Published online Dec 24, 2015. doi: 10.5500/wjt.v5.i4.320
Peer-review started: June 28, 2015
First decision: August 26, 2015
Revised: October 6, 2015
Accepted: November 23, 2015
Article in press: November 25, 2015
Published online: December 24, 2015
Processing time: 180 Days and 7.9 Hours
AIM: To describe our single-centre experience in liver transplantation (LT) with the infusion of high perioperative thymoglobulin doses. The optimal dosage and timing of thymoglobulin® [antithymocyte globulin (ATG)] administration during LT remains controversial. Cytokine release syndrome, haemolytic anaemia, thrombocytopenia, neutropenia, fever and serum sickness are potential adverse effects associated with ATG infusion.
METHODS: Between December 2009 and December 2010, 16 adult non-randomized patients (ATG group), receiving a liver graft from a deceased donor, received an intraoperative infusion (4-6 h infusion) of thymoglobulin (3 mg/kg, ATG: Thymoglobuline®). These patients were compared (case control approach) with 16 patients who had a liver transplant without ATG treatment (control group) to evaluate the possible effects of intraoperative ATG infusion. The matching parameters were: Sex, recipient age (± 5 years), LT indication including viral status, MELD score (± 5 points), international normalized ratio and platelet count (as close as possible). The exclusion criteria for both groups included the following: Multi-organ or living donor transplant, immunosuppressive therapy before transplantation, contraindications to the administration of any thymocyte globulin, human immunodeficiency virus seropositivity, thrombocytopenia [platelet < 50000/μL] or leukopenia [white blood cells < 1000/μL]. The perioperative side effects (haemodynamic alterations, core temperature variations, colloids and crystalloids requirements, and surgical time) possibly related to ATG infusion and the thromboelastographic (TEG) evaluation of the ATG effects on coagulation, blood loss and blood product transfusion were analysed during the operation and the first three postoperative days.
RESULTS: Intraoperative ATG administration was associated with longer surgical procedures [560 ± 88 min vs 480 ± 83 min (control group), P = 0.013], an intraoperative core temperature more than 37 °C (50% of ATG patients vs 6.2% of control patients, P = 0.015), major intraoperative blood loss [3953 ± 3126 mL vs 1419 ± 940 mL (control group), P = 0.05], higher red blood cell [2092 ± 1856 mL ATG group vs 472 ± 632 mL (control group), P = 0.02], fresh frozen plasma [671 ± 1125 mL vs 143 ± 349 mL (control group), P = 0.015], and platelet [374 ± 537 mL vs 15.6 ± 62.5 mL (control group), P = 0.017] transfusion, and a higher requirement for catecholamines (0.08 ± 0.07 μg/kg per minutes vs 0.01 ± 0.38 μg/kg per minutes, respectively, in the ATG and control groups) for haemodynamic support. The TEG tracings changed to a straight line during ATG infusion (preanhepatic and anhepatic phases) in 81% of the patients from the ATG group compared to 6.25% from the control group (P < 0.001). Patients from the ATG group compared to controls had higher post-op core temperatures (38 °C ± 1.0 °C vs 37.3 °C ± 0.5 °C; P = 0.02), an increased need of noradrenaline (43.7% vs 6.25%, P = 0.037), received more platelet transfusions (31.5% vs 0%, P = 0.04) and required continuous renal replacement therapy (4 ATG patients vs none in the control group; P = 0.10). ATG infusion was considered the cause of a fatal anaphylactic shock and of a suspected adverse reaction that led to intravascular haemolysis and acute renal failure.
CONCLUSION: The side effects and the coagulation imbalance observed in patients receiving a high dosage of ATG suggest caution in the use of thymoglobulin during LT.
Core tip: The optimal management, in terms of dosing and timing of thymoglobulin® [antithymocyte globulin (ATG)] administration, during liver transplantation (LT) remains controversial. Several adverse effects associated with ATG infusion have been described, but the perioperative effects of ATG administration, with particular regard to coagulation and haemodynamic consequences, in patients who received a LT have never been described. Perioperative ATG administration was associated with a significantly longer surgical procedure, higher core temperature, blood loss, blood product transfusion, a higher requirement for catecholamines and continuous renal replacement therapy. The side effects and the coagulation imbalance observed in patients receiving a high dosage of ATG suggest caution in the use of thymoglobulin during LT.
- Citation: De Pietri L, Serra V, Preziosi G, Rompianesi G, Begliomini B. Perioperative effects of high doses of intraoperative thymoglobulin induction in liver transplantation. World J Transplant 2015; 5(4): 320-328
- URL: https://www.wjgnet.com/2220-3230/full/v5/i4/320.htm
- DOI: https://dx.doi.org/10.5500/wjt.v5.i4.320
Immunomodulation is a challenging aspect of organ transplantation. Polyclonal antithymocyte globulin (ATG) preparations have been used since the late 1960s[1] for the prevention and treatment of acute rejection in solid organ transplantation[2-4]. Thymoglobulin (thymoglobulin®), a rabbit-derived polyclonal antibody, belongs to this category of agents[5-9].
The polyclonal antithymocyte antibodies (“induction agents”), administered preoperatively, allow doctors to minimize the use of the nephrotoxic calcineurin inhibitors[6], to reduce overall steroid use[10] and to eliminate the need for maintenance immunosuppression[8], promoting tolerance in organ recipients by donor leucocyte augmentation. Despite extensive clinical use, its pharmacology and mechanisms of action in vivo remain mostly unknown[11]. ATG produces a variety of biological effects that go beyond T-cell depletion; it is not specific for T-cells, and its antibodies are directed against different blood cell types (B-cells, natural killer, platelets, and erythrocytes)[2]. The optimal dosage and duration of treatment are still uncertain[12]. Cytokine release syndrome, haemolytic anaemia, thrombocytopenia, neutropenia, fever and serum sickness are potential adverse effects, and the associated short-term costs need to be planned[2,11,13]. For these reasons, many centres are hesitant to routinely treat transplant recipients with polyclonal antibody induction[14].
From the available literature we know that the incidence of adverse effects after ATG administration has not been evaluated in the intra and immediate postoperative period, and this is the reason why our understanding of the role of thymoglobulin as an induction therapy in liver transplantation (LT) is still evolving. Immunosuppressive agents can induce thrombocytopenia, worsening a perioperative coagulation imbalance in patients whose bleeding control is already compromised because of end-stage liver disease. For this reason, the present study has been designed to evaluate the perioperative effects of ATG administration during a liver transplant with particular regard to ATG effects on coagulation.
Between December 2009 and December 2010, 16 consecutive non-randomized adult patients (ATG group), receiving a liver graft from a deceased donor, were treated intraoperatively with the immunosuppressive induction agent thymoglobulin (ATG: Thymoglobuline®). These patients were retrospectively compared (case control approach) with 16 patients who had a liver transplant without ATG treatment (control group) to evaluate the possible effects of intraoperative ATG infusion. All of the patients provided a written informed consent. The study protocol was approved by the Institutional Review Board of Azienda Ospedaliera-Universitaria, Modena (N°:23/2009) and was conducted in accordance with provisions of the Declaration of Helsinki and good clinical practice guidelines. The matching parameters were: Sex, recipient age (± 5 years), LT indication including viral status, MELD score (± 5 points), INR and platelet count (as close as possible).
Exclusion criteria for both groups were: Multi-organ or living donor transplant, immunosuppressive therapy before transplantation, contraindications to the administration of any thymocyte globulin, HIV seropositivity, thrombocytopenia [platelet (PLT) < 50.000/μL] or leukopenia [white blood cells (WBC) < 1000/μL].
In the ATG group, thymoglobulin (3 mg/kg) was administered as a continuous infusion between the induction of anaesthesia and graft reperfusion (usually a 4-6 h period). All of the patients of this group were given paracetamol (500 mg), chlorphenamine (10 mg) and methylprednisolone (500 mg) 45-60 min before starting ATG infusion to prevent cytokine release syndrome. Tacrolimus (Advagraf®, 0.1 mg/kg once a day) and everolimus (Certican®, 0.25 mg twice daily) were started on the first postoperative day in 9 patients and tacrolimus (Advagraf® 0.1 mg/kg once a day) alone in 7 patients. All of the 16 patients also received prednisone (5 mg a day) for 12 mo.
The patients in the control group received methylprednisolone (1000 mg) at the end of the anhepatic phase and were treated according to our standard immunosuppression protocol (tacrolimus (Advagraf®) 0.1 mg/kg from the first postoperative day and prednisone 5 mg a day for 12 mo).
The data analysis included the intraoperative time and the first three postoperative days.
The primary endpoint of this study was the evaluation of the side effects possibly related to ATG infusion during the surgical procedure and intensive care treatment (postoperative days 1 to 3). Thromboelastographic (TEG) evaluation of the effects of ATG on coagulation, blood loss and blood product transfusion was another aim of our study.
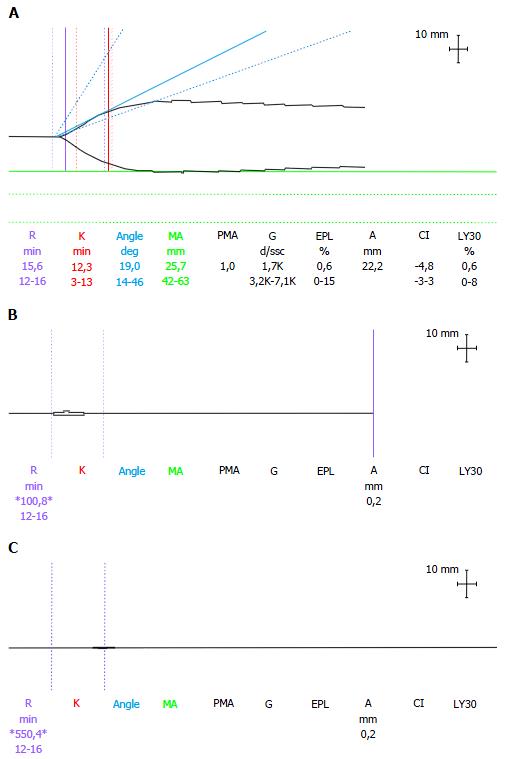
Arterial blood samples and TEG tracing were scheduled at the following points: Induction of anaesthesia (baseline), laparotomy, end of the pre-anhepatic and anhepatic phase, and 30, 60 and 120 min post-reperfusion. The TEG variables analysed were reaction time (R-time: 12-26 min), clot formation time (K-time: 3-13 min), α angle (14°-46°) and maximum amplitude (MA: 42-63 mm). The normal ranges, for native whole-blood samples, were derived from the observed values in our population of cirrhotic patients. Extremely long (> 60 min) or not detectable (n/a) values for the R-time and K-time, and values of 0° for the α-angle and 0 mm for the MA, were read arbitrarily as straight line traces. Clot formation was triggered by contact activation. Cups containing heparinase were used after reperfusion to avoid interference from heparin from the liver graft.
Other recorded variables were: Operative time, pulmonary arterial blood temperature (°C), amounts of fluids and blood products infused (or processed and re-infused by the cell-saver), estimated blood loss (mL), and the use of fibrinogen (g), tranexamic acid (mg), and bicarbonate (mEq of HCO3- 8.4%). The haemodynamic variables considered were: Mean arterial pressure (MAP), the systemic vascular resistance index (SVRI) and the end diastolic volume index (EDVI). In addition, the noradrenaline and/or adrenaline requirements before and after reperfusion and the total urine output were recorded.
Where applicable, the same variables were recorded from the first (POD1) to the third (POD3) post-operative day. The blood samples were collected daily to determine haemoglobin, haematocrit, full laboratory coagulation and liver profile, urea and creatinine. The intensive care unit (ICU) stay, need for invasive ventilation (hours), renal replacement therapy and return to the operating room were also recorded.
During hospitalization, complete laboratory investigations and screening for viral, bacterial or fungal infections were performed during the follow-up period (1 mo).
The diagnosis of acute rejection had to be proven by histological investigation during the hospital stay as well as during the follow-up.
The results of the comparison of the different variables, if not differently specified in the text or in the tables, were not significant.
Continuous data were reported as the mean ± SD and were compared by using the Wilcoxon matched pairs test. Comparisons between groups for categorical variables were performed using the χ2 test with Yates’ correction or the Fisher’s exact test when appropriate. The statistical significance was set at P < 0.05. IBM© SPSS© Statistics Version 19.0 was used to perform the statistical analysis. The statistical review of the study was performed by a biomedical statistician.
Table 1 shows the preoperative characteristics of the two groups, which did not show any significant differences in terms of age, sex, clinical features, MELD score and clotting parameters. The donor characteristics for both groups were similar in regard to age, gender, cause of death and steatosis.
| Characteristics | ATG group | Control group | P | |
| Gender | Male | 12 (75%) | 15 (93.8%) | 0.33 |
| Female | 4 (25%) | 1 (6.2%) | ||
| Age | yr | 59.8 ± 8.3 | 55.9 ± 8.8 | 0.08 |
| Cause of liver disease | Viral cirrhosis | 10 (56.3%) | 10 (56.3%) | 1 |
| Alcoholic | 4 (25%) | 4 (25%) | ||
| Cholestatic | 1 (6.3%) | 1 (6.3%) | ||
| Hemochromatosis | 1 (6.3%) | 1 (6.3%) | ||
| HCV | Pos | 7 (43.8%) | 6 (37.5%) | 1 |
| HBV | Pos | 4 (25%) | 3 (18.8%) | 1 |
| HCC | Yes | 6 (37.5%) | 7 (43.8%) | 1 |
| MELD score | 11.6 ± 6.5 | 11.6 ± 4.6 | 0.95 | |
| INR | 1.29 ± 0.34 | 1.44 ± 0.59 | 0.57 | |
| PLT | 103/μL | 97.2 ± 50 | 94.1 ± 37.1 | 0.3 |
Patients in the ATG group underwent longer surgical operations [560 ± 88 min (ATG) vs 480 ± 83 min (control); P = 0.013] had major blood loss (P=0.05) and received more red blood cell (RBC) (P = 0.02), fresh frozen plasma (P = 0.015), and PLT (P = 0.017) transfusions. The patients were haemodynamically unstable after thymoglobulin infusion, requiring more crystalloid and catecholamine infusion, and had a higher central blood temperature (P = 0.015) compared to the control group (Table 2). In the ATG group, the pH was lower (P = 0.01) and the base excess more negative before (P = 0.005) and after (P = 0.03) reperfusion (Table 2). Marked reductions of the MAP and the SVRI were not related to the decreased EDVI and were treated with higher dosages of inotropes at all stages of the operation (Table 3).
| Intraoperative parameters recorded | ATG group | Control group | P |
| No. of patients transfused with RBC | 16 (100%) | 7 (43.8%) | 0.01 |
| Intraoperative blood loss (mL) | 3953 ± 3126 | 1419 ± 940 | 0.05 |
| Omologous blood transfused (mL) | 2092 ± 1856 | 472 ± 632 | 0.02 |
| Crystalloids (mL) | 11356 ± 4419 | 6771 ± 2416 | 0.008 |
| PLT transfused (g) | 374 ± 537 | 15.6 ± 62.5 | 0.017 |
| FFP transfused (g) | 671 ± 1125 | 143 ± 349 | 0.015 |
| No. of patients transfused with PLT | 8 (50%) | 1 (6.2%) | 0.015 |
| No. of patients transfused with FFP | 9 (56.2%) | 3 (18.8%) | 0.07 |
| No. of patients with core temperature > 37 °C | 8 (50%) | 1 (6.2%) | 0.015 |
| No. of patients who received Noradrenaline before reperfusion | 11 (68.8%) | 3 (18.8%) | 0.013 |
| Mean dosage of noradrenaline infused before reperfusion (μg/kg per minute) | 0.08 ± 0.07 | 0.01 ± 0.38 | 0.03 |
| Mean dosage of noradrenaline infused after reperfusion (μg/kg per minute) | 0.2 ± 0.07 | 0.10 ± 0.11 | 0.029 |
| No. of patients who received noradrenaline before and after reperfusion | 11 (68.8%) | 3 (18.8%) | 0.013 |
| Mean dosage of adrenaline infused at reperfusion (g) | 106 ± 86 | 28 ± 21 | 0.047 |
| pH before reperfusion | 7.29 ± 0.85 | 7.36 ± 0.5 | 0.01 |
| BE before reperfusion | -7.74 ± 4 | -4.7 ± 2.6 | 0.005 |
| pH after reperfusion | 7.24 ± 0.7 | 7.3 ± 0.5 | 0.03 |
| BE after reperfusion | -8.7 ± 3.2 | -6.5 ± 2.4 | 0.02 |
| Phases of liver transplantation and post-op days 1 and 2 | Groups | MAP | CI | SVRI | RVEDVI | ||||
| mmHg | P | L/min per square meter | P | dyne/s/cm5 per square meter | P | mL/m2 | P | ||
| Basal | ATG | 75 ± 10 | 0.18 | 5.1 ± 3.5 | 0.91 | 1100 ± 215 | 0.31 | 219 ± 75 | 0.12 |
| Control | 70 ± 8 | 4.9 ± 2.8 | 1150 ± 324 | 222 ± 58 | |||||
| Laparotomy | ATG | 67 ± 24 | 0.16 | 5.7 ± 3.4 | 0.41 | 730 ± 337 | < 0.01 | 259 ± 120 | 0.21 |
| Control | 70 ± 31 | 5.8 ± 2 | 1235 ± 488 | 262 ± 98 | |||||
| Pre-anhepatic | ATG | 52 ± 19 | < 0.01 | 6.5 ± 2.9 | 0.3 | 540 ± 188 | < 0.01 | 340 ± 99 | 0.65 |
| Control | 69 ± 25 | 5.9 ± 3.1 | 1145 ± 338 | 300 ± 78 | |||||
| Anhepatic | ATG | 68 ± 12 | 0.16 | 7.5 ± 3.6 | 0.69 | 598 ± 213 | < 0.01 | 335 ± 98 | 0.61 |
| Control | 73 ± 23 | 6.3 ± 3.9 | 988 ± 238 | 308 ± 115 | |||||
| 30’ post-reperfusion | ATG | 48 ± 24 | 0.04 | 7.1 ± 3.1 | 0.7 | 510 ± 343 | < 0.05 | 368 ± 75 | 0.63 |
| Control | 59 ± 31 | 6.6 ± 3.8 | 855 ± 417 | 355 ± 58 | |||||
| 60’ post-reperfusion | ATG | 47 ± 33 | < 0.01 | 7.2 ± 4.1 | 0.61 | 521 ± 243 | < 0.001 | 300 ± 100 | 0.78 |
| Control | 66 ± 28 | 6.6 ± 3.8 | 945 ± 301 | 289 ± 125 | |||||
| 120’ post-reperfusion | ATG | 55 ± 38 | 0.07 | 7.6 ± 4.3 | 0.04 | 788 ± 306 | 0.06 | 345 ± 107 | 0.005 |
| Control | 65 ± 23 | 5.9 ± 3.8 | 1100 ± 398 | 268 ± 99 | |||||
| POD1 | ATG | 60 ± 32 | < 0.05 | 6.8 ± 4.1 | 0.51 | 795 ± 341 | 0.01 | 305 ± 106 | 0.01 |
| Control | 71 ± 40 | 6 ± 3.5 | 1100 ± 287 | 233 ± 102 | |||||
| POD2 | ATG | 67 ± 26 | 0.15 | 6.1 ± 2.5 | 0.43 | 1130 ± 438 | < 0.08 | 238 ± 143 | 0.33 |
The thromboelastograph tracings were similar in both groups at baseline. In the ATG group, worsening hypocoagulability became evident on the TEG from laparotomy to graft reperfusion (Table 4). During thymoglobulin infusion, the TEG changed to a straight line in 13 (81%) patients in the ATG group, but only one patient (6.25%) in the control group showed the same trace, which was limited to the postreperfusion phase (P < 0.001, Figure 1). Five (31%) patients of the ATG group, and none of the control group, had K, α and MA values not detectable at one or more of the scheduled times of observation (mainly from laparotomy to the anhepatic phase). Eight (50%) patients from the ATG group, compared to only one (6.25%) from the control group (after reperfusion), had an undetectable K value at one or more of the scheduled times of observation, beginning from laparotomy and continuing after the reperfusion phase.
| Measurements available for comparison of TEG variables (ATG vs control) | Groups | P | ||
| ATG | Control | |||
| R basal (min) | 16 vs 16 | 17.7 ± 9.6 | 29.9 ± 20.5 | 0.059 |
| K basal (min) | 16 vs 16 | 9.7 ± 3.39 | 18.7 ± 11.7 | 0.007 |
| α basal (degrees) | 16 vs 16 | 25 ± 9.8 | 16.2 ± 10.2 | 0.02 |
| MA basal (mm) | 16 vs 16 | 41.7 ± 8.8 | 40.2 ± 12.7 | 0.06 |
| MA laparotomy (mm) | 14 vs 16 | 31.5 ± 20.4 | 49.2 ± 11.4 | 0.002 |
| R pre-anhepatic (min) | 16 vs 16 | 41.4 ± 49.4 | 15.9 ± 6.0 | 0.017 |
| α pre-anhepatic (degrees) | 13 vs 16 | 12.4 ± 13.8 | 25.9 ± 11.5 | 0.007 |
| MA pre-anhepatic (mm) | 13 vs 16 | 21.2 ± 19 | 42.2 ± 12.7 | 0.002 |
| R anhepatic (min) | 16 vs 16 | 79.6 ± 117.7 | 14.7 ± 4.1 | 0.02 |
| K anhepatic (min) | 6 vs 16 | 4.6 ± 8.3 | 9 ± 3.5 | 0.01 |
| α anhepatic (degrees) | 13 vs 16 | 11.9 ± 14.4 | 26.0 ± 8.5 | 0.017 |
| MA anhepatic (mm) | 13 vs 16 | 17.7 ± 19.8 | 41.5 ± 9.5 | 0.004 |
| K 30’ post-reperfusion (min) | 8 vs 15 | 3.8 ± 6.3 | 8.0 ± 3.3 | 0.012 |
| MA 30’ post-reperfusion (mm) | 15 vs 16 | 20.4 ± 17.8 | 40.0 ± 8.9 | 0.003 |
| K 60’ post-reperfusion (min) | 9 vs 16 | 5.3 ± 6.7 | 9.1 ± 2.2 | 0.05 |
| MA 60’ post-reperfusion (mm) | 15 vs 16 | 24.1 ± 16.8 | 41.7 ± 6.2 | 0.02 |
| MA 120’ post-reperfusion (mm) | 16 vs 16 | 25.3 ± 12.4 | 40.4 ± 8.7 | 0.008 |
Tranexamic acid (328 ± 394 mg) was given to nine patients (56%) in the ATG group, but none of the controls, to treat thromboelatographic and clinical signs of fibrinolysis (P = 0.001).
After the surgical procedure, all of the variables examined, with the exception of the number of patients transfused with RBC and fresh frozen plasma, were significantly worse in ATG patients than in controls on POD1. The WBC counts and PLT numbers remained statistically lower in the ATG group until POD2 (P = 0.009) and POD3 (P = 0.02) (Table 5).
| Parameters | ATG group | Control group | P | ||||||
| POD1 | POD2 | POD3 | POD1 | POD2 | POD3 | P1 | P2 | P3 | |
| Maximum core temperature (°C) | 38 ± 1.0 | 37.3 ± 0.77 | 37 ± 0.43 | 37.3 ± 0.5 | 37.1 ± 0.48 | 37.0 ± 0.62 | 0.02 | 0.4 | 1 |
| Creatinine (mg/dL) | 1.13 ± 0.56 | 1.22 ± 0.68 | 1.52 ± 1.02 | 0.77 ± 0.14 | 0.81 ± 0.8 | 0.96 ± 0.55 | 0.03 | 0.6 | 0.7 |
| WBC (103×μL) | 6.9 ± 2.9 | 8.2 ± 2.7 | 8.2 ± 3.4 | 10.9 ± 3.6 | 12.6 ± 6.5 | 12.3 ± 6.3 | 0.01 | 0.009 | 0.9 |
| PLT (103×μL) | 37 ± 15 | 38 ± 18 | 22 ± 8 | 72 ± 37 | 60 ± 31 | 35 ± 19 | 0.001 | 0.01 | 0.02 |
| HCT (%) | 26.3 ± 5.6 | 27.3 ± 4.2 | 25.7 ± 3.1 | 32.4 ± 6.3 | 30.3 ± 7.6 | 28.5 ± 3.9 | 0.02 | 0.1 | 0.1 |
| aPTT | 1.8 ± 0.67 | 1.5 ± 0.29 | 1.2 ± 0.16 | 1.5 ± 0.49 | 1.3 ± 0.27 | 1.3 ± 0.19 | 0.04 | 0.33 | 0.46 |
| Fibrinogen (mg/dL) | 135 ± 40 | 171 ± 44 | 195 ± 73 | 193 ± 82 | 216 ± 76 | 239 ± 88 | 0.007 | 0.1 | 0.1 |
| No. of patients who received noradrenaline | 7 (43.7%) | 4 (25%) | 2 (12.5%) | 1 (6.25%) | 0 | 0 | 0.037 | 0.1 | 0.48 |
| No. of patients transfused with RBC | 6 (37.5%) | 8 (50%) | 1 (6.25%) | 2 (12.5%) | 2 (12.5%) | 0 | 0.22 | 0.06 | 1 |
| No. of patients transfused with PLT | 5 (31.2%) | 5 (31.2%) | 4 (25%) | 0 | 0 | 0 | 0.04 | 0.04 | 0.1 |
| No. of patients transfused with FFP | 5 (31.2%) | 3 (18.7%) | 0 | 1 (6.25%) | 1 (6.25%) | 0 | 0.17 | 0.6 | NA |
| Albumin administered (mL) | 243 ± 156 | 200 ± 164 | 161 ± 253 | 78 ± 140 | 135 ± 133 | 55 ± 88 | 0.014 | 0.23 | 0.1 |
Eight patients (50%) from the ATG group and 3 (18.7%) from the control group had a central blood temperature higher than 38 °C from the admission to ICU until POD1 (not significant, P = 0.14). The patients treated with ATG had an unstable haemodynamic profile (mainly on POD1) requiring noradrenaline infusion to keep the MAP over 60 mmHg in a greater number of patients compared to the controls (P = 0.037). The duration (hours) of the ICU stay and of mechanical ventilation were similar, while 4 (25%) patients from the ATG group and none from the control group required continuous renal replacement therapy while in the ICU (P = 0.10). As shown in Table 5, five patients (31.2%) in the ATG group (none in the control group) were transfused with PLT on POD1 and POD2 (P = 0.04), and more albumin was infused in the ATG patients than in the controls on POD1 (P = 0.014).
The incidence of rejection was 0% in the ATG group and 6.25% in the control group (P = 0.1).
In the perioperative phase, 10 patients (62.5%) in the ATG group had one or more bacterial and/or fungal infection, whereas the infection rate in the control group was 43.7% (n = 7; P = 0.36). During the observation period, the rate of viral infection was 6.25% (n = 1) after ATG induction, while no cases of viral infection were detected in the control group (P = 0.97).
One patient in the ATG group had anaphylactic shock and died on POD3. The anaphylactic status was confirmed by serological exams showing a high presence of IgE antibodies to cross-reactive carbohydrate determinants (CCD).
Another patient in the ATG group had a possible cytokine release syndrome episode with a temperature up to 39 °C since the admission to the ICU. He developed intravascular haemolysis and oliguria with a rapidly increasing serum creatinine requiring continuous renal replacement therapy until discharge.
The induction of immunosuppression by a single administration of ATG during LT (3 mg/kg infusion, from laparotomy to anhepatic stage) was chosen to provide a significantly more effective and sustained T-cell clearance, with a consequent reduction in long-term immunosuppressive treatment. As previously described by Starzl et al[8], pre-treatment with polyclonal ATG aims to exhaust the host vs graft response, resulting in a tolerogenic effect and making it possible to use less post-transplantation immunosuppression.
The present case-control study, designed to evaluate the effects of ATG in the perioperative period, showed that ATG infusion was associated with an increase in core temperature, worsening of the haemostatic, acid-base and haemodynamic balance and higher requirements for blood products. Signs related to cytokine syndrome[15,16] were observed, mixed with, and intensified by, the haemodynamic imbalances related to caval and portal clamping and the metabolic profile of liver dysfunction.
Both during the surgical procedure and the ICU stay, an increase in the central blood temperature, which is unusual during LT despite the use of devices for heating the patient and fluid infusions, was observed in eight patients from the ATG group. In the same group, a higher number of patients were haemodynamically unstable, requiring increased amounts of noradrenaline and adrenaline to maintain a MAP of 60 mmHg. We are inclined to attribute the severe hypotensive episodes, observed before reperfusion, to a decrease in systemic vascular resistance due to the vasodilator effect of cytokine release because the stability of EDVI, observed at the same time, makes it unlikely that an inadequate intake of fluids and the resulting reduced blood volume was the cause of hypotension.
Patients from the ATG group received a much larger amount of crystalloids (1.2 L/h vs 800 mL/h, P = 0.008) and blood products during the surgical procedure because the vasodilator effect and the coagulation impairment induced by thymoglobulin caused a relative hypovolemia and because the circulating volume had to be maintained.
Similar observations were made in a case report published by our group, wherein Busani et al[17] described the side effects of ATG infusion during LT. In the perioperative period their patient, receiving a higher dosage (5 mg/kg) of polyclonal antibody compared to our study, showed hyperthermia, hypotension and haemolysis, but no observations were made about the effect of ATG on coagulation. After this experience, the LT surgeons decided to propose a new thymoglobulin protocol which involved a lower dosage of ATG (3 mg/kg instead of 5 mg/kg as suggested by Starzl et al[8]), described in this study as a pre-treatment.
Regarding the coagulation status, the basal TEG variables showed a better coagulation balance (shorter R, K and higher α values) in the patients receiving ATG. Subsequently, during the pre-anhepatic and anhepatic stages (time interval of ATG infusion), these patients showed an early and intense fibrinolysis, TEG changes which are usually observed after reperfusion of the graft. The MA values (expression of platelet activity and clot strength) were lower in the ATG group from laparotomy to the end of surgery. A possible explanation for this observation could be the low specificity of thymoglobulin for T-cells. This drug is a carrier of antibodies which can cross-react with different blood cell types[18,19] such as platelets, causing thrombocytopenia and an impairment of platelet function. Other antibodies can cause other side effects, such as haemolytic anaemia, neutropenia, hyperthermia and cytokine release syndrome[2,11,13,14].
The use of ATG for induction therapy during LT was investigated with specific attention to graft function and survival. During the follow-up period, one episode of rejection was observed in the control group (none in ATG group), which appears consistent with the aim of the treatment, but the low number of patients involved in the study did not provide statistical strength.
Malignancies and infections were reported as the main adverse effects of ATG treatment in the field of solid organ transplants. Kamar et al[20] found that ATG induction therapy was safe, reliable and effective in HCV-positive liver recipients, but no comment was made about the early effects of this treatment on haemostasis, blood loss and blood product requirements.
In contrast to Kamar et al[20], the ATG treated patients of our study had a higher, even if not statistically significant, incidence of bacterial/fungal infections compared to the patients not treated with this antibody. The perioperative infection rates were higher than those recorded by Soliman et al[21] as well. A possible explanation of this observation could be the high incidence of transfusions observed in the ATG group. Several studies have shown that intraoperative blood loss and a large amount of RBC, platelet and plasma transfusions have a negative impact on outcome after LT and are a strong independent risk factor for patient infections and survival after LT[22,23].
During our study, two serious adverse events were attributed to ATG infusion. A haemolytic anaemia requiring renal replacement treatment was diagnosed immediately after LT in one patient. A presumptive diagnosis of an adverse reaction to ATG infusion (first dose side effect) and cytokine release syndrome was made because the liver transplant was not complicated by haemodynamic instability, and no other new drugs were administered during the operation because the patient had been previously exposed to all of them without any side effects.
In our study, a large number of patient required continuous renal replacement therapy after LT, while Soliman et al[21] reported a positive effect of thymoglobulin in preventing early renal impairment by suppressing the release of proinflammatory mediators. We can assume that cytokine release, related to the ATG infusion and the associated bleeding, induced a severe hypotension (often causing a decrease in the MAP below 60 mmHg notwithstanding noradrenaline infusion) responsible for the poor kidney perfusion and failure, which could explain the absence of the beneficial effects reported by Soliman et al[21].
Another patient died of anaphylactic shock, and high titres of IgE antibodies to CCD were found in his blood, as reported in recently observed cases of IgE-mediated anaphylaxis[18,19]. ATG can induce an anaphylactic response because it contains complex oligosaccharides acting as epitopes for specific CCD antibodies.
Notwithstanding these interesting observations, our study has some limitations, including its retrospective nature, the small number of patients and the differences in postoperative immunosuppressive therapy, which could be responsible for interpretation bias in the postoperative variables examined.
However, it is likely that the adverse events observed in the study group can be associated with the administration of thymoglobulin, and the dosage chosen, as well as the short term infusion (4-6 h), can be further justification of our comments. It is our belief that appropriate dosage and a longer timeframe of administration could help to avoid complications associated with ATG use, but further study would be necessary to prove this hypothesis.
The retrospective nature of the study and the small sample size make our conclusions weaker than desired, even though the observed events should be appreciated.
In spite of the many potential benefits of this potent antibody as induction therapy, we suggest that the side effects observed in the ATG group should justify caution in the use of thymoglobulin for single, high dosage, intraoperative administration during LT. The two adverse events observed in our study make this therapeutic approach to LT less desirable. A more thorough investigation and larger population samples are needed to define better protocols with a safer drug dosage and timing of administration.
Polyclonal antithymocyte globulin (ATG) preparations have been used for the prevention and treatment of acute rejection in solid organ transplantation. ATG administration preoperatively as an “induction agent” allows doctors to minimize the use of the nephrotoxic calcineurin inhibitors, to reduce overall steroids use and to eliminate the need for maintenance immunosuppression, promoting tolerance in organ recipients by donor leucocyte augmentation. ATG produces a variety of biological effects that go beyond T-cell depletion, and its antibodies are directed against different blood cell types (B-cells, natural killer, platelets, and erythrocytes). Cytokine release syndrome, haemolytic anaemia, thrombocytopenia, neutropenia, fever and serum sickness are potential adverse effects, and the associated short-term costs need to be planned. The optimal dosage and duration of treatment are still uncertain.
The potential for adverse events after ATG administration has not been evaluated so far in the intra and immediate postoperative period, and the authors’ understanding of the role of thymoglobulin as an induction therapy in liver transplantation (LT) is still evolving. Because of the ATG T-cell selectivity shortage, ATG can induce thrombocytopenia, worsening a perioperative coagulation status which is already compromised because of end stage liver disease and causing haemodynamic alterations because of cytokine release. For this reason, the present study has been designed to evaluate the perioperative effects of ATG administration during a liver transplant with particular regard to ATG effects on coagulation.
In the previous literature, immunosuppressive ATG therapy was mainly described as a method to provide a more effective and sustained T-cell clearance, with a consequent reduction of long-term immunosuppressive treatment. The use of thymoglobuline as an agent of immunological tolerance has never been analysed in terms of the haemodynamic, coagulation and biochemical repercussions during a LT. In particular, the effects of the short term infusion of high ATG doses have never been studied before. Moreover, whether the administration of ATG could have negative repercussions on the coagulation status of cirrhotic patients with an already compromised coagulation balance had never been verified by the use of thromboelastography.
Thanks to the observations in this study, the authors can predict any negative effects associated with the preoperative administration of high-dose ATG. This awareness may enable doctors to better treat any complications associated with the administration of this immunosuppressant, applying renal preservation strategies, coagulation control and adjustment strategies, and haemodynamic support. Based on the authors’ observations and previous literature reports, it may be safer to start administration of this drug earlier and at lower doses in order to mitigate any adverse impacts.
Polyclonal ATG preparations are immunosuppressants used for the prevention and treatment of acute rejection in solid organ transplantation. Thymoglobulin, a rabbit-derived polyclonal antibody, is a polyclonal antithymocyte antibody (“induction agents”) which is administered preoperatively in order to minimize the use of nephrotoxic calcineurin inhibitors, to reduce overall steroid use and to eliminate the need for maintenance immunosuppression, promoting tolerance in organ recipients by donor leucocyte augmentation. LT is the only therapeutic approach for end stage liver disease. It is a surgical procedure characterized by significant haemodynamic, coagulation and biochemical repercussions which are different depending on the surgical stage (laparotomy, pre-anhepatic, anhepatic, and reperfusion phase).
It’s a well performed and thought out study with many relevant findings.
P- Reviewer: Al-Shamma S, Mittal C
S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Storb R, Gluckman E, Thomas ED, Buckner CD, Clift RA, Fefer A, Glucksberg H, Graham TC, Johnson FL, Lerner KG. Treatment of established human graft-versus-host disease by antithymocyte globulin. Blood. 1974;44:56-75. [PubMed] |
| 2. | Beiras-Fernandez A, Thein E, Chappel D, Gallego R, Fernandez-Roel D, Kemming G, Hammer C. Polyclonal anti-thymocyte globulins influence apoptosis in reperfused tissues after ischaemia in a non-human primate model. Transpl Int. 2004;17:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Filo RS, Smith EJ, Leapman SB. Reversal of acute renal allograft rejection with adjunctive AG therapy. Transplant Proc. 1981;13:482-490. [PubMed] |
| 4. | Zietse R, van Steenberge EP, Hesse CJ, Vaessen LB, IJzermans JN, Weimar W. Single-shot, high-dose rabbit ATG for rejection prophylaxis after kidney transplantation. Transpl Int. 1993;6:337-340. [PubMed] |
| 5. | Weimer R, Staak A, Süsal C, Streller S, Yildiz S, Pelzl S, Renner F, Dietrich H, Daniel V, Rainer L. ATG induction therapy: long-term effects on Th1 but not on Th2 responses. Transpl Int. 2005;18:226-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 6. | Tector AJ, Fridell JA, Mangus RS, Shah A, Milgrom M, Kwo P, Chalasani N, Yoo H, Rouch D, Liangpunsakul S. Promising early results with immunosuppression using rabbit anti-thymocyte globulin and steroids with delayed introduction of tacrolimus in adult liver transplant recipients. Liver Transpl. 2004;10:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Eason JD, Nair S, Cohen AJ, Blazek JL, Loss GE. Steroid-free liver transplantation using rabbit antithymocyte globulin and early tacrolimus monotherapy. Transplantation. 2003;75:1396-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 134] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Starzl TE, Murase N, Abu-Elmagd K, Gray EA, Shapiro R, Eghtesad B, Corry RJ, Jordan ML, Fontes P, Gayowski T. Tolerogenic immunosuppression for organ transplantation. Lancet. 2003;361:1502-1510. [PubMed] |
| 9. | Wiesner RH, Fung JJ. Present state of immunosuppressive therapy in liver transplant recipients. Liver Transpl. 2011;17 Suppl 3:S1-S9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Eason JD, Loss GE, Blazek J, Nair S, Mason AL. Steroid-free liver transplantation using rabbit antithymocyte globulin induction: results of a prospective randomized trial. Liver Transpl. 2001;7:693-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 93] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Préville X, Flacher M, LeMauff B, Beauchard S, Davelu P, Tiollier J, Revillard JP. Mechanisms involved in antithymocyte globulin immunosuppressive activity in a nonhuman primate model. Transplantation. 2001;71:460-468. [PubMed] |
| 12. | Goggins WC, Pascual MA, Powelson JA, Magee C, Tolkoff-Rubin N, Farrell ML, Ko DS, Williams WW, Chandraker A, Delmonico FL. A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients. Transplantation. 2003;76:798-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 169] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 13. | Tchervenkov J, Flemming C, Guttmann RD, des Gachons G. Use of thymoglobulin induction therapy in the prevention of acute graft rejection episodes following liver transplantation. Transplant Proc. 1997;29:13S-15S. [PubMed] |
| 14. | Hardinger KL, Schnitzler MA, Koch MJ, Labile E, Stirnemann PM, Miller B, Enkvetchakul D, Brennan DC. Thymoglobulin induction is safe and effective in live-donor renal transplantation: a single center experience. Transplantation. 2006;81:1285-1289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 15. | Dhir V, Fort M, Mahmood A, Higbee R, Warren W, Narayanan P, Wittman V. A predictive biomimetic model of cytokine release induced by TGN1412 and other therapeutic monoclonal antibodies. J Immunotoxicol. 2012;9:34-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Maggi E, Vultaggio A, Matucci A. Acute infusion reactions induced by monoclonal antibody therapy. Expert Rev Clin Immunol. 2011;7:55-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Busani S, Rinaldi L, Begliomini B, Pasetto A, Girardis M. Thymoglobulin-induced severe cardiovascular reaction and acute renal failure in a patient scheduled for orthotopic liver transplantation. Minerva Anestesiol. 2006;72:243-248. [PubMed] |
| 18. | Mehrabi A, Mood ZhA, Sadeghi M, Schmied BM, Müller SA, Welsch T, Kuttymuratov G, Wente MN, Weitz J, Zeier M. Thymoglobulin and ischemia reperfusion injury in kidney and liver transplantation. Nephrol Dial Transplant. 2007;22 Suppl 8:viii54-viii60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Gaber AO, Monaco AP, Russell JA, Lebranchu Y, Mohty M. Rabbit antithymocyte globulin (thymoglobulin): 25 years and new frontiers in solid organ transplantation and haematology. Drugs. 2010;70:691-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 20. | Kamar N, Ribes D, Sandres-Saune K, Suc B, Barange K, Cointault O, Lavayssiere L, Durand D, Izopet J, Rostaing L. Efficacy and safety of induction therapy with rabbit antithymocyte globulins in liver transplantation for hepatitis C. Transplant Proc. 2004;36:2757-2761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 21. | Soliman T, Hetz H, Burghuber C, Györi G, Silberhumer G, Steininger R, Mühlbacher F, Berlakovich GA. Short-term induction therapy with anti-thymocyte globulin and delayed use of calcineurin inhibitors in orthotopic liver transplantation. Liver Transpl. 2007;13:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Brand A. Immunological aspects of blood transfusions. Transpl Immunol. 2002;10:183-190. [PubMed] |
| 23. | Massicotte L, Sassine MP, Lenis S, Seal RF, Roy A. Survival rate changes with transfusion of blood products during liver transplantation. Can J Anaesth. 2005;52:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |