Published online Mar 24, 2016. doi: 10.5500/wjt.v6.i1.215
Peer-review started: April 10, 2015
First decision: September 2, 2015
Revised: October 31, 2015
Accepted: November 24, 2015
Article in press: November 25, 2015
Published online: March 24, 2016
Processing time: 343 Days and 23.8 Hours
AIM: To evaluate frequency and temporal relationship between pulmonary nodules (PNs) and transbronchial biopsy (TBBx) among lung transplant recipients (LTR).
METHODS: We retrospectively reviewed 100 records of LTR who underwent flexible bronchoscopy (FB) with TBBx, looking for the appearance of peripheral pulmonary nodule (PPN). If these patients had chest radiographs within 50 d of FB, they were included in the study. Data was compared with 30 procedures performed among non-transplant patients. Information on patient’s demographics, antirejection medications, anticoagulation, indication and type of lung transplantation, timing of the FB and the appearance and disappearance of the nodules and its characteristics were gathered.
RESULTS: Nineteen new PN were found in 13 procedures performed on LTR and none among non-transplant patients. Nodules were detected between 4-47 d from the procedure and disappeared within 84 d after appearance without intervention.
CONCLUSION: FB in LTR is associated with development of new, transient PPN at the site of TBBx in 13% of procedures. We hypothesize that these nodules are related to local hematoma and impaired lymphatic drainage. Close observation is a reasonable management approach.
Core tip: Transbronchial biopsy (TBBx) is routinely performed in lung transplant recipients (LTR). The development of pulmonary nodules (PNs) in this population is common. We investigated LTR who developed PNs post TBBx to determine the temporal relationship between the procedure and the timing of appearance and disappearance of these nodules. Our conclusion is that TBBx in LTR is associated with development of transient nodules at the site of TBBx in 13% of procedures. We hypothesize that these nodules are related to local hematoma and impaired lymphatic drainage. Close observation is a reasonable management approach.
- Citation: Mehta AC, Wang J, Abuqayyas S, Garcha P, Lane CR, Tsuang W, Budev M, Akindipe O. New Nodule-Newer Etiology. World J Transplant 2016; 6(1): 215-219
- URL: https://www.wjgnet.com/2220-3230/full/v6/i1/215.htm
- DOI: https://dx.doi.org/10.5500/wjt.v6.i1.215
Lung transplantation (LTx) is a well-accepted treatment modality for end stage pulmonary diseases such as interstitial lung disease (ILD), cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD) and pulmonary artery hypertension (PAH). Since the mid-80s more than 51000 patients have undergone lung transplantation (http://www.ishlt.org/). Flexible bronchoscopy (FB) is routinely performed in this population based on clinical grounds and/or as a surveillance to rule out subclinical rejection. LTx is being performed at our institution for over 25 years and over 1500 procedures have been performed. For the last five years we have performed an average of 900 bronchoscopies per year on this group of patients.
Peripheral pulmonary nodule (PPN) is a common clinical challenge for the pulmonologist given that it presents with a wide range of differential diagnosis. When present in the LTR, these nodules represent even a greater challenge due to the possibilities of opportunistic infection, post-transplant lymphoproliferative disorder (PTLD) and other malignancies[1].
Prompt evaluation and appropriate treatment for the PPN are essential in this high-risk population. Recently we have noticed transient appearance of PPNs in lung transplant recipients (LTR) who underwent FB with a transbronchial biopsy (TBBx). These nodules prompted diagnostic workup in some individuals but were eventually thought to be related to the procedure. The following study was carried out to evaluate the relationship between FB with TBBx and the new PPN in this group focusing on the nodule’s characteristics and the temporal relationship with the procedure.
We retrospectively reviewed 100 bronchoscopy records of LTR who underwent FB with TBBx between January 2013 and March 2014 at our institution. If either a chest X-ray or a computed tomography (CT) was performed within 50 d of the procedure on these patients they were considered for the study. Patients with preexisting lung nodule of known or unknown etiology prior to the FB were excluded from the study.
PPN was defined as a focal pulmonary lesion or opacity, round or oval in shape, which measured less than 3 cm in diameter and appeared within 50 d after the bronchoscopy.
Data collection included patient demographics, antirejection and anticoagulation medication used, indication and type of lung transplantation (single vs bilateral), timing of the FB in relation to the transplantation, site of the TBBx, bronchoscopy complications, histological findings and microbiological culture results, number of the nodules, site, shape, size and presence or absence of cavitation. Once a nodule was detected all available post-bronchoscopy radiographic studies were reviewed to judge the outcome of the nodule and/or the day of disappearance. The day of appearance and disappearance of the nodule was also tabulated. The patient’s clinical status was noted and was correlated with the appearance and disappearance of the nodules from the available medical records.
A control group was created by reviewing bronchoscopy records of non-transplant patients who underwent FB with TBBx during the same period and had a chest radiograph performed within 50 d of the procedure. Similar data as in the LTR was collected from these patients if they were found to have a PPN.
A surveillance bronchoscopy is routinely performed at our institution among the LTR at 3, 6 and 12 wk, and 6, 9 and 12 mo following the LTx. If rejection is detected, a follow-up bronchoscopy is performed 3 wk following the completion of appropriate treatment. A clinical bronchoscopy is performed on an as needed basis. All bronchoscopies are performed under conscious sedation and fluoroscopic guidance. A bronchoalveolar lavage (BAL) is obtained from a non-dependent portion of the lung in all patients to stain and/or culture for opportunistic infections.
For the surveillance procedure, our common practice is to obtain a total of 6 pieces of tissue in a single lung transplant (SLTx) recipient and 8 pieces of tissue in recipients of bilateral transplant (BLTx). All the biopsies are obtained from either a single segment or two separate segments of the dependent lobe of the lung at the discretion of the bronchoscopist. All tissue specimens are processed for histological examination in an usual fashion. When antibody mediated rejection (AMR) is suspected, biopsies are sent for C3d and C4d immunofluorescent staining.
The Institutional Review Board of the Cleveland Clinic, Cleveland, Ohio, approved the study. Due to the retrospective nature of the study, there was no need to obtain patient consent.
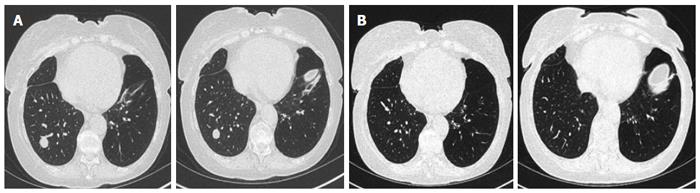
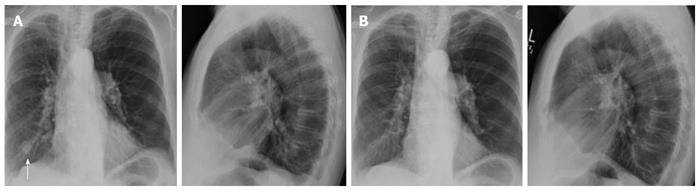
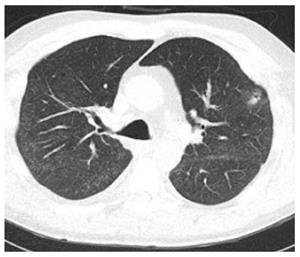
In the LTR group, we found 19 new nodules after 13 procedures performed on 10 LTR patients (Tables 1 and 2). All nodules were found at the same site of the TBBx (Figures 1 and 2). Nine of these nodules were rounded (47%) and 10 were oval in shape (53%). Fourteen nodules were solid (74%) and 5 were cavitary in nature (26%) (Figure 3). Nodule size (greatest diameter) ranged between 0.4 to 3 cm with a mean of 1.4 cm. Nodules were detected within 4 to 47 d (mean 25 d) after the FB with TBBx and they disappeared within 9 to 84 d (mean: 38.3).
| Patient | Sex | Age | Indication for LTX | Type of LTX | Anticoagulation |
| 1 | M | 71 | IPF/UIP | Right | Warfarin |
| 2 | F | 42 | COPD | Right | |
| 3 | F | 60 | CB | Bil | |
| 4 | F | 54 | PVOD | Bil | LMWH |
| 5 | M | 62 | COPD | Bil | |
| 6 | M | 69 | IPF | Left | |
| 7 | M | 29 | PVOD | Bil | |
| 8 | F | 50 | ILD/MCTD/PSS | Bil | |
| 9 | M | 32 | ILD/PSS with PHTN | Bil | |
| 10 | M | 31 | CF | Bil |
| FB | DOA | DOD | n | Size (cm) | Shape | Nature | Location |
| 1 | 21 | 71 | 1 | 1.1 | Round | Solid | RML |
| 2 | 17 | 84 | 1 | 2.3 | Round | Solid | RLL |
| 3 | 16 | 12 | 2 | 1.2, 2.2 | Round oval | Solid | RLL |
| 4 | 13 | 60 | 2 | 1.1, 3 | Round oval | Solid | RLL |
| 5 | 27 | 25 | 2 | 1 × 0.7, 0.5 × 0.4 | Oval | Solid | LUL, LLL |
| 6 | 14 | 33 | 1 | 1 × 1.1 | Oval | Solid | RML |
| 7 | 4 | 9 | 1 | 1.5 × 2.5 | Oval | Cavitary | LUL |
| 8 | 21 | 33 | 1 | 1 × 1.1 | Oval | Solid | LUL |
| 9 | 10 | 19 | 1 | 2.2 | Round | Solid | LLL |
| 10 | 8 | 53 | 1 | 1.4 × 1.1 | Oval | Cavitary | LUL |
| 11 | 4 | 37 | 4 | 2, 2, 2, 1.2 | Round | Cavitary | LUL, LLL 3 |
| Solid | |||||||
| 12 | 28 | 48 | 1 | 0.4 | Round | Cavitary | LLL |
| 13 | 47 | 35 | 1 | 0.7 | Round | Cavitary | RLL |
The male to female ratio was (1.5:1), age ranged between 29 to 71 years with a mean of 39.3 years. In these patients, LTx was performed for different indications, IPF in two patients, COPD in two patients, constrictive bronchiolitis in one patient, CF in one patient, pulmonary veno-occlusive disease in two patients, interstitial lung disease due to progressive systemic sclerosis in one patient and mixed connective tissue disease in one patient. Seven of these patients had BLTx (70%) and 3 SLTx (30%). Eight of them were on antirejection medication, Tacrolimus. Two patients were on chronic anticoagulation with either warfarin or low molecular weight heparin (LMWH) in which the therapy was appropriately stopped prior to the procedure. Two patients were on aspirin. Complications reported included minimal bleeding of less than 40 mL in seven procedures, one procedure had more than 40 mL blood loss.
In five patients, no acute or chronic rejection was found. Mild acute vascular rejection was found in two patients, mild acute rejection in three patients, chronic airway rejection in one and in one more patient scattered giant cells were found on the biopsy.
Other associated radiographic findings that were reported included blunting of the right costophrenic angle in one patient, mosaic attenuation and scattered ground glass opacities in another patient.
In all 13 procedures, the results of BAL were negative for viral, bacterial, mycobacterial and fungal infections.
In the control group, there were 30 patients. The indications for the FB with TBBx included (many of them did have confirmed diagnosis): Sarcoidosis, cryptogenic organizing pneumonia (COP), ILD, MCTD, bronchiolitis, asthma and COPD. No new nodules were detected in this group of patients.
Part of the success of lung transplant is attributed to the flexible bronchoscopy. Most patients either undergo surveillance or require a clinical bronchoscopy with TBBx to rule out rejection, infection or malignancy. Even though there is no proven benefit of surveillance bronchoscopy over clinically indicated procedures, the former has been accepted as a common practice for early detection of subclinical rejection[1-3].
It is a conservative estimate that over 200000 pulmonary nodules will be detected in year 2014 in the United States, outside the lung cancer screening program[4].
PPNs are a common radiographic finding, and are still considered a clinical dilemma. The PPN among LTR is of added significance as it involves differential diagnosis such as PTLD (39%), Invasive Pulmonary Aspergillosis (IAP) (37%) and other opportunistic infections[5-8].
Our study revealed that LTRs are also at risk of developing PPN nodule following a TBBx. This finding is rarely reported in the literature[9-11].
This finding is unique to the transplant population as it was not detected in our control group. These nodules can develop in 13% of the procedures performed on LTR. The location suggests that they developed directly as a result of the TBBx and are most likely due to a local hematoma and impaired pulmonary lymphatic drainage in the LTR[12]. We speculate that size of the nodule may depend upon the number of samples obtained from a single location.
The nodules could be single, multiple, solid, round, oval solid or cavitating. They seem to be associated with neither infection nor rejection and not related to the type of transplantation. They could appear as early as 4 d after the FB and may take up to 86 d to resolve. Given the fact that they resolve spontaneously, their diagnosis and management require only a good temporal relationship and a close follow-up.
As compared to the early 80s a larger number of lung transplants are being performed today including in patients with selected co-morbidities. Besides, today we rely on chest CT scans more than on plain chest X-rays. These may be the reasons behind the delayed recognition of these iatrogenic pulmonary nodules.
The weakness of our study is that we could recruit very few patients in our control group as rarely non-transplant recipients underwent radiographic studies following the bronchoscopy. We sincerely doubt that this would have affected our observations as TBBxs have been performed in non-transplant recipients for over 40 years and no PPN have been reported in this group.
All physicians involved in caring for LTRs should be cognizant of this newer iatrogenic etiology of a PPN. The awareness will avoid unnecessary, expensive work up in this unique group of patients.
Peripheral pulmonary nodule (PPN) is a common clinical challenge. This entity is even more challenging when detected in lung transplant recipients (LTR). Flexible bronchoscopy (FB) is routinely performed following lung transplantation. The authors incidentally noted development of new PPN in LTR following a FB with a transbronchial biopsy (TBBx). This finding has a potential to initiate unnecessary diagnostic work-up. Purpose of the study was to evaluate frequency and the temporal relationship between the nodule and the TBBx among the LTR, with an intention to avoid unwarranted testing.
Lung nodules are commonly found in LTR. Previous reports have focused on infection, malignancy and rejection as potential causes. The study revealed that LTRs are also at risk of developing PPN nodule following a TBBx. The authors aim to raise the awareness of such nodules with a goal to avoid unwarranted testing.
In this study, the occurrence of PPN following TBBx in LTR was 13% compared to 0% in non LTR. The focus of our study, in comparison to others, was to investigate these temporary nodules (size, time of appearance and disappearance, shape and consistency).
All physicians involved in caring for LTRs should be cognizant of this newer iatrogenic etiology of a PPN. The awareness will avoid unnecessary, expensive work up in this unique group of patients.
FB with TBBx: Flexible bronchoscopy with the application of transbronchial biopsy, is a commonly used method for routine surveillance as well as clinically indicated procedures in LTR.
This is a well organized manuscript. The authors incidentally noted development of new PPN in LTR following a FB with a TBBx. This finding has a potential to initiate unnecessary diagnostic work-up.
P- Reviewer: Deng B S- Editor: Ji FF L- Editor: A E- Editor: Wang CH
| 1. | Lee P, Minai OA, Mehta AC, DeCamp MM, Murthy S. Pulmonary nodules in lung transplant recipients: etiology and outcome. Chest. 2004;125:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Trulock EP, Ettinger NA, Brunt EM, Pasque MK, Kaiser LR, Cooper JD. The role of transbronchial lung biopsy in the treatment of lung transplant recipients. An analysis of 200 consecutive procedures. Chest. 1992;102:1049-1054. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 141] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 3. | Glanville AR. The role of bronchoscopic surveillance monitoring in the care of lung transplant recipients. Semin Respir Crit Care Med. 2006;27:480-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | McWilliams TJ, Williams TJ, Whitford HM, Snell GI. Surveillance bronchoscopy in lung transplant recipients: risk versus benefit. J Heart Lung Transplant. 2008;27:1203-1209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 5. | Stoller JK, Ahmad M, Rice TW. Solitary pulmonary nodule. Cleve Clin J Med. 1988;55:68-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Millet B, Higenbottam TW, Flower CD, Stewart S, Wallwork J. The radiographic appearances of infection and acute rejection of the lung after heart-lung transplantation. Am Rev Respir Dis. 1989;140:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 57] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Davies B, Ghosh S, Hopkinson D, Vaughan R, Rocco G. Solitary pulmonary nodules: pathological outcome of 150 consecutively resected lesions. Interact Cardiovasc Thorac Surg. 2005;4:18-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Narula T, Machuzak MS, Mehta AC. Newer modalities in the work-up of peripheral pulmonary nodules. Clin Chest Med. 2013;34:395-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Root JD, Molina PL, Anderson DJ, Sagel SS. Pulmonary nodular opacities after transbronchial biopsy in patients with lung transplants. Radiology. 1992;184:435-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Mehta AC, Wang J, Singh J, Cicenia J. Iatrogenic pulmonary nodule in a heart transplant recipient. Case Rep Pulmonol. 2014;2014:546209. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Daly BD, Martinez FJ, Brunsting LA, Deeb GM, Cascade PN, Lynch JP. High-resolution CT detection of lacerations in the transplanted lung after transbronchial biopsy. J Thorac Imaging. 1994;9:160-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Wagner EM, Blosser S, Mitzner W. Bronchial vascular contribution to lung lymph flow. J Appl Physiol (1985). 1998;85:2190-2195. [PubMed] |