INTRODUCTION
Chronic kidney disease (CKD) in all its stages bears an intimate relationship with cardiovascular disease (CVD) in conjunction with other risk factors[1]. Patients with advanced CKD being considered for kidney transplantation are no exception[2]. Kidney transplantation is the preferred therapy for advanced CKD including end-stage kidney disease (ESKD), since it provides improved quality of life and extends life expectancy. However, the CVD-CKD relationship extends to the post-transplant phase of CKD as well[2], and as part of the overall improvement in CKD prognosis expected as a result of successful transplantation[3], clinicians seek to mitigate the risk for significant cardiovascular events and mortality by restoring a sufficient amount of kidney function. However, despite a reduction in overall long-term mortality with transplantation, an increased short-term post-transplant mortality risk has been well-documented[4]. Selection biases that operate in pre-transplant selection[4] reduce this short-term mortality to some extent, but it remains unclear which pre-transplant screening tests and interventions are most appropriate for transplant candidates as a whole as well as for specific candidate subgroups. Since CVD remains a major contributor to the overall post-transplant co-morbidity burden, pre-transplant cardiac screening has garnered particular attention as a preemptive approach to improving post-transplant success. Pre-transplant cardiac screening tests and procedures can be both labor-intensive and expensive, and must therefore be employed judiciously, while still preserving the expectation that the transplant candidate will benefit from receiving the allograft.
Further adding to the complexity surrounding questions regarding pre-transplant cardiac screening is that a major cardiac event in a transplant candidate can be a faster moving, and therefore more difficult target to “hit and prevent” than in the general population due to uremia (which itself varies in severity) and other accelerators of CKD. The clinician also has a duty not just to the candidate’s health but to the success of the donated allograft. Few CVD conditions detected by screening require immediate attention; there is a trade-off between the risks from a given procedure that are immediate and the benefits from that procedure which are more remote and for a given patient remain for the time being hypothetical. Finally, CKD itself is a heterogeneous entity since its etiology is multifactorial, its treatment (both medication and renal replacement therapy) is varied, and there are numerous competing risks for CVD in CKD patients. These nuances make prescribing a standardized approach to pre-transplant cardiac screening very difficult.
With this background, the purpose of this review is to highlight the significance of specific pre-transplant cardiac screening procedures in kidney transplant candidate management prior to transplantation. Particular emphasis will be placed on practical considerations in pre-transplant screening rather than on statistical comparisons among screening tests or consequent management either before or after the transplant. Our goal is to supplement such information already contained in excellent consensus statements[2] and recent reviews[5], among others on these topics.
SCREENING FOR CORONARY ARTERY DISEASE BEFORE KIDNEY TRANSPLANTATION
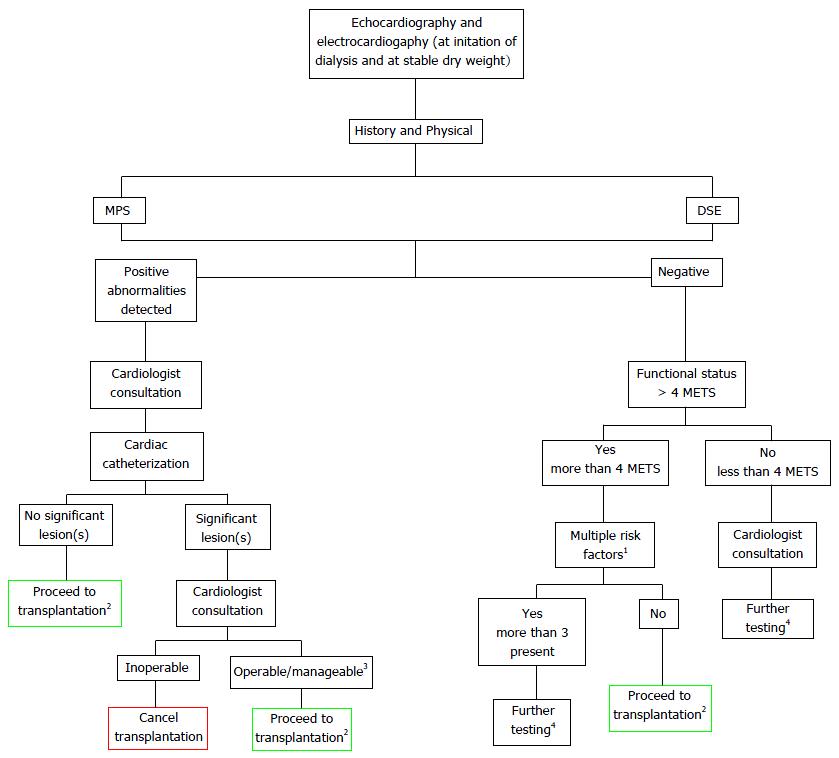
As mentioned, the bulk of the published literature on the screening and management of pre-transplant CVD screening pertains to CAD. All non-cardiac surgery candidates require preoperative screening with a focus on detecting significant CAD[10]. The evidence for and against particular tests has been subjected to extensive review[2]. Screening for CAD serves two major purposes: To more accurately inform transplant candidates of their risk for a coronary ischemic event both before and after the transplant and thereby helping to inform their decision about proceeding with transplantation, and to guide pre-transplant management so that post-transplant success can be optimized. Less commonly, if a candidate is turned down for transplantation, the information obtained from pre-transplant screening can also be used to guide management on dialysis. Transplant candidates on dialysis are more likely to be screened for CAD than those not being considered for transplantation, although it remains unclear if they experience any long-term benefits as a result of screening per se, while still remaining on dialysis. A clinical trial of a screening vs no-screening policy for CAD in transplant candidates will likely never be performed due to the uniform perception that CKD patients are at high risk for CAD. A suggested algorithm for screening in transplant candidates is provided in Figure 1 and will now be discussed.
Figure 1 Suggested pre-transplant screening algorithm for cardiovascular disease.
1Diabetes mellitus, prior CVD, > 1 year on dialysis, LVH, age > 60 years, smoking, hypertension, and dyslipidemia; 2Proceed to transplantation with standard screening and frequency of testing [for patients with no abnormalities at start, standard frequency is evaluation once dry weight is achieved (1-3 mo) and prior to transplantation date]; 3Abnormalities that pose limited threat to the success of the allograft and patient’s health; 4Per cardiologist recommendation. CVD: Cardiovascular disease; MPS: Myocardial perfusion scintigraphy; DSE: Dobutamine stress echocardiography; LVH: Left ventricular hypertrophy; METS: Metabolic equivalents.
History and physical examination
Thorough history taking and a physical examination by the transplant clinician prior to diagnostic testing for CAD is commonly employed. Some centres may choose to prepare a package of CAD screening tests in advance of an interview with the clinician, although this approach could conceivably affect the approach to subsequent testing. A history of chest pain and impaired exercise tolerance to 4 metabolic equivalents are important considerations, but these indicators of CAD can be very deceptive. For example, chest pain at the time of presentation with acute myocardial infarction (MI) is much less common in patients on dialysis[11] although shortness of breath (SOB) is more common[12]. Both chest pain and SOB are especially valued by cardiologists when making decisions about proceeding with invasive testing in the general population, since they are important to enhancing pre-test probabilities. Both these symptoms however require an initiating event; this may be lacking in dialysis patients. The ability to walk four blocks and climb two flights of stairs is one proposed criterion for pre-operative cardiac fitness[13]. While chest pain and/or SOB may limit the ability to perform these tasks, an assessment for this at the time of a patient interview may portend erroneous CAD risk classification since they lack both sufficient sensitivity and specificity.
Guidelines acknowledge[2] that further work is required to determine the ability of “functional status” to identify significant CAD in transplant candidates. Transplant candidates may be motivated to downplay symptoms of chest pain or SOB by sincerely believing that these are due to non-CAD etiologies such as acid reflux or asthma. Dialysis patients may also be quite deconditioned. They may learn to successfully avoid chest pain or SOB by not exerting themselves to the required extent for long periods of time and translate this for the clinician into a negative history. Musculoskeletal conditions may further impair mobility. Tools such as the Framingham risk score severely underestimate cardiac risk post-transplant in almost all patient subgroups[14] and so categorizing a transplant candidate as low cardiac risk based on symptoms alone may not always be appropriate. Nonetheless, recording all the traditional Framingham risk factors such as diabetes and hyperlipidemia, as well as factors such as prior CVD and a family history of CVD may be informative to guiding both pre- and post-transplant management. Ethnicity of the candidate is also not typically considered by consensus group guidelines although some ethnic groups may be at higher early and late post-transplant CVD risk[15] and therefore these ethnic groups need to be considered for more thorough screening regardless of history or current physical findings. A history of unexplained recurrent hypotension on hemodialysis, persistent volume overload resulting from intolerance to fluid removal, and claudication without chest pain or SOB may all point towards significant underlying CAD. Despite a lack of evidence-based consensus recommendations on this topic, it seems reasonable to pursue at least some investigations in almost all patients, independent of history.
Similar to taking a thorough medical history, the time invested in physical examination of the transplant candidate may be quite rewarding despite lack of documented evidence or consensus around physical examinations. As one example, peripheral arterial disease (PAD) may be associated with CAD in some candidates[16]. PAD also affects post-transplant mortality[17]. Uncontrolled hypertension, hypotension or a wide pulse pressure on sphygmomanometry, elevated central venous pressure, atrial fibrillation if new-onset[18], edema not related to nephrotic syndrome, and abdominal obesity particularly when associated with the metabolic syndrome[1] may all serve to heighten the level of awareness of possible underlying CAD. Non-diabetic transplant candidates may particularly benefit from risk stratification for CAD[19] and so a case can be made that this should be pursued even in the absence of worrisome physical findings. On the other hand, transplant candidates with diabetes should uniformly benefit from intensive screening.
Resting electrocardiography
Tests for CAD may be broadly classified as non-invasive and invasive, with non-invasive tests typically ordered first. Among the non-invasive tests, 12-lead electrocardiography (ECG) is a simple, widely available, non-invasive test that can be used to screen for preexisting CAD in almost all patient populations, but ECG also attracts less attention from guideline committees and in systematic reviews perhaps for these reasons. An abnormal ECG is predictive of cardiac death in renal transplant candidates, even if LVH is excluded[20]. “Non-specific” ST-T wave changes on ECG in conjunction with other risk factors necessarily including diabetes is associated with a higher probability of underlying CAD[21]. Features on resting ECG such as pathological Q waves, ST segment depression or elevation exceeding 1 mm, T wave inversion, and bundle branch block all point towards significant CAD[22]. Resting ECG relates often correlate with results from more sophisticated non-invasive tests as well as angiographic CAD[23] in some populations. Although there are no supporting data, it is reasonable to consider obtain a resting ECG annually in transplant candidates but especially within 30 d of surgery[24]. Serial ECG testing allows for the detection of evolving new abnormalities, thereby allowing for timely further investigation prior to transplantation.
Resting echocardiography
Many kidney transplant candidates will often have a resting transthoracic echocardiogram already performed by virtue of the fact that this test is routinely recommended in all patients within a few months of starting dialysis[25]. It is more expensive and labor-intensive than an ECG, but is still convenient for the patient. Although resting echocardiography is not typically used as a screening test for CAD per se, some parameters provided by this test can be useful. Increased LV size, decreased LV ejection fraction, and resting wall motion abnormalities may all point towards significant underlying CAD[22]. Resting wall motion abnormalities in particular are associated with reduced CAD event-free survival in the presence of diabetes[26]. In patients without known CAD or a previous MI, the finding of resting wall motion abnormalities correlates with perfusion abnormalities on pharmacologic stress testing[27]. Many CKD patients have hypertension and/or diabetes, and findings from resting echocardiography such as reduced coronary sinus flow may predict CAD with good sensitivity and specificity[28]. While all these findings do not especially pertain to transplant candidates, they may provide sufficient reason to obtain resting echocardiography in all transplant candidates and then act upon the detection of resting wall motion abnormalities as candidates proceed towards transplant listing. It is less clear, however, if patients require this pre-transplant screening procedure repeated in the absence of other specific indications.
Exercise stress testing
Recent studies examining the utility of exercise stress testing in transplant candidates are remarkably scarce. The availability of other types of stress tests that provide a greater degree of sensitivity and specificity for CAD, while at the same time also supplying information beyond what a clinical assessment of functional capacity can provide, may preclude a decision to order an exercise stress test. Concerns about safety in performing the test in transplant candidates with limited mobility may further limit its utilization. Failure to achieve the target heart rate impairs the capture of sufficient diagnostic information[29], and ambiguity in correlating ECG findings to territorial ischemia will lead to a decision to pursue additional forms of stress testing in most cases. Testing for functional cardiovascular reserve[30,31] instead may be superior for prognostication although these tests have not been widely adapted. Nonetheless, exercise stress testing is reasonable to pursue in young, otherwise apparently healthy transplant candidates by serving as a positive reinforcement to them. It also prevents radiation exposure, and in some parts of the world exercise stress testing may be the only form of cardiac stress testing available to kidney transplant programs.
Myocardial perfusion scintigraphy
Myocardial perfusion scintigraphy (MPS) is commonly employed as a screening test by transplant programs as the major alternative to exercise stress testing. MPS utilizes the principle of cardiac single photon emission computed tomography, or “SPECT”. Various forms of MPS are usually available to transplant programs. MPS is the preferred test if blood pressure is uncontrolled or a cardiac arrhythmia is present[5]. Dipyradimole is the pharmacologic agent typically used to increase endogenous adenosine levels, which in turn results in vasodilation and stress to cardiac muscle as a result of challenging the flow reserve. MPS has moderate sensitivity and specificity in detecting significant CAD[6] but CKD itself remains a significant cardiac risk factor even with a normal MPS result[32]. A major concern with MPS is that in CKD there is already higher resting blood flow due to higher basal adenosine levels[33]. As a result, the challenge induced to flow reserve from pharmacologic stimulation is attenuated, so that any measurable difference in uptake of the administered radioisotope in different myocardial regions will be attenuated as well[34]. Particular caution in interpreting results from MPS is warranted in CKD patients with PAD[35]. There is also concern that various antihypertensive and anti-anginal agents, commonly used in dialysis patients, further decrease the sensitivity of MPS[32]. The radiation dose received also needs to be considered. An average dose of 15 milliSievert corresponds to approximately 750 chest X-rays[36]. Nonetheless, based on a recent systematic review[5], MPS does have predictive value for major adverse cardiac events (based on 19 studies) but not all-cause mortality (based on 11 studies). “Fixed” perfusion defects, for which intervention is not usually pursued, also have prognostic value[5]. MPS may reveal no discernible distinct territory with ischemia, but global ischemia may still be present as a result of balancing large vessels being occluded or as a result of diffuse microvascular occlusion from conditions like diabetes. All MPS indicates in this situation is that there is no unbalanced large vessel occlusion amenable to a revascularization procedure or otherwise. As a result, MPS-detectable ischemia but a subsequently ascertained inability to perform revascularization based on MPS results will factor in to decisions about proceeding with transplantation without the possible benefit of revascularization.
Dobutamine stress echocardiography
Dobutamine stress echocardiography (DSE) is gaining in popularity as a screening test for CAD, after having been shown to improve risk stratification in vascular surgery, in which it has good negative predictive value[37]. A discussion of DSE mandates comparisons to MPS. Unlike MPS, DSE does not depend on heterogeneity of myocardial blood flow, thus making DSE a more specific test than MPS. Instead, DSE depends on the occurrence of reversible systolic dysfunction occurring as a result of an underlying perfusion abnormality. However, its value for detecting CAD is limited when a target heart rate response is not achieved, particularly in CKD and long-standing hypertension which are frequently accompanied by LVH[38]. Furthermore, when the intra-cavitary volume is small at peak stress, subtle wall motion abnormalities can be missed[39]. DSE may induce atrial fibrillation. Nonetheless, DSE may be preferred in transplant candidates who have a low blood pressure or reactive airways disease[5]. A widely discussed topic in the current literature is the comparison of DSE to MPS in kidney transplant candidates. A systematic review of 5 studies of DSE indicates a significant risk of excess all-cause mortality with an abnormal DSE result[5]. Similarly, the pooled result of 10 studies demonstrated an increased risk of major adverse cardiac events with an abnormal DSE result[5]. These data do not necessarily indicate superiority of DSE over MPS, and any estimated superior sensitivity or specificity of DSE over MPS has not reached statistical significance, even though DSE may have greater test accuracy because result interpretation is less subjective[6]. Which test (DSE or MPS) is ultimately pursued then becomes a matter of transplant clinician or cardiologist comfort, or preference and various logistical concerns, despite at least one recommendation of DSE over MPS[6]. Nonetheless, with DSE unlike MPS any radiation exposure can be avoided, so DSE may therefore be especially helpful in candidates who require repeat assessments.
Cardiac computed tomography
The use of cardiac computed tomography (CCT) scanning without the use of contrast in order to assess calcification in the coronary arteries has been evaluated in recent guidelines[2]. The rationale of CCT is that elevated calcium scores are common in hemodialysis patients[40] and these may independently predict mortality[41]. The value of CCT for determining coronary risk with transplantation is controversial[42] and has even been described as “questionable”[43], since the poor correlation of coronary artery wall calcification with occlusive CAD indicates that the calcification seen with CT is more medial in location than intimal. CT angiography which uses contrast is also not recommended by guidelines[2], despite the claim that a negative result (a zero calcium score) effectively excludes significant CAD and prevents the need for repeat DSE when the response to dobutamine is submaximal[33]. With coronary CT angiography, loss of residual renal function may have substantial impact on subsequent dialysis efficiency especially in those receiving peritoneal dialysis (PD). The additional burden of radiation exposure for little additional diagnostic or prognostic yield further imbalances the risk-benefit ratio of these procedures, so CCT is yet to gain in popularity.
Coronary angiography
This invasive, contrast-based screening procedure of the coronary arteries is typically pursued when sufficient clinical suspicion of vascular occlusion has been raised by prior non-invasive screening tests. Coronary angiography is usually pursued only when there is intent for potential revascularization, but the decision in ESKD patients may be influenced by other needs as described below. A clear outline of the major epicardial artery anatomy with the sites and their severity of obstruction can be obtained, and this allows for subsequent referral of candidates towards angioplasty or bypass surgery. Coronary angiography is often considered to be a “gold” standard for CAD detection for these reasons, although recent systematic reviews do not demonstrate its superiority over noninvasive tests previously discussed[5]. Moreover, the burden of radiation is also a consideration when performing coronary angiography, and there is also the loss of residual renal function in candidates who are on PD or have not yet commenced dialysis. Comorbid conditions that increase the pretest probability for detecting significant CAD include diabetes[33]. Other comorbidities used to shepherd candidates towards coronary angiography include age over 50 years, symptoms relatable to ischemia, abnormal stress test results, and a depressed LV ejection fraction[44]. Additional criteria for coronary angiography include known prior CAD with or without intervention, multiple CVD risk factors including PAD[16] and cardiologist discretion[45]. Significant stenosis requiring revascularization in ESKD patients is often set at a 70% occlusion threshold based on the practice in the general population, although it needs to be recognized that lesions under this threshold may still progress while patients are waiting for an available organ. There are no data to indicate the particular value of intervention in one epicardial vessel over another, except perhaps for the left main coronary artery. As with decisions for noninvasive screening, patient symptoms can be unreliable. One approach is to attempt timing the planned revascularization based on an angiography result closer to the estimated date of transplant, but this is difficult to achieve when waiting times for an organ are quite variable. Even if intervention is not at all pursued thereafter, results from coronary angiography may ultimately spur the optimization of medical therapies in dialysis patients, as well as motivate closer clinical follow-up during the often lengthy pre-transplant waiting period. In other non-cardiac surgical populations (such as in PAD)[46] the role of revascularization as a result of angiography remains controversial, and this uncertainty extends to transplant candidates as well. Uncertainty exists because justification for the initial coronary angiogram itself becomes unclear when the intent is risk stratification more than revascularization per se. As a result, clinical judgment becomes the deciding factor for pursuing coronary angiography.
It is important to recognize that the mere absence of a lesion amenable to revascularization does not mean the absence of an increased coronary risk. This fact may be a source of serious misunderstanding between clinicians and patients. Diffuse microvascular disease not amenable to operative intervention, particularly in diabetes, may lead to demonstrable ischemia on noninvasive testing that is real and could lead to adverse post-transplant outcomes. The effect of ethnicity may also be important in determining higher risk, for example in South Asians[15] even after coronary angiography and subsequent intervention is performed. Published guidelines typically are authored only from certain countries and are based largely on the population characteristics of those countries, and so it is important for transplant centres to recognize their own unique dialysis and general population characteristics, then custom-design their approach to diagnostic testing accordingly. Despite no clear message provided by the literature on the effectiveness of coronary angiography at ultimately reducing post-transplant mortality, clinicians can be comforted by the finding that transplantation may be associated with better survival in all candidates regardless of CAD severity[47].
SCREENING FOR OTHER FORMS OF HEART DISEASE BEFORE KIDNEY TRANSPLANTATION
A significant amount of cardiac morbidity and mortality around transplantation is not directly related to CAD. LVH and dysfunction, valve disease, and pulmonary hypertension are important disease considerations for screening in renal transplant candidates. The role of screening tests will be discussed in the context of these conditions.
History and physical examination
As with CAD, a thorough history-taking and physical assessment can be valuable in determining the presence of, and risks associated with LV pathology and valve disease. Besides a long-standing history of hypertension and CKD, a history of childhood rheumatic fever is important to obtain from patients born in endemic areas since this could point to significant cardiac valve abnormalities. A prior history of endocarditis, symptoms of paroxysmal nocturnal dyspnea, edema, and signs of atrial fibrillation or increased central venous pressure, displacement of the cardiac apex, adventitious heart sounds unrelated to an arteriovenous fistula, wide pulse pressure, and PAD may all point towards serious non-coronary heart disease that merits attention prior to transplantation. Since none of these findings are either necessary or sufficient for diagnosis or prognosis of any condition, further screening tests are almost always indicated. However, the order of performing these tests may be appropriately informed.
Resting ECG
This inexpensive, non-invasive test can be used to detect LVH by voltage criteria, as well as right ventricular hypertrophy, previously unrecognized cardiac arrhythmias such as atrial fibrillation, and conduction delays or blocks, particularly in previously unscreened pre-dialysis transplant candidates. Kidney transplantation before dialysis is needed remains a “gold standard” for timing since this will lead to superior post-transplant outcomes. Resting ECG is insufficient as a stand-alone test but may help expedite cardiologist consultation for pre-dialysis candidates before other screening tests have actually been performed.
Resting echocardiography
As in the case with CAD, resting echocardiography can be used to detect other forms of cardiac disease. Trans-thoracic echocardiography is typically sufficient to detect significant LVH and enlargement, abnormalities in the other cardiac chambers, valve abnormalities including mitral stenosis and aortic stenosis, and pulmonary hypertension. Aortic and mitral valve calcification is strongly associated with CKD[48] Current guidelines[2] indicate value to initial screening for LV function by echocardiography in renal transplant candidates, but not for repeated assessments after listing. When used for transplant candidacy purposes, it is important to perform testing for patients on hemodialysis only after a dry weight has been achieved and there is no clinical evidence of congestive heart failure. Serial echocardiography to measure valve diameter in known aortic stenosis is important to determine timing for aortic valve replacement prior to transplantation, especially since aortic stenosis progresses more rapidly in ESKD than in the general population[49]. Likewise, the presence of an elevated pulmonary artery pressure is associated with adverse post-transplant outcomes with respect to both graft function[50] and patient survival[51]. The finding of pulmonary hypertension by echocardiography may in turn lead to further investigations such as sleep studies for obstructive sleep apnea and right heart catheterization[2].
Cardiac biomarkers
Biomarker measurement is commonly employed as part of the management of acute coronary syndrome, but may be helpful for non-invasive, non-coronary risk stratification in asymptomatic renal transplant candidates. Amongst cardiac biomarkers the measurement of cardiac troponins particularly cardiac troponin T (cTnT) has received recent attention. Elevation in cTnT in the non-acute setting could be from LVH, volume overload, or even uncontrolled hypertension[52]. An elevated cTnT level has been associated with post-transplant cardiac events[53] as well as reduced patient survival[54,55]. Cardiac troponin T has been labeled by guidelines as an “additional” prognostic marker[2]. The measurement of brain natriuretic peptide can also be considered. Other biomarkers have been regularly evaluated in the post-transplant setting and in the dialysis population, for example the calcium-phosphate product and C-reactive protein, but these and other biomarkers such as markers of blood glucose control, lipid profiles, or electrolyte and acid-base balance have not been systematically evaluated as prognostic markers in kidney transplant candidates although they may be helpful to individual transplant centres. It is also unclear if the finding of abnormal biomarker levels can help guide decisions for pre-transplant interventions that will alter post-transplant outcomes.
Magnetic resonance imaging
Cardiovascular magnetic resonance imaging (CMR) is a well-established and actively evolving area in diagnostic medical imaging[56]. In CMR, the magnetic field is generated from superconducting, liquid-helium cooled electromagnets that affect hydrogen nuclei. These nuclei in turn are manipulated by radiofrequency pulses in different planes, generating electromagnetic signals that are transformed into images[56]. Moving (cine) images can also be obtained and stacked. CMR can be used to assess LVH and left atrial volume in kidney transplant candidates[57], and is useful for distinguishing CAD from nonischemic cardiomyopathies, and for diagnosing hypertrophic cardiomyopathy and infiltrative heart conditions. Gadolinium contrast, normally used for angiography and tissue enhancement exposure is typically avoided in transplant candidates due to the risk of nephrogenic systemic fibrosis. Imaging by CMR is independent of chamber volume and is accurate at assessing both cardiac structure and function even without the use of contrast. There is no radiation exposure involved. CMR is generally safe even in the presence of coronary or peripheral artery stents, sternal wires, and prosthetic cardiac valves, but is generally unsafe with pacemakers or internal defibrillators and ocular metal shavings[56]. There may be less underestimation of LV mass compared to echocardiography because erroneous mathematical assumptions inherent to mass calculations in echocardiography can be avoided[58]. CMR also helps with the assessment of valvular heart disease[56], such as in determining volumes in valve regurgitation and gradients in valve stenosis[59]. However, besides eliciting claustrophobia in some patients, CMR is expensive, time consuming, and not widely available, but can be used for more detailed evaluation of important cardiac structure and function parameters when other non-invasive tests such as MPS and DSE yield conflicting information.
CMR is not addressed as a potential diagnostic tool in current screening guidelines, probably due to an insufficient supportive literature for its use. None-theless, fewer patients are required for CMR studies than echocardiography studies because of greater measurement accuracy and precision in measurement, and so CMR may become more widely accepted as a screening tool for kidney transplant candidates in the future. CMR represents the frontier of research progress in cardiac diagnostic screening for both CAD and other forms of heart disease. The impact of further developments such as three-dimensional single inversion-recovery prepared steady-state free precession[60], in which the coronary artery wall and plaque can be visualized will be followed with interest.
SCREENING OF PATIENTS WHILE ON THE WAITING LIST
Cardiac screening tests performed just once in living donor kidney transplant candidates can be reasonably timed so as to stay current at the time of transplantation. It is possible that a more accurate assessment of cardiovascular health in living donor transplant recipients contributes significantly to the superior long-term success of these patients compared to those who receive kidneys from waiting lists in whom test results may become outdated. In the case of patients waiting for a deceased donor kidney on a waiting list, questions arise about the need to repeat tests, and the frequency of their repetition. Many screening tests are simply too involved to perform at short notice when the patient is actually called in for transplantation.
A standard recommendation is to repeat cardiac stress testing that involves imaging once annually, particularly in those patients with diabetes[2]. However, testing once every two years regardless of diabetes status may be a more reasonable approach based on general population data, especially when a scan is normal[61]. A screening frequency of up to once every three years has also been suggested by some groups[62]. As preventative CVD management for patients on dialysis improves, it may be possible to screen all candidates even less frequently. However, there always remains the possibility that tests that were previously negative could later become positive, owing to the natural history of progressive conditions such as CAD and LVH in the context of uremia. Such “conversion” always remains a possibility[63] even in low-risk patients. Candidates may experience frustration if their “progress” towards a transplant is halted by newly-diagnosed cardiac disease, but it would be worth emphasizing to patients that a perioperative cardiac event may be quite deleterious to allograft health. One administrative option in waitlist management to address this concern is to maintain the candidate’s relative position on the waitlist, so they are not penalized with extra waiting time after the cardiac condition has been addressed. Frequent cardiac screening also reduces surprises to the clinician on the day of the transplant by providing valuable supplementary information to the admission history and physical examination. Based on available information, the overall recommendation from guidelines is that the utility of periodic screening is uncertain[2] since post-transplant outcomes may not be altered as a result. A pathophysiological explanation for why periodic screening is not obviously valuable is unavailable, and so physician discretion in pursuing repeat testing for individual candidates is warranted.
Unfortunately, some transplant candidates may be turned down for transplantation based on the results of cardiac screening procedures, or may be removed from a deceased donor transplant waitlist after the accumulation of cardiac morbidity over time. Intervening acute coronary syndromes leading to loss of myocardial contractility, severe aortic stenosis, or congestive heart failure with a severely depressed LV ejection fraction (such as below 40%) that cannot be improved by revascularization may effectively preclude transplantation. In such instances cardiologist consultation is required to ensure that patient safety is not compromised regardless of transplantation decisions, and at the same time transplant centres can avoid the possibility of depriving candidates who might have become eligible with coronary intervention a chance at transplantation. For such candidates who are eventually waitlisted, periodic screening becomes especially important. In the event of an acute coronary syndrome, it may be advisable to suspend a patient from the waitlist and repeat cardiac screening tests after a period of time has lapsed, for example six months, and again seek cardiologist consultation prior to placement back on the waitlist.
Some patients may also indicate that they have a very high quality of life on dialysis despite their cardiac comorbidities, and so transplantation in a setting of an increased cardiac risk will not provide them with the incremental improvement in quality of life that transplantation seeks to provide. If such patients have already been listed for transplantation, their overall ESKD management plan also requires reevaluation.