the plain application of the Spanish model to other national donation systems would not necessarily lead to increased donation rates due to several socio-economic and cultural differences between countries, nonetheless the Spanish experience in recent decades and published studies from other countries indicate that the donor potential is probably not fully exploited. A few definitions currently used in the organ donation literature and protocols are reported in Table 2.
| Donor | A person from whom at least one organ was procured for the purpose of transplant, regardless of whether the organ was transplanted |
| Eligible death | Death of a person aged 70 yr or younger, legally declared brain dead according to hospital policy and without exclusions listed in OPTN policy |
| Imminent neurological death | 70 yr or younger, ventilated, with severe brain injury and without exclusion criteria, lacking 3 brain stem reflexes but not fulfilling BD criteria |
| Potential donor | Patient with devastating irreversible brain injury apparently medically suitable for organ donation and suspected to fulfill BD criteria |
Although with different definitions, the number of potential donors has been estimated in previous studies. According to the IoM report, the number of donor-eligible deaths has been estimated in the range between 10500 and 16800 per year, significantly higher than the actual 8500-9000 deceased donors/year over the last two years[8,15]. In other reports, the potential for brain dead donors has been estimated between 10000 and 26000 per year, depending on the study modality based on either mortality records or hospital chart review[16-20]. In 2010 the Health Resources and Service Administration of the Department of Health and Human Services commissioned UNOS to conduct the Deceased Donor Potential Study to estimate the number of potential donors in the United States. According to the results of this study, the pool of potential donors is larger than previously estimated with as many as 35000 to 40000 potential donors each year meeting basic criteria for donation[21]. Although the true potential could have been over-estimated due to the lack of more detailed medical information, nevertheless this study confirms that there is an untapped pool of potential donors. Another interesting finding in this study was that, among people who met basic medical criteria for deceased donation, the actual donation rate was considerably lower (10%) in the age group 50 to 75 years compared to those age 18 to 34 (50%), implying that more donors could be potentially obtained in the age group 50-75 years.
The potential for donation varies across geographic areas of the United States with a four-fold difference in eligible death/million population reported to OPTN by OPOs (national mean 31 eligible death/million population, ranging from 15 to 61) based on the existing geographical variability in mortality (91-229 deaths/million population from cerebro-vascular accident and trauma)[2]. Importantly, this study highlighted that the number of eligible deaths is correlated to the number of deaths from cerebro-vascular accidents and trauma in that specific area (r square = 0.79).
Outside the United States, studies from Europe, Canada and other countries have documented similar findings regarding potential donors. In Belgium, Roels et al[22] found that 57% of deceased potential donors were missed along the process due to non-identification or missed referral or lack of consent. Likewise, a study from Canada based on discharge data submitted to the Hospital Morbidity Database reported that only 1 in 6 potential donors (17%) became actual donor[23]. Even assuming that the study methodology overestimated the number of potential donors due to the limitations of analyzing abstract data rather than actual patient chart review, nevertheless this study confirms that the potential to increase the number of deceased donors exists. Regardless of the definition of potential donor, it is evident from several studies that the number of actual donors represents only a small proportion of the pool of potential donors (Figure 2).
Therefore, a major challenge to increase donation rates would consist of expanding the pool of actual donors to include potential donors. The process of organ donation and potential strategies to expand the pool of actual donors will be discussed below.
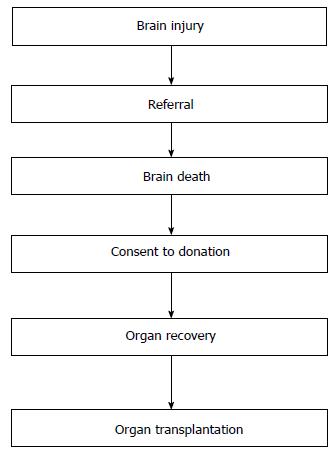
Currently, organs for transplant are recovered after determination of the donor’s death. This standard practice, commonly known as the “dead donor rule”, requires that the intended donor be declared dead before the removal of any life-sustaining organs[24]. This rule was introduced to protect the person’s life before death and to prevent that lives were ended for the purpose of procuring organs. This rule is important to maintain the public trust in organ donation and transplantation and to avoid the misconception that care is withdrawn from potential donors in order to expedite death for the purpose of organ recovery. Recently, however, the dead donor rule has been reconsidered[25]. In the opinion of some ethicists, while the “dead donor rule” assures patients, families and health professionals that a patient is dead before removing organs, therefore making organ transplantation legally and ethically acceptable, on the other hand it may jeopardize donation in selected cases. As an example, it is quoted the case of a DCD potential donor with prolonged agonal phase (the interval between withdrawal of support and cardiac arrest) that prevented organ recovery and transplantation due to prolonged ischemia. It is argued by some that, after the decision of withdrawing support has been reached, organs be procured without waiting for the declaration of death by circulatory criteria (i.e., cardiac arrest). The advantage of this pathway would be to give patients the opportunity to donate even before death is declared, when death is imminent (“near death”) and donation is desirable, in order not to jeopardize the viability of donated organs for transplant. It is argued that, when death is very near, some patients may want to die in the process of helping others to live, even if that means altering the timing or manner of their death. Regardless of this debate about the dead donor rule, it is important that ICU physicians, transplant professionals and organ procurement organizations make every effort towards maintaining public trust. Mistrust from the general public regarding the procurement of organs will likely result in reduced consent rates for donation based on the perceived fear by the donor’s family that treatment is withdrawn from their loved one in order to obtain organs. In other words, fearful people will assume that physicians care more about obtaining organs than saving the patient’s life. In addition, this debate on the dead donor rule emphasizes the importance of a previous recommendation by the IoM about the integration of organ donation with end-of-life care. By this integration, the donation process starts before the occurrence of the donor’s death, at the time when the potential donor with irreversible devastating brain injury is referred but is not yet declared dead. Since every actual donor has been a potential donor sometime before in the process, it is likely that the coordination of end-of-life care and organ donation would allow to identify and manage potential donors early in the process, increasing the chances of donation. The process leading from donation to transplantation can be described in the following 6 steps: Brain injury, referral, brain death, consent, organ recovery and organ transplantation (Figure 3).
The process of organ donation for transplantation has been described before[11]. In this review we will limit our considerations to deceased organ donation in the United States.
Organ donors are patients with extensive brain injury resulting, most commonly, from cerebro-vascular accident or trauma or anoxia. Only a small proportion of those patients who suffered extensive and irreversible brain injury become actual organ donors because of the variable impact, in terms of intensity and timing, of brain injury on neurological functions and on brain stem activity. As a result, the occurrence of brain death is more or less likely and more or less rapid in different patients. As an example, a patient with large intra-cerebral hemorrhage or a bilateral pontine hemorrhage is more likely to progress to brain death within a relatively short timeframe than a patient with diffuse anoxic injury without intracranial hypertension[26]. Consequently, the time interval between brain injury and brain death varies, impacting on the management of the potential donor and costs. In addition, during the time interval between brain injury and brain death the patient is exposed to the systemic adverse effects of brain injury, including hemodynamic instability, diabetes insipidus, and others. In this context, the management of the potential donor while in ICU is paramount and has been described elsewhere[27].
Among all patients with brain injury as described above, the medical suitability for organ donation is determined according to established criteria and represents the second step of the process leading to the referral of the potential donor. Federal rules require hospitals to notify the OPO of an individual whose death is imminent or who has died in the hospital[28]. A network of 58 OPOs constitutes the liaison system designated by the United States federal government to coordinate the organ donation process. The criteria (or triggers) for referral from the hospital to the local OPO are reported in Table 3.
| Every ventilated patient with |
| Glasgow coma scale of 5 or less without sedation |
| Brain death testing being considered/pursued |
| Do-not-resuscitate or comfort care being considered |
| Withdrawal of support being considered |
| Family initiates conversation about donation |
| Every cardiac death within 1 h |
The referral of a potential donor to the OPO can occur as early as on patient presentation to the Emergency department[29]. After referral, the OPO is involved with the management of the potential donor by coordinating the logistic, medical and regulatory aspects of donation. Importantly, an OPO representative approaches the family of the donor providing support from the time of referral through donation and after donation. The potential donor is considered medically suitable for donation based on established criteria of transplantability of the organs except in cases with potentially transmittable diseases, such as infections or cancer, as indicated in the UNOS policy[30].
