Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.69
Peer-review started: August 27, 2015
First decision: September 28, 2015
Revised: November 2, 2015
Accepted: December 18, 2015
Article in press: December 20, 2015
Published online: March 24, 2016
Processing time: 206 Days and 22.9 Hours
At present, proven clinical treatments but no cures are available for diabetes, a global epidemic with a huge economic burden. Transplantation of islets of Langerhans by their infusion into vascularized organs is an experimental clinical protocol, the first approach to attain cure. However, it is associated with lifelong use of immunosuppressants. To overcome the need for immunosuppression, islets are encapsulated and separated from the host immune system by a permselective membrane. The lead material for this application is alginate which was tested in many animal models and a few clinical trials. This review discusses all aspects related to the function of transplanted encapsulated islets such as the basic requirements from a permselective membrane (e.g., allowable hydrodynamic radii, implications of the thickness of the membrane and relative electrical charge). Another aspect involves adequate oxygen supply, which is essential for survival/performance of transplanted islets, especially when using large retrievable macro-capsules implanted in poorly oxygenated sites like the subcutis. Notably, islets can survive under low oxygen tension and are physiologically active at > 40 Torr. Surprisingly, when densely crowded, islets are fully functional under hyperoxic pressure of up to 500 Torr (> 300% of atmospheric oxygen tension). The review also addresses an additional category of requirements for optimal performance of transplanted islets, named auxiliary technologies. These include control of inflammation, apoptosis, angiogenesis, and the intra-capsular environment. The review highlights that curing diabetes with a functional bio-artificial pancreas requires optimizing all of these aspects, and that significant advances have already been made in many of them.
Core tip: Replacing standard insulin therapy for patients with type I diabetics with a cell-based cure is yet to be achieved. Assuming unlimited supply of beta cells, allogeneic or xenogeneic cells should be separated from the host immune system by a permselective membrane that still allows insulin egress. In addition, a mandatory requirement for such a cure in a poorly oxygenated environment includes adequate oxygen supply. In addition, to optimize islet functionality, control over inflammation, cell apoptosis, angiogenesis, and the close environment of the transplanted cells must be accomplished.
- Citation: Barkai U, Rotem A, de Vos P. Survival of encapsulated islets: More than a membrane story. World J Transplant 2016; 6(1): 69-90
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/69.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.69
Diabetes is considered an epidemic with global prevalence of 9% [based on World Health Organization (WHO) data from 01/2015] and a huge economic burden[1]. Type I diabetes, consists of 10% of the total diabetic population. Prevalence of clinical diabetes is predicted to double in the next 20 years[2].
Transplantation of cadaveric islets of Langerhans (IOL) by their infusion into vascularized organs, preferentially the liver, is an experimental clinical protocol which was first established in Edmonton in 2000[3]. Since then, 2000 allogeneic transplantations are estimated to have been performed worldwide. A report published by the Collaborative Islet Transplant Registry at the end of 2013 summarized clinical data from 864 such recipients[4]. Despite the promise, clinical application of islet transplantation is limited due to short organ supply, inefficient use of organs (approximately 2.5 donors are required per recipient), low reproducibility of quantity and quality of the isolated IOL, and the obligatory use of life-long immunosuppressive therapy. Thus, the current global research focuses on resolving all bottlenecks in the pathway to successful clinical application. These include addressing the limited supply of β-cells by using juvenile/adult porcine IOL[5-8] and β-cells derived from renewable sources (e.g., stem cells[9-11]); development of efficient and reproducible protocols for isolating donor IOL[12-14]; and development of efficient encapsulation technologies in order to allow immunosuppression-free procedures. These encapsulation approaches, which include macro, micro, and nano-encapsulations were tested in animal models and a few clinical trials (for reviews, see[15-18]). To date, the least developed niche in the IOL transplantation approach is the use of active oxygen supply and auxiliary technologies to provide “friendly microenvironment” to the transplanted islets.
This article reviews the various aspects related to optimizing cell-based curing product for diabetes and highlights the achievements made to date.
For clinical islet transplantation, systemic administration of immunosuppressive drugs has remained the foundation for preventing graft rejection. However, chronic immunosuppressive therapy is associated with loss of islet mass as well as with significant risk for higher rates of malignancies and opportunistic infections. The risk of these serious side effects is inherent, as it is currently impossible to block rejection of foreign tissues without simultaneously suppressing necessary immune functions. Cell encapsulation is an alternative technology. It creates a passive barrier between the implanted graft and the hostile immune system using a permselective membrane. The membrane must be discriminative in terms of molecular diffusivity, allowing for free ingress and egress of low molecular-weight nutrients such as glucose, amino acids, and insulin. Diffusion of small molecules, such as oxygen, glucose, and L-tryptophan, has been shown to be only marginally affected by hydrogel like alginate and agarose[19-25]. At the same time, the permselective membrane must create impassable barrier for host immune effectors in order to efficiently prevent graft rejection. The immune system uses plethora of mechanisms to reject grafts, most of them are dependent on cell-to-cell contacts and effector macromolecules. Therefore, diffusion resistance constitutes the foundation of all immunoisolation strategies.
The cellular arm of the immune rejection is mediated by cytotoxic T-cells and the process requires direct representation of donor MHC class I molecules to recipient CD8 T cells. This mechanism, however, has only a minor impact on encapsulated grafted cells because the membrane physically separates donor cells from recipient cells[26].
Humoral rejection does not require cell-to-cell contact and is operable via mechanisms activated by the indirect recognition pathway. Antibody-complement mediated rejection is a major contributor to this pathway. A cascade of biochemical reactions, termed the complement cascade, follows the binding of either IgG or IgM paratopes to their matching epitopes. Eventually, this cascade leads to the formation of membrane attack complexes (MAC), which are 100-nm diameter transmembrane channels characterized by a hydrophilic internal surface. MACs are integrated across the cell plasma membrane thus allowing for free 2-way passage of water and molecules. Loss of essential differential concentrations of ions between the intra- and extracellular compartments is fatal and induces necrosis (e.g., as demonstrated by Papadimitriou et al[27]). With respect to this type of rejection, the merit of inserting a separating membrane between the donor and recipient depends on the permeability indices of the membrane, the dimension of the solutes, and their hydrodynamic radius (RH). IgG (a pivotal activator of the complement cascade), IgM, C1q (the rate-limiting activator of the complement cascade), and transferrin (a molecular chaperone transporting iron to the graft), vary in their molecular dimensions (Table 1)[28-38]. In order to concomitantly prevent damage to encapsulated cells and allow essential nutrition, the permselective membrane should permit free diffusion of molecules with RH < 4 nm (i.e., molecular chaperones such as transferrin) while preventing ingress of molecules with RH≥ 12 nm (i.e., IgM, C1q). Notably, even if the intermediate size IgG passes the membrane, it is an inefficient cell killer on its own.
| Effector | Molecular weight, kDa | Crystal dimensions, nm | Hydrodynamic radius, nm | Ref. |
| IgG | 150 | 15 × 6 × 2 | 5.4 | [32-36] |
| IgM | > 900 | 30 × 13 | 12.7 | [35,213] |
| C1q | > 400 | 30 × 33 | 12.8 | [28-31] |
| Transferrin | 80 | 5 × 10 | 3.7 | [37,38] |
The third path to rejection involves inflammation-type reactions. Surgical incision, preceding any type of graft implantation damages the vascular bed and irritates the tissue, while insertion of any artificial device into an interior site enhances the magnitude of this reaction. The process induces inflammatory responses immediately. These are manifested by cross activation of immune cells of the innate system (neutrophils, basophils, and macrophages[39]). Once activated, these cells release bioactive cytokines[40-42] in the vicinity of the graft that aim to heal the wound. However, some of these cytokines are destructive to the grafted cells. Indeed, studies in a model of syngeneic islet transplantation demonstrated that damage to islet grafts is primarily due to nonspecific inflammatory response[43,44]. This effect is aggravated when allotype or xenotype islets are being transplanted. Although the inflammation lasts less than 2 wk, up to 60% of islet cells may be lost in this timeframe[45].
The 3 major effectors that damage islets include: Interleukin (IL)-1β, interferon (INF)-γ, and tumor necrosis factor (TNF)-α[46-52]. These cytokines also play a major role in the neutrophils-macrophage activation cascade. Their apparent molecular masses differ (17 kDa for IL-1β, 47 kDa for dimeric glycosylated INFγ and 52 kDa for trimeric TNF-α); however, their RH are similar (2.2, 3.1, and 3 nm, respectively)[53,54]. This range of radii is well below the minimal threshold required for immunoisolating membranes (12 nm), but is close to the RH value of transferrin. Therefore, reducing the size of membrane pores to approximately 4 nm, and the fact that the pores are geometrically inhomogeneous may attenuate ingress of the pro-inflammatory cytokines TNF-α and INF-γ but at the expense of transferrin. Still, no permselective membrane can prevent IL-1β diffusion. In summary, based on pore size only, permselective membranes are effective against cell-mediated and complement-mediated cytotoxicity; however, they are less helpful against harmful cytokines.
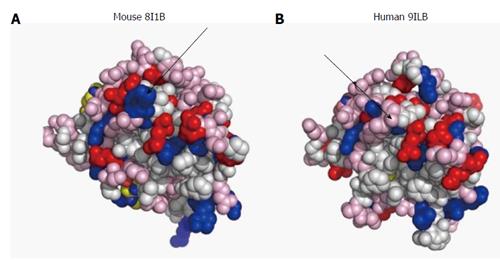
Besides pore size, the physical makeup of permselective membranes also affects their permeability properties. In water, diffusion of a solute is a process of random movement of molecules across concentration gradient and is quantitatively portrayed by a diffusion coefficient. In a typical hydrogel, the void volume is > 95%; however, diffusion of a solute across a hydrogel is not determined solely by its diffusion coefficient. Permeability of larger molecules is also controlled by slow transfer across lengthy path of traversing pores, hydrodynamic drag on the moving solute, and polar or hydrophobic interactions between the membrane material and the traversing macromolecule. Crosslinking of acidic alginate polymers by divalent ions creates an “eggs-in-a-box” hydrogel scaffold that is never saturated by the divalent cross-linker. Therefore, under physiological environment (pH = 7.35), alginate hydrogel is negatively charged in its core and even more at the exposed surfaces. Proteins usually have hydrophobic core and hydrophilic surfaces. Therefore, electrical repulsion between negatively-charged domains on protein surfaces and the exterior of the hydrogel is expected[55] and may play a role in selective permeability of polypeptides. This hypothesis could be tested for IL-1β, the most devastating interleukin. This cytokine, despite extensive sequence homology and similar biological activity, has a range of isoelectric points (pI) across species. On one side, porcine IL-1β (NP_001005149.1) has an acidic pI of approximately 5.5, whereas rat IL-1β (NP_113700) is characterized by a basic pI (> 8.5). Local surface charges may also make a difference. The exposed amino acid shells of human (PDB 9ILB; pI = 5.92) and mouse (PDB 8I1B; pI = 8.30) IL-1β shown in Figure 1 clearly demonstrate enhanced electronegativity of the human compared with the murine molecule. Therefore, the transfer rate of these cytokines across alginate hydrogels may provide insights into the role of electrical charges in differential permeability, and may help in the design of better protecting membranes.
Concentration of local cytokines is a balance between synthesis and degradation at inflammation sites. Proteolysis of IL-1β is controlled by a plethora of matrix metalloproteinases (e.g., as described by Ito et al[56]). In addition, a group of serine proteases (e.g., cathepsin G and elastase) are capable of cleaving nearly all proteins in an unspecific manner. Most cytokines contain many cleavage sites for serine proteases. Activated macrophages and neutrophils, major producers of these proteases, co-localize with inflammatory cytokines at implantation sites. As such, direct restrictive effect of proteases on the lifetime of cytokines is envisaged and was shown for TNF-α which is rapidly degraded by supernatant of activated neutrophils and by elastase[57,58]. Some membrane design, including those with extended width of the membrane, has been shown to partially protect encapsulated cells against cytokines[59-62]. Therefore, attenuation of ingress of cytokines may expose them to enhanced degradation by resident proteases thereby reducing the necessity to completely prevent their ingress.
Following islet transplantation, nitric oxide (NO) and reactive oxygen species (ROS) are released by cells of the innate immunity, responding to the insult[63,64]. Working independently or as effectors of IL-1β, they contribute to the loss of functionality and viability of encapsulated islets soon after implantation[65-68]. Likewise, hydrogen peroxide, an abundant ROS, impairs glucose-induced insulin secretion in β-cells[69,70]. ROS are constantly produced in living systems but are kept by homeostatic mechanisms at relatively low levels. Upon transplantation of IOLs, this balanced state is deranged. Oxidative stress is much enhanced, but is not countered by efficient antioxidant machinery as islets contain ineffective antioxidant protection system. Consequently, transplanted islets are prone to destruction by NO and ROS[71-74].
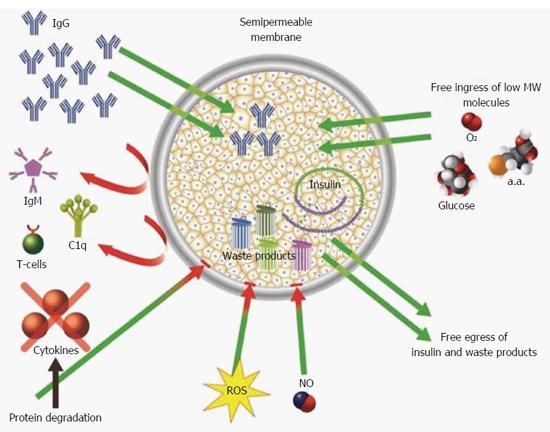
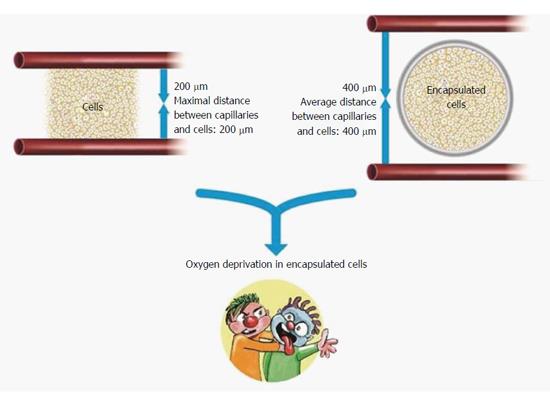
Due to their miniaturized molecular dimension, none of the permselective membranes can prevent free passage of NO and ROS. This inherited challenge may be solved using a different approach. It is based on the short half-lives of these molecules (seconds for NO and even shorter for ROS), and consequently their limited radii of effectiveness (approximately 200 μm for NO and < 100 μm for ROS)[75-77]. Thus, increasing the distance between the cells that are generating ROS and NO and the transplanted islets may decrease the deleterious effect of the formers. Figure 2 summarizes proven and putative mechanisms by which permselective membrane protect grafted cells from the host immune system.
In order for the separating membrane to be functional, it should also protect the graft without impacting the viability/functionality of the grafted cells, be biocompatible to the host, flexible, and mechanically stable. Collectively, immune barrier could replace immunosuppressive therapy only when the size of the graft is small and internal re-vascularization is not mandatory for its proper function (e.g., IOLs).
Several strategies for islet microencapsulation were developed to protect grafted islets from the host immune system. These are described in several excellent review articles[15,18,45,78-81]. This paper focuses on retrievable devices, for which hollow fiber and flat geometry configurations are practical solutions.
Two major classes of natural polymers are being used for cell encapsulation: Polysaccharides and polypeptides. Polysaccharides gained widespread use because they are simple to use, allow hydrogel formation under mild conditions (gentle heat or presence of divalent cations), and because they do not interfere with cell viability and functional performance. Alginate, the most studied polymer, which was tested in many animal models and even in clinical trials (for example, see Matsumoto et al[5]), is the leading biomaterial for cell encapsulation. Other polysaccharides are also being used (e.g., chitosan, agarose, and cellulose). Alginate is a natural product mainly extracted from seaweeds. It is chemically composed of two monomers: Guluronic (G) and mannuronic (M) acid. These form linear polymers with a wide distribution of molecular masses, different ratios of G to M, and various combinations of homo- and hetero-polymer blocks. Therefore, inter-lot variability in the chemical composition of the polymer is inevitable. This variability is an advantage for facilitating selection of an optimal variation of the polymer but once chosen, it presents a disadvantage, as the specific chemical composition of every alginate lot is unique. Currently, no practical method for producing lots with identical chemistry exists. Only 3 variables in the final makeup of an alginate hydrogel are controllable: The G to M ratio, dry matter composition and the type/concentration of the divalent cation used for crosslinking. To a minimal extent, physical parameters of the final hydrogel (e.g., viscosity) can be adjusted by varying these parameters. At present, the field of alginate-based cell encapsulation is in urgent need for an industrialized source of controlled and reproducible raw material. A group of epimerase enzymes[82-84], converting G to M, thus providing tailor-made alginates form the first step in addressing this critical need.
Agarose has also been tested as an encapsulating hydrogel for cells. Its use for islet encapsulation started in the late 80’s[85,86] and was subsequently broadened[87-91]. Other natural polysaccharides used for encapsulation of cells/islets include chitosan and cellulose. The data generated for chitosan as an encapsulating hydrogel are limited and chitosan is usually formulated as part of a more complex membrane that also includes alginate or methacrylated glycol[92-94]. Also, its application is rather limited because it binds crosslinking molecules at acidic pH and does not bind them at physiological pH[95]. Cellulose was also tested for encapsulation; however, it never reached animal testing[96,97]. PharmaCyte Biotech, Inc. (Silver Spring, MD) is planning to use cellulose sulfate and polydiallyldimethyl ammonium chloride, known as “Cell-in-a-Box®” as an immune barrier for β-cell transplantation. Chitosan and cellulose were both found to be inferior to agarose and alginate (reviewed by de Vos et al[45]).
In 1996, French scientists published a design of a planar bioartificial pancreas (BAP) that used a synthetic membrane developed for dialysis of blood (AN69) to create an immune barrier between grafted islets and the host immune system. Normoglycemia of diabetic mice implanted with this device lasted 30 d[98]. A variation of this membrane is now a part of a new medical device, MAILPLAN (Defymed; Strasbourg, France), which is scheduled to start clinical trials in 2016. No preclinical data supporting this claim have been published so far. In 2001, Islet Sheet Medical (San Francisco, CA) presented an advanced planar BAP generated by encapsulating donor islets in a thin sheet of alginate[99]. At a dose of approximately 10000 islet equivalent (IEQ)/kg, a diabetic dog was cured for 84 d. Five years later, a Belgian group reported six-month normoglycemia in diabetic Cynomoglus monkeys[100]. Xenotype islets were encapsulated in a planar monolayer cellular device consisting of 2-sided collagen matrix enveloped in 3% (w/v) high mannuronic acid alginate (US patent 2008/0050417).
TheraCyte Inc. (Laguna Hills, CA) also attempted to macroencapsulate islets in a minimally invasive device based on technology developed by Baxter Healthcare (Round lake, IL)[101]. It is a robust, mesh-supported, and retrievable planar device consisting of a 3-layer membrane. An outer layer of woven polyester mesh supports a 5 μm pore size polytetrafluoroethylene (PTFE) leaf and an inner PTFE leaf with nominal pore size of 0.45 μm[102]. The 3-layer approach is designed to allow for development of dense vascularization on the outer part of the membrane in order to reduce the diffusion distance of nutrients and waste products from the vascular bed and the encapsulated cells. The most inner leaf of this structure is supposed to create an immune barrier between the graft and the host immune system although the nominal pore size seems to be inadequate for this purpose. Rat islets implanted within this device were functional for 4 wk in immunocompromized mouse recipients[103], for > 6 mo in allogeneic rat recipients[104] and for 30 d in a mouse model resembling autoimmune diabetes[105]. Also, reversal of diabetes for a 16-wk period was reported when neonatal porcine islets were transplanted subcutaneously in nonobese diabetic mice[106]. Successful reversal of diabetes by this device is currently limited to rodent recipients. Data on successful transplantation of donor islets into larger animal models are limited. Nonetheless, the device was transplanted in non-human primates, including a 3-mo trial with xenogeneic porcine islets[106], and up to 12-mo trial with allogeneic NHP islets[107]. However, cell doses in these studies were minimal (substantially below curing doses). ViaCyte, Inc. (San Diego, CA) is using a modified TheraCyte membrane (Encaptra) as an immune barrier in order to protect stem cells-derived β-cells from the host immune system. Preclinical data on the efficacy of Encaptra as an immune barrier are yet to be published, but the company launched a phase I/II clinical trial in September 2014 (NCT 02239354). Practically, neovascularized devices are not easily retrievable because of bleeding and hematoma[108].
A quite different macroencapsulation method was developed at the Rogosin institute (Xenia, OH)[90,91]. Donor islets are encapsulated in double layer agarose macrobeads; a 5% external agarose film functions as the immune barrier. Using this method, porcine islets were shown to lower blood glucose in diabetic rats and reduce their insulin requirements for > 6 mo[91,109]. Similar results were obtained when porcine islets encapsulated in these macrobeads were implanted into diabetic dogs; however, no complete remission of diabetic state was evident even with high islet dose[110,111]. This macroencapsulation technology is currently awaiting regulatory approval for initiating Phase I studies.
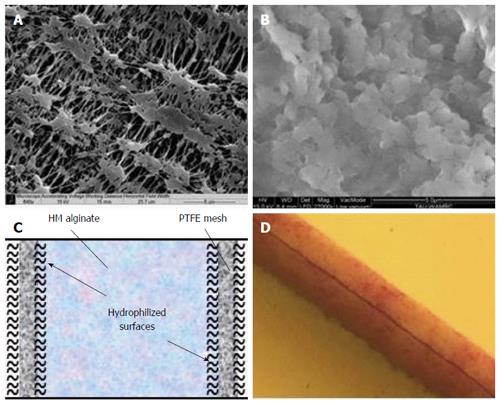
Beta O2 Technologies (Rosh Ha’ayin, Israel) developed the β-Air device which includes a composite membrane serving as an immune barrier (Figure 3). This barrier includes 2 (25 μm each) hydrophilized PTFE membranes with pore size of 0.45 μm, similar to the inner leaf of the TheraCyte membrane. High viscous high mannuronic (HM) acid alginate (G = 0.46) at 6% (w/v) is impregnated into the membrane pores using mild vacuum[112]. The β-Air composite membrane is strong but quasi-flexible. It does not allow host cells to permeate into the device (e.g., CD3 cells; Barkai et al, unpublished data), and is also impermeable to viruses, C1q and IgG molecules, while allowing free diffusion of glucose and insulin both inwards and outwards[112].
The vasculature of the pancreas consists of a complex network differentially adopted for the distinctive needs of the endocrine and exocrine parts of the organ. Pancreatic islets possess an autonomous mechanism of blood flow regulation, independent of that of the exocrine pancreas. The endocrine tissue, which in humans includes approximately 1 million IOL, is scattered in the exocrine pancreas and constitutes only 1%-2% of its biomass, while utilizing 10%-20% of the total blood flow into the organ[113-115]. The proportion of arteriole endings and of vascular density in IOL and exocrine tissue is similar[116,117]. IOL are supplied with arterial blood via one or more arterioles which, after penetrating the capsule, form dense, glomerular-like network of capillaries. They are wider than their exocrine counterparts and have much more fenestrae[118]. The sinusoidal capillaries are drained via several efferent venules. Figure 4 (courtesy of Dr. Bonner-Weir[119]) demonstrates the complexity of single islet vascularization. Vascular density is such that all endocrine cells are no more than one cell away from arterial blood[120]. This architecture is dramatically changed following transplantation. Capillary densities of rodent islet grafts implanted under the kidney capsule average 500-700 capillaries/mm2[121-124], which is approximately half the density of native pancreatic islets (1300 capillaries/mm2)[123] and vascular density of murine islets transplanted into the liver is not more than 20% of the original density[117]. The vascular density of the human subcutis is lower by an additional order of magnitude averaging only 60-100 capillaries/mm2[125-127]. The density of local vasculature should be reflected in the perfusion characteristics of these organs. Basic pancreatic blood perfusion is measured at 200-300 mL/100 g per minute[128-130]. So far, perfusion values for islet blood flow were not reported but they are expected to be higher than the average pancreatic values. Notably, subcutaneous blood flow is lower by 2 orders of magnitude[131-133]. Thus, when addressing the question of islet transplantation into the subcutis, these differential values should be considered.
Oxygen supply to cells in tissues/organs is driven by a concentration gradient. Oxygen is solubilized from oxygenated hemoglobin on plasma membrane of red blood cells into the plasma, further diffuses into the interstitial space and then through the cell plasma membrane into the mitochondria. As it diffuses, a pressure gradient is formed. The oxygen transfer rate (flux) from the plasma to the mitochondria is dictated by the oxygen gradient, the distance it has to cross, and the diffusion coefficients in the various tissues being crossed. When oxygen consumption rate (OCR) of the mitochondria increases, local oxygen concentration decreases. Similarly, as distance between blood plasma and target mitochondria increases, the flux of oxygen decreases.
In the normal blood circulation, oxygen partial pressure (PO2) in the large arteries starts at > 100 Torr. It then decreases to approximately 65 Torr in the smallest arterioles and further decreases to 40 Torr in the venous system. In pancreatic IOL, the average PO2 measured in situ in anesthetized animals is 35-40 Torr[134,135]. This level is slightly higher in healthy, wake animals and comparable to the PO2 values measured in the hepatic portal vein used for clinical islet transplantation[136]. However, following isolation and transplantation of IOL, this level changes dramatically. As IOLs are cut from their blood supply, oxygen is supplied from the periphery solely by diffusion and quickly becomes a rate-limiting nutrient. Transplantation is followed by neo-vascularization and IOLs transplanted into the subcapsular space of the kidney or into the hepatic sinusoids undergo a similar neovascularization process. Finally, they almost reach level of vascular density of normal pancreatic islets[137]. However, the anatomy of this vascular bed is completely different than that of the native complex; blood is supplied from the periphery inside instead of the original core-shell direction. Consequently, under the kidney capsule, PO2 of transplanted IOL is only 10 Torr[134] and values in diabetic animals are even lower (5-6 Torr[138]). This is also the level recorded for islets transplanted into the liver or spleen[134,138]. Pimonidazole is an oxygen tension indicator signaling at ambient pressure of ≤ 10 Torr. In the native pancreas, approximately one third of the islets are pimonidazole positive. This proportion is doubled in islets isolated from a donor and infused into the liver of diabetic recipients[139].
While transplantation of islets into vascularized spaces presents perfusion limitation, encapsulation just aggravates this situation (Figure 5). As no revascularization process is allowed, the distance of these islet cells from the nearest capillary is extended substantially. A mathematical model developed by Johnson et al[140] predicts that whereas islets transplanted under the kidney capsule or into the portal venous system are exposed to ambient PO2 of 40-50 Torr, encapsulation (in standard 500 μm width microspheres or planar macrocapsules) reduces the PO2 to 25 Torr. Under these conditions, cells in a 50 μm cores of these islets are exposed to PO2 < 10 Torr. Most encapsulation methods use an enveloping hydrogel with a width of 500-800 μm. If positioned at the geometric center of the capsule, the innermost islets cells are up to 400 μm away from the host vascular bed. To provide sufficient oxygen to mitochondria inside a cell, the maximal distance between capillary and the cell must not exceed 200 μm[141]. Cancer cells have relatively high OCR but OCR of cancer cell lines[142] is only one third of that of islet cells. Even though, cancer cells placed > 100 μm away from capillaries become necrotic[143]. Evidently, following encapsulation, the distance between the islets and the vascular bed becomes a major impediment for their normal physiological performance and even for their ability to survive.
Several mathematical models were developed in order to simulate oxygen transfer to encapsulated islets. In a detailed analysis, Dulong and Legallaise[144] presented pessimistic data on the feasibility of producing a BAP device using microencapsulated islets or islets encapsulated in hollow fibers. Based on oxygen transfer parameters, efficient performance of a human-type BAP requires a minimum of 570000 IEQ. These should be encapsulated in narrow, 250 μm diameter, hollow fiber measuring 270 cm. Under the same conditions, planar encapsulation is preferred. A sheet of 240 cm2 surface area and 300-μm width containing 420000 IEQ suffices the needs but, increasing the width to only 500 μm, which is desirable to protect the islets from the host immune system, makes this design impractical. About 1 million islets have to be encapsulated in a sheet of 600 cm2 surface area. Another model by Johnson et al[140] predicts that even at surface density of 500 IEQ/cm2, the core of a standard encapsulated IEQ becomes hypoxic. These findings were confirmed in an independent mathematical model[145]. Islets cultured under normoxic conditions in 1 mm high standard culture medium at density of 1600 IEQ/cm2 present hypoxic core when their size exceed a diameter of 100 μm.
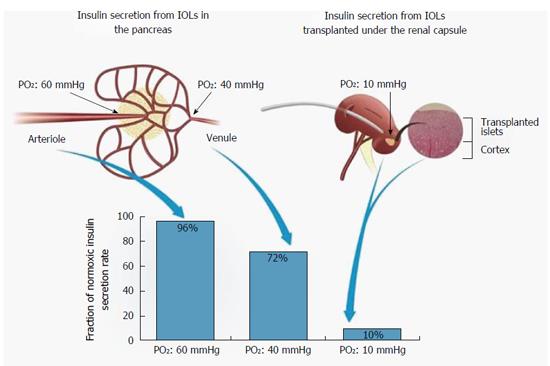
A BAP device should continuously sense ambient glucose concentrations and respond to a glucose concentration change by releasing adequate amounts of insulin. This process is also PO2-dependent[146,147]. Fractional secretion of islets decreases at PO2 below 60 Torr and reaches 50% efficiency at 27 Torr. At PO2 of 10 Torr, fractional secretion is only 10% of the normoxic level (Figure 6).
In their native environment, islets enjoy surface PO2 of 40-60 Torr and the efficiency of insulin secretion is predicted to be high (> 75% of the normoxic level; Figure 6). In contrast, islets transplanted under the kidney capsule or into the hepatic sinusoids, as practiced in clinical transplantations, are exposed to PO2 of ≤ 10 Torr[134]. Diabetes and encapsulation just worsen this situation. Under surface PO2 of ≤ 10 Torr, insulin secretion is expected to be reduced by an order of magnitude compared with physiological conditions. Also, short distance from capillaries and high perfusion rate which are characteristic of native islets are obstructed following encapsulation. As such, protection against the host immune system imparted by a standard permselective membrane is traded for low efficiency of insulin secretion.
A simple solution to this apparent oxygen deficiency is active delivery of oxygen by generating it in situ or using stored reservoirs. Some solutions were experimentally tested including a direct supply of oxygen to cultured cells using decomposition of solid calcium peroxide[148], electrochemical generator[149] (USP 8368592), or local photosynthesis[150,151]. Unfortunately, none of these systems generated enough oxygen to maintain clinical doses of islet graft viable and functional for long periods of time. Recently, we published a series of manuscripts describing active oxygen supply to encapsulated islets from internal storage. The islets were packed in a planar slab at a very high surface density, 1400-3600 IEQ/cm2 (5%-13% v/v). The device, β-Air), was implanted under the skin or into the pre-peritoneal space of diabetic recipients and gaseous oxygen was injected daily into a gas chamber that is an integral part of the device[24,112,152-154].
Hypoxia adversely affects the functionality of donor islets transplanted into a recipient and has emerged as the bottleneck in the development of efficient BAP devices. The role played by hyperoxia is less explored. In culture, IOL exposed to atmospheric air survive and function properly for extended periods of time. Higher PO2 levels, on the other hand, were reported to be toxic[155-157], but the levels used in these experiments were extremely high (680-1300 Torr, 5-9 times the atmospheric pressure). We hypothesized that some degree of hyperoxia could be beneficial to implanted islets as high PO2 at the surface of the encapsulated graft is necessary to fuel islet cells across the entire width of the capsule and all the way to the islet core. Also, hyperoxia may allow the use of denser islet grafts which may contribute to decreased device volume.
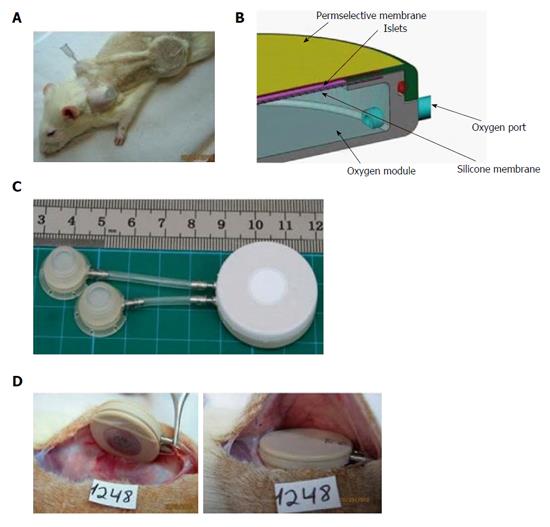
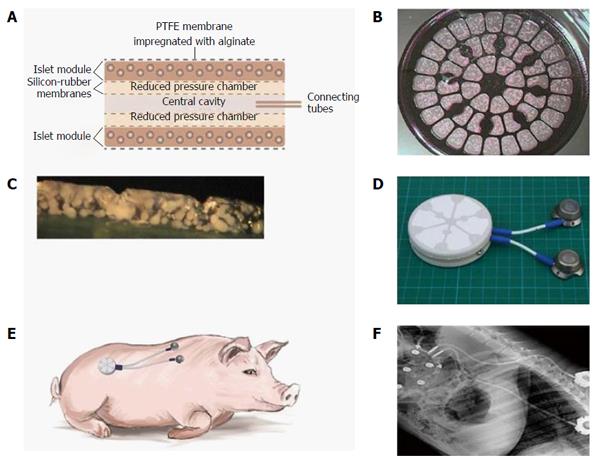
β-Air is a BAP device implanted under the skin or into the pre-peritoneal cavity, both of which are easily accessed by minimal surgical intervention. The rat variant of this device is composed of an integral macrochamber, access ports and connecting tubing (Figure 7). The device also holds an islet module containing 2400 IEQ [approximately 8000 IEQ/kg body weight (BW)] separated from an integral gas chamber by a rubber silicone membrane (Figure 8). Gas blend is infused into the gas chamber every 2 h (first prototype) or once a day using the access ports and a manual injector.
Using this device we exposed the islet module to increasing levels of PO2 and tested the effect of hyperoxia on their functional performance under culture conditions and following implantation of the BAP into diabetic animals. At a dose of 2400 IEQ/device and surface density of 1000 IEQ/cm2, none of 10 devices implanted in the subcutis without direct oxygen supply were functional for more than 3 d. On the other hand, refueling of 15 min every 2 h with atmospheric air was sufficient to maintain normoglycemia in diabetic recipients through the end of the experiments (up to 240 d)[24]. Surprisingly, all the devices equipped with the same islet dose but at increased surface density (2400 IEQ/cm2) failed to cure diabetic animals for > 1 wk when refueled alike the former group. Similar negative results were obtained when β-Air devices were refueled once a day with a gas blend at PO2 of 230 Torr (30% O2; Barkai et al, unpublished data). As the null hypothesis was that this failure stemmed from under and not hyper oxygenation of islets, PO2 in the gas chamber was raised further to 304, 456, and 570 Torr. Most of the diabetic animals implanted with β-Air devices and refueled as such were cured from the disease for the entire study period (Evron, Barkai et al, unpublished data). Notably, no signs of oxygen toxicity to the islets were observed in devices refueled with oxygen at 304 and 456 Torr at surface densities of 2400 or even at 3600 IEQ/cm2. Raising PO2 level to 570 Torr led to inconclusive observations, with part of the animals refueled at this level failing to achieve normoglycemia for more than a month. Therefore, we concluded that any PO2 < 550 Torr at the islet module-gas chamber interface is safe and maintains normoglycemia in implanted animals for long periods of time. These results also explain the toxic effects of oxygen observed at higher PO2 (> 680 Torr) reported by others[155,157,158].
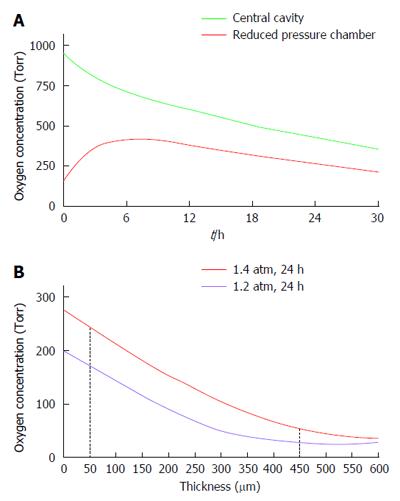
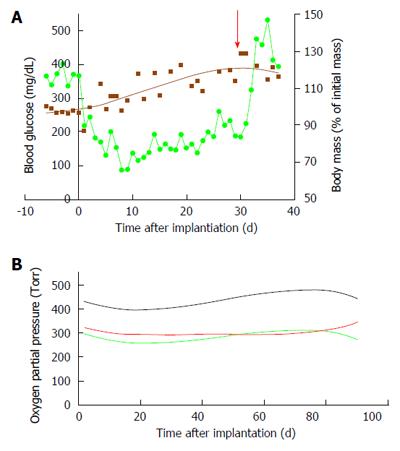
The data collected with the rat-type β-Air device were used to design a larger, porcine-type device (Figure 9), which can maintain up to 180000 IEQ and is, theoretically, capable of supporting glycemic demands of diabetic animals of 25-30 kg at a dose of 6000-7500 IEQ/kg. The porcine-type device (Figure 9A and B) is a disc-shaped structure composed of 2 opposing islet modules attached to a gas chamber. The islet modules are composed of a planar, 600-μm thick, alginate hydrogel encapsulating donor islets at surface density of 3600 IEQ/cm2 (approximately 11% v/v). They are separated from the gas chamber by a porous gas-permeable membrane. The gas chamber is a 3-compartment structure. A central cavity is separated from 2 “reduced pressure chambers” by a pair of porous membranes. It is connected by polyurethane tubes to subcutaneous access ports (Figure 9D). These ports allow direct injection of oxygen-enriched gas mixture (95% oxygen at 1.4 ATM; 1011 Torr) into the central cavity. Oxygen is diffusing from the central cavity into the “reduce pressure chambers” and from these chambers into the islet module where it is being dissolved in the aqueous environment of the hydrogel. The role of the 2 silicone membrane pairs separating the central cavity from the side chambers and the side chambers from the islet module is to reduce the PO2 at the chamber-islet module boundary to < 550 Torr. A mathematical model developed for this purpose (Lorber, Barkai et al, unpublished data) predicted that this level is never crossed during a standard refueling cycle and that refueling every 24 h ensures minimal PO2 at a critical value of 60 Torr, even at a depth of 450 μm from this boundary (Figure 10). Porcine-type β-Air devices, equipped with xenogeneic rat islets, were implanted into 4 diabetic Sinclair mini swine with fasting blood glucose levels of > 350 mg/dL (Figure 11A). The device maintained close to normal blood glucose levels in the diabetic animals and was functional for 1 mo. The islet dose was 6700 ± 600 IEQ/kg at the onset of the experiment and 5500 ± 500 at time of explantation. When implantation time was extended to 90 d, BW increased by more than 60%, islet dose decreased to < 4000 IEQ/kg and, eventually, glycemic control was lost by day 75[112]. These results clearly demonstrate that under proper oxygenation regime, xenogeneic islets dosed at 6000 IEQ/kg (half of the standard clinical dose) are curative[112].
