Published online Dec 24, 2013. doi: 10.5500/wjt.v3.i4.99
Revised: November 15, 2013
Accepted: December 9, 2013
Published online: December 24, 2013
Processing time: 124 Days and 17.9 Hours
Hematopoietic stem cell transplant (HSCT) is a standard treatment for many hematological malignancies. Three different sources of stem cells, namely bone marrow (BM), peripheral blood stem cells (PBSC) and cord blood (CB) can be used for HSCT, and each has its own advantages and disadvantages. Randomized controlled trials (RCTs) suggest that there is no significant survival advantage of PBSC over BM in Human Leukocyte Antigen-matched sibling transplant for adult patients with hematological malignancies. PBSC transplant probably results in lower risk of relapse and hence better disease-free survival, especially in patients with high risk disease at the expense of higher risks of both severe acute and chronic graft-versus-host disease (GVHD). In the unrelated donor setting, the only RCT available suggests that PBSC and BM result in comparable overall and disease-free survivals in patients with hematological malignancies; and PBSC transplant results in lower risk of graft failure and higher risk of chronic GVHD. High level evidence is not available for CB in comparison to BM or PBSC. The risks and benefits of different sources of stem cells likely change with different conditioning regimen, strategies for prophylaxis and treatment of GVHD and manipulation of grafts. The recent success and rapid advance of double CB transplant and haploidentical BM and PBSC transplants further complicate the selection of stem cell source. Optimal selection requires careful weighing of the risks and benefits of different stem cell source for each individual recipient and donor. Detailed counseling of patient and donor regarding risks and benefits in the specific context of the patient and transplant method is essential for informed decision making.
Core tip: Randomized controlled trials (RCTs) suggest no difference in survival between peripheral blood stem cell (PBSC) and bone marrow (BM) in matched sibling transplant for patients with hematological malignancies. PBSC may result in fewer relapse in high risk patients but more severe graft-versus-host disease (GVHD). For unrelated donor, the only RCT suggests PBSC and BM result in comparable survivals, with PBSC resulting in fewer graft failure but more chronic GVHD. RCT is not available to compare cord blood with BM or PBSC. The risks and benefits of different sources of stem cells likely change with transplant methods and manipulation of grafts.
- Citation: Cheuk DK. Optimal stem cell source for allogeneic stem cell transplantation for hematological malignancies. World J Transplant 2013; 3(4): 99-112
- URL: https://www.wjgnet.com/2220-3230/full/v3/i4/99.htm
- DOI: https://dx.doi.org/10.5500/wjt.v3.i4.99
Hematopoietic stem cell transplantation (HSCT) is now established as a standard therapeutic modality for a variety of malignant and non-malignant diseases. The first successful allogeneic HSCT was done with bone marrow (BM) as the source of hematopoietic stem cells in 1968[1]. In the subsequent 2 decades only bone marrow was used as the source of stem cells for transplantation. In the 1960s, experiments have shown that peripheral blood contains a small number of stem cells[2], which can be enriched by pre-treatment with certain chemotherapeutic drugs and hematopoietic growth factors[3-5]. Therefore mobilized peripheral blood stem cells (PBSC) became another stem cell source for HSCT and PBSC has been increasingly used as it has certain advantages compared with BM. In 1978, cord blood (CB) was found to be a rich source of stem cells[6] and was later successfully used for allogeneic HSCT[7] at a lower cell dose infused compared with BM or PBSC.
Nowadays transplant physicians are faced with 3 viable choices of stem cells for allogeneic HSCT, namely BM, PBSC and CB and clinicians have to face the challenges of selecting the optimal stem cell source. Although all 3 sources of stem cells are capable of reconstituting the hematopoietic system in recipient after transplant, they have many inherent differences in cellular constituents and biological and immunological properties. In this article we shall review the advantages and disadvantages of different sources of stem cells and the available clinical evidence that helps clinicians to make decision.
Although BM, PBSC and CB all contain hematopoietic stem cells, other constituents present in the harvest products before additional manipulation are quite different. Compared with unmanipulated BM, Granulocyte colony stimulating factor-mobilized PBSC and cord blood contain significantly lower amount of red blood cells (RBC) and plasma. This has certain impact on the choice of stem cell source when there is mismatch in blood group between the donor and the recipient, as harvested donor BM must be processed to deplete RBC or plasma or both before infusion to recipient. However, depletion of RBC or plasma is not required for PBSC or cord blood transplants even when blood group is mismatched, as the relatively low amount of RBC and RBC antibodies present in these products are unlikely to cause significant hemolysis. Another important difference among the sources of stem cell is the amount of mature T cells present. PBSC usually contains a lot more mature T cells compared to BM, which in turn contains more T cells compared to CB, and this partly explains the differences in the risk of graft rejection and graft-versus-host disease (GVHD). Depletion of T cells is associated with increased risk of graft rejection and disease relapse, but lower risk of GVHD. The comparison of the characteristics of the 3 different sources of stem cells is presented in Table 1.
| BM | PBSC | CB | |
| Typical time frame from initiation of search to transplantation | 3-6 mo | 3-6 mo | 2-4 wk |
| Usual volume | 500-2000 mL | 50-300 mL | 25-150 mL |
| Adverse effects for donor | Risks of wound infection, bleeding, general anesthesia, etc. | Risks of bleeding, infection, thrombosis, hypotension, electrolyte disturbance, etc. | No |
| Minimal cell dose for transplant | Total nucleated cell: 2 x 108/kg | Total CD34+ cell: 2 x 106/kg | Total nucleated cell: 2.5 x 107/kg |
| Red blood cell content | High | Low | Low |
| Possibility to give additional stem cell dose | Possible | Possible | Impossible |
| Exposure to dimethyl sulfoxide | No if fresh | No if fresh | Yes |
| HLA matching requirement | More stringent (7-8 out of 8 matched) | More stringent (7-8 out of 8 matched) | Less stringent (4-6 out of 6 matched) |
| Speed of neutrophil engraftment | About 3 wk | About 2 wk | About 4 wk |
| Speed of immune reconstitution | Faster | Faster | Slower |
| Risk of graft-versus-host disease | Medium | Highest | Lowest |
| Risk of post-transplant infections | Lower | Lower | Higher |
| Risk of latent virus transmission | Higher | Higher | Lower |
| Possibility of CMV transmission | Higher as most donors are CMV seropositive | Higher as most donors are CMV seropositive | Lower as most CB units do not harbor CMV |
| Risk of relapse for high risk patients | Higher | Lower | Higher |
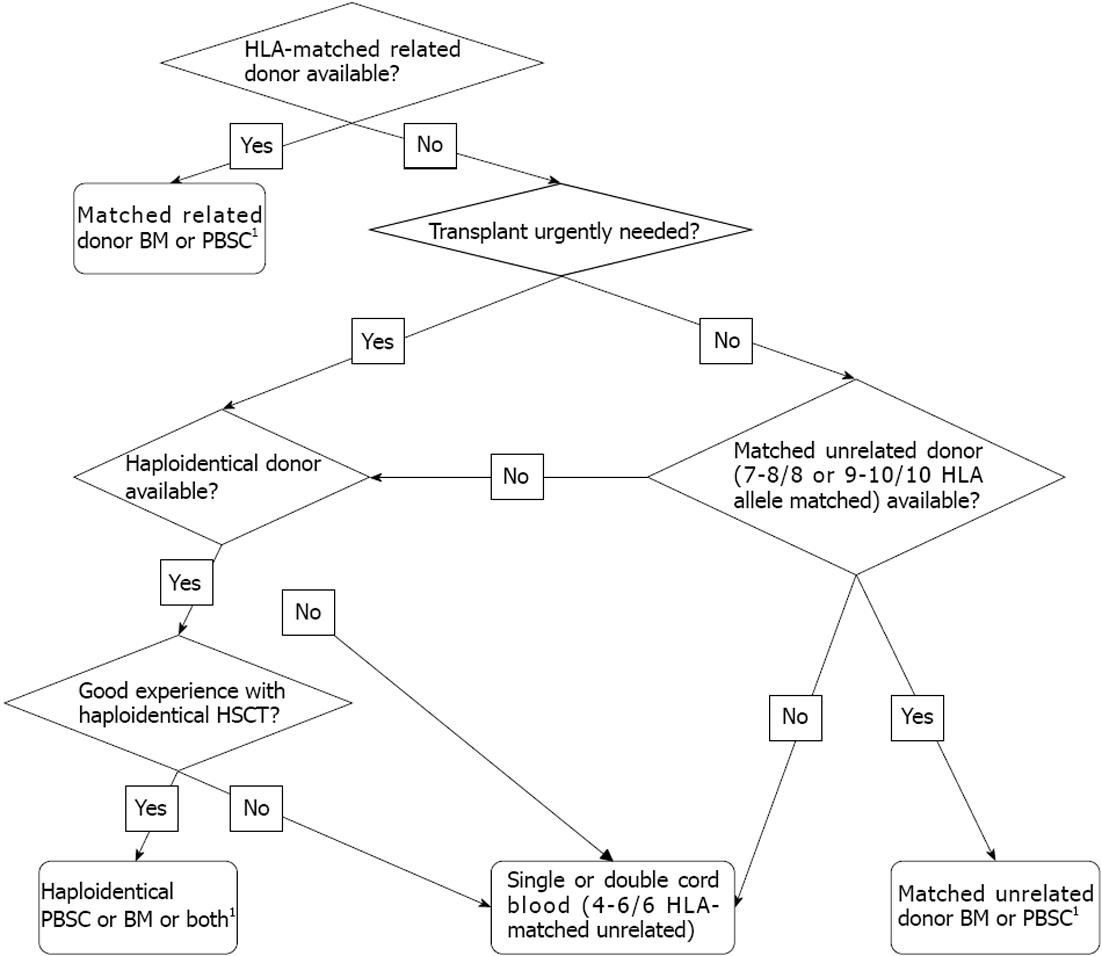
We often have to consider and weigh the relative benefits and risks before decision on the source of stem cells for allogeneic HSCT. The selection of stem cell source is often intertwined with the selection of donor. A suggested algorism for selection of donor and stem cell source is given in Figure 1. One of the basic considerations for allogeneic HSCT is whether a Human Leukocyte Antigen (HLA)-matched related donor is available. Although currently results of unrelated donor transplants of many transplant centres are similar to that of matched related donor transplants, the latter is still considered the first choice for most allogeneic HSCTs, as the donor is readily available for initial donation and subsequent back-up, and might be associated with a lower risk of GVHD and transplant-related mortality (TRM). Therefore, if a matched related donor is available, the choice of stem cell source is simpler and often remains BM versus PBSC, as related donor CB is unlikely to be available. The transplant physician has to weigh the risks and benefits to both the donor and the recipient, explain the different procedures and experiences of stem cell collection to the donor and help the donor to make informed choices. Clinical evidence on different outcomes of recipients transplanted with BM or PBSC presented below will form important basis for the selection.