Once exclusion criteria have been ruled out, the potential donor becomes eligible for donation after declaration of brain death, which is the third step of the process. Established neurologic tests allow the determination of death by neurologic criteria (brain death tests) and therefore determine eligibility for donation. According to UNOS definition (see above), an eligible death for organ donation is defined as the death of a patient 70 years old or younger, without any exclusion criteria for donation, legally declared brain dead according to hospital policy independent of family decision regarding donation or availability of next-of-kin, independent of medical examiner or coroner involvement in the case, and independent of local acceptance criteria or transplant hospital practice.
The concept of brain death has been introduced in 1968 following the proposal by an Ad Hoc Committee that a person could be declared dead after irreversible cessation of the function of the entire brain[10]. Before the introduction of this concept, the death of a person was declared after irreversible cessation of circulatory and respiratory function. After the introduction of brain death, it became accepted that a person requiring mechanical ventilation can be declared dead even while maintaining heart beating. This is an important aspect to discuss with the donor’s family given that the concept of death in the public opinion is mainly associated with arrest of cardio-circulatory activity.
After brain death, in observance of the principles of autonomy and non-maleficence, the consent to donation is sought from the patient, the family or the next of kin before proceeding with organ recovery. This represents the fourth step in the process and an important focus for future strategies to increase donation (see below). Several aspects of the step of obtaining consent to donation are crucial, including the timing, the method and the approach. Usually, the donor’s family is approached after declaration of brain death. However, in selected cases it may be indicated to approach the family before brain death, as in the case of an unstable donor where rapid deterioration of organ function may occur. This critical step of communicating with the family highlights the importance of effective coordination of end of life care between ICU providers and OPO personnel. In some countries outside the United States, regulations allow the procurement of organs based on the presumed consent of the donor in absence of documented objection to donation. In the United States system, which is based on explicit rather than presumed consent, it is important that the approach to the family and the process of obtaining consent for donation is conducted in a culturally-sensitive way. It is becoming increasingly clear that a better understanding of the donor’s family language, culture, faith, and values is critically important to increase consent rates[31]. The current consent rate is on average 76% ranging between 62% and 93% across OPOs[32]. Little is known about the factors associated with such variability across regions. In addition, the reasons for denied consent to donation by the donor’s family are still poorly understood and represent an opportunity for action in order to increase deceased donation (see below).
After consent is obtained, the OPO, in collaboration with the donor hospital, allocates suitable organs and arranges for the operation of organ recovery, which represents the fifth step of the process. Typically, multiple organs are procured in different combinations including heart, lungs, liver, kidneys, pancreas and intestine from the same deceased donor during a multi-team operation lasting several hours. Each team carries the burden of recovering the respective organ in the best possible condition for their intended recipient. Therefore excellent communication and coordination between teams is essential during procurement. Typically the teams recovering the thoracic organs and the abdominal organs proceed simultaneously. The intra-operative management of the donor during organ recovery has been reviewed elsewhere[33]. It is critical to assess and correct, when necessary, the hemodynamic, metabolic, hormonal and pro-inflammatory alterations occurring in the setting of brain death. Studies have documented that the quality of donor management impacts on the quality of the procured grafts and on graft function[34]. The different techniques of multi-organ procurement have been described extensively and vary among countries.
The allocation and transplantation of the procured organs represents the final step of the process. In the United States organ allocation is regulated by organ-specific policies following the criteria of urgency as indicated by the degree of disease severity of transplant candidates. Although the vast majority of recovered organs are subsequently transplanted, not all recovered organs are always transplanted. The reasons for failure to transplant procured organs are multiple and include damage to the organ during procurement, organ unsuitability discovered during or after procurement, sudden unsuitability of the intended recipient to receive the allocated organ and others. Regardless, to maximize the use of this scarce resource it is important to prevent organ “discard” after recovery. The conversion rate, which reflects the proportion of eligible donors that becomes actual donors and is one of the parameters monitored by the OPO, is an indirect way to assess discard rate of procured organs. Accordingly, actual donors are considered those in which at least one organ has been successfully transplanted. Multiple factors impact on conversion rates and are beyond the scope of this review. Each step of the process of organ donation from deceased donors as outlined above can potentially be the target of strategies to increase donation rates, as discussed below.
