INTRODUCTION
When a foreign organ, such as a kidney, is transplanted into a non-identical individual of the same species, the organ is called an allograft. The immune response from the recipient to the allograft is termed an alloimmune response, which is initiated by T-cell recognition of alloantigens (commonly known as allorecognition). Allorecognition is the first step of a series of complex events that leads to T-cell activation, antibody production, and allograft rejection[1-3]. This review will summarize the key concepts of transplant immunology and modern immunological assays, which are essential in our clinical practice.
MAJOR HISTOCOMPATIBILITY COMPLEX/HUMAN LEUKOCYTE ANTIGENS MOLECULES
The major histocompatibility complex (MHC) genes code the strongest transplant antigens. In humans, these MHC molecules are called human leukocyte antigens (HLA) and the genetic region is located on the short arm of chromosome 6. Each parent provides a haplotype (a linked set of MHC genes) to each offspring in Mendelian co-dominant inheritance. There are two classes of MHC or HLA molecules, viz. Class I molecules and Class II molecules. Class I molecules (HLA-A, -B, and -C) are composed of a polymorphic heavy chain (α chain, 44 kDa) and a non-polymorphic light chain (β2 microglobulin, 12 kDa). They are expressed on all nucleated cells and generally present endogenous small antigens (typically 9 to 11 amino acids), such as viruses and self-protein fragments, in the context of self-MHC to CD8+ T. Class II molecules (HLA-DP, -DQ, and -DR) are composed of a polymorphic α chain (35 kDa) and a β chain (31 kDa). They are constitutively expressed only on professional antigen-presenting cells (APC), including dendritic cells, macrophages, and B-cells. Their expression may be upregulated on epithelial and vascular endothelial cells after exposure to pro-inflammatory cytokines. Class II molecules present relatively larger antigens (12 to 28 amino acids), derived from extracellular proteins to CD4+ T-cells[1-4]. The degree of HLA mismatch between donor and recipient plays a role in determining the risk of chronic rejection and graft loss. HLA-A, -B, and -DR (3 pairs, 6 antigens) are traditionally used for typing and matching before kidney or pancreas transplant. HLA-Cw, -DP, and -DQ are now increasingly typed and used in many transplant centers. For kidney transplants, the long-term graft survival is best in HLA-identical living related kidney transplants. The major impact comes from the match of the DR antigen, and the order of importance for HLA match in kidney transplant is DR > B > A[1,3,4].
NON-HLA ANTIGENS/ANTIBODIES
Acute and chronic graft rejection can occur in HLA-identical sibling transplants, indicating the presence of immune response to non-HLA antigens. There are several non-HLA antigens and their antibodies derived from either alloimmunity or autoimmunity have been reported[5,6].
ABO blood group antigens
ABO blood group antigens are not only expressed on red blood cells, but also on vascular endothelial cells and other cells. ABO incompatible organ transplants cause hyperacute rejection due to the presence of the preformed hemagglutinin A and/or B antibody. ABO compatibility between donor and recipient are essential for organ transplant, similar to red blood cell transfusion. Desensitization protocols to remove the preformed hemagglutinin A and/or B from recipient circulation have been used for ABO incompatible kidney transplants[1,7]. The rhesus factor and other red cell antigens are not relevant to organ transplant, as they are not expressed on endothelium.
Minor histocompatibility antigens
Minor histocompatibility antigens (MiHA) are small endogenous peptides that occupy the antigen-binding site of donor MHC molecules. They are generally recognized by CD8+ cytotoxic T-cells in the context of self-MHC, which leads to graft rejection. In bone marrow transplant, MiHA play an important role in graft-vs-host disease in patients who have received HLA-matched cells[8]. H-Y MiHA is encoded by the Y chromosome in males and can induce alloimmune response when a male organ is transplanted into a female recipient[9]. MHC class 1 related chain A and B (MICA and MICB) are also expressed on endothelial cells. Antibodies against MICA and/or MICB can cause antibody-mediated rejection (AMR) and graft loss[10].
Other reported antibodies causing graft rejection include anti-angiotensin-2 receptor, anti-glutathione S-transferase T1, and anti-endothelial antibodies[11-13]. Anti-endothelial antibody can be detected by using donor monocytes for crossmatch[13]. Some minor transplant antigens may come from mitochondrial proteins and enzymes. As our knowledge in transplant immunology advances, there will likely be more alloreactive and autoreactive antibodies to uncover.
ALLORECOGNITION PATHWAYS
Allorecognition can occur by one of three pathways: direct, indirect, and semi-direct[14-16]. In the direct pathway, recipient’s T-cells recognize intact allogeneic HLAs expressed by donor cells. In the indirect pathway, T-cells recognize peptides derived from donor HLAs presented by recipient APC. In the semi-direct pathway, recipient dendritic cells or other APC acquire intact HLAs from donor cells and present them to recipient T cells. The direct and indirect pathways are well understood in organ transplantation; the semi-direct pathway is not of clinical importance. The direct pathway is very important in the immediate post transplant period. Without appropriate immunosuppression, a strong and effective alloresponse would follow, which is primarily due to the high number of recipient T-cells that will recognize the graft antigens and cause acute cellular rejection. While the indirect pathway of allorecognition may also participate in acute rejection, it is usually predominant in the late onset of rejection, and especially chronic rejection[14-16]. As long as the allograft is present in the host, the recipient APCs can pick up the alloantigen shed from the graft and start alloimmune response. Therefore, maintenance immunosuppression is required for the lifetime of the allograft to prevent late rejection and chronic rejection.
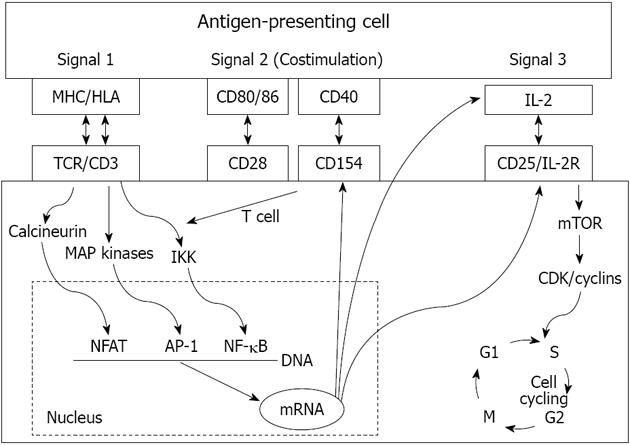
THREE-SIGNAL MODEL OF T-CELL ACTIVATION
T-cell activation is the key process of allograft rejection. T-cells recognize alloantigen through T-cell receptors (TCR). The initiation of intracellular signaling requires additional peptides known as CD3 complex, and the antigen-specific signal (signal 1) is transduced through the TCR-CD3 complex[1-3]. Two signals are needed for complete T-cell activation (Figure 1). The second co-stimulatory signal depends on the receptor-ligand interactions between T-cells and APCs (signal 2). Numerous co-stimulatory pathways have been described and blockage of these pathways can lead to antigen-specific inactivation or death of T-cells[17-19]. The best-studied ones are the CD28-B7 and CD154-CD40 pathways. CD28 and CD154 are expressed on T-cells, and their ligands B7 and CD40 are expressed on APCs. CD28 has two ligands, B7-1 (CD80) and B7-2 (CD86). T-cells also express cytotoxic T-lymphocyte associated antigen-4 (CTLA-4), which is homologous to CD28 and has a higher affinity than CD28 to bind B7. However, when CTLA-4 binds B7 (both CD80 and CD86), it produces an inhibitory signal to terminate T-cell response. This unique interaction leads to the clinical development of a fusion protein CTLA-4-Ig (belatacept) as a novel immunosuppressive medication[19]. CD154-CD40 blockages have also been shown to prevent allograft rejection in animal models, including anti-CD154 antibody and molecules that target CD40[18].
Figure 1 The 3-signal model of T cell activation.
MHC: Major histocompatibility complex; HLA: Human leukocyte antigens; IL: Interleukin; TCR: T-cell receptors; NFAT: Nuclear factor of activated T cells; mTOR: Mechanistic target of rapamycin.
The combination of signal 1 and 2 activates three downstream signal transduction pathways: the calcium-calcineurin pathway, the RAS-mitogen activated protein kinase pathway, and the IKK-nuclear factor κB (NF-κB) pathway. These three pathways further activate transcription factors including the nuclear factor of activated T cells, activated protein-1, and NF-κB, respectively. Several new molecules and cytokines including CD25, CD154, interleukin (IL)-2, and IL-15 are subsequently expressed[1-3]. IL-2 and IL-15 deliver growth signals (signal 3) through the mammalian target of rapamycin pathway and phosphoinositide-3-kinase pathway, which subsequently trigger the T-cell cycle and proliferation (Figure 1). The fully activated T-cells undergo clonal expansion and produce a large number of cytokines and effector T-cells, which eventually produce CD8+ T-cell mediated cytotoxicity, help macrophage-induced delayed type hypersensitivity response (by CD4+Th1), and help B cells for antibody production (by CD4+Th2). A subset of activated T-cells becomes the alloantigen-specific memory T-cells[20,21].
B LYMPHOCYTES
B-cells express clonally restricted antigen-specific receptors as immunoglobulins on their surfaces. When these receptors bind donor HLA antigens in the context of assistance from helper T-cells (CD4+Th2), B-cells are activated. They then divide, differentiate, and become plasma cells to secret antibodies. Some activated B-cells become memory B-cells[22-24]. The helper T-cells may facilitate B-cell activation either through intimate membrane contact involving a variety of receptors and ligands (such as CD40:CD154) or through the secreted soluble cytokines (such as IL-4)[18,23,24]. These HLA antibodies bind antigens and can cause graft injury either by activating the complement cascade [complement-dependent cytotoxicity (CDC)] or via Fc receptor on natural killer (NK) cells, neutrophils, and eosinophils (antibody-dependent cellular cytotoxicity)[1,14]. In addition producing antibodies, B-cells are also APCs. B-cells can present allograft-derived antigens to T-cells for T cell activation through the indirect pathway of allorecognition[2-4].
INNATE AND ADAPTIVE IMMUNE RESPONSES IN GRAFT REJECTION
Innate immunity refers to the nonspecific natural immune system that involves macrophages, neutrophils, NK cells, cytokines, toll-like receptors, and complement components[25]. Alloimmune is an adaptive immunity that involves recognition of alloantigen and confers antigen specificity and memory by T and B cells as discussed above. However, alloimmune response not only produces specific effector T cells and antibodies, but also secretes chemokines and cytokines, which recruit components of the innate immune system, such as complement activation and leukocyte migration from the circulation into a site of inflammation[1-4]. On the other hand, ischemic injury of the allograft initially activates the innate immune response, which leads to increased antigen presentation to T-cells by up-regulating the expression of class II HLAs, adhesion molecules, and cytokines[2-4]. Therefore, the innate and adaptive immune responses are closely interrelated and both play important roles in allograft rejection and rejection-associated tissue damage.
SENSITIZATION AND PANEL REACTIVE ANTIBODY
Human sensitization is defined by the presence of antibodies in the recipient’s blood against a panel of selected HLA antigens representing donor population. It is reported as the percent panel reactive antibody (PRA). PRA estimates the likelihood of positive crossmatches to potential donors[1,14]. The higher the PRA level, the lower the chance of receiving a compatible kidney and longer the waiting time on the kidney waitlist, previous exposure to HLA antigens. Sensitization is caused by previous exposure to HLA antigens, usually through previous organ transplant(s), pregnancy or blood transfusion particularly relevant is the exposure of women to their partner’s HLA during pregnancy. This results in direct sensitization against the partner, potentially making the partner and/or their child an unsuitable donor. The percent PRA in an individual patient may vary from one testing date to another secondary to either a change in antibody titers, or a change in the usage of HLA antigens in the assay. The technology of PRA assay has advanced from the initial CDC assay, to the enzyme-linked immunoabsorption (ELISA), to the current multiplexed particle-based flow cytometry (Luminex). Single antigen beads are increasingly used to characterize the preformed HLA antibodies before transplant as well as any de novo development of HLA antibodies (donor-specific antibodies, DSA) after transplant[1,26].
CROSSMATCH AND DSA
Solid phase based ELISA or Luminex assay can detect and characterize the preformed HLA antibodies in an individual patient. The corresponding antigens are considered unacceptable for that patient, and in the unites states of America (United States), they are listed into the United Network of Organ Sharing database. A patient will not be offered a kidney from the deceased donor who expresses an unacceptable HLA antigen (positive virtual crossmatch). Only those patients whose HLA antibodies are not donor directed will appear on the match run (negative virtual crossmatch). Such “virtual crossmatch” can improve efficiency of organ allocation by decreasing the risk of positive crossmatch before transplant[26]. When a potential donor is identified, a final crossmatch with fresh serum from recipient and lymphocytes from donor has to be performed to rule out any preformed DSA, which can produce hyperacute AMR. The final crossmatch must be negative to proceed with kidney transplantation. The two commonly used tests for evaluation of kidney transplant eligibility are CDC crossmatch and flow cytometry crossmatch (FCXM). The choice of which crossmatch test to perform remains a controversial issue. Individual transplant programs, according to center experience and availability, usually determine it.
T-cells express HLA class I antigens only, while B-cells express both HLA class I and class II antigens. Furthermore, B-cells express HLA class I antigens at quantitatively greater level than on T-cells. T-cell positive crossmatch is considered as true and significant sensitization with DSA against HLA class I antigens. T-cell negative/B-cell positive crossmatches may represent either HLA class II antibodies or low titers of HLA class I antibodies. T-cell positive/B-cell negative results are likely due to presence of non-HLA antibodies[1,3].
CDC CROSSMATCH
The donor lymphocytes (T-cells, B-cells, or mixed) are isolated from blood or lymph nodes, and placed in wells. The recipient serum is then added along with rabbit complement. The cytotoxicity is determined by counting the lyses of lymphocytes compared with a control. It is usually modified by addition of antihuman globulin to increase the sensitivity (AHG-CDC), as antihuman globulin can induce cross-linking of antibodies and increase the visual cytotoxicity. If the initial CDC crossmatch is positive, it will be repeated with the addition of dithiothreitol (DTT), which reduces the disulfide bonds of immunoglobulin (Ig)M if it is present. Initial positive and repeated DTT positive tests indicate the presence of DSA of IgG rather than IgM. IgM antibodes are generally not considered to be real sensitization. Kidney transplantation should not proceed if there is evidence of a positive crossmatch secondary to a cytotoxic IgG anti-HLA antibody. However, there are various desensitization protocols that can be used to remove the preformed DSA to achieve negative final crossmatch for HLA incompatible transplants if a living donor is involved[27-31].
FCXM
Donor T and B-lymphocytes are isolated and mixed with recipient serum. A fluorescence labeled antihuman IgG is then added. The cells that bind any recipient antibodies are stained with fluorescence labeled antihuman IgG and cause the channel shifts in fluorescent intensity. FCXM is much more sensitive than CDC or AHG-CDC in detecting low level of antibodies. Non-cytotoxic antibodies can also be detected with FCXM since it does not depend on the complement activation of antibody. The significance of non-complement activating or non-cytotoxic antibodies in-vivo is unclear. Single antigen bead (Luminex) can be used to further characterize any DSA presence and to determine if the DSA is responsible for the channel shift in the flow crossmatch[1-3].
Again, these two crossmatches differ in the degree of sensitivity. Conservative transplant programs may choose sensitive FCXM, which will significantly reduce the incidence of post-transplant AMR. However, it may also be too sensitive in that clinically irrelevant antibodies are detected. Consequently, some viable transplant opportunities are potentially lost. Crossmatch tests can also be performed with the recipient’s previous sera. The scenario of current sera negative, historical sera positive suggests previous antibodies may have waned in titer. But the specific memory B-cells could rapidly expand and produce the antibodies when re-exposed to the specific alloantigen. Although this is not considered as a contraindication for transplantation, it does increase the risk of AMR after transplant. Close monitoring of DSA titer and more immunosuppression is usually recommended.
CONCLUSION
The alloimmune response is initiated by T-cell recognition of alloantigens through direct or indirect pathways. Three signal models have been established during T-cell activation, which subsequently produces various effector T-cells and antibody production. Sensitive crossmatch is routinely performed before kidney transplant to detect any significant DSA, so that hyperacute rejection can be eliminated. Solid phase based Luminex assay can further characterize HLA antibodies before and after kidney transplant to guide our clinical practice. In addition to the traditional anti-HLA antibodies, alloreactive and autoreactive antibodies against non-HLA antigens have now been increasingly recognized to play an important role in humoral rejection of allograft.
ACKNOWLEDGMENTS
We thank Michael Yatsevitch for assisting us in preparing the figure.
P- Reviewers: Rodriguez DC, Sun XY S- Editor: Gou SX L- Editor: A E- Editor: Zheng XM