Published online Dec 24, 2014. doi: 10.5500/wjt.v4.i4.276
Revised: August 12, 2014
Accepted: September 16, 2014
Published online: December 24, 2014
Processing time: 203 Days and 1.9 Hours
Coronary allograft vasculopathy remains one of the leading causes of death beyond the first year post transplant. As a result of denervation following transplantation, patients lack ischaemic symptoms and presentation is often late when the graft is already compromised. Current diagnostic tools are rather invasive, or in case of angiography, significantly lack sensitivity. Therefore a non-invasive tool that could allow early diagnosis would be invaluable.This paper review the disease form its different diagnosis techniques,including new and less invasive diagnostic tools to its pharmacological management and possible treatments.
Core tip: Coronary allograft vasculopathy remains the leading cause of great loss in children after the first year post transplant. This paper offers a state of the art review of the disease from diagnosis including most recent and less invasive tools to management.
- Citation: Dedieu N, Greil G, Wong J, Fenton M, Burch M, Hussain T. Diagnosis and management of coronary allograft vasculopathy in children and adolescents. World J Transplant 2014; 4(4): 276-293
- URL: https://www.wjgnet.com/2220-3230/full/v4/i4/276.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i4.276
In children, coronary allograft vasculopathy (CAV) remains the main limiting survival factor after heart transplantation and the major cause of mortality after the first year post transplant leading ultimately to graft loss[1,2].
One elegant etiological description of CAV is that of “immunologic mechanisms operating in a milieu of non-immunologic risk factors”[3]. The process is believed to start off as a response to endothelial injury in the graft, originated by a complex interaction of multiple donor and recipient factors. The resulting endothelial dysfunction, leads to altered endothelial permeability and subsequent intimal hyperplasia as a consequence of the vascular remodeling originated by the inflammatory response. The immunologic events constitute the original trigger and non-inflammatory events such as cytomegalovirus infection, ischemic time (reperfusion injury), increased donor age and classical cardiovascular risk factors (i.e., diabetes, dyslipidemias, smoking and hypertension), perpetuate the inflammatory response and increase the endothelial injury[4].
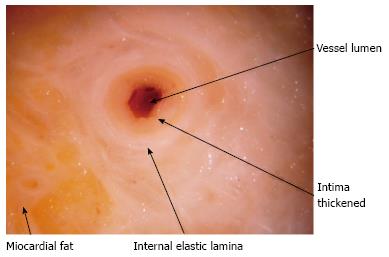
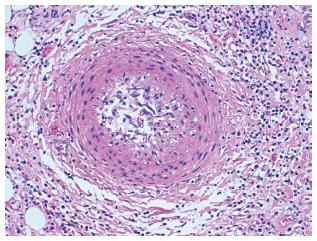
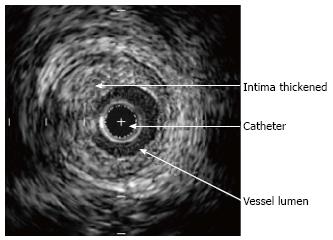
Typical lesions (Figures 1 and 2) consist of diffuse intimal proliferation leading to the development of luminal stenosis and small vessels occlusion which then limits blood supply to the graft causing chronic vascular injury and ultimately myocardial ischemia[5]. The lesions develop earlier and quicker than atherosclerotic lesions. In addition, progression is often silent due to the lack of ischemic symptoms from the denervated heart and often, the first clinical manifestation is an adverse cardiac event[6].
The real incidence of CAV among the pediatric population remains unknown, with a reported incidence varying between studies from 3% to 43%[7]. According to an angiographic multicenter study, the incidence of CAV would be 2%, 9% and 17% at 1, 3 and 5 years[1]. Looking at the incidence reported in studies that use intravascular ultrasound (IVUS), this is even higher, with 75% incidence of detectable intimal thickening at 5 years, with half of these representing at least mild disease[8]. The most current angiographic data estimates the incidence of CAV in the pediatric cohort of 13% at 5 years, 25% at 10 years and 54% at 15 years[9].
According to the ISHLT registry, using angiographic definitions, 65% of recipients are free of CAV at 10 years, but after a diagnosis of CAV, the 2-year graft survival rate is less than 50%[2].
Age at transplantation has a strong influence on survival with a 74% 8-year freedom of CAV in younger recipients compared to 56% in recipients older than 10 years[10].
As CAV lesions are preceded by endothelial dysfunction, it is essential to identify and characterize this as early as possible for targeted therapy and ultimately to improve patient survival.
The diagnosis of CAV is challenging. As a result of the denervation inherent to heart transplantation, patients fail to display classical clinical warning signs of angina[11]. The ability of early diagnosis is essential but unfortunately, the majority of the diagnostic techniques lack sensitivity or are rather invasive. A reliable and repeatable non-invasive method that detects CAV and its functional significance would have a huge impact on the follow up of heart transplant recipients. However, sensitivity and specificity of the currently available non-invasive tests remain limited.
Screening protocols vary among centers and the majority of units use a combination of diagnostic modalities, depending mainly on local preferences and expertise.
For many years, until the introduction of IVUS, this has been the cornerstone of CAV diagnosis[12,13]. Despite its relatively low sensitivity[14,15] and resulting delay in diagnosis, coronary angiography remains the most widely used diagnostic technique for CAV in the majority of transplant centers.
Angiography is known to underestimate the disease[16]. Adults series display a low sensitivity and negative predictive value. St Goar et al[14] found that 50% of patients with normal angiographies had moderate to severe intima thickening on IVUS. In a series by Tuzcu et al[17] the sensitivity of angiography for CAV detection (defined by maximal intimal thickness > 0.5 mm) was 43%, specificity was however high with 95%.
Similarly in a most recent paper, Gregory et al[18], using the same definition, showed a sensitivity even lower of 11% with a negative predictive value of 57%. Defining CAV as mean intimal thickness > 0.3 mm, Störk et al[19] found a sensitivity of 44% and a negative predictive value of 28% when compared to the IVUS data.
Its main limitation arises from the fact that it assesses the vessel lumen. The contrast fills the patent lumen without direct visualisation of the vessel wall. By the time a filling defect appears and there is significant stenosis, the graft is already compromised. CAV tends to be diffuse and concentric affecting large and medium size vessels as well as the microvasculature[14,20]. Typically there is initial vessel expansion: as the intima thickens, the external elastic membrane expands preserving initially the lumen area (Glagov-type positive remodeling)[21-24]. This explains why the coronary angiography result can be normal in the presence of significant disease demonstrated by IVUS. Nevertheless, angiography is inexpensive, readily available across centers and findings have proven prognostic implications regarding graft survival and adverse cardiac events[25,26].
One of the largest experiences in pediatric patients has been published by Pahl et al[1] in 2005 and included multicenter data proceeding from the Pediatric Heart Transplant Study database. Two thousand and forty-nine angiograms from 751 patients were analysed. The incidence of angiographic abnormalities at 5 years was 17%. However, moderate-to-severe disease occurred in only 6% at 5 years[1]. The use of IVUS in children is limited and they showed a sensitivity of angiography to detect CAV when compared to IVUS data between 18% and 30%[8,27].
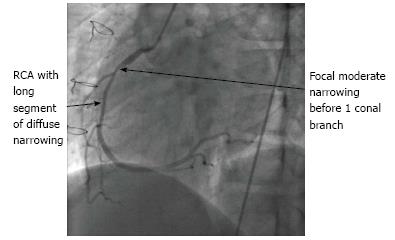
In 2010, the ISHLT published new guidelines for CAV including a new classification (Table 1) in view to provide a more refined definition and prognostic value[13]. Figures 3 and 4 showed angiography of two grats with severe disease.
| Grade | |
| 0 (Not significant) | No detectable angiographic lesion |
| I (Mild) | Angiographic LM < 50% stenosis, or primary vessel with maximal lesion of < 70%, or any branch stenosis of < 70% ( including diffuse narrowing) |
| II (Moderate) | Angiographic LM 50%-69% stenosis, a single primary vessel ≥ 70% stenosis, or isolated branch stenosis of ≥ 70%in branches of 2 systems |
| III (Severe) | Angiographic LM ≥ 70%, or 2 or more primary vessels ≥ 70% stenosis, or isolated branch stenosis of ≥ 70% in all 3 systems, or mild/moderate angiographic disease with LVEF < 45% or evidence of significant restrictive physiology (i.e., symptomatic heart failure with echocardiographic E to A velocity ratio > 2 (> 1.5 in children), shortened isovolumetric relaxation time (< 60 ms), shortened deceleration time (< 150 ms), or restrictive hemodynamic values (Right Atrial Pressure > 12 mmHg, Pulmonary Capillary Wedge Pressure > 25 mmHg, Cardiac Index < 2l min/m2) |
IVUS is more sensitive than angiography for early CAV detection and allows delineation of the vessel wall as well as measurement of intimal thickness[14]. Even if it might provide an oversimplified picture of the disease process, the intimal thickening measured via IVUS remains the most sensitive diagnostic modality available[13].
As mentioned above, Glagov-type positive remodeling occurs in response to the vessel wall disease. This serves to maintain initial lumen patency and the angiographic appearance of the vessel can therefore be normal despite significant CAV. This is particularly significant in the first year post transplantation. Later on in the disease process, constrictive negative remodeling of the vessel will occur and lead to the stenosis of the vessel[23].
IVUS parameters reported in the literature include: intimal thickness, mean intimal index (ratio of the mean intimal area to the sum of the mean intimal and luminal areas), total atheroma volume and percentage of atheroma volume. In 1995, the Rickenbacher et al[28] demonstrated that, in an adult cohort, moderate to severe intimal thickening diagnosed by IVUS was predictive of the future development of angiographically detectable disease (Table 2). This article describes CAV as being present when maximal intimal thickness is ≥ 0.3 mm. A further finding was that maximal intimal thickness (MIT) ≥ 0.3 at 1 year was associated with a 4 year survival of 73% compared to 96% within the group of MIT < 0.3 mm[28]. Two more recent studies published in 2005[17,29] reported that a change of MIT ≥ 0.5 mm over the first year post-transplant was an independent predictor for subsequent angiographic development of CAV; for myocardial infarction and for all-cause death at 5-years post-transplant. Patients with a change in MIT > 0.5 mm had a 5-year incidence of 21% for death or graft loss, 46% for all major adverse events and 65% for the development of subsequent angiographic disease compared to 6%, 17% and 35% respectively for patients without a 0.5 mm change[29].
| Grade | Severity | Intimal thickness |
| I | Minimal | < 0.3 mm and < 180 degrees |
| II | Mild | < 0.3 mm and > 180 degrees |
| II | Moderate | 0.3-0.5 mm |
| OR | ||
| 0.5-1 mm and < 180 degrees | ||
| IV | Severe | > 1 mm |
| OR | ||
| 0.5-1 mm and > 180 degrees |
Interestingly, however, intimal proliferation evaluated in IVUS does not always correlate with microvascular or small artery disease in biopsies specimens[13,30]. Looking specifically at Pediatric data, IVUS has not shown yet impact on prognosis[27] and this probably relates to the limited number of studies, each with differing analysis methodology.
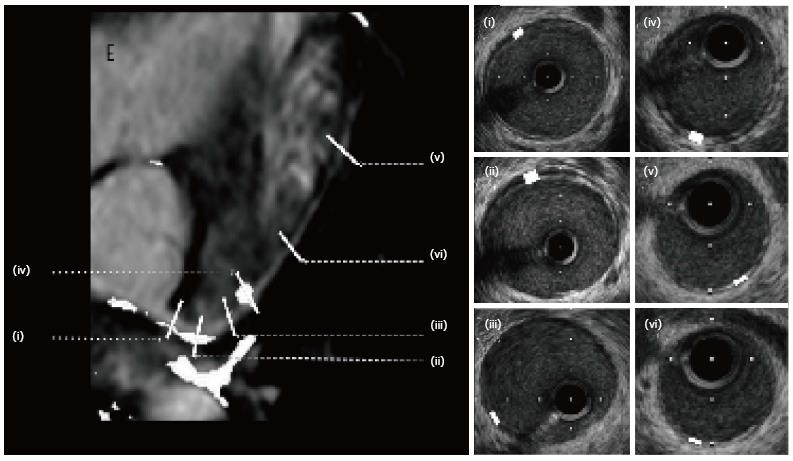
According to published data, sensitivity increases with the number of vessels imaged[31]. However, our experience in children suggests that this is not the case and multi-vessel imaging increases risk without substantially altering sensitivity. Therefore, in our usual practice, we only image the left main and proximal left anterior descending. We use automatic pullback to enhance consistent sampling and identification of branch vessels that are used as landmarks in order to be able to compare serial investigations. We analyze 30 cross-section images taken at 1.5 mm intervals and identified (as mentioned above) by branch points. Additionally, image analysis is performed during mid-diastolic rest period for consistency. In addition to maximal intimal thickness, mean intimal thickness, and mean intimal index, Stanford grading score (Table 2) and percentage of atheroma are recorded. We also use a semi-automatic interactive edge detection software (QIVUS) to improve reproducibility of measurements[32] (Figure 5).
Unfortunately, IVUS remains rather unused in clinical routine: the higher cost and potential morbidity added to the requirement of a trained operator, limits its use currently. This is particularly true in the pediatric population, where the size of the patient is an additional limitation. Nicolas et al[27], have reported feasibility in patients ≥ 10 kg but in our institution, we normally do not proceed in patients under 10 years of age[8,27].
The usefulness and accuracy of several echocardiographic techniques, as diagnostic methods for CAV have been explored. Published data have shown disparate results but more recent reports involving dobutamine stress echocardiography have demonstrated greater prognostic value[33-40].
Dobutamine stress echocardiography (DSE) allows assessment of wall motion, inducible ischemia and viability. Nevertheless, the sensitivity, specificity positive predictive value and negative predictive value vary significantly among these studies. Despite these limitations, Spes et al[35] noted, in an adult cohort, that in patients with abnormal DSE, 90% had significant CAV by IVUS, but only 49% by angiography again demonstrating the relative insensitivity of angiography. Furthermore, they showed that a normal pharmacological stress echocardiography after heart transplantation has a high negative predictive value for any major adverse cardiovascular event. This suggests that if a strict DSE protocol is followed, a selective invasive angiography/IVUS policy may be adopted[34,35,37,38,41]. This was corroborated in a Pediatric cohort by Pahl et al[39]. Some authors have pointed out that endothelial dysfunction might be the cause of abnormal wall motion detected by DSE and normal angiography[42].
In children, the variability when compared to angiography, is even higher than that showed in adult series. Sensitivity rates vary between 35% and 71%, specificity between 80% and 94%, positive predictive value between 45% and 91% and negative predictive value between 81% and 92%[37,43,44]. If reliability within a given department is established, then it certainly appears to be an attractive option for children due to its non-invasive nature. However, it does require a good set up, effective sedation, expertise in images acquisition, expertise in interpretation and a standardised, reproducible protocol.
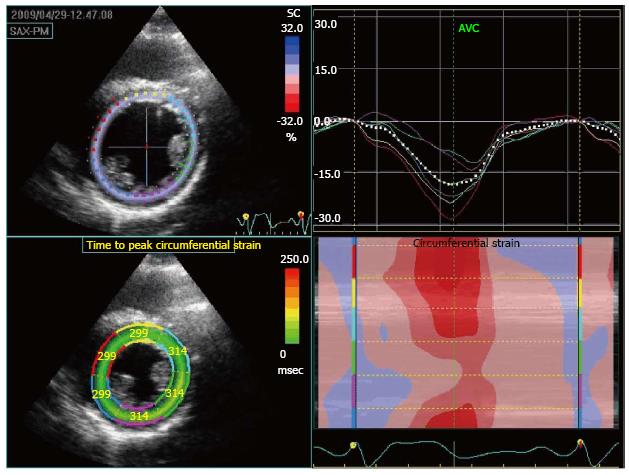
Sensitivity and specificity of stress echocardiography techniques can be improved by quantitative analysis using strain imaging. This modality can quantify regions of wall motion abnormality, (i.e., a reduction in peak systolic strain % will be seen in LV segments associated with inducible ischemia and accurate measurements of time to peak strain may also give information on regional wall motion abnormalities). Eroglu et al[40], showed that, in adults, the accuracy of DSE can be improved using strain analysis (Figure 6).
Combined use of contrast-enhanced echocardiography with adenosine mediated hyperemia in order to assess coronary flow reserve has shown encouraging results in adults. Tona et al[45] demonstrated feasibility and prognostic value of coronary flow reserve measured by contrast enhanced echocardiography with good correlation with major acute cardiac events. Severe Coronary Flow Reserve (CFR) alteration was shown to precede acute cardiac event onset. On a more recent study, the same group, showed high sensitivity and specificity for this technique in the detection of significant CAV (defined by Media Intimal Thickness > 0.5 in IVUS)[46]. Although these results are really encouraging, more studies are needed to establish the reproducibility. Interestingly, a separate small study in adults showed that transesophageal echocardiographic measurement of CFR impairment could identify CAV but it did not allow grading of severity[47]. However, this approach will be more difficult to implement in children, owing to difficulty in imaging due to the small size of the coronary arteries and the need for sedation in many patients.
The application of tissue Doppler techniques to the transplant population is also worth mentioning. Dandel et al[48,49] showed the utility of power Doppler TDI for the diagnosis of CAV in adults. Systolic Tissue Doppler Imaging (TDI) parameters at basal lateral LV wall level showed the highest diagnostic accuracy. Peak systolic motion velocity (Sm) and time to peak systole (TSm) differed significantly between patients with and without CAV as identified by IVUS. Furthermore, with Sm > 11 cm/s and TSm > 110 cm/s2, angiographic disease can be excluded and, in the absence of any rejection, an Sm < 10 cm/s has a positive predictive value of over 97% for CAV (as detected by IVUS or angiography)[48,49]. The main limitations for the widespread use of this technique arise from the inter-observer and inter-departmental variability. These techniques have been applied to adult cohorts mainly and the available literature in the pediatric population is still very limited. One small retrospective study has shown that tricuspid annulus velocity was the best predictor of graft failure in pre-terminal patients. However, conventional echocardiographic parameters such as increase in tricuspid regurgitation severity and a reduction in left ventricular ejection fraction were also associated with increased mortality[50]. However, another recent study in a pediatric cohort showed poor correlation between TDI and hemodynamics parameters[51], highlighting the need for further confirmatory studies in children.
Exercise stress echocardiography (ESE) in adult patients was initially found to have unacceptably low sensitivity for the detection of CAV[33,52]. However, Chen et al[53] showed recently a sensitivity higher than 88% with almost 92% specificity in detecting significant epicardial angiographic CAD among pediatric heart transplant recipients. The positive predictive value of ESE was 72.7%, and the negative predictive value was 97.1%[53]. These results need wider confirmation prior to consideration as a screening tool.
CAV is a complex and diffuse process that leads to concentric luminal stenosis and occlusion of epicardial large and medium sized vessels. It also affects the intramyocardial microvasculature. Microvascular disease is present in heart transplant recipients early after transplant, even in asymptomatic patients[20,54] and it is known to be associated with CAV, ischemia and death.
Fractional flow reserve (FFR) is defined as the ratio of maximum flow in the presence of a stenosis to normal maximum flow. It is a lesion-specific index of stenosis severity that can be calculated by simultaneous measurement of mean arterial, distal coronary, and central venous pressure, during pharmacological vasodilation. FFR is a well established tool to assess hemodynamic significance of coronary focal stenosis and has been recommended since 2010 by European Society of Cardiology for the physiological assessment of moderate coronary stenosis when functional information is lacking[55] in atherosclerotic disease.
In such cases, pressure gradients and FFR are recorded throughout the length of the artery through a pull back of the wire during maximum pharmacologically-induced hyperemia.
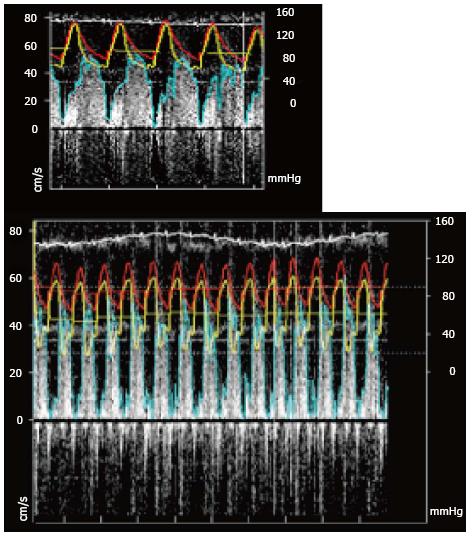
The Combowire® XT also allows simultaneous measurement of flow and pressure and FFR simultaneously to the coronary flow reserve (CFR) (Figure 7).
In transplanted patients, the exact value of FFR to determine epicardial disease is difficult to establish and results have been inconsistent between series[56,57]. In a publication by Hirohata et al[20], FFR improved as the microvascular disease deteriorated and therefore, due to the particular interaction between microvascular and epicardial disease that occurs in CAV, FFR might not be the best reflection of epicardial affectation in this situation.
CFR reflects the ability of the myocardium to increase blood flow in response to maximal exercise or stress. It is expressed by the ratio of the myocardial blood flow at peak stress, or maximal vasodilatation, to the flow at rest. Decrease in CFR, after Adenosine administration to achieve maximum vasodilation, in the absence of significant epicardial stenosis (normal fractional flow reserve) indicates microvascular dysfunction[58]. If the significance of decreased CFR is well established in the atherosclerotic population[59,60]. Although theoretically more important for CAV, the exact significance of CFR measurement remains to be determined. Using acetylcholine-mediated, endothelium-dependent, coronary vasodilatation measurement of CFR, Hollenberg et al[61] showed that endothelial microvascular dysfunction was more common in the group suffering adverse outcomes (death or angiographic evidence of CAV) than in those without adverse outcome. However, published data are not consistent between studies. Kübrich et al[62], in a larger cohort, found no correlation between epicardial and microvascular disease and found that, whilst microvascular dysfunction demonstrated by CFR was a predictor of outcome (death or adverse cardiovascular event) in the univariate analysis, it did not predict outcome in the multivariate analysis.
The pediatric population offers very limited data for CFR. In a small cohort, a decrease in CFR correlated with microvasculopathy seen in endomyocardial biopsy specimens[63]. The invasive nature of Doppler wire flow measurements to determine CFR makes it an unattractive tool for children.
Several groups have presented data of CFR quantified by CMRI of the coronary sinus showing good correlation with PET or flow phantoms[64-66]. More recently, Ishida et al[67] presented data on CFR as independent predictor of MACE in patients with known or suspected CAD. Kennedy et al[68] have translated this idea into the transplant population: they found that CFR determination by Cardiac Magnetic Resonance Imaging (CMRI) in the coronary sinus, was significantly decreased inpatients with severe CAV and therefore, it may be a useful tool in non-invasively evaluating coronary allograft vasculopathy in heart transplant recipients.
Single photon emission computed tomography (SPECT) is a useful clinical tool for myocardial perfusion imaging to detect and risk-stratify of coronary atherosclerotic disease for management guidance[69]. Either exercise or pharmacological stress can be employed and, most commonly, one of theTc-99m-labeled tracers is used. Numerous studies in adult population with coronary atherosclerotic disease have assessed the relative accuracies of stress imaging using nuclear cardiology techniques: for stress SPECT, sensitivity is around 87% with a specificity of 73% (compared to coronary angiography)[70]. Recently, it has been recognized that some patients with non-critical coronary artery stenosis can have abnormal stress perfusion imaging. This is due to microvascular and endothelial dysfunction causing abnormal flow reserve[71].
When applied to CAV, SPECT has a high negative predictive value in adults[72-77]. When using Dobutamine stress and 99m technetium tetrofosmin, abnormal perfusion is associated to a risk ratio of 3.5 in predicting cardiac death[78-80]. A reversible perfusion defect on stress SPECT is an independent predictor of mortality or graft loss[72,81-83] and it seems that stress SPECT at one year post transplantation could be an earlier prognostic indicator[84].
In Pediatrics, the experience with SPECT is largely anecdotal. The small size of the heart might be a limiting technical factor and the radiation related to the technique itself makes it a rather unattractive diagnostic tool.
Positron emission tomography (PET) has established itself as the gold standard for noninvasive assessment of myocardial perfusion measuring myocardial blood flow at rest and during stress. As well as myocardial perfusion reserve, perfusion of the epicardial arteries and the microvasculature can be determined[85,86]. In patients after heart transplantation, myocardial perfusion reserve measured with PET has been performed in a few studies[87-89]. Wu et al[88] found good correlation between IVUS and myocardial perfusion reserve even in the absence of angiographic lesions. Published data is very limited even in adults, related to the limited availability of the technique and the expertise required.
In the atherosclerotic population, multidetector computed tomography (MDCT) has shown high sensitivity and specificity in the diagnosis of angiographic coronary arteriopathy and characterization of the stenotic disease[90]. Recent studies also indicate that detection and characterization of the plaque is possible although challenging[91,92] increasing potential value as a diagnostic tool.
The literature provides some data regarding the heart transplant population: Sigurdsson et al[93] used a 16-detector MDCT to identify coronary stenosis and compared to angiographic disease (defined by luminal stenosis > 95%). Sensitivity, specificity, positive and negative predictive values were 86%, 99%, 81% and 99% respectively, unfortunately only a few subgroup of patients underwent IVUS.
Gregory et al[18], on the other hand, did use IVUS to compare 64-slice MDCT results in 20 patients at 1 year post-transplant. They defined CAV as maximal intimal thickness > 0.5 mm and found that MDCT has a sensitivity of 70% and a specificity of 92% with a positive predictive value of 89% and negative predictive value of 77%. However, in this study, slightly less than 20% of coronary segments (mainly distal) could not been analysed due to poor image quality (probably in relation with elevated heart rate)[18].
Recent studies showed that dual source MDCT allows good image quality of vessel lumen[94,95] and, when validated against IVUS, high diagnostic accuracy[96]. A small study, just under 20 patients, demonstrated that MDCT, using 64-slices, was superior to angiography for the identification of non-obstructive vessel wall disease. However, they did not use IVUS for comparison[94]. Schepis et al[96] compared 64 channels dual source MDCT with IVUS to look at vessel wall thickness. Defining CAV as intimal thickness > 0.5 mm on IVUS they established sensitivity, specificity, negative predictive value and positive predictive value of MDCT of 85%, 84%, 76% and 91% respectively.
Therefore, MDCT appears to be a useful tool for CAV screening. Although not as sensitive as IVUS, it is non-invasive and clearly superior to angiography. However, the elevated heart rate post-transplantation, especially in pediatric patients, compromises image quality and the need for potentially nephro-toxic contrast agent adds concern for heart transplant recipients, for whom renal impairment is a frequent comorbidity[97,98].
There is preliminary data available in children using MDCT compared to angiography and IVUS to identify coronary luminal stenosis, although the size of the series was very small[99] and results would require further studies to be validated.
Again, the implied repeated radiation dosage makes it a less attractive screening option in children.
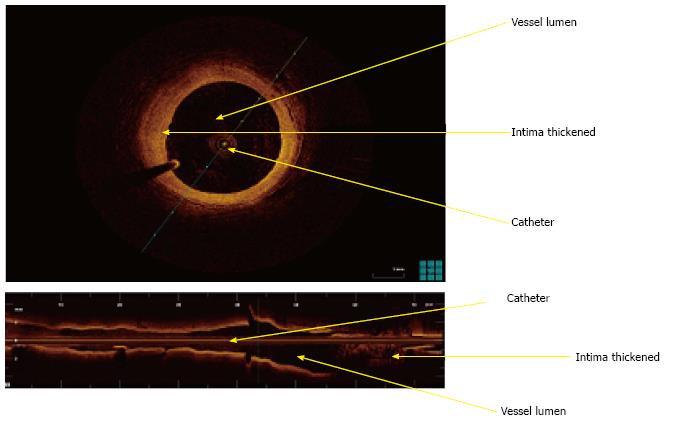
Optical coherence tomography (OCT) is an intravascular high resolution imaging modality that measures reflected light waves intensity and converts these into a high resolution tomographic image[100]. In CAD patients, OCT has been used to characterize plaque composition and differentiate between intimal hyperplasia, fibrous plaque, lipid-rich plaque or calcifications[101,102] (Figure 8).
Recent studies have evaluated the use of OCT in heart transplant recipients with promising results. The OCTAV study demonstrated, in 15 patients early post-transplant (with no angiographic evidence of CAV), that early quantification of intima-media ratio and characterization of the plaque is possible. There was no IVUS performed for comparison[103]. Garrido et al[104] compared OCT to IVUS in 21 patients, later post-transplant, and not only found good correlation with IVUS but also postulated that OCT offers better plaque characterization and less inter-observer variability.
Cassar et al[105] compared OCT to IVUS and angiography in 53 patients, showing that OCT was superior to angiography but not to IVUS. IVUS and OCT were strongly correlated with 100% agreement.
Further prospective and larger studies are needed to define the exact role of OCT in the diagnosis of CAV and, more importantly, to define its prognostic implications.
Cardiac magnetic resonance imaging (CMRI) coronary angiography in the context of CAD has proved its capacity to detect atherosclerotic plaque and proximal to mid-coronary artery stenoses[106-108]. Uribe et al[109] have demonstrated the feasibility and accuracy of MR coronary angiography in the detection of coronary anomalies in children, despite elevated heart rates with whole heart dual phase cardiac imaging[109,110]. Greil et al[111], have also previously shown the utility of coronary magnetic resonance angiography (CMRA) in patients with Kawasaki disease.
These studies undoubtedly open the door for the application of CMRA in CAV including in pediatric cohorts. Unfortunately, when compared to MDCT, CMRA does not seem to be as sensitive or robust in the detection of coronary stenoses, although limited studies have been done. CMRI offers several advantages: it provides functional information on myocardial characterization and contractility as well as wall motion performance; it allows quantitative measurements of ventricular volumes and it is radiation-free, which is especially valuable in a population already exposed to repeated X-ray angiography.
In conventional atherosclerosis, perfusion imaging has shown to be effective in detecting myocardial ischemia and to assess microvascular dysfunction as it detects downstream microvacular blood flow within the myocardium. The MR-IMPACT study demonstrated that CMRI is superior to SPECT in identifying perfusion defects within the myocardium for atherosclerotic patients[112]. Perfusion stress with adenosine also provides prognostic data: a normal CMR stress perfusion scan showed 99% event free survival at 3 years[113].
The use of adenosine for myocardium stress perfusion after heart transplantation has not been widely reported. Nevertheless, Muehling et al[114] showed a reduced myocardial perfusion reserve in patients with CAV with good correlation between MRI and invasive measurements. Unfortunately, microvascular disease in this study could not be assessed. They also demonstrated that patients with CAV have a reduced myocardial perfusion even during rest conditions[114].
In regards to CMR tissue characterization, Steen et al[115] showed that more than 80% of patients with severe angiographic CAV had a late gadolinium enhancement pattern suggesting subendocardial infarction with a distribution consistent with the angiographic pattern. Furthermore, he was able to identify silent myocardial infraction in otherwise apparently event-free patients (Figure 9). In a more recent publication, the same group looked at infarct-atypical myocardial involvement that they were not able to correlate with coronary angiographic pattern in the prior study. According to their findings, within the 4 different patterns of infarct-atypical LGE-CMR, only the diffuse form was significantly higher patients early post transplantation, but they could not establish a definite reason for the findings[116].
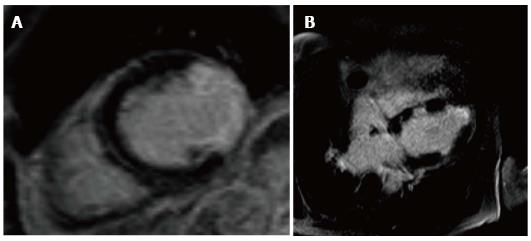
Hussain et al[117], have taken this technique further showing that high resolution late gadolinium enhancement (LGE) can be used to show vessel wall disease in CAV with good correlation with IVUS Figure 10. LGE scores correlated well with the maximal intimal thickness and mean intimal index [Pearson coefficient 0.80 (P < 0.001) and 0.92 (P < 0.001), respectively]. An enhancement diameter > 7.5 mm gave promising sensitivity and specificity values of 86% and 93%, respectively, for the detection of significant CAV.
A recently published paper, evaluated in 48 transplanted patients both epicardial and microvacular disease concomitantly. The patients underwent coronary angiography, invasive coronary physiological assessment, IVUS and multi-parametric cardiac MRI that includes, tissue characterization, perfusion analysis and tissue tagging. They found that cardiac MRI-based myocardial perfusion reserve was independently predictive of both epicardial and microvacular components of CAV and furthermore that diagnostic performance was significantly higher than angiography[118].
More studies are needed to establish CMRI as a reliable non-invasive tool for CAV diagnostic but certainly the latest data are encouraging and more work needs to be achieved in this direction.
Rapid progression of CAV within the first year post transplant is a strong indicator of severe CAV, graft loss and mortality[17]. Therefore, prophylactic strategies are paramount and must been introduced early to improve long-term outcomes and prognosis.
Similar to native coronary disease, primary prevention includes control of traditional cardiovascular risk factors such as hypertension, smoking, diabetes and hyperlipidemia. This can be challenging, as many of these factors are also side effects of the immunosuppressive therapy. Tobacco should be avoided and care should be taken to avoid passive smoking in children. Modifications of specific risk factors related to the transplant include prevention and aggressive treatment in case of cytomegalovirus (CMV) sero-conversion[118]. In addition, it is essential to treat any episode of rejection early and aggressively.
Psychological support is crucial in transplanted children and their families throughout all the transplant journey: Leaving with a reduced life expectancy when compared to peers is often complicated and despite good quality of life can be a source of distress for the recipients. In the context of CAV psychological support is especially important: Prevention is paramount; and, if it is essential to treat aggressively any rejection episode, it is also vital for the patients to be compliant with the antirejection therapy. However, it is well known that often therapy compliance declines in adolescence and case of sudden death have been reported related to antirejection treatment discontinuation. In these patients psychological support is essential to ensure therapy obeisance. In cases of advanced CAV the ineluctability of the graft loss and its implication lead to severe depression and negation that also frequently required psychological input.
Most transplant protocols nowadays include statin, independently of the lipid level. Several studies have highlighted their benefits beyond lipid lowering effects[119-121]; including reduced incidence of severe rejection episodes, reduced CAV progression and improved long term survival[122-124]. Consensus guidelines unequivocally recommend statin therapy[125].
CMV infection results in acceleration of CAV as the result of the host immune response. Aggressive treatment with ganciclovir reduces progression of CAV[126] and the lack of prophylaxis is associated with increased lumen loss[127]. Our institution, as with most of the transplant centers, uses acyclovir for CMV prophylaxis during the first 3 mo post-transplantation.
A few reports indicate a potential role for vasodilators in preventing and slowing CAV progression. Calcium channels blockers and ACE inhibitors have been reported in the literature to be beneficial but large prospective trials are needed to determine their exact role[128-130]. Most transplant institutions use both of these to treat hypertension, which develops frequently as side effect of calcineurin inhibitors therapy.
Most of the data are from adult studies with limited evidence in the pediatric population.
Tacrolimus not only offers better protection against acute rejection compared to cyclosporine[131-133], but it is also superior against CAV[134]. Moreover, Petrakopoulou et al[135] showed that tacrolimus is better than cyclosporine in the prevention of microvascular endothelial dysfunction.
Mycophenolate mofetil (MMF) has demonstrated superiority to azathioprine in mortality and graft loss[136]. In the re-analysis of the same study, it also showed less intimal thickening and wider lumen area[137]. Finally, Kaczmarek et al[138], in 2006 demonstrated that MMF decreased CAV incidence.
Contrary to Calcineurin Inhibitors (CNIs) that blocks T-cell activation and proliferation by suppressing lymphokines production, proliferation signal inhibitors (PSIs) inhibit Tcell and B cell proliferation by impairing their response to growth promoting lymphokines[139]. In addition, PSIs have also a significant cytostatic effect on the immune system[134,140]. In 2003, Eisen et al[141] published the first data in favor of PSIs, using everolimus de novo after heart transplantation. They showed preservation of the coronary lumen at 1 year with significant lower incidence of CAV in the everolimus group compared to the aziathropine group. A sub-study published in 2007 confirmed the results at 24 mo[142] and the same group has also shown reduced incidence of cardiovascular events in the everolimus group[143]. Nevertheless, despite the promising results of these studies, all of them compared PSIs to Azathioprine, which is not used as first line therapy anymore and known to be associated to higher rate of rejection than newer immunosuppressive agents. The results of an eagerly awaited clinical trial comparing everolimus de novo to cyclosporine has been recently published showing marked improvement in renal function at 12 mo in the everolimus group without increased of adverse events as well as demonstrated, via IVUS, significantly reduced CAV progression at 12 mo in the everolimus group[144].
Mancini et al[145], in a randomized study reported that sirolimus (as a secondary immunosuppressant) slows progression of CAV and reduces the incidence of clinically significant events, such as death or graft failure. Keogh et al[146], using randomized de novo treatment between sirolimus or azathioprine reported significantly reduced progression in intimal and medial proliferation at 6 mo post-transplant and a reduction in the number of acute rejection episodes of around 50%. The effect was sustained at 2 years post transplant using IVUS to quantify vessel wall proliferation.
Although a combined regime CNIs + PSIs appears to be attractive in preventing and slowing CAV, serious concerns with this regimen should be raised regarding nephrotoxicity. PSIs have shown in several studies to increase side effects of CNIs, especially for nephropathy[147-151].
Raichlin et al[152] have published encouraging data with sirolimus-based immunosuppression, and even postulated that a CNI free regimen would be safe, well tolerated and associated with less CAV progression, coronary events and graft failure, when initiated beyond the first year (and within the first 2 years).
In a more recent study, the same group showed that early conversion to sirolimus attenuated plaque progression, improved overall survival, and increased freedom from cardiac events. However, the retrospective nature of the design and the differences in criteria for the therapy changes, make the results less generalizable[153]. Moreover, a recent study reported that late conversion to PSIs is associated with necrotic plaque core and calcification of the plaque[154].
Hence, safety of early CNI withdrawal with PSI conversion remains uncertain, especially in the first year post-transplant with concerns also raised about acute rejection. Therefore, many continue to recommend against withdrawal of CNIs during the first 12 mo post transplantation[155].
Side effects from PSIs are not infrequent: anemia, dyslipidemia, increased incidence of bacterial infections, peripheral oedema, pericardial or pleural effusion, pneumonitis and delayed wound closure. They seem to be dose-related and reversed by discontinuation of the drug, although most can be controlled with dose adjustments[155].
PSIs have also been attributed with a reduction in CMV infections and an inhibition of Epstein-Barr virus-infected tumorigenic cell lines[156-158]. In the Pediatric population, PSI use is still limited to a rescue therapy for post-transplant complications such as CAV or renal impairment secondary to therapy.
In contrast to native coronary disease, CAV is progressive and revascularization procedures are only palliative with no survival benefit[159,160]. Moreover, the concentric, diffuse and distal nature of CAV precludes the majority of patients for revascularization procedures.
Percutaneous intervention in transplanted patients are characterized by good short term results but high restenosis rates[160-165].
Unfortunately, stents do not offer better long-term results with a late re-stenosis rate around 70%. Drug eluting stents appear to have slightly better results with less restenosis[57,166]. However, only the minority of CAV lesions are amenable for percutaneous revascularization as outlined above and stent angioplasty might only be an option in selected patients.
Surgical revascularization is associated with a very high mortality (up to 40%)[162,167,168] and limited success. Indication is then reserved to highly selected patients.
Re-transplantation is the only definitive treatment for CAV. Unfortunately it is associated with lower survival than with the primary graft[169] (relative risk for 10 years mortality according to ISHLT 2012 data is 1.56) and the probability of CAV recurrence is higher (50% at 3 years)[167,170].
The scarcity of donors, and prior antigen sensitization means that, in practice, re-transplantation occurs infrequently.
Despite a wide range of new diagnostic techniques, angiography remains, to date, the most commonly used diagnostic tool for CAV. Not only is it invasive, costly and radiation-prone but it also fails to identify the disease in its early phase. IVUS is the most sensitive technique but requires trained operators and it is, again, an invasive technique requiring ionizing radiation.
Overall, the available published evidence support a role for MDCT or DSE as non-invasive screening test to reduce the number of invasive angiograms (and IVUS). However, an accurate and reproducible non-invasive diagnostic tool is yet to be widely established. CMR offers anatomical, histological and physiological assessments and, in the future, it could be valuable in the detection and grading of CAV.
Early detection is paramount but remains challenging. It may allow us to identify those requiring modification in immunosuppression, such as early introduction of PSIs for those with more aggressive CAV.
Unfortunately CAV remains the primary cause of graft failure after the first year post-transplantation and the only definitive treatment is re-transplantation.
P- Reviewer: Izawa KP, Petix NR, Said SAM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Pahl E, Naftel DC, Kuhn MA, Shaddy RE, Morrow WR, Canter CE, Kirklin J. The impact and outcome of transplant coronary artery disease in a pediatric population: a 9-year multi-institutional study. J Heart Lung Transplant. 2005;24:645-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 2. | Kirk R, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Dobbels F, Rahmel AO, Stehlik J, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: thirteenth official pediatric heart transplantation report--2010. J Heart Lung Transplant. 2010;29:1119-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 104] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 3. | Rahmani M, Cruz RP, Granville DJ, McManus BM. Allograft vasculopathy versus atherosclerosis. Circ Res. 2006;99:801-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 4. | Colvin-Adams M, Harcourt N, Duprez D. Endothelial dysfunction and cardiac allograft vasculopathy. J Cardiovasc Transl Res. 2013;6:263-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Valantine H. Cardiac allograft vasculopathy after heart transplantation: risk factors and management. J Heart Lung Transplant. 2004;23:S187-S193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 193] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 6. | Raichlin ER, McConnell JP, Lerman A, Kremers WK, Edwards BS, Kushwaha SS, Clavell AL, Rodeheffer RJ, Frantz RP. Systemic inflammation and metabolic syndrome in cardiac allograft vasculopathy. J Heart Lung Transplant. 2007;26:826-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 48] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Conway J, Dipchand AI. Heart transplantation in children. Pediatr Clin North Am. 2010;57:353-373, table of contents. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Dent CL, Canter CE, Hirsch R, Balzer DT. Transplant coronary artery disease in pediatric heart transplant recipients. J Heart Lung Transplant. 2000;19:240-248. [PubMed] |
| 9. | Kobayashi D, Du W, L’ecuyer TJ. Predictors of cardiac allograft vasculopathy in pediatric heart transplant recipients. Pediatr Transplant. 2013;17:436-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Kirk R, Edwards LB, Aurora P, Taylor DO, Christie JD, Dobbels F, Kucheryavaya AY, Rahmel AO, Stehlik J, Hertz MI. Registry of the International Society for Heart and Lung Transplantation: Twelfth Official Pediatric Heart Transplantation Report-2009. J Heart Lung Transplant. 2009;28:993-1006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Gao SZ, Schroeder JS, Hunt SA, Billingham ME, Valantine HA, Stinson EB. Acute myocardial infarction in cardiac transplant recipients. Am J Cardiol. 1989;64:1093-1097. [PubMed] |
| 12. | Schmauss D, Weis M. Cardiac allograft vasculopathy: recent developments. Circulation. 2008;117:2131-2141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 311] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 13. | Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, Madsen J, Parameshwar J, Starling RC, Uber PA. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 563] [Cited by in RCA: 693] [Article Influence: 46.2] [Reference Citation Analysis (0)] |
| 14. | St Goar FG, Pinto FJ, Alderman EL, Valantine HA, Schroeder JS, Gao SZ, Stinson EB, Popp RL. Intracoronary ultrasound in cardiac transplant recipients. In vivo evidence of “angiographically silent” intimal thickening. Circulation. 1992;85:979-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 321] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | O'Neill BJ, Pflugfelder PW, Singh NR, Menkis AH, McKenzie FN, Kostuk WJ. Frequency of angiographic detection and quantitative assessment of coronary arterial disease one and three years after cardiac transplantation. Am J Cardiol. 1989;63:1221-1226. [PubMed] |
| 16. | Johnson DE, Alderman EL, Schroeder JS, Gao SZ, Hunt S, DeCampli WM, Stinson E, Billingham M. Transplant coronary artery disease: histopathologic correlations with angiographic morphology. J Am Coll Cardiol. 1991;17:449-457. [PubMed] |
| 17. | Tuzcu EM, Kapadia SR, Sachar R, Ziada KM, Crowe TD, Feng J, Magyar WA, Hobbs RE, Starling RC, Young JB. Intravascular ultrasound evidence of angiographically silent progression in coronary atherosclerosis predicts long-term morbidity and mortality after cardiac transplantation. J Am Coll Cardiol. 2005;45:1538-1542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 205] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 18. | Gregory SA, Ferencik M, Achenbach S, Yeh RW, Hoffmann U, Inglessis I, Cury RC, Nieman K, McNulty IA, Laffan JA. Comparison of sixty-four-slice multidetector computed tomographic coronary angiography to coronary angiography with intravascular ultrasound for the detection of transplant vasculopathy. Am J Cardiol. 2006;98:877-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Störk S, Behr TM, Birk M, Uberfuhr P, Klauss V, Spes CH, Angermann CE. Assessment of cardiac allograft vasculopathy late after heart transplantation: when is coronary angiography necessary? J Heart Lung Transplant. 2006;25:1103-1108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Hirohata A, Nakamura M, Waseda K, Honda Y, Lee DP, Vagelos RH, Hunt SA, Valantine HA, Yock PG, Fitzgerald PJ. Changes in coronary anatomy and physiology after heart transplantation. Am J Cardiol. 2007;99:1603-1607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2687] [Cited by in RCA: 2405] [Article Influence: 63.3] [Reference Citation Analysis (0)] |
| 22. | Tsutsui H, Schoenhagen P, Ziada KM, Crowe TD, Klingensmith JD, Vince DG, Bott-Silverman C, Starling R, Hobbs RE, Young J. Early constriction or expansion of the external elastic membrane area determines the late remodeling response and cumulative lumen loss in transplant vasculopathy: an intravascular ultrasound study with 4-year follow-up. J Heart Lung Transplant. 2003;22:519-525. [PubMed] |
| 23. | Tsutsui H, Ziada KM, Schoenhagen P, Iyisoy A, Magyar WA, Crowe TD, Klingensmith JD, Vince DG, Rincon G, Hobbs RE. Lumen loss in transplant coronary artery disease is a biphasic process involving early intimal thickening and late constrictive remodeling: results from a 5-year serial intravascular ultrasound study. Circulation. 2001;104:653-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 111] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 24. | Wong C, Ganz P, Miller L, Kobashigawa J, Schwarzkopf A, Valantine von Kaeper H, Wilensky R, Ventura H, Yeung AC. Role of vascular remodeling in the pathogenesis of early transplant coronary artery disease: a multicenter prospective intravascular ultrasound study. J Heart Lung Transplant. 2001;20:385-392. [PubMed] |
| 25. | Costanzo MR, Naftel DC, Pritzker MR, Heilman JK, Boehmer JP, Brozena SC, Dec GW, Ventura HO, Kirklin JK, Bourge RC. Heart transplant coronary artery disease detected by coronary angiography: a multiinstitutional study of preoperative donor and recipient risk factors. Cardiac Transplant Research Database. J Heart Lung Transplant. 1998;17:744-753. [PubMed] |
| 26. | Barbir M, Lazem F, Banner N, Mitchell A, Yacoub M. The prognostic significance of non-invasive cardiac tests in heart transplant recipients. Eur Heart J. 1997;18:692-696. [PubMed] |
| 27. | Nicolas RT, Kort HW, Balzer DT, Trinkaus K, Dent CL, Hirsch R, Canter CE. Surveillance for transplant coronary artery disease in infant, child and adolescent heart transplant recipients: an intravascular ultrasound study. J Heart Lung Transplant. 2006;25:921-927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Rickenbacher PR, Pinto FJ, Lewis NP, Hunt SA, Alderman EL, Schroeder JS, Stinson EB, Brown BW, Valantine HA. Prognostic importance of intimal thickness as measured by intracoronary ultrasound after cardiac transplantation. Circulation. 1995;92:3445-3452. [PubMed] |
| 29. | Kobashigawa JA, Tobis JM, Starling RC, Tuzcu EM, Smith AL, Valantine HA, Yeung AC, Mehra MR, Anzai H, Oeser BT. Multicenter intravascular ultrasound validation study among heart transplant recipients: outcomes after five years. J Am Coll Cardiol. 2005;45:1532-1537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 305] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 30. | Clausell N, Butany J, Molossi S, Lonn E, Gladstone P, Rabinovitch M, Daly PA. Abnormalities in intramyocardial arteries detected in cardiac transplant biopsy specimens and lack of correlation with abnormal intracoronary ultrasound or endothelial dysfunction in large epicardial coronary arteries. J Am Coll Cardiol. 1995;26:110-119. [PubMed] |
| 31. | Kapadia SR, Ziada KM, L’Allier PL, Crowe TD, Rincon G, Hobbs RE, Bott-Silverman C, Young JB, Nissen SE, Tuzcu EM. Intravascular ultrasound imaging after cardiac transplantation: advantage of multi-vessel imaging. J Heart Lung Transplant. 2000;19:167-172. [PubMed] |
| 32. | Koning G, Dijkstra J, von Birgelen C, Tuinenburg JC, Brunette J, Tardif JC, Oemrawsingh PW, Sieling C, Melsa S, Reiber JH. Advanced contour detection for three-dimensional intracoronary ultrasound: a validation--in vitro and in vivo. Int J Cardiovasc Imaging. 2002;18:235-248. [PubMed] |
| 33. | Smart FW, Ballantyne CM, Cocanougher B, Farmer JA, Sekela ME, Noon GP, Young JB. Insensitivity of noninvasive tests to detect coronary artery vasculopathy after heart transplant. Am J Cardiol. 1991;67:243-247. [PubMed] |
| 34. | Derumeaux G, Redonnet M, Soyer R, Cribier A, Letac B. Assessment of the progression of cardiac allograft vasculopathy by dobutamine stress echocardiography. J Heart Lung Transplant. 1998;17:259-267. [PubMed] |
| 35. | Spes CH, Klauss V, Mudra H, Schnaack SD, Tammen AR, Rieber J, Siebert U, Henneke KH, Uberfuhr P, Reichart B. Diagnostic and prognostic value of serial dobutamine stress echocardiography for noninvasive assessment of cardiac allograft vasculopathy: a comparison with coronary angiography and intravascular ultrasound. Circulation. 1999;100:509-515. [PubMed] |
| 36. | Bacal F, Moreira L, Souza G, Rodrigues AC, Fiorelli A, Stolf N, Bocchi E, Bellotti G, Ramires JA. Dobutamine stress echocardiography predicts cardiac events or death in asymptomatic patients long-term after heart transplantation: 4-year prospective evaluation. J Heart Lung Transplant. 2004;23:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Larsen RL, Applegate PM, Dyar DA, Ribeiro PA, Fritzsche SD, Mulla NF, Shirali GS, Kuhn MA, Chinnock RE, Shah PM. Dobutamine stress echocardiography for assessing coronary artery disease after transplantation in children. J Am Coll Cardiol. 1998;32:515-520. [PubMed] |
| 38. | Lewis JF, Selman SB, Murphy JD, Mills RM, Geiser EA, Conti CR. Dobutamine echocardiography for prediction of ischemic events in heart transplant recipients. J Heart Lung Transplant. 1997;16:390-393. [PubMed] |
| 39. | Pahl E, Crawford SE, Swenson JM, Duffy CE, Fricker FJ, Backer CL, Mavroudis C, Chaudhry FA. Dobutamine stress echocardiography: experience in pediatric heart transplant recipients. J Heart Lung Transplant. 1999;18:725-732. [PubMed] |
| 40. | Eroglu E, D’hooge J, Sutherland GR, Marciniak A, Thijs D, Droogne W, Herbots L, Van Cleemput J, Claus P, Bijnens B. Quantitative dobutamine stress echocardiography for the early detection of cardiac allograft vasculopathy in heart transplant recipients. Heart. 2008;94:e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 41. | Akosah KO, McDaniel S, Hanrahan JS, Mohanty PK. Dobutamine stress echocardiography early after heart transplantation predicts development of allograft coronary artery disease and outcome. J Am Coll Cardiol. 1998;31:1607-1614. [PubMed] |
| 42. | Jackson PA, Akosah KO, Kirchberg DJ, Mohanty PK, Minisi AJ. Relationship between dobutamine-induced regional wall motion abnormalities and coronary flow reserve in heart transplant patients without angiographic coronary artery disease. J Heart Lung Transplant. 2002;21:1080-1089. [PubMed] |
| 43. | Dipchand AI, Bharat W, Manlhiot C, Safi M, Lobach NE, McCrindle BW. A prospective study of dobutamine stress echocardiography for the assessment of cardiac allograft vasculopathy in pediatric heart transplant recipients. Pediatr Transplant. 2008;12:570-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 44. | Di Filippo S, Semiond B, Roriz R, Sassolas F, Raboisson MJ, Bozio A. Non-invasive detection of coronary artery disease by dobutamine-stress echocardiography in children after heart transplantation. J Heart Lung Transplant. 2003;22:876-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 45. | Tona F, Caforio AL, Montisci R, Angelini A, Ruscazio M, Gambino A, Ramondo A, Thiene G, Gerosa G, Iliceto S. Coronary flow reserve by contrast-enhanced echocardiography: a new noninvasive diagnostic tool for cardiac allograft vasculopathy. Am J Transplant. 2006;6:998-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 46. | Tona F, Osto E, Tarantini G, Gambino A, Cavallin F, Feltrin G, Montisci R, Caforio AL, Gerosa G, Iliceto S. Coronary flow reserve by transthoracic echocardiography predicts epicardial intimal thickening in cardiac allograft vasculopathy. Am J Transplant. 2010;10:1668-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Rodrigues AC, Frimm Cde C, Bacal F, Andreolli V, Tsutsui JM, Bocchi EA, Mathias W, Lage SG. Coronary flow reserve impairment predicts cardiac events in heart transplant patients with preserved left ventricular function. Int J Cardiol. 2005;103:201-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Dandel M, Hummel M, Müller J, Wellnhofer E, Meyer R, Solowjowa N, Ewert R, Hetzer R. Reliability of tissue Doppler wall motion monitoring after heart transplantation for replacement of invasive routine screenings by optimally timed cardiac biopsies and catheterizations. Circulation. 2001;104:I184-I191. [PubMed] |
| 49. | Dandel M, Wellnhofer E, Hummel M, Meyer R, Lehmkuhl H, Hetzer R. Early detection of left ventricular dysfunction related to transplant coronary artery disease. J Heart Lung Transplant. 2003;22:1353-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 50. | Fyfe DA, Ketchum D, Lewis R, Sabatier J, Kanter K, Mahle W, Vincent R. Tissue Doppler imaging detects severely abnormal myocardial velocities that identify children with pre-terminal cardiac graft failure after heart transplantation. J Heart Lung Transplant. 2006;25:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 30] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 51. | Savage A, Hlavacek A, Ringewald J, Shirali G. Evaluation of the myocardial performance index and tissue doppler imaging by comparison to near-simultaneous catheter measurements in pediatric cardiac transplant patients. J Heart Lung Transplant. 2010;29:853-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 52. | Collings CA, Pinto FJ, Valantine HA, Popylisen S, Puryear JV, Schnittger I. Exercise echocardiography in heart transplant recipients: a comparison with angiography and intracoronary ultrasonography. J Heart Lung Transplant. 1994;13:604-613. [PubMed] |
| 53. | Chen MH, Abernathey E, Lunze F, Colan SD, O’Neill S, Bergersen L, Geva T, Blume ED. Utility of exercise stress echocardiography in pediatric cardiac transplant recipients: a single-center experience. J Heart Lung Transplant. 2012;31:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 54. | Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Oberoi M, Johnson MR, Costanzo MR. Coronary endothelial dysfunction after heart transplantation predicts allograft vasculopathy and cardiac death. Circulation. 2001;104:3091-3096. [PubMed] |
| 55. | Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J. Guidelines on myocardial revascularization. Eur Heart J. 2010;31:2501-2555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1660] [Cited by in RCA: 1715] [Article Influence: 114.3] [Reference Citation Analysis (0)] |
| 56. | Fearon WF, Hirohata A, Nakamura M, Luikart H, Lee DP, Vagelos RH, Hunt SA, Valantine HA, Fitzgerald PJ, Yock PG. Discordant changes in epicardial and microvascular coronary physiology after cardiac transplantation: Physiologic Investigation for Transplant Arteriopathy II (PITA II) study. J Heart Lung Transplant. 2006;25:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 57. | Lee CM, Wu YW, Jui HY, Yen RF, Tzen KY, Chou NK, Wang SS. Intravascular ultrasound correlates with coronary flow reserve and predicts the survival in angiographically normal cardiac transplant recipients. Cardiology. 2008;109:93-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 58. | Fearon WF, Nakamura M, Lee DP, Rezaee M, Vagelos RH, Hunt SA, Fitzgerald PJ, Yock PG, Yeung AC. Simultaneous assessment of fractional and coronary flow reserves in cardiac transplant recipients: Physiologic Investigation for Transplant Arteriopathy (PITA Study). Circulation. 2003;108:1605-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | Suwaidi JA, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948-954. [PubMed] |
| 60. | Schächinger V, Britten MB, Zeiher AM. Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation. 2000;101:1899-1906. [PubMed] |
| 61. | Hollenberg SM, Klein LW, Parrillo JE, Scherer M, Burns D, Tamburro P, Bromet D, Satran A, Costanzo MR. Changes in coronary endothelial function predict progression of allograft vasculopathy after heart transplantation. J Heart Lung Transplant. 2004;23:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 62. | Kübrich M, Petrakopoulou P, Kofler S, Nickel T, Kaczmarek I, Meiser BM, Reichart B, von Scheidt W, Weis M. Impact of coronary endothelial dysfunction on adverse long-term outcome after heart transplantation. Transplantation. 2008;85:1580-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 63. | Schubert S, Abdul-Khaliq H, Wellnhofer E, Hiemann NE, Ewert P, Lehmkuhl HB, Meyer R, Miera O, Peters B, Hetzer R. Coronary flow reserve measurement detects transplant coronary artery disease in pediatric heart transplant patients. J Heart Lung Transplant. 2008;27:514-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 64. | van Rossum AC, Visser FC, Hofman MB, Galjee MA, Westerhof N, Valk J. Global left ventricular perfusion: noninvasive measurement with cine MR imaging and phase velocity mapping of coronary venous outflow. Radiology. 1992;182:685-691. [PubMed] |
| 65. | Koskenvuo JW, Sakuma H, Niemi P, Toikka JO, Knuuti J, Laine H, Komu M, Kormano M, Saraste M, Hartiala JJ. Global myocardial blood flow and global flow reserve measurements by MRI and PET are comparable. J Magn Reson Imaging. 2001;13:361-366. [PubMed] |
| 66. | Schwitter J, DeMarco T, Kneifel S, von Schulthess GK, Jörg MC, Arheden H, Rühm S, Stumpe K, Buck A, Parmley WW. Magnetic resonance-based assessment of global coronary flow and flow reserve and its relation to left ventricular functional parameters: a comparison with positron emission tomography. Circulation. 2000;101:2696-2702. [PubMed] |
| 67. | Ishida M, Ito T, Shiraishi Y, Kitagawa K, Dohi K, Nakajima H, Ito M, Sakuma H. Impaired coronary flow reserve determined by MR measurement of coronary sinus flow predicts adverse outcome in patients with known or suspected coronary artery disease. J Cardiovasc Magn Reson. 2013;15:O91. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 68. | Kennedy K, Dick A, Drangova M, Raval A, Mahoney C, Karlik S, Pflugfelder PW. Magnetic resonance measurements of coronary flow reserve in heart transplant recipients: an exploratory study of the relationship to coronary angiographic findings. J Cardiovasc Magn Reson. 2007;9:701-707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 69. | Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O’Rourke RA, Dada M, Spertus JA. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1215] [Cited by in RCA: 1162] [Article Influence: 68.4] [Reference Citation Analysis (0)] |
| 70. | Klocke FJ, Baird MG, Lorell BH, Bateman TM, Messer JV, Berman DS, O’Gara PT, Carabello BA, Russell RO, Cerqueira MD. ACC/AHA/ASNC guidelines for the clinical use of cardiac radionuclide imaging--executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/ASNC Committee to Revise the 1995 Guidelines for the Clinical Use of Cardiac Radionuclide Imaging). Circulation. 2003;108:1404-1418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 404] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 71. | Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med. 2007;356:830-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1412] [Cited by in RCA: 1266] [Article Influence: 70.3] [Reference Citation Analysis (0)] |
| 72. | Wu YW, Yen RF, Lee CM, Ho YL, Chou NK, Wang SS, Huang PJ. Diagnostic and prognostic value of dobutamine thallium-201 single-photon emission computed tomography after heart transplantation. J Heart Lung Transplant. 2005;24:544-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 73. | Carlsen J, Toft JC, Mortensen SA, Arendrup H, Aldershvile J, Hesse B. Myocardial perfusion scintigraphy as a screening method for significant coronary artery stenosis in cardiac transplant recipients. J Heart Lung Transplant. 2000;19:873-878. [PubMed] |
| 74. | Ciliberto GR, Mangiavacchi M, Banfi F, Massa D, Danzi G, Cataldo G, Cipriani M, Piccalò G, Dabalà A, Gronda E. Coronary artery disease after heart transplantation: non-invasive evaluation with exercise thallium scintigraphy. Eur Heart J. 1993;14:226-229. [PubMed] |
| 75. | Rodney RA, Johnson LL, Blood DK, Barr ML. Myocardial perfusion scintigraphy in heart transplant recipients with and without allograft atherosclerosis: a comparison of thallium-201 and technetium 99m sestamibi. J Heart Lung Transplant. 1994;13:173-180. [PubMed] |
| 76. | Howarth DM, Forstrom LA, Samudrala V, Sinak LJ, McGregor CG, Rodeheffer RJ. Evaluation of 201Tl SPET myocardial perfusion imaging in the detection of coronary artery disease after orthotopic heart transplantation. Nucl Med Commun. 1996;17:105-113. [PubMed] |
| 77. | Ambrosi P, Habib G, Kreitman B, Metras D, Riberi A, Faugère G, Bernard P, Luccioni R. Thallium perfusion and myocardial hypertrophy in transplanted heart recipients with normal or near-normal coronary arteriograms. Eur Heart J. 1994;15:1119-1123. [PubMed] |
| 78. | Elhendy A, Sozzi FB, van Domburg RT, Vantrimpont P, Valkema R, Krenning EP, Roelandt JR, Maat LP, Balk AH. Accuracy of dobutamine tetrofosmin myocardial perfusion imaging for the noninvasive diagnosis of transplant coronary artery stenosis. J Heart Lung Transplant. 2000;19:360-366. [PubMed] |
| 79. | Verhoeven PP, Lee FA, Ramahi TM, Franco KL, Mendes de Leon C, Amatruda J, Gorham NA, Mattera JA, Wackers FJ. Prognostic value of noninvasive testing one year after orthotopic cardiac transplantation. J Am Coll Cardiol. 1996;28:183-189. [PubMed] |
| 80. | Elhendy A, van Domburg RT, Vantrimpont P, Poldermans D, Bax JJ, van Gelder T, Baan CC, Schinkel A, Roelandt JR, Balk AH. Prediction of mortality in heart transplant recipients by stress technetium-99m tetrofosmin myocardial perfusion imaging. Am J Cardiol. 2002;89:964-968. [PubMed] |
| 81. | Ciliberto GR, Ruffini L, Mangiavacchi M, Parolini M, Sara R, Massa D, De Maria R, Gronda E, Vitali E, Parodi O. Resting echocardiography and quantitative dipyridamole technetium-99m sestamibi tomography in the identification of cardiac allograft vasculopathy and the prediction of long-term prognosis after heart transplantation. Eur Heart J. 2001;22:964-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 82. | Hacker M, Tausig A, Romüller B, Hoyer X, Klauss V, Stempfle U, Reichart B, Hahn K, Tiling R. Dobutamine myocardial scintigraphy for the prediction of cardiac events after heart transplantation. Nucl Med Commun. 2005;26:607-612. [PubMed] |
| 83. | Manrique A, Bernard M, Hitzel A, Bubenheim M, Tron C, Agostini D, Cribier A, Véra P, Bessou JP, Redonnet M. Diagnostic and prognostic value of myocardial perfusion gated SPECT in orthotopic heart transplant recipients. J Nucl Cardiol. 2010;17:197-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 84. | Lenihan DJ, Rosenbaum AF, Burwinkel P, Tseng CY, Bhat G, Wagoner L, Walsh RA, Gerson MC. Prediction of human transplantation arteriopathy and coronary events with lung/heart count ratios during intravenous dipyridamole thallium-201 imaging. Am Heart J. 1999;137:942-948. [PubMed] |
| 85. | Bengel FM, Higuchi T, Javadi MS, Lautamäki R. Cardiac positron emission tomography. J Am Coll Cardiol. 2009;54:1-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 187] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 86. | Uren NG, Melin JA, De Bruyne B, Wijns W, Baudhuin T, Camici PG. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N Engl J Med. 1994;330:1782-1788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 652] [Cited by in RCA: 594] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 87. | Allen-Auerbach M, Schöder H, Johnson J, Kofoed K, Einhorn K, Phelps ME, Kobashigawa J, Czernin J. Relationship between coronary function by positron emission tomography and temporal changes in morphology by intravascular ultrasound (IVUS) in transplant recipients. J Heart Lung Transplant. 1999;18:211-219. [PubMed] |
| 88. | Wu YW, Chen YH, Wang SS, Jui HY, Yen RF, Tzen KY, Chen MF, Lee CM. PET assessment of myocardial perfusion reserve inversely correlates with intravascular ultrasound findings in angiographically normal cardiac transplant recipients. J Nucl Med. 2010;51:906-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 89. | Preumont N, Berkenboom G, Vachiery J, Jansens J, Antoine M, Wikler D, Damhaut P, Degré S, Lenaers A, Goldman S. Early alterations of myocardial blood flow reserve in heart transplant recipients with angiographically normal coronary arteries. J Heart Lung Transplant. 2000;19:538-545. [PubMed] |
| 90. | Mollet NR, Cademartiri F, van Mieghem CA, Runza G, McFadden EP, Baks T, Serruys PW, Krestin GP, de Feyter PJ. High-resolution spiral computed tomography coronary angiography in patients referred for diagnostic conventional coronary angiography. Circulation. 2005;112:2318-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 790] [Cited by in RCA: 753] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 91. | Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, Wintersperger B, Reiser M, Becker CR, Steinbeck G. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J Am Coll Cardiol. 2005;46:147-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 870] [Cited by in RCA: 826] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 92. | Hoffmann U, Moselewski F, Nieman K, Jang IK, Ferencik M, Rahman AM, Cury RC, Abbara S, Joneidi-Jafari H, Achenbach S. Noninvasive assessment of plaque morphology and composition in culprit and stable lesions in acute coronary syndrome and stable lesions in stable angina by multidetector computed tomography. J Am Coll Cardiol. 2006;47:1655-1662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 435] [Cited by in RCA: 436] [Article Influence: 22.9] [Reference Citation Analysis (0)] |
| 93. | Sigurdsson G, Carrascosa P, Yamani MH, Greenberg NL, Perrone S, Lev G, Desai MY, Garcia MJ. Detection of transplant coronary artery disease using multidetector computed tomography with adaptative multisegment reconstruction. J Am Coll Cardiol. 2006;48:772-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 94. | Iyengar S, Feldman DS, Cooke GE, Leier CV, Raman SV. Detection of coronary artery disease in orthotopic heart transplant recipients with 64-detector row computed tomography angiography. J Heart Lung Transplant. 2006;25:1363-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 95. | Romeo G, Houyel L, Angel CY, Brenot P, Riou JY, Paul JF. Coronary stenosis detection by 16-slice computed tomography in heart transplant patients: comparison with conventional angiography and impact on clinical management. J Am Coll Cardiol. 2005;45:1826-1831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Schepis T, Achenbach S, Weyand M, Raum P, Marwan M, Pflederer T, Daniel WG, Tandler R, Kondruweit M, Ropers D. Comparison of dual source computed tomography versus intravascular ultrasound for evaluation of coronary arteries at least one year after cardiac transplantation. Am J Cardiol. 2009;104:1351-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 97. | Bogot NR, Durst R, Shaham D, Admon D. Cardiac CT of the transplanted heart: indications, technique, appearance, and complications. Radiographics. 2007;27:1297-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 18] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 98. | Nunoda S, Machida H, Sekikawa A, Shitakura K, Okajima K, Kubo Y, Ueno E, Otsuka K. Evaluation of cardiac allograft vasculopathy by multidetector computed tomography and whole-heart magnetic resonance coronary angiography. Circ J. 2010;74:946-953. [PubMed] |
| 99. | Bae KT, Hong C, Takahashi N, Gutierrez F, Sharkey AM, Hirsch R, Canter CE. Multi-detector row computed tomographic angiography in pediatric heart transplant recipients: Initial observations. Transplantation. 2004;77:599-602. [PubMed] |
| 100. | Pinto TL, Waksman R. Clinical applications of optical coherence tomography. J Interv Cardiol. 2006;19:566-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 101. | Suter MJ, Nadkarni SK, Weisz G, Tanaka A, Jaffer FA, Bouma BE, Tearney GJ. Intravascular optical imaging technology for investigating the coronary artery. JACC Cardiovasc Imaging. 2011;4:1022-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 102. | Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058-1072. [PubMed] |
| 103. | Khandhar SJ, Yamamoto H, Teuteberg JJ, Shullo MA, Bezerra HG, Costa MA, Selzer F, Lee JS, Marroquin OC, McNamara DM. Optical coherence tomography for characterization of cardiac allograft vasculopathy after heart transplantation (OCTCAV study). J Heart Lung Transplant. 2013;32:596-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 104. | Garrido IP, García-Lara J, Pinar E, Pastor-Pérez F, Sánchez-Mas J, Valdés-Chavarri M, Pascual-Figal DA. Optical coherence tomography and highly sensitivity troponin T for evaluating cardiac allograft vasculopathy. Am J Cardiol. 2012;110:655-661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 105. | Cassar A, Matsuo Y, Herrmann J, Li J, Lennon RJ, Gulati R, Lerman LO, Kushwaha SS, Lerman A. Coronary atherosclerosis with vulnerable plaque and complicated lesions in transplant recipients: new insight into cardiac allograft vasculopathy by optical coherence tomography. Eur Heart J. 2013;34:2610-2617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 88] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 106. | Kim WY, Danias PG, Stuber M, Flamm SD, Plein S, Nagel E, Langerak SE, Weber OM, Pedersen EM, Schmidt M. Coronary magnetic resonance angiography for the detection of coronary stenoses. N Engl J Med. 2001;345:1863-1869. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 577] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 107. | Botnar RM, Kim WY, Börnert P, Stuber M, Spuentrup E, Manning WJ. 3D coronary vessel wall imaging utilizing a local inversion technique with spiral image acquisition. Magn Reson Med. 2001;46:848-854. [PubMed] |
| 108. | Botnar RM, Stuber M, Kissinger KV, Kim WY, Spuentrup E, Manning WJ. Noninvasive coronary vessel wall and plaque imaging with magnetic resonance imaging. Circulation. 2000;102:2582-2587. [PubMed] |
| 109. | Uribe S, Hussain T, Valverde I, Tejos C, Irarrazaval P, Fava M, Beerbaum P, Botnar RM, Razavi R, Schaeffter T. Congenital heart disease in children: coronary MR angiography during systole and diastole with dual cardiac phase whole-heart imaging. Radiology. 2011;260:232-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 110. | Tangcharoen T, Bell A, Hegde S, Hussain T, Beerbaum P, Schaeffter T, Razavi R, Botnar RM, Greil GF. Detection of coronary artery anomalies in infants and young children with congenital heart disease by using MR imaging. Radiology. 2011;259:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 111. | Greil GF, Stuber M, Botnar RM, Kissinger KV, Geva T, Newburger JW, Manning WJ, Powell AJ. Coronary magnetic resonance angiography in adolescents and young adults with kawasaki disease. Circulation. 2002;105:908-911. [PubMed] |
| 112. | Schwitter J, Wacker CM, van Rossum AC, Lombardi M, Al-Saadi N, Ahlstrom H, Dill T, Larsson HB, Flamm SD, Marquardt M. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008;29:480-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 456] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 113. | Jahnke C, Nagel E, Gebker R, Kokocinski T, Kelle S, Manka R, Fleck E, Paetsch I. Prognostic value of cardiac magnetic resonance stress tests: adenosine stress perfusion and dobutamine stress wall motion imaging. Circulation. 2007;115:1769-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 405] [Cited by in RCA: 355] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 114. | Muehling OM, Wilke NM, Panse P, Jerosch-Herold M, Wilson BV, Wilson RF, Miller LW. Reduced myocardial perfusion reserve and transmural perfusion gradient in heart transplant arteriopathy assessed by magnetic resonance imaging. J Am Coll Cardiol. 2003;42:1054-1060. [PubMed] |
| 115. | Steen H, Merten C, Refle S, Klingenberg R, Dengler T, Giannitsis E, Katus HA. Prevalence of different gadolinium enhancement patterns in patients after heart transplantation. J Am Coll Cardiol. 2008;52:1160-1167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 116. | Braggion-Santos MF, Andre F, Lossnitzer D, Hofmann E, Simpfendörfer J, Dösch A, Katus HA, Steen H. Prevalence of different forms of infarct-atypical late gadolinium enhancement in patients early and late after heart transplantation. Clin Res Cardiol. 2014;103:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 117. | Hussain T, Burch M, Fenton MJ, Whitmore PM, Rees P, Elliott M, Aurora P. Positive pretransplantation cytomegalovirus serology is a risk factor for cardiac allograft vasculopathy in children. Circulation. 2007;115:1798-1805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 118. | Miller CA, Sarma J, Naish JH, Yonan N, Williams SG, Shaw SM, Clark D, Pearce K, Stout M, Potluri R. Multiparametric cardiovascular magnetic resonance assessment of cardiac allograft vasculopathy. J Am Coll Cardiol. 2014;63:799-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 119. | Blum A, Shamburek R. The pleiotropic effects of statins on endothelial function, vascular inflammation, immunomodulation and thrombogenesis. Atherosclerosis. 2009;203:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 219] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 120. | Anderson TJ, Uehata A, Gerhard MD, Meredith IT, Knab S, Delagrange D, Lieberman EH, Ganz P, Creager MA, Yeung AC. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235-1241. [PubMed] |
| 121. | Treasure CB, Klein JL, Weintraub WS, Talley JD, Stillabower ME, Kosinski AS, Zhang J, Boccuzzi SJ, Cedarholm JC, Alexander RW. Beneficial effects of cholesterol-lowering therapy on the coronary endothelium in patients with coronary artery disease. N Engl J Med. 1995;332:481-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 919] [Cited by in RCA: 861] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 122. | Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Krobot K, Steinbeck G, Seidel D, Reichart B. Simvastatin initiated early after heart transplantation: 8-year prospective experience. Circulation. 2003;107:93-97. [PubMed] |
| 123. | Mehra MR, Uber PA, Vivekananthan K, Solis S, Scott RL, Park MH, Milani RV, Lavie CJ. Comparative beneficial effects of simvastatin and pravastatin on cardiac allograft rejection and survival. J Am Coll Cardiol. 2002;40:1609-1614. [PubMed] |
| 124. | Mehra MR, Raval NY. Metaanalysis of statins and survival in de novo cardiac transplantation. Transplant Proc. 2004;36:1539-1541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 125. | Costanzo MR, Dipchand A, Starling R, Anderson A, Chan M, Desai S, Fedson S, Fisher P, Gonzales-Stawinski G, Martinelli L. The International Society of Heart and Lung Transplantation Guidelines for the care of heart transplant recipients. J Heart Lung Transplant. 2010;29:914-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1100] [Cited by in RCA: 1218] [Article Influence: 81.2] [Reference Citation Analysis (0)] |
| 126. | Valentine VG, Weill D, Gupta MR, Raper B, Laplace SG, Lombard GA, Bonvillain RW, Taylor DE, Dhillon GS. Ganciclovir for cytomegalovirus: a call for indefinite prophylaxis in lung transplantation. J Heart Lung Transplant. 2008;27:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 127. | Fearon WF, Potena L, Hirohata A, Sakurai R, Yamasaki M, Luikart H, Lee J, Vana ML, Cooke JP, Mocarski ES. Changes in coronary arterial dimensions early after cardiac transplantation. Transplantation. 2007;83:700-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 128. | Mehra MR, Ventura HO, Smart FW, Collins TJ, Ramee SR, Stapleton DD. An intravascular ultrasound study of the influence of angiotensin-converting enzyme inhibitors and calcium entry blockers on the development of cardiac allograft vasculopathy. Am J Cardiol. 1995;75:853-854. [PubMed] |
| 129. | Schroeder JS, Gao SZ, Alderman EL, Hunt SA, Johnstone I, Boothroyd DB, Wiederhold V, Stinson EB. A preliminary study of diltiazem in the prevention of coronary artery disease in heart-transplant recipients. N Engl J Med. 1993;328:164-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 216] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 130. | Erinc K, Yamani MH, Starling RC, Crowe T, Hobbs R, Bott-Silverman C, Rincon G, Young JB, Feng J, Cook DJ. The effect of combined Angiotensin-converting enzyme inhibition and calcium antagonism on allograft coronary vasculopathy validated by intravascular ultrasound. J Heart Lung Transplant. 2005;24:1033-1038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 131. | Meiser BM, Groetzner J, Kaczmarek I, Landwehr P, Müller M, Jung S, Uberfuhr P, Fraunberger P, Stempfle HU, Weis M. Tacrolimus or cyclosporine: which is the better partner for mycophenolate mofetil in heart transplant recipients? Transplantation. 2004;78:591-598. [PubMed] |
| 132. | Grimm M, Rinaldi M, Yonan NA, Arpesella G, Arizón Del Prado JM, Pulpón LA, Villemot JP, Frigerio M, Rodriguez Lambert JL, Crespo-Leiro MG. Superior prevention of acute rejection by tacrolimus vs. cyclosporine in heart transplant recipients--a large European trial. Am J Transplant. 2006;6:1387-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 133. | Kobashigawa JA, Miller LW, Russell SD, Ewald GA, Zucker MJ, Goldberg LR, Eisen HJ, Salm K, Tolzman D, Gao J. Tacrolimus with mycophenolate mofetil (MMF) or sirolimus vs. cyclosporine with MMF in cardiac transplant patients: 1-year report. Am J Transplant. 2006;6:1377-1386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 246] [Cited by in RCA: 225] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 134. | Segovia J, Gómez-Bueno M, Alonso-Pulpón L. Treatment of allograft vasculopathy in heart transplantation. Expert Opin Pharmacother. 2006;7:2369-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 135. | Petrakopoulou P, Anthopoulou L, Muscholl M, Klauss V, von Scheidt W, Uberfuhr P, Meiser BM, Reichart B, Weis M. Coronary endothelial vasomotor function and vascular remodeling in heart transplant recipients randomized for tacrolimus or cyclosporine immunosuppression. J Am Coll Cardiol. 2006;47:1622-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 136. | Eisen HJ, Kobashigawa J, Keogh A, Bourge R, Renlund D, Mentzer R, Alderman E, Valantine H, Dureau G, Mancini D. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24:517-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 137. | Kobashigawa JA, Tobis JM, Mentzer RM, Valantine HA, Bourge RC, Mehra MR, Smart FW, Miller LW, Tanaka K, Li H. Mycophenolate mofetil reduces intimal thickness by intravascular ultrasound after heart transplant: reanalysis of the multicenter trial. Am J Transplant. 2006;6:993-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 138. | Kaczmarek I, Ertl B, Schmauss D, Sadoni S, Knez A, Daebritz S, Meiser B, Reichart B. Preventing cardiac allograft vasculopathy: long-term beneficial effects of mycophenolate mofetil. J Heart Lung Transplant. 2006;25:550-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 139. | Sehgal SN. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant Proc. 2003;35:7S-14S. [PubMed] |
| 140. | Raichlin E, Kushwaha SS. Proliferation signal inhibitors and cardiac allograft vasculopathy. Curr Opin Organ Transplant. 2008;13:543-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 141. | Eisen HJ, Tuzcu EM, Dorent R, Kobashigawa J, Mancini D, Valantine-von Kaeppler HA, Starling RC, Sørensen K, Hummel M, Lind JM. Everolimus for the prevention of allograft rejection and vasculopathy in cardiac-transplant recipients. N Engl J Med. 2003;349:847-858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 889] [Article Influence: 40.4] [Reference Citation Analysis (0)] |
| 142. | Viganò M, Tuzcu M, Benza R, Boissonnat P, Haverich A, Hill J, Laufer G, Love R, Parameshwar J, Pulpón LA. Prevention of acute rejection and allograft vasculopathy by everolimus in cardiac transplants recipients: a 24-month analysis. J Heart Lung Transplant. 2007;26:584-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 89] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 143. | Eisen H. Long-term cardiovascular risk in transplantation--insights from the use of everolimus in heart transplantation. Nephrol Dial Transplant. 2006;21 Suppl 3:iii9-ii13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 144. | Andreassen AK, Andersson B, Gustafsson F, Eiskjaer H, Rådegran G, Gude E, Jansson K, Solbu D, Sigurdardottir V, Arora S. Everolimus Initiation and Early Calcineurin Inhibitor Withdrawal in Heart Transplant Recipients: A Randomized Trial. Am J Transplant. 2014;14:1828-1838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 145. | Mancini D, Pinney S, Burkhoff D, LaManca J, Itescu S, Burke E, Edwards N, Oz M, Marks AR. Use of rapamycin slows progression of cardiac transplantation vasculopathy. Circulation. 2003;108:48-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 330] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 146. | Keogh A, Richardson M, Ruygrok P, Spratt P, Galbraith A, O’Driscoll G, Macdonald P, Esmore D, Muller D, Faddy S. Sirolimus in de novo heart transplant recipients reduces acute rejection and prevents coronary artery disease at 2 years: a randomized clinical trial. Circulation. 2004;110:2694-2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 337] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 147. | Lyster H, Panicker G, Leaver N, Banner NR. Initial experience with sirolimus and mycophenolate mofetil for renal rescue from cyclosporine nephrotoxicity after heart transplantation. Transplant Proc. 2004;36:3167-3170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 148. | Rothenburger M, Teerling E, Bruch C, Lehmkuhl H, Suwelack B, Bara C, Wichter T, Hinder F, Schmid C, Stypmann J. Calcineurin inhibitor-free immunosuppression using everolimus (Certican) in maintenance heart transplant recipients: 6 months’ follow-up. J Heart Lung Transplant. 2007;26:250-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 149. | Groetzner J, Kaczmarek I, Meiser B, Müller M, Daebritz S, Reichart B. Sirolimus and mycophenolate mofetil as calcineurin inhibitor-free immunosuppression in a cardiac transplant patient with chronic renal failure. J Heart Lung Transplant. 2004;23:770-773. [PubMed] |
| 150. | Groetzner J, Kaczmarek I, Landwehr P, Mueller M, Daebritz S, Lamm P, Meiser B, Reichart B. Renal recovery after conversion to a calcineurin inhibitor-free immunosuppression in late cardiac transplant recipients. Eur J Cardiothorac Surg. 2004;25:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 151. | Hunt J, Lerman M, Magee MJ, Dewey TM, Herbert M, Mack MJ. Improvement of renal dysfunction by conversion from calcineurin inhibitors to sirolimus after heart transplantation. J Heart Lung Transplant. 2005;24:1863-1867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 60] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 152. | Raichlin E, Bae JH, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, Rihal C, Lerman A. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726-2733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 153. | Topilsky Y, Hasin T, Raichlin E, Boilson BA, Schirger JA, Pereira NL, Edwards BS, Clavell AL, Rodeheffer RJ, Frantz RP. Sirolimus as primary immunosuppression attenuates allograft vasculopathy with improved late survival and decreased cardiac events after cardiac transplantation. Circulation. 2012;125:708-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 154. | Arora S, Erikstad I, Ueland T, Sigurdardottir V, Ekmehag B, Jansson K, Eiskjaer H, Bøtker HE, Mortensen SA, Saunamaki K. Virtual histology assessment of cardiac allograft vasculopathy following introduction of everolimus--results of a multicenter trial. Am J Transplant. 2012;12:2700-2709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 155. | Zuckermann A, Manito N, Epailly E, Fiane A, Bara C, Delgado JF, Lehmkuhl H, Ross H, Eisen H, Chapman J. Multidisciplinary insights on clinical guidance for the use of proliferation signal inhibitors in heart transplantation. J Heart Lung Transplant. 2008;27:141-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 156. | Demopoulos L, Polinsky M, Steele G, Mines D, Blum M, Caulfield M, Adamkovic A, Liu Q, Harler MB, Hahn C. Reduced risk of cytomegalovirus infection in solid organ transplant recipients treated with sirolimus: a pooled analysis of clinical trials. Transplant Proc. 2008;40:1407-1410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 157. | Hill JA, Hummel M, Starling RC, Kobashigawa JA, Perrone SV, Arizón JM, Simonsen S, Abeywickrama KH, Bara C. A lower incidence of cytomegalovirus infection in de novo heart transplant recipients randomized to everolimus. Transplantation. 2007;84:1436-1442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 158. | Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472-4480. [PubMed] |
| 159. | Benza RL, Zoghbi GJ, Tallaj J, Brown R, Kirklin JK, Hubbard M, Rayburn B, Foley B, McGiffin DC, Pinderski LJ. Palliation of allograft vasculopathy with transluminal angioplasty: a decade of experience. J Am Coll Cardiol. 2004;43:1973-1981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 160. | Simpson L, Lee EK, Hott BJ, Vega DJ, Book WM. Long-term results of angioplasty vs stenting in cardiac transplant recipients with allograft vasculopathy. J Heart Lung Transplant. 2005;24:1211-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 161. | Jonas M, Fang JC, Wang JC, Giri S, Elian D, Har-Zahav Y, Ly H, Seifert PA, Popma JJ, Rogers C. In-stent restenosis and remote coronary lesion progression are coupled in cardiac transplant vasculopathy but not in native coronary artery disease. J Am Coll Cardiol. 2006;48:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 162. | Halle AA, DiSciascio G, Massin EK, Wilson RF, Johnson MR, Sullivan HJ, Bourge RC, Kleiman NS, Miller LW, Aversano TR. Coronary angioplasty, atherectomy and bypass surgery in cardiac transplant recipients. J Am Coll Cardiol. 1995;26:120-128. [PubMed] |
| 163. | Schnetzler B, Drobinski G, Dorent R, Camproux AC, Ghossoub J, Thomas D, Gandjbakhch I. The role of percutaneous transluminal coronary angioplasty in heart transplant recipients. J Heart Lung Transplant. 2000;19:557-565. [PubMed] |
| 164. | Doshi AA, Rogers J, Kern MJ, Hauptman PJ. Effectiveness of percutaneous coronary intervention in cardiac allograft vasculopathy. Am J Cardiol. 2004;93:90-92. [PubMed] |
| 165. | Fernández-Yáñez J, Palomo J, Elízaga J, Moreno J, Sánchez A, Almendral J. Results of percutaneous revascularization in cardiac allograft vascular disease. Transplant Proc. 2003;35:1996-1998. [PubMed] |
| 166. | Bader FM, Kfoury AG, Gilbert EM, Barry WH, Humayun N, Hagan ME, Thomas H, Renlund D. Percutaneous coronary interventions with stents in cardiac transplant recipients. J Heart Lung Transplant. 2006;25:298-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 167. | Musci M, Loebe M, Wellnhofer E, Meyer R, Pasic M, Hummel M, Bocksch W, Grauhan O, Weng Y, Hetzer R. Coronary angioplasty, bypass surgery, and retransplantation in cardiac transplant patients with graft coronary disease. Thorac Cardiovasc Surg. 1998;46:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 168. | Patel VS, Radovancevic B, Springer W, Frazier OH, Massin E, Benrey J, Kadipasaoglu K, Cooley DA. Revascularization procedures in patients with transplant coronary artery disease. Eur J Cardiothorac Surg. 1997;11:895-901. [PubMed] |
| 169. | Topkara VK, Dang NC, John R, Cheema FH, Barbato R, Cavallo M, Liu JF, Liang LM, Liberman EA, Argenziano M. A decade experience of cardiac retransplantation in adult recipients. J Heart Lung Transplant. 2005;24:1745-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 170. | Srivastava R, Keck BM, Bennett LE, Hosenpud JD. The results of cardiac retransplantation: an analysis of the Joint International Society for Heart and Lung Transplantation/United Network for Organ Sharing Thoracic Registry. Transplantation. 2000;70:606-612. [PubMed] |