The number of deceased organ donors per year has remained relatively stable over the last decade with only a small annual increase over the years from 8016 deceased organ donors in the year 2006 to 8143 in 2012 and 8596 in 2014[35]. At the same time, the number of patients added to the wait list has increased at a faster pace every year, making the gap between need and supply of organs wider every year (Figure 1). One of the strategies to narrow this gap is to increase the number of donors for transplant, especially deceased donors. Being the pool of potential donors larger than the number of actual donors, as outlined above, and considering that all donors were “potential” at some point during the process, it is reasonable to focus efforts on identifying and managing potential donors in order to increase donation rates. This would require a novel and broader approach to deceased donation to include not only those fulfilling brain death criteria (eligible deaths) but also those closed to it (“near brain death” or “imminent death”). According to OPTN, imminent donor is a potential donor who is imminent to fulfill the criteria for the determination of death by neurologic criteria (BD). Currently, imminent deaths are being monitored by OPOs, although their definition varies among regions and hospitals. It would be important to have a uniform characterization of imminent deaths and, more importantly, to have a better understanding of their evolution in terms of progression to BD.
Several challenges have been identified at each step of the process of deceased organ donation that could potentially be the target of action to improve donation rates. These include: Missed clinical triggers for referral, premature withdrawal of support before BD testing, cardiac death during evaluation, lack of consent, donor instability and death during organ recovery, organ damage at procurement or organ unsuitability discovered after recovery and others. At the very beginning of the process of organ donation from deceased donors it is crucial that the potential donor is recognized early after presentation to hospital and referred promptly to the local OPO. The determination of the suitability for donation based on initial demographic (age) or clinical parameters and co-morbidities of patients with devastating brain injury should be deferred to the OPO representative rather than to the primary ICU team. An early referral allows the OPO sufficient time to evaluate the potential donor for medical suitability and to approach the family[36].
The donor’s family plays a key role in the donation process. Within the OPO, a dedicated team of trained personnel approaches the family in a sensitive way. Even in case of registered donors, the family is always consulted before organ procurement. Although legally the donor’s consent is sufficient to allow organ recovery, nevertheless the wishes of the family are always taken in consideration and usually organ recovery is not pursued in case of opposition from the donor’s family. Respect for the donor’s family is important to maintain the public trust: It would be deleterious to pursue organ donation against the family wishes, even in presence of donor’s consent. In addition, it is important to understand the motivations behind the declined consent by the donor’s family. Factors associated with declined consent include donor age (older), ethnic minority, time interval between certification of brain death and approach to the family and the amount of time spent by the coordinator with the family[37,38]. The education of families from ethnical minorities using a culturally-sensitive approach seems particularly important, since minority groups are disproportionally represented on the transplant waiting list and unfortunately also suffer from disparities in deceased and living donation. Barriers to donation in minority groups include decreased awareness of transplantation, religious or cultural distrust of the medical community, fear of medical abandonment and fear of racism[39]. Culturally sensitive communication and interventions are needed to overcome these barriers[40].
After referral, the ideal management of the potential donor involves both ICU team and OPO personnel. This combined approach provides the best chances to effectively integrate organ donation as part of end of life care, as recommended by the Institute of Medicine. Although prognostic factors have been studied and identified[41], still the likelihood and timing of progression to BD in patient with brain injury remains incompletely understood. Further studies are needed to better identify early predictors of brain death.
BD is associated with a plethora of systemic manifestations including hemodynamic, metabolic and endocrine disturbances. Guidelines have been developed to assist the donor management before organ recovery. Occasionally, eligible donors are lost due to intercurrent hemodynamic instability and cardiac arrest. As part of the integration of end-of-life care with organ donation, it would be important to identify risk factors for cardiac arrest, treat disimbalances and discuss with the donor’s family the code status of the donor, including the possibility of hemodynamic support and, if necessary, cardio-pulmonary resuscitation in order to maintain organ perfusion until organ recovery occurs.
An increase in deceased organ donation is necessary to make organ transplantation accessible to more candidates. Among others, new strategies to manage the pool of potential donors are needed in order to increase donation rates.
I am indebted to the Washington Regional Transplant Community staff, especially Lori Brigham, Emily Johnson and Carlos Fernandez-Bueno, MD, for fruitful collaboration.
