Published online Jun 24, 2014. doi: 10.5500/wjt.v4.i2.141
Revised: April 16, 2014
Accepted: May 16, 2014
Published online: June 24, 2014
Processing time: 235 Days and 3.5 Hours
AIM: To determine the impact of transplant nephrectomy on peak panel reactive antibody (PRA) levels, patient and graft survival in kidney re-transplants.
METHODS: From 1969 to 2006, a total of 609 kidney re-transplantations were performed at the University of Freiburg and the Campus Benjamin Franklin of the University of Berlin. Patients with PRA levels above (5%) before first kidney transplantation were excluded from further analysis (n = 304). Patients with graft nephrectomy (n = 245, NE+) were retrospectively compared to 60 kidney re-transplants without prior graft nephrectomy (NE-).
RESULTS: Peak PRA levels between the first and the second transplantation were higher in patients undergoing graft nephrectomy (P = 0.098), whereas the last PRA levels before the second kidney transplantation did not differ between the groups. Age adjusted survival for the second kidney graft, censored for death with functioning graft, were comparable in both groups. Waiting time between first and second transplantation did not influence the graft survival significantly in the group that underwent nephrectomy. In contrast, patients without nephrectomy experienced better graft survival rates when re-transplantation was performed within one year after graft loss (P = 0.033). Age adjusted patient survival rates at 1 and 5 years were 94.1% and 86.3% vs 83.1% and 75.4% group NE+ and NE-, respectively (P < 0.01).
CONCLUSION: Transplant nephrectomy leads to a temporary increase in PRA levels that normalize before kidney re-transplantation. In patients without nephrectomy of a non-viable kidney graft timing of re-transplantation significantly influences graft survival after a second transplantation. Most importantly, transplant nephrectomy is associated with a significantly longer patient survival.
Core tip: In our paper, presented as “poster of distinction” at the ATC, we show that graft nephrectomy of a first non-functioning kidney graft leads to an increase in peak panel reactive antibody that normalizes before re-transplantation. In 305 low-risk patients who underwent re-transplantation, graft survival did not differ between those with or without prior nephrectomy. Interestingly, patient survival was significantly better in patients with nephrectomy. This supports the findings of Ayus et al, who investigated patients staying on maintenance dialysis after graft failure. Therefore graft nephrectomy should be considered in patients returning to dialysis after failure of a kidney transplant.
- Citation: Tittelbach-Helmrich D, Pisarski P, Offermann G, Geyer M, Thomusch O, Hopt UT, Drognitz O. Impact of transplant nephrectomy on peak PRA levels and outcome after kidney re-transplantation. World J Transplant 2014; 4(2): 141-147
- URL: https://www.wjgnet.com/2220-3230/full/v4/i2/141.htm
- DOI: https://dx.doi.org/10.5500/wjt.v4.i2.141
Kidney transplantation is the therapy of choice for patients suffering from end-stage renal failure. Due to improvements in immunosuppressive therapy and operative technique, contemporary graft survival rates in first deceased donor transplants have reached 90% after one year and 68% after five years, respectively[1]. Patients returning to dialysis after failure of the primary graft have a significantly higher mortality rate compared to patients awaiting their first kidney graft[2]. Repeat kidney transplantation has been shown to offer a significant survival benefit in these cases[3,4]. However, the outcome of repeat kidney transplantation is known to be inferior to primary transplantation[1]. In 2005 18.7% of patients on the waiting list in the United States had been transplanted previously (OPTN/SRTR Annual report 1995-2004) and recent research indicates that the number of patients undergoing kidney retransplantation is growing rapidly[1].
The indication and timing of primary allograft nephrectomy in patients awaiting a secondary renal transplant are still a matter of debate[5]. Graft nephrectomy is a safe procedure in experienced centers. It is associated with perioperative morbidity that depends on the surgical technique used (e.g., extra- vs intracapsular) and the indication for nephrectomy. Morbidity ranges from 4% to 48% and encompasses bleeding, infection or, less frequently, injury of iliac vessels[6,7]. Due to perioperative complications some authors recommend not to remove the non-functional kidney until graft associated complications occur[8-11]. However, others advise the routine removal of the failed graft to avoid infection, bleeding, hypertension or erythropoietin resistance due to chronic inflammation[10,11]. The most common practice seems to be nephrectomy after early graft loss, while in patients with graft failure after more than one year, nephrectomy is often exclusively reserved for cases experiencing complications[12-15].
The impact of a non-functioning kidney graft left in situ or graft nephrectomy on antibody production and outcome after secondary renal transplantation remains unclear, although PRA levels in patients undergoing nephrectomy seem to be higher than in patients in which the graft is not removed[16,17].
The aim of this study was to determine the influence of nephrectomy on PRA levels and the outcome after secondary renal transplantations.
The records of all retransplant renal allograft recipients at the University of Freiburg and the University of Berlin, Campus Benjamin Franklin, between 1969 and 2006 were reviewed.
In total 609 re-transplantations were performed, of which 305 (50.1%) were included in our study. Inclusion criteria were as follows: second renal transplantation (third or fourth transplantations were excluded from analysis), PRA prior to first kidney transplantation ≤ 5%, available data on nephrectomy and a minimum of three documented PRA values (before first, between first and second and immediately before second transplantation). Of 305 patients meeting these criteria, 245 patients underwent nephrectomy (NE+) and 60 patients retained their failed first graft (NE-).
The mean age at the time of the first kidney transplantation was 35.5 ± 13.9 years and 39.3 ± 12.8 years for NE+ and NE- patients, respectively (P = 0.056). At the time of second transplantation patients were 41.6 ± 13.3 years old in group NE+ and 47.2 ± 13.3 years in the group NE- (P = 0.004). Demographic data of patients are shown in Table 1.
| Characteristics | NE+ | NE | P |
| n | 245 | 60 | |
| Sex (M/F) | 158/87 | 41/19 | 0.650 |
| Age at 1. Tx (yr; mean) | 35.5 ± 13.9 | 39.2 ± 12.9 | 0.056 |
| Age at 2. Tx (yr; mean) | 41.6 ± 13.3 | 47.2 ± 13.3 | 0.004 |
| Date of 1. Tx | 09/1969-03/2005 | 10/1979-09/2002 | |
| Date of 2. Tx | 09/1981-12/2005 | 04/1991-09/2006 |
The immunosuppressive regimen included steroids plus cyclosporin A (CsA; n = 175), CsA plus azathioprine or mycophenolate mofetil (n = 106) or other regimens containing tacrolimus or an induction therapy with antibodies (n = 22). All patients in the group NE- received CsA for maintenance therapy.
Graft failure was defined as the irreversible loss of graft function with the need to resume dialysis. Immunosuppression (prednisone 5 mg per day) was continued as long as diuresis exceeded 500 mL/d. If urine production fell below 500 mL/d, immunosuppression was discontinued. In group NE-, the non-functioning kidney graft remained in situ, unless patients developed complications (e.g., infections, bleeding or hypertension). Patients in the group NE+ underwent nephrectomy soon after resuming dialysis. Transplant nephrectomy was performed according to the technique described by Rosenthal et al[6].
Perioperative and follow-up data of patients were gained retrospectively from electronic health care records or from Eurotransplant Network Information System. Statistical analysis was performed using SPSS. Values are expressed as mean ± SD unless otherwise stated. Patient and graft survival rates were calculated according to the Kaplan-Meier method. Survival rates among both groups were compared using the univariate log-rank analysis. Group comparisons were calculated by independent Students t tests. P values of < 0.05 were considered significant. Non-significant differences are indicated as ns.
Follow-up data were available for all patients. Mean follow-up was 7.9 years (range 0.3-22.8 years) in the group NE+ and 6.2 years (range 0.4-19.3 years) in the group NE-. Mean waiting time from graft loss to re-transplantation was 3.44 ± 2.68 years in the group NE+ and 2.55 ± 2.55 years in the group NE- (P = 0.021). In the group NE+, nephrectomy was performed 0.53 ± 1.47 years after graft loss and 3.05 ± 2.57 years before second transplantation.
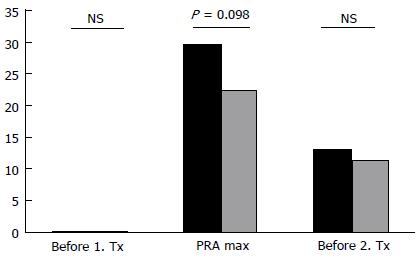
The last recorded PRA levels before second transplantation did not differ between groups (Figure 1). In contrast, the mean maximum PRA levels were higher in the group NE+ than in the group NE- (29.7% vs 22.5%), although this difference did not reach statistical significance (P = 0.09). When comparing the median, the difference in maximum PRA levels reached statistical significance (18.5 in NE+ vs 9 in NE-; P = 0.038). The maximum PRA level was detected 1.6 ± 1.9 years (NE+) and 0.5 ± 2.9 years (NE-) after graft loss and 2.2 ± 2.3 years (NE+) vs 2.1 ± 3.4 years (NE-) before re-transplantation. Maximum PRA levels in the group NE+ were observed at an average of one year after nephrectomy (1.0 ± 2.2 years).
The uni- and multivariate analysis of potential risk factors show that PRA levels measured directly before transplantations were the only factor being associated with a significantly higher risk of graft loss (Table 2).
| OR | 95%CI | P | |
| NE+/- | 1.06 | 0.71-1.56 | 0.79 |
| PRA before 1. Tx | 1.59 | 1.11-2.30 | 0.01 |
| PRA max | 0.99 | 0.98-1.00 | 0.18 |
| PRA before 2. Tx | 1.02 | 1.00-1.04 | 0.01 |
| Time from 1. Tx to graft loss | 0.96 | 0.88-1.05 | 0.37 |
| Time from graft loss to nephrectomy | 0.89 | 0.76-1.07 | 0.22 |
| Time from nephrectomy to 2. Tx | 0.89 | 0.79-1.02 | 0.09 |
| Time from graft loss to 2. Tx | 0.98 | 0.91-1.06 | 0.65 |
| Age at 1. Tx | 1.01 | 0.99-1.03 | 0.45 |
| Age at 2. Tx | 1.01 | 0.99-1.03 | 0.13 |
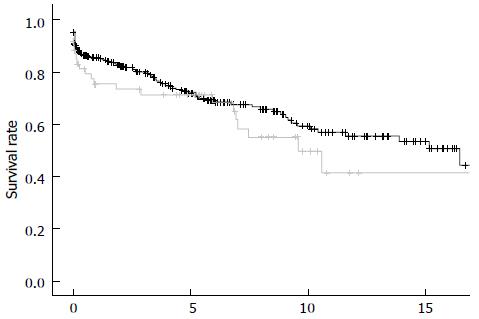
Graft survival for the entire cohort differed significantly with 1, 5 and 10-year graft survival rates of 81.4%, 62.4% and 46.3% vs 66.8%, 59.0% and 30.2% for patients of the groups NE+ and NE- (P = 0.01), respectively. However, this advantage disappeared when the analysis was censored for death with a functioning graft (Figure 2).
Graft survival rates after the second kidney transplantation did not differ between patients with early failure of the first graft (within 6 mo) and patients with graft loss occurring later than 6 mo.
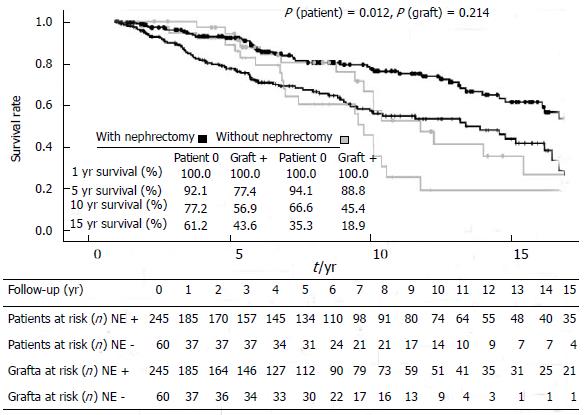
To further exclude potential confounding variables, any failure of the second graft within one year after re-transplantation, which is mainly related to technical or early immunological complications, was censored (Figure 3). Graft survival rates at 5 and 10 years did not differ and were 77.4% and 56.9% in the group NE+ and 88.8% and 45.4% in group NE- (P = 0.214).
In addition, we evaluated the influence of center-specific factors on graft survival rates due to different immunosuppressive regimens. According to our data, patients on triple immunosuppressive regimens using calcineurin inhibitors (mainly CsA) and azathioprine or MMF and steroids experienced significantly better graft survival rates if compared to patients using only CsA and steroids. The graft survival rates of patients in the groups NE+ and NE-, respectively, receiving the same immunosuppressive regimen did not differ between the two centers.
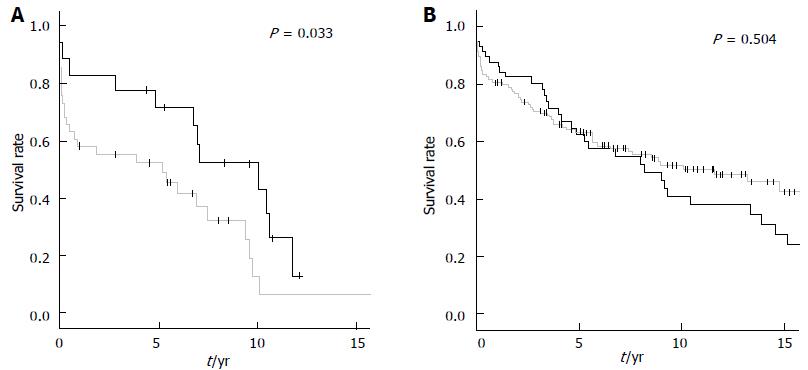
Interestingly, in patients undergoing nephrectomy prior to re-transplantation (NE+) the timing of second kidney transplantation (within one year after graft loss vs later than one year) did not significantly influence the outcome. In contrast, patients without nephrectomy experienced better graft survival rates when re-transplantation was performed within one year after graft loss (P = 0.033) (Figure 4).
Patient survival rates according to the Kaplan-Meier method at 1, 5 and 10 years were 94.1%, 86.3%, 72.2% and 79.2%, 73.1%, 44.1% in the group NE+ and in the group NE-, respectively (P < 0.01). Since patients of the group NE- were significantly older than patients in the group NE+, patient survival may have been influenced by differences in age at the time of second transplantation. However, Log-rank analysis of age-adjusted patient survival rates after the exclusion of all patients older than 65 years at time of second transplantation, still revealed a significant survival benefit for patients in the nephrectomy group, compared to patients without nephrectomy (94.1% and 86.3% vs 83.1% and 75.4% at 1 and 5 years; P = 0, < 0.01)
Therapeutic strategies for patients having lost a primary kidney graft and awaiting re-transplantation differ from center to center. Until now, there is no consensus regarding the indication and timing of the removal of a nonviable graft.
It is known that graft and patient survival is worse after second kidney transplantation compared to the first transplantation[1]. Several factors may contribute to this finding: Kidney re-transplants acquire additional waiting time on dialysis after failure of the first transplant which in itself is well known to increase morbidity and mortality after re-transplantation[2,18]. Moreover, patients who undergo repeat renal transplantation are older than at the time of first transplantation and often receive grafts from extended-criteria donors[19-22].
The main finding of our study was a significantly increased patient survival in those second graft recipients who had undergone nephrectomy of their first nonviable graft before receiving a repeat transplantation. This striking effect was observed despite a lack of difference in second kidney graft survival rates between patients who had their first transplant removed before re-transplantation and those who retained their failed graft.
The reasons for the improved survival of repeat transplant candidates who had undergone prior nephrectomy are unclear. However, patients staying on maintenance dialysis after failure of a first kidney graft also show improved survival after graft nephrectomy[23]. The residual non-functioning graft in patients not undergoing nephrectomy may thus be a source of complications in itself or through the need for continued immunosuppressive therapy (e.g., infections or a chronic inflammatory condition).
By analyzing graft survival rates censored for death with functioning graft or graft loss within one year, our results revealed no differences for patients with or without nephrectomy, which is in accordance with recent literature[24]. Therefore, nephrectomy of the failed first kidney graft does not influence survival of the second graft.
Patients considered for re-transplantation are often immunized or even highly immunized due to the development of HLA-specific antibodies to previous transplant antigens. Yong Won Cho showed that panel reactive antibodies are observed more often after graft loss than after blood transfusions or prior pregnancies[25]. Therefore, even with negative complement dependent cytotoxicity crossmatch, these patients are more likely to develop acute humoral rejection episodes[25-27]. This correlates with our findings that higher PRA values before first and second transplantation are associated with an increased risk of graft loss. The impact of the elevated PRA levels in the group NE+ remains unclear but was also observed in other studies[5,28,29]. Our study design precluded information on presensitized patients. Schleicher et al[29] could show that in their collective patients undergoing nephrectomy had significantly higher PRA levels at the time of retransplantation, which led to a worse graft survival in that group. Especially a PRA level > 70% was an independent risk factor for graft loss. In our study, graft survival did not differ between the groups. This may be due to similar PRA levels before retransplantation. Lucarelli et al[30] also did not find a difference in second graft survival in patients with or without prior nephrectomy. They also observed comparable PRA levels in both groups at the time of retransplantation[30].
Intensified immunosuppression may therefore improve graft and patient survival in patients with elevated PRA after a first graft nephrectomy and can also be found in our data considering the different immunosuppressive regimens.
Other authors state that the rise in HLA antibodies after nephrectomy is an expression of the capacity of even a nonfunctional graft to absorb donor specific antibodies or mount an immune response to the donor’s MHC antigens. This may protect a second renal graft[31,32]. The graft intolerance syndrome, which leads to chronic inflammatory disease that can be treated by embolization of nonfunctioning renal allografts[33-35], favors the aforementioned hypothesis. However,, neither murine and nor human studies could proof these findings[36].
By analyzing graft survival rates censored for death with functioning graft or graft loss within one year, we observed no difference for patients with or without nephrectomy. Therefore, nephrectomy of the failed first kidney graft does not influence survival of the second graft.
Although we observed no influence of prior graft survival, we could confirm the importance of waiting time to retransplantation. In patients undergoing nephrectomy prior to re-transplantation, no difference was evident. In contrast, in patients without nephrectomy, a survival benefit was evident when re-transplantation was performed within one year after graft loss. In our patient group waiting time to retransplantation was about two to three years; in the United States waiting times of more than five years are common[35]. This also needs to be taken into account when considering a graft nephrectomy with its associated perioperative risk.
This study is limited by its retrospective design and the long timeframe in which patients have undergone transplantation. It still offers novel insights into the advantages of graft nephrectomy on the outcome of secondary kidney transplantation.
In a conclusion, Nephrectomy of a nonfunctioning kidney graft prior to re-transplantation is a save procedure in experienced centers that, despite a temporary increase in PRA levels, results in significantly better patient survival. Therefore transplant nephrectomy should be considered in all patients awaiting a kidney re-transplantation.
Kidney transplantation is the treatment of choice for patients with end-stage renal disease. Despite excellent results, the half-live after deceased or living donor transplantation was 8.8 and 11.9 years after transplantation in 2005 in the United States, the management of patients with graft failure is still under debate. Some authors favor removal of the non viable kidney to prevent complications such as infection or chronic inflammatory response, others recommend to leave the nonfunctioning kidney in order to prevent surgery associated complications and a rise in panel reactive antibodies.
There are many studies showing that panel reactive antibodies rise after the removal of a non viable kidney transplant. The long-term outcome concerning morbidity and mortality of patients as well as the outcome of a second kidney transplant after graft nephrectomy remains unclear. Prior studies demonstrated controversial results regarding complication rates and mortality with or without nephrectomy in patients staying on dialysis after graft failure or undergoing secondary renal transplantation.
Nephrectomy of a non-viable kidney graft leads to a temporary increase in panel reactive antibodies (PRA) level which equalizes before the time of retransplantation. Graft survival after a second kidney transplantation is not influenced by nephrectomy of the first graft. If nephrectomy is not performed, re-transplantation should be undertaken within one year after graft loss due to significantly better graft survival rates. Most importantly, patient survival one or five years after a second kidney transplantation is significantly better in patients undergoing nephrectomy of the first failed graft.
The study results suggest that in patients with graft failure nephrectomy should be considered due to a better patient survival after a second renal transplantation.
Kidney or renal transplantation is the process of transferring a kidney of a deceased or living donor to a patient with end-stage renal disease, including not only the surgical procedure but also the immunological management. Graft survival is the rate of kidney transplants that remains with good function after a certain time period. PRA are pre-existing antibodies against cell proteins, which present, if elevated, a risk factor for rejection after organ transplantation.
The article aims to determine the impact of transplant nephrectomy on peak panel reactive antibody levels and patient and graft survival in kidney re-transplants. It is conducted as a retrospective study in a large patient cohort and with a long follow-up. The article is very interesting for anybody involved in the care of renal transplant patients since it offers new insights into in the dilemma of management of patients with graft loss and the usefulness of transplant nephrectomy prior to re-transplantation.
P- Reviewers: D'Elia C, Markic D, Martins LSS S- Editor: Wen LL L- Editor: A E- Editor: Wu HL
| 1. | Magee JC, Barr ML, Basadonna GP, Johnson MR, Mahadevan S, McBride MA, Schaubel DE, Leichtman AB. Repeat organ transplantation in the United States, 1996-2005. Am J Transplant. 2007;7:1424-1433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 2. | Rao PS, Schaubel DE, Jia X, Li S, Port FK, Saran R. Survival on dialysis post-kidney transplant failure: results from the Scientific Registry of Transplant Recipients. Am J Kidney Dis. 2007;49:294-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 3. | Rao PS, Schaubel DE, Wei G, Fenton SS. Evaluating the survival benefit of kidney retransplantation. Transplantation. 2006;82:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 99] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 4. | Ojo A, Wolfe RA, Agodoa LY, Held PJ, Port FK, Leavey SF, Callard SE, Dickinson DM, Schmouder RL, Leichtman AB. Prognosis after primary renal transplant failure and the beneficial effects of repeat transplantation: multivariate analyses from the United States Renal Data System. Transplantation. 1998;66:1651-1659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 201] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Johnston O, Rose C, Landsberg D, Gourlay WA, Gill JS. Nephrectomy after transplant failure: current practice and outcomes. Am J Transplant. 2007;7:1961-1967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Rosenthal JT, Peaster ML, Laub D. The challenge of kidney transplant nephrectomy. J Urol. 1993;149:1395-1397. [PubMed] |
| 7. | Secin FP, Rovegno AR, del Rosario Brunet M, Marrugat RE, Dávalos Michel M, Fernandez H. Cumulative incidence, indications, morbidity and mortality of transplant nephrectomy and the most appropriate time for graft removal: only nonfunctioning transplants that cause intractable complications should be excised. J Urol. 2003;169:1242-1246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 8. | Martínez Urrutia MJ, López Pereira P, Perdiguero M, Gutiérrez E, Gar Jaureguizar Monereo E. [Problems associated with nephrectomy of transplanted kidneys]. Cir Pediatr. 1995;8:151-154. [PubMed] |
| 9. | O’Sullivan DC, Murphy DM, McLean P, Donovan MG. Transplant nephrectomy over 20 years: factors involved in associated morbidity and mortality. J Urol. 1994;151:855-858. [PubMed] |
| 10. | Gregoor PJ, Kramer P, Weimar W, van Saase JL. Infections after renal allograft failure in patients with or without low-dose maintenance immunosuppression. Transplantation. 1997;63:1528-1530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 39] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | López-Gómez JM, Pérez-Flores I, Jofré R, Carretero D, Rodríguez-Benitez P, Villaverde M, Pérez-García R, Nassar GM, Niembro E, Ayus JC. Presence of a failed kidney transplant in patients who are on hemodialysis is associated with chronic inflammatory state and erythropoietin resistance. J Am Soc Nephrol. 2004;15:2494-2501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Casali R, Simmons RL, Ferguson RM, Mauer MM, Kjellstrand CM, Buselmeier TJ, Najarian JS. Factors related to success or failure of second renal transplants. Ann Surg. 1976;184:145-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Cacciarelli T, Sumrani N, Delaney V, Hong JH, DiBenedetto A, Sommer BG. The influence of delayed renal allograft function on long-term outcome in the cyclosporine era. Clin Nephrol. 1993;39:335-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Chiverton SG, Murie JA, Allen RD, Morris PJ. Renal transplant nephrectomy. Surg Gynecol Obstet. 1987;164:324-328. [PubMed] |
| 15. | Zargar MA, Kamali K. Reasons for transplant nephrectomy: a retrospective study of 60 cases. Transplant Proc. 2001;33:2655-2656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Douzdjian V, Rice JC, Carson RW, Gugliuzza KK, Fish JC. Renal retransplants: effect of primary allograft nephrectomy on early function, acute rejection and outcome. Clin Transplant. 1996;10:203-208. [PubMed] |
| 17. | Abouljoud MS, Deierhoi MH, Hudson SL, Diethelm AG. Risk factors affecting second renal transplant outcome, with special reference to primary allograft nephrectomy. Transplantation. 1995;60:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 56] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Gill JS, Abichandani R, Kausz AT, Pereira BJ. Mortality after kidney transplant failure: the impact of non-immunologic factors. Kidney Int. 2002;62:1875-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 160] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Gjertson DW. A multi-factor analysis of kidney regraft outcomes. Clin Transpl. 2002;335-349. [PubMed] |
| 20. | Miles CD, Schaubel DE, Jia X, Ojo AO, Port FK, Rao PS. Mortality experience in recipients undergoing repeat transplantation with expanded criteria donor and non-ECD deceased-donor kidneys. Am J Transplant. 2007;7:1140-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Howard RJ, Reed AI, Van Der Werf WJ, Hemming AW, Patton PR, Scornik JC. What happens to renal transplant recipients who lose their grafts? Am J Kidney Dis. 2001;38:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Kaplan B, Meier-Kriesche HU. Death after graft loss: an important late study endpoint in kidney transplantation. Am J Transplant. 2002;2:970-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 208] [Cited by in RCA: 221] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 23. | Ayus JC, Achinger SG, Lee S, Sayegh MH, Go AS. Transplant nephrectomy improves survival following a failed renal allograft. J Am Soc Nephrol. 2010;21:374-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 113] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 24. | Surga N, Viart L, Wetzstein M, Mazouz H, Collon S, Tillou X. Impact of renal graft nephrectomy on second kidney transplant survival. Int Urol Nephrol. 2013;45:87-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 25. | Bryan CF, Baier KA, Nelson PW, Luger AM, Martinez J, Pierce GE, Ross G, Shield CF, Warady BA, Aeder MI. Long-term graft survival is improved in cadaveric renal retransplantation by flow cytometric crossmatching. Transplantation. 1998;66:1827-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Mahoney RJ, Norman DJ, Colombe BW, Garovoy MR, Leeber DA. Identification of high- and low-risk second kidney grafts. Transplantation. 1996;61:1349-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Scornik JC. The flow cytometry crossmatch in second kidney grafts. Transplantation. 1996;62:1698-1700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Khakhar AK, Shahinian VB, House AA, Muirhead N, Hollomby DJ, Leckie SH, McAlister VC, Chin JL, Jevnikar AM, Luke PP. The impact of allograft nephrectomy on percent panel reactive antibody and clinical outcome. Transplant Proc. 2003;35:862-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 29. | Schleicher C, Wolters H, Kebschull L, Anthoni C, Suwelack B, Senninger N, Mersfeld B, Palmes D. Impact of failed allograft nephrectomy on initial function and graft survival after kidney retransplantation. Transpl Int. 2011;24:284-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 30. | Lucarelli G, Vavallo A, Bettocchi C, Losappio V, Gesualdo L, Grandaliano G, Selvaggi FP, Battaglia M, Ditonno P. Impact of transplant nephrectomy on retransplantation: a single-center retrospective study. World J Urol. 2013;31:959-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Lucchiari N, Panajotopoulos N, Xu C, Rodrigues H, Ianhez LE, Kalil J, Glotz D. Antibodies eluted from acutely rejected renal allografts bind to and activate human endothelial cells. Hum Immunol. 2000;61:518-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 41] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Martin L, Guignier F, Bocrie O, D’Athis P, Rageot D, Rifle G, Justrabo E, Mousson C. Detection of anti-HLA antibodies with flow cytometry in needle core biopsies of renal transplants recipients with chronic allograft nephropathy. Transplantation. 2005;79:1459-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 33. | Pérez Martínez J, Gallego E, Juliá E, Llamas F, López A, Palao F, Lorenzo I, López E, Illescas ML, Gómez Roldán C. Embolization of non-functioning renal allograft: efficacy and control of systemic inflammation. Nefrologia. 2005;25:422-427. [PubMed] |
| 34. | Solinas A, De Giorgi F, Frongia M. Ablation of non functioning renal allograft by embolization: a valid alternative to graft nephrectomy? Arch Ital Urol Androl. 2005;77:99-102. [PubMed] |
| 35. | Morales A, Gavela E, Kanter J, Beltrán S, Sancho A, Escudero V, Crespo J, Pallardó LM. Treatment of renal transplant failure. Transplant Proc. 2008;40:2909-2911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 36. | Lair D, Coupel S, Giral M, Hourmant M, Karam G, Usal C, Bignon JD, Brouard S, Soulillou JP. The effect of a first kidney transplant on a subsequent transplant outcome: an experimental. Kidney International. 2005;67:2368-2375. [RCA] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |