Revised: November 15, 2012
Accepted: December 1, 2012
Published online: March 24, 2013
Processing time: 199 Days and 8.9 Hours
AIM: To compare urological infections in patients with or without stents following transplantation and to determine the effect of such infections on graft function.
METHODS: All 285 recipients of kidney transplantation at our centre between 2006 and 2010 were included in the study. Detailed information including stent use and transplant function was collected prospectively and analysed retrospectively. The diagnosis of urinary tract infection was made on the basis of compatible symptoms supported by urinalysis and/or microbiological culture. Graft function, estimated glomerular filtration rate and creatinine at 6 mo and 12 mo, immediate graft function and infection rates were compared between those with a stent or without a stent.
RESULTS: Overall, 196 (183 during initial procedure, 13 at reoperation) patients were stented following transplantation. The overall urine leak rate was 4.3% (12/277) with no difference between those with or without stents - 7/183 vs 5/102, P = 0.746. Overall, 54% (99/183) of stented patients developed a urological infection compared to 38.1% (32/84) of those without stents (P = 0.0151). All 18 major urological infections occurred in those with stents. The use of stent (Wald χ2 = 5.505, P = 0.019) and diabetes mellitus (Wald χ2 = 5.197, P = 0.023) were found to have significant influence on urological infection rates on multivariate analysis. There were no deaths or graft losses due to infection. Stenting was associated with poorer transplant function at 12 mo.
CONCLUSION: Stents increase the risks of urological infections and have a detrimental effect on early to medium term renal transplant function.
- Citation: Akoh JA, Rana T. Effect of ureteric stents on urological infection and graft function following renal transplantation. World J Transplant 2013; 3(1): 1-6
- URL: https://www.wjgnet.com/2220-3230/full/v3/i1/1.htm
- DOI: https://dx.doi.org/10.5500/wjt.v3.i1.1
Stents are used to protect the ureter-bladder anastomosis when performing renal transplantation in order to avoid or reduce urological complications[1-5]. The insertion of a stent does not eliminate the risk of complications, particularly urinary leak but may alter the approach to managing them[6]. Due to immunosuppression, stenting in transplant patients increases the risk of urological or blood stream infections[7,8]. As a result, opinion continues to be divided between those who routinely stent and those who only do so selectively on the basis of clear indications[2,9,10-13]. Proponents of selective stenting state that the associated risks are high enough to avoid routine stenting and advocate that careful surgical technique with selective stenting of problematic anastomoses yields similar results[12,13].
The key question is to determine what effect the increased risk of urological infection with stenting has on the early and medium term outcome of renal transplantation. This study was carried out to compare the incidence of urological infection in patients with or without stents inserted at transplantation and to determine the effect of urinary tract infections (MUI) in the early post transplantation period on short and medium term graft function.
All recipients of kidney transplantation at the South West Transplant Centre (SWTC), Derriford Hospital, Plymouth between January 2006 and December 2010 were included in the study. Patient data was entered prospectively into the renal computer database (PROTON Information System, Clinical Computing PLC, London, United Kingdom) that was also used for information on patients handed over to other centres for follow up. Patients who developed significant urological complications after hand back to their home units were referred back to the SWTC for management and included in this analysis. Transplant nurses at peripheral centres were contacted to provide information on those patients whose data were incomplete. The duration of follow up ranged from 12 mo to 72 mo.
Patients were managed according to the standard protocol of the SWTC. Immunosuppression comprised basiliximab (induction), tacrolimus (0.1 mg/kg per day), mycophenolic acid (2 g/d) and prednisolone. Antibiotic prophylaxis included a single intravenous dose of augumentin 1.2 g at anaesthetic induction and a daily dose of co-trimoxazole 480 mg for 3 mo. At surgery, a 6-French, 12 cm, double pigtail ureteral stent (Cook Medical) was inserted at the discretion of the operating surgeon to establish internal drainage from the uretero-pelvic junction to the bladder. The transplant nurse practitioner would identify patients requiring stent removal and refer them to the urology nurse practitioners as soon as possible following transplantation. The stent was removed by flexible cystoscopy under local anaesthetic on a day case basis by a urologist. The duration of retention of routinely placed stents was progressively decreased from six weeks (initially) to two weeks in the latter phase of the study. Selectively inserted stents were removed after the duration advised by the transplant surgeon (usually 4-6 wk). In the latter part of the study period, a single intravenous prophylactic dose of antibiotics was administered prior to stent removal - usually gentamicin 3 mg/kg (rounded to the nearest convenient multiple of 40 and a maximum dose of 160 mg). If there were serious difficulties with venous access, the dose was given intramuscularly 30 min before the procedure. A mid stream specimen of urine was sent 48 h prior to removal of stent and this was repeated if blood or protein was present in urine or the patient was symptomatic.
The diagnosis of UTI was made on the basis of compatible symptoms supported by urinalysis and/or microbiological culture. Major urological infections (MUIs) included complicated UTI, pyelonephritis and urosepsis with or without bacteraemia. Delayed graft function (DGF) was defined as requirement for dialysis within the first week of transplantation. Primary non function (PNF) was defined as a graft that never worked or that never allowed the recipient to come off dialysis.
Relevant data including age, type and date of transplant, recognised risk factors for urological complications (stripped ureter, damaged renal arteries/bench surgery, multiple renal arteries, cold ischaemic time greater than 24 h, lower urinary tract obstruction and bladder abnormality) or risk factors for infection such as diabetes, reoperation and peritoneal dialysis associated peritonitis were entered into proforma sheets. This data was then transferred to an Microsoft Excel worksheet and analysed using SPSS 17® for Windows (SPSS Inc, Chicago, IL).
Early and late graft function, estimated glomerular filtration rate (eGFR) and creatinine (Cr) at 6 mo (Cr6, eGFR6) and 12 mo (Cr12, eGFR12), immediate graft function, graft outcome, infection rates, type of infection and urine leak were compared between those with a stent (ST) or without a stent (WST). Differences between groups were tested by the χ2 statistic. Correlation between duration of stenting, interval to infection after transplantation, number of infection episodes and Cr and eGFR at 6 mo and 12 mo were tested using Pearson’s correlation statistics. Also, the General Linear Modelling multivariate analysis of categorical variables [stent use; type of transplant - donation after circulatory death (DCD), donation after brain death (DBD) or living donation (LD); transplant number - whether first, second or third; diabetes; ureteric reflux; body mass index (BMI) > 30 kg/m2; and early transplant outcome - immediate function, DGF and PNF] affecting urological infection following transplantation was performed. A P value of < 0.05 was taken as significant.
A total of 285 renal transplants were performed during the period comprising 181 males (age, mean ± SE: 52.1 ± 1.0 years; median: 53.5 years) and 104 females (age, mean ± SE: 49.2 ± 1.2 years; median: 50.4 years) giving a male to female ratio of 1.7:1. The commonest causes of established renal failure were glomerulonephritis (14.7%), cystic kidney disease (14%), immunoglobulin A nephropathy (13.3%) and diabetic nephropathy (6.7%). The overwhelming majority of transplants (189, 66.3%) were from DCD donors, with living donors LD constituting 28%. Also, 240 of the 285 patients (84%) were undergoing their first transplants whereas 36 (13%) and 9 (3%) were having their second and third transplants respectively. Information about use of antibiotic prophylaxis prior to implantation was unavailable in 23 cases (8.1%) but of the remaining 262, 86% (226) had appropriate prophylaxis. Ninety seven percent received prophylactic co-trimoxazole for 3 mo after transplantation.
One hundred and two patients (35.8%) did not have a ureteric stent inserted during their initial transplant operation. The indications for stenting in the remaining 183 (64.2%) are shown in Table 1. The demographic and other characteristics of the ST and WST groups are compared in Table 2. Thirteen of the WST group were stented at subsequent re-exploration and re-implantation of the transplant ureter (stenosis/stricture in ten, urine leak in two and negative exploration in one). Five patients in the ST group received a stent at subsequent re-operation for a urological complication. Overall, 196 (68.8%) patients received a stent following renal transplantation, with 159 (81%) of these inserted routinely. The proportion of patients receiving stents at transplantation (irrespective of whether inserted during the initial operation or at re-operation) varied with the type of organ donor (DCD 54.5%; DBD 58.8% and LD 70.9%), the differences were statistically significant - Pearson χ2 = 6.202; df = 2; P = 0.045. The mean ± SE duration of stenting was 46.99 ± 7.6 d, which was lower for routine than selective indication (39 ± 4.4 d vs 83.4 ± 33.2 d, respectively).
| Reason | Comments | |
| Routine | 159 (86.9) | |
| Ureter related | 9 (4.9) | e.g., Stripped ureter |
| Poor kidney perfusion | 6 (3.3) | |
| Contracted/thin bladder | 3 (1.6) | Compliance mismatch |
| Technical factors | 3 (1.6) | e.g., intra-abdominal implantation |
| Ileal conduit | 1 (0.5) | |
| Small kidney | 1 (0.5) | Concern about size of renal artery |
| Long cold ischaemia time (> 24 h) | 1 (0.5) | |
| Total | 183 (99.8) |
| Parameter | Stented group (n = 183) | Without stent group (n = 102) | P value |
| Gender (male) | 118 (65) | 63 (62) | 0.648 |
| Age (yr), mean ± SE | 52.4 ± 1.0 (53.7)1 | 49.1 ± 1.3 (50.6)1 | 0.035 |
| Diabetes | 31 (17) | 13 (13) | 0.347 |
| BMI > 30 kg/m2 | 39 (21) | 21 (21) | 0.531 |
| Vesico-ureteric reflux | 11 (6) | 14 (14) | 0.031 |
| First transplant | 151 (83) | 89 (87) | 0.315 |
| Type of transplant | |||
| DCD | 111 (61) | 78 (77) | 0.023 |
| DBD | 12 (7) | 5 (5) | |
| LD | 60 (33) | 19 (19) | |
| Delayed graft function | 55 (30) | 35 (34) | 0.595 |
| Septrin | 173 (99) | 91 (93) | 0.002 |
| Urine leak | 7 (4) | 5 (5) | 0.746 |
| Ureter stenosis | 6 (3) | 7 (7) | 0.359 |
| Ureter necrosis | 1 (0.5) | 0 (0) | |
| Infection | 91 (53) | 40 (42) | 0.075 |
| Operation-infection interval (d) | |||
| mean ± SE | 28.1 ± 3.7 | 33.3 ± 6.2 | 0.451 |
| Median | 10.5 | 11.0 | |
If eight patients with no data regarding urine leak were excluded from analysis, then the overall urine leak rate was 4.3% (12/277). Five of 100 patients (5%) not having a stent inserted during their initial transplant suffered urine leak whereas seven of 177 (4%) in the ST group leaked - the difference in leak rates between the two groups was not statistically significant (Table 2). Similarly, the difference in the distribution of ureteric stenosis or necrosis between groups was not statistically significant (Table 2).
Excluding 18 patients with missing information regarding infection, 49% (131/267) of the patients had infection after transplantation, with the majority (87%) being UTI. Five patients (1.9%) had miscellaneous (non urological) infections. Micro-organisms were isolated in 131 (46%) patients. Infection was caused by multiple organisms in 32% (42/131) but Escherichia coli (21%) was the commonest single isolate. Other coliforms amounted to 23%, whereas Candida was cultured in 1.5% cases. Overall, 54% (99/183) of ST patients developed a urological infection compared to 38.1% (32/84) of the WST group and the difference was statistically significant (χ2 = 5.900; df = 1; P = 0.0151). However, with respect to the initial transplant procedure, the difference in infection rates between ST and WST groups was not statistically significant (Table 2). The difference in the distribution of infection types (UTI or MUI) between the ST and WST groups was statistically significant (Yate’s χ2 = 6.027; df = 1; P = 0.0141). All 18 MUI (9 with urosepsis, 6 with pyelonephritis and 3 with bacteraemia) occurred in those with stents. Ureteric stenting was associated with poorer transplant function at 6 mo and 12 mo (Table 3).
| Cr6 | Cr12 | eGFR6 | eGFR12 | |
| Stent | ||||
| Yes | ||||
| mean ± SE | 137.5 ± 4.6 | 139.7 ± 4.7 | 50.9 ± 1.3 | 49.9 ± 1.3 |
| Median | 123.5 | 125 | 52 | 49.5 |
| n | 180 | 176 | 180 | 176 |
| No | ||||
| mean ± SE | 132.4 ± 5.6 | 124.4 ± 5.4 | 52.1 ± 1.9 | 54.8 ± 2.1 |
| Median | 120.5 | 120 | 52 | 55 |
| n | 78 | 74 | 77 | 73 |
| F-statistic | 0.42 | 3.603 | 0.256 | 4.047 |
| P value | 0.517 | 0.059 | 0.614 | 0.045 |
| Parameters | ||||
| Infection | ||||
| Yes | ||||
| mean ± SE | 143.1 ± 6.2 | 144.0 ± 6.2 | 49.5 ± 1.6 | 48.9 ± 1.7 |
| n | 121 | 118 | 120 | 117 |
| No | ||||
| mean ± SE | 128.0 ± 4.0 | 125.5 ± 4.3 | 53.4 ± 1.4 | 54.4 ± 1.4 |
| n | 123 | 119 | 123 | 119 |
| F-statistic | 2.232 | 3.123 | 2.154 | 4.038 |
| P value | 0.109 | 0.046 | 0.118 | 0.019 |
| Infection type | ||||
| UTI | ||||
| mean ± SE | 140.6 ± 6.9 | 139.8 ± 6.8 | 50.2 ± 1.8 | 50.3 ± 1.8 |
| n | 105 | 104 | 104 | 103 |
| MUI | ||||
| mean ± SE | 156.4 ± 11.5 | 167.6 ± 11.6 | 45.8 ± 3.8 | 41.3 ± 3.7 |
| n | 18 | 16 | 18 | 16 |
| F-statistic | 0.558 | 1.299 | 0.524 | 1.783 |
| P value | 0.574 | 0.277 | 0.593 | 0.173 |
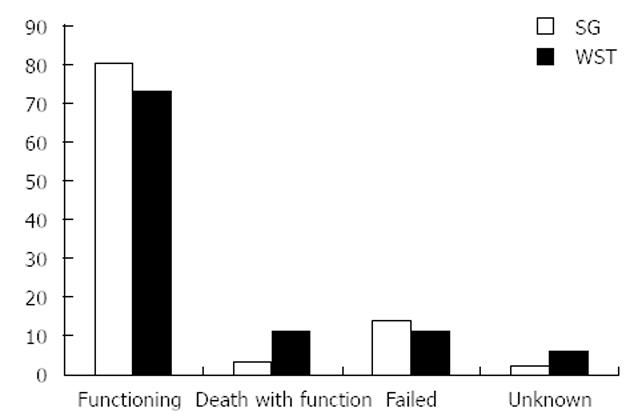
One hundred and eighty three (64.2%) patients achieved immediate allograft function whereas 90 (31.6%) had DGF and 12 (4.2%) had PNF. There was no difference in the rate of DGF between the ST and WST groups (Table 2). By the end of the follow up period, 17 patients had died with a functioning graft and 37 allografts had failed (Figure 1). Although the cause of death was undetermined in six, none of the deaths were directly related to urological infection (cardiac in four, cancer in three, bowel infarction in two, cytomegalovirus infection and trauma in one case respectively.
Infection was more likely to occur in ST patients with DGF (73.7%; 42/57) than in those with immediate allograft function (45%; 54/120) and the difference was statistically significant (χ2 =12.810; df = 1; P = 0.0003). Irrespective of stenting, the association between infection and immediate allograft function [41% (71/173)] or DGF [65% (56/86)] was found to be statistically significant (Fisher’s exact test, P = 0.0003). However, the distribution of UTI and MUI between patients with DGF and immediate function were not statistically significant (Yate’s χ2 = 0.054, df = 1, P = 0.8165). Only the use of stent (Wald χ2 = 5.505, df = 1, P = 0.019), diabetes mellitus (Wald χ2 =5.197, df = 1, P = 0.023) and a BMI > 30 kg/m2 (Wald χ2 =3.801, df = 1, P = 0.051) were shown to have significant influence on urological infection rates on multivariate analysis.
Stents inserted for ≤ 30 d were associated with a higher infection rate of 58.3 % (49/84) compared to a rate of 48% (47/98) for those with stents longer than 30 d (χ2 = 1.953, df = 1, P = 0.163). The median time to infection in the ST group was 10.5 d (Table 2) with 75% of infections occurring by the 38th day postoperatively. The duration of ureteric stenting and transplant - infection interval had no significant correlation with Cr6, Cr12, eGFR6 and eGFR12. However, the number of infection episodes had a significant level of correlation with Cr6, Cr12, eGFR6 and eGFR12 [Pearson correlation (PC) = 0.175, P = 0.008; PC = 0.210, P = 0.002; PC = -0.174, P = 0.009; and PC = -0.231, P = 0.001 respectively]. Patients who developed urological infection had worse allograft function at 6 and 12 mo after transplantation with the differences reaching statistical significance at 12 mo (Table 3). Although patients with MUI had worse Cr and eGFR at 6 mo and 12 mo post transplantation, the differences were not statistically significant (Table 3).
This observational study demonstrates the higher risk of infection in patients with ureteric stenting compared to those without (54% vs 38%) during renal transplantation - rates that are similar to other reports[7,8,12,14] but much higher than the 12% reported by Ashraf et al[15]. Branitz et al[16] and Ranganathan et al[1] not only showed a much higher infection rate in the stented group (76% vs 45% and 71% vs 39%, respectively), but also noted that patients who suffered a UTI while they had a stent in place were more likely to get further episodes of UTI after stent removal. In our study, all eighteen cases of MUI occurred in the stented group. This is similar to the finding by Branitz et al[16] of all 10 episodes of severe infection in their ST group. Though there were no graft losses or patient deaths secondary to MUIs and the rate of DGF in the patients with MUIs was not significantly different to other UTIs, MUIs were associated with poorer transplant function at 12 mo (Table 3). In a Cochrane review of seven randomised controlled trials (1154 patients), Wilson et al[2] found an increased risk of UTIs in stented patients (RR = 1.49, 95%CI: 1.04-2.15; with two kidneys lost to infections), but noted that this effect was neutralised by co-trimoxazole 480 mg daily. Argani et al[17] also demonstrated the role of prophylactic cotrimoxazole in reducing the incidence of UTI in stented patients. Prophylaxis with co-trimoxazole is standard practice at the author’s centre (99% of the ST group received it) but no such beneficial effect was evident. However, there are several reports of a lower UTI rate in the ST group[18-20] or a similar infection rate in both groups[6].
The optimal duration of stenting in renal transplantation is not known. In this study the average duration for stenting over the period under consideration was 46 d. Although the duration of stenting did not significantly correlate with the risk of infection and had no statistically significant impact on Cr levels at 6 and 12 mo in our study, based on a median time to infection of 10.5 d, it would seem reasonable to remove all stents by 2 wk after insertion. This approach is similar to Verma et al[21] who reported from a case controlled study that stenting for two weeks avoided the complications associated with prolonged stenting without compromising the benefits. Also, Tavakoli et al[14] showed that the rate of UTIs was increased, especially if stents were left in for more than 30 d although they advocated stent removal by 4 wk. Based on the understanding that routine placement of stents is aimed at keeping the ureteric anastomosis patent in the postoperative phase when inflammatory oedema is common, there is now a general trend towards early stent removal in order to avoid complications like infections. Dong et al[22] have reported a UTI rate of 4% (3/70) achieved by removing the stent along with the bladder catheter between the seventh and tenth post operative day. Sansalone et al[23] joined the stent and urinary catheter and removed both at 10 d post operatively demonstrating a lower complication rate when compared to those without stents (1.5% vs 4.1% P < 0.0001). The issue about how long to leave a stent in situ is an important one and possibly requires a randomized controlled trial to properly address it. Perhaps another way of reducing the infection complications of stents is through technological development of better materials to reduce or prevent bacterial adherence to the stents. Whether antibacterial coating/impregnation of stents would work is another question.
The finding that urine leak rate was not affected by the placement of ureteric stents (Table 2) in this series is similar to the report by Dharnidharka et al[8] who showed that stents offered no benefit in preventing ureteric stenosis or leaks, nor in improving graft survival. Some studies have demonstrated lower leak rates in the stented group[14,18,19,23,24] whereas Osman et al[12] found a small increase in leakage in the stented group (4% vs 0%) and a significant increase in UTIs (39.6% vs 18%, P = 0.02). Perhaps factors like stripping of the ureter, ureteric injury, multiple renal arteries, damage to lower polar artery, operative technique, cold ischaemia time and donor vascular disease are more important in determining whether urine leak or ureteric necrosis occurs or not. DuBay et al[25] while arguing the case that routine stent placement was inexpensive due to reduction in ureteric complications failed to consider the additional cost of infection related complications.
Review of the literature revealed a dearth of information on the effect of urological infections on subsequent transplant function, although bacteraemia in transplant recipients frequently originates in the urinary tract. An important finding in this study is the deleterious effect of multiple urological infections on transplant function. Whether this negative effect which was demonstrated even at 12 mo impacts on long term function as well needs to be studied in in a larger trial. In light of the fact that stents increase the rate of repeat UTIs[1,16] and the almost exclusive occurrence of MUIs in the stented group, stents may be exerting a harmful effect on graft function. This may in itself be a strong argument in favour of selective placement of stents and needs to be looked at in a larger randomised controlled trial.
A study of this nature has several limitations. The retrospective nature of this study limits its usefulness somewhat, but all the data were collected prospectively and recorded in a designated renal electronic database. In addition there were some gaps in the data, especially in the length of hospital stay, readmission rate, and the incidence of UTI prior to transplantation. This is partly due to the loss of patients to follow up and despite exhaustive efforts to individually chase all cases, data was unavailable from some of the outlying hospitals in the fairly large region covered by our centre. Also, it was not possible to determine the quantitative effect of infection on length of hospital stay or readmissions to hospital.
Notwithstanding the retrospective nature of this study, stents increase the risks of urological infections and appear to have a detrimental effect on early to medium term renal transplant function. Whether stents are used routinely or selectively, there is need to remove them early (< 2 wk) in order to reduce the risk of infection.
We acknowledge with thanks the contribution of Dr. Eugenia Lam, Eleanor Gaff and Anna Wamsley who helped with data collection.
Stents are used to protect the joining between the transplant ureter and the recipient’s bladder when performing kidney transplantation in order to avoid or reduce complications. It is thought that using a stent in this way does not eliminate the risk of complications, particularly urinary leak may in fact increases the risk of urological or blood stream infections. As a result, opinion continues to be divided between those who routinely stent and those who only do so selectively on the basis of clear indications.
There are several reports on the effect of ureter stenting for kidney transplant recipients but the key issues such as how long it should be retained in the body before removal, its effect on kidney function remain unanswered. There are also no well conducted randomised controlled trials to assess the effect of stents.
Proponents of selective stenting state that the associated risks are high enough to avoid routine stenting and advocate that careful surgical technique with selective stenting of problematic anastomoses yields similar results. The key question is to determine what effect the increased risk of urological infection with stenting has on the early and medium term outcome of renal transplantation. In the present study, authors compared the incidence of urological infection in patients with or without stents inserted at transplantation and report the effect of urinary tract infections (UTIs) in the early post transplantation period on short and medium term graft function
This study suggests that stents increase the risks of urological infections and have a detrimental effect on early to medium term kidney transplant function. It calls for a controlled trial to determine the optimum duration of retaining stents following insertion.
A ureteric stent used for the purpose of kidney transplantation is a 6-French, 12 cm, double pigtail ureteral plastic tubing inserted to establish internal drainage from the ureter in to the bladder.The diagnosis of UTI was made on the basis of compatible symptoms such as discomfort during urination, urinary discharge, lower abdominal pain and fever supported by findings on urine strip test and/or microbiological culture. Major urological infections included complicated UTI, pyelonephritis (infection extending to the kidneys) and urosepsis with or without bacteraemia (bacteria multiplying in the blood stream).
Although there are minor recommendations that would be good for the authors if they revise the manuscript accordingly, the manuscript can also be published in this original form as well given the nature of the study which is not randomized.
P- Reviewer Altaca G S- Editor Wen LL L- Editor A E- Editor Xiong L
| 1. | Ranganathan M, Akbar M, Ilham MA, Chavez R, Kumar N, Asderakis A. Infective complications associated with ureteral stents in renal transplant recipients. Transplant Proc. 2009;41:162-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 2. | Wilson CH, Bhatti AA, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. 2005;CD004925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Mangus RS, Haag BW. Stented versus nonstented extravesical ureteroneocystostomy in renal transplantation: a metaanalysis. Am J Transplant. 2004;4:1889-1896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 138] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Nicholson ML, Veitch PS, Donnelly PK, Bell PR. Urological complications of renal transplantation: the impact of double J ureteric stents. Ann R Coll Surg Engl. 1991;73:316-321. [PubMed] |
| 5. | Nicol DL, P’Ng K, Hardie DR, Wall DR, Hardie IR. Routine use of indwelling ureteral stents in renal transplantation. J Urol. 1993;150:1375-1379. [PubMed] |
| 6. | Giakoustidis D, Diplaris K, Antoniadis N, Papagianis A, Ouzounidis N, Fouzas I, Vrochides D, Kardasis D, Tsoulfas G, Giakoustidis A. Impact of double-j ureteric stent in kidney transplantation: single-center experience. Transplant Proc. 2008;40:3173-3175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Silva M, Marra AR, Pereira CA, Medina-Pestana JO, Camargo LF. Bloodstream infection after kidney transplantation: epidemiology, microbiology, associated risk factors, and outcome. Transplantation. 2010;90:581-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 82] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 8. | Dharnidharka VR, Araya CE, Wadsworth CS, McKinney MC, Howard RJ. Assessing the value of ureteral stent placement in pediatric kidney transplant recipients. Transplantation. 2008;85:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Mongha R, Kumar A. Transplant ureter should be stented routinely. Indian J Urol. 2010;26:450-453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Pleass HC, Clark KR, Rigg KM, Reddy KS, Forsythe JL, Proud G, Taylor RM. Urologic complications after renal transplantation: a prospective randomized trial comparing different techniques of ureteric anastomosis and the use of prophylactic ureteric stents. Transplant Proc. 1995;27:1091-1092. [PubMed] |
| 11. | Benoit G, Blanchet P, Eschwege P, Alexandre L, Bensadoun H, Charpentier B. Insertion of a double pigtail ureteral stent for the prevention of urological complications in renal transplantation: a prospective randomized study. J Urol. 1996;156:881-884. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Osman Y, Ali-El-Dein B, Shokeir AA, Kamal M, El-Din AB. Routine insertion of ureteral stent in live-donor renal transplantation: is it worthwhile? Urology. 2005;65:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Dominguez J, Clase CM, Mahalati K, MacDonald AS, McAlister VC, Belitsky P, Kiberd B, Lawen JG. Is routine ureteric stenting needed in kidney transplantation? A randomized trial. Transplantation. 2000;70:597-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 120] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Tavakoli A, Surange RS, Pearson RC, Parrott NR, Augustine T, Riad HN. Impact of stents on urological complications and health care expenditure in renal transplant recipients: results of a prospective, randomized clinical trial. J Urol. 2007;177:2260-224; discussion 2264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 87] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 15. | Ashraf HS, Khan MU, Hussain I, Hyder I. Urological complications in ureteric stenting live related renal transplantation. J Coll Physicians Surg Pak. 2011;21:34-36. [PubMed] |
| 16. | Branitz BH, Veith FJ, Freed SZ, Tellis V, Gliedman ML. Effect of ureteral stent on urinary tract infections in renal transplantation. Urology. 1975;6:687-928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Argani H, Rahnama AM, Amjadi M, Ghafari A, Bahlooli A. The role of stent and cotrimoxazole in prevention of UTI after kidney transplantation. Transplant Proc. 2001;33:2667. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 18. | Briones Mardones G, Burgos Revilla FJ, Pascual Santos J, Marcen Letosa R, Pozo Mengual B, Arambarri Segura M, Fernández Fernández E, Escudero Barrilero A, Ortuño Mirete J. [Comparative study of ureteral anastomosis with or without double-J catheterization in renal transplantation]. Actas Urol Esp. 2001;25:499-503. [PubMed] |
| 19. | Guvence N, Oskay K, Karabulut I, Ayli D. Effects of ureteral stent on urologic complications in renal transplant recipients: a retrospective study. Ren Fail. 2009;31:899-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Luján S, García-Fadrique G, Budía A, Broseta E, Jiménez-Cruz F. [Should ureteral catheterization be systematically used in kidney transplants?]. Actas Urol Esp. 2011;35:213-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Verma BS, Bhandari M, Srivastava A, Kapoor R, Kumar A. Optimum duration of J.J. stenting in live related renal transplantation. Indian J Urol. 2002;19:54-57. |
| 22. | Dong J, Lu J, Zu Q, Yang S, Sun S, Cai W, Zhang L, Zhang X. Routine short-term ureteral stent in living donor renal transplantation: introduction of a simple stent removal technique without using anesthesia and cystoscope. Transplant Proc. 2011;43:3747-3750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 23. | Sansalone CV, Maione G, Aseni P, Mangoni I, Soldano S, Minetti E, Radaelli L, Civati G. Advantages of short-time ureteric stenting for prevention of urological complications in kidney transplantation: an 18-year experience. Transplant Proc. 2005;37:2511-2515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Salahi H, Malek-Hosseini SA, Ghahramani N, Ahmad E, Bahador A, Momtahan S, Karbasi A, Jan-Ghorban P. The efficacy of ureteral stents in prevention of urological complications in renal transplantation. Transplant Proc. 2001;33:2668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | DuBay DA, Lynch R, Cohn J, Ads Y, Punch JD, Pelletier SJ, Campbell DA, Englesbe MJ. Is routine ureteral stenting cost-effective in renal transplantation? J Urol. 2007;178:2509-213; discussion 2513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |