Published online Oct 24, 2012. doi: 10.5500/wjt.v2.i5.74
Revised: August 8, 2012
Accepted: October 20, 2012
Published online: October 24, 2012
Cancer after transplantation is the third cause of death and one of the more relevant comorbidities. Aim of this review is to verify the role of different pathogenetic mechanisms in cancer development in transplant patients and in general population as well. In particular has been outlined the different role exerted by two different families of drug as calcineurin inhibitor and mammalian target of rapamycin (mTOR) inhibitor. The role of mTOR pathways in cell homeostasis is complex but enough clear. As a consequence the mTOR pathway deregulation is involved in the genesis of several cancers. Hence the relevant role of mTOR inhibitors. The authors review the complex mechanism of action of mTOR inhibitors, not only for what concerns the immune system but also other cells as endothelial, smooth muscle and epithelial cells. The mechanism of action is still now not completely defined and understood. It implies the inhibition of mTOR pathway at different levels, but mainly at level of the phosphorylation of several intracellular kinases that contribute to activate mTOR complex. Many prospective and retrospective studies in transplant patients document the antineoplastic role of mTOR inhibition. More recently mTOR inhibitors proven to be effective in the treatment of some cancers also in general population. Kidney cancers, neuroendocrine tumors and liver cancers seem to be the most sensitive to these drugs. Best results are obtained with a combination treatment, targeting the mTOR pathway at different levels.
- Citation: Salvadori M. Antineoplastic effects of mammalian target of rapamycine inhibitors. World J Transplant 2012; 2(5): 74-83
- URL: https://www.wjgnet.com/2220-3230/full/v2/i5/74.htm
- DOI: https://dx.doi.org/10.5500/wjt.v2.i5.74
Cancer after renal transplantation is one of the main morbidity and is the third cause of death after cardiovascular diseases and infections. Cancers account for 7.5% of deaths of patients with a functioning graft and overall for 15% of deaths after renal transplantation, including patients with non functioning graft.
According to different registries, the prevalence of cancer after renal transplantation with respect to general population is mainly higher for non melanoma skin cancer, bladder, kidney, vulvovaginal cancer and non Hodgkin’s lymphoma[1]. The relative risk of cancer after renal transplantation comparing patients on waiting list is 2.55 for skin cancer, 1.12 for bladder, 1.39 for kidney, 2.19 for vulvovaginal and 3.29 for non Hodgkin lymphoma. The Australian and New Zealand registry comparing the ratio of observed vs expected incidence reports a standardized incidence ratio (excluding nonmelanocytic skin cancer) of 435.6 for vulva, 36.7 for vagina, 26.44 for Kaposi’s sarcoma, 10.16 for lymphomas[2]. Besides conventional risk factors such as advanced age and cigarette smoking, peculiar factors of transplant patients seem to be length of dialysis, chronic viral infections, genetic and immunosuppression[3]. Table 1 shows the cancers with an incidence after renal transplantation 5-fold higher with respect to general population, compared with cancers that do not show any significant increase. Table 2 shows that the vast majority of malignancies occurring in transplant patients is linked to chronic viral infections also independently from the type of immunosuppressive therapy.
| Increased≥5 fold | Little/no increase |
| Skin | Breast |
| Vulvovaginal | Prostate |
| Cervix/uterus | Testicular |
| Lymphoma | Ovarian |
| Liver | Lung |
| Kidney/Bladder | Colon |
| Virus | Malignancy |
| EBV | Lymphoma (PTLD) |
| HHV-8 | Kaposi’s sarcoma |
| HPV | Cervical, vulvar cancer |
| HPV-58 | Bowen disease |
| HPV 8, 19 | Non melanoma skin cancer |
| HPV 16, 20 | Skin and tonsillar carcinoma |
| HCV, HBV | Hepatocellular carcinoma |
The early demonstration of immunological rejection of donor transmitted malignancies after discontinuation of immunosuppressive therapy was the first indication of the role of immunosuppressive therapy in transplant related malignancies. The cancer enhancing role of drugs was further supported by Starzl’s report of the regression of lymphomas and lymphoproliferative lesions after the reduction or discontinuation of immunosuppressive drug therapy [4]. The role of immunosuppression was further amplified by the work of Dantal that prospectively compared the cancer incidence with a low cyclosporine (CsA) regimen with that of a standard dose CsA regimen[5]. The normal dose group had a significantly higher incidence of any cancer (P < 0.034) and of skin cancer (P < 0.05). Malignancy-inducing effects of immunosuppressive drugs initially thought to result from drug-induced T-lymphocyte dysfunction i.e., immune surveillance[6]. As in that period calcineurin inhibitors (CNIs) and in particular CsA represented the cornerstone of immunosuppressive therapy, cancer incidence in transplant patients was thought to be related to immunosuppression and CsA in particular. Later on has been documented that the malignancy-inducing effects may primarily result from direct pro-cancer effects independent of host immune system. These factors include the autonomous proliferation, lack of response to antiproliferative signals, evasion of apoptosis, angiogenesis, tissue invasion and metastasis, replicative immortality. These characteristics may be due to activation of oncogenes or inactivation of cancer suppressor genes that modify regulatory “check points” in cell growth[7]. Indeed the relationship between immunosuppressive drugs and malignancies is more complex than thought in the past and immune impairment is only one factor, probably not the most relevant in cancer development. Infections, DNA repair, cancer cellular growth and angiogenesis seem to have a relevant role in cancer development in immunosuppressed patients[8].
As a proof that immunosuppressive agents play only a role, probably not the most important, in cancer development after transplantation is the fact that not all immunosuppressive agents have the same oncogenic activity.
Indeed while in the past the risk of cancer morbidity and mortality has largely been attributed to long-term immunosuppressive drug therapy, which remains necessary to prevent organ allograft rejection, recent studies challenge the premise that all immunosuppressive drugs necessarily promote cancer. A particular class of immunosuppressants referred to as mammalian target of rapamycin (mTOR) inhibitors (mTORIs), has been shown to have potent anti-cancer effects that are now being tested in clinical studies[9].
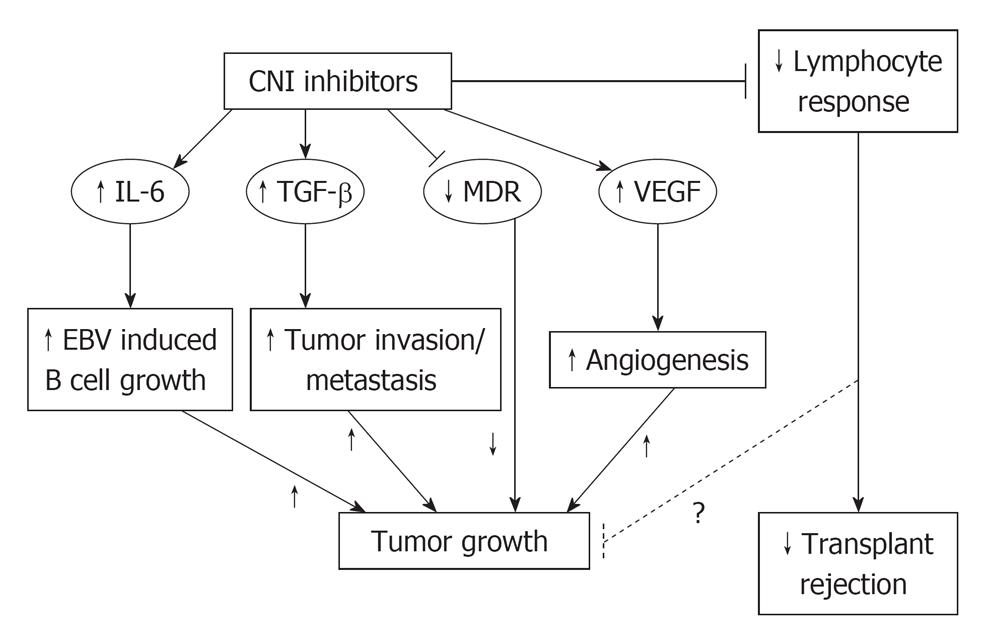
The aforementioned Dantal paper documented the pro-oncogenic activity of CNIs as cyclosporine. CNIs may possibly generate cancer growth via reduction of lymphocyte response, but more probably by interleukin 6 (IL-6) increase, transforming growth factor β (TGF-β) increase and vascular endothelial growth factor (VEGF) increase (Figure 1).
IL-6 promotes B-cell activation, growth and possibly immortalization. This fact could favour post-transplant lymphoproliferative disorders development.
TGF-β increase mediates phenotypic changes by a cell autonomous mechanism, including invasiveness of nontransformed cells[10].
CNIs also increase the production of VEGF that is a powerful agent of angiogenesis, strictly linked to cancer cell development and cancer increase[11]. Indeed CNIs effect on the expression of VEGF leads to an angiogenic milieu that favors cancer growth[12].
The mTORI besides their antirejection effect, seem to have a different profile with respect to malignancies when compared with CNIs.
mTORIs are immunosuppressive agents, widely used in transplantation. They form a complex with the FK binding protein complex (FKBP-12). This complex binds with high affinity to mTOR. Rapamycin and derivatives, including CCI-779 and RAD001, inhibit mTOR, down-regulating p70S6 kinase activity and subsequent translation of specific mRNAs required for cell-cycle progression from the G1 to S phase. In transplantation, everolimus (EVL) and or sirolimus (SRL) demonstrate immunosuppressive properties and has been used to prevent acute rejection in cardiac[13], liver[14], lung and renal transplant recipients. It appears that this agent may be potent enough to allow for the minimization or removal of calcineurin inhibitors in the long term of renal transplant recipients.
Due to their action on different kind of cells, besides the action on lymphocytes these drugs have also other effects. Because the action on endothelial and vascular smooth muscle cells in cardiology, EVL is available as a drug-coated stent and is used in percutaneous coronary interventions for prevention of restenosis[15,16]. Because of the antiproliferative action on fibroblast, rapamycins can cause delay in wound healing and lymphoceles. The same action on fibroblast has also positive effects in liver transplantation, attenuating liver fibrosis[17,18].
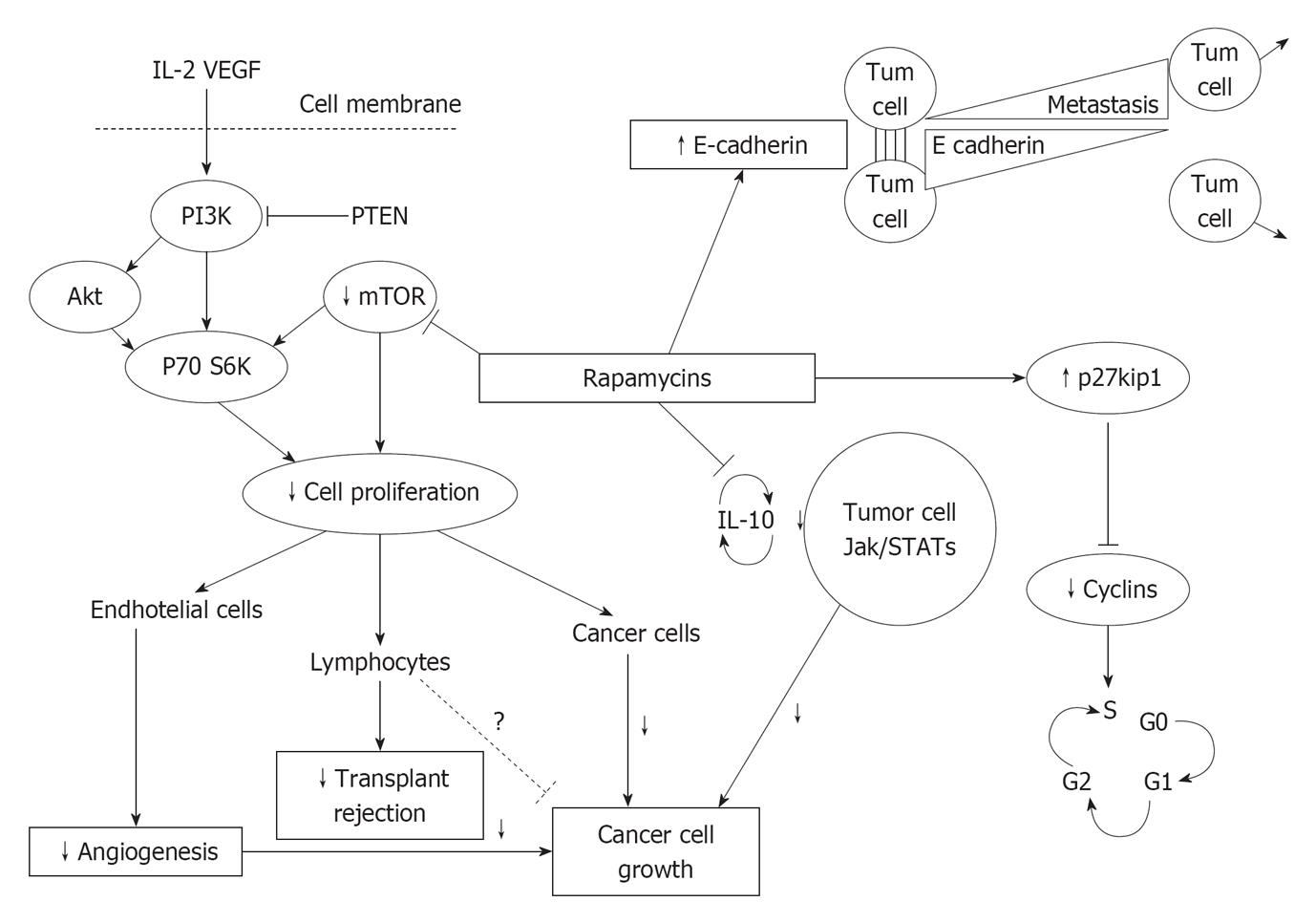
mTORIs could have a protective action against malignancies at least by four different pathways (Figure 2): (1) The increase of E-cadherin levels favors cellular adhesion and blocks neoplastic cells migration[19]; (2) The increase of p-27kip-1 kinase inhibits cyclins, needed for cell cycle[20]; (3) The reduction of IL-10 inhibits cellular Janus kinase- Signal transducer and activator of transcription (Jak-Stat) transcription and cell growth[21]; and (4) The inhibition of the serine-threonine kinase mTOR reduces proliferation of different kind of cells, as (a) Endothelial and smooth muscle cells (angiogenesis); (b) T lymphocytes (antirejection activity); and (c) Neoplastic cells.
Such biological insights on the protective effect of mTORIs on new malignancies after renal transplantation are confirmed by several retrospective and prospective studies[22].
The vast majority of these studies compare, as maintenance immunosuppressive therapy, therapies mainly based on CNIs with therapy based on mTORIs without CNIs or with CNIs minimization.
In a randomized prospective trial, patients who received rapamycin-based, calcineurin inhibitor-free therapy after CsA withdrawal at month 3 had a reduced incidence of both skin and non-skin malignancies at 5 years after renal transplantation compared with those who received rapamycin therapy combined with CsA[23]. In the CONVERT study renal transplant patients were randomized after transplantation either to receive rapamycin or to continue CsA therapy. About 10.2% patients on CNI had malignancies vs 3.4% patients converted to rapamycin (P < 0.001). The effect was similar for skin cancers (6.9% vs 1.8%, P < 0.001) and non skin cancers (4.4% vs 1.1%; P = 0.004)[24]. In a systematic review and meta-analysis of randomized trials, 33 studies were included (27 trials of SRL, 5 of EVL and 1 head-to-head). The relative risk to have malignancy was lower in any kind of comparison and in favor of mTORIs[25].
A retrospective study of the OPTN/UNOS database on 33 249 deceased donor kidney transplants revealed that 504 patients received either SRL or EVL without a calcineurin inhibitor, 2321 received either SRL or EVL in combination with a calcineurin inhibitor, and 30 424 received a calcineurin inhibitor without a mTOR inhibitor. Data were censored at 963 d to allow comparable follow up among the treatment groups. The incidence of any malignancy was 0.60% for both SRL/EVL alone and SRL/EVL plus a calcineurin inhibitor and was 1.81% for calcineurin inhibitors (P < 0.00001). The incidence rates for de novo solid malignancies were 0% for SRL/EVL alone, 0.47% for SRL/EVL plus calcineurin inhibitor, and 1.0% for calcineurin inhibitors. Multivariate analysis indicated that mTOR inhibitor maintenance immunosuppression was associated with a 60% reduced risk of any post-transplant malignancy and a 55% reduced risk of solid malignancy[26].
As aforementioned, rapamycins (a group of parental compounds), block an intracellular serine-threonine kinase called mTOR.
Extracellular signal regulated kinase pathway, c-Jun terminal kinase pathway, p38 pathway and mTOR pathway regulate cell survival, proliferation, apoptosis resistance, angiogenesis and metastasis diffusion[27]. Therefore is not surprising that such protein deregulation could be involved in cancer development representing a target in the treatment of solid tumors.
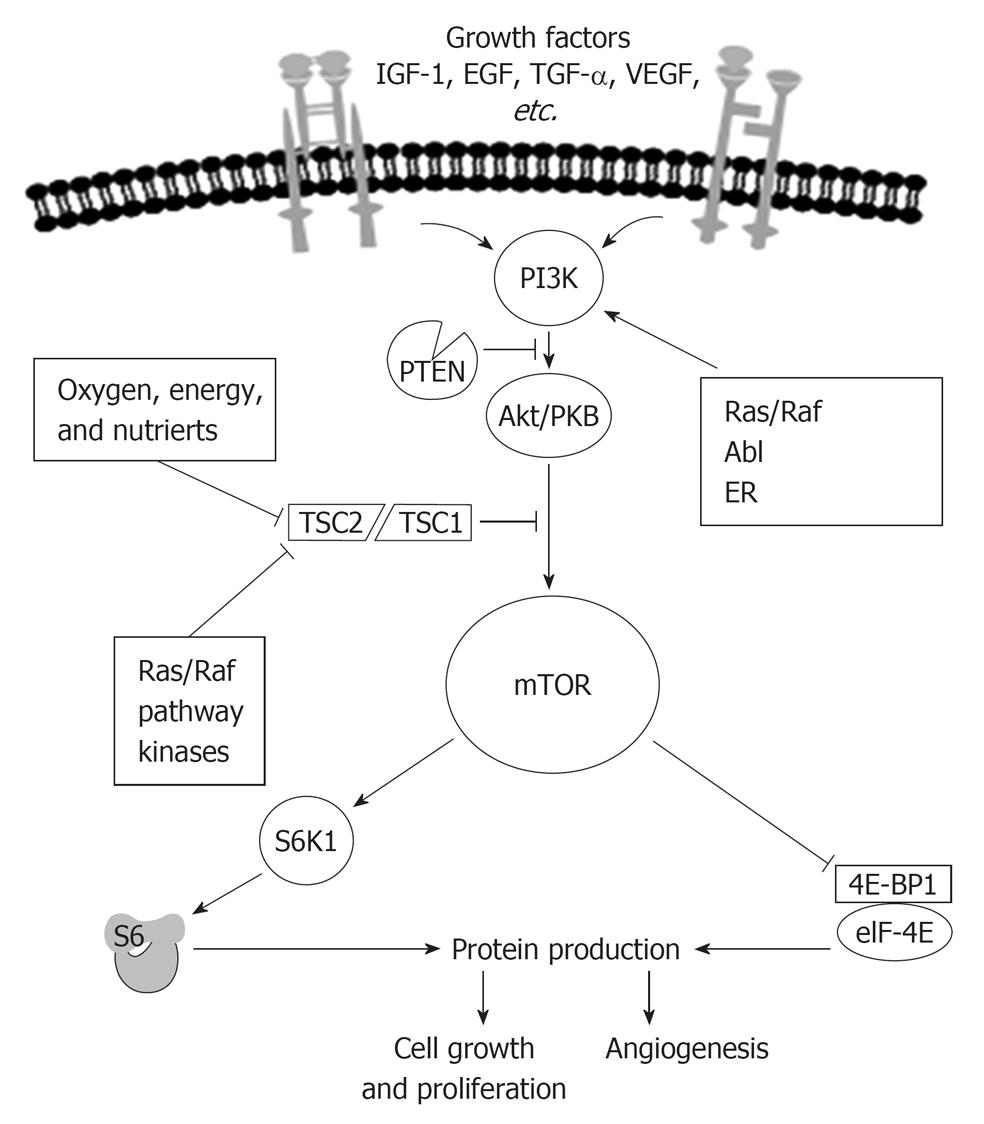
Which is exactly the role of the serine threonine kinase mTOR in cancer development mTOR is a central controller of cell growth and proliferation in normal cells. mTOR integrates signals from a variety of sources, including nutrients and growth factors. mTOR acts to induce protein synthesis of molecules necessary for angiogenesis[28], cell growth, and nutrient uptake[29] for cell survival[30].
Growth factors, such as insulin like growth factor (IGF), epidermal growth factor (EGF)[31], platelet derived growth factor, and VEGF, bind to and activate receptors located on the cell surface[32].
Receptors activate intracellular signaling cascades that regulate cell growth, angiogenesis, and nutrient uptake[33].
mTOR is a key integration point for information received from upstream receptors[34].
The mTOR pathway is fully operating in a nutrient rich environment[35,36]. Indeed the activity of mTOR is regulated by stress-inducing conditions associated to the microenvironment. Suboptimal conditions for cellular anabolic metabolism (e.g., amino acid starvation, hypoxia or glucose deficiency) or cell progression (e.g., growth factor deprivation) inactivate mTOR by several pathways converging on the tuberous sclerosis complex (TSC), which, through its Ras homologue enriched in brain (Rheb-GAP) activity, inactivates Rheb, thereby suppressing mTOR[37]. Subsequently, energy consumption is reduced through a reduction in protein synthesis, cell delay G1-S progression and cell viability is maintained through autophagic recycling of cellular macromolecules[24,38].
Figure 3 shows how in normal conditions growth-stimulating signals originating within and outside the cell act on growth factor receptors and sequentially on signaling molecules as phosphoinositide 3-kinase (PI3K), that, when not blocked by phosphatase and tensin homologue delated on chromosome 10 (PTEN), phosphorylates and activates Akt, that, when not blocked by TSC1/2 activates mTOR.
One of the most interesting recent findings is that mTOR itself is part of two distinct signaling complexes; one complex, mTOR complex 1 (mTORC1) receives a signal from an upstream molecule AKT and is rapamycin sensitive, the second complex, mTOR Complex 2 (mTORC2) regulates AKT through Ser473 phosphorylation, mTORC2 is rapamycin insensitive. AKT is activated by phosphoinositide-dependent kinase 1 (PDK1) phosphorylation at a second site, Thr308, with phosphorylation at both sites required for maximal AKT activity[39]. Growth factors binding to their cognate receptors results in a recruitment of receptor substrate (e.g., insulin receptor substrate; IRS-1), and binding of PI3K. PI3K converts phosphatidylinositiol-4,5-phosphate (PIP2) to phosphatidylinositiol-3,4,5-phosphate (PIP3), which recruits PDK1 that phosphorylates AKT in the site Thr308. mTORC2 regulates also AKT through Ser473 phosphorylation. Importantly, phosphatase and tensin homologue deleted on chromosome 10 (PTEN), a tumor suppressor, reverses the PIP2-to-PIP3 reaction, thus reducing AKT activity. When activated, AKT mediates mTORC1 activation via inhibition of a tumor suppressor complex made up the two tuberous sclerosis proteins, hamartin (TSC1) and tuberin (TSC2). The TSC1/2 complex functions as a GTPase-activating protein, resulting in inactivation of Ras homolog enriched in brain (Rheb). In the GTP-bound state, Rheb selectively complexes with and activates mTORC1[40,41].
From another perspective has been recently discovered that inhibitory κB kinase β, a kinase downstream of tumor necrosis factor α, integrates with mTORC1 by inhibiting TSC1/2, thus providing a new molecular link between inflammation and cancer[42].
The importance of mTOR in regulating normal cell growth, cell division and angiogenesis is highlighted by the number of proteins involved in its activation or inhibition[43,44].
mTOR is deregulated in cancer by increased upstream signaling, loss-of-function mutations in upstream inhibitors, and activating mutations in mTOR activators.
Increased mTOR activity results in the increased protein synthesis of more than 100 genes and proteins involved in cellular responses. Many of the proteins that are regulated by mTOR support the growth, metabolic requirements, and survival of cancer cells.
Deregulation of the mTOR-linked pathways increase the risk of developing cancer or have been identified in many cancers[45,46].
mTOR activation, beside other activities, stimulates translation of hypoxia inducible factor-1α (HIF-1α), which ultimately increases production of proangiogenic factors such as VEGF-A and other molecules such as those involved in glucose transport. In well-oxygenated cells, HIF-1α is degraded by the von Hippel-Lindau (VHL) protein, which binds and targets it for destruction by the proteasome; loss of the VHL protein is a driving force in the development of some cancers, such as clear cell renal cancer[47,48].
In hypoxic cells, such as those found in cancers, HIF-1α translocates to the nucleus and combines with HIF-1β, ultimately initiating the transcription of hypoxia-regulated genes, such as those for VEGF-A and inducible nitric oxide synthetase, which promote cell survival under anaerobic conditions, angiogenesis, metastasis[49].
Overexpression of HIF-1α has been associated with cancers of the breast, ovary, cervix, esophagus, brain, and head and neck higher aggressive and with a poorer prognosis; loss of HIF-1 activity decreases tumor growth, vascularization, and energy metabolism. mTOR inhibition can decrease HIF-1α levels and inhibits VEGF production[32-33,50].
Finally mTORC1 has a role also in regulating DNA damage caused by agents such as cisplatin. DNA damage activates p53. p53 triggers DNA repair, which allows the cell to survive, or, failing that, p53 initiates cell death. mTOR regulates production of p21, a cell cycle inhibitor that allows DNA repair. mTOR inhibition blocks p21 translation, forcing cell death even when the DNA damage is otherwise nonlethal. By this way mTOR inhibition can enhance the activity of certain drugs such as cisplatin and other platinum derivatives[51].
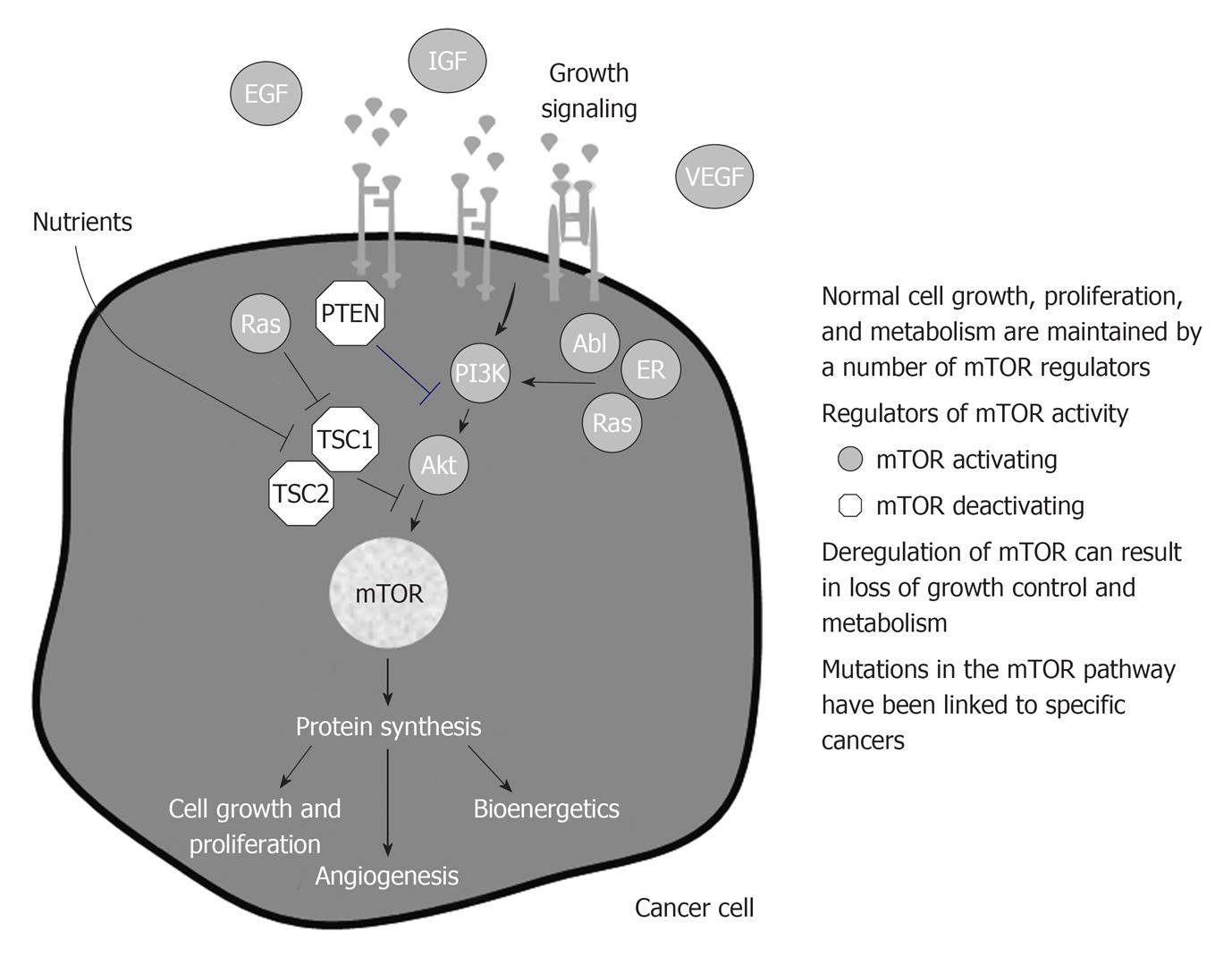
Deregulation of the mTOR-linked pathways increases the risk of developing cancer or have been identified in many cancers (Figure 4).
As a consequence the role of genetics in cancer development both in transplant patients and in general population is easy to be understood.
Growth factor receptors are altered in many cancers. Frequently, a deletion of the ligand-binding domain of EGF receptor (EGFR), a transmembrane protein with tyrosine kinase activity, causes constitutive activation of the receptor in the absence of ligand binding[52].
Some of mTOR inhibitors as p53 and PTEN are often deleted or mutated in human cancer. Such kind of tumor cells are extremely sensible to the effect of mTOR inhibitors[53,54].
In detail deregulation of the pathway can include overexpression of growth factors, overexpression or mutations of growth factor receptors, loss of tumor suppressor genes, and gain-of function mutations in mTOR-linked pathways, such as: (1) Inappropriate signaling through members of the human epidermal growth factor receptor (HER/EGFR) family in lung, colon, and breast cancers[55-57]; (2) Activation of the estrogen receptor through ligand independent pathways linked to mTOR in breast cancer[58]; (3) High levels of IGF-1 or expression of IGF-1 receptor in breast, prostate, lung, thyroid, and kidney cancers, melanoma, and sarcoma[59-65]; (4) Increase Ras or PI3K signaling through activating mutations or loss-of-function mutations in tumor suppressor genes in pancreas, colon, thyroid, lung, leukemia, brain, gastric, breast, ovarian, prostate, endometrial, and oral squamous cancers and melanoma[66-75]; (5) Formation of the Bcr-Abl fusion gene, which causes Ph+ chronic myelogenous leukemia[76]; and (6) Deregulated signaling or cross-talk through mTOR linked pathways can increase mTOR activity; mTOR inhibition could counteract this deregulated signaling. This represents the rationale because combining an agent that directly targets mTOR with an agent that targets a deregulation in an mTOR-linked pathway could produce more profound anticancer activity than either agent alone, particularly in cancers that have lost function of the tumor suppressor gene, PTEN[77].
In the last decade a number of mTORC1 activating molecules have been found abnormally high (gain of function) and linked to specific cancers. PI3K is abnormally high in a variety of human cancers, as well as AKT[78]. Rheb and ras have similarly been found abnormally high in human cancers[79]. Also the effectors of mTOR have been found overexpressed in human cancers, in breast cancers and correlate with poor prognosis[80]. Similarly, among tumor suppressors, mutations linked to tumor development have been found. Loss of PTEN is linked to hamartoma syndromes, as well as TSC and the serine threonine kinase 11 (LKB1)[81]. P53 is mutated in the majority of human tumors. NF1 mutation is associated to neurofibromatosis type 1[82].
In selected human cancer mTOR linked pathway deregulations has been found as shown in Table 3: Lung cancer: deregulation of EGFR, AKT, Ras, PTEN and PI3K in a range from 4% to 60%; Kidney cancer: deregulation of TGF, VHL, IGF-1/IGF-1R, AKT, PTEN TSC1/2 in a range from 31% to 100%; Breast cancer: deregulation of AKT, PTEN, PI3K, EGFR in a range from 6% to 42%; Neuroendocrine tumors: VHL, IGF-1/IGF-1R, TSC1/2; and colon carcinoma: EGFR, PI3K, PTEN, AKT, Ras in a range from 3% to 50%.
| Lung | EGFR | 32-60 |
| p-AKT | 23-50 | |
| Ras | 30 | |
| PTEN | 24 | |
| HER2 | 5 | |
| PI3K | 4 | |
| Kidney | TGF-α/TGF-β1 | 60-100 |
| VHL | 30-50 | |
| IGF-1/IGF-1R | 39-69 | |
| p-AKT | 38 | |
| PTEN | 31 | |
| TSC1/TSC2 | ||
| Breast | p-Akt | 42 |
| PTEN | 15-41 | |
| HER2 | 30-36 | |
| PI3K | 18-26 | |
| EGFR | 6 | |
| NET | TSC1/TSC2 | |
| IGF-1/IGF-1R | ||
| VHL | ||
| Colon | Ras | 50 |
| p-Akt | 46 | |
| PTEN | 35 | |
| PI3K | 20-32 | |
| EGFR | 8 | |
| HER2 | 3 |
In summary aberrant signaling through upstream pathways can activate mTOR inappropriately: (1) Abnormal cell growth, proliferation, and angiogenesis; and (2) Survival of cancer cells in the nutrient- and oxygen-depleted tumor environment.
Targeting deregulated pathways has been a successful clinical strategy in cancer and a combination therapy targeting mTOR and deregulated pathways may provide enhanced anticancer activity [56,58].
As already outlined and as a consequence of the aforementioned pathogenesis, a two hits therapy or a combination therapy targeting both upstream signaling and mTORC1 is a highly promising strategy[31,77].
Overall four groups of agents have been developed for targeting solid cancers, alone or in combination: (1) Agents targeting EGFR; (2) Agents targeting IGF-1R; (3) Agents targeting VEGF/VEGFR; and (4) Agents targeting multi-kinase, among which the mTORIs have a prevalent role[71,83].
The beneficial effect of mTORIs on cancer prevention in transplant patients has been documented in the aforementioned clinical trials.
Recently a beneficial effect of rapamycin for Kaposi’s sarcoma in renal-transplant recipients has been reported in 15 patients[84].
In a different study a switch from CNIs to mTORIs has been performed in 53 renal transplant recipients developing non melanoma skin cancer after transplantation. A remission was observed in 37 patients with minimal adverse events reported[85].
EVL has been used in liver transplant patients with de novo hepatocellular carcinoma after liver transplantation. The probability of survival in 10 patients of the EVL group was significantly greater than the observed in a historical cohort of 14 similar patients who did not receive EVL (HR = 4.6, P = 0.008)[86].
Thinking with the old concept that reduction in immune surveillance is the main factor in cancer genesis, could seem paradoxical the use of immunosuppressive agents like mTORIs in the treatment of cancer in patients not needing immunosuppressive therapy. It is not so if we look to the proven involvement of mTOR pathways in cancer development. The block of abnormal mTOR pathways united with other antineoplastic agents seems now the best therapeutic approach.
Indeed mTORIs and EVL in particular have proven be effective in targeting cancer also in general population, independently from transplantation.
Many hematological malignancies have aberrant activation of the mTOR and related signaling pathways. Accordingly mTOR inhibitors, a class of signal transduction inhibitors, originally developed as immunosuppressive agents, are being investigated in preclinical models and clinical trials for a number of hematological malignancies[50,87,88].
Several data indicate that pharmacological agents that target PI3K, AKT, or FRAP in prostate cancer cells, inhibit HIF-1α expression and that such inhibition may contribute to therapeutic efficacy[89,90].
Recently FDA approved EVL in metastatic renal cell carcinoma after a trial documenting in 272 patients affected by such carcinoma the efficacy of EVL with respect to standard therapy[91].
In a recent study (RADIANT-3) 410 patients with low grade or intermediate grade pancreatic neuroendocrine tumors were randomized to receive EVL or placebo. EVL significantly prolonged progression-free survival and was associated with a low rate of severe adverse events[92].
In the BOLERO-2 trial mTOR inhibitor EVL, added to endocrine therapy showed antitumor activity. In such patients indeed the resistance to endocrine therapy in breast cancer is associated with activation of the mTOR intracellular signaling pathway[93].
Recently a synergistic effect of mTOR inhibitor and chemotherapy in a rat model of hepatocellular carcinoma has been found[94]. Many clinical trials now at their final or preliminary publication, are planned or are actively recruiting patients for treatment of liver carcinoma with mTOR inhibitors.
mTOR inhibitors are a group of parent drugs with a well defined immunosuppressive property and are widely used as immunosuppressant drugs in kidney, liver, lung and heart transplantation. Thanks to their mechanism of action, favouring apoptosis and inhibiting proliferation of both immune and non immune cells, such drugs have a documented antineoplastic action in transplant patients. Some of them as SRL (rapamycine), EVL (afinitor/certican), temSRL (torisel) and deforolimus are either launched or in advanced development stage in cancer therapy also outside transplantation.
As aforementioned their mechanism of action is still now not fully understood and most probably this group of drugs will prove to be effective in controlling some types of cancer, and other not. The complexity of the mTOR pathway, mainly considering the negative feedback loops that exist, suggests that only properly designed clinical trials will provide the final answer. Nonetheless, our present knowledge of the mTOR pathway supports such trials.
Peer reviewers: Andrea De Gottardi, MD, PhD, Assistant Professor, Clinic of Visceral Surgery and Medicine, Hepatology, Freiburgstrasse, CH-3010 Berne, Inselspital, Switzerland; Caigan Du, PhD, Assistant Professor, Department of Urologic Sciences, University of British Columbia, Jack Bell Research Centre, 2660 Oak Street, Vancouver, BC V6H 3Z6, Canada
S- Editor Wang JL L- Editor A E- Editor Zheng XM
| 1. | Kasiske BL, Snyder JJ, Gilbertson DT, Wang C. Cancer after kidney transplantation in the United States. Am J Transplant. 2004;4:905-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 772] [Cited by in RCA: 763] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 2. | Buell JF, Gross TG, Woodle ES. Malignancy after transplantation. Transplantation. 2005;80:S254-S264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 3. | Morath C, Mueller M, Goldschmidt H, Schwenger V, Opelz G, Zeier M. Malignancy in renal transplantation. J Am Soc Nephrol. 2004;15:1582-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Kauffman HM, Cherikh WS, McBride MA, Cheng Y, Hanto DW. Post-transplant de novo malignancies in renal transplant recipients: the past and present. Transpl Int. 2006;19:607-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 5. | Dantal J, Hourmant M, Cantarovich D, Giral M, Blancho G, Dreno B, Soulillou JP. Effect of long-term immunosuppression in kidney-graft recipients on cancer incidence: randomised comparison of two cyclosporin regimens. Lancet. 1998;351:623-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 572] [Cited by in RCA: 537] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 6. | Guba M, Graeb C, Jauch KW, Geissler EK. Pro- and anti-cancer effects of immunosuppressive agents used in organ transplantation. Transplantation. 2004;77:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 7. | Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19834] [Cited by in RCA: 19515] [Article Influence: 780.6] [Reference Citation Analysis (0)] |
| 8. | Geissler EK, Schlitt HJ. The relation between immunosuppressive agents and malignancy. Curr Opin Organ Transplant. 2004;9:394-399. [RCA] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Gaumann A, Schlitt HJ, Geissler EK. Immunosuppression and tumor development in organ transplant recipients: the emerging dualistic role of rapamycin. Transpl Int. 2008;21:207-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 39] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, Shimbo T, Suthanthiran M. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999;397:530-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 852] [Cited by in RCA: 827] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 11. | Guba M, von Breitenbuch P, Steinbauer M, Koehl G, Flegel S, Hornung M, Bruns CJ, Zuelke C, Farkas S, Anthuber M. Rapamycin inhibits primary and metastatic tumor growth by antiangiogenesis: involvement of vascular endothelial growth factor. Nat Med. 2002;8:128-135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1347] [Cited by in RCA: 1301] [Article Influence: 56.6] [Reference Citation Analysis (0)] |
| 12. | Basu A, Contreras AG, Datta D, Flynn E, Zeng L, Cohen HT, Briscoe DM, Pal S. Overexpression of vascular endothelial growth factor and the development of post-transplantation cancer. Cancer Res. 2008;68:5689-5698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 13. | Viganò M, Tuzcu M, Benza R, Boissonnat P, Haverich A, Hill J, Laufer G, Love R, Parameshwar J, Pulpón LA. Prevention of acute rejection and allograft vasculopathy by everolimus in cardiac transplants recipients: a 24-month analysis. J Heart Lung Transplant. 2007;26:584-592. [PubMed] |
| 14. | Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, Kam I. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008;14:633-638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Gabardi S, Baroletti SA. Everolimus: a proliferation signal inhibitor with clinical applications in organ transplantation, oncology, and cardiology. Pharmacotherapy. 2010;30:1044-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 86] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Huang S, Bjornsti MA, Houghton PJ. Rapamycins: mechanism of action and cellular resistance. Cancer Biol Ther. 2003;2:222-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 236] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 17. | Biecker E, De Gottardi A, Neef M, Unternährer M, Schneider V, Ledermann M, Sägesser H, Shaw S, Reichen J. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J Pharmacol Exp Ther. 2005;313:952-961. [PubMed] |
| 18. | McKenna GJ, Trotter JF, Klintmalm E, Onaca N, Ruiz R, Jennings LW, Neri M, O'Leary JG, Davis GL, Levy MF. Limiting hepatitis C virus progression in liver transplant recipients using sirolimus-based immunosuppression. Am J Transplant. 2011;11:2379-2387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 19. | Luan FL, Hojo M, Maluccio M, Yamaji K, Suthanthiran M. Rapamycin blocks tumor progression: unlinking immunosuppression from antitumor efficacy. Transplantation. 2002;73:1565-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 187] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 20. | Nourse J, Firpo E, Flanagan WM, Coats S, Polyak K, Lee MH, Massague J, Crabtree GR, Roberts JM. Interleukin-2-mediated elimination of the p27Kip1 cyclin-dependent kinase inhibitor prevented by rapamycin. Nature. 1994;372:570-573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 701] [Cited by in RCA: 722] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 21. | Nepomuceno RR, Balatoni CE, Natkunam Y, Snow AL, Krams SM, Martinez OM. Rapamycin inhibits the interleukin 10 signal transduction pathway and the growth of Epstein Barr virus B-cell lymphomas. Cancer Res. 2003;63:4472-4480. [PubMed] |
| 22. | Mathew T, Kreis H, Friend P. Two-year incidence of malignancy in sirolimus-treated renal transplant recipients: results from five multicenter studies. Clin Transplant. 2004;18:446-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 226] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 23. | Campistol JM, Eris J, Oberbauer R, Friend P, Hutchison B, Morales JM, Claesson K, Stallone G, Russ G, Rostaing L. Sirolimus therapy after early cyclosporine withdrawal reduces the risk for cancer in adult renal transplantation. J Am Soc Nephrol. 2006;17:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 379] [Cited by in RCA: 354] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 24. | Schena FP, Pascoe MD, Alberu J, del Carmen Rial M, Oberbauer R, Brennan DC, Campistol JM, Racusen L, Polinsky MS, Goldberg-Alberts R. Conversion from calcineurin inhibitors to sirolimus maintenance therapy in renal allograft recipients: 24-month efficacy and safety results from the CONVERT trial. Transplantation. 2009;87:233-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 455] [Cited by in RCA: 434] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 25. | Webster AC, Lee VW, Chapman JR, Craig JC. Target of rapamycin inhibitors (sirolimus and everolimus) for primary immunosuppression of kidney transplant recipients: a systematic review and meta-analysis of randomized trials. Transplantation. 2006;81:1234-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 26. | Kauffman HM, Cherikh WS, Cheng Y, Hanto DW, Kahan BD. Maintenance immunosuppression with target-of-rapamycin inhibitors is associated with a reduced incidence of de novo malignancies. Transplantation. 2005;80:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 464] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 27. | Giamas G, Man YL, Hirner H, Bischof J, Kramer K, Khan K, Ahmed SS, Stebbing J, Knippschild U. Kinases as targets in the treatment of solid tumors. Cell Signal. 2010;22:984-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 28. | Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 2002;16:771-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 304] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 29. | Edinger AL, Thompson CB. Akt maintains cell size and survival by increasing mTOR-dependent nutrient uptake. Mol Biol Cell. 2002;13:2276-2288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 464] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 30. | Jaeschke A, Dennis PB, Thomas G. mTOR: a mediator of intracellular homeostasis. Curr Top Microbiol Immunol. 2004;279:283-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 31. | Mitsudomi T, Yatabe Y. Epidermal growth factor receptor in relation to tumor development: EGFR gene and cancer. FEBS J. 2010;277:301-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 32. | Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424-430. [PubMed] |
| 33. | Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. Physiology (Bethesda). 2006;21:362-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 478] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 34. | Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596-603. [PubMed] |
| 35. | Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J Clin Invest. 2006;116:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 258] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 36. | Motoshima H, Goldstein BJ, Igata M, Araki E. AMPK and cell proliferation--AMPK as a therapeutic target for atherosclerosis and cancer. J Physiol. 2006;574:63-71. [PubMed] |
| 37. | Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2185] [Cited by in RCA: 2296] [Article Influence: 127.6] [Reference Citation Analysis (0)] |
| 38. | Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets. 2008;12:209-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 74] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 39. | Plas DR, Thompson CB. Akt-dependent transformation: there is more to growth than just surviving. Oncogene. 2005;24:7435-7442. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 40. | Geissler EK, Schlitt HJ, Thomas G. mTOR, cancer and transplantation. Am J Transplant. 2008;8:2212-2218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | Manning BD, Cantley LC. Rheb fills a GAP between TSC and TOR. Trends Biochem Sci. 2003;28:573-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 377] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 42. | Lee DF, Kuo HP, Chen CT, Hsu JM, Chou CK, Wei Y, Sun HL, Li LY, Ping B, Huang WC. IKK beta suppression of TSC1 links inflammation and tumor angiogenesis via the mTOR pathway. Cell. 2007;130:440-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 489] [Cited by in RCA: 510] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 43. | Averous J, Proud CG. When translation meets transformation: the mTOR story. Oncogene. 2006;25:6423-6435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Mamane Y, Petroulakis E, LeBacquer O, Sonenberg N. mTOR, translation initiation and cancer. Oncogene. 2006;25:6416-6422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 491] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 45. | Ellisen LW. Growth control under stress: mTOR regulation through the REDD1-TSC pathway. Cell Cycle. 2005;4:1500-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 96] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 46. | Kaper F, Dornhoefer N, Giaccia AJ. Mutations in the PI3K/PTEN/TSC2 pathway contribute to mammalian target of rapamycin activity and increased translation under hypoxic conditions. Cancer Res. 2006;66:1561-1569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Powis G, Kirkpatrick L. Hypoxia inducible factor-1alpha as a cancer drug target. Mol Cancer Ther. 2004;3:647-654. [PubMed] |
| 48. | Vaupel P. The role of hypoxia-induced factors in tumor progression. Oncologist. 2004;9 Suppl 5:10-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 595] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 49. | Stoeltzing O, McCarty MF, Wey JS, Fan F, Liu W, Belcheva A, Bucana CD, Semenza GL, Ellis LM. Role of hypoxia-inducible factor 1alpha in gastric cancer cell growth, angiogenesis, and vessel maturation. J Natl Cancer Inst. 2004;96:946-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 195] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 50. | Haase VH. Hypoxia-inducible factors in the kidney. Am J Physiol Renal Physiol. 2006;291:F271-F281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 51. | Beuvink I, Boulay A, Fumagalli S, Zilbermann F, Ruetz S, O'Reilly T, Natt F, Hall J, Lane HA, Thomas G. The mTOR inhibitor RAD001 sensitizes tumor cells to DNA-damaged induced apoptosis through inhibition of p21 translation. Cell. 2005;120:747-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 384] [Cited by in RCA: 407] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 52. | Croce CM. Oncogenes and cancer. N Engl J Med. 2008;358:502-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 643] [Article Influence: 37.8] [Reference Citation Analysis (0)] |
| 53. | Sansal I, Sellers WR. The biology and clinical relevance of the PTEN tumor suppressor pathway. J Clin Oncol. 2004;22:2954-2963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 684] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 54. | Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci USA. 2001;98:10314-10319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 768] [Cited by in RCA: 789] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 55. | Cappuzzo F, Hirsch FR, Rossi E, Bartolini S, Ceresoli GL, Bemis L, Haney J, Witta S, Danenberg K, Domenichini I. Epidermal growth factor receptor gene and protein and gefitinib sensitivity in non-small-cell lung cancer. J Natl Cancer Inst. 2005;97:643-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1267] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 56. | Cunningham D, Humblet Y, Siena S, Khayat D, Bleiberg H, Santoro A, Bets D, Mueser M, Harstrick A, Verslype C. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3767] [Cited by in RCA: 3708] [Article Influence: 176.6] [Reference Citation Analysis (1)] |
| 57. | Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783-792. [PubMed] |
| 58. | Ali S, Coombes RC. Endocrine-responsive breast cancer and strategies for combating resistance. Nat Rev Cancer. 2002;2:101-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 622] [Cited by in RCA: 658] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 59. | Hankinson SE, Willett WC, Colditz GA, Hunter DJ, Michaud DS, Deroo B, Rosner B, Speizer FE, Pollak M. Circulating concentrations of insulin-like growth factor-I and risk of breast cancer. Lancet. 1998;351:1393-1396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1264] [Cited by in RCA: 1199] [Article Influence: 44.4] [Reference Citation Analysis (0)] |
| 60. | Chan JM, Stampfer MJ, Giovannucci E, Gann PH, Ma J, Wilkinson P, Hennekens CH, Pollak M. Plasma insulin-like growth factor-I and prostate cancer risk: a prospective study. Science. 1998;279:563-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1302] [Article Influence: 48.2] [Reference Citation Analysis (0)] |
| 61. | Minuto F, Del Monte P, Barreca A, Fortini P, Cariola G, Catrambone G, Giordano G. Evidence for an increased somatomedin-C/insulin-like growth factor I content in primary human lung tumors. Cancer Res. 1986;46:985-988. [PubMed] |
| 62. | Belfiore A, Pandini G, Vella V, Squatrito S, Vigneri R. Insulin/IGF-I hybrid receptors play a major role in IGF-I signaling in thyroid cancer. Biochimie. 1999;81:403-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 73] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 63. | Schips L, Zigeuner R, Ratschek M, Rehak P, Rüschoff J, Langner C. Analysis of insulin-like growth factors and insulin-like growth factor I receptor expression in renal cell carcinoma. Am J Clin Pathol. 2004;122:931-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 64. | All-Ericsson C, Girnita L, Seregard S, Bartolazzi A, Jager MJ, Larsson O. Insulin-like growth factor-1 receptor in uveal melanoma: a predictor for metastatic disease and a potential therapeutic target. Invest Ophthalmol Vis Sci. 2002;43:1-8. [PubMed] |
| 65. | Burrow S, Andrulis IL, Pollak M, Bell RS. Expression of insulin-like growth factor receptor, IGF-1, and IGF-2 in primary and metastatic osteosarcoma. J Surg Oncol. 1998;69:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 66. | Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682-4689. [PubMed] |
| 67. | Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2599] [Cited by in RCA: 2761] [Article Influence: 131.5] [Reference Citation Analysis (0)] |
| 68. | Levine DA, Bogomolniy F, Yee CJ, Lash A, Barakat RR, Borgen PI, Boyd J. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin Cancer Res. 2005;11:2875-2878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 348] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 69. | Itoh N, Semba S, Ito M, Takeda H, Kawata S, Yamakawa M. Phosphorylation of Akt/PKB is required for suppression of cancer cell apoptosis and tumor progression in human colorectal carcinoma. Cancer. 2002;94:3127-3134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 70. | Zhou X, Tan M, Stone Hawthorne V, Klos KS, Lan KH, Yang Y, Yang W, Smith TL, Shi D, Yu D. Activation of the Akt/mammalian target of rapamycin/4E-BP1 pathway by ErbB2 overexpression predicts tumor progression in breast cancers. Clin Cancer Res. 2004;10:6779-6788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 232] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 71. | Mandal M, Kim S, Younes MN, Jasser SA, El-Naggar AK, Mills GB, Myers JN. The Akt inhibitor KP372-1 suppresses Akt activity and cell proliferation and induces apoptosis in thyroid cancer cells. Br J Cancer. 2005;92:1899-1905. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 72. | David O, Jett J, LeBeau H, Dy G, Hughes J, Friedman M, Brody AR. Phospho-Akt overexpression in non-small cell lung cancer confers significant stage-independent survival disadvantage. Clin Cancer Res. 2004;10:6865-6871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 160] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 73. | Dai DL, Martinka M, Li G. Prognostic significance of activated Akt expression in melanoma: a clinicopathologic study of 292 cases. J Clin Oncol. 2005;23:1473-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 222] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 74. | Lim J, Kim JH, Paeng JY, Kim MJ, Hong SD, Lee JI, Hong SP. Prognostic value of activated Akt expression in oral squamous cell carcinoma. J Clin Pathol. 2005;58:1199-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 75. | Soria JC, Lee HY, Lee JI, Wang L, Issa JP, Kemp BL, Liu DD, Kurie JM, Mao L, Khuri FR. Lack of PTEN expression in non-small cell lung cancer could be related to promoter methylation. Clin Cancer Res. 2002;8:1178-1184. [PubMed] |
| 76. | Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, Niederwieser D, Resta D, Capdeville R, Zoellner U. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346:645-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1503] [Cited by in RCA: 1404] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 77. | Hynes NE, Lane HA. ERBB receptors and cancer: the complexity of targeted inhibitors. Nat Rev Cancer. 2005;5:341-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2397] [Cited by in RCA: 2487] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 78. | Vivanco I, Sawyers CL. The phosphatidylinositol 3-Kinase AKT pathway in human cancer. Nat Rev Cancer. 2002;2:489-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4468] [Cited by in RCA: 4588] [Article Influence: 199.5] [Reference Citation Analysis (0)] |
| 79. | Coleman ML, Marshall CJ, Olson MF. RAS and RHO GTPases in G1-phase cell-cycle regulation. Nat Rev Mol Cell Biol. 2004;5:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 272] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 80. | Bjornsti MA, Houghton PJ. The TOR pathway: a target for cancer therapy. Nat Rev Cancer. 2004;4:335-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1042] [Cited by in RCA: 1040] [Article Influence: 49.5] [Reference Citation Analysis (0)] |
| 81. | Inoki K, Ouyang H, Li Y, Guan KL. Signaling by target of rapamycin proteins in cell growth control. Microbiol Mol Biol Rev. 2005;69:79-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 259] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 82. | Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4260] [Cited by in RCA: 4434] [Article Influence: 233.4] [Reference Citation Analysis (0)] |
| 83. | Höpfner M, Schuppan D, Scherübl H. Growth factor receptors and related signalling pathways as targets for novel treatment strategies of hepatocellular cancer. World J Gastroenterol. 2008;14:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 74] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 84. | Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, Ranieri E, Gesualdo L, Schena FP, Grandaliano G. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317-1323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 677] [Article Influence: 33.9] [Reference Citation Analysis (0)] |
| 85. | de Fijter JW. Use of proliferation signal inhibitors in non-melanoma skin cancer following renal transplantation. Nephrol Dial Transplant. 2007;22 Suppl 1:i23-i26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Gomez-Camarero J, Salcedo M, Rincon D, Lo Iacono O, Ripoll C, Hernando A, Sanz C, Clemente G, Bañares R. Use of everolimus as a rescue immunosuppressive therapy in liver transplant patients with neoplasms. Transplantation. 2007;84:786-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 87. | Shi Y, Gera J, Hu L, Hsu JH, Bookstein R, Li W, Lichtenstein A. Enhanced sensitivity of multiple myeloma cells containing PTEN mutations to CCI-779. Cancer Res. 2002;62:5027-5034. [PubMed] |
| 88. | Teachey DT, Grupp SA, Brown VI. Mammalian target of rapamycin inhibitors and their potential role in therapy in leukaemia and other haematological malignancies. Br J Haematol. 2009;145:569-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 93] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 89. | Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, Manola J, Brugarolas J, McDonnell TJ, Golub TR. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 748] [Cited by in RCA: 767] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 90. | Zhong H, Chiles K, Feldser D, Laughner E, Hanrahan C, Georgescu MM, Simons JW, Semenza GL. Modulation of hypoxia-inducible factor 1alpha expression by the epidermal growth factor/phosphatidylinositol 3-kinase/PTEN/AKT/FRAP pathway in human prostate cancer cells: implications for tumor angiogenesis and therapeutics. Cancer Res. 2000;60:1541-1545. [PubMed] |
| 91. | Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2365] [Cited by in RCA: 2374] [Article Influence: 139.6] [Reference Citation Analysis (0)] |
| 92. | Yao JC, Shah MH, Ito T, Bohas CL, Wolin EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2039] [Cited by in RCA: 2117] [Article Influence: 151.2] [Reference Citation Analysis (0)] |
| 93. | Baselga J, Campone M, Piccart M, Burris HA, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2021] [Cited by in RCA: 2220] [Article Influence: 170.8] [Reference Citation Analysis (0)] |
| 94. | Treiber G. mTOR inhibitors for hepatocellular cancer: a forward-moving target. Expert Rev Anticancer Ther. 2009;9:247-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.6] [Reference Citation Analysis (0)] |