Published online Jun 24, 2016. doi: 10.5500/wjt.v6.i2.356
Peer-review started: October 27, 2015
First decision: December 28, 2015
Revised: January 6, 2016
Accepted: March 9, 2016
Article in press: March 14, 2016
Published online: June 24, 2016
Processing time: 241 Days and 17.5 Hours
AIM: To investigate possible disparities in perioperative morbidity and mortality among different body mass index (BMI) groups and to simulate the impact that these differences might have had on the cohort of patients undergoing cadaveric liver transplantation (LT).
METHODS: All adult recipients undergoing first time LT for benign conditions and receiving a whole graft from brain-dead donors were selected from the united network of organ sharing registry. From January 1994 to June 2013, 48281 patients satisfied the inclusion criteria and were stratified by their BMI. The hypothesis that abnormal BMIs were independent predictors of inferior outcomes was tested with univariate and multivariate regression analyses.
RESULTS: In comparison to normal weight recipients, underweight and morbidly obese recipients had increased 90-d mortality (adjusted OR = 1.737; 95%CI: 1.185-2.548, P = 0.005) (adjusted OR = 1.956; 95%CI: 1.473-2.597, P = 0.000) respectively and inferior patients’ survivals (adjusted HR = 1.265; 95%CI: 1.096-1.461, P = 0.000) (adjusted HR = 1.157; 95%CI: 1.031-1.299, P = 0.013) respectively. Overall, patients’ 5-year survival were 73.9% for normal-weight, 71.1% for underweight, 74.0% for overweight, 74.4% for class I obese, 75.0% for class II obese and 71.5% for class III obese recipients. Analysis of hypothetical exclusion of underweight and morbidly obese patients from the pool of potential LT candidates would have improved the overall survival of the entire cohort by 2.7% (95%CI: 2.5%-3.6%).
CONCLUSION: Selected morbidly obese patients undergoing LT for benign conditions had 5-year survival rates clinically comparable to normal weight recipients. Impact analysis showed that exclusion of high-risk recipients (underweight and morbid obese patients) would not significantly improve the overall survival of the entire cohort of patients requiring LT.
Core tip: Obesity has become a prevalent condition in many part of the world. Yet, evaluation of its impact on patients requiring liver transplantation is limited. Analysis of united network of organ sharing data of 48281 patients undergoing first time cadaveric liver transplantation has shown that, 5-year survival rates for selected underweight and morbidly obese patients were clinically comparable to normal weight recipients as 5-year survival for class III obese recipients was 71.5% vs 73.9% for normal weight patients. Impact analysis showed that exclusion of morbidly obese and underweight recipients would not significantly improve the overall survival of the entire cohort of patients undergoing liver transplant.
- Citation: Ayloo S, Hurton S, Cwinn M, Molinari M. Impact of body mass index on outcomes of 48281 patients undergoing first time cadaveric liver transplantation. World J Transplant 2016; 6(2): 356-369
- URL: https://www.wjgnet.com/2220-3230/full/v6/i2/356.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i2.356
Since 1980, the incidence of obesity in the adult population has more than doubled in many countries[1,2]. Obesity might cause a spectrum of disorders such as non-alcoholic fatty liver disease and non-alcoholic steatohepatitis (NASH) that can lead to cirrhosis and hepatocellular carcinoma[3]. Data from the United States have shown that during the last decade, the indication for liver transplantation (LT) for NASH has risen from 1.2% to 9.7%. Currently it represents the third most common cause of liver failure but it is expected to be the leading indication by year 2025 if the current trends of obesity remain unchanged[4].
Some studies have reported that obese recipients have worse outcomes than normal weight counterparts[4-7]. However, some other investigators did not find any significant differences[8,9]. One of the shortcomings of these studies is the lack of adjustment for known effect modifiers such as coexisting comorbidities. Therefore, the controversy around the issue whether obesity itself is an independent predictor of poorer outcomes after LT still remains. In vision of these conflicting results, we reviewed the outcomes of a large cohort of adult patients who underwent LT in the United States with the intent of assessing if abnormal body mass index (BMI) was an independent predictor for patients’ and grafts’ survival after adjusting for clinical and demographic characteristics selected a priori. Secondary outcomes of this study were to investigate possible disparities in perioperative morbidity and mortality among different BMI groups and to simulate the impact that these differences might have had on the cohort of patients undergoing LT.
Data of this study were extracted from United Network for Organ Sharing (UNOS) Standard Transplant Analysis and Research (STAR) Files that included socio-demographic and clinical variables of every donor and recipient of solid organ transplants performed in the United States during the period between January 1, 1994 and June 30, 2013. For each recipient, BMI was calculated using the formula: Weight (kg)/height (m)[2]. The World Health Organization definitions were used to classify recipients in six categories: Underweight (BMI < 18.5), normal weight (BMI 18.5-24.9), overweight (BMI 25-29.9), class I obese (BMI 30-34.9), class II obese (BMI 35-39.9) and class III obese patients (BMI ≥ 40)[10]. Data for different BMI classes were not adjusted for ascites because the volume of peritoneal fluid drained during LT was not recorded in the STAR Files.
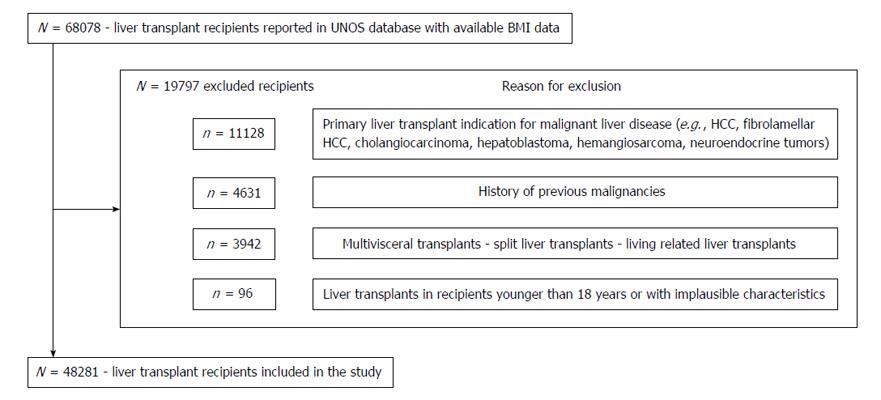
Every adult (age ≥ 18 years) undergoing a LT was considered a potential candidate without restriction of race, citizenship or UNOS region where surgery was performed. Recipients who underwent LT for known primary or secondary liver malignancies (e.g., hepatocellular carcinoma, cholangiocarcinoma, neuroendocrine metastases, etc.) and recipients who had a malignancy found in their explanted livers were excluded to avoid confounders related to the neoplastic nature of their disease. Other exclusion criteria were transplants using grafts harvested from living or non-heart beating donors, split grafts, multivisceral or redo transplants and transplant performed across ABO incompatible blood groups as those recipients had increased risk of non-functioning grafts, perioperative morbidity and mortality. Additional exclusion criteria were missing data on recipients’ weight or height, lack of records on short and long-term outcomes, or the presence of variables that were deemed implausible for an adult recipient[11]. Cutoffs for these variables were: Recipient height ≤ 120 cm or ≥ 240 cm, recipient weight ≤ 30 kg or ≥ 250 kg, BMI ≤ 13 or ≥ 80, cold ischemia time ≤ 1 h or ≥ 24 h and warm ischemia time ≤ 10 min or ≥ 120 min. No imputations of missing data were performed and recipients who had more than 10% of omitted information were excluded.
For the purpose of this study, variables included in the final analysis were recipients’ age at the time of transplant, sex, ethnicity, primary cause of liver disease, height and weight or BMI when available, presence of renal failure requiring hemodialysis before surgery, history of diabetes (type I or II), presence of chronic obstructive pulmonary disease (COPD), hypertension, model for end-stage liver disease score after its implementation in 2002 and beforehand when serum creatinine, bilirubin and INR were available for calculation, perioperative complications, perioperative mortality and overall patients’ and grafts’ survival. Donors’ variables included age, gender, height and weight or BMI if available, primary cause of death and ethnicity. Intra-operative variables included warm ischemia time measured in minutes and cold ischemia times measured in hours.
Recipient overall survival was estimated by calculating the difference between the date of transplantation and the date of death from any cause. Censoring was used for recipients who were still alive at the end of the time interval of this study or who were alive at the time of their last available follow-up or at the time of re-transplantation.
Graft survival was calculated by the difference between the date of transplantation and the date of recipient death or the first date that recorded graft failure or the date when the recipient underwent a redo LT. Perioperative adverse events leading to death were grouped in the following categories: Hemorrhagic (e.g., intraoperative or postoperative bleeding), vascular (either arterial or venous thrombosis), biliary (anastomotic strictures or leaks), infections, acute cellular rejection, cerebrovascular complications (ischemic or hemorrhagic strokes) and primary graft non function defined as irreversible graft function requiring emergency liver replacement within the first 2 wk after LT. The remaining less common complications were categorized as others or unknown if the cause of death was not reported in the UNOS files.
Primary outcomes of this study included patient and graft survival, and secondary outcomes were perioperative causes of morbidity and mortality stratified by recipients’ BMI groups.
Late causes of death (≥ 12 mo after LT) were grouped in the following categories: Infections, cardiopulmonary (e.g., ischemic cardiomyopathy, embolism, insufficiency), renal failure, cerebrovascular events (ischemic or hemorrhagic strokes), malignancies (any type of cancer), graft failure (e.g., recurrent disease or chronic rejection), and hemorrhagic (any cause). The remaining infrequent causes of death were grouped together under the category named “other”, and if there was no recorded cause of death, patients were entered in the group named “unknown”. This study was conducted and reported according to recommendations from the STROBE statement[12] and did not require approval by the local ethic review board.
Sample size of this retrospective analysis was fixed. All variables of first time cadaveric LTs performed over a 19-year period in the United States had been captured in an electronic healthcare database prospectively maintained by UNOS and provided to the authors upon their request.
The cohort was described using estimates of central tendency (means, medians) and spread (standard deviation, interquartile range) for continuous data and frequency and percentages for categorical data.
Etiologies of end stage liver disease (ESLD) were grouped as follows: Hepatitis C virus (HCV), alcohol, alcohol and HCV, other viral hepatitis in combination with HCV, primary sclerosing cholangitis (PSC), primary biliary cirrhosis (PBC), congenital or metabolic diseases (e.g., alpha-1-antitrypsin deficiency, Budd-Chiari syndrome, hemocrhomatosis, polycystic liver disease, etc.), NASH, hepatitits B virus (HBV), autoimmune, acute liver failure and other rare conditions.
The primary end points were overall patient and graft survivals stratified by recipients’ BMI at time of transplant. Kaplan-Meier method was used to calculate survival estimates and analyzed with two-sided log-rank test, with the hazard ratio and two-sided 95%CIs. All hazard ratios (HR) and adjusted HR (AHR) involving patients’ and grafts’ survivals are reported with normal weight recipients as the reference group. The median follow-up time for both patients’ and grafts’ survival were estimated by means and medians of the reverse Kaplan-Meier method. Multiple clinically relevant two-way interactions were evaluated in the multivariable Cox model and included in the final model if significant at a P-value < 0.05. The proportional hazard assumption of the final adjusted model was tested visually by plotting the scaled Schoenfeld residuals of time and BMI, the main predictor of interest. Departure from linearity was assessed by plotting scatterplot smooth curves through residuals[13,14]. Time-dependent covariates such as recipients’ age, which allowed for a change in the hazard ratio over time, were considered and used in the model when appropriate.
To account for the cohort effects, all analyses were adjusted for year of transplantation. Univariate and multivariate Cox regression analyses were performed to test the null hypothesis that recipients’ BMI was a predictor of patients’ and graft survival. Only pre-transplant characteristics were used in the models and all confounders entered in the regression models were selected a priori as they had been shown to be correlated with patients’ and grafts’ survival in earlier studies: Year of transplantation, patients’ and recipients’ characteristics (age, gender, BMI), recipients’ comorbidities (renal insufficiency, diabetes, COPD, hypertension), primary indication for LT, warm and cold ischemia times.
Secondary outcomes were perioperative morbidity and mortality. For these analyses, proportions were compared using the χ2 test and continuous variables were compared using ANOVA test across multiple BMI groups. Perioperative mortality was calculated during the index admission, at 30, 60 and 90 d and at 1-year post LT. Unadjusted and adjusted risk estimates of perioperative mortality were calculated as odds ratios (OR) and adjusted OR (AOR) with 95%CI using logistic regression analysis. Risk estimates were adjusted for patients’ and donors’ BMI (six categories: Underweight, normal weight, overweight, class I obese, class II obese and class III obese), recipients’ and donors’ age (six categories: 18-45, 46-55, 56-65, 66-75, ≥ 76), recipients’ and donors’ sex, year of transplantation, recipients’ comorbidities (four categories: Renal insufficiency requiring dialysis, diabetes type I and II, COPD, hypertension), warm and cold ischemia time, and primary indication for LT (twelve categories: HCV, Alcohol and HCV, HCV and other viral hepatitis, Alcohol, HBV, PSC, PBC, NASH, autoimmune, acute liver failure, congenital or metabolic disease, other).
All statistical analyses were performed using IBM SPSS Statistics version 20 (SPSS Inc. Chicago, IL, United States). Statistical significance was identified by two-tailed P-values of less than 0.05 and 95%CI.
Impact analysis of the potential benefit of allocating grafts to patients with BMIs that had the longest survival and lowest perioperative mortality risk was performed using estimates of central tendency and 95%CI. Microsoft® excel 2008 was used to calculate the overall number and 95%CI of preventable perioperative deaths and the number and 95%CI of life-years that could have been saved by allocation grafts to low-risk recipients.
Among 68078 LT recipients recorded in the UNOS registry, a total of 48281 (70.9%) met eligibility criteria (Figure 1). Of these, 914 (1.89%) were underweight, 14529 (30%) had normal BMI, 16724 (34.6%) were overweight and 16114 were obese (33.3%). Within the group of obese recipients, 9944 (61.7%) were class I obese, 4438 (27.5%) class II and 1732 (10.3%) satisfied class III criteria (Table 1). Demographic and clinical characteristics of the donors are summarized in Table 2.
| WHO BMI classification | P value | |||||||
| Obese recipients | ||||||||
| Variable | Total number of patients (n = 48281) (100%) | Underweight (n = 914) (1.9%) | Normal weight (n = 14529) (30.0%) | Overweight (n = 16724) (34.6%) | Class I (n = 9944) (20.5%) | Class II (n = 4438) (9.2%) | Class III (n = 1732) (3.5%) | |
| Age in years, median (25th, 75th) | 53 (46, 59) | 50 (40, 57.2) | 52 (44, 58) | 53 (46, 58) | 53 (46, 58) | 53 (46, 58) | 52 (47, 58) | 0.000 |
| Gender, n (%) | ||||||||
| Male | 30250 (62.7) | 407 (44.5) | 8573 (59.0) | 11336 (67.8) | 6489 (65.3) | 2609 (58.8) | 836 (48.3) | 0.000 |
| Female | 18.031 (37.3) | 507 (55.5) | 5956 (41.0) | 5388 (32.2) | 3455 (34.7) | 1829 (41.2) | 896 (51.7) | |
| Recipient living status, n (%) | ||||||||
| Alive | 27552 (57.1) | 496 (54.3) | 8040 (55.4) | 9534 (57.1) | 5825 (58.6) | 2642 (59.6) | 1015 (58.6) | 0.000 |
| Dead | 16689 (34.6) | 346 (37.9) | 5123 (35.3) | 5804 (34.7) | 3344 (33.7) | 1471 (33.2) | 601 (34.7) | |
| Lost at follow-up | 3987 (8.3) | 72 (7.9) | 1348 (9.3) | 1370 (8.2) | 765 (7.7) | 317 (7.2) | 115 (6.6) | |
| Race, n (%) | ||||||||
| Non-hispanic white | 36809 (76.2) | 664 (72.6) | 10850 (74.7) | 12820 (76.7) | 7687 (77.3) | 3475 (78.3) | 1313 (75.8) | 0.000 |
| Non-hispanic black | 3962 (8.2) | 92 (10.1) | 1237 (8.5) | 1277 (7.6) | 815 (8.2) | 371 (8.4) | 170 (9.8) | |
| Hispanic | 5535 (11.5) | 73 (8.0) | 1534 (10.6) | 2020 (12.1) | 1191 (12.0) | 500 (11.3) | 217 (12.5) | |
| Asian | 1446 (3.0) | 78 (8.5) | 782 (5.4) | 415 (2.5) | 131 (1.3) | 30 (0.7) | 10 (0.6) | |
| Other | 529 (1.1) | 7 (0.7) | 126 (0.8) | 192 (1.1) | 120 (1.2) | 62 (1.3) | 22 (1.2) | |
| Recipient BMI, median (25th, 75th) | 27.05 (23.8, 31.1) | 17.63 (17.0, 18.1) | 22.73 (21.2, 23.9) | 27.26 (26.0, 28.5) | 32.01 (30.3, 33.3) | 36.85 (35.8, 38.1) | 42.24 (41.1, 44.2) | 0.000 |
| Primary indication for liver transplantation, n (%) | ||||||||
| HCV | 13838 (28.7) | 176 (19.3) | 3538 (24.4) | 5248 (31.4) | 3101 (31.2) | 1302 (29.3) | 473 (27.3) | 0.000 |
| Alcohol | 8111 (16.8) | 163 (17.8) | 2543 (17.5) | 2909 (17.4) | 1686 (17.0) | 622 (14.0) | 188 (10.9) | |
| Idiopathic | 5073 (10.5) | 77 (8.4) | 1179 (8.1) | 1656 (9.9) | 1270 (12.8) | 628 (14.2) | 263 (15.2) | |
| Alcohol + HCV | 3601 (7.5) | 44 (4.8) | 1033 (7.1) | 1370 (8.2) | 762 (7.7) | 310 (7.0) | 82 (4.7) | |
| PSC | 2799 (5.8) | 101 (11.1) | 1396 (9.6) | 903 (5.4) | 282 (2.8) | 92 (2.1) | 25 (1.4) | |
| Congenital/metabolic disease | 2567 (5.3) | 80 (8.8) | 870 (6.0) | 826 (4.9) | 455 (4.6) | 215 (4.8) | 121 (7.0) | |
| PBC | 2485 (5.1) | 92 (10.1) | 1122 (7.7) | 765 (4.6) | 342 (3.4) | 116 (2.6) | 48 (2.8) | |
| NASH | 2247 (4.7) | 13 (1.4) | 226 (1.6) | 585 (3.5) | 660 (6.6) | 522 (11.8) | 241 (13.9) | |
| HBV | 1896 (3.9) | 33 (3.6) | 798 (5.5) | 646 (3.9) | 291 (2.9) | 92 (2.1) | 36 (2.1) | |
| Other | 5664 (11.7) | 135 (14.7) | 1824 (12.5) | 1816 (10.8) | 1095 (11.0) | 539 (12.1) | 291 (14.7) | |
| MELD score, median (25th, 75th) | 21 (16, 28) | 22 (16, 28) | 21 (16, 29) | 20 (15, 28) | 21 (16, 28) | 21 (15, 29) | 22 (16, 31) | 0.000 |
| Cold ischemia time, hours, median (25th, 75th) | 7.0 (5.4, 9.2) | 7.1 (5.3, 9.0) | 7.0 (5.3, 9.1) | 7.0 (5.3, 9.1) | 7.1 (5.4, 9.2) | 7.2 (5.5, 9.3) | 7.3 (5.7, 9.4) | 0.288 |
| Warm ischemia time, minutes, median (25th, 75th) | 44 (34, 55) | 44 (35, 55) | 43 (34, 55) | 44 (35, 55) | 44 (35, 56) | 45 (35, 57) | 41 (31, 50) | 0.000 |
| Waiting time, days (including days on hold), median (25th, 75th) | 120 (27, 335) | 91 (21, 286) | 106 (24, 308) | 124 (28, 336) | 131 (30, 358) | 134 (31, 355.5) | 86 (12, 314) | 0.000 |
| Hospital stay after liver transplant, days, median (25th, 75th) | 11 (8, 19) | 13 (7, 23) | 11 (8, 19) | 11 (8, 18) | 11 (8, 18) | 12 (8, 19) | 13 (9, 22) | 0.000 |
| Preoperative comorbidities, n (%) | ||||||||
| Diabetes type I | 536 (1.1) | 9 (1.7) | 108 (0.8) | 181 (1.1) | 155 (1.6) | 60 (1.4) | 23 (1.3) | 0.000 |
| Diabetes type II | 8541 (18.0) | 81 (9.1) | 1827 (12.9) | 2896 (17.6) | 2179 (22.2) | 1105 (25.2) | 453 (26.6) | |
| Dialysis | 3538 (7.3) | 80 (8.8) | 1059 (7.3) | 1160 (6.9) | 693 (7.0) | 366 (8.2) | 180 (5.1) | 0.000 |
| Hypertension | 4124 (8.5) | 54 (5.9) | 989 (6.8) | 1424 (8.5) | 990 (10.0) | 481 (10.8) | 186 (10.7) | 0.000 |
| Chronic obstructive pulmonary disease | 447 (0.9) | 13 (1.4) | 133 (0.9) | 153 (0.9) | 86 (0.9) | 45 (1.0) | 17 (1.0) | 0.000 |
| WHO recipients’ BMI | P value | |||||||
| Obese recipients | ||||||||
| Donor variable | Total number of donors (n = 48281) (100%) | Underweight (n = 914) (1.9%) | Normal weight (n = 14529) (30.0%) | Overweight (n = 16724) (34.6%) | Class I (n = 9944) (20.5%) | Class II (n = 4438) (9.2%) | Class III (n = 1732) (3.5%) | |
| Age in years, median (25th, 75th) | 40 (24, 53) | 37 (20, 52) | 39 (22, 53) | 40 (24, 53) | 41 (24, 53) | 41 (25, 53) | 41 (25, 54) | 0.000 |
| BMI, median (25th, 75th) | 25.2 (22.3, 29.0) | 23.6 (20.7, 27.1) | 24.6 (21.7, 28.2) | 25.3 (22.3, 29.0) | 25.7 (22.7, 29.8) | 25.9 (23.0, 29.9) | 25.8 (22.8, 30.0) | 0.000 |
| Gender, n (%) | ||||||||
| Male | 29034 (60.1) | 486 (53.2) | 8171 (56.2) | 10219 (61.1) | 6280 (63.2) | 2801 (63.1) | 1077 (62.2) | 0.000 |
| Female | 19247 (39.8) | 428 (46.8) | 6358 (43.8) | 6505 (38.9) | 3664 (36.8) | 1637 (36.9) | 655 (37.8) | |
| Primary cause of death, n (%) | ||||||||
| Anoxia | 6895 (14.3) | 163 (17.8) | 2004 (13.8) | 2337 (33.9) | 1465 (14.7) | 675 (15.2) | 251 (14.5) | 0.040 |
| Cerebrovascular | 19840 (41.1) | 355 (38.8) | 5980 (41.2) | 6869 (41.1) | 4079 (41.0) | 1826 (41.1) | 731 (42.3) | |
| Head trauma | 20273 (42.0) | 372 (40.7) | 6161 (42.4) | 7098 (42.5) | 4110 (41.4) | 1831 (41.3) | 701 (40,5) | |
| Central nervous system tumor | 357 (0.7) | 8 (0.9) | 107 (0.7) | 116 (0.7) | 92 (0.9) | 24 (0.5) | 10 (0.6) | |
| Other | 892 (1.8) | 16 (1.8) | 272 (1.9) | 292 (1.7) | 193 (1.9) | 82 (1.8) | 37 (2.1) | |
| Race, n (%) | ||||||||
| Non-hispanic white | 34907 (72.3) | 639 (69.9) | 10488 (72.2) | 12078 (72.2) | 7196 (72.4) | 3264 (73.5) | 1242 (71.7) | 0.000 |
| Non-hispanic black | 6758 (14.0) | 127 (13.9) | 1938 (13.3) | 2334 (14.0) | 1458 (14.7) | 640 (14.4) | 261 (15.1) | |
| Hispanic | 5118 (10.6) | 109 (11.9) | 1623 (11.2) | 1790 (10.7) | 994 (10.0) | 427 (9.6) | 175 (10.1) | |
| Asian | 885 (1.8) | 28 (3.1) | 299 (2,1) | 309 (1.8) | 162 (1,6) | 55 (1.2) | 32 (1.8) | |
| Other | 673 (1.3) | 11 (1.2) | 241 (1.6) | 213 (1.2) | 134 (1.3) | 52 (1.1) | 22 (1.2) | |
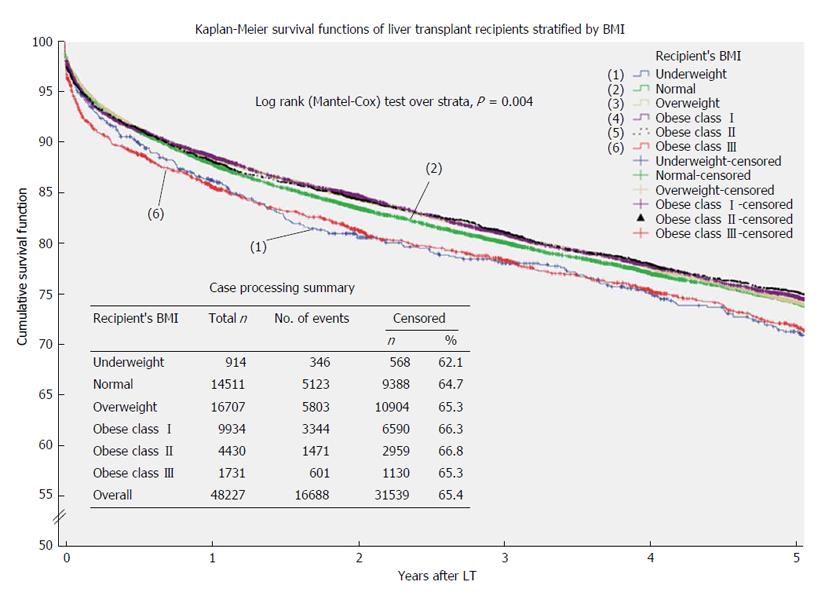
Overall survival: During the study period, 16689 patients (34.6%) died while 31539 were alive or censored. Median overall survival for the entire cohort was 12.7 years (95%CI: 12.5-12.9). Normal weight patients had the longest median survival (13.1 years; 95%CI: 12.6-13.6 years) while the shortest survival was observed in class III obese recipients (11.3 years; 95%CI: 10.3-12.3) and underweight patients (11.5 years; 95%CI: 10.4-12.7) (Table 3).
| Means and medians for survival time (yr) | ||||||||
| Recipient’s BMI | Mean1 | Median | ||||||
| Estimate | Std. error | 95%CI | Estimate | Std. error | 95%CI | |||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| Underweight | 10.8 | 0.3 | 10.2 | 11.4 | 11.5 | 0.6 | 10.4 | 12.7 |
| Normal | 11.6 | 0.1 | 11.4 | 11.7 | 13.1 | 0.2 | 12.6 | 13.6 |
| Overweight | 11.5 | 0.1 | 11.4 | 11.7 | 12.8 | 0.2 | 12.5 | 13.2 |
| Obese-class I | 11.3 | 0.1 | 11.2 | 11.5 | 12.4 | 0.2 | 11.9 | 12.8 |
| Obese-class II | 11.1 | 0.1 | 10.8 | 11.4 | 12.2 | 0.3 | 11.6 | 12.9 |
| Obese-class III | 10.7 | 0.2 | 10.2 | 11.1 | 11.3 | 0.5 | 10.3 | 12.3 |
| Overall | 11.5 | 0.0 | 11.4 | 11.5 | 12.7 | 0.1 | 12.5 | 12.9 |
Kaplan-Meier functions, stratified by recipients’ BMI, are reported in Figure 2. Logrank test showed a significant survival difference across BMI groups (P = 0.004) and pairwise comparisons showed that underweight (P = 0.034) and class III obese patients (P = 0.001) experienced significant lower survivals compared to normal weight counterparts.
At multivariate cox regression analysis, after adjusting for recipients’ and donors’ characteristics (age, gender, BMI, primary cause of end-stage liver disease, comorbidities), cold and warm ischemia times and year of transplantation, underweight status (AHR = 1.265; 95%CI: 1.096-1.461; P = 0.001) and class III obesity (AHR = 1.157; 95%CI: 1.031-1.299; P = 0.013) remained significant predictors for shorter survival in comparison to normal weight recipients (Table 4). On the other hand, being overweight appeared to have modest protective effect (AHR = 0.908; 95%CI: 0.864-0.954; P = 0.000).
| Univariate analysis | Multivariate analysis | |||
| Variable | Hazard rate (95%CI) | P value | Hazard rate (95%CI) | P value |
| Recipient BMI | 0.003 | 0.000 | ||
| Normal weight (reference) | 1 | 1 | ||
| Underweight | 1.125 (1.009-1.255) | 0.034 | 1.265 (1.096-1.461) | 0.001 |
| Overweight | 1.005 (0.968-1.043) | 0.807 | 0.908 (0.864-0.954) | 0.000 |
| Obese class I | 1.024 (0.980-1.070) | 0.284 | 0.947 (0.893-1.004) | 0.067 |
| Obese class II | 1.042 (0.983-1.104) | 0.169 | 0.971 (0.898-1.051) | 0.470 |
| Obese class III | 1.163 (1.069-1.266) | 0.000 | 1.157 (1.031-1.299) | 0.013 |
| Donor BMI | 0.001 | 0.000 | ||
| Normal weight (reference) | 1 | 1 | ||
| Underweight | 0.962 (0.897-1.033) | 0.288 | 1.017 (0.928-1.114) | 0.716 |
| Overweight | 1.060 (1.023-1.098) | 0.001 | 1.009 (0.962-1.057) | 0.717 |
| Obese class I | 1.049 (0.998-1.102) | 0.059 | 0.986 (0.921-1.057) | 0.695 |
| Obese class II | 1.129 (1.045-1.220) | 0.002 | 1.020 (0.912-1.140) | 0.729 |
| Obese class III | 0.988 (0.999-1.112) | 0.988 | 0.889 (0.765-1.034) | 0.128 |
| Recipient age | 0.000 | 0.000 | ||
| 18-45 (reference) | 1 | 1 | ||
| 46-55 | 1.264 (1.212-1.319) | 0.000 | 1.207 (1.143-1.276) | 0.000 |
| 56-65 | 1.536 (1.471-1.603) | 0.000 | 1.490 (1.405-1.580) | 0.000 |
| 66-75 | 2.005 (1.887-2.130) | 0.000 | 2.069 (1.904-2.247) | 0.000 |
| ≥ 76 | 3.224 (2.099-4.951) | 0.000 | 2.476 (1.462-4.194) | 0.001 |
| Donor age | 0.000 | 0.000 | ||
| 0-17 (reference) | 1 | 1 | ||
| 18-45 | 1.107 (1.050-1.166) | 0.000 | 1.066 (0.996-1.141) | 0.066 |
| 46-55 | 1.297 (1.223-1.376) | 0.000 | 1.266 (1.170-1.370) | 0.000 |
| 56-65 | 1.502 (1.411-1.598) | 0.000 | 1.413 (1.300-1.537) | 0.000 |
| 66-75 | 1.706 (1.583-1.840) | 0.000 | 1.609 (1.453-1.782) | 0.000 |
| ≥ 76 | 1.661 (1.448-1.883) | 0.000 | 1.609 (1.340-1.932) | 0.000 |
| Recipient sex (male) | 1.063 (1.030-1.097) | 0.000 | 1.025 (0.979-1.073) | 0.297 |
| Donor sex (male) | 0.951 (0.922-0.980) | 0.001 | 0.967 (0.926-1.008) | 0.967 |
| Cold ischemia time (h) | 1.010 (1.006-1.013) | 0.000 | 1.008 (1.003-1.013) | 0.001 |
| Warm ischemia time (min) | 1.002 (1.001-1.003) | 0.000 | 1.002 (1.001-1.003) | 0.000 |
| Year of transplantation | 0.996 (0.992-0.999) | 0.017 | 0.987 (0.980-0.993) | 0.000 |
| Dialysis | 1.507 (1.422-1.598) | 0.000 | 1.492 (1.367-1.629) | 0.000 |
| Diabetes | 1.406 (1.355-1.460) | 0.000 | 1.314 (1.248-1.383) | 0.000 |
| COPD | 1.384 (1.218-1.573) | 0.000 | 1.250 (1.075-1.454) | 0.004 |
| Hypertension | 1.207 (1.150-1.267) | 0.000 | 1.057 (0.998-1.120) | 0.059 |
| Primary indication | 0.000 | 0.000 | ||
| HCV | 1.356 (1.313-1.400) | 0.000 | 1.429 (1.335-1.530) | 0.000 |
| Alcohol + HCV | 1.214 (1.152-1.281) | 0.000 | 1.477 (1.351-1.616) | 0.000 |
| HCV + other viral hepatitis | 1.093 (0.932-1.283) | 0.274 | 1.342 (1.098-1.638) | 0.004 |
| Other | 1.020 (0.951-1.094) | 0.583 | 1.111 (0.993-1.244) | 0.067 |
| Alcohol | 1.060 (1.018-1.103) | 0.005 | 1.188 (1.102-1.282) | 0.000 |
| HBV | 0.669 (0.613-0.729) | 0.000 | 0.782 (0.691-0.883) | 0.000 |
| PSC | 0.597 (0.554-0.644) | 0.000 | 0.709 (0.634-0.792) | 0.000 |
| PBC | 0.670 (0.623-0.721) | 0.000 | 0.715 (0.641-0.797) | 0.000 |
| NASH | 0.906 (0.821-1.001) | 0.051 | 0.953 (0.783-1.160) | 0.630 |
| Autoimmune | 0.807 (0.742-0.878) | 0.000 | 0.916 (0.810-1.036) | 0.164 |
| Acute liver failure | 0.801 (0.689-0.931) | 0.004 | 1.049 (0.822-1.339) | 0.701 |
| Congenital or metabolic disease | 0.758 (0.703-0.817) | 0.000 | 0.825 (0.736-0.926) | 0.001 |
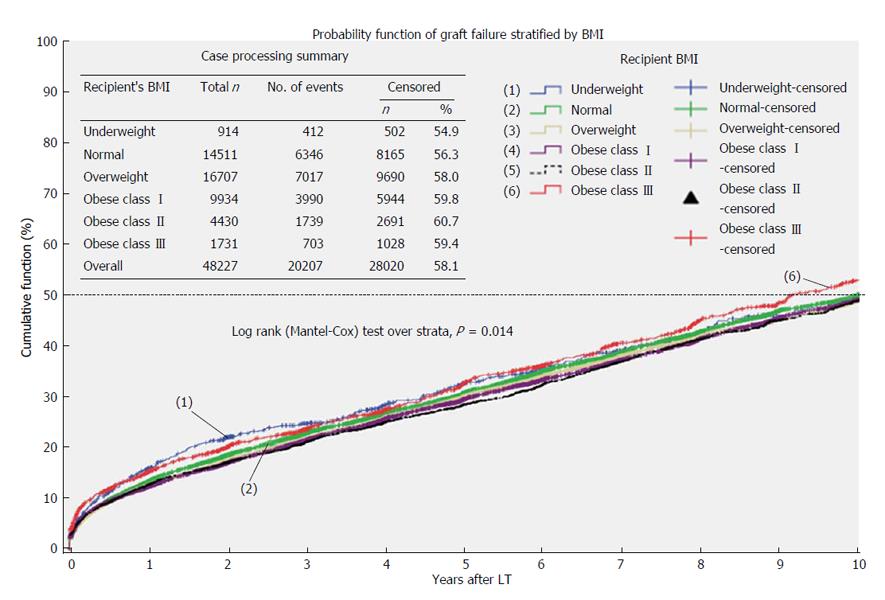
Graft survival: During the study period, 20207 grafts failed (41.9%) and median graft survival was 11.8 years (95%CI: 11.6-12.0) (Table 5).
| Means and medians for graft survival time (yr) | ||||||||
| Recipient’s BMI | Mean1 | Median | ||||||
| Estimate | Std. error | 95%CI | Estimate | Std. error | 95%CI | |||
| Lower bound | Upper bound | Lower bound | Upper bound | |||||
| Underweight | 10.4 | 0.3 | 9.8 | 11 | 11.1 | 0.4 | 10.1 | 12.1 |
| Normal | 11.1 | 0.07 | 10.9 | 11.3 | 12 | 0.2 | 11.6 | 12.5 |
| Overweight | 11.1 | 0.07 | 11 | 11.3 | 12 | 0.1 | 11.6 | 12.4 |
| Obese-class I | 11 | 0.09 | 10.8 | 11.2 | 11.7 | 0.2 | 11.3 | 12.1 |
| Obese-class II | 10.8 | 0.14 | 10.5 | 11.1 | 11.7 | 0.3 | 11.1 | 12.3 |
| Obese-class III | 10.3 | 0.24 | 9.8 | 10.8 | 10.7 | 0.5 | 9.7 | 11.7 |
| Overall | 11 | 0.04 | 11 | 11.1 | 11.8 | 0.1 | 11.6 | 12 |
Figure 3 represents Kaplan-Meier probability functions for graft failure stratified by recipients’ BMI. Underweight (11.1 years; 95%CI: 10.1-12.1, P = 0.034) and class III obese patients (10.7 years; 95%CI: 9.7-11.7, P = 0.001) had significant shorter median survivals when compared to normal weight recipients (12.0 years; 95%CI: 11.6-12.5).
The most frequent causes of graft failure were recipients’ death (60.9%), recurrent disease (4.9%), primary graft non-function (3.5%), infections (3.5%), and unknown reasons (23.2%) (Table 6).
| WHO recipients’ BMI class | P value | |||||||
| Obese recipients | ||||||||
| All recipients (n = 16715) | Underweight (n = 346) | Normal weight (n = 5129) | Overweight (n = 5813) | Class I (n = 3351) | Class II (n = 1473) | Class III (n = 603) | ||
| Primary cause of graft failure | (I) | (II) | (III) | (IV) | (V) | (VI) | ||
| Primary graft non-function | 603 (3.6) | 11 (3.2) | 178 (3.5) | 221 (3.8) | 128 (3.8) | 48 (3.3) | 17 (2.8) | ≥ 0.05 |
| Biliary complications | 89 (0.5) | 2 (0.6) | 18 (0.4) | 34 (0.6) | 23 (0.7) | 6 (0.4) | 6 (1.0) | ≥ 0.05 |
| Vascular thrombosis | 119 (0.7) | 2 (0.6) | 45 (0.9) | 39 (0.7) | 16 (0.5) | 11 (0.7) | 6 (1.0) | ≥ 0.05 |
| Recurrent disease | 829 (4.9) | 14 (4.0) | 240 (4.6) | 306 (5.2) | 172 (5.1) | 75 (5.0) | 22 (3.6) | ≥ 0.05 |
| Acute rejection | 158 (0.9) | 2 (0.6) | 57 (1.1) | 55 (0.9) | 27 (0.8) | 10 (0.7) | 7 (1.2) | ≥ 0.05 |
| Chronic rejection | 270 (1.6) | 7 (2.0) | 104 (2.0) | 82 (1.4) | 50 (1.5) | 17 (1.1) | 10 (1.6) | ≥ 0.05 |
| Infection | 589 (3.5) | 11 (3.2) | 163 (3.2) | 216 (3.7) | 119 (3.6) | 53 (3.6) | 27 (4.5) | ≥ 0.05 |
| Recipient death | 10172 (60.9) | 224 (64.7) | 3107 (60.6) | 3480 (59.9) | 2037 (60.8) | 945 (64.2) | 379 (62.9) | ≥ 0.05 |
| Unknown | 3886 (23.2) | 73 (21.1) | 1217 (23.7) | 1380 (23.7) | 779 (23.2) | 308 (20.9) | 129 (21.4) | ≥ 0.05 |
Cox-regression multivariate analysis showed that underweight status (AHR = 1.315; 95%CI: 1.129-1.531; P = 0.000) and class III obesity (AHR = 1.156; 95%CI: 1.021-1.309; P = 0.022) remained significant predictors for shorter graft survival in comparison to normal weight recipients after adjusting for both recipients’ and donors’ characteristics (age, gender, BMI, primary cause of end-stage liver disease, comorbidities), cold and warm ischemia times and year of transplantation. On the other hand, grafts transplanted in overweight recipients had lower risk of failure with AHR of 0.931 (95%CI: 0.882-0.981; P = 0.008) in comparison to normal weight recipients.
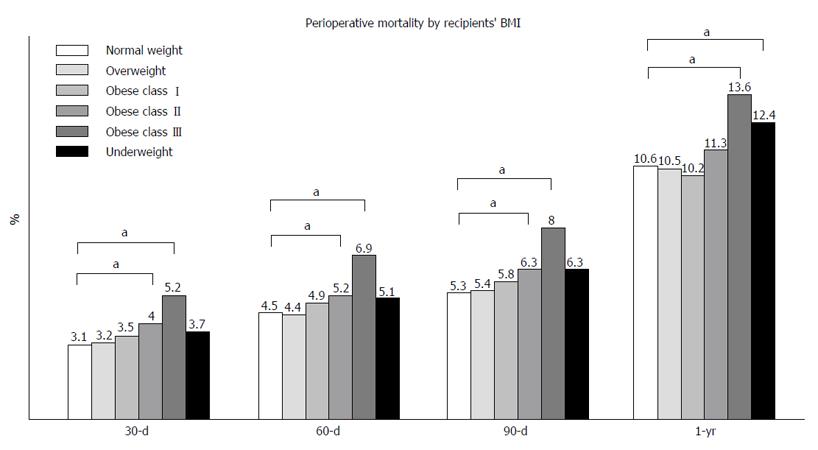
Perioperative mortality: Statistical significant differences in perioperative mortality were identified between normal weight and class II and III obese patients at 30, 60, 90-d and at 1-year after LT (Figure 4).
Analysis of the most common causes of perioperative deaths during the index admission is summarized in Table 7. Overall, in hospital mortality was observed in 4.6% of the entire cohort and sepsis and multiorgan failure represented 31.9% of all causes of death. Comparison across BMI categories showed that only cardiovascular ischemic or embolic events were significantly higher in class II obese patients vs normal weight recipients (0.2% vs 0.05%).
| WHO recipients' MBI class | Group comparisons | P value | |||||||
| Obese recipients | |||||||||
| Primary cause of perioperative mortality | All recipients (n = 48281) | Underweight (n = 914) | Normal weight (n = 14529) | Overweight (n = 16724) | Class I (n = 9944) | Class II (n = 4438) | Class III (n = 1732) | ||
| (I) | (II) | (III) | (IV) | (V) | (VI) | ||||
| Infections or multiorgan failure | 718 (1.4) | 22 (2.4) | 202 (1.3) | 229 (1.3) | 155 (1.5) | 67 (1.5) | 43 (2.4) | ≥ 0.05 | |
| Cerebrovascular complication | 155 (0.3) | 4 (0.4) | 47 (0.3) | 60 (0.3) | 25 (0.2) | 14 (0.3) | 5 (0.2) | ≥ 0.05 | |
| Hemmorrhagic | 128 (0.2) | 3 (0.3) | 38 (0.2) | 41 (0.2) | 32 (0.3) | 11 (0.2) | 3 (0.1) | ≥ 0.05 | |
| Single organ failure | 102 (0.2) | 1 (0.1) | 25 (0.1) | 35 (0.2) | 21 (0.2) | 13 (0.2) | 7 (0.4) | ≥ 0.05 | |
| Intraoperative complications | 75 (0.1) | 2 (0.2) | 30 (0.2) | 21 (0.1) | 12 (0.1) | 4 (0.09) | 6 (0.3) | ≥ 0.05 | |
| Cardiovascular or embolic event | 53 (0.1) | 1 (0.1) | 8 (0.05) | 16 (0.09) | 13 (0.13) | 12 (0.2) | 3 (0.17) | (II) vs (V) | ≤ 0.05 |
| Vascular thrombosis | 22 (0.04) | 0 | 5 (0.03) | 9 (0.05) | 2 (0.02) | 4 (0.09) | 2 (0.1) | ≥ 0.05 | |
| Biliary complication | 2 (0.004) | 0 | 0 | 1 (0.005) | 1 (0.01) | 0 | 0 | ≥ 0.05 | |
| Primary graft non-function | 62 (0.12) | 1 (0.1) | 11 (0.07) | 27 (0.16) | 12 (0.12) | 5 (0.1) | 6 (0.3) | ≥ 0.05 | |
| Rejection | 9 (0.01) | 0 | 3 (0.02) | 3 (0.01) | 2 (0.02) | 1 (0.02) | 0 | ≥ 0.05 | |
| Other causes | 751 (1.5) | 12 (1.3) | 203 (1.3) | 235 (1.4) | 177 (1.7) | 91 (2.0) | 33 (1.9) | ≥ 0.05 | |
| Unknown | 172 (0.3) | 1 (0.1) | 57 (0.3) | 61 (0.3) | 32 (0.3) | 13 (0.2) | 8 (0.4) | ≥ 0.05 | |
| Total | 2249 (4.6) | 47 (5.1) | 629 (4.3) | 738 (4.4) | 484 (4.8) | 235 (5.2) | 116 (6.6) | ||
At multivariate logistic regression analysis, recipients’ BMI category remained a significant predictor for in-hospital, 90 d and 1 year mortality after adjusting for cold and warm ischemia time, donors’ characteristics, primary indication for LT and recipients’ comorbidities (Table 8). Specifically, when compared to normal weight recipients, class III obesity was a predictor for in-hospital mortality (AOR = 1.749; 95%CI: 1.276-2.397; P = 0.001), 90 d mortality (AOR = 1.956; 95%CI: 1.473-2.597; P = 0.000) and 1 year mortality (AOR = 1.458; 95%CI: 1.154-1.842; P = 0.002). Also, being underweight was a risk factor for 90 d mortality (AOR = 1.737; 95%CI: 1.185-2.548; P = 0.005) and 1-year mortality (AOR = 1.505; 95%CI: 1.105-2.048; P = 0.009) while being overweight was protective (AOR = 0.886 at 1-year post LT; 95%CI: 0.792-0.992; P = 0.036).
| Multivariate analysis: In hospital mortality | Multivariate analysis: 90-d mortality | Multivariate analysis: 1-yr mortality | ||||
| Variable | Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value | Odds ratio (95%CI) | P value |
| Recipient BMI | ||||||
| Normal weight | 1 | 1 | 1 | |||
| Underweight | 1.359 (0.865-2.135) | 0.184 | 1.737 (1.185-2.548) | 0.005 | 1.505 (1.105-2.048) | 0.009 |
| Overweight | 0.942 (0.798-1.112) | 0.481 | 0.995 (0.856-1.157) | 0.950 | 0.886 (0.792-0.992) | 0.036 |
| Obese class I | 1.171 (0.974-1.408) | 0.094 | 1.185 (1.000-1.430) | 0.050 | 0.900 (0.792-1.028) | 0.120 |
| Obese class II | 1.135 (0.889-1.450) | 0.309 | 1.197 (0.959-1.495) | 0.112 | 1.004 (0.846-1.193) | 0.960 |
| Obese class III | 1.749 (1.276-2.397) | 0.001 | 1.956 (1.473-2.597) | 0.000 | 1.458 (1.154-1.842) | 0.002 |
| Donor BMI | ||||||
| Normal weight | 1 | 1 | 1 | |||
| Underweight | 1.581 (1.214-2.060) | 0.001 | 1.449 (1.131-1.857) | 0.003 | 1.200 (0.978-1.472) | 0.080 |
| Overweight | 1.025 (0.881-1.194) | 0.746 | 1.008 (0.878-1.158) | 0.907 | 1.025 (0.922-1.140) | 0.647 |
| Obese class I | 0.983 (0.788-1.226) | 0.878 | 1.016 (0.832-1.239) | 0.879 | 1.061 (0.913-1.233) | 0.437 |
| Obese class II | 1.217 (0.878-1.686) | 0.239 | 1.189 (0.880-1.606) | 0.259 | 1.223 (0.973-1.536) | 0.996 |
| Obese class III | 1.013 (0.647-1.585) | 0.955 | 0.952 (0.626-1.448) | 0.817 | 0.999 (0.731-1.365) | 0.996 |
| Recipient age | ||||||
| 18-45 | 1 | 1 | 1 | |||
| 46-55 | 1.305 (1.089-1.563) | 0.004 | 1.324 (1.122-1.583) | 0.001 | 1.302 (1.146-1.479) | 0.000 |
| 56-65 | 1.563 (1.292-1.891) | 0.000 | 1.719 (1.447-2.041) | 0.000 | 1.650 (1.443-1.888) | 0.000 |
| 66-75 | 2.251 (1.737-2.917) | 0.000 | 2.451 (1.941-3.094) | 0.000 | 2.570 (2.146-3.078) | 0.000 |
| ≥ 76 | 5.081 (1.410-18.316) | 0.013 | 6.345 (2.060-20.105) | 0.001 | 2.694 (0.871-8.328) | 0.344 |
| Donor age | ||||||
| 0-17 | 1 | 1 | 1 | |||
| 18-45 | 1.207 (0.961-1.516) | 0.106 | 1.216 (0.988-1.496) | 0.065 | 1.138 (0.968-1.337) | 0.118 |
| 46-55 | 1.492 (1.154-1.929) | 0.002 | 1.411 (1.117-1.784) | 0.004 | 1.464 (1.221-1.755) | 0.000 |
| 56-65 | 1.357 (1.027-1.793) | 0.032 | 1.388 (1.079-1.785) | 0.011 | 1.365 (1.123-1.660) | 0.002 |
| 66-75 | 1.308 (0.929-1.843) | 0.124 | 1.379 (1.014-1.875) | 0.041 | 1.550 (1.230-1.954) | 0.000 |
| ≥ 76 | 0.639 (0.291-1.403) | 0.265 | 0.864 (0.456-1.638) | 0.654 | 1.527 (1.034-2.253) | 0.033 |
| Recipient sex (male) | 0.909 (0.787-1.050) | 0.194 | 0.966 (0.847-1.102) | 0.608 | 1.051 (0.948-1.164) | 0.344 |
| Donor sex (male) | 1.017 (0.888-1.164) | 0.807 | 1.009 (0.892-1.142) | 0.888 | 0.993 (0.904-1.092) | 0.888 |
| Cold ischemia time (h) | 1.033 (1.019-1.047) | 0.000 | 1.021 (1.007-1.035) | 0.003 | 1.024 (1.013-1.035) | 0.000 |
| Warm ischemia time (min) | 1.008 (1.005-1.011) | 0.000 | 1.007 (1.005-1.010) | 0.000 | 1.006 (1.004-1.008) | 0.000 |
| Year of transplantation | 0.987 (0.968-1.006) | 0.182 | 0.970 (0.953-0.987) | 0.000 | 1.003 (0.989-1.016) | 0.686 |
| Dialysis | 2.922 (2.378-3.590) | 0.000 | 2.824 (2.326-3.429) | 0.000 | 2.436 (2.071-2.865) | 0.000 |
| Diabetes | 1.165 (0.990-1.371) | 0.065 | 1.149 (0.992-1.331) | 0.063 | 1.226 (1.095-1.374) | 0.000 |
| COPD | 1.276 (0.803-2.027) | 0.303 | 1.155 (0.996-1.341) | 0.057 | 1.233 (0.883-1.720) | 0.219 |
| Hypertension | 0.957 (0.796-1.151) | 0.642 | 0.959 (0.810-1.135) | 0.624 | 1.027 (0.905-1.166) | 0.680 |
| Primary indication for transplant | ||||||
| HCV | 1.369 (1.114-1.682) | 0.003 | 1.215 (1.049-1.408) | 0.009 | 0.980 (0.847-1.135) | 0.791 |
| Alcohol + HCV | 1.307 (0.976-1.752) | 0.073 | 1.038 (0.808-1.332) | 0.772 | 0.961 (0.788-1.173) | 0.698 |
| HCV + Other viral hepatitis | 1.307 (0.976-1.752) | 0.073 | 0.895 (0.509-1.575) | 0.701 | 0.876 (0.564-1.362) | 0.558 |
| Other | 0.730 (0.549-0.970) | 0.030 | 0.693 (0.544-0.883) | 0.003 | 0.843 (0.677-1.050) | 0.128 |
| Alcohol | 1.380 (1.095-1.740) | 0.006 | 1.172 (0.988-1.391) | 0.068 | 1.366 (1.153-1.618) | 0.000 |
| HBV | 1.230 (0.871-1.737) | 0.239 | 1.263 (0.912-1.747) | 0.159 | 1.326 (1.023-1.719) | 0.033 |
| PSC | 1.749 (1.229-2.489) | 0.002 | 1.608 (1.190-2.172) | 0.002 | 1.777 (1.377-2.293) | 0.000 |
| PBC | 1.989 (1.394-2.837) | 0.000 | 1.834 (1.352-2.487) | 0.000 | 1.754 (1.365-2.252) | 0.000 |
| NASH | 1.564 (0.899-2.722) | 0.113 | 1.043 (0.660-1.649) | 0.857 | 1.298 (0.902-1.869) | 0.160 |
| Autoimmune | 1.179 (0.825-1.686) | 0.365 | 0.879 (0.648-1.191) | 0.405 | 1.027 (0.794-1.329) | 0.840 |
| Acute liver failure | 0.839 (0.462-1.522) | 0.839 | 0.797 (0.450-1.410) | 0.435 | 1.183 (0.712-1.964) | 0.517 |
| Congenital or metabolic disease | 1.139 (0.832-1.522) | 0.563 | 1.094 (0.821-1.458) | 0.540 | 1.162 (0.919-1.469) | 0.211 |
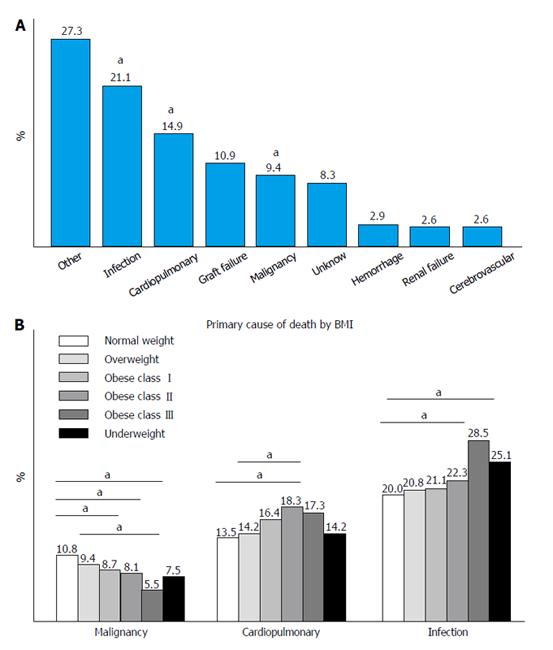
All causes of death: Analysis of all primary causes of mortality after LT is reported in Figure 5. Infections were responsible for 21.1% of all deaths, cardiopulmonary complications for 14.9%, and graft failure for 10.9%. Other main causes of mortality were malignant diseases (9.4%), unknown causes (8.3%) and other less common causes that represented 27.3% of all deaths when grouped together (Figure 5A).
Compared to other BMI groups, class III obese patients died more frequently form infections and cardiopulmonary complications. On the other hand, normal weight and overweight patients experienced a higher rate of malignant diseases (Figure 5B).
Analysis of the hypothetical number of lives that could have been saved within one-year post LT by allocating grafts only to low risk groups (normal weight, overweight and obese class I recipients) was performed using observed values and ranges of this study. If no transplants had been performed for class III obese patients, 55 deaths could have been avoided, 38 if no transplants had been done for class II obese and 18 if no transplants had been done for underweight recipients. These results were equivalent to 2.38% of deaths for the entire cohort.
Analysis of the long-term impact of allocating grafts to underweight recipients showed a potential loss of 1009 life-years (95%CI: 390-1627 years), equivalent to 80 grafts (95%CI: 29-133 grafts). Allocation of grafts to obese class II recipients resulted in a potential loss of 2311 life-years (95%CI: 1690-2932 years) or equivalent to 183 grafts (95%CI: 129-240 grafts). Allocation of grafts to obese class III recipients resulted in a potential loss of 2056 life-years (95%CI: 1319-2793 years) or equivalent to 163 grafts (95%CI: 101-229 grafts). Overall, we estimated that avoiding LT for the two highest risk BMI groups (underweight and class III obese recipients) would have saved 3065 life-years (95%CI: 1710-4421 years) that were equivalent to 243 extra grafts (95%CI: 131-363 grafts).
In the best hypothetical scenario where all the extra-grafts were allocated to patients with the longest median survival (normal weight recipients), the net gain for the entire cohort was 15921 life-years (95%CI: 15375-22754 life-years) that corresponded to a 2.7% (95%CI: 2.5%-3.6%) improvement in overall survival for the entire cohort.
The main findings of this study were that class III obesity and underweight status were associated with higher perioperative mortality and inferior patient and graft survival in comparison to normal weight recipients.
To our knowledge, this is the largest multicentric retrospective observational study on the impact of BMI in LT recipients. One of its strengths is the fact that its sample size allowed us to adjust the analysis of primary and secondary outcomes for several confounders. Our study corroborated the results of several other investigators[4,15-19] but it went against the findings of other groups[8,9,18,20,21] including a recent meta-analysis[9] of 13 studies involving 76620 LT recipients that found that obesity did not impact survival of patients undergoing LT.
In 2008, Segev et al[22] found that in the United States, obese and morbidly obese patients were more likely turned down for a LT in comparison to normal weight candidates. A possible explanation for this is finding that LTs for obese patients can be challenging and require more resources in comparison to recipients with lower BMI indices[6,7,23]. Yet, transplant centers are dealing with obese patients with increasing frequency because obesity is prevalent in many countries[24] and in the contest of insufficient number of grafts, this creates a unique ethical dilemma[25,26]. One of the possible strategies is to deny LT to certain groups of high risk patients based on the utilitarian principle of maximizing results by transplanting only patients who have the best potential outcomes, and to accept the fact that patients who do not receive a LT would have significant shorter lives. In our study, 5-year survival for class III obese recipients was 71.5% vs 73.9% for normal weight patients. Although statistically significant, the absolute difference was clinically irrelevant. Therefore, the exclusion of patients based only on their BMI might be unethical in vision of the fact that 5-year survival of obese and underweight LT recipients was higher than 50% conventionally considered the minimum survival benefit to justify allocation of liver grafts to patients with ESLD[27,28].
One of the most pressing questions we wanted to address in this study was to quantify the impact of abnormal BMIs on the overall survival of the entire cohort of patients waiting for a LT. Therefore, we simulated clinical scenarios where different graft allocation policies were implemented. By excluding underweight and morbidly obese recipients (the two highest-risk categories for perioperative mortality but representing only 5.4% of the entire cohort), an extra 243 grafts (95%CI: 131-363) could have been used to transplant low risk patients. This strategy would result in an overall 5-year survival improvement of 0.5% (95%CI: 0.27%-0.75%) for the entire cohort. The main reasons for this marginal increase were the fact that underweight and class III obese patients represented only a very small percentage of the cohort, and the fact that the absolute difference in median survival between normal weight recipients and class III obese and underweight patients was only 1.8 and 1.6 years respectively. These relatively small differences are most likely due to the fact that LT recipients undergo rigorous cardiopulmonary testing prior to listing, and only the healthiest of the morbidly obese patients are cleared for transplantation with overall acceptable results.
Our study has several limitations. One of the most important is its retrospective design. Therefore, confounders like immunosuppression protocols, surgical skills and pre and postoperative care provided by so many transplant centers could not be controlled in the final analysis. Another main finding of this study was that the proportion of patients who died from malignant diseases was inversely correlated with their BMI. This phenomenon was observed also by Valentijn et al[29] in patients undergoing non-transplant related surgeries where 52% of underweight patients died of cancer-related deaths in comparison to 24% for class III obese. This might be due to different factors (e.g., smoking habits), or to the fact that obese patients might have lower risk of developing cancer[30]. Further investigations are needed to test if obesity is indeed a protective condition against malignancies after transplantation as one of the most important limitations of this study is its retrospective design.
Another limitation was our inability to adjust for the amount of ascites that often affects patients with ESLD. Therefore, the true incidence of obesity might have been overestimated. In addition, we intentionally included recipients transplanted over a long period of time to increase the study population. The advantage of having a large number of patients had to be weighed against the fact that over the study period, there have been significant changes such as immunosuppresssion protocols, perioperative care and patient selection with significant decrease in morbidity and mortality for obese patients undergoing LT during the last ten years. These improvements might have decreased our ability to detect any clinically significant differences in overall survivals across different BMI categories.
Despite these limitations, our study has the strength of including a very large number of patients that allowed us to perform multivariate analyses to test if selected obese patients have significant worse outcomes than normal weight patients after LT. The results suggested that even for very selected class III obese and underweight recipients, perioperative morbidity and mortality are higher than normal-weight recipients. However, these differences are clinically inconsequential as these patients have good long-term outcomes and their exclusion has a minimal survival benefit for the entire cohort of patients waiting for LT. These finding might be of some help to clinicians and policy makers who deal with the ethical dilemma of allocating liver grafts to recipients with abnormal BMI. The biggest challenge ahead of transplant programs remains the selection of those recipients who, despite their abnormal BMI, will have good outcomes and long-term benefit from LT.
Liver transplantation (LT) is the only treatment that can save patients’ lives in the presence of irreversible liver failure. There has been a persistent discrepancy between the number of patients who are waiting for a liver transplant and the number of available livers. Several strategies have been used to increase the number of donors, but despite all the best efforts, a significant proportion of patients affected by end-stage liver disease still die while waiting for a suitable organ. Since organs are limited, the transplant community has used some criteria to prioritize the allocation of livers grafts to patients who are in urgent need of a transplant. The main reason for these criteria is to maximize the benefits and minimize the potential risks associated with such extensive surgeries. One of the emerging controversies in the field of transplantation is the allocation of livers to patients who are obese as they are considered at high risk of developing serious complications that can lead to death after LT. Therefore, there is evidence that obesity might be a negative factor that disadvantages some groups of patients who have lower probabilities of being selected for LT.
The authors’ group analyzed a very large database containing data prospectively collected from patients who underwent LT in the United States to assess if abnormal body mass index (BMI) was a negative predictor for survival after LT. Previous studies, using different databases, had conflicting results and controversy regarding LT, especially for obese patients, still persists.
This paper found that, although underweight and morbid obese patients had increased risks for perioperative complications and lower long term survival in comparison to normal weight recipients of liver transplants, the absolute differences were clinically negligible. In addition, impact analysis revealed that exclusion of high risk patients from undergoing LT did not improve the overall results for the entire group of patients who needed a LT.
Selected obese and underweight patients affected by end-stage liver disease should not be excluded from LT as their overall outcomes are clinically comparable to normal weight patients.
BMI is the ratio between a person’s stature and respective weight. In most cases, the higher is the BMI, the higher is the concentration of fat in the body. Persons with BMI higher than 30 are considered obese and individuals with BMI higher than 40 are considered morbidly obese. Obesity is associated with increased risks for metabolic derangements such as diabetes, hypertension, hypercholesterolemia and atherovascular diseases. Because of this association, obese patients are considered at higher risk of developing cardiopulmonary complications after LT and they absorb more resources when undergoing complex surgical interventions like LT.
This is a large retrospective study to attempt to answer if BMI affect outcomes of liver transplant patients. The study is well designed, performed, and written.
P- Reviewer: Chiu KW, Marino IR, Qin JM, Xia V S- Editor: Qiu S L- Editor: A E- Editor: Liu SQ
| 1. | Popkin BM, Adair LS, Ng SW. Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev. 2012;70:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2709] [Cited by in RCA: 2392] [Article Influence: 184.0] [Reference Citation Analysis (0)] |
| 2. | World Health Organization. Obesity and overweight. [Accessed 2015 Jan]. Available from: http//www.who.int/mediacentre/factsheets/fs311/en. |
| 3. | Agopian VG, Kaldas FM, Hong JC, Whittaker M, Holt C, Rana A, Zarrinpar A, Petrowsky H, Farmer D, Yersiz H. Liver transplantation for nonalcoholic steatohepatitis: the new epidemic. Ann Surg. 2012;256:624-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 184] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 4. | Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 835] [Cited by in RCA: 855] [Article Influence: 61.1] [Reference Citation Analysis (0)] |
| 5. | Hillingsø JG, Wettergren A, Hyoudo M, Kirkegaard P. Obesity increases mortality in liver transplantation--the Danish experience. Transpl Int. 2005;18:1231-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Nair S, Verma S, Thuluvath PJ. Obesity and its effect on survival in patients undergoing orthotopic liver transplantation in the United States. Hepatology. 2002;35:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 7. | Hakeem AR, Cockbain AJ, Raza SS, Pollard SG, Toogood GJ, Attia MA, Ahmad N, Hidalgo EL, Prasad KR, Menon KV. Increased morbidity in overweight and obese liver transplant recipients: a single-center experience of 1325 patients from the United Kingdom. Liver Transpl. 2013;19:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Leonard J, Heimbach JK, Malinchoc M, Watt K, Charlton M. The impact of obesity on long-term outcomes in liver transplant recipients-results of the NIDDK liver transplant database. Am J Transplant. 2008;8:667-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Saab S, Lalezari D, Pruthi P, Alper T, Tong MJ. The impact of obesity on patient survival in liver transplant recipients: a meta-analysis. Liver Int. 2015;35:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Lai JC, Feng S, Roberts JP, Terrault NA. Gender differences in liver donor quality are predictive of graft loss. Am J Transplant. 2011;11:296-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. PLoS Med. 2007;4:e297. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2871] [Cited by in RCA: 3484] [Article Influence: 193.6] [Reference Citation Analysis (0)] |
| 13. | Vittinghoff EGD, Shiboski S, McCulloch C. Regression Methods in Biostatistics: Linear, Logistic, Survival and Repeated Measures Models. New York, NY: Springer Science Business Media 2005; . |
| 14. | Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233-2247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 373] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 15. | Nair S, Cohen DB, Cohen MP, Tan H, Maley W, Thuluvath PJ. Postoperative morbidity, mortality, costs, and long-term survival in severely obese patients undergoing orthotopic liver transplantation. Am J Gastroenterol. 2001;96:842-845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 106] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | LaMattina JC, Foley DP, Fernandez LA, Pirsch JD, Musat AI, D’Alessandro AM, Mezrich JD. Complications associated with liver transplantation in the obese recipient. Clin Transplant. 2012;26:910-918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 17. | Braunfeld MY, Chan S, Pregler J, Neelakanta G, Sopher MJ, Busuttil RW, Csete M. Liver transplantation in the morbidly obese. J Clin Anesth. 1996;8:585-590. [RCA] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Perez-Protto SE, Quintini C, Reynolds LF, You J, Cywinski JB, Sessler DI, Miller C. Comparable graft and patient survival in lean and obese liver transplant recipients. Liver Transpl. 2013;19:907-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 19. | Conzen KD, Vachharajani N, Collins KM, Anderson CD, Lin Y, Wellen JR, Shenoy S, Lowell JA, Doyle MB, Chapman WC. Morbid obesity in liver transplant recipients adversely affects longterm graft and patient survival in a single-institution analysis. HPB (Oxford). 2015;17:251-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 20. | Pelletier SJ, Maraschio MA, Schaubel DE, Dykstra DM, Punch JD, Wolfe RA, Port FK, Merion RM. Survival benefit of kidney and liver transplantation for obese patients on the waiting list. Clin Transpl. 2003;77-88. [PubMed] |
| 21. | Singhal A, Wilson GC, Wima K, Quillin RC, Cuffy M, Anwar N, Kaiser TE, Paterno F, Diwan TS, Woodle ES. Impact of recipient morbid obesity on outcomes after liver transplantation. Transpl Int. 2015;28:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 22. | Segev DL, Thompson RE, Locke JE, Simpkins CE, Thuluvath PJ, Montgomery RA, Maley WR. Prolonged waiting times for liver transplantation in obese patients. Ann Surg. 2008;248:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Siegel AB, Lim EA, Wang S, Brubaker W, Rodriguez RD, Goyal A, Jacobson JS, Hershman DL, Verna EC, Zaretsky J. Diabetes, body mass index, and outcomes in hepatocellular carcinoma patients undergoing liver transplantation. Transplantation. 2012;94:539-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 24. | Meulenbroek RA, Sargent KL, Lunde J, Jasmin BJ, Parks RJ. Use of adenovirus protein IX (pIX) to display large polypeptides on the virion--generation of fluorescent virus through the incorporation of pIX-GFP. Mol Ther. 2004;9:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238-1245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3857] [Cited by in RCA: 3383] [Article Influence: 161.1] [Reference Citation Analysis (0)] |
| 26. | Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, Marks JS. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3786] [Cited by in RCA: 3674] [Article Influence: 167.0] [Reference Citation Analysis (0)] |
| 27. | Neuberger J, James O. Guidelines for selection of patients for liver transplantation in the era of donor-organ shortage. Lancet. 1999;354:1636-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 128] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 28. | Freeman RB, Jamieson N, Schaubel DE, Porte RJ, Villamil FG. Who should get a liver graft? J Hepatol. 2009;50:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 29. | Valentijn TM, Galal W, Hoeks SE, van Gestel YR, Verhagen HJ, Stolker RJ. Impact of obesity on postoperative and long-term outcomes in a general surgery population: a retrospective cohort study. World J Surg. 2013;37:2561-2568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Bays H, Rodbard HW, Schorr AB, González-Campoy JM. Adiposopathy: treating pathogenic adipose tissue to reduce cardiovascular disease risk. Curr Treat Options Cardiovasc Med. 2007;9:259-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |