Published online Sep 24, 2014. doi: 10.5500/wjt.v4.i3.206
Revised: July 9, 2014
Accepted: July 18, 2014
Published online: September 24, 2014
Processing time: 288 Days and 8.3 Hours
AIM: To hypothesize that the product of calculated Model for End-Stage Liver Disease score excluding exception points and donor age (D-MELD) risk capping ± Rule 14 could improve post liver transplant and overall survival after listing.
METHODS: Probabilities derived from the United Network for Organ Sharing database between 2002 and 2004 were used to simulate potential outcomes for all patients listed for transplantation. The Markov simulation was then modified by screening matches using a 1200 or 1600 D-MELD risk cap ± allowing transplants for Model for End-Stage Liver Disease (MELD) ≤ 14 (Rule 14). The differential impact of the rule changes was assessed.
RESULTS: The Markov simulation accurately reproduced overall and post transplant survival. A 1200 D-MELD risk cap improved post-transplant survival. Both the 1200 and 1600 risk caps improved overall survival for waitlisted patients. The addition of Rule 14 further improved post transplant and overall survival by redistribution of donor livers to recipients in higher MELD subgroups. The mechanism for improved overall and post-transplant survival after listing was due to shifting a larger percentage of transplants to the moderate MELD score subgroup (MELD 15-29) while also ensuring that high MELD recipients have livers of high quality to achieve excellent post transplant survival.
CONCLUSION: A 1200 D-MELD risk cap + Rule 14 provided the greatest overall benefit primarily by focusing liver transplantation towards the moderate MELD recipient.
Core tip: Optimal matching between donor livers and recipients balances recipient need with optimal utility. The product of calculated Model for End-Stage Liver Disease score excluding exception points and donor age (D-MELD) risk cap uniquely utilizes ethically neutral donor/recipient factors while maintaining predictive power, making it useful for donor/recipient matching paradigms aimed at improving utility. Described is a novel utilization of the D-MELD risk cap as an aid for donor/recipient matching. Markov modeling suggests that this paradigm improves outcomes both by decreasing futile transplantations but also by focusing the majority of transplantation on moderate Model for End-Stage Liver Disease (MELD) recipients while continuing to provide younger donor livers for the fewer number of patients transplanted at high MELD.
- Citation: Halldorson JB, Jr RLC, Bhattacharya R, Bakthavatsalam R, Liou IW, Dick AA, Reyes JD, Perkins JD. D-MELD risk capping improves post-transplant and overall mortality under markov microsimulation. World J Transplant 2014; 4(3): 206-215
- URL: https://www.wjgnet.com/2220-3230/full/v4/i3/206.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i3.206
As a response to waitlist mortality from the cadaveric donor shortage, the once homogenous pool of younger, donation after brain death donors has now become a heterogeneous mix of standard and extended criteria donors. By far the most significant change is greater use of older donors aged greater than 50 who now may comprise over half of liver donor populations[1-3]. The older donor has now become a dominant risk factor for poorer graft and patient survival after liver transplantation[4,5].
The older donor pool in combination with sickest first allocation policies based on Model for End-Stage Liver Disease (MELD) scoring often produces match offers between higher risk donors and the sickest patients. Although it can be demonstrated that the sickest (highest MELD) patients derive survival benefit from transplantation even when matching with higher risk donors, overall survival is inferior to that of lower risk donor/recipient combinations[6]. While good results can be obtained with older liver donors, high risk donor/recipient matches have poorer survival and therefore decrease the utility obtained from the scarce resource of donor livers for transplantation[7].
Since post transplant outcome is not formally considered in current allocation policy, matching higher risk older donors with appropriate risk recipients is often improvised at the time of liver offer. With intent to maximize the benefit from the severely limited medical resource of cadaveric organs, modifications have been called for to optimize matching between donors and recipients at the time of allocation[8-10].
One proposed modification raises the minimal MELD score for listing to 14 (Rule 14) with the intent to ensure that recipients are sick enough to realize survival benefit from transplantation. Prior modeling work has demonstrated Rule 14 to improve overall survival for the population of waitlisted patients, with a particular benefit for patients listed at low MELD who may have higher expected survival without transplantation[11].
Another potential area to maximize utility in liver transplantation is decreasing the number of transplantations with low expected post transplant survival. Survival after transplantation is largely dependent on the risk combination between donor quality and recipient medical condition, and survival is poorer when higher risk donors are placed into the sickest recipients[12]. Since the dominant donor risk factor is age, and recipient medical condition is accurately measured by MELD score, Model for End-Stage Liver Disease score excluding exception points and donor age (D-MELD) (the product of donor age and recipient MELD excluding United Network for Organ Sharing exception points) was developed as an ethically appropriate tool to improve donor/recipient matching during allocation[13]. As a product of two continuous variables, D-MELD produces a risk gradient from which a cap can be instituted to decrease the rate of futile donor/recipient matches. Importantly, D-MELD was designed to avoid controversial discriminator variables such as gender, recipient age, race or subjective assessments of “organ quality” that make other donor/recipient matching systems impractical or ethically controversial[14]. Prior work demonstrated D-MELD scores greater than 1200 or 1600 to be significantly associated with poorer post transplant survival[13].
Based on D-MELD risk cap thresholds ± Rule 14, the experimental model evaluates the potential utility of modifications in donor/recipient matching as follows: (1) donor/recipient matches with D-MELD risk caps above threshold (e.g., D-MELD scores greater than 1200 or 1600) would continue waiting for a better match; (2) Matches below the D-MELD threshold (e.g., D-MELD scores less than 1600) would then be offered based on urgency prioritized by MELD score; and (3) A minimum MELD score of 14 would be required for listing. We hypothesize that elimination of donor/recipient matching at high D-MELD and low MELD would focus the majority of liver matches in waitlisted patients with moderate (15-29) MELD scores, improving both post transplant survival and pre-transplant mortality by reducing the need for terminal disease progression in order to obtain an offer.
While it is intuitive that a risk cap could improve post transplant outcomes, an overly restrictive limit might result in prolonged waiting times for the sickest patients, thereby increasing waitlist deaths and outweighing any gains from increased post transplant survival. In addition, redistribution of older livers originally designated for high MELD recipients could potentially worsen post transplant survival for lower MELD subpopulation recipients, lessening the expected benefit from transplantation.
Markov decision analysis can be used to estimate the cumulative impact of decisions involving many potential outcome states in a complex system[15]. In order to understand the potential impact of proposed allocation changes, a Markov microsimulation was designed based on outcomes from all patients listed for liver transplantation between 2002-2004 and followed until 2008. The impact of both more (D-MELD 1200) and less (D-MELD 1600) restrictive risk caps with and without Rule 14 was examined for its potential impact on post transplant survival, overall survival and MELD subgroup at transplantation.
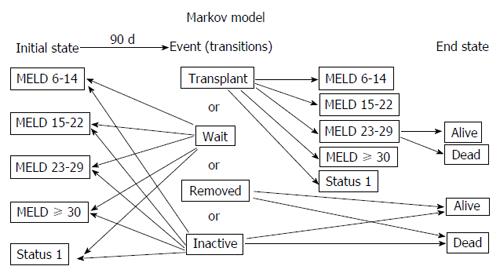
To model the multiple potential sequential events following liver transplant listing, we devised a Markov model assigning potential states as derived from the database (Figure 1). Event probabilities and expected survival probabilities for different end states were derived from records of all adult patients listed between January 1, 2002 and December 31, 2004, as recorded in the scientific registry of transplant recipients (SRTR) database with follow-up until February 6, 2008. Actively listed patients were evaluated for MELD score at listing, MELD score at removal, time waiting, post transplant survival, and waitlist events translated from the database. Overall survival was defined as the interval between listing date until death. Survival was assessed as “intention to treat” and included mortality with or without receiving a liver transplant as well as patients removed from the list or made inactive. Post transplant survival was assessed from the time of liver transplantation until death at the time of follow-up. Events after listing were assessed at 90-d intervals. Patients with missing MELD scores or donor age were excluded (< 1%). There were 24295 patients who met the study criteria. D-MELD was calculated as the product of donor age and MELD score excluding United Network for Organ Sharing exception points, and capped at 40. Patients were sub-stratified into 5 MELD categories (6-14, 15-22, 23-29, ≥ 30 and Status 1) based on initial and end state as recorded in the database. Final end state measured was death or survival. Patients who were made inactive on the list were given the same survival as patients removed from the list; however, survivors after 90 d were allowed to re-enter at the same MELD as they had at time of removal. Analysis of events for the years 2002-2004 did not allow assessment of changes in MELD category for patients remaining waitlisted (e.g., movement from MELD 6-14 to MELD 15-22). To estimate change in MELD for each 90-d period for patients who did not die or receive a transplant, we analyzed the most recent complete 90-d time period between September 31, 2007 and December 31, 2007. Rates of change between MELD subgroups were then used to predict MELD progression for the study period 2002-2004. A boot strap cohort was then modeled with initial MELD and donor age distributions derived from the database.
To confirm accuracy of the model, we compared the model’s predictions for both overall and post transplant survival with actual survival for all patients as a whole and then by MELD category. After accuracy of the model was confirmed, we analyzed the impact of proposed D-MELD risk caps with and without Rule 14 as compared to the model. Using the D-MELD risk cap, donor/recipient matches with a score above a 1200 or 1600 threshold remained on the waiting list until a suitable donor was found. Donor livers that were prevented from matching under the D-MELD cap were redistributed to patients with matches below the risk cap using the formula [D-MELD (1200 or 1600)]/(donor age in years) = maximum acceptable MELD at transplant. The liver was then allocated to the highest MELD subgroup allowable. For example, allocating a 61-year-old liver with a D-MELD 1600 filter would give a maximum tolerated MELD of 1600/61 = 26.2. This liver then would be allocated to the subgroup MELD 23-29. Survival for the donor/recipient match was predicted from the D-MELD stratification curves for each MELD subgroup derived from SRTR data.
To analyze the impact of Rule 14, matches were not allowed for patients with a MELD score of 14 or below. The livers from this group were re-distributed as before without surpassing the proposed 1200 or 1600 D-MELD cap. Because status 1 listed patients might suffer from increased waiting times under a D-MELD risk cap, allocation to this subgroup was not limited by D-MELD. Redistributed livers from Rule 14 and D-MELD risk caps were allocated to Status 1 listed patients in the same proportion as actual data from 2002-2004 SRTR data.
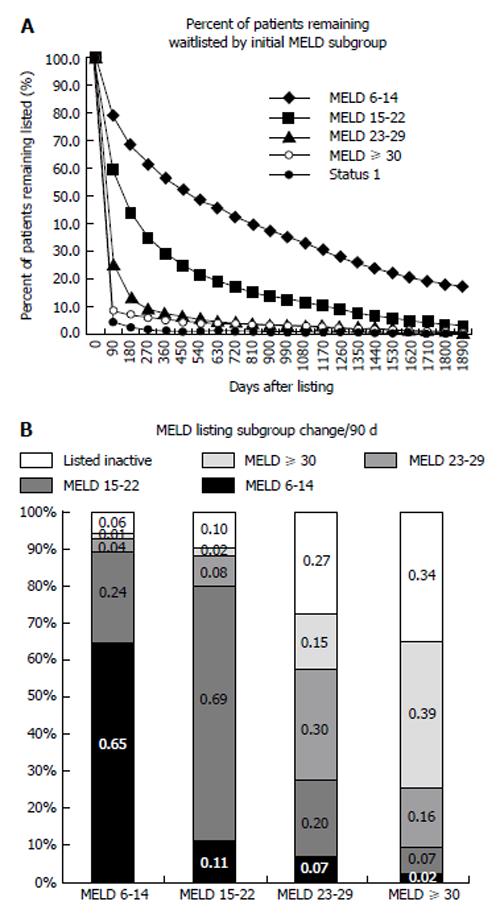
A Markov model encompassing all events (transitions) potentially occurring after listing (initial state) was created (Figure 1). Events (list removal or inactivation, transplantation or active listing) by MELD subgroup were calculated directly from actual data. Rate to first event and list removal was dependent on MELD subgroup. As expected, the MELD 6-14 subgroup was slowest to leave the list, with 55% of patients remaining waitlisted at one year. The percentage of patients remaining waitlisted over time decreased dramatically as MELD increased. In the sickest subgroups, MELD ≥ 30 and Status 1, less than 10% of patients remained listed after the first 90-d period (Figure 2A). Analysis of movement between MELD subgroups for patients remaining waitlisted demonstrated the lower MELD subgroups (6-14, 65% and 15-22, 69%) to be most likely to remain waitlisted at the same MELD after 90 d (Figure 2B). Patients at moderate MELD (MELD 15-22 and MELD 23-29) who remained waitlisted were slightly more likely to move to a lower rather than a higher MELD subgroup per 90-d period if still waiting. The small percentage of patients who remained waitlisted in the subgroup MELD ≥ 30 were most likely to wait at the same MELD followed by inactive listing.
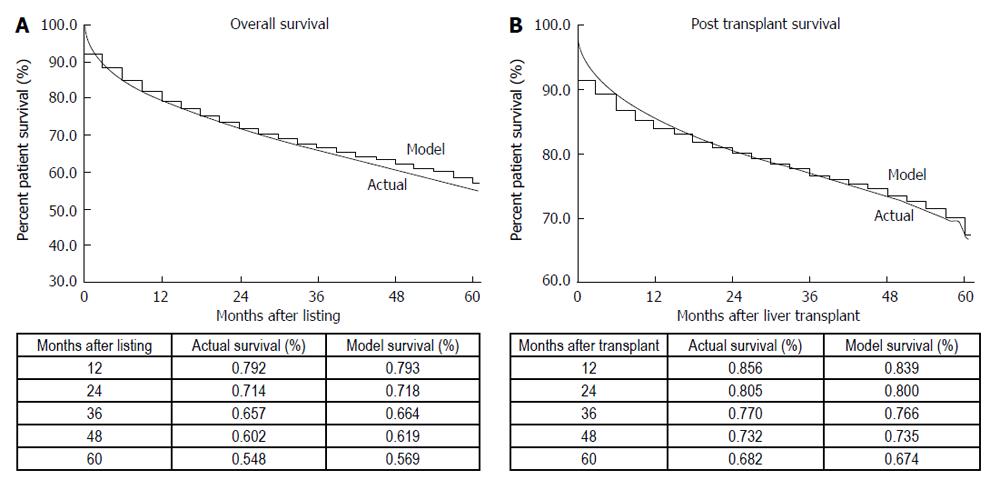
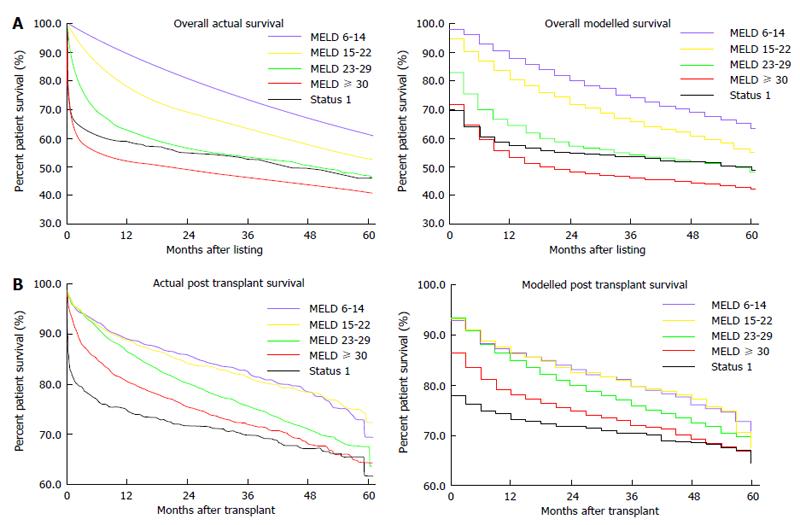
The Markov microsimulation correlated well with post transplant and overall survival when compared to actual data (Figure 3). Comparison of predicted overall survival by individual MELD subgroup demonstrated no greater than 1.8% and 2.3% variation between model and actual data for the years 2002 at 1 year and 4 years, respectively (Figure 4A). Comparison of predicted post transplant survival by MELD subgroup to actual data demonstrated no greater than 2.3% variation between model and actual at 1 year and no greater than 2.4% variation at 4 years (Figure 4B).
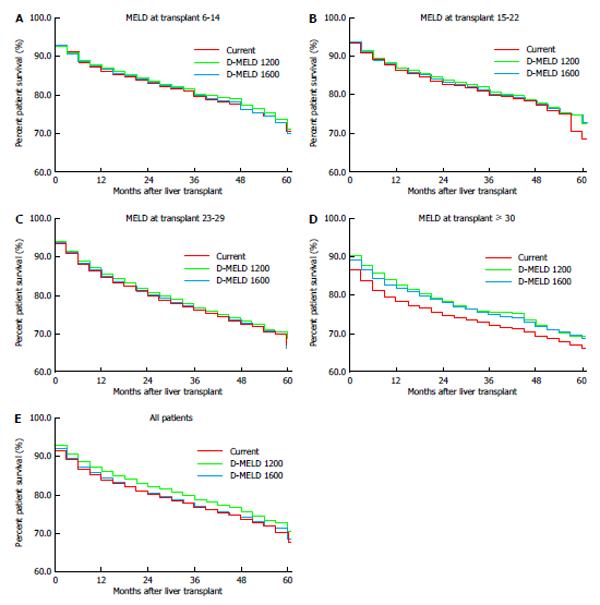
The impact of D-MELD risk caps was analyzed (Figure 5). A D-MELD risk cap of 1600 demonstrated no overall improvement in post transplant survival. A D-MELD risk cap of 1200 improved post transplant survival by 1.6% at one year and by 2.0% at 4 years (Figure 5E). Examination of post transplant survival by MELD subgroup at transplant demonstrated a similar benefit for both D-MELD risk caps for the MELD ≥ 30 subgroup, with an approximately 4% improvement in one-year patient survival for both the 1200 and 1600 risk caps and approximately 2.9% improvement for both groups at 4 years (Figure 5D).
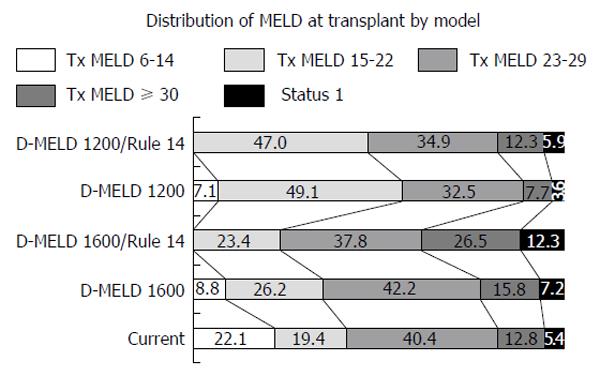
The MELD distribution at transplant was examined for both the 1200 and 1600 risk caps ± Rule 14 (Figure 6). Institution of the 1600 risk cap shifted allocation slightly in favor of the MELD 15-22 and MELD 23-29 subgroups. The 1200 risk cap had an expectedly greater effect, shifting greater than 90% of transplants to patients with MELD scores of ≤ 29.
Addition of Rule 14 to either of the risk caps resulted in an increased percentage of transplants for the MELD ≥ 30 subgroup with a greater number of livers redistributing to high MELD under the D-MELD 1600 risk cap compared to the 1200 risk cap (Figure 6). For example, the D-MELD risk cap in combination with Rule 14 resulted in 26.5% of transplants performed at MELD ≥ 30% vs 12.8% for the model. Addition of Rule 14 to the D-MELD risk cap of 1200 resulted in 47% of transplants performed at MELD 15-22 compared to 19.4% in the model.
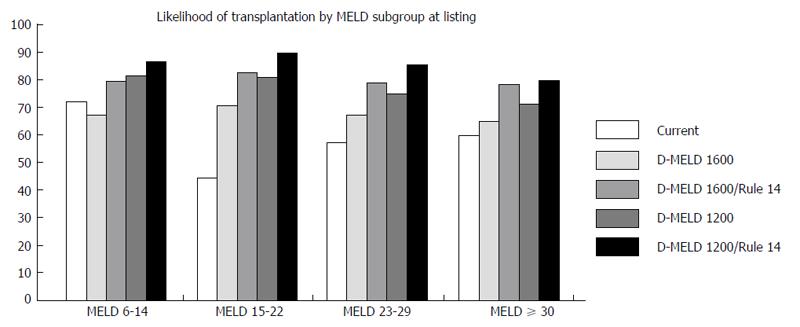
Likelihood of transplant by MELD subgroup at listing was examined for each model. For the proposals including Rule 14, patients initially listed in the group 6-14 required progression to a higher MELD to obtain a match. A D-MELD risk cap of 1200 in combination with Rule 14 resulted in the highest likelihood of receiving a transplant for all MELD sub-groups (Figure 7).
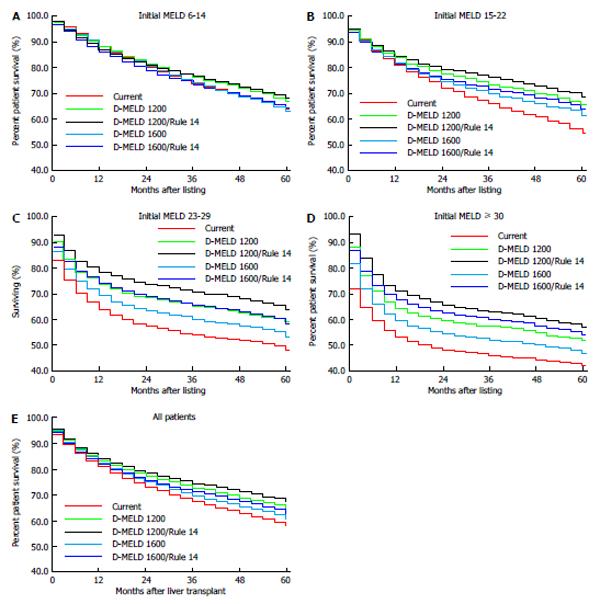
Analysis of overall survival from the time of listing was examined for all patients together as well as by initial MELD subgroup at listing. All proposals resulted in improved survival from the time of listing when compared to the modeled current survival (Figure 8). The proposed D-MELD 1200/Rule 14 rule change provided the greatest improvement for the whole waitlisted population, increasing overall survival by 3.6% at one year and by 8.5% at 4 years (Figure 8E). Examination by MELD subgroup at listing demonstrated a similar pattern, with the most significant improvement in overall survival for the most restrictive risk cap of 1200 in combination with Rule 14. The subgroups gaining the greatest survival benefit were patients listed at MELD 23-29 and MELD ≥ 30 (Figure 8C and D).
Currently, liver allocation based on the MELD system is urgency-based without regard for post transplant survival or consideration of the potential benefit of transplantation at a less advanced disease state. The merits of the current system are simplicity, objectivity, and accuracy predicting waiting list mortality, as well as equity since disease severity is the only prioritizing factor[16,17]. Disadvantages of the current system include a continued pressure toward poorer outcomes since the sickest (highest MELD) recipients often match with higher risk donors. This pressure towards transplantation at higher MELD worsens as transplant centers “compete” for scarce donor livers under a matching system solely prioritizing disease severity (MELD)[18]. The desire for a better balance between urgency and utility in organ allocation has been an elusive goal[10].
In order to better balance the risk for individual donor recipient combinations in a manner ethically acceptable for allocation, we developed a statistic, D-MELD, designed to account for the two dominant variables in donor/recipient matching, patient physiologic condition at transplantation as measured by MELD score and donor quality which is most significantly related to donor age[13,14]. As the product of two continuous variables, donor age and MELD (excluding exception points), D-MELD calculation produces a continuous gradient of risk for increased post-operative mortality and length of stay. Strata from the gradient can be defined and risk caps can be assigned to optimize distribution of organs for the population.
While the impact of risk capping has an intuitive benefit for improving post-transplant survival, the impact on overall survival for all waitlisted patients is less intuitive since the D-MELD risk cap would prohibit transplants for some high MELD recipients who might incur higher waitlist mortality. Conversely, re-distribution of livers to recipients within the moderate MELD subgroup would also decrease the number of patients progressing to high MELD prior to transplant. Whether the redistribution would have an overall positive or negative effect likely depends on the relative availability of donor livers, the rate of disease progression and the ability of patients to wait for a higher quality donor offer. An additional concern is whether redistribution of older donors to moderate MELD recipients would worsen post-transplant outcome for this subgroup and negate the benefit of greater liver availability.
Using a model derived from SRTR data, D-MELD risk capping in combination with Rule 14 provided a survival benefit for the waitlisted population as a whole without harming any subpopulation of listed patients. The more restrictive risk cap of 1200 had a greater impact on MELD at transplant, shifting a greater proportion of transplants to an earlier stage of disease thereby preventing progression to high MELD prior to transplant for many recipients. As the high MELD subgroup decreased in size, the D-MELD risk capping actually increased the likelihood of transplantation for patients with MELD ≥ 30, with the added potential post transplant survival advantage that the highest quality young donors were reserved for the sickest patients. Addition of Rule 14 further redistributed livers disproportionally towards higher MELD recipients, providing additional insurance against prolonged waiting times at high MELD and improving overall survival by approximately 2%-3% for both 1200 and 1600 risk caps. This improvement in survival for the Rule 14 is consistent with prior modeling experiments[11].
Our model suffers from a number of limitations that require further study prior to any recommendation to modify allocation policy. We chose a Markov microsimulation paradigm to model survival given the data available from the SRTR database. We considered initial MELD, end MELD, time on the list, deaths while waiting and survival after transplant to be the most robust information available from the database. Certain information was not directly obtainable from analysis of the database for the time period 2002-2004, and modifications were required to attain accuracy of the model.
The first modification was necessary to understand the expected changes in MELD over each 90-d period. In order to correct this deficit, we analyzed data from the most recent complete 90-d time period available, September-December 2007, comparing initial MELD to final MELD for patients remaining waitlisted. These probabilities were then used to estimate 90-d changes in MELD for the period 2002-2004. The second modification was required to estimate survival for patients listed inactive. Patients in this group were given the same survival probability as those removed from the list for other reasons by MELD subgroup. Similar model calibrations have been required for accuracy in described modeling experiments[19-22].
Using the Markov microsimulation based on probabilities alone also limited the range of output data, and we were not able to assess, for example, changes in waiting time by MELD subgroup. Although our model is simpler than other described simulations, it was able to achieve accuracy predicting overall and post transplant survival for all subgroups for the time period studied.
A second limitation of our model is the assumption of minimal friction in organ redistribution across the whole waitlisted population. Allocation practice during the time period of the study were quite limited by geographic and political considerations, so that other than for status 1 recipients, sharing of organs outside of local service areas was only practiced for livers that did not match locally. The current environment, in which regional sharing is now also mandated for recipients with MELD > 35, now matches our model more closely. Nevertheless, as survival in our model improved comparing D-MELD risk caps of 1200 and 1600, the beneficial effect of the model cannot result from sharing alone. Certainly, any negative effect from increased cold ischemia times that would result if livers were re-distributed over long distances might counter benefits gained from better matching and would need to be assessed as part of any increased sharing paradigm.
Given the real world limitations inherent to the current allocation system, implementation of a D-MELD risk cap at the regional level may require optimization to achieve optimal impact on survival[13,23]. For example, in regions 4, 6 and 10, which have a higher donor/recipient ratio and higher likelihood of transplantation, a D-MELD score of 1200 may be optimal. In contrast, in regions 5 and 9, which have a higher average MELD at transplantation and higher average D-MELD, a higher cap might produce optimal survival. Further modeling is required before any final conclusions should be drawn.
Instituting risk caps would require a change in the ethics of liver allocation by introducing societal limitations on the amount of acceptable risk for an individual patient. Given the knowledge that matches between high risk donors and high risk recipients still provide a survival benefit for the recipient, some individuals who would otherwise have survival benefit from a transplant would remain waitlisted losing preference to a match with a better predicted outcome. This introduces a utilitarian ethic into the current urgency only liver allocation policy. Utilitarian considerations are only appropriate when medical resources are limited. According to the American Medical Association Code of Medical Ethics, Opinion 2.03-Allocation of Limited Medical Resources, only “ethically appropriate”, criteria may be used for allocation decisions pertaining to “likelihood of benefit, urgency of need, change in quality of life, duration of benefit and resources required for treatment”. Furthermore, the Code states that physician must “remain the patient advocate” and “should not make these allocation decisions”[24]. Policy favoring utility such as D-MELD risk caps and Rule 14 would help remove the caregiving physician from the potentially conflicted task of patient advocate and resource manager by displacing bypass decisions for unfavorable donor/recipient matches to the allocation mechanism.
Currently a risk cap paradigm is already in practiced in liver allocation in the form of an exception point system for hepatocellular carcinoma[25]. While patients with cirrhosis alone are prioritized solely by their risk for waiting list mortality (calculated MELD score), those with hepatocellular carcinoma are prioritized by a combination of their risk of death from cancer and expected survival after transplantation[26,27]. Since the risk for post transplant mortality from tumor recurrence can be predicted pre-operatively in an ethically neutral manner by measuring tumor size and number, the risk cap allows MELD exception points only for patients falling within an acceptable risk threshold based on the Milan criteria[28]. Risk capping for donor/recipient matching could be based follow a similar paradigm to better balance risk of death and optimal utility.
In summary, modeling suggests that adoption of D-MELD risk caps in combination with Rule 14 would improve both post transplant and overall survival for the population of patients with liver disease awaiting transplantation. This modification works by redistributing donor livers toward patients with MELD scores between 15-29 when transplantation is most effective thereby decreasing the number of futile transplants and reducing the severity of disease progression prior to a liver offer. Institution of risk caps could improve outcomes for the whole population of patients waiting for transplantation, but would require consensus among stakeholders given the potential negative consequences for individual patients.
The authors thank Marilyn Carlson for editorial assistance and Alfonso Avolio, MD, Division of General Surgery and Transplantation, Deparment of Surgery, Catholic University, Rome, Italy for critical reading, intellectual support and suggested improvements for the manuscript.
As a response to waitlist mortality, liver transplantation has witnessed an increasing use of marginal organs previously thought to have excessive risk for poor post-operative outcome (older donors, fatty liver donors, donors after cardiac death, donors with history of malignancy, etc.); the predominant risk assumed being the increased use of older donors. While this effort to increase the size of the donor pool was successful, donor risk has increased and is now a dominant risk factor when predicting post transplant survival. As patient survival after liver transplantation is dependent on the combination of both donor and recipient risk, poor donor/recipient matches in which total risk is excessive result in futile transplantations, an inefficient use of the critically limited resource of donor livers. The Model for End-Stage Liver Disease score excluding exception points and donor age (D-MELD) statistic was developed as a donor/recipient “risk capping” strategy with an eye to improve donor/recipient matching and reduce futile transplantations.
Markov decision analysis can be used to estimate the cumulative impact of decisions involving many potential outcome states in a complex system. In order to understand the potential impact of proposed allocation changes utilizing D-MELD risk capping, a Markov microsimulation was designed and validated based on outcomes from all patients listed for liver transplantation between 2002-2004 and followed until 2008. The impact of both more (D-MELD 1200) and less (D-MELD 1600) restrictive risk caps with and without Rule 14 was examined for its potential impact on post transplant survival, overall survival and Model for End-Stage Liver Disease (MELD) subgroup at transplantation.
This study demonstrates that allocation rule changes designed to cap combined donor/recipient risk may improve utility in liver transplantation by decreasing the frequency of futile transplantations and by decreasing average disease severity at transplantation.
This study examines the potential impact of universal adoption of D-MELD risk caps. The D-MELD statistic is calculated as the product of donor age and the laboratory MELD score excluding United Network for Organ Sharing (UNOS) exception points. Importantly, the laboratory-based MELD score (excluding UNOS exception points) is utilized for the calculation as a measure of recipient physiologic condition. As the product of two continuous variables and laboratory MELD produces a risk gradient predicting postoperative mortality and length of stay. This gradient can then be used to identify strata where donor and recipient risk combine to result in inferior outcomes.
Markov models are increasingly used in economic evaluations of population impacts of medical treatments. In this article described and validate is an original Markov microsimulation model designed to accurately assess the potential impact of risk limitation based allocation of donor livers.
P- Reviewer: Lai Q, Rostami K S- Editor: Wen LL L- Editor: A E- Editor: Liu SQ
| 1. | Waki K. UNOS Liver Registry: ten year survivals. Clin Transpl. 2006;29-39. [PubMed] |
| 2. | Renz JF, Kin C, Kinkhabwala M, Jan D, Varadarajan R, Goldstein M, Brown R, Emond JC. Utilization of extended donor criteria liver allografts maximizes donor use and patient access to liver transplantation. Ann Surg. 2005;242:556-563; discussion 563-565. [PubMed] |
| 3. | Volk ML, Lok AS, Pelletier SJ, Ubel PA, Hayward RA. Impact of the model for end-stage liver disease allocation policy on the use of high-risk organs for liver transplantation. Gastroenterology. 2008;135:1568-1574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Mutimer DJ, Gunson B, Chen J, Berenguer J, Neuhaus P, Castaing D, Garcia-Valdecasas JC, Salizzoni M, Moreno GE, Mirza D. Impact of donor age and year of transplantation on graft and patient survival following liver transplantation for hepatitis C virus. Transplantation. 2006;81:7-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 5. | Lake JR, Shorr JS, Steffen BJ, Chu AH, Gordon RD, Wiesner RH. Differential effects of donor age in liver transplant recipients infected with hepatitis B, hepatitis C and without viral hepatitis. Am J Transplant. 2005;5:549-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 131] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 6. | Schaubel DE, Sima CS, Goodrich NP, Feng S, Merion RM. The survival benefit of deceased donor liver transplantation as a function of candidate disease severity and donor quality. Am J Transplant. 2008;8:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 7. | Segev DL, Maley WR, Simpkins CE, Locke JE, Nguyen GC, Montgomery RA, Thuluvath PJ. Minimizing risk associated with elderly liver donors by matching to preferred recipients. Hepatology. 2007;46:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Kim WR, Kremers WK. Benefits of “the benefit model” in liver transplantation. Hepatology. 2008;48:697-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Dawwas MF, Gimson AE. Candidate selection and organ allocation in liver transplantation. Semin Liver Dis. 2009;29:40-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Avolio AW, Halldorson JB, Burra P, Dutkowski P, Agnes S, Clavien PA. Balancing utility and need by means of donor-to-recipient matching: a challenging problem. Am J Transplant. 2013;13:522-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Perkins JD, Halldorson JB, Bakthavatsalam R, Fix OK, Carithers RL, Reyes JD. Should liver transplantation in patients with model for end-stage liver disease scores < or = 14 be avoided? A decision analysis approach. Liver Transpl. 2009;15:242-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Rana A, Hardy MA, Halazun KJ, Woodland DC, Ratner LE, Samstein B, Guarrera JV, Brown RS, Emond JC. Survival outcomes following liver transplantation (SOFT) score: a novel method to predict patient survival following liver transplantation. Am J Transplant. 2008;8:2537-2546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 350] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 13. | Halldorson JB, Bakthavatsalam R, Fix O, Reyes JD, Perkins JD. D-MELD, a simple predictor of post liver transplant mortality for optimization of donor/recipient matching. Am J Transplant. 2009;9:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 14. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1486] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 15. | Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11:91-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 378] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Coombes JM, Trotter JF. Development of the allocation system for deceased donor liver transplantation. Clin Med Res. 2005;3:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 55] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 17. | Lake JR. MELD--an imperfect, but thus far the best, solution to the problem of organ allocation. J Gastrointestin Liver Dis. 2008;17:5-7. [PubMed] |
| 18. | Halldorson JB, Paarsch HJ, Dodge JL, Segre AM, Lai J, Roberts JP. Center competition and outcomes following liver transplantation. Liver Transpl. 2013;19:96-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 19. | Shechter SM, Bryce CL, Alagoz O, Kreke JE, Stahl JE, Schaefer AJ, Angus DC, Roberts MS. A clinically based discrete-event simulation of end-stage liver disease and the organ allocation process. Med Decis Making. 2005;25:199-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Freeman RB, Harper AM, Edwards EB. Redrawing organ distribution boundaries: results of a computer-simulated analysis for liver transplantation. Liver Transpl. 2002;8:659-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Thompson D, Waisanen L, Wolfe R, Merion RM, McCullough K, Rodgers A. Simulating the allocation of organs for transplantation. Health Care Manag Sci. 2004;7:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Ratcliffe J, Young T, Buxton M, Eldabi T, Paul R, Burroughs A, Papatheodoridis G, Rolles K. A simulation modelling approach to evaluating alternative policies for the management of the waiting list for liver transplantation. Health Care Manag Sci. 2001;4:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 23. | Roberts JP, Dykstra DM, Goodrich NP, Rush SH, Merion RM, Port FK. Geographic differences in event rates by model for end-stage liver disease score. Am J Transplant. 2006;6:2470-2475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | American Medical Association. Opinion 2.03-Allocation of Limited Medical Resources. 2009; Available from: http; //www.ama-assn.org/ama/pub/physician-resources/medical-ethics/code-medical-ethics/opinion203.shtml. |
| 25. | United Network for Organ Sharing. Policy 3.6.4. 4: Liver transplant candidates with hepatocellular carcinoma (HCC) 2009; Available from: http: //www.unos.org/PoliciesandBylaws2/policies/pdfs/po9licy_8.pdf.. |
| 26. | Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, Camerini T, Roayaie S, Schwartz ME, Grazi GL. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009;10:35-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1267] [Cited by in RCA: 1570] [Article Influence: 92.4] [Reference Citation Analysis (1)] |
| 27. | Yao FY. Liver transplantation for hepatocellular carcinoma: beyond the Milan criteria. Am J Transplant. 2008;8:1982-1989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 28. | Mazzaferro V, Rondinara GF, Rossi G, Regalia E, De Carlis L, Caccamo L, Doci R, Sansalone CV, Belli LS, Armiraglio E. Milan multicenter experience in liver transplantation for hepatocellular carcinoma. Transplant Proc. 1994;26:3557-3560. [PubMed] |