The donor’s perspective should be given due consideration. A prospective study on donors’ experience of BM or PBSC donation found that before donation, BM donors had lower confusion, fewer concerns, and were more prepared for donation compared with PBSC donors[8]. Shortly after donation, BM donors experienced more physical side effects than PBSC donors[8]. BM donors also reported greater impact on their social activities, but had better psychological status and were more likely to indicate that the donation made their lives more meaningful[8]. However, there were no significant longer-term differences between BM and PBSC donors including recovery time[8].
In case HLA-matched related donor CB with adequate cell dose is available, relative benefits and risks of CB in comparison to BM or PBSC also need to be considered. If an HLA-matched related donor is not available; we have to find an alternative donor, the choice of which often includes mismatched family donor (including HLA-haploidentical donor), unrelated donor, or unrelated CB. The selection usually depends heavily on the urgency of transplant, HLA matching and cell dose of CB available, and preference and experience of the transplant centre. Unrelated CB and mismatched family donor (BM or PBSC) are usually more readily available compared to unrelated donor and therefore if transplant needs to be done urgently, CB or mismatched family donor is sometimes preferable. If HSCT is not urgently required, unrelated donor BM or PBSC should be given due consideration. Since the requirement for HLA matching is less stringent for unrelated CB compared to BM or PBSC, unrelated CB is preferable to unrelated donor BM or PBSC if no 7-8/8 allele-matched unrelated donor (or 9-10/10 allele-matched unrelated donor which may be associated with even lower risks of TRM and GVHD) is available, provided that the CB is at least 4/6 HLA-matched with adequate cell dose. If there is no single CB with sufficient cell dose, use of double CB can be considered. If transplant is not urgently required and both good matched unrelated donor and unrelated CB with adequate cell dose are available, other considerations prevail, including the preference and experience of the transplant centre, the patient’s disease status, the speed of engraftment, risks of infections and GVHD, age, gender and location of donor, ABO blood group matching, and cytomegalovirus (CMV) status, etc. If the recipient is CMV seronegative, CB transplant might be preferred as it is less likely to transmit CMV infection and CMV seronegative donor might not be easily available. Good clinical evidence guiding selection of stem cells for HSCT in patients with hematological malignancies is summarized in the following section.
There were a number of randomized controlled trials (RCTs) comparing PBSC and BM as stem cell source in transplants using HLA-matched related donor for patients with hematological malignancies. They are summarized in Table 2. There were no clinical trials comparing HLA-matched related CB with either BM or PBSC.
| Ref. | No.ofpatients | Age of patients (yr) | Underlying diseases | Conditioning | Overall survival (BM vs PBSC) | Disease-free survival (BM vs PBSC) | Relapse (BM vs PBSC) | Transplant-related mortality (BM vs PBSC) | Acute graft-versus host disease (BM vs PBSC) | Chronic graft-versus host disease (BM vs PBSC) | Median time of neutrophil engraftment (d) (BM vs PBSC) | Median time of platelet engraftment (d) (BM vs PBSC) |
| [23,29] | 56 | 7-59 | Acute leukemias, CML, MDS, MM, NHL | Myeloablative1 | 48% vs 56%2 (2000 d) | 50 vs 60%2 (2000 d) | NA | NA | 23% vs 26%2 (grades 2-4) | 61% vs 77%2 (extensive cGVHD) | 18 vs 15a | 18 vs 12b |
| [9] | 39 | 22-51 | Acute leukemias, CML, CLL, MDS, MM, NHL | Myeloablative1 | 63% vs 70%2 (2 yr) | NA | 37% vs 0%a (2 yr) | 32% vs 35%2 | 58% vs 68%2 (grades 1-4) | 40% vs 44%2 (All cGVHD) | 23 vs 17.5b | 18 vs 11d |
| [25] | 61 | 15-62 | Acute leukemias, CML, MDS, PMF | Bu/Cy | 73% vs 80%2 (4 yr) | 55% vs 80%2 (4 yr) | 30% vs 3%2 | 10% vs 17%2 | 10% vs 21%2 (grades 2-4) | 27% vs 56%2 (All cGVHD) | 23 vs 17d | 21 vs 13d |
| [10,19] | 101 | Mean 37 | Acute leukemias, CML | Myeloablative1 | 65% vs 67%2 (2 yr) | 66% vs 67%2 (2 yr) | 15% vs 6%2 | 21% vs 25%2 | 42% vs 44%2 (grades 2-4) | 36% vs 65%b (All cGVHD) 17% vs 44%b (extensive cGVHD) | 21 vs 153 | 21 vs 13d |
| [15-18] | 329 | 19-58 | Acute leukemias, CML, MDS | Myeloablative1 | 65% vs 65%2 (2 yr) 65% vs 58%2 (3 yr) 57% vs 49%2 (10 yr) | 60% vs 56%2 (3 yr) 46% vs 42%2 (10 yr) | 24% vs 20%2 (10 yr) | 32% vs 24%2 | 42% vs 44%a (grades 2-4) | 56% vs 74%b (All cGVHD) 19% vs 36%b (extensive cGVHD) | 15 vs 12d | 20 vs 15d |
| [12,13,20] | 172 | 12-55 | Acute leukemias, CML, CLL, MDS, MM, lymphomas | Myeloablative1 | 54% vs 66%2 (2 yr) 52% vs 55%2 (10 yr) | 45% vs 65%a (2 yr) 40% vs 50%a (10 yr) | 25% vs 14%a (2 yr) 32% vs 20%a (10 yr) | 30% vs 21%2 | 57% vs 64%2 (grades 2-4) | 52% vs 63%2 (extensive cGVHD) | 21 vs 16d | 19 vs 13d |
| [11] | 227 | 19-64 | AML, CML, MDS | Bu/Cy | 60% vs 68%a (30 mo) | NA | 9% vs 9%2 | 32% vs 21%2 | 44% vs 44%2 (grades 2-4) | 69% vs 85%2 (All cGVHD) 30% vs 40%2 (extensive cGVHD) | 23 vs 19d | 22 vs 16d |
| [30] | 110 | 15-62 | Acute leukemias, MDS, MM, lymphomas | Myeloablative1 | 60% vs 34%a (4 yr) | NA | 13% vs 18%2 | 28% vs 41%2 | 37% vs 52%2 (grades 2-4) | 45% vs 61%2 (All cGVHD) 16% vs 28%2 (extensive cGVHD) | 20 vs 15d | 38 vs 25d |
| [14] | 72 | 18-61 | CML | Myeloablative1 | 72% vs 81%2 (3 yr) | 65% vs 81%2 (3 yr) | 15% vs 0%a (3 yr) | 20% vs 19%2 | 49% vs 55%2 (grades 2-4) | 50% vs 59%2 (extensive cGVHD) | 22 vs 17a | 21 vs 142 |
Most of the RCTs comparing matched related donor BM and PBSC transplantation for patients with hematological malignancies found no significant differences between the two stem cell source in important outcomes including overall survival, disease-free survival, transplant-related mortality, relapse, acute GVHD and chronic GVHD. However, all trials showed significantly faster neutrophil engraftment in PBSC transplants, and all but one trial showed significantly faster platelet engraftment in PBSC transplants, which may result in earlier hospital discharge for PBSC recipients[9,10] and lower cost for PBSC transplantation[10]. Lymphocyte recovery was also found to be better in the PBSC group in one trial[9].
There was one trial showing significantly better overall survival at 30 mo in patients who received PBSC compared with BM[11]. Yet another trial showed opposite result, with better overall survival in BM recipients. However, in this trial CD34 selection was done before stem cell infusion in both BM and PBSC products and PBSC recipients happened to receive more CD34+ cells and T cells. Overall survival at 4 years was significantly worse in the PBSC group compared with the BM group, largely due to increased GVHD and TRM in PBPC recipients receiving T-cells greater than 2 × 105/kg. Acute GVHD appeared strongly associated with increased TRM. Higher number of CD34+ cells was associated with less TRM.
Some trials showed significantly higher probability of relapse in BM recipients than in PBSC recipients[9,12-14], which might translate into better disease-free survival in PBSC transplants compared with BM transplants[12,13]. The differences in disease-free survival appeared more pronounced among patients with higher risk malignancies[12]. “High risk” or “late stage” hematological malignancies usually include patients with acute leukemia in second or later remission, CML in blastic transformation, refractory anemia with excess of blasts in transformation, and lymphoma heavily pretreated with chemotherapy or autologous transplants.
Some trials showed PBSC recipients had significantly more grade 2-4 acute GVHD[15-18], chronic GVHD[15-19] and extensive chronic GVHD[15-19] compared with BM recipients, which resulted in significantly more patients who underwent PBPC transplant needed immunosuppressive treatment[18,20], and longer periods of corticosteroid use and hospitalization[19]. There was no difference in performance status, return to work, incidence of bronchiolitis obliterans, hematopoietic function, and secondary malignancies between the two groups in the long term in one trial[18]. In contrast, another trial showed that late mortality due to chronic GVHD was more frequent in PBSC recipients compared with BM recipients[13].
There were 2 more RCTs that included a few patients with severe aplastic anemia in addition to patients with hematological malignancies[21,22]. One small trial of 30 patients found that PBSC transplant resulted in significantly faster hematopoietic reconstitution, fewer days with neutropenic fever, shorter hospital stay and fewer acute GVHD (6.7% vs 46.7%)[21]. Another trial of 57 patients found that the PBSC and the BM groups had similar overall survival at 18 mo (64% vs 67%), speed to neutrophil and platelet engraftment, and grade 2-4 acute GVHD (54% vs 52%)[22]. However, PBSC transplant resulted in significantly more steroid refractory acute GVHD (32% vs 0%), chronic GVHD (90% vs 47%), extensive chronic GVHD (80% vs 22%) and longer requirement for immunosuppressive therapy[22].
A meta-analysis of 5 RCTs[9-12,16,23] showed that PBSC transplant had significantly higher risk of acute GVHD (RR = 1.23, 95%CI: 1.05-1.45) and chronic GVHD (RR = 1.37, 95%CI: 1.08-1.74) compared with BM transplant[24]. A newer meta-analysis of 7 of RCTs[9-12,16,23,25] showed no difference in mortality between PBSC and BM transplants (OR = 0.81, 95%CI: 0.62-1.05)[26]. However, mortality was significantly lower in PBSC recipients compared with BM recipients in studies that included more patients with intermediate or advanced disease (OR = 0.64, 95%CI: 0.45-0.91)[26]. Subgroup analysis revealed no significant association between mortality and CD34+ cell dose[26].
Another meta-analysis of individual data of 1111 patients from 9 RCTs (both published and unpublished) found that there was no significant difference in overall survival between the PBSC and the BM groups but disease-free survival was significantly higher in the PBSC group (OR = 0.80, 95%CI: 0.67-0.97)[27]. Subgroup analyses showed that both overall survival (OR = 0.64, 95%CI: 0.46-0.90) and disease-free survival (OR = 0.63, 95%CI: 0.45-0.87) were significantly better in patients with late stage disease who received PBSC compared with BM[27]. PBSC transplant led to significantly faster neutrophil engraftment (OR = 0.31, 95%CI: 0.25-0.38) and platelet engraftment (OR = 0.52, 95%CI: 0.44-0.61) compared with BM transplant[27]. PBSC transplant was associated with a significant increase in grade 3-4 acute GVHD (OR = 1.39, 95%CI: 1.03-1.88), chronic GVHD (OR = 1.92, 95%CI: 1.47-2.49), and extensive chronic GVHD (OR = 1.89, 95%CI: 1.47-2.42), but a significant decrease in relapse (OR = 0.71, 95%CI: 0.54-0.93) in both late stage disease (OR = 0.59, 95%CI: 0.38-0.93) and early stage disease (OR = 0.69, 95%CI: 0.49-0.98)[27]. Non-relapse mortality was not significantly different between the PBSC and the BM groups[27]. A decision analysis based on meta-analysis results[27] demonstrated the superiority of PBSC over BM in both overall and quality-adjusted life expectancy[28]. However, BM was found to be the more appropriate strategy if the 1-year relapse probability was below 5%[28].
The most recent meta-analysis which included 11 RCTs[9-11,14,18,20-22,25,29,30] found that PBSC and BM transplants had comparable overall survival (HR = 1.06, 95%CI: 0.81-1.39), disease-free survival (HR =1.04, 95%CI: 0.83-1.30), and TRM (HR = 1.08, 95%CI: 0.56-2.10)[31]. PBSC transplant resulted in significantly better neutrophil engraftment (HR = 2.08, 95%CI: 1.80-2.42) and platelet engraftment (HR = 2.77, 95%CI: 1.78-4.30), but significantly more grade 2-4 acute GVHD (HR = 0.75, 95%CI: 0.63-0.90), grade 3-4 acute GVHD (HR = 0.63, 95%CI: 0.47-0.84), chronic GVHD (HR = 0.70, 95%CI: 0.59-0.83), and extensive chronic GVHD (HR = 0.60, 95%CI: 0.39-0.91). PBSC recipients had significantly lower incidence of relapse (HR = 1.91, 95%CI: 1.34-2.74). A significant inverse relationship was observed between acute GVHD and overall survival.
There was an RCT comparing PBSC and BM transplants using HLA-matched unrelated donors after myeloablative or reduced intensity conditioning in 551 patients with hematological malignancies. There was no significant difference between the PBSC and the BM groups in 2-year overall survival (51% vs 46%), 2-year disease-free survival, relapse, or acute GVHD[32]. However, PBSC transplant resulted in significantly lower risk of graft failure (3% vs 9%) and higher risk of chronic GVHD (53% vs 41%), especially extensive chronic GVHD (48% vs 32%)[32]. However, another recent non-randomized study found that children who received PBSC or BM did not differ significantly in the incidence of acute and chronic GVHD, which might be related to the use of anti-thymocyte globulin as GVHD prophylaxis[33]. The result indicates that more intensive GVHD prophylaxis is required in PBSC transplant and this might abrogate the difference in GVHD risk between PBSC and BM transplants.
There was no RCT comparing unrelated CB with either BM or PBSC but many non-randomized comparative studies were available. In a meta-analysis[34] of 10 non-randomized clinical trials[35-44] comparing unrelated BM and unrelated CB for HSCT in children and adults with malignant and non-malignant hematological diseases, it was found that BM transplant resulted in significantly better overall survival (HR = 1.28, 95%CI: 1.13-1.44) and TRM (RR = 1.28, 95%CI: 1.03-1.58)[34]. However, CB transplant resulted in significantly lower grade 2-4 acute GVHD (RR = 0.73, 95%CI: 0.64-0.82) and chronic GVHD (RR = 0.70, 95%CI: 0.51-0.97) compared with BM transplant[34]. There was no significant difference in the risk of relapse.
There was a large non-randomized study not included in the above meta-analysis comparing unrelated CB with BM and PBSC in 1525 patients with acute leukemia[45]. Leukemia-free survival in CB transplant was comparable with that after 7-8/8 allele-matched BM or PBSC transplant[45]. However, TRM was significantly higher after CB transplant than after 8/8 allele-matched BM transplant (HR = 1.69, 95%CI: 1.19-2.39) or PBPC transplant (HR = 1.62, 95%CI: 1.18-2.23)[45]. Grade 2-4 acute and chronic GVHD were significantly lower in CB recipients compared with 7-8/8 allele-matched PBPC recipients (HR = 0.57, 95%CI: 0.42-0.77 and HR = 0.38, 95%CI: 0.27-0.53, respectively)[45]. Chronic but not acute GVHD was significantly lower after CB transplant than after 8/8 allele-matched BM transplant (HR = 0.63, 95%CI: 0.44-0.90)[45]. There was no difference among the stem cell sources in the rate of relapse[45].
One comparative study performed disease-specific analysis of the difference between CB transplant and BM transplant in 484 patients with AML and 336 patients with ALL after myeloablative conditioning[44]. Among AML patients, CB recipients had significantly lower overall survival (HR 1.5, 95%CI: 1.0-2.0) and leukemia-free survival (HR = 1.5, 95%CI: 1.1-2.0) compared with BM recipients[44]. TRM and relapse did not differ significantly[44]. Among ALL patients, there was no significant difference between the groups in overall survival, leukemia-free survival, TRM, and relapse[44].
Another study compared unrelated CB transplants with unrelated donor BM or PBSC transplants in adults with ALL in first or second complete remission[46]. This study found no significant differences in the 3-year overall survival between CB (44%), matched (44%) and mismatched (43%) unrelated donor transplants. CB transplants had significantly slower engraftment and less grade 2-4 acute but similar chronic GVHD, disease-free survival, TRM, and relapse[46].
In case a single CB unit has insufficient cell dose, 2 CB units can be used, but both are preferably at least 4/6 HLA-matched with the recipient and with each other, and together provide sufficient cell dose. Non-randomized studies comparing double CB transplant with single CB transplant in patients with hematological malignancies usually found that double CB transplant was associated with higher incidence of grade 2 acute GVHD[47-52] and lower incidence of leukemia relapse[48-51,53-55], but there was no significant difference in overall survival, disease-free survival, chronic GVHD and engraftment times[50,52,53,56-59]. However, recently one study found superior overall survival and disease-free survival in addition to lower relapse in patients who received double CB compared with single CB transplant, although TRM and chronic GVHD were not significantly different[60]. Double CB transplant was also found to be more cost-effective in terms of quality adjusted life years in adults with acute leukemia in first remission in France[60]. On the other hand, intrabone injection of single CB might be associated with faster engraftment (median 23 vs 28 d) and lower cumulative incidence of relapse (25% vs 29%) compared with intravenous double CB transplant[61].
There were some non-randomized studies comparing double CB transplant with BM or PBSC transplant from other donors. One study on 536 patients with hematological malignancies transplanted with myeloablative conditioning found that 5-year leukemia-free survival was similar in double CB transplant (51%) and other types of donors (either BM or PBSC), including matched related donor (33%), matched unrelated donor (48%), and mismatched unrelated donor (38%)[62]. Non-relapse mortality was highest for double CB (34%), compared with matched related donor (24%), matched unrelated donor (14%), or mismatched unrelated donor (27%)[62]. However, the risk of relapse was lowest in recipients of double CB (15%), compared with matched related donor (43%), matched unrelated donor (37%), or mismatched unrelated donor (35%)[62]. The risks of grade 2-4 acute GVHD and chronic GVHD were also the lowest for double CB (60% and 26%), compared with matched related donor (65% and 47%), matched unrelated donor (80% and 43%), or mismatched unrelated donor (85% and 48%)[62].
Another study on 367 patients with hematological malignancies after myeloablative or non-myeloablative conditioning found that 2-year overall survival, progression-free survival, TRM and grade 2-4 acute GVHD were not significantly different in double CB transplant (65%, 55%, 25% and 43%) as compared to related donor transplant (70%, 66%, 15%, and 27%) and unrelated donor transplant (62%, 55%, 27%, and 39%)[63]. However, late acute or chronic GVHD was significantly lower in double CB transplant (28%) as compared to related donor transplant (31%) and unrelated donor transplant (44%)[63].
A third study compared double CB transplant with 9/10 mismatched unrelated donor BM or PBSC transplants with reduced intensity conditioning for patients with hematological malignancies and found that double CB transplant was associated with lower incidence of extensive chronic GVHD at 2 years compared with unrelated donor transplant (6.4% vs 21.4%)[64]. However, both groups were comparable for 2-year overall survival (47.9% vs 52.3%), progression-free survival (43.3% vs 38.3%), TRM (26% vs 24.2%), relapse (34.3% vs 37.6%), grade 3-4 acute GVHD (19.1% vs 21.4%), and neutrophil engraftment time (median 17 vs 16 d)[64].
There were 3 studies comparing double CB transplant with unrelated donor PBSC transplants after reduced intensity conditioning for adult patients with hematological malignancies. The study by Le Bourgeois found that the 2 groups had similar 2-year overall survival (61% vs 62%), disease-free survival (50.5% vs 59.0%), relapse incidence (23.0% vs 35.5%), cumulative incidences of engraftment, grade 2-4 acute and chronic GVHD[65]. However, double CB recipients had significantly higher median time to platelet recovery (38 vs 0 d), early mortality before day +100 (20.5% vs 4.0%), and 2-year TRM (26.5% vs 6.0%) compared with PBSC recipients[65]. The presence of a lymphoid disorder was associated with a significantly higher overall survival[65]. The study by Chen found that the 3-year overall survival and progression-free survival were comparable between double CB and PBSC transplant (46% vs 50% and 30% vs 40%, respectively), but the cumulative incidence of TRM was significantly higher in double CB transplant (26.9% vs 10.4%)[66]. The cumulative incidence of grade 2-4 acute GVHD was not significantly different but the 2-year cumulative incidence of chronic GVHD was significantly lower in double CB transplant compared with PBSC transplant (21.9% vs 53.9%)[66]. The study by Jacobson found that there was no significant difference between double CB transplant and PBSC transplant in 2-year overall survival (66% vs 68%), progression-free survival (49% vs 57%), TRM (11% vs 11%), relapse (40% vs 32%) and grade 2-4 acute GVHD (21% vs 12%)[67]. Double CB recipients had significantly more infections (69% vs 33%), both viral (29% vs 1%) and bacterial (50% vs 8%) infections, but significantly less chronic GVHD (24% vs 54%)[67]. Reconstitution of T cells was significantly delayed in double CB recipients compared with PBSC recipients for 1-6 mo post-transplant, including naive and memory CD4+ T cells, regulatory T cells, and CD8+ T cells[67]. In contrast, B cells recovered more rapidly in double CB recipients and B cell number remained significantly greater at 3-24 mo post-transplant[67]. Natural killer (NK) cells also recovered more rapidly in double CB recipients and remained significantly greater at 1-24 mo post-transplant[67].
HLA-haploidentical related donor is an important alternative if no matched related donor is available[68]. Either PBSC or BM can be the stem cell source for haploidentical transplant. Positive selection of CD34+ stem cells from harvested PBSC and infusion of high doses of stem cells successfully overcame HLA barrier with good engraftment rate and low incidence of GVHD[69-78]. Leukemia-free survivals and relapses were better in transplants performed in larger centers[79], and in transplants with natural killer cell killer immunoglobulin like receptor (KIR) mismatch[80]. However, infection risk was high as immunoreconstitution was slow with purified CD34+ cells. Subsequently, negative stem cell selection with depletion of CD3+ T cells with or without depletion of CD19+ B cells achieved similar success of engraftment without excessive GVHD, with myeloablative or reduced intensity conditioning[81-85]. Immune recovery with this method was notably faster with reduced infections[81,85,86]. Unmanipulated T cell replete PBSC and/or BM products could also achieve reasonably good results with intensive GVHD prophylaxis or post-transplant cyclophosphamide, despite presence of large amount of T cells[87-96]. A non-randomized comparative study of T cell depleted with T cell replete haploidentical transplants for adult patients with hematological malignancies found that T cell replete transplant resulted in significantly better 1-year overall survival (64% vs 30%), progression-free survival (50% vs 21%), lower TRM (16% vs 42%), chronic GVHD (7% vs 18%), and infections, with better reconstitution of T cell subsets[97].
Evolving modifications might further improve outcomes of haploidentical HSCT, such as post-transplant CD8-depleted donor lymphocyte infusion, which could promote immune reconstitution[98]. Post-transplant infusion of regulatory T cells could also promote lymphoid reconstitution with improved immunity to opportunistic pathogens, while preventing GVHD in the absence of any post-transplant immunosuppression, and preserving the graft-versus-leukemia effect[99,100]. Coinfusion of mesenchymal stromal cells could facilitate engraftment without increasing leukemia recurrence after haploidentical HSCT[101,102]. Combining PBSC and BM might also improve engraftment, and reduce TRM[103] and relapse[104]. Suicide-gene-engineered donor lymphocytes might accelerate immune reconstitution while limiting GVHD[105-107]. Selective photodepletion of alloreactive T cells could also enhance immunoreconstitution while preventing GVHD[108]. Ex vivo induction of anergy to recipient alloantigen by costimulation blockade was another strategy to limit GVHD[109]. Depletion of T cell receptor alphabeta positive T cells while retaining gammadelta T cells may reduce GVHD while preserving anti-infective and anti-tumor effects[110]. A two-step approach in which the lymphoid and myeloid portions of the graft are given in two separate steps to control and optimize T cell dosing may further improve results with robust immunoreconstitution, low GVHD and better disease control[111,112].
There are some non-randomized studies comparing haploidentical PBSC or BM transplants with other types of donor or stem cell source. The Blood and Marrow Transplant Clinical Trials Network conducted 2 multicentre trials for patients with leukemia or lymphoma undergoing reduced intensity conditioning allogeneic transplants and found that haploidentical transplant and double CB transplant had comparable 1-year overall survival (62% vs 54%), 1-year progression-free survival (48% vs 46%), neutrophil engraftment (96% vs 94%), and grade 2-4 acute GVHD (32% vs 40%)[113]. One-year cumulative TRM was lower in haploidentical transplant compared with double CB transplant (7% vs 24%), but relapse rate was higher (45% vs 31%)[113].
In conclusion, existing high level evidence suggest that there is no significant advantage of PBSC over BM in HLA-matched sibling transplant for patients with hematological malignancies. PBSC transplant probably results in lower risk of relapse and hence better disease-free survival, especially in patients with high risk or late stage disease at the expense of higher risks of both severe acute and chronic GVHD. Existing data are insufficient or inconclusive for firm conclusions in specific subgroups such as a particular disease entity, conditioning regimen or in children. High level evidence is scarce in the unrelated donor setting. The only RCT available suggests that PBSC and BM result in comparable overall and disease-free survivals in patients with hematological malignancies; and PBSC transplant results in lower risk of graft failure but higher risk of chronic GVHD. High level evidence is lacking for CB in comparison to BM or PBSC. The risks and benefits of different sources of stem cells likely change with different conditioning regimen, strategies for prophylaxis and treatment of GVHD and manipulation of grafts. The recent success and rapid advance of double CB transplant and haploidentical BM and PBSC transplants further complicate the selection of optimal stem cell source. Novel therapies for treatment and prophylaxis of GVHD also minimize the key differences between stem cell sources. Advances in graft manipulation and cellular therapies might change the whole paradigm making stem cell source selection less critical, e.g., stem cell enrichment could facilitate engraftment, specific and highly selective depletion of certain lymphocyte subsets and alloreactive cells could minimize GVHD, infusion of mesenchymal stem cells could facilitate engraftment and reduce GVHD, titrated T cell dosing and NK cell therapy might reduce relapse. Detailed counseling of patient and donor regarding risks and benefits in the specific context of the patient and transplant method is of paramount importance for informed decision making.
P- Reviewers: Goebel WS, Lee ACW, Tommasini A S- Editor: Ma YJ L- Editor: A E- Editor: Yan JL
| 1. | Gatti RA, Meuwissen HJ, Allen HD, Hong R, Good RA. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 715] [Cited by in RCA: 613] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 2. | Körbling M, Freireich EJ. Twenty-five years of peripheral blood stem cell transplantation. Blood. 2011;117:6411-6416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 3. | Richman CM, Weiner RS, Yankee RA. Increase in circulating stem cells following chemotherapy in man. Blood. 1976;47:1031-1039. [PubMed] |
| 4. | Socinski MA, Cannistra SA, Elias A, Antman KH, Schnipper L, Griffin JD. Granulocyte-macrophage colony stimulating factor expands the circulating haemopoietic progenitor cell compartment in man. Lancet. 1988;1:1194-1198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 343] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 5. | Dührsen U, Villeval JL, Boyd J, Kannourakis G, Morstyn G, Metcalf D. Effects of recombinant human granulocyte colony-stimulating factor on hematopoietic progenitor cells in cancer patients. Blood. 1988;72:2074-2081. [PubMed] |
| 6. | Prindull G, Prindull B, Meulen N. Haematopoietic stem cells (CFUc) in human cord blood. Acta Paediatr Scand. 1978;67:413-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Gluckman E, Broxmeyer HA, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174-1178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1444] [Cited by in RCA: 1237] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 8. | Switzer GE, Bruce JG, Harrington D, Haagenson M, Drexler R, Foley A, Confer D, Bishop M, Anderlini P, Rowley S. Health-related quality of life of bone marrow versus peripheral bood stem cell donors: a prespecified subgroup analysis from a phase III RCT-BMTCTN protocol 0201. Biol Blood Marrow Transplant. 2013;Nov 1; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 9. | Powles R, Mehta J, Kulkarni S, Treleaven J, Millar B, Marsden J, Shepherd V, Rowland A, Sirohi B, Tait D. Allogeneic blood and bone-marrow stem-cell transplantation in haematological malignant diseases: a randomised trial. Lancet. 2000;355:1231-1237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 265] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Blaise D, Kuentz M, Fortanier C, Bourhis JH, Milpied N, Sutton L, Jouet JP, Attal M, Bordigoni P, Cahn JY. Randomized trial of bone marrow versus lenograstim-primed blood cell allogeneic transplantation in patients with early-stage leukemia: a report from the Société Française de Greffe de Moelle. J Clin Oncol. 2000;18:537-546. [PubMed] |
| 11. | Couban S, Simpson DR, Barnett MJ, Bredeson C, Hubesch L, Howson-Jan K, Shore TB, Walker IR, Browett P, Messner HA. A randomized multicenter comparison of bone marrow and peripheral blood in recipients of matched sibling allogeneic transplants for myeloid malignancies. Blood. 2002;100:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 235] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Bensinger WI, Martin PJ, Storer B, Clift R, Forman SJ, Negrin R, Kashyap A, Flowers ME, Lilleby K, Chauncey TR. Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N Engl J Med. 2001;344:175-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 763] [Cited by in RCA: 699] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 13. | Mielcarek M, Storer B, Martin PJ, Forman SJ, Negrin RS, Flowers ME, Inamoto Y, Chauncey TR, Storb R, Appelbaum FR. Long-term outcomes after transplantation of HLA-identical related G-CSF-mobilized peripheral blood mononuclear cells versus bone marrow. Blood. 2012;119:2675-2678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 14. | Oehler VG, Radich JP, Storer B, Blume KG, Chauncey T, Clift R, Snyder DS, Forman SJ, Flowers ME, Martin P. Randomized trial of allogeneic related bone marrow transplantation versus peripheral blood stem cell transplantation for chronic myeloid leukemia. Biol Blood Marrow Transplant. 2005;11:85-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Schmitz N, Bacigalupo A, Hasenclever D, Nagler A, Gluckman E, Clark P, Bourquelot P, Greinix H, Frickhofen N, Ringdén O. Allogeneic bone marrow transplantation vs filgrastim-mobilised peripheral blood progenitor cell transplantation in patients with early leukaemia: first results of a randomised multicentre trial of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 1998;21:995-1003. [PubMed] |
| 16. | Schmitz N, Beksac M, Hasenclever D, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, Russell N, Apperley JF, Gorin NC. Transplantation of mobilized peripheral blood cells to HLA-identical siblings with standard-risk leukemia. Blood. 2002;100:761-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 186] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 17. | Schmitz N, Beksac M, Bacigalupo A, Ruutu T, Nagler A, Gluckman E, Russell N, Apperley J, Szerm J, Bradstock K. Filgrastim-mobilized peripheral blood progenitor cells versus bone marrow transplantation for treating leukemia: 3-year results from the EBMT randomized trial. Haematologica. 2005;90:643-648. [PubMed] |
| 18. | Friedrichs B, Tichelli A, Bacigalupo A, Russell NH, Ruutu T, Shapira MY, Beksac M, Hasenclever D, Socié G, Schmitz N. Long-term outcome and late effects in patients transplanted with mobilised blood or bone marrow: a randomised trial. Lancet Oncol. 2010;11:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 19. | Mohty M, Kuentz M, Michallet M, Bourhis JH, Milpied N, Sutton L, Jouet JP, Attal M, Bordigoni P, Cahn JY. Chronic graft-versus-host disease after allogeneic blood stem cell transplantation: long-term results of a randomized study. Blood. 2002;100:3128-3134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 144] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Flowers ME, Parker PM, Johnston LJ, Matos AV, Storer B, Bensinger WI, Storb R, Appelbaum FR, Forman SJ, Blume KG. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100:415-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 301] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 21. | Mahmoud H, Fahmy O, Kamel A, Kamel M, El-Haddad A, El-Kadi D. Peripheral blood vs bone marrow as a source for allogeneic hematopoietic stem cell transplantation. Bone Marrow Transplant. 1999;24:355-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 55] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 22. | Morton J, Hutchins C, Durrant S. Granulocyte-colony-stimulating factor (G-CSF)-primed allogeneic bone marrow: significantly less graft-versus-host disease and comparable engraftment to G-CSF-mobilized peripheral blood stem cells. Blood. 2001;98:3186-3191. [PubMed] |
| 23. | Vigorito AC, Azevedo WM, Marques JF, Azevedo AM, Eid KA, Aranha FJ, Lorand-Metze I, Oliveira GB, Correa ME, Reis AR. A randomised, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of haematological malignancies. Bone Marrow Transplant. 1998;22:1145-1151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 146] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Cutler C, Giri S, Jeyapalan S, Paniagua D, Viswanathan A, Antin JH. Acute and chronic graft-versus-host disease after allogeneic peripheral-blood stem-cell and bone marrow transplantation: a meta-analysis. J Clin Oncol. 2001;19:3685-3691. [PubMed] |
| 25. | Heldal D, Tjønnfjord G, Brinch L, Albrechtsen D, Egeland T, Steen R, Solheim BG, Evensen SA. A randomised study of allogeneic transplantation with stem cells from blood or bone marrow. Bone Marrow Transplant. 2000;25:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 91] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 26. | Horan JT, Liesveld JL, Fernandez ID, Lyman GH, Phillips GL, Lerner NB, Fisher SG. Survival after HLA-identical allogeneic peripheral blood stem cell and bone marrow transplantation for hematologic malignancies: meta-analysis of randomized controlled trials. Bone Marrow Transplant. 2003;32:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Stem Cell Trialists' Collaborative Group. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074-5087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 374] [Cited by in RCA: 337] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 28. | Pidala J, Anasetti C, Kharfan-Dabaja MA, Cutler C, Sheldon A, Djulbegovic B. Decision analysis of peripheral blood versus bone marrow hematopoietic stem cells for allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2009;15:1415-1421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 44] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 29. | Vigorito AC, Marques Júnior JF, Aranha FJ, Oliveira GB, Miranda EC, De Souza CA. A randomized, prospective comparison of allogeneic bone marrow and peripheral blood progenitor cell transplantation in the treatment of hematologic malignancies: an update. Haematologica. 2001;86:665-666. [PubMed] |
| 30. | Cornelissen JJ, van der Holt B, Petersen EJ, Vindelov L, Russel CA, Höglund M, Maertens J, Schouten HC, Braakman E, Steijaert MM. A randomized multicenter comparison of CD34(+)-selected progenitor cells from blood vs from bone marrow in recipients of HLA-identical allogeneic transplants for hematological malignancies. Exp Hematol. 2003;31:855-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 31. | Chang YJ, Weng CL, Sun LX, Zhao YT. Allogeneic bone marrow transplantation compared to peripheral blood stem cell transplantation for the treatment of hematologic malignancies: a meta-analysis based on time-to-event data from randomized controlled trials. Ann Hematol. 2012;91:427-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 32. | Anasetti C, Logan BR, Lee SJ, Waller EK, Weisdorf DJ, Wingard JR, Cutler CS, Westervelt P, Woolfrey A, Couban S. Peripheral-blood stem cells versus bone marrow from unrelated donors. N Engl J Med. 2012;367:1487-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 643] [Cited by in RCA: 681] [Article Influence: 52.4] [Reference Citation Analysis (0)] |
| 33. | Meisel R, Klingebiel T, Dilloo D. Peripheral blood stem cells versus bone marrow in pediatric unrelated donor stem cell transplantation. Blood. 2013;121:863-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Wang J, Zhan P, Ouyang J, Chen B, Zhou R, Yang Y. Unrelated donor umbilical cord blood transplantation versus unrelated donor bone marrow transplantation in adult and pediatric patients: A meta-analysis. Leuk Res. 2010;34:1018-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Rocha V, Cornish J, Sievers EL, Filipovich A, Locatelli F, Peters C, Remberger M, Michel G, Arcese W, Dallorso S. Comparison of outcomes of unrelated bone marrow and umbilical cord blood transplants in children with acute leukemia. Blood. 2001;97:2962-2971. [PubMed] |
| 36. | Barker JN, Davies SM, DeFor T, Ramsay NK, Weisdorf DJ, Wagner JE. Survival after transplantation of unrelated donor umbilical cord blood is comparable to that of human leukocyte antigen-matched unrelated donor bone marrow: results of a matched-pair analysis. Blood. 2001;97:2957-2961. [PubMed] |
| 37. | Dalle JH, Duval M, Moghrabi A, Wagner E, Vachon MF, Barrette S, Bernstein M, Champagne J, David M, Demers J. Results of an unrelated transplant search strategy using partially HLA-mismatched cord blood as an immediate alternative to HLA-matched bone marrow. Bone Marrow Transplant. 2004;33:605-611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Jacobsohn DA, Hewlett B, Ranalli M, Seshadri R, Duerst R, Kletzel M. Outcomes of unrelated cord blood transplants and allogeneic-related hematopoietic stem cell transplants in children with high-risk acute lymphocytic leukemia. Bone Marrow Transplant. 2004;34:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 39. | Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, Stevens C, Barker JN, Gale RP, Lazarus HM. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265-2275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 823] [Article Influence: 39.2] [Reference Citation Analysis (0)] |
| 40. | Rocha V, Labopin M, Sanz G, Arcese W, Schwerdtfeger R, Bosi A, Jacobsen N, Ruutu T, de Lima M, Finke J. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276-2285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 906] [Cited by in RCA: 829] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 41. | Takahashi S, Iseki T, Ooi J, Tomonari A, Takasugi K, Shimohakamada Y, Yamada T, Uchimaru K, Tojo A, Shirafuji N. Single-institute comparative analysis of unrelated bone marrow transplantation and cord blood transplantation for adult patients with hematologic malignancies. Blood. 2004;104:3813-3820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 250] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 42. | Barker JN, Hough RE, van Burik JA, DeFor TE, MacMillan ML, O’Brien MR, Wagner JE. Serious infections after unrelated donor transplantation in 136 children: impact of stem cell source. Biol Blood Marrow Transplant. 2005;11:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 101] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 43. | Eapen M, Rubinstein P, Zhang MJ, Stevens C, Kurtzberg J, Scaradavou A, Loberiza FR, Champlin RE, Klein JP, Horowitz MM. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: a comparison study. Lancet. 2007;369:1947-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 584] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 44. | Atsuta Y, Suzuki R, Nagamura-Inoue T, Taniguchi S, Takahashi S, Kai S, Sakamaki H, Kouzai Y, Kasai M, Fukuda T. Disease-specific analyses of unrelated cord blood transplantation compared with unrelated bone marrow transplantation in adult patients with acute leukemia. Blood. 2009;113:1631-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 45. | Eapen M, Rocha V, Sanz G, Scaradavou A, Zhang MJ, Arcese W, Sirvent A, Champlin RE, Chao N, Gee AP. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol. 2010;11:653-660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 502] [Cited by in RCA: 453] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 46. | Marks DI, Woo KA, Zhong X, Appelbaum FR, Bachanova V, Barker JN, Brunstein CG, Gibson J, Kebriaei P, Lazarus HM. Unrelated umbilical cord blood transplant for adult acute lymphoblastic leukemia in first and second complete remission: a comparison with allografts from adult unrelated donors. Haematologica. 2013;Sep 20; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | MacMillan ML, Weisdorf DJ, Brunstein CG, Cao Q, DeFor TE, Verneris MR, Blazar BR, Wagner JE. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: analysis of risk factors. Blood. 2009;113:2410-2415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 158] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, McGlave PB, Wagner JE. Umbilical cord blood transplantation after nonmyeloablative conditioning: impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064-3070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 49. | Verneris MR, Brunstein CG, Barker J, MacMillan ML, DeFor T, McKenna DH, Burke MJ, Blazar BR, Miller JS, McGlave PB. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood. 2009;114:4293-4299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 223] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 50. | Sideri A, Neokleous N, Brunet De La Grange P, Guerton B, Le Bousse Kerdilles MC, Uzan G, Peste-Tsilimidos C, Gluckman E. An overview of the progress on double umbilical cord blood transplantation. Haematologica. 2011;96:1213-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 51. | Sanz J, Wagner JE, Sanz MA, Defor T, Montesinos P, Bachanova V, Lorenzo I, Warlick E, Sanz GF, Brunstein C. Myeloablative cord blood transplantation in adults with acute leukemia: comparison of two different transplant platforms. Biol Blood Marrow Transplant. 2013;19:1725-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Ruggeri A, Sanz G, Bittencourt H, Sanz J, Rambaldi A, Volt F, Yakoub-Agha I, Ribera JM, Mannone L, Sierra J. Comparison of outcomes after single or double cord blood transplantation in adults with acute leukemia using different types of myeloablative conditioning regimen, a retrospective study on behalf of Eurocord and the Acute Leukemia Working Party of EBMT. Leukemia. 2013;Sep 5; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 53. | Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, Margevicius S, Fu P, van Heeckeren W, Lazarus HM, Cooper BW, Gerson SL, Barr P. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47:924-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 54. | Rodrigues CA, Sanz G, Brunstein CG, Sanz J, Wagner JE, Renaud M, de Lima M, Cairo MS, Fürst S, Rio B. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: a study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 150] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 55. | Avery S, Barker JN. Cord blood transplants: one, two or more units? Curr Opin Hematol. 2010;17:531-537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 56. | Scaradavou A, Brunstein CG, Eapen M, Le-Rademacher J, Barker JN, Chao N, Cutler C, Delaney C, Kan F, Isola L. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood. 2013;121:752-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 136] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 57. | Goldstein G, Elhasid R, Bielorai B, Shimoni A, Yerushalmi R, Kassis I, Nagler A. Adults requiring cord blood transplants but have insufficient cell doses from a single cord blood unit can receive two units with successful engraftment kinetics similar to those of children receiving a single unit. Leuk Lymphoma. 2011;52:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 58. | Yoo KH, Lee SH, Sung KW, Koo HH, Chung NG, Cho B, Kim HK, Kang HJ, Shin HY, Ahn HS. Current status of pediatric umbilical cord blood transplantation in Korea: a multicenter retrospective analysis of 236 cases. Am J Hematol. 2011;86:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 59. | Robin M, Sanz GF, Ionescu I, Rio B, Sirvent A, Renaud M, Carreras E, Milpied N, Mohty M, Beguin Y. Unrelated cord blood transplantation in adults with myelodysplasia or secondary acute myeloblastic leukemia: a survey on behalf of Eurocord and CLWP of EBMT. Leukemia. 2011;25:75-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 60. | Labopin M, Ruggeri A, Gorin NC, Gluckman E, Blaise D, Mannone L, Milpied N, Yakoub-Agha I, Deconinck E, Michallet M. Cost-effectiveness and clinical outcomes of double versus single cord bloodtransplantation in adults with acute leukemia in France. Haematologica. 2013;Oct 18; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 61. | Rocha V, Labopin M, Ruggeri A, Podestà M, Gallamini A, Bonifazi F, Sanchez-Guijo FM, Rovira M, Socie G, Baltadakis I. Unrelated cord blood transplantation: outcomes after single-unit intrabone injection compared with double-unit intravenous injection in patients with hematological malignancies. Transplantation. 2013;95:1284-1291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, Verneris MR, Appelbaum FR, Wagner JE, Delaney C. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693-4699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 63. | Ponce DM, Zheng J, Gonzales AM, Lubin M, Heller G, Castro-Malaspina H, Giralt S, Hsu K, Jakubowski AA, Jenq RR. Reduced late mortality risk contributes to similar survival after double-unit cord blood transplantation compared with related and unrelated donor hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2011;17:1316-1326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Malard F, Fürst S, Loirat M, Chevallier P, El-Cheikh J, Guillaume T, Delaunay J, Le Gouill S, Moreau P, Blaise D. Effect of graft source on mismatched unrelated donor hemopoietic stem cell transplantation after reduced intensity conditioning. Leukemia. 2013;27:2113-2117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 65. | Le Bourgeois A, Mohr C, Guillaume T, Delaunay J, Malard F, Loirat M, Peterlin P, Blin N, Dubruille V, Mahe B. Comparison of outcomes after two standards-of-care reduced-intensity conditioning regimens and two different graft sources for allogeneic stem cell transplantation in adults with hematologic diseases: a single-center analysis. Biol Blood Marrow Transplant. 2013;19:934-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 66. | Chen YB, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G, Liney D, Bourdeau G, Alyea EP, Armand P. Reduced-intensity conditioning stem cell transplantation: comparison of double umbilical cord blood and unrelated donor grafts. Biol Blood Marrow Transplant. 2012;18:805-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Jacobson CA, Turki AT, McDonough SM, Stevenson KE, Kim HT, Kao G, Herrera MI, Reynolds CG, Alyea EP, Ho VT. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:565-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 138] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 68. | Di Bartolomeo P, Santarone S, De Angelis G, Picardi A, Cudillo L, Cerretti R, Adorno G, Angelini S, Andreani M, De Felice L. Haploidentical, unmanipulated, G-CSF-primed bone marrow transplantation for patients with high-risk hematologic malignancies. Blood. 2013;121:849-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 69. | Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, Felicini R, Falcinelli F, Velardi A, Ruggeri L. Full haplotype-mismatched hematopoietic stem-cell transplantation: a phase II study in patients with acute leukemia at high risk of relapse. J Clin Oncol. 2005;23:3447-3454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 551] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 70. | Ciurea SO, Saliba R, Rondon G, Pesoa S, Cano P, Fernandez-Vina M, Qureshi S, Worth LL, McMannis J, Kebriaei P. Reduced-intensity conditioning using fludarabine, melphalan and thiotepa for adult patients undergoing haploidentical SCT. Bone Marrow Transplant. 2010;45:429-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 71. | Aversa F, Terenzi A, Felicini R, Tabilio A, Falzetti F, Carotti A, Falcinelli F, Sodani P, Amici A, Zucchetti P. Mismatched T cell-depleted hematopoietic stem cell transplantation for children with high-risk acute leukemia. Bone Marrow Transplant. 1998;22 Suppl 5:S29-S32. [PubMed] |
| 72. | Handgretinger R, Klingebiel T, Lang P, Schumm M, Neu S, Geiselhart A, Bader P, Schlegel PG, Greil J, Stachel D. Megadose transplantation of purified peripheral blood CD34(+) progenitor cells from HLA-mismatched parental donors in children. Bone Marrow Transplant. 2001;27:777-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 211] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 73. | Peters C, Matthes-Martin S, Fritsch G, Holter W, Lion T, Witt V, Höcker P, Fischer G, Dieckmann K, Handgretinger R. Transplantation of highly purified peripheral blood CD34+ cells from HLA-mismatched parental donors in 14 children: evaluation of early monitoring of engraftment. Leukemia. 1999;13:2070-2078. [PubMed] |
| 74. | Ortín M, Raj R, Kinning E, Williams M, Darbyshire PJ. Partially matched related donor peripheral blood progenitor cell transplantation in paediatric patients adding fludarabine and anti-lymphocyte gamma-globulin. Bone Marrow Transplant. 2002;30:359-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 75. | Lang P, Greil J, Bader P, Handgretinger R, Klingebiel T, Schumm M, Schlegel PG, Feuchtinger T, Pfeiffer M, Scheel-Walter H. Long-term outcome after haploidentical stem cell transplantation in children. Blood Cells Mol Dis. 2004;33:281-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 76. | Klingebiel T, Handgretinger R, Lang P, Bader P, Niethammer D. Haploidentical transplantation for acute lymphoblastic leukemia in childhood. Blood Rev. 2004;18:181-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Marks DI, Khattry N, Cummins M, Goulden N, Green A, Harvey J, Hunt LP, Keen L, Robinson SP, Steward CG. Haploidentical stem cell transplantation for children with acute leukaemia. Br J Haematol. 2006;134:196-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Reisner Y, Martelli MF. Tolerance induction by ‘megadose’ transplants of CD34+ stem cells: a new option for leukemia patients without an HLA-matched donor. Curr Opin Immunol. 2000;12:536-541. [PubMed] |
| 79. | Klingebiel T, Cornish J, Labopin M, Locatelli F, Darbyshire P, Handgretinger R, Balduzzi A, Owoc-Lempach J, Fagioli F, Or R. Results and factors influencing outcome after fully haploidentical hematopoietic stem cell transplantation in children with very high-risk acute lymphoblastic leukemia: impact of center size: an analysis on behalf of the Acute Leukemia and Pediatric Disease Working Parties of the European Blood and Marrow Transplant group. Blood. 2010;115:3437-3446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 142] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 80. | Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2010;16:533-542. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 142] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 81. | Lang P, Schumm M, Greil J, Bader P, Klingebiel T, Müller I, Feuchtinger T, Pfeiffer M, Schlegel PG, Niethammer D. A comparison between three graft manipulation methods for haploidentical stem cell transplantation in pediatric patients: preliminary results of a pilot study. Klin Padiatr. 2005;217:334-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 82. | Federmann B, Bornhauser M, Meisner C, Kordelas L, Beelen DW, Stuhler G, Stelljes M, Schwerdtfeger R, Christopeit M, Behre G. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica. 2012;97:1523-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 87] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 83. | Palma J, Salas L, Carrión F, Sotomayor C, Catalán P, Paris C, Turner V, Jorquera H, Handgretinger R, Rivera GK. Haploidentical stem cell transplantation for children with high-risk leukemia. Pediatr Blood Cancer. 2012;59:895-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 84. | Chen DF, Prasad VK, Broadwater G, Reinsmoen NL, DeOliveira A, Clark A, Sullivan KM, Chute JP, Horwitz ME, Gasparetto C. Differential impact of inhibitory and activating Killer Ig-Like Receptors (KIR) on high-risk patients with myeloid and lymphoid malignancies undergoing reduced intensity transplantation from haploidentical related donors. Bone Marrow Transplant. 2012;47:817-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 85. | Handgretinger R, Chen X, Pfeiffer M, Mueller I, Feuchtinger T, Hale GA, Lang P. Feasibility and outcome of reduced-intensity conditioning in haploidentical transplantation. Ann N Y Acad Sci. 2007;1106:279-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 86. | Chen X, Hale GA, Barfield R, Benaim E, Leung WH, Knowles J, Horwitz EM, Woodard P, Kasow K, Yusuf U. Rapid immune reconstitution after a reduced-intensity conditioning regimen and a CD3-depleted haploidentical stem cell graft for paediatric refractory haematological malignancies. Br J Haematol. 2006;135:524-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 87. | Raiola AM, Dominietto A, Ghiso A, Di Grazia C, Lamparelli T, Gualandi F, Bregante S, Van Lint MT, Geroldi S, Luchetti S. Unmanipulated haploidentical bone marrow transplantation and posttransplantation cyclophosphamide for hematologic malignancies after myeloablative conditioning. Biol Blood Marrow Transplant. 2013;19:117-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 88. | Solomon SR, Sizemore CA, Sanacore M, Zhang X, Brown S, Holland HK, Morris LE, Bashey A. Haploidentical transplantation using T cell replete peripheral blood stem cells and myeloablative conditioning in patients with high-risk hematologic malignancies who lack conventional donors is well tolerated and produces excellent relapse-free survival: results of a prospective phase II trial. Biol Blood Marrow Transplant. 2012;18:1859-1866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 243] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 89. | Wang HX, Yan HM, Wang ZD, Xue M, Liu J, Guo ZK. Haploidentical hematopoietic stem cell transplantation in hematologic malignancies with G-CSF mobilized bone marrow plus peripheral blood stem cells grafts without T cell depletion: a single center report of 29 cases. Leuk Lymphoma. 2012;53:654-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 90. | Munchel AT, Kasamon YL, Fuchs EJ. Treatment of hematological malignancies with nonmyeloablative, HLA-haploidentical bone marrow transplantation and high dose, post-transplantation cyclophosphamide. Best Pract Res Clin Haematol. 2011;24:359-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 91. | Chen Y, Liu K, Xu L, Chen H, Liu D, Zhang X, Shi H, Han W, Wang Y, Zhao T. HLA-mismatched hematopoietic SCT without in vitro T-cell depletion for myelodysplastic syndrome. Bone Marrow Transplant. 2010;45:1333-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 92. | Wang HX, Yan HM, Duan LN, Wang ZD, Zhu L, Xue M, Liu J, Hu LD, Guo ZK. Haploidentical hematopoietic stem cell transplantation in child hematologic malignancies with G-CSF-mobilized marrow grafts without T-cell depletion: a single-center report of 45 cases. Pediatr Hematol Oncol. 2009;26:119-128. [PubMed] |
| 93. | Luznik L, O’Donnell PV, Symons HJ, Chen AR, Leffell MS, Zahurak M, Gooley TA, Piantadosi S, Kaup M, Ambinder RF. HLA-haploidentical bone marrow transplantation for hematologic malignancies using nonmyeloablative conditioning and high-dose, posttransplantation cyclophosphamide. Biol Blood Marrow Transplant. 2008;14:641-650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1597] [Cited by in RCA: 1471] [Article Influence: 86.5] [Reference Citation Analysis (0)] |
| 94. | Liu D, Huang X, Liu K, Xu L, Chen H, Han W, Chen Y, Zhang X, Jiang Q. Haploidentical hematopoietic stem cell transplantation without in vitro T cell depletion for treatment of hematological malignancies in children. Biol Blood Marrow Transplant. 2008;14:469-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 95. | Yoshihara T, Okada K, Kobayashi M, Kikuta A, Kato K, Adachi N, Kikuchi A, Ishida H, Hirota Y, Kuroda H. Outcome of non-T-cell-depleted HLA-haploidentical hematopoietic stem cell transplantation from family donors in children and adolescents. Int J Hematol. 2007;85:246-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 96. | Bashey A, Zhang X, Sizemore CA, Manion K, Brown S, Holland HK, Morris LE, Solomon SR. T-cell-replete HLA-haploidentical hematopoietic transplantation for hematologic malignancies using post-transplantation cyclophosphamide results in outcomes equivalent to those of contemporaneous HLA-matched related and unrelated donor transplantation. J Clin Oncol. 2013;31:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 404] [Article Influence: 33.7] [Reference Citation Analysis (0)] |
| 97. | Ciurea SO, Mulanovich V, Saliba RM, Bayraktar UD, Jiang Y, Bassett R, Wang SA, Konopleva M, Fernandez-Vina M, Montes N. Improved early outcomes using a T cell replete graft compared with T cell depleted haploidentical hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2012;18:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 198] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 98. | Dodero A, Carniti C, Raganato A, Vendramin A, Farina L, Spina F, Carlo-Stella C, Di Terlizzi S, Milanesi M, Longoni P. Haploidentical stem cell transplantation after a reduced-intensity conditioning regimen for the treatment of advanced hematologic malignancies: posttransplantation CD8-depleted donor lymphocyte infusions contribute to improve T-cell recovery. Blood. 2009;113:4771-4779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 99. | Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, Del Papa B, Zei T, Ostini RI, Cecchini D. Tregs prevent GVHD and promote immune reconstitution in HLA-haploidentical transplantation. Blood. 2011;117:3921-3928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 779] [Cited by in RCA: 833] [Article Influence: 59.5] [Reference Citation Analysis (0)] |
| 100. | Di Ianni M, Falzetti F, Carotti A, Terenzi A, Del Papa B, Perruccio K, Ruggeri L, Sportoletti P, Rosati E, Marconi P. Immunoselection and clinical use of T regulatory cells in HLA-haploidentical stem cell transplantation. Best Pract Res Clin Haematol. 2011;24:459-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 101. | Liu K, Chen Y, Zeng Y, Xu L, Liu D, Chen H, Zhang X, Han W, Wang Y, Zhao T. Coinfusion of mesenchymal stromal cells facilitates platelet recovery without increasing leukemia recurrence in haploidentical hematopoietic stem cell transplantation: a randomized, controlled clinical study. Stem Cells Dev. 2011;20:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, Locatelli F, Fibbe WE. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110:2764-2767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 399] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 103. | Xu LP, Liu KY, Liu DH, Chen H, Han W, Chen YH, Wang Y, Huang XJ. The inferiority of G-PB to rhG-CSF-mobilized blood and marrow grafts as a stem cell source in patients with high-risk acute leukemia who underwent unmanipulated HLA-mismatched/haploidentical transplantation: a comparative analysis. Bone Marrow Transplant. 2010;45:985-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 104. | Yan HM, Xue M, Wang ZD, Zhu L, Liu J, Ding L, Pan SP, Duan LN, Wang HX. [Haploidentical allogeneic bone marrow stem cell transplantation combined with peripheral blood stem cells for therapy of leukemia]. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2009;17:1330-1334. [PubMed] |
| 105. | Vago L, Oliveira G, Bondanza A, Noviello M, Soldati C, Ghio D, Brigida I, Greco R, Lupo Stanghellini MT, Peccatori J. T-cell suicide gene therapy prompts thymic renewal in adults after hematopoietic stem cell transplantation. Blood. 2012;120:1820-1830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 106. | Ciceri F, Bonini C, Stanghellini MT, Bondanza A, Traversari C, Salomoni M, Turchetto L, Colombi S, Bernardi M, Peccatori J. Infusion of suicide-gene-engineered donor lymphocytes after family haploidentical haemopoietic stem-cell transplantation for leukaemia (the TK007 trial): a non-randomised phase I-II study. Lancet Oncol. 2009;10:489-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 414] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 107. | Di Stasi A, Tey SK, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, Straathof K, Liu E, Durett AG, Grilley B. Inducible apoptosis as a safety switch for adoptive cell therapy. N Engl J Med. 2011;365:1673-1683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1077] [Cited by in RCA: 1176] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 108. | Bastien JP, Roy J, Roy DC. Selective T-cell depletion for haplotype-mismatched allogeneic stem cell transplantation. Semin Oncol. 2012;39:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 109. | Guinan EC, Boussiotis VA, Neuberg D, Brennan LL, Hirano N, Nadler LM, Gribben JG. Transplantation of anergic histoincompatible bone marrow allografts. N Engl J Med. 1999;340:1704-1714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 358] [Cited by in RCA: 324] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 110. | Handgretinger R. New approaches to graft engineering for haploidentical bone marrow transplantation. Semin Oncol. 2012;39:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 111. | Grosso D, Flomenberg N. A two-step approach to allogeneic haploidentical hematopoietic stem cell transplantation. Semin Oncol. 2012;39:694-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 112. | Grosso D, Carabasi M, Filicko-O’Hara J, Kasner M, Wagner JL, Colombe B, Cornett Farley P, O’Hara W, Flomenberg P, Werner-Wasik M. A 2-step approach to myeloablative haploidentical stem cell transplantation: a phase 1/2 trial performed with optimized T-cell dosing. Blood. 2011;118:4732-4739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 113. | Brunstein CG, Fuchs EJ, Carter SL, Karanes C, Costa LJ, Wu J, Devine SM, Wingard JR, Aljitawi OS, Cutler CS. Alternative donor transplantation after reduced intensity conditioning: results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 455] [Article Influence: 32.5] [Reference Citation Analysis (0)] |