Manuscript source: Invited manuscript
Specialty type: Transplantation
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Eghtesad B, Kin T S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Organ procurement and transplantation network. [accessed 2016 Feb 29]. Available from: https: //optn.transplant.hrsa.gov/. |
| 2. | Sheehy E, O’Connor KJ, Luskin RS, Howard RJ, Cornell D, Finn J, Mone T, Selck FW, Delmonico FL. Investigating geographic variation in mortality in the context of organ donation. Am J Transplant. 2012;12:1598-1602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 3. | Matesanz R, Soratti C, Pérez-Rosales MD. Regional Perspective: The Iberoamerican Network/Council on Donation and Transplantation. Transplantation. 2015;99:1739-1742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Knoll GA, Tinckam KJ. Organ Donation and Transplantation: The View From Canada. Transplantation. 2015;99:2231-2233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 5. | Min SI, Ha J. Recent Progresses in Organ Donation and Transplantation in Korea. Transplantation. 2015;99:2431-2433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. WHO guiding principles on human cell, tissue and organ transplantation. Transplantation. 2010;90:229-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (1)] |
| 7. | The Madrid resolution on organ donation and transplantation: national responsibility in meeting the needs of patients, guided by the WHO principles. Transplantation. 2011;91 Suppl 11:S29-S31. [PubMed] |
| 8. | Committee on Increasing Rates of Organ Donation, Board on Health Sciences Policy, Institute of Medicine. Organ donation: Opportunities for action. Washington, D.C. : National Academies Press 2006; 358. |
| 9. | Data on donation and transplantation, AOPO. [accessed 2016 Feb 29]. Available from: http://www.aopo.org/related-links-data-on-donation-and-transplantation/. |
| 10. | A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA. 1968;205:337-340. [PubMed] |
| 11. | Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, Neuberger J, Coene L, Morel P, Corinne A, Muehlbacher F, Brezovsky P, Costa AN. Current situation of donation after circulatory death in European countries. Transpl Int. 2011;24:676-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 199] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 12. | Nelson HM, Glazier AK, Delmonico FL. Changing Patterns of Organ Donation: Brain Dead Donors Are Not Being Lost by Donation After Circulatory Death. Transplantation. 2016;100:446-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 13. | de la Rosa G, Domínguez-Gil B, Matesanz R, Ramón S, Alonso-Álvarez J, Araiz J, Choperena G, Cortés JL, Daga D, Elizalde J. Continuously evaluating performance in deceased donation: the Spanish quality assurance program. Am J Transplant. 2012;12:2507-2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | OPTN policies. [accessed 2016 Feb 29]. Available from: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf#nameddest=Policy_01. |
| 15. | US Department of Health & Human Services. National data [accessed 2016 Mar 9]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. |
| 16. | Nathan HM, Jarrell BE, Broznik B, Kochik R, Hamilton B, Stuart S, Ackroyd T, Nell M. Estimation and characterization of the potential renal organ donor pool in Pennsylvania. Report of the Pennsylvania Statewide Donor Study. Transplantation. 1991;51:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 108] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 17. | Evans RW, Orians CE, Ascher NL. The potential supply of organ donors. An assessment of the efficacy of organ procurement efforts in the United States. JAMA. 1992;267:239-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 175] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 18. | Gortmaker SL, Beasley CL, Brigham LE, Franz HG, Garrison RN, Lucas BA, Patterson RH, Sobol AM, Grenvik NA, Evanisko MJ. Organ donor potential and performance: size and nature of the organ donor shortfall. Crit Care Med. 1996;24:432-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 138] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 19. | Ojo AO, Wolfe RA, Leichtman AB, Dickinson DM, Port FK, Young EW. A practical approach to evaluate the potential donor pool and trends in cadaveric kidney donation. Transplantation. 1999;67:548-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Sheehy E, Conrad SL, Brigham LE, Luskin R, Weber P, Eakin M, Schkade L, Hunsicker L. Estimating the number of potential organ donors in the United States. N Engl J Med. 2003;349:667-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 322] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 21. | Organ procurement and transplantation network deceased donor potential study (DDPS). [accessed 2016 Apr 6]. Available from: https://optn.transplant.hrsa.gov/media/1161/ddps_03-2015.pdf. |
| 22. | Roels L, Smits J, Cohen B. Potential for deceased donation not optimally exploited: donor action data from six countries. Transplantation. 2012;94:1167-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 23. | Canadian institute for health information, deceased organ donor potential in canada. [accessed 2016 Apr 6]. Available from: https://www.cihi.ca/web/resource/en/organdonorpotential_2014_en.pdf. |
| 24. | Robertson JA. The dead donor rule. Hastings Cent Rep. 1999;29:6-14. [PubMed] |
| 25. | Sade RM, Boan A. The paradox of the dead donor rule: increasing death on the waiting list. Am J Bioeth. 2014;14:21-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 26. | Weiss N, Galanaud D, Carpentier A, Tezenas de Montcel S, Naccache L, Coriat P, Puybasset L. A combined clinical and MRI approach for outcome assessment of traumatic head injured comatose patients. J Neurol. 2008;255:217-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Dictus C, Vienenkoetter B, Esmaeilzadeh M, Unterberg A, Ahmadi R. Critical care management of potential organ donors: our current standard. Clin Transplant. 2009;23 Suppl 21:2-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Department of Health and Human Services. Medicare and medicaid programs; hospital conditions of participation: Identification of potential organ, tissue and eye donors and transplant hospitals’ provision of transplant-related data. Final Rule 63 Federal Register 119 (1998) (codified at 42 CFR §482 45). |
| 29. | Michael GE, O’Connor RE. The importance of emergency medicine in organ donation: successful donation is more likely when potential donors are referred from the emergency department. Acad Emerg Med. 2009;16:850-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 30. | US Department of Health & Human Services. OPTN policies [accessed 2016 Apr 18]. Available from: https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. |
| 31. | Goldberg DS, French B, Abt PL, Gilroy RK. Increasing the Number of Organ Transplants in the United States by Optimizing Donor Authorization Rates. Am J Transplant. 2015;15:2117-2125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Association of Organ Procurment Organizations. Data on donation and transplantation [accessed 2016 Apr 18]. Available from: http: //www.aopo.org/related-links-data-on-donation-and-transplantation/. |
| 33. | McKeown DW, Bonser RS, Kellum JA. Management of the heartbeating brain-dead organ donor. Br J Anaesth. 2012;108 Suppl 1:i96-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Oto T, Excell L, Griffiths AP, Levvey BJ, Bailey M, Marasco S, Macdonald P, Snell GI. Association between primary graft dysfunction among lung, kidney and heart recipients from the same multiorgan donor. Am J Transplant. 2008;8:2132-2139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 35. | Organ Procurement and Transplantation Network. National data [accessed 2016 Apr 18]. Available from: https://optn.transplant.hrsa.gov/data/view-data-reports/national-data/#. |
| 36. | Kotloff RM, Blosser S, Fulda GJ, Malinoski D, Ahya VN, Angel L, Byrnes MC, DeVita MA, Grissom TE, Halpern SD. Management of the Potential Organ Donor in the ICU: Society of Critical Care Medicine/American College of Chest Physicians/Association of Organ Procurement Organizations Consensus Statement. Crit Care Med. 2015;43:1291-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 37. | Brown CV, Foulkrod KH, Dworaczyk S, Thompson K, Elliot E, Cooper H, Coopwood B. Barriers to obtaining family consent for potential organ donors. J Trauma. 2010;68:447-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 38. | Ebadat A, Brown CV, Ali S, Guitierrez T, Elliot E, Dworaczyk S, Kadric C, Coopwood B. Improving organ donation rates by modifying the family approach process. J Trauma Acute Care Surg. 2014;76:1473-1475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 39. | Bratton C, Chavin K, Baliga P. Racial disparities in organ donation and why. Curr Opin Organ Transplant. 2011;16:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 40. | Davis BD, Norton HJ, Jacobs DG. The Organ Donation Breakthrough Collaborative: has it made a difference? Am J Surg. 2013;205:381-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 41. | Galbois A, Boëlle PY, Hainque E, Raynal M, Cazejust J, Baudel JL, Ait-Oufella H, Alves M, Bigé N, Maury E. Prediction of evolution toward brain death upon admission to ICU in comatose patients with spontaneous intracerebral hemorrhage using simple signs. Transpl Int. 2013;26:517-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |