Published online Dec 24, 2014. doi: 10.5500/wjt.v4.i4.294
Revised: October 21, 2014
Accepted: October 28, 2014
Published online: December 24, 2014
Processing time: 139 Days and 8 Hours
Hepatoblastoma (HB) is the most common primary liver tumor in children and accounts for two-thirds of all malignant liver neoplasms in the pediatric population. For patients with advanced HB (unresectable or unresponsive to chemotherapy), combined treatment with chemotherapy and liver transplantation is an excellent option. The etiology of HB is mostly obscure because of its extreme rarity although some inherited syndromes and very low birth weight have been associated with it. The prognosis for children with HB has significantly improved in the past three decades thanks to advancements in chemotherapy, surgical resection and postoperative care. In 2002 a surgical staging system called pretreatment extent of disease (PRETEXT) was designed to allow a universal, multidisciplinary approach to patients with HB. Between one-third to two-thirds of patients initially present with unresectable tumors or distant metastases, but up to 85% of these tumors become operable after neoadjuvant chemotherapy. Patients with PRETEXT categories 1, 2, and some 3 are referred for neoadjuvant chemotherapy followed by surgical resection with the goal of complete tumor removal. Classic treatments regimens include a combination of cisplatin, fluorouracil, and vincristine or cisplatin and doxorubicin. Liver transplantation is the only treatment option for unresectable HB. In 2010 the pediatric end-stage liver disease, a pediatric-specific scoring system that determines a patient’s ranking on the liver transplant list, began to award additional “exception” points for patients with HB. We analyzed the Standard Transplant Analysis and Research dataset to assess the impact of changes in exception point criteria for HB on outcomes after liver transplantation at Texas Children’s Hospital in Houston, Texas. We found that patients who were listed for transplantation with current HB exception criteria experienced a shorter waitlist time but survival was similar between the two eras.
Core tip: Hepatoblastoma (HB) is the most common primary liver tumor in children. Between one-third to two-thirds of patients present with unresectable tumors or distant metastases, but up to 85% of these tumors become operable after neoadjuvant chemotherapy. Liver transplantation is the only treatment option for unresectable HB. In 2010 the pediatric end-stage liver disease scoring system began to award additional “exception” points for patients with HB. We analyzed the Standard Transplant Analysis and Research dataset and found that patients who were listed for transplantation with current HB exception criteria experienced a shorter waitlist time but survival was similar between the two eras.
- Citation: Khaderi S, Guiteau J, Cotton RT, O’Mahony C, Rana A, Goss JA. Role of liver transplantation in the management of hepatoblastoma in the pediatric population. World J Transplant 2014; 4(4): 294-298
- URL: https://www.wjgnet.com/2220-3230/full/v4/i4/294.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i4.294
Hepatoblastoma (HB) is the most common primary liver tumor in children and accounts for two-thirds of all malignant liver neoplasms in the pediatric population[1]. Standard treatment of HB includes neoadjuvant chemotherapy and surgical resection followed by adjuvant chemotherapy. For patients with advanced HB (unresectable or unresponsive to chemotherapy), combined treatment with chemotherapy and liver transplantation is an excellent option[2]. This article briefly reviews the epidemiology and treatment of HB in the pediatric population with an emphasis on the role of orthotopic liver transplantation (OLT).
The etiology of HB is mostly obscure because of its extreme rarity. The rate of HB in the United States Surveillance, Epidemiology, and End Results (SEER) from 2002-2008 was 10.5 cases per million in children less than one year of age and 5.2 cases per million in children 1 through 4 years of age[3]. It is assumed the tumor originates in utero for two reasons. Histologically HB cells resemble embryonal liver cells and the incidence is highest at birth suggesting the process is initiated during gestation[4].
Some inherited syndromes have been associated with HB. Incidence of HB among children with Familial Adenomatous Polyposis was found to be 847 times the incidence in the SEER population[5]. Those with the Beckwith-Wiedemann overgrowth syndrome had an incidence 2280 times that of the United States population of the same age[6]. Although these inherited conditions raise the risk of HB, they account for only a few cases overall.
Very low birth weight (< 1500 g) increases the risk of HB in children 20-fold and moderate low birth weight (1500-2500 g) doubles the risk[7]. The association of low birth weight and HB has two explanations. HB may be initiated or promoted by iatrogenic hazards in the neonatal intensive care units[8] in combination with decreased antioxidant defense mechanisms of pre-term infants[9]. Alternatively, HB and very low birth weight may share a common mechanism and the increase in survival of these patients has made the association more apparent.
The prognosis for children with HB has significantly improved in the past three decades thanks to advancements in chemotherapy, surgical resection and postoperative care[10]. Prior to the discovery of effective chemotherapy, cure was limited to completely resectable tumors and overall survival was dismal[10].
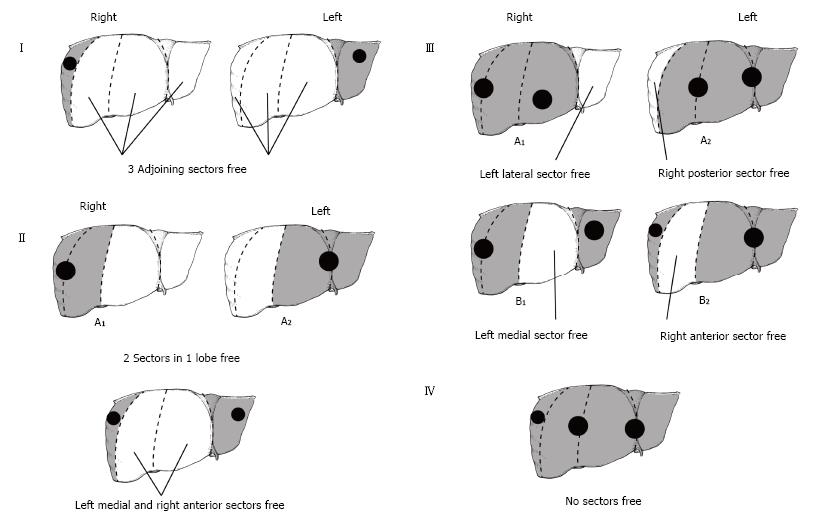
Early experiences with successful cure were sporadic at best and were limited to lesions that could be completely resected. In 2002 a staging system called the PRETreatment EXTent of disease (PRETEXT) was designed to allow a universal, multidisciplinary approach to patients with HB (Figure 1). The main aim of PRETEXT grouping was to identify patients in whom complete tumor resection was possible with a partial hepatectomy. Physicians placed patients in one of four PRETEXT categories based on the extent of their tumor on imaging. The liver is divided into four sectors in the PRETEXT system - anterior and posterior on the right and a medial and lateral sector on the left. Four groups were identified based on tumor extension: PRETEXT I, tumor only in one sector; PRETEXT II, tumor involves two sectors; PRETEXT III, tumor involves three sectors or two non-adjoining sectors; and PRETEXT 4, tumor involves all four sectors[11]. These categories are further characterized by describing extrahepatic spread: V for involvement of the hepatic veins and/or inferior vena cava, P for involvement of the portal vein, E for extrahepatic tumor extension, and M for distant metastases[11].
Between one-third to two-thirds of patients initially present with unresectable tumors or distant metastases, but up to 85% of these tumors become operable after neoadjuvant chemotherapy[12]. Preoperative chemotherapy has many advantages. It is responsible for making tumors smaller and more demarcated from the surrounding liver. Most surgeons agree that operating on tumors that shrink with chemotherapy is easier because the tumor is more defined and less prone to bleeding. It also exposes metastases (both visible and micrometastases) to chemotherapy earlier. In one trial, up to 52% of patients with initial lung metastases achieved complete remission with chemotherapy alone[13]. Classic treatment regimens include a combination of cisplatin, fluorouracil, and vincristine or cisplatin and doxorubicin. Although an effective agent, patients treated with doxorubicin can have a higher incidence of treatment complications and toxic death-especially from heart failure[14]. More recent studies have shown the effectiveness of single-agent cisplatin treatment in both standard and high-risk patients with HB[15,16] decreasing the likelihood of chemotherapy-induced toxicity.
Patients with PRETEXT categories 1, 2, and some 3 are referred for neoadjuvant chemotherapy followed by surgical resection with the goal of complete tumor removal. Current chemotherapy at our institution consists of cisplatin, 5-fluorouracil, and vincristine or vincristine and doxorubicin. Patients will undergo four rounds of chemotherapy prior to resection and two rounds after resection. Disease-free survival following partial liver resection under these circumstances has been reported to be greater than 70%[2]. It has been argued that tumors with favorable prognostic factors, such as pure fetal histology and low mitotic rate, may not require toxic chemotherapy and should be treated with surgical resection only[17]. However, the treatment regimen at our institution closely follows the precedent set forth in European studies which emphasize the use of neoadjuvant chemotherapy in all HB patients because of the high frequency of HB chemosensitivity[11,18].
Liver transplantation is the only treatment option for unresectable HB. Transplant should be considered in the following cases: multifocal disease (PRETEXT IV), PRETEXT III with the tumor in close proximity to major vessels, and tumor extension into major vessels. Patients that fall into these categories at our institution are listed for OLT immediately after the diagnosis of HB is confirmed and undergo chemotherapy while they await transplantation. Overall patient survival at 6 years has been reported to be over 80% making OLT the preferred treatment modality in this group[19]. Patients with intrahepatic recurrence or residual tumor after resection are rarely candidates for transplant because of poor outcomes[19].
There are few contraindications to OLT for unresectable HB. Patients with persistent pulmonary metastases despite neoadjuvant therapy and those with viable extrahepatic tumor not amenable to resection are not candidates for OLT. Patients that present with lung metastases are candidates for OLT if their lung metastases resolve with chemotherapy or with resection. Those with extrahepatic disease that remains viable after full chemotherapy and not amenable to surgical resection represent the only absolute contraindication to OLT in patients with HB[19].
Organ allocation rules for children with HB have changed over the past decade. The pediatric end-stage liver disease (PELD) is a pediatric-specific scoring system that was adopted in 2002 to help determine a patient’s ranking on the liver transplant list. The effect of the system has been to decrease the rate of death and removal from the transplant list and increase the percentage of children who receive a deceased donor organ. The score is based on total bilirubin, coagulopathy, serum albumin, age < 1 year and growth failure, but additional “exception” points may be awarded for risk factors not represented by the PELD equation. For example, patients with unresectable HB are listed with a PELD score of 30 for 30 d and are increased to status 1B if they have not been transplanted.
We analyzed the Standard Transplant Analysis and Research dataset to assess the impact of changes in exception point criteria for HB on outcomes after liver transplantation at Texas Children’s Hospital in Houston, Texas. Patients who underwent orthotopic liver transplant in our center from 1987-2014 with recipient diagnosis of either HB, cirrhosis post-resection of HB, or for whom a MELD exception was granted for non-metastatic HB were selected for analysis. Patients were grouped based on date of initial listing for transplantation. The 1987-2009 era preceded the current policy for HB exception while the 2010-2014 era followed its implementation. Differences in age at listing, recipient gender, waitlist time, and post-transplant patient survival between the two groups were calculated. To examine the difference between the number of patients listed in each era, a one-sample binomial test was used. Independent samples Mann-Whitney U testing was performed to compute differences in means between the two groups, while Pearson’s Chi-Squared was employed for differences in frequencies. Actuarial survival was assessed via the Kaplan-Meier Method. All statistical computations were performed with SPSS version 22 (IBM Armonk, New York).
Descriptive statistics for patients transplanted in each era are displayed in Table 1. A statistically similar number of patients were transplanted in each group (7 vs 14, P = 0.189). Similarly, there was no significant difference in gender, age at listing, and age at transplantation between the two eras. Patients listed for transplantation with the current HB exception criteria experienced a shorter waitlist time (45.5 d vs 25.4 d, P = 0.025).
| Era 1 (1987-2009) | Era 2 (2010-2014) | Significance (P-value) | |
| Total patients listed for transplantation | 7 | 14 | 0.189 |
| Gender | 0.557 | ||
| Male | 57.10% | 35.70% | |
| Female | 42.90% | 64.30% | |
| Age at listing (yr) | 5.4 | 2 | 0.094 |
| Age at transplant (yr) | 5.6 | 2.1 | 0.110 |
| Waitlist time (d) | 45.6 | 25.4 | 0.025 |
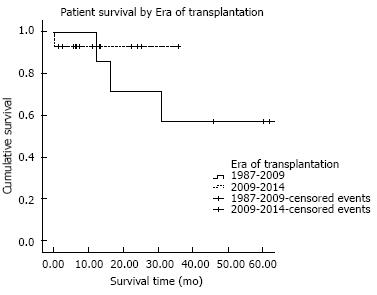
Figure 2 demonstrates patient survival in our center before and after implementation of the revised HB exception policy. From 1987-2009, 30-d, one-year, and five-year survival following liver transplant in our center was 98.6%, 87.0%, and 77.4%, respectively. In comparison, 30-d, one-year survival following transplantation from 2010-2014 was 97.1% and 90.5%. Statistically, patient survival is similar between the two eras (P = 0.7).
In conclusion, standard treatment with neoadjuvant chemotherapy, surgical resection followed by adjuvant chemotherapy is a good option for most pediatric and adolescent patients with HB. For those with tumors that are unresectable or unresponsive to chemotherapy, combined treatment with chemotherapy and liver transplantation is an excellent option. PELD exception points for HB have decreased the wait time for most patients listed for transplant but it is too soon to determine if this translates into increased survival for the group.
We would like to thank Scott Holmes, board certified medical illustrator with Baylor College of Medicine, for his help with the illustration.
P- Reviewer: Hori T, Qin JM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Ries LAG, Smith MA, Gurney JG, Linet M, Tamra T, Young JL, Bunin GR (eds). Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. National Cancer Institute, SEER Program. 1999;99-4649 Available from: http://seer.cancer.gov/archive/publications/childhood/. |
| 2. | Ismail H, Broniszczak D, Kaliciński P, Dembowska-Bagińska B, Perek D, Teisseyre J, Kluge P, Kościesza A, Lembas A, Markiewicz M. Changing treatment and outcome of children with hepatoblastoma: analysis of a single center experience over the last 20 years. J Pediatr Surg. 2012;47:1331-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z. SEER Cancer Statistics Review, 1975-2009 (Vintage 2009 Populations). National Cancer Institute. Based on November. 2011;SEER data submission, posted to the SEER web site, April 2012 [Updated August 20, 2012] Available from: http://seer.cancer.gov/archive/csr/1975_2009_pops09/. |
| 4. | Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008;13:812-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 139] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 5. | Giardiello FM, Offerhaus GJ, Krush AJ, Booker SV, Tersmette AC, Mulder JW, Kelley CN, Hamilton SR. Risk of hepatoblastoma in familial adenomatous polyposis. J Pediatr. 1991;119:766-768. [PubMed] |
| 6. | DeBaun MR, Tucker MA. Risk of cancer during the first four years of life in children from The Beckwith-Wiedemann Syndrome Registry. J Pediatr. 1998;132:398-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 288] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 7. | Spector LG, Birch J. The epidemiology of hepatoblastoma. Pediatr Blood Cancer. 2012;59:776-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 222] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 8. | Lai TT, Bearer CF. Iatrogenic environmental hazards in the neonatal intensive care unit. Clin Perinatol. 2008;35:163-181, ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
| 9. | Weinberger B, Laskin DL, Heck DE, Laskin JD. Oxygen toxicity in premature infants. Toxicol Appl Pharmacol. 2002;181:60-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Davies JQ, de la Hall PM, Kaschula RO, Sinclair-Smith CC, Hartley P, Rode H, Millar AJ. Hepatoblastoma--evolution of management and outcome and significance of histology of the resected tumor. A 31-year experience with 40 cases. J Pediatr Surg. 2004;39:1321-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Brown J, Perilongo G, Shafford E, Keeling J, Pritchard J, Brock P, Dicks-Mireaux C, Phillips A, Vos A, Plaschkes J. Pretreatment prognostic factors for children with hepatoblastoma-- results from the International Society of Paediatric Oncology (SIOP) study SIOPEL 1. Eur J Cancer. 2000;36:1418-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 204] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Pham TH, Iqbal CW, Grams JM, Zarroug AE, Wall JC, Ishitani MB, Nagorney DM, Moir C. Outcomes of primary liver cancer in children: an appraisal of experience. J Pediatr Surg. 2007;42:834-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 40] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Zsíros J, Maibach R, Shafford E, Brugieres L, Brock P, Czauderna P, Roebuck D, Childs M, Zimmermann A, Laithier V. Successful treatment of childhood high-risk hepatoblastoma with dose-intensive multiagent chemotherapy and surgery: final results of the SIOPEL-3HR study. J Clin Oncol. 2010;28:2584-2590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 213] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 14. | Ortega JA, Douglass EC, Feusner JH, Reynolds M, Quinn JJ, Finegold MJ, Haas JE, King DR, Liu-Mares W, Sensel MG. Randomized comparison of cisplatin/vincristine/fluorouracil and cisplatin/continuous infusion doxorubicin for treatment of pediatric hepatoblastoma: A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol. 2000;18:2665-2675. [PubMed] |
| 15. | Perilongo G, Maibach R, Shafford E, Brugieres L, Brock P, Morland B, de Camargo B, Zsiros J, Roebuck D, Zimmermann A. Cisplatin versus cisplatin plus doxorubicin for standard-risk hepatoblastoma. N Engl J Med. 2009;361:1662-1670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 192] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 16. | Zhang YT, Feng LH, Zhong XD, Wang LZ, Chang J. Single-agent cisplatin treatment of children with high-risk hepatoblastoma. J Pediatr Hematol Oncol. 2014;36:271-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Finegold MJ. Chemotherapy for suspected hepatoblastoma without efforts at surgical resection is a bad practice. Med Pediatr Oncol. 2002;39:484-486. [PubMed] |
| 18. | Carceller A, Blanchard H, Champagne J, St-Vil D, Bensoussan AL. Surgical resection and chemotherapy improve survival rate for patients with hepatoblastoma. J Pediatr Surg. 2001;36:755-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Otte JB, Pritchard J, Aronson DC, Brown J, Czauderna P, Maibach R, Perilongo G, Shafford E, Plaschkes J. Liver transplantation for hepatoblastoma: results from the International Society of Pediatric Oncology (SIOP) study SIOPEL-1 and review of the world experience. Pediatr Blood Cancer. 2004;42:74-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 206] [Article Influence: 9.8] [Reference Citation Analysis (0)] |