INTRODUCTION
As the number of kidney transplants grows and the survival of patients who have received a kidney graft improves, new challenges in managing their long-term complications appear. Disorders of bone and mineral metabolism are common in these patients, causing high morbidity and affecting the quality of life in transplant recipients.
The type and severity of bone lesions in the renal transplant recipients are determined by factors such as pre-transplant bone disease, renal graft function, effect of immunosuppressive agents on the bone, menopause, age, and comorbidities, such as diabetes, among the most important ones[1,2].
The progressive loss of kidney function leads to the gradual development of bone and mineral metabolism disorders, and bone disease is almost universally present in all patients with less than 60 mL/min of glomerular filtration rate. The pathophysiology of bone disorders is complex, predominantly related to increased bone turnover as it occurs in secondary hyperparathyroidism (HPT) or with low bone turnover, as seen in osteomalacia and adynamic bone disease. These patients have reduced bone strength, and increasing the risk of fractures in presence of renal osteodystrophy, with a prevalence of hip fractures four times compared to the general population[3].
INFLUENCE OF PARATHYROID FUNCTION BEFORE RENAL TRANSPLANTATION
Secondary HPT and its complications are frequently found in chronic kidney disease (CKD), especially in patients with dialysis therapy[4,5]. Data obtained in Latin America from bone biopsies show that about half of them present osteitis fibrosa due to secondary HPT[6]. Similar findings were shown in a prevalent hemodialysis population[6], but they differ from those found in some European countries, where low levels of parathyroid hormone (PTH) prevail over secondary HPT[7,8].
The development of secondary HPT is related to the presence of hyperphosphatemia, low levels of vitamin D, hypocalcemia, time on dialysis, parathyroid gland size and the presence of parathyroid adenomas[6,9]. If secondary HPT is not prevented or treated early during the period of dialysis, the patient will receive a renal transplantation having a disorder in the parathyroid function that is difficult to revert.
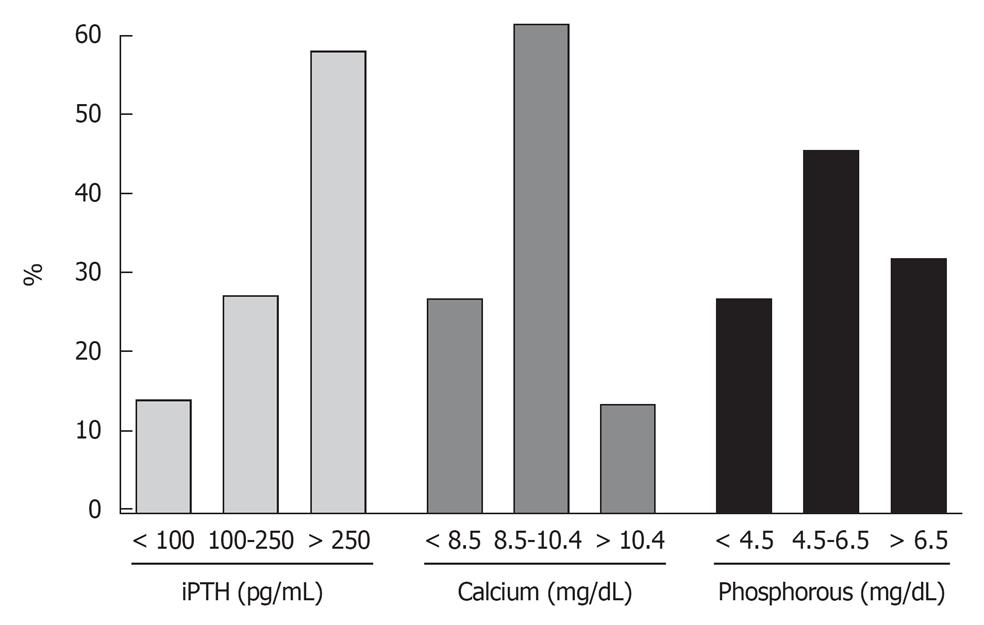
A high number of patients who receive renal grafts present altered calcium-phosphate metabolism and abnormal levels of PTH. Data from our own center on levels of markers of mineral metabolism in 365 patients at the time of renal transplantation, showed that 58% had intact PTH levels > 250 pg/mL, 12.4% had hypercalcemia and 27.9% presented severely elevated levels of serum phosphorus (Figure 1). The mean level of intact PTH was 518.5 ± 520.4 pg/mL, serum calcium of 9.2 ± 1.3 mg/dL and phosphorus 5.6 ± 2.0 mg/dL. These data highlight the prevalence of secondary HPT with hyperphosphatemia at the time of renal transplantation and match with the finding of patients undergoing bone biopsy[6].
Figure 1 Markers of mineral metabolism immediately before renal transplantation.
Data from Hospital Privado Centro-Médico de Córdoba.
PERSISTENT SECONDARY HPT AFTER RENAL TRANSPLANTATION
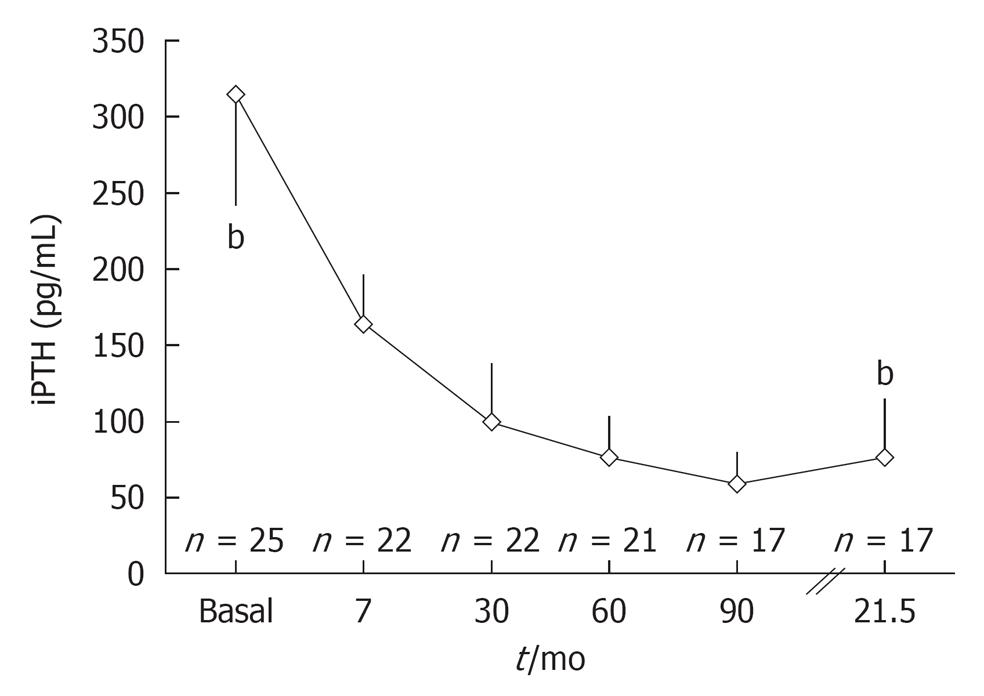
A successful renal transplantation normalise most of the endocrine and metabolic imbalances since the beginning of recovery of renal function[1]. Thus, with renal function the capacity to produce 1-25 dihydroxyvitamin D is recovered, phosphate levels are corrected due to the improvement of renal tubular function and the levels of serum calcium are normalized. The consequence of these changes is the spontaneous decrease in PTH levels in most patients[10]. In our experience, intact PTH levels showed a significant decrease from a mean value of 353 pg/mL to 77 pg/mL in an average time of 2 years from transplantation (Figure 2). However, in 56% of registered cases, intact PTH levels remained high at the end of follow-up, 32% had hypercalcemia and 28% had hypophosphatemia. According to Bertoni et al[11], serum PTH levels remain above 100 pg/mL in one-third of the patients at 6 mo of a functioning kidney transplant while in 20% of cases PTH levels remain abnormally high at 5 years of transplantation. Our data are consistent with those published by Heaf et al[12] where 50% of patients achieved spontaneous recovery of PTH levels 1 year after transplantation, while 21% remained with high levels 15 years after successful kidney transplant.
Figure 2 Changes in iPTH levels in post-renal transplantation.
Data from Hospital Privado-Centro Médico de Córdoba. bP < 0.001.
The persistence and severity of HPT in post-renal transplantation is primarily associated with its duration and magnitude in the pre-transplant period and with the presence of parathyroid adenomas[1,2]. Although the pathophysiology of persistent HPT after transplantation is not fully elucidated, it is likely related to the presence of parathyroid adenomas in the pre-transplant phase, whose cells have low density of calcitriol receptors (Vitamin D receptors, or VDR), calcium-sensing receptor in the plasma membrane and receptors for phosphatonin FGF23 (FGFR)[13-15]. Thus, proliferating cells in the adenomatous tissue do not respond to circulating calcitriol, calcium or FGF23[15].
HPT after renal transplantation is clinically manifested with hypercalcemia, hypophosphatemia, bone pain, fractures, and in more serious cases with cardiovascular calcifications that determine the survival of patients with CKD.
MANAGEMENT OF BONE DISEASE AFTER TRANSPLANTATION
Although advances in the management of transplantation have improved its outcomes, still exist a high morbidity rate that affects the survival of the graft and the patient, and that is mainly related to cardiovascular disease, rejections, infections, cancer and bone disease[16].
Overall, there is little consensus about how to manage post-transplant osteodystrophy and how long it should be waited for an adequate renal function that influences on the correction of the deviations of bone and mineral metabolism from the stage of dialysis. Therapeutic options at this stage include optimizing the dosage of immunosuppressive drugs, particularly corticosteroids, calcium or vitamin D supplements, hormone replacement, correction of hypophosphatemia and the use of bisphosphonates[16,17].
The recommendations for this population indicate that all transplant patients should be assessed and eventually treated for their bone disease due to its high prevalence and the implications on morbidity and mortality rates in this population. This includes measures to improve skeletal health such as promoting mobilization, controlling excessive alcohol intake or smoking and correcting levels of gonadotropic hormones and negative balance of calcium and vitamin D[18]. KDIGO guidelines on the management of bone disease in patients with CKD recommend for post-renal transplantation, periodic measurement of serum calcium, phosphorus, vitamin D and PTH levels with a frequency that will vary according to the severity of bone disease and function of the transplanted kidney[17]. In patients with renal function grades 1 to 5, it is advisable to measure levels of 25-OH vitamin D and correct them by implementing similar measures to those of the general population, if deficiency or insufficiency were detected (< 10 vs 10-30 ng/mL respectively).
Perhaps one of the conditions that more questions arise when deciding the type and length of therapy is the correction of persistent secondary HPT after renal transplantation and the presence of hypercalcemia.
MEDICAL MANAGEMENT OF HPT AFTER RENAL TRANSPLANTATION
The primary clinical objective for patients with secondary HPT after renal transplantation is to obtain a level of PTH adequate to the graft function and to normalize levels of calcium, phosphorus and vitamin D. It is a common practice to follow an expectant course with regard to serum PTH levels until adequate renal function, allowing the normalization of calcemia, phosphatemia and increasing the production of calcitriol to control HPT.
In many cases during this period, the development of hypercalcemia and/or hypophosphatemia makes it necessary to take different therapeutic measures. The use of vitamin D or its analogues has been extrapolated from the management of pre-transplant HPT to the period after renal graft obtaining variable outcomes. The use of calcitriol allowed the decrease of PTH levels in normocalcemic recipients with post-transplant HPT[19] although its use is limited by its capacity to produce hypercalcemia similarly to the pre-transplant period[20]. For the presence of persistent post-transplant HPT with low 25-hydroxyvitamin D levels, even with normal 1-25 dihydroxyvitamin D levels, the use of nutritional supplements of vitamin D may be an adequate therapy to restore the levels of 25-hydroxyvitamin D[21]. However, despite the many studies of vitamin D or its analogues, the presence of autonomous parathyroid adenomas with decreased calcitriol, FGF23 or calcium-sensing receptor expression makes it unlikely to obtain a successful outcome and many post-transplant HPT becomes refractory to the medical treatment.
Calcimimetics are drugs that have proven effective in reducing PTH levels in patients with HPT on dialysis through modulating the activity of the calcium sensing receptor (CaR) on the plasma membrane of parathyroid cells[22]. Cinacalcet has been effective in reducing up to 50% PTH levels in moderate to severe HPT in post-renal transplantation[23,24]. In addition to the effective decrease of PTH levels, Cinacalcet could control two of the major problems of post-transplant HPT such as, hypercalcemia and hypophosphatemia[25]. As it also happens in the treatment with Cinacalcet in the HPT of patients on dialysis, might produce hypocalcemia[25] and PTH may return to pre-treatment levels after discontinuation of this drug, especially in patients with persistent adenomas or tertiary HPT.
Other treatments such as bisphosphonates or calcitonin have proven efficacy in controlling some of the disorders of HPT such as loss of bone mass or hypocalcemia but without effects on PTH levels[26,27].
When HPT persists after renal transplantation and does not respond to medical treatment, invasive management by percutaneous ethanol injection therapy (PEIT) of parathyroid glands or parathyroidectomy should be considered.
INDICATIONS FOR PARATHYROIDECTOMY IN POST-RENAL TRANSPLANTATION
The decision to perform a parathyroidectomy in a patient with a functioning renal transplant is rare and is usually taken when HPT leads to mineral disorders that cannot be controlled with medication. The prevalence of this interventons in post-renal transplantation ranges from 0.6% to 5.6%[28]. The indications tend to come from a combination of clinical, imaging and laboratory abnormalities, as the presence of hypercalcemia and/or severe hypophosphatemia, calciphylaxis, progressive vascular calcification, symptomatic and severe bone disease and spontaneous fractures. A retrospective study of 90 renal transplant patients who underwent parathyroidectomy determined the factors that significantly influenced on the decision to perform surgery were the highest pre-transplant PTH levels, female sex and hypercalcemia[28]. Parathyroidectomy resulted in decreased levels of PTH and calcium, increased serum phosphate and improving blood pressure and serum lipids[29,30].
Some studies have associated post-transplant parathyroidectomy with subsequent decreasing renal function[30,31], but this effect could not be confirmed in subsequent studies[28]. While this finding not had a definitive explanation so far, some researchers link it to specific effects of surgery and anaesthesia rather than changes related to parathyroid function[32]. The surgery-related risks and the potential development of low-turnover bone disease indicate the need for further assessments to determine risks and benefits of surgery or to postpone surgical treatment.
MANAGEMENT OF HPT AFTER RENAL TRANSPLANTATION BY USING PEIT
An alternative for these patients is to use PEIT into parathyroid adenomas with 95% ethanol solution[32,33]. This technique is indicated for patients with elevated PTH levels (300 to 1500 pg/mL), recurrent post-parathyroidectomy HPT[34], clinical contraindication to parathyroid surgery, and with 1 or 2 nodular parathyroid glands are observed by ultrasonography[32,33,35].
PEIT of the parathyroid gland is a low-risk technique that requires the use of a needle with small side holes to inject 95% ethanol solution into parathyroid nodules under ultrasonographic guidance with local anaesthesia in an outpatient basis[35].
We have recently showed that patients with persistent post-transplant HPT can be successfully managed by using PEIT[36]. These represent the first published data on the use of PEIT in post-renal transplantation.
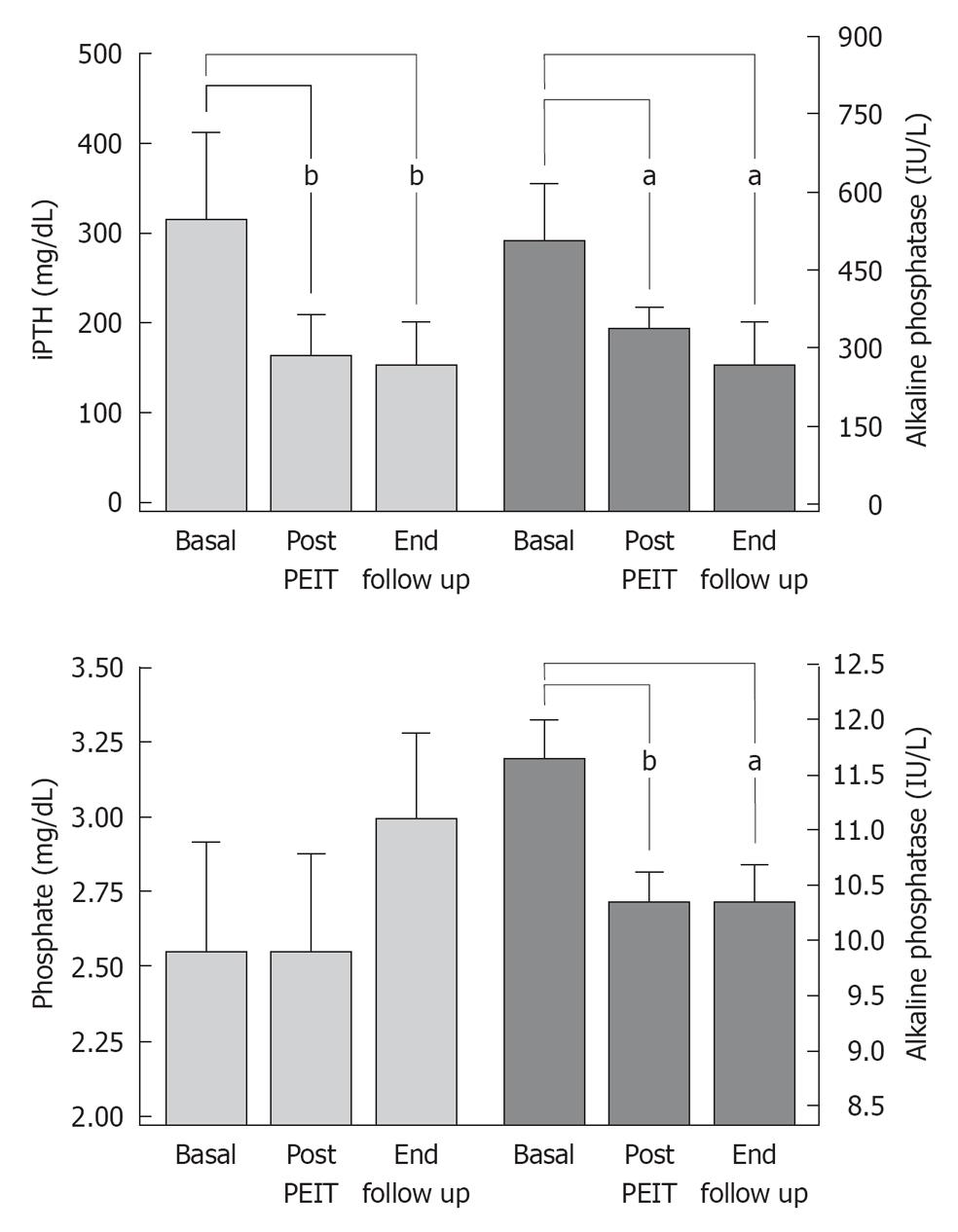
Intact PTH levels decreased an average of 36.5% ± 9.5% in patients who underwent PEIT (286.9 ± 107.2 to 154.6 ± 42.2 pg/mL), with a marked long-term improvement of calcemia and phosphatemia without major complications after PEIT[36] (Figure 3). Although this was a small series of patients, PEIT has proved to be a safe and useful method to manage HPT after transplantation and avoid expensive and risky surgeries such as parathyroidectomy.
Figure 3 Markers of mineral metabolism before and after percutaneous ethanol injection therapy of parathyroid gland in renal transplant patients with hyperparathyroidism at Hospital Privado, Córdoba, Argentina.
PEIT: Percutaneous ethanol injection therapy. aP < 0.05, bP < 0.01.
CONCLUSION
The high prevalence of bone diseases in post-transplantation of solid organs, especially osteoporosis and secondary HPT, should promote a more rigorous emphasis on early diagnosis and management of these conditions. At present, more data are required on the effect of immunosuppressive drugs on bone health and the use of antiresorptive agents in transplantation in order to prevent or treat bone mass defects. The emergence of new methods for the management of HPT such as PEIT of the parathyroid gland expands the availability of therapeutic tools for transplant patients.
Peer reviewers: Kais Harzallah, Associate Professor, Unit of Organ Transplantation, Military hospital of Tunis, Tunis, 1008, Tunisia; Yang-Jen Chiang, MD, Division of Urology, Department of Surgery, Chang Gung Memorial Hospital, Gueishan, Taoyuan 333, Taiwan, China
S- Editor Cheng JX L- Editor A E- Editor Zheng XM