Our mathematical model predicted that upon refueling with oxygen at pressure of >1000 Torr, the PO2 obtained at the “reduced pressure chamber” measured at the end of 24-h cycles (just before the next refueling), remains at > 100 Torr but never > 550 Torr. Actual measurements were made in 3 devices implanted in diabetic pigs for 90 d and are illustrated in Figure 11B. At the central cavity, oxygen tension was between 400 and 450 Torr and in both “reduced pressure chambers” it was approximately 300 Torr. These values are consistent with our mathematical model and also proved that the stored oxygen in this device is sufficient to maintain the demands of a graft comprising > 80000 IEQ for > 24 h.
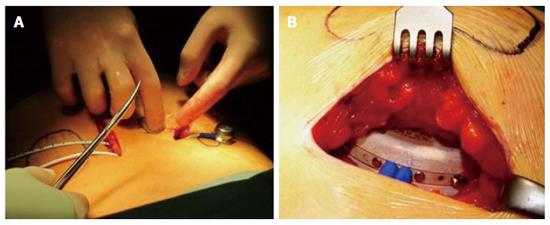
A porcine-type β-Air device equipped with human donor islets was tested in first-in-human clinical trial[154]. Images from the surgical procedure used for implantation are shown in Figure 12. Although the dose of donor islets used was < 20% of the standard clinical dose (approximately 2100 IEQ/kg), efficacy was clearly demonstrated. Ten months after implantation, the daily insulin requirement was reduced by approximately 15%, HbA1c decreased from 7.4% to 6.4%, and explanted islets stained for insulin and glucagon. The same device is now tested in a registered open labeled, pilot investigation clinical trial (NCT02064309).
In summary, the negative outcome of hypoxia on cultured or transplanted islets is a well-documented phenomenon. Shortage in oxygen supply must be resolved before long-term functional performance of macro-encapsulated islets graft is obtained. The studies described herein also set an upper level for long-term islet hyperoxia. Evidently, islets tolerate and are functional when directly exposed to PO2 < 300 Torr, about 2 times the PO2 in atmospheric air. Using these PO2 levels, we were able to maintain isogeneic, allogeneic, and xenogeneic islet grafts in animal models and human diabetic recipients for extended periods of time.
Most of the BAP devices use physical encapsulation as a way to introduce donor islets into a recipient body. This approach is promising; yet, many unresolved obstacles still exist before a long-term functional BAP could be established. Auxiliary complementary technologies, especially introduced during the period immediately after transplantation, are needed to create a “friendly environment” and prevent loss of transplanted islets. In the previous chapter we provided evidence that hyperoxic oxygen supply is beneficial to graft function. However, parameters such as chronic inflammation and biocompatibility, uncontrolled loss of viable cells, distance from the vascular bed to support readily exchange of glucose, insulin, and nutrients and supportive microenvironment are still considerable hurdles to get over in order to optimize graft function.
Implantation of a medical device is a 3-tier irritation process including: the surgical procedure; the chemistry and size of the implanted device; and the type and amount of contained cells. A tissue repair process is inevitable with any surgical procedure. It is aggravated by inserting an artificial device into the open wound and further intensified if the device includes cells. Inflammation during tissue repair process is a protective attempt of the immune system to remove the injurious stimuli and to initiate a healing process. It is a short-term process including vascular changes such as increased blood flow, vasodilation, infiltration of blood cells, and augmented permeability of plasma proteins. Inflammatory cytokines, prostaglandins, NO and ROS molecules that are locally produced by resident and imported immune cells are the major effectors of this response.
Primary malfunction of transplanted islets accounts for the bulk of graft losses (for example, see[45,159,160]). The aforementioned encapsulation of islets in hydrogels, practiced for many years by many laboratories, is only a partial solution to this problem. Overgrowth of activated macrophages on just a fraction of implanted islet capsules negatively affects glucose responsiveness of the entire graft[161]. Therefore, strategies to reduce inflammation are expected to improve long-term survival and proper operation of islet grafts. A pivotal approach in this direction involves using the protective mechanisms of immunomodulatory cells-Sertoli and mesenchymal stem cells (MSCs). MSCs are described as an “injury drugstore” having antibacterial, immunomodulatory and trophic activities[162]. They produce a curtain of activities behind which tissue regeneration is operable. These range of activities led Arnold Caplan to suggest changing the “MSC” acronym to “medicinal signaling cells”[163]. Co-transplantation of islets and MSCs seeded on naked scaffold enhanced islet function[164,165], and similar advantage were demonstrated following co-encapsulation of islets and MSCs[166,167]. In our hands, rat islets co-encapsulated with marginal mass of pancreatic MSCs and cultured for 2 wk demonstrated enhanced insulin secretion capacity and better survival rate (Barkai et al, unpublished data). Sertoli cells have similar effect on survival and functioning of islet graft in rodents[168,169] and co-aggregates of core Sertoli cells and mantle β-cells promoted close-to-normal glycemic control in allogeneic recipients for > 100 d[170]. Sertoli cells were also able to enhance survival of islets graft in xenogeneic recipients[171-173]. Finally, co-encapsulated porcine islets and Sertoli cells were implanted into human subjects in a controversial Mexican clinical trial[8,174,175]. Some of the transplanted patients experienced reduction in their requirements for insulin therapy for up to 3 years.
Acute phase proteins, a group of circulating plasma proteins, rapidly respond to inflammation. Hepatic alpha-1 antitrypsin (AAT), a member of this class, is in abundant in the plasma and its level increases many-folds in response to inflammation. AAT protects tissues from proteases released from inflammatory cells. It also exhibits protease-independent anti-inflammatory activities against these cells and against the soluble effectors they release[176,177]. Unlike immunosuppressive drugs, AAT helps the immune system to distinguish between desired responses against authentic threats and unwanted responses fueled by positive feedback loops[178], thereby transforming devastating inflammation into beneficial immune tolerance. AAT was shown to prolong survival of transplanted islets in rodents[179-181] and in non-human primates[182]. It also induces immune tolerance in animals receiving transplantation of multiple allografts[183]. We showed that, in diabetic animals implanted with β-Air devices, a week treatment with systemic AAT resulted in improved survival of islet cells (Barkai et al, unpublished data). Collectively, the findings suggest that proper control of inflammation may improve transplantation outcome of islets grafts.
Cysteine-aspartic proteases (caspases) play a pivotal role in apoptosis. Cell-permeable apoptosis inhibitors pentapeptides (V5 and DHMEQ) were shown to improve transplantation outcomes when used throughout the islet isolation process[184,185]. Similar improvements in yield and quality of rat and porcine islets were obtained when the tetra-peptide z-DEVD-FMK (caspase 3 inhibitor) was included in the enzymatic blend used to digest the pancreas (Barkai et al, unpublished data). With all the promise, there is only one anti-apoptotic drug, an orally delivered pan-caspase inhibitor (Emricasan, Conatus Pharmaceuticals Inc., San Diego, CA) that is currently evaluated as islet transplantation adjuvant therapy in a phase I/II clinical study (NCT01653899).
A subgroup of G-protein coupled receptors (GPCR) is the B-family GPCR consisting 15 members[186], which bind relatively short peptides (20-50 amino acids long). A subset of this family of effectors includes incretin hormones (GLP-1, GIP), growth hormone releasing hormone (GHRH), and corticotropin-releasing hormone (CRH), all of which augment insulin secretion. GLP-1 was shown to inhibit apoptosis of pancreatic β-cells[187-189], to reduce inflammation[190], and is clinically used to treat type 2 diabetes. Less known are GHRH and CRH. Both ligands as well as their cognate receptors are expressed in pancreatic β-cells of rat and human[191-194]. Upon binding, these ligands increases cell proliferation and decreases β-cells apoptotic rate. Both peptides also change the intracellular balance between the active and inactive glucocorticoid molecules in favor of the inactive form, thereby increasing insulin sensitivity[191]. We tested one of these effectors in diabetic rats using the β-Air BAP. Devices loaded with islets pretreated with a GHRH agonist significantly enhanced graft function by improving glucose tolerance and β-cell insulin reserve[153].
BAP macro-devices are usually inserted under the skin. This site is characterized by poor vascularization to begin with, and adding the enveloping capsule creates a large diffusion distance between the capillary bed and the graft. Inducing dense angiogenesis at close proximity to the graft capsule may create a more supportive environment. Such induction attempts included temporal placement of pro-angiogenic membrane or mesh[195,196], slow release of pro-angiogenic factors[197-200], and using both these strategies concurrently[201]. Enhanced angiogenesis that promoted long-term islet function occurred, but was validated only in rodent models. Also, from a regulatory perspective, the use of pure pro-angiogenic factors that may promote growth of malignant cells may be problematic.
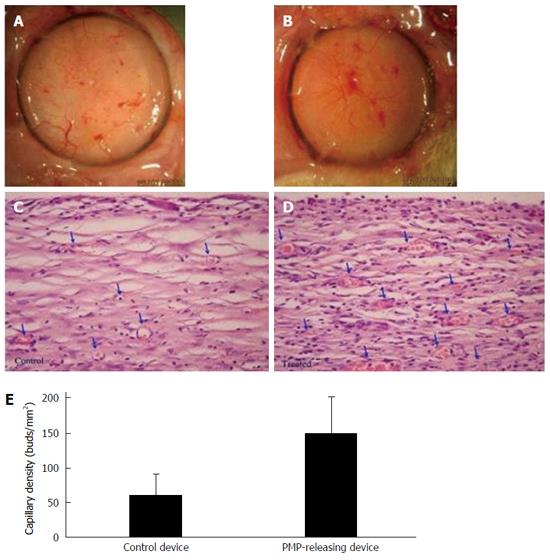
Many cells shed small (0.1-1 μm) fragments of their plasma membranes into the circulation. Platelet micro-particles (PMP) derived from megakaryocytes are the most abundant circulating micro-particle subtype. PMP contain broad spectrum of bioactive molecules including a concentrated set of cytokines and signaling proteins. PMP are postulated to play a key role in angiogenesis[202-204] and to treat hypoxia (WO patent 2006059329). Notably, PMP are regulated as a blood product. When freely mixed with the encapsulating hydrogel of β-Air devices and implanted for 3 wk in rats, PMP promoted denser and more mature angiogenesis of the capsule formed around the devices (Figure 13).
Research has focused on the inflammatory and immune responses against the capsule polymers, whereas the research on the compatibility of the intra-capsular milieu with the contained islets remains insufficient. Islets are very sensitive clumps of cells requiring nutritional factors, hormones, extracellular matrix (ECM), and a relative pliable microenvironment. Islets undergo a cellular transition immediately after encapsulation, during which islet cells are very sensitive to changes in the rigidity of the microenvironment and may die by a mechanotransduction process[205]. The exact threshold at which islet cells are sensitive to mechanotransduction is unknown. Therefore, cell lines were used to explore whether increase in alginate-concentration in microcapsules could induce mechanotransduction-mediated cell-death. The study showed that the concentration as well as the type of alginate were critical in activating mechanotransduction[206]. Alginates that are high in guluronic acid form stiffer gels and are associated with massive cell death as of a concentration of 2% while alginates containing more mannuronic acid exhibited optimal survival up to alginate concentrations of 3.4%[206]. The contribution of micro-environmental rigidity to the enormous inter-lab variability in survival of encapsulated islets remains to be established and warrants further investigation and standardization.
Engineering the intra-capsular milieu with ECM molecules may decrease the effects of mechanotransduction. It has been suggested that integrins are the sensors of the cells for mechanical stress. A synthetic peptide RGD, mimicking the original tri-peptide part on the ECM molecule fibronectin is now being sold by Novamatrix (Sandvika, Norway). It binds and prevents clustering of integrins which form an essential step in mechanotranduction[207,208]. Some groups have added RGD or IKVAV (another integrin binding epitope) to the intracapsular environment and demonstrated improved viability and functionality under culture conditions (for examples, see[209,210]) and in animal models[211]. However, ECM molecules may be necessary for additional processes contributing to prevention of anoikis and prolonging survival of islet cells as they are anchoring sites for many essential growth factors. To date, only little is known on the role played by the lack of specific ECM components on islet longevity[45].
The quality of the intra-capsular milieu is far more than a step towards survival of more functional cells. It also contributes to prevention of pro-inflammatory immune responses against the grafts. Human encapsulated islets regularly undergo 4 processes of cell death: Necrosis, apoptosis, autophagy and necroptosis (de Vos et al, unpublished data). In islets, all these cell-death processes ended with the release of significant amounts of danger-associated molecular patterns (DAMPs), which even in small amount activate immune cells. Microcapsules retain part of the DAMPs, however significant amounts are still released. Adding NEC-1, an inhibitor of necroptosis reduced DAMP release and activation of immune cells and rescued larger part of the islet cells[212]. Combined, these data highlight that the adequacy of the intracapsular microenvironment should be taken into consideration.
Encapsulation in permselective membrane is experimentally used in diabetes for progressing from drug- and standard cell-based therapy to immunosuppressive-free cell-based therapy. Cell encapsulation is a mandatory but not a sufficient requirement for an efficient curing technology. Adequate oxygen supply to the grafted cells constitutes the second tier of mandatory requirements. Fulfilling these requirements should enhance the practicability of clinical islet transplantation; however, successful implementation of a cell-based cure also depends on auxiliary technologies, some of which are portrayed in this review.
The authors thank Dr. Zohar Biron-Sorek for providing the images appearing in Figure 1.
P- Reviewer: Mitnala S, Sumi S S- Editor: Song XX L- Editor: A E- Editor: Wang CH
| 1. | Dall TM, Yang W, Halder P, Pang B, Massoudi M, Wintfeld N, Semilla AP, Franz J, Hogan PF. The economic burden of elevated blood glucose levels in 2012: diagnosed and undiagnosed diabetes, gestational diabetes mellitus, and prediabetes. Diabetes Care. 2014;37:3172-3179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Ehehalt S, Dietz K, Willasch AM, Neu A; DIARY-Group Baden-Wuerttemberg. Prediction model for the incidence and prevalence of type 1 diabetes in childhood and adolescence: evidence for a cohort-dependent increase within the next two decades in Germany. Pediatr Diabetes. 2012;13:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, Kneteman NM, Rajotte RV. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3996] [Cited by in RCA: 3827] [Article Influence: 153.1] [Reference Citation Analysis (0)] |
| 4. | Collaborative Island Transplant Registry (CITR) eighth annual report 2013. [Accessed 2015 Aug 1]. Available from: http://www.citregistry.org. |
| 5. | Matsumoto S, Tan P, Baker J, Durbin K, Tomiya M, Azuma K, Doi M, Elliott RB. Clinical porcine islet xenotransplantation under comprehensive regulation. Transplant Proc. 2014;46:1992-1995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 102] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 6. | Krishnan R, Buder B, Alexander M, Foster CE, Lakey JR. Juvenile porcine islets can restore euglycemia in diabetic athymic nude mice after xenotransplantation. Transplantation. 2015;99:710-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 7. | DuBois DL. Problematic consequences of using standard errors rather than standard deviations: calculation of effect sizes. JAMA Pediatr. 2015;169:95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Valdes-Gonzalez R, Rodriguez-Ventura AL, White DJ, Bracho-Blanchet E, Castillo A, Ramírez-González B, López-Santos MG, León-Mancilla BH, Dorantes LM. Long-term follow-up of patients with type 1 diabetes transplanted with neonatal pig islets. Clin Exp Immunol. 2010;162:537-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 9. | Russ HA, Parent AV, Ringler JJ, Hennings TG, Nair GG, Shveygert M, Guo T, Puri S, Haataja L, Cirulli V. Controlled induction of human pancreatic progenitors produces functional beta-like cells in vitro. EMBO J. 2015;34:1759-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 461] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 10. | Pagliuca FW, Millman JR, Gürtler M, Segel M, Van Dervort A, Ryu JH, Peterson QP, Greiner D, Melton DA. Generation of functional human pancreatic β cells in vitro. Cell. 2014;159:428-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1321] [Cited by in RCA: 1519] [Article Influence: 151.9] [Reference Citation Analysis (1)] |
| 11. | Rezania A, Bruin JE, Arora P, Rubin A, Batushansky I, Asadi A, O’Dwyer S, Quiskamp N, Mojibian M, Albrecht T. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat Biotechnol. 2014;32:1121-1133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 1152] [Article Influence: 104.7] [Reference Citation Analysis (0)] |
| 12. | Balamurugan AN, Loganathan G, Bellin MD, Wilhelm JJ, Harmon J, Anazawa T, Soltani SM, Radosevich DM, Yuasa T, Tiwari M. A new enzyme mixture to increase the yield and transplant rate of autologous and allogeneic human islet products. Transplantation. 2012;93:693-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 13. | Iglesias I, Valiente L, Shiang KD, Ichii H, Kandeel F, Al-Abdullah IH. The effects of digestion enzymes on islet viability and cellular composition. Cell Transplant. 2012;21:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Balamurugan AN, Breite AG, Anazawa T, Loganathan G, Wilhelm JJ, Papas KK, Dwulet FE, McCarthy RC, Hering BJ. Successful human islet isolation and transplantation indicating the importance of class 1 collagenase and collagen degradation activity assay. Transplantation. 2010;89:954-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 15. | Robles L, Storrs R, Lamb M, Alexander M, Lakey JR. Current status of islet encapsulation. Cell Transplant. 2014;23:1321-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Cras P, Federsppiel SS, Gheuens J, Martin JJ, Lowenthal A. Demonstration of neuron-specific enolase in nonneuronal tumors using a specific monoclonal antibody. Ann Neurol. 1986;20:106-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Scharp DW, Marchetti P. Encapsulated islets for diabetes therapy: history, current progress, and critical issues requiring solution. Adv Drug Deliv Rev. 2014;67-68:35-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 18. | Yang HK, Yoon KH. Current status of encapsulated islet transplantation. J Diabetes Complications. 2015;29:737-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 19. | Lundberg P, Kuchel PW. Diffusion of solutes in agarose and alginate gels: 1H and 23Na PFGSE and 23Na TQF NMR studies. Magn Reson Med. 1997;37:44-52. [PubMed] |
| 20. | Mehmetoglu U, Ates S, Berber R. Oxygen diffusivity in calcium alginate gel beads containing Gluconobacter suboxydans. Artif Cells Blood Substit Immobil Biotechnol. 1996;24:91-106. [PubMed] |
| 21. | Zhang T, Fang HH. Effective diffusion coefficients of glucose in artificial biofilms. Environ Technol. 2005;26:155-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Tanaka H, Matsumura M, Veliky IA. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol Bioeng. 1984;26:53-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 528] [Cited by in RCA: 444] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 23. | Oyaas J, Storrø I, Svendsen H, Levine DW. The effective diffusion coefficient and the distribution constant for small molecules in calcium-alginate gel beads. Biotechnol Bioeng. 1995;47:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Barkai U, Weir GC, Colton CK, Ludwig B, Bornstein SR, Brendel MD, Neufeld T, Bremer C, Leon A, Evron Y. Enhanced oxygen supply improves islet viability in a new bioartificial pancreas. Cell Transplant. 2013;22:1463-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 25. | Weng L, Liang S, Zhang L, Zhang XJX. Transport of glucose and poly(ethylene glycol)s in agarose gels studied by the refractive index method. Macromolecules. 2005;38:5236-5242. |
| 26. | Gray DW. An overview of the immune system with specific reference to membrane encapsulation and islet transplantation. Ann N Y Acad Sci. 2001;944:226-239. [PubMed] |
| 27. | Papadimitriou JC, Drachenberg CB, Shin ML, Trump BF. Ultrastructural studies of complement mediated cell death: a biological reaction model to plasma membrane injury. Virchows Arch. 1994;424:677-685. [PubMed] |
| 28. | Perkins SJ. Molecular modelling of human complement subcomponent C1q and its complex with C1r2C1s2 derived from neutron-scattering curves and hydrodynamic properties. Biochem J. 1985;228:13-26. [PubMed] |
| 29. | Perkins SJ, Nealis AS. The quaternary structure in solution of human complement subcomponent C1r2C1s2. Biochem J. 1989;263:463-469. [PubMed] |
| 30. | Perkins SJ. Models for the C1 complex determined by physical techniques. Behring Inst Mitt. 1989;129-141. [PubMed] |
| 31. | Liberti PA, Paul SM. Gross conformation of C1q: a subcomponent of the first component of complement. Biochemistry. 1978;17:1952-1958. [PubMed] |
| 32. | Hanley BP, Xing L, Cheng RH. Variance in multiplex suspension array assays: microsphere size variation impact. Theor Biol Med Model. 2007;4:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 33. | Wadu-Mesthrige K, Amro NA, Garno JC, Xu S, Liu G. Fabrication of nanometer-sized protein patterns using atomic force microscopy and selective immobilization. Biophys J. 2001;80:1891-1899. [PubMed] |
| 34. | Silverton EW, Navia MA, Davies DR. Three-dimensional structure of an intact human immunoglobulin. Proc Natl Acad Sci USA. 1977;74:5140-5144. [PubMed] |
| 35. | Armstrong JK, Wenby RB, Meiselman HJ, Fisher TC. The hydrodynamic radii of macromolecules and their effect on red blood cell aggregation. Biophys J. 2004;87:4259-4270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 579] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 36. | Jøssang T, Feder J, Rosenqvist E. Photon correlation spectroscopy of human IgG. J Protein Chem. 1988;7:165-171. [PubMed] |
| 37. | Gorinsky B, Horsburgh C, Lindley PF, Moss DS, Parkar M, Watson JL. Evidence for the bilobal nature of diferric rabbit plasma transferrin. Nature. 1979;281:157-158. [PubMed] |
| 38. | Kilár F, Simon I. The effect of iron binding on the conformation of transferrin. A small angle x-ray scattering study. Biophys J. 1985;48:799-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 58] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 39. | de Vos P, van Hoogmoed CG, de Haan BJ, Busscher HJ. Tissue responses against immunoisolating alginate-PLL capsules in the immediate posttransplant period. J Biomed Mater Res. 2002;62:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 40. | Parameswaran N, Patial S. Tumor necrosis factor-α signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87-103. [PubMed] |
| 41. | Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2289] [Cited by in RCA: 2616] [Article Influence: 163.5] [Reference Citation Analysis (0)] |
| 42. | Beuscher HU, Günther C, Röllinghoff M. IL-1 beta is secreted by activated murine macrophages as biologically inactive precursor. J Immunol. 1990;144:2179-2183. [PubMed] |
| 43. | Alejandro R, Cutfield RG, Shienvold FL, Polonsky KS, Noel J, Olson L, Dillberger J, Miller J, Mintz DH. Natural history of intrahepatic canine islet cell autografts. J Clin Invest. 1986;78:1339-1348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 119] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 44. | Nagata M, Mullen Y, Matsuo S, Herrera M, Clare-Salzler M. Destruction of islet isografts by severe nonspecific inflammation. Transplant Proc. 1990;22:855-856. [PubMed] |
| 45. | de Vos P, Lazarjani HA, Poncelet D, Faas MM. Polymers in cell encapsulation from an enveloped cell perspective. Adv Drug Deliv Rev. 2014;67-68:15-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 194] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 46. | Montolio M, Biarnés M, Téllez N, Escoriza J, Soler J, Montanya E. Interleukin-1beta and inducible form of nitric oxide synthase expression in early syngeneic islet transplantation. J Endocrinol. 2007;192:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 47. | Baker MS, Chen X, Rotramel A, Nelson J, Kaufman DB. Proinflammatory cytokines induce NF-kappaB-dependent/NO-independent chemokine gene expression in MIN6 beta cells. J Surg Res. 2003;110:295-303. [PubMed] |
| 48. | Gysemans CA, Waer M, Valckx D, Laureys JM, Mihkalsky D, Bouillon R, Mathieu C. Early graft failure of xenogeneic islets in NOD mice is accompanied by high levels of interleukin-1 and low levels of transforming growth factor-beta mRNA in the grafts. Diabetes. 2000;49:1992-1997. [PubMed] |
| 49. | King A, Andersson A, Sandler S. Cytokine-induced functional suppression of microencapsulated rat pancreatic islets in vitro. Transplantation. 2000;70:380-383. [PubMed] |
| 50. | el-Ouaghlidi A, Jahr H, Pfeiffer G, Hering BJ, Federlin K, Bretzel RG. Cytokine transcripts in peripheral blood cells during immunosuppressive induction therapy in allogeneic human islet transplantation. Transplant Proc. 1997;29:2154. [PubMed] |
| 51. | Xenos ES, Stevens RB, Sutherland DE, Lokeh A, Ansite JD, Casanova D, Gores PF, Platt JL. The role of nitric oxide in IL-1 beta-mediated dysfunction of rodent islets of Langerhans. Implications for the function of intrahepatic islet grafts. Transplantation. 1994;57:1208-1212. [PubMed] |
| 52. | Cetkovic-Cvrlje M, Eizirik DL. TNF-alpha and IFN-gamma potentiate the deleterious effects of IL-1 beta on mouse pancreatic islets mainly via generation of nitric oxide. Cytokine. 1994;6:399-406. [PubMed] |
| 53. | Uthaiah RC, Praefcke GJ, Howard JC, Herrmann C. IIGP1, an interferon-gamma-inducible 47-kDa GTPase of the mouse, showing cooperative enzymatic activity and GTP-dependent multimerization. J Biol Chem. 2003;278:29336-29343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | Lu C, Davalos RV, Li L. The effect of interleukin-1 (IL-1) concentration on single cell NF-κB activation in a gradient-generating microfluidic device. MSc thesis. BioMedical Engineering: Virginia Polytechnic Institute and State University 2011; . |
| 55. | Smidsrød O, Skjåk-Braek G. Alginate as immobilization matrix for cells. Trends Biotechnol. 1990;8:71-78. [PubMed] |
| 56. | Ito A, Mukaiyama A, Itoh Y, Nagase H, Thogersen IB, Enghild JJ, Sasaguri Y, Mori Y. Degradation of interleukin 1beta by matrix metalloproteinases. J Biol Chem. 1996;271:14657-14660. [PubMed] |
| 57. | Scuderi P, Nez PA, Duerr ML, Wong BJ, Valdez CM. Cathepsin-G and leukocyte elastase inactivate human tumor necrosis factor and lymphotoxin. Cell Immunol. 1991;135:299-313. [PubMed] |
| 58. | van Kessel KP, van Strijp JA, Verhoef J. Inactivation of recombinant human tumor necrosis factor-alpha by proteolytic enzymes released from stimulated human neutrophils. J Immunol. 1991;147:3862-3868. [PubMed] |
| 59. | Nafea EH, Marson A, Poole-Warren LA, Martens PJ. Immunoisolating semi-permeable membranes for cell encapsulation: focus on hydrogels. J Control Release. 2011;154:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 60. | Basta G, Sarchielli P, Luca G, Racanicchi L, Nastruzzi C, Guido L, Mancuso F, Macchiarulo G, Calabrese G, Brunetti P. Optimized parameters for microencapsulation of pancreatic islet cells: an in vitro study clueing on islet graft immunoprotection in type 1 diabetes mellitus. Transpl Immunol. 2004;13:289-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 61. | de Vos P, Smedema I, van Goor H, Moes H, van Zanten J, Netters S, de Leij LF, de Haan A, de Haan BJ. Association between macrophage activation and function of micro-encapsulated rat islets. Diabetologia. 2003;46:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 62. | Kulseng B, Thu B, Espevik T, Skjåk-Braek G. Alginate polylysine microcapsules as immune barrier: permeability of cytokines and immunoglobulins over the capsule membrane. Cell Transplant. 1997;6:387-394. [PubMed] |
| 63. | Jang JY, Lee DY, Park SJ, Byun Y. Immune reactions of lymphocytes and macrophages against PEG-grafted pancreatic islets. Biomaterials. 2004;25:3663-3669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 82] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 64. | Kröncke KD, Rodriguez ML, Kolb H, Kolb-Bachofen V. Cytotoxicity of activated rat macrophages against syngeneic islet cells is arginine-dependent, correlates with citrulline and nitrite concentrations and is identical to lysis by the nitric oxide donor nitroprusside. Diabetologia. 1993;36:17-24. [PubMed] |
| 65. | Quintana-Lopez L, Blandino-Rosano M, Perez-Arana G, Cebada-Aleu A, Lechuga-Sancho A, Aguilar-Diosdado M, Segundo C. Nitric oxide is a mediator of antiproliferative effects induced by proinflammatory cytokines on pancreatic beta cells. Mediators Inflamm. 2013;2013:905175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 66. | Holohan C, Szegezdi E, Ritter T, O’Brien T, Samali A. Cytokine-induced beta-cell apoptosis is NO-dependent, mitochondria-mediated and inhibited by BCL-XL. J Cell Mol Med. 2008;12:591-606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 67. | de Groot M, Schuurs TA, Leuvenink HG, van Schilfgaarde R. Macrophage overgrowth affects neighboring nonovergrown encapsulated islets. J Surg Res. 2003;115:235-241. [PubMed] |
| 68. | Marquet RL, Bonthuis F, van IJken M, Bouwman E, Wolvekamp MC, van Rooijen N, Scheringa M, IJzermans JN. Primary nonfunction of islet xenografts: the role of macrophages. Transpl Int. 1994;7 Suppl 1:S660-S662. [PubMed] |
| 69. | Maechler P, Jornot L, Wollheim CB. Hydrogen peroxide alters mitochondrial activation and insulin secretion in pancreatic beta cells. J Biol Chem. 1999;274:27905-27913. [PubMed] |
| 70. | Krippeit-Drews P, Kramer C, Welker S, Lang F, Ammon HP, Drews G. Interference of H2O2 with stimulus-secretion coupling in mouse pancreatic beta-cells. J Physiol. 1999;514:471-481. [PubMed] |
| 71. | Lortz S, Tiedge M. Sequential inactivation of reactive oxygen species by combined overexpression of SOD isoforms and catalase in insulin-producing cells. Free Radic Biol Med. 2003;34:683-688. [PubMed] |
| 72. | Xu B, Moritz JT, Epstein PN. Overexpression of catalase provides partial protection to transgenic mouse beta cells. Free Radic Biol Med. 1999;27:830-837. [PubMed] |
| 73. | Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med. 1996;20:463-466. [PubMed] |
| 74. | Malaisse WJ, Malaisse-Lagae F, Sener A, Pipeleers DG. Determinants of the selective toxicity of alloxan to the pancreatic B cell. Proc Natl Acad Sci USA. 1982;79:927-930. [PubMed] |
| 75. | Mikkelsen RB, Wardman P. Biological chemistry of reactive oxygen and nitrogen and radiation-induced signal transduction mechanisms. Oncogene. 2003;22:5734-5754. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 414] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 76. | Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424-C1437. [PubMed] |
| 77. | Lancaster JR. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc Natl Acad Sci USA. 1994;91:8137-8141. [PubMed] |
| 78. | Kozlovskaya V, Zavgorodnya O, Kharlampieva E. Encapsulation and surface engineering of pancreatic islets: Advances and challenges. Biomedicine. In: Lin C, editor. InTech 2012; 1-32. |
| 79. | Qi M. Transplantation of Encapsulated Pancreatic Islets as a Treatment for Patients with Type 1 Diabetes Mellitus. Adv Med. 2014;2014:429710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 80. | Sakata N, Sumi S, Yoshimatsu G, Goto M, Egawa S, Unno M. Encapsulated islets transplantation: Past, present and future. World J Gastrointest Pathophysiol. 2012;3:19-26. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 68] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 81. | de Vos P, Faas MM, Strand B, Calafiore R. Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials. 2006;27:5603-5617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 361] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 82. | Mørch YA, Holtan S, Donati I, Strand BL, Skjåk-Braek G. Mechanical properties of C-5 epimerized alginates. Biomacromolecules. 2008;9:2360-2368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 83. | Donati I, Draget KI, Borgogna M, Paoletti S, Skjåk-Braek G. Tailor-made alginate bearing galactose moieties on mannuronic residues: selective modification achieved by a chemoenzymatic strategy. Biomacromolecules. 2005;6:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Strand BL, Mørch YA, Syvertsen KR, Espevik T, Skjåk-Braek G. Microcapsules made by enzymatically tailored alginate. J Biomed Mater Res A. 2003;64:540-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 85. | Iwata H, Amemiya H, Matsuda T, Takano H, Hayashi R, Akutsu T. Evaluation of microencapsulated islets in agarose gel as bioartificial pancreas by studies of hormone secretion in culture and by xenotransplantation. Diabetes. 1989;38 Suppl 1:224-225. [PubMed] |
| 86. | Gin H, Dupuy B, Baquey C, Ducassou D, Aubertin J. Agarose encapsulation of islets of Langerhans: reduced toxicity in vitro. J Microencapsul. 1987;4:239-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Xu B, Iwata H, Miyamoto M, Balamurugan AN, Murakami Y, Cui W, Imamura M, Inoue K. Functional comparison of the single-layer agarose microbeads and the developed three-layer agarose microbeads as the bioartificial pancreas: an in vitro study. Cell Transplant. 2001;10:403-408. [PubMed] |
| 88. | Iwata H, Takagi T, Kobayashi K, Oka T, Tsuji T, Ito F. Strategy for developing microbeads applicable to islet xenotransplantation into a spontaneous diabetic NOD mouse. J Biomed Mater Res. 1994;28:1201-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 89. | Iwata H, Kobayashi K, Takagi T, Oka T, Yang H, Amemiya H, Tsuji T, Ito F. Feasibility of agarose microbeads with xenogeneic islets as a bioartificial pancreas. J Biomed Mater Res. 1994;28:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 90. | Holdcraft RW, Gazda LS, Circle L, Adkins H, Harbeck SG, Meyer ED, Bautista MA, Martis PC, Laramore MA, Vinerean HV. Enhancement of in vitro and in vivo function of agarose-encapsulated porcine islets by changes in the islet microenvironment. Cell Transplant. 2014;23:929-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 91. | Gazda LS, Vinerean HV, Laramore MA, Diehl CH, Hall RD, Rubin AL, Smith BH. Encapsulation of porcine islets permits extended culture time and insulin independence in spontaneously diabetic BB rats. Cell Transplant. 2007;16:609-620. [PubMed] |
| 92. | Bačáková L, Novotná K, Pařízek M. Polysaccharides as cell carriers for tissue engineering: the use of cellulose in vascular wall reconstruction. Physiol Res. 2014;63 Suppl 1:S29-S47. [PubMed] |
| 93. | Sakai S, Ono T, Ijima H, Kawakami K. Control of molecular weight cut-off for immunoisolation by multilayering glycol chitosan-alginate polyion complex on alginate-based microcapsules. J Microencapsul. 2000;17:691-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 94. | Chandy T, Mooradian DL, Rao GH. Evaluation of modified alginate-chitosan-polyethylene glycol microcapsules for cell encapsulation. Artif Organs. 1999;23:894-903. [PubMed] |
| 95. | Sobol M, Bartkowiak A, de Haan B, de Vos P. Cytotoxicity study of novel water-soluble chitosan derivatives applied as membrane material of alginate microcapsules. J Biomed Mater Res A. 2013;101:1907-1914. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 96. | Risbud MV, Bhargava S, Bhonde RR. In vivo biocompatibility evaluation of cellulose macrocapsules for islet immunoisolation: Implications of low molecular weight cut-off. J Biomed Mater Res A. 2003;66:86-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 17] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 97. | Schaffellner S, Stadlbauer V, Stiegler P, Hauser O, Halwachs G, Lackner C, Iberer F, Tscheliessnigg KH. Porcine islet cells microencapsulated in sodium cellulose sulfate. Transplant Proc. 2005;37:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 36] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 98. | Jesser C, Kessler L, Lambert A, Belcourt A, Pinget M. Pancreatic islet macroencapsulation: a new device for the evaluation of artificial membrane. Artif Organs. 1996;20:997-1007. [PubMed] |
| 99. | Storrs R, Dorian R, King SR, Lakey J, Rilo H. Preclinical development of the Islet Sheet. Ann N Y Acad Sci. 2001;944:252-266. [PubMed] |
| 100. | Dufrane D, Goebbels RM, Gianello P. Alginate macroencapsulation of pig islets allows correction of streptozotocin-induced diabetes in primates up to 6 months without immunosuppression. Transplantation. 2010;90:1054-1062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 101. | Geller RL, Loudovaris T, Neuenfeldt S, Johnson RC, Brauker JH. Use of an immunoisolation device for cell transplantation and tumor immunotherapy. Ann N Y Acad Sci. 1997;831:438-451. [PubMed] |
| 102. | Brauker JH, Carr-Brendel VE, Martinson LA, Crudele J, Johnston WD, Johnson RC. Neovascularization of synthetic membranes directed by membrane microarchitecture. J Biomed Mater Res. 1995;29:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 333] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 103. | Sörenby AK, Kumagai-Braesch M, Sharma A, Hultenby KR, Wernerson AM, Tibell AB. Preimplantation of an immunoprotective device can lower the curative dose of islets to that of free islet transplantation: studies in a rodent model. Transplantation. 2008;86:364-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 104. | Kumagai-Braesch M, Jacobson S, Mori H, Jia X, Takahashi T, Wernerson A, Flodström-Tullberg M, Tibell A. The TheraCyte™ device protects against islet allograft rejection in immunized hosts. Cell Transplant. 2013;22:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 102] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 105. | Boettler T, Schneider D, Cheng Y, Kadoya K, Brandon EP, Martinson L, Herrath MV. Pancreatic tissue transplanted in TheraCyte™ encapsulation devices are protected and prevent hyperglycemia in a mouse model of immune-mediated diabetes. Cell Transplant. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 106. | Elliott RB, Escobar L, Calafiore R, Basta G, Garkavenko O, Vasconcellos A, Bambra C. Transplantation of micro- and macroencapsulated piglet islets into mice and monkeys. Transplant Proc. 2005;37:466-469. [PubMed] |
| 107. | Sasikala M, Rao GV, Vijayalakshmi V, Pradeep R, Pothani S, Kumar PP, Gaddipati R, Sirisha G, Cheemalakonda R, Tandan M. Long-term functions of encapsulated islets grafted in nonhuman primates without immunosuppression. Transplantation. 2013;96:624-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 108. | King SR, Dorian R, Storrs RW. Requirements for encapsulation technology and the challenges for transplantation of islets of Langerhans. Graft. 2001;4:491-499. |
| 109. | Jain K, Asina S, Yang H, Blount ED, Smith BH, Diehl CH, Rubin AL. Glucose control and long-term survival in biobreeding/Worcester rats after intraperitoneal implantation of hydrophilic macrobeads containing porcine islets without immunosuppression. Transplantation. 1999;68:1693-1700. [PubMed] |
| 110. | Gazda LS, Vinerean HV, Laramore MA, Hall RD, Carraway JW, Smith BH. No evidence of viral transmission following long-term implantation of agarose encapsulated porcine islets in diabetic dogs. J Diabetes Res. 2014;2014:727483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 111. | Gazda LS, Vinerean HV, Laramore MA, Hall RD, Carraway JW, Smith BH. Pravastatin improves glucose regulation and biocompatibility of agarose encapsulated porcine islets following transplantation into pancreatectomized dogs. J Diabetes Res. 2014;2014:405362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 112. | Neufeld T, Ludwig B, Barkai U, Weir GC, Colton CK, Evron Y, Balyura M, Yavriyants K, Zimermann B, Azarov D. The efficacy of an immunoisolating membrane system for islet xenotransplantation in minipigs. PLoS One. 2013;8:e70150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 113. | Lifson N, Kramlinger KG, Mayrand RR, Lender EJ. Blood flow to the rabbit pancreas with special reference to the islets of Langerhans. Gastroenterology. 1980;79:466-473. [PubMed] |
| 114. | Jansson L. The regulation of pancreatic islet blood flow. Diabetes Metab Rev. 1994;10:407-416. [PubMed] |
| 115. | Carlsson PO, Andersson A, Jansson L. Pancreatic islet blood flow in normal and obese-hyperglycemic (ob/ob) mice. Am J Physiol. 1996;271:E990-E995. [PubMed] |
| 116. | Ohtani O, Ushiki T, Kanazawa H, Fujita T. Microcirculation of the pancreas in the rat and rabbit with special reference to the insulo-acinar portal system and emissary vein of the islet. Arch Histol Jpn. 1986;49:45-60. [PubMed] |
| 117. | Henriksnäs J, Lau J, Zang G, Berggren PO, Köhler M, Carlsson PO. Markedly decreased blood perfusion of pancreatic islets transplanted intraportally into the liver: disruption of islet integrity necessary for islet revascularization. Diabetes. 2012;61:665-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 118. | Henderson JR, Moss MC. A morphometric study of the endocrine and exocrine capillaries of the pancreas. Q J Exp Physiol. 1985;70:347-356. [PubMed] |
| 119. | Bonner-Weir S, Orci L. New perspectives on the microvasculature of the islets of Langerhans in the rat. Diabetes. 1982;31:883-889. [PubMed] |
| 120. | Bonner-Weir S. Morphological evidence for pancreatic polarity of beta-cell within islets of Langerhans. Diabetes. 1988;37:616-621. [PubMed] |
| 121. | Vajkoczy P, Menger MD, Simpson E, Messmer K. Angiogenesis and vascularization of murine pancreatic islet isografts. Transplantation. 1995;60:123-127. [PubMed] |
| 122. | Beger C, Cirulli V, Vajkoczy P, Halban PA, Menger MD. Vascularization of purified pancreatic islet-like cell aggregates (pseudoislets) after syngeneic transplantation. Diabetes. 1998;47:559-565. [PubMed] |
| 123. | Mattsson G, Jansson L, Carlsson PO. Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes. 2002;51:1362-1366. [PubMed] |
| 124. | Carlsson PO, Palm F, Mattsson G. Low revascularization of experimentally transplanted human pancreatic islets. J Clin Endocrinol Metab. 2002;87:5418-5423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 133] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 125. | Jaap AJ, Shore AC, Stockman AJ, Tooke JE. Skin capillary density in subjects with impaired glucose tolerance and patients with type 2 diabetes. Diabet Med. 1996;13:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 126. | Malik RA, Metcalfe J, Sharma AK, Day JL, Rayman G. Skin epidermal thickness and vascular density in type 1 diabetes. Diabet Med. 1992;9:263-267. [PubMed] |
| 127. | van der Veldt AA, de Boer MP, Boven E, Eringa EC, van den Eertwegh AJ, van Hinsbergh VW, Smulders YM, Serné EH. Reduction in skin microvascular density and changes in vessel morphology in patients treated with sunitinib. Anticancer Drugs. 2010;21:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 128. | Bali MA, Metens T, Denolin V, De Maertelaer V, Devière J, Matos C. Pancreatic perfusion: noninvasive quantitative assessment with dynamic contrast-enhanced MR imaging without and with secretin stimulation in healthy volunteers--initial results. Radiology. 2008;247:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 129. | Schraml C, Schwenzer NF, Martirosian P, Claussen CD, Schick F. Perfusion imaging of the pancreas using an arterial spin labeling technique. J Magn Reson Imaging. 2008;28:1459-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 130. | Cox EF, Smith JK, Chowdhury AH, Lobo DN, Francis ST, Simpson J. Temporal assessment of pancreatic blood flow and perfusion following secretin stimulation using noninvasive MRI. J Magn Reson Imaging. 2015;42:1233-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 131. | Bülow J, Tøndevold E. Blood flow in different adipose tissue depots during prolonged exercise in dogs. Pflugers Arch. 1982;392:235-238. [PubMed] |
| 132. | Heinonen I, Bucci M, Kemppainen J, Knuuti J, Nuutila P, Boushel R, Kalliokoski KK. Regulation of subcutaneous adipose tissue blood flow during exercise in humans. J Appl Physiol (1985). 2012;112:1059-1063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 133. | Heinonen I, Kemppainen J, Kaskinoro K, Knuuti J, Boushel R, Kalliokoski KK. Capacity and hypoxic response of subcutaneous adipose tissue blood flow in humans. Circ J. 2014;78:1501-1506. [PubMed] |
| 134. | Carlsson PO, Liss P, Andersson A, Jansson L. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes. 1998;47:1027-1032. [PubMed] |
| 135. | Carlsson PO, Palm F, Andersson A, Liss P. Chronically decreased oxygen tension in rat pancreatic islets transplanted under the kidney capsule. Transplantation. 2000;69:761-766. [PubMed] |
| 136. | Martinez I, Nedredal GI, Øie CI, Warren A, Johansen O, Le Couteur DG, Smedsrød B. The influence of oxygen tension on the structure and function of isolated liver sinusoidal endothelial cells. Comp Hepatol. 2008;7:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 137. | Barbu A, Jansson L, Sandberg M, Quach M, Palm F. The use of hydrogen gas clearance for blood flow measurements in single endogenous and transplanted pancreatic islets. Microvasc Res. 2015;97:124-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 138. | Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489-495. [PubMed] |
| 139. | Olsson R, Olerud J, Pettersson U, Carlsson PO. Increased numbers of low-oxygenated pancreatic islets after intraportal islet transplantation. Diabetes. 2011;60:2350-2353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 140. | Johnson AS, Fisher RJ, Weir GC, Colton , CK . Oxygen consumption and diffusion in assemlages of reprising spheres: Performance enhancement of a bioartificial pancreas. Chem Eng Sci. 2009;64:4470-4487. |
| 141. | Chow DC, Wenning LA, Miller WM, Papoutsakis ET. Modeling pO(2) distributions in the bone marrow hematopoietic compartment. I. Krogh’s model. Biophys J. 2001;81:675-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 191] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 142. | Herst PM, Berridge MV. Cell surface oxygen consumption: a major contributor to cellular oxygen consumption in glycolytic cancer cell lines. Biochim Biophys Acta. 2007;1767:170-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 130] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 143. | Yeom CJ, Goto Y, Zhu Y, Hiraoka M, Harada H. Microenvironments and cellular characteristics in the micro tumor cords of malignant solid tumors. Int J Mol Sci. 2012;13:13949-13965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 144. | Dulong JL, Legallais C. A theoretical study of oxygen transfer including cell necrosis for the design of a bioartificial pancreas. Biotechnol Bioeng. 2007;96:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 145. | Buchwald P. FEM-based oxygen consumption and cell viability models for avascular pancreatic islets. Theor Biol Med Model. 2009;6:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 146. | Dionne KE, Colton CK, Yarmush ML. Effect of hypoxia on insulin secretion by isolated rat and canine islets of Langerhans. Diabetes. 1993;42:12-21. [PubMed] |
| 147. | Buchwald P. A local glucose-and oxygen concentration-based insulin secretion model for pancreatic islets. Theor Biol Med Model. 2011;8:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 148. | Pedraza E, Coronel MM, Fraker CA, Ricordi C, Stabler CL. Preventing hypoxia-induced cell death in beta cells and islets via hydrolytically activated, oxygen-generating biomaterials. Proc Natl Acad Sci USA. 2012;109:4245-4250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 294] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 149. | Wu H, Avgoustiniatos ES, Swette L, Bonner-Weir S, Weir GC, Colton CK. In situ electrochemical oxygen generation with an immunoisolation device. Ann N Y Acad Sci. 1999;875:105-125. [PubMed] |
| 150. | Bloch K, Papismedov E, Yavriyants K, Vorobeychik M, Beer S, Vardi P. Photosynthetic oxygen generator for bioartificial pancreas. Tissue Eng. 2006;12:337-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 151. | Evron Y, Zimermann B, Ludwig B, Barkai U, Colton CK, Weir GC, Arieli B, Maimon S, Shalev N, Yavriyants K. Oxygen supply by photosynthesis to an implantable islet cell device. Horm Metab Res. 2015;47:24-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 152. | Ludwig B, Zimerman B, Steffen A, Yavriants K, Azarov D, Reichel A, Vardi P, German T, Shabtay N, Rotem A. A novel device for islet transplantation providing immune protection and oxygen supply. Horm Metab Res. 2010;42:918-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 153. | Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, Neufeld T, Block NL, Yavriyants K, Steffen A. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci USA. 2012;109:5022-5027. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 131] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 154. | Ludwig B, Reichel A, Steffen A, Zimerman B, Schally AV, Block NL, Colton CK, Ludwig S, Kersting S, Bonifacio E. Transplantation of human islets without immunosuppression. Proc Natl Acad Sci USA. 2013;110:19054-19058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 202] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 155. | Ma Z, Moruzzi N, Catrina SB, Grill V, Björklund A. Hyperoxia inhibits glucose-induced insulin secretion and mitochondrial metabolism in rat pancreatic islets. Biochem Biophys Res Commun. 2014;443:223-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 156. | Bertrand B, Mason A, Jacobs JB. A simple apparatus and a novel method of cell collection for combined light microscopic and scanning electron microscopic exfoliative cytology. Acta Cytol. 1979;23:427-428. [PubMed] |
| 157. | Chase HP, Ocrant I, Talmage DW. The effects of different conditions of organ culture on the survival of the mouse pancreas. Diabetes. 1979;28:990-993. [PubMed] |
| 158. | Mandel TE, Koulmanda M. Effect of culture conditions on fetal mouse pancreas in vitro and after transplantation in syngeneic and allogeneic recipients. Diabetes. 1985;34:1082-1087. [PubMed] |
| 159. | Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77:587-597. [PubMed] |
| 160. | Eizirik DL, Flodström M, Karlsen AE, Welsh N. The harmony of the spheres: inducible nitric oxide synthase and related genes in pancreatic beta cells. Diabetologia. 1996;39:875-890. [PubMed] |
| 161. | de Vos P, de Haan BJ, de Haan A, van Zanten J, Faas MM. Factors influencing functional survival of microencapsulated islet grafts. Cell Transplant. 2004;13:515-524. [PubMed] |
| 162. | Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell. 2011;9:11-15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1372] [Cited by in RCA: 1222] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 163. | Caplan AI, Sorrell JM. The MSC curtain that stops the immune system. Immunol Lett. 2015;168:136-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 164. | Hamilton DC, Shih HH, Schubert RA, Michie SA, Staats PN, Kaplan DL, Fontaine MJ. A silk-based encapsulation platform for pancreatic islet transplantation improves islet function in vivo. J Tissue Eng Regen Med. 2015;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 165. | Davis NE, Beenken-Rothkopf LN, Mirsoian A, Kojic N, Kaplan DL, Barron AE, Fontaine MJ. Enhanced function of pancreatic islets co-encapsulated with ECM proteins and mesenchymal stromal cells in a silk hydrogel. Biomaterials. 2012;33:6691-6697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 166. | Borg DJ, Weigelt M, Wilhelm C, Gerlach M, Bickle M, Speier S, Bonifacio E, Hommel A. Mesenchymal stromal cells improve transplanted islet survival and islet function in a syngeneic mouse model. Diabetologia. 2014;57:522-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 73] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 167. | Kerby A, Jones ES, Jones PM, King AJ. Co-transplantation of islets with mesenchymal stem cells in microcapsules demonstrates graft outcome can be improved in an isolated-graft model of islet transplantation in mice. Cytotherapy. 2013;15:192-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 168. | Luca G, Calafiore R, Basta G, Ricci M, Calvitti M, Neri L, Nastruzzi C, Becchetti E, Capitani S, Brunetti P. Improved function of rat islets upon co-microencapsulation with Sertoli’s cells in alginate/poly-L-ornithine. AAPS PharmSciTech. 2001;2:E15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 169. | Korbutt GS, Elliott JF, Rajotte RV. Cotransplantation of allogeneic islets with allogeneic testicular cell aggregates allows long-term graft survival without systemic immunosuppression. Diabetes. 1997;46:317-322. [PubMed] |
| 170. | Takemoto N, Liu X, Takii K, Teramura Y, Iwata H. Transplantation of co-aggregates of Sertoli cells and islet cells into liver without immunosuppression. Transplantation. 2014;97:287-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 171. | Ramji QA, Bayrack K, Arefanian H, Marcet-Palacios M, Bleackley RC, Rajotte RV, Rayat GR. Protection of porcine islet xenografts in mice using sertoli cells and monoclonal antibodies. Transplantation. 2011;92:1309-1315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 172. | Yin Z, Chen D, Hu F, Ruan Y, Li J, Wang L, Xiang Y, Xie L, Wang X, Ichim TE. Cotransplantation with xenogenetic neonatal porcine sertoli cells significantly prolongs islet allograft survival in nonimmunosuppressive rats. Transplantation. 2009;88:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 173. | Yang H, Wright JR. Co-encapsulation of Sertoli enriched testicular cell fractions further prolongs fish-to-mouse islet xenograft survival. Transplantation. 1999;67:815-820. [PubMed] |
| 174. | Valdés-González RA, White DJ, Dorantes LM, Terán L, Garibay-Nieto GN, Bracho-Blanchet E, Dávila-Pérez R, Evia-Viscarra L, Ormsby CE, Ayala-Sumuano JT. Three-yr follow-up of a type 1 diabetes mellitus patient with an islet xenotransplant. Clin Transplant. 2007;21:352-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 175. | Valdés-González RA, Dorantes LM, Garibay GN, Bracho-Blanchet E, Mendez AJ, Dávila-Pérez R, Elliott RB, Terán L, White DJ. Xenotransplantation of porcine neonatal islets of Langerhans and Sertoli cells: a 4-year study. Eur J Endocrinol. 2005;153:419-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 144] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 176. | Jonigk D, Al-Omari M, Maegel L, Müller M, Izykowski N, Hong J, Hong K, Kim SH, Dorsch M, Mahadeva R. Anti-inflammatory and immunomodulatory properties of α1-antitrypsin without inhibition of elastase. Proc Natl Acad Sci USA. 2013;110:15007-15012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 207] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 177. | Bergin DA, Hurley K, McElvaney NG, Reeves EP. Alpha-1 antitrypsin: a potent anti-inflammatory and potential novel therapeutic agent. Arch Immunol Ther Exp (Warsz). 2012;60:81-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 115] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 178. | Guttman O, Baranovski BM, Schuster R, Kaner Z, Freixo-Lima GS, Bahar N, Kalay N, Mizrahi MI, Brami I, Ochayon DE. Acute-phase protein α1-anti-trypsin: diverting injurious innate and adaptive immune responses from non-authentic threats. Clin Exp Immunol. 2015;179:161-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 179. | Koulmanda M, Bhasin M, Fan Z, Hanidziar D, Goel N, Putheti P, Movahedi B, Libermann TA, Strom TB. Alpha 1-antitrypsin reduces inflammation and enhances mouse pancreatic islet transplant survival. Proc Natl Acad Sci USA. 2012;109:15443-15448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 180. | Shahaf G, Moser H, Ozeri E, Mizrahi M, Abecassis A, Lewis EC. α-1-antitrypsin gene delivery reduces inflammation, increases T-regulatory cell population size and prevents islet allograft rejection. Mol Med. 2011;17:1000-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 181. | Lewis EC, Shapiro L, Bowers OJ, Dinarello CA. Alpha1-antitrypsin monotherapy prolongs islet allograft survival in mice. Proc Natl Acad Sci USA. 2005;102:12153-12158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 153] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 182. | Koulmanda M, Sampathkumar RS, Bhasin M, Qipo A, Fan Z, Singh G, Movahedi B, Duggan M, Chipashvili V, Strom TB. Prevention of nonimmunologic loss of transplanted islets in monkeys. Am J Transplant. 2014;14:1543-1551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 183. | Lewis EC, Mizrahi M, Toledano M, Defelice N, Wright JL, Churg A, Shapiro L, Dinarello CA. alpha1-Antitrypsin monotherapy induces immune tolerance during islet allograft transplantation in mice. Proc Natl Acad Sci USA. 2008;105:16236-16241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 157] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 184. | Takahashi T, Matsumoto S, Matsushita M, Kamachi H, Tsuruga Y, Kasai H, Watanabe M, Ozaki M, Furukawa H, Umezawa K. Donor pretreatment with DHMEQ improves islet transplantation. J Surg Res. 2010;163:e23-e34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 185. | Rivas-Carrillo JD, Soto-Gutierrez A, Navarro-Alvarez N, Noguchi H, Okitsu T, Chen Y, Yuasa T, Tanaka K, Narushima M, Miki A. Cell-permeable pentapeptide V5 inhibits apoptosis and enhances insulin secretion, allowing experimental single-donor islet transplantation in mice. Diabetes. 2007;56:1259-1267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 186. | Poyner DR, Hay DL. Secretin family (Class B) G protein-coupled receptors - from molecular to clinical perspectives. Br J Pharmacol. 2012;166:1-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 187. | Linnemann AK, Neuman JC, Battiola TJ, Wisinski JA, Kimple ME, Davis DB. Glucagon-Like Peptide-1 Regulates Cholecystokinin Production in β-Cells to Protect From Apoptosis. Mol Endocrinol. 2015;29:978-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 188. | Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149-5158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 461] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 189. | Farilla L, Hui H, Bertolotto C, Kang E, Bulotta A, Di Mario U, Perfetti R. Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology. 2002;143:4397-4408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 190. | Cechin SR, Pérez-Álvarez I, Fenjves E, Molano RD, Pileggi A, Berggren PO, Ricordi C, Pastori RL. Anti-inflammatory properties of exenatide in human pancreatic islets. Cell Transplant. 2012;21:633-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 191. | Schmid J, Ludwig B, Schally AV, Steffen A, Ziegler CG, Block NL, Koutmani Y, Brendel MD, Karalis KP, Simeonovic CJ. Modulation of pancreatic islets-stress axis by hypothalamic releasing hormones and 11beta-hydroxysteroid dehydrogenase. Proc Natl Acad Sci USA. 2011;108:13722-13727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 192. | Ludwig B, Ziegler CG, Schally AV, Richter C, Steffen A, Jabs N, Funk RH, Brendel MD, Block NL, Ehrhart-Bornstein M. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci USA. 2010;107:12623-12628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 193. | Huising MO, van der Meulen T, Vaughan JM, Matsumoto M, Donaldson CJ, Park H, Billestrup N, Vale WW. CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc Natl Acad Sci USA. 2010;107:912-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 194. | Rivier J, Spiess J, Thorner M, Vale W. Characterization of a growth hormone-releasing factor from a human pancreatic islet tumour. Nature. 1982;300:276-278. [PubMed] |
| 195. | Pepper AR, Gala-Lopez B, Pawlick R, Merani S, Kin T, Shapiro AM. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat Biotechnol. 2015;33:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 285] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 196. | Pileggi A, Molano RD, Ricordi C, Zahr E, Collins J, Valdes R, Inverardi L. Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation. 2006;81:1318-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 197. | Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, Bellocq JP, Pinget M, Kessler L. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transplant. 2003;12:627-635. [PubMed] |
| 198. | Kawakami Y, Iwata H, Gu YJ, Miyamoto M, Murakami Y, Balamurugan AN, Imamura M, Inoue K. Successful subcutaneous pancreatic islet transplantation using an angiogenic growth factor-releasing device. Pancreas. 2001;23:375-381. [PubMed] |
| 199. | Kawakami Y, Iwata H, Gu Y, Miyamoto M, Murakami Y, Yamasaki T, Cui W, Ikada Y, Imamura M, Inoue K. Modified subcutaneous tissue with neovascularization is useful as the site for pancreatic islet transplantation. Cell Transplant. 2000;9:729-732. [PubMed] |
| 200. | Trivedi N, Steil GM, Colton CK, Bonner-Weir S, Weir GC. Improved vascularization of planar membrane diffusion devices following continuous infusion of vascular endothelial growth factor. Cell Transplant. 2000;9:115-124. [PubMed] |
| 201. | Luan NM, Iwata H. Long-term allogeneic islet graft survival in prevascularized subcutaneous sites without immunosuppressive treatment. Am J Transplant. 2014;14:1533-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 202. | Hayon Y, Shai E, Varon D, Leker RR. The role of platelets and their microparticles in rehabilitation of ischemic brain tissue. CNS Neurol Disord Drug Targets. 2012;11:921-925. [PubMed] |
| 203. | Varon D, Hayon Y, Dashevsky O, Shai E. Involvement of platelet derived microparticles in tumor metastasis and tissue regeneration. Thromb Res. 2012;130 Suppl 1:S98-S99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 204. | Varon D, Shai E. Role of platelet-derived microparticles in angiogenesis and tumor progression. Discov Med. 2009;8:237-241. [PubMed] |
| 205. | Liu YS, Lee OK. In search of the pivot point of mechanotransduction: mechanosensing of stem cells. Cell Transplant. 2014;23:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 206. | Bhujbal SV, de Haan B, Niclou SP, de Vos P. A novel multilayer immunoisolating encapsulation system overcoming protrusion of cells. Sci Rep. 2014;4:6856. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 42] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 207. | Gilbert HT, Nagra NS, Freemont AJ, Millward-Sadler SJ, Hoyland JA. Integrin - dependent mechanotransduction in mechanically stimulated human annulus fibrosus cells: evidence for an alternative mechanotransduction pathway operating with degeneration. PLoS One. 2013;8:e72994. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 208. | Teräväinen TP, Myllymäki SM, Friedrichs J, Strohmeyer N, Moyano JV, Wu C, Matlin KS, Muller DJ, Manninen A. αV-integrins are required for mechanotransduction in MDCK epithelial cells. PLoS One. 2013;8:e71485. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 209. | Weber LM, Anseth KS. Hydrogel encapsulation environments functionalized with extracellular matrix interactions increase islet insulin secretion. Matrix Biol. 2008;27:667-673. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 106] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 210. | Weber LM, Hayda KN, Haskins K, Anseth KS. The effects of cell-matrix interactions on encapsulated beta-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials. 2007;28:3004-3011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 162] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 211. | Johansson U, Ria M, Åvall K, Dekki Shalaly N, Zaitsev SV, Berggren PO, Hedhammar M. Pancreatic Islet Survival and Engraftment Is Promoted by Culture on Functionalized Spider Silk Matrices. PLoS One. 2015;10:e0130169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 212. | Paredes-Juarez GA, Sahasrabudhe NM, Tjoelker RS, de Haan BJ, Engelse MA, de Koning EJ, Faas MM, de Vos P. DAMP production by human islets under low oxygen and nutrients in the presence or absence of an immunoisolating-capsule and necrostatin-1. Sci Rep. 2015;5:14623. [PubMed] |
| 213. | Czajkowsky DM, Shao Z. The human IgM pentamer is a mushroom-shaped molecule with a flexural bias. Proc Natl Acad Sci USA. 2009;106:14960-14965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 155] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 214. | Ahmad S, Gromiha M, Fawareh H, Sarai A. ASAView: database and tool for solvent accessibility representation in proteins. BMC Bioinformatics. 2004;5:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 213] [Article Influence: 10.1] [Reference Citation Analysis (0)] |