Revised: December 20, 2011
Accepted: February 23, 2012
Published online: February 24, 2012
Live liver donor transplantation to adult recipients is becoming a common practice, increasing the organ pool and providing an alternative to whole cadaveric liver transplantation. These patients are healthy adults without serious medical conditions and typically have normal coagulation profiles preoperatively. Right hepatic lobectomy is usually performed for adult recipients, while left hepatic lobectomy is performed for pediatric recipients. Removal of the whole right lobe from the donors may expose theses patients to multiple intraoperative and postoperative complications. Hypercoagulability has been identified as a serious complication which leads to thromboembolic phenomena with potential fatal consequences. The primary aim of this review is to look at possible changes in post-operative coagulation dynamics that may increase the risk for development of thromboembolic complications in live liver donors. In this article, we stress the importance of addressing the issue that conventional clotting tests (PT, INR, PTT) are unable to detect a hypercoagulable state, and therefore, we should examining alternative laboratory tests to improve diagnosis and early detection of thrombotic complications. Measurement of natural anticoagulant/procoagulant biomarkers combined with conventional coagulation studies and thromboelastography offers a more accurate assessment of coagulation disorders. This allows earlier diagnosis, permitting appropriate intervention sooner, hence avoiding potential morbidity and mortality. Biomarkers that may be evaluated include, but are not limited to: protein C, soluble P-selectin, antithrombin III, thrombin-antithrombin complex, and thrombin generation complex.
- Citation: Hilmi IA, Planinsic RM. Live liver donors: Are they at a higher risk for post-operative thrombotic complications? World J Transplant 2012; 2(1): 1-4
- URL: https://www.wjgnet.com/2220-3230/full/v2/i1/1.htm
- DOI: https://dx.doi.org/10.5500/wjt.v2.i1.1
As the result of an increasing number of patients with end-stage liver disease awaiting liver transplantation, an increased number of centers performing liver transplantations and due to the encouraging results from living-related pediatric transplantation, adult to adult living donor liver transplantation is becoming an increasingly popular option. However, in pediatric live-donor liver transplantation typically uses the donor liver’s left lobe or fewer segments resulting in less dramatic effects on the post-resection donor’s hepatic functions. The overall immediate postoperative complications which are related to the surgery (bleeding, bile leak) are very low and mostly have been encountered in centers that perform few of these procedures[1]. However, as documented by recent review of donor’s data the overall morbidity rate was 31% for the first year after surgery[2]. Critical analysis of surgical outcomes would suggest that there is under-reporting and under-estimation of the frequency and severity of such complications[3-5].
The lack of standardization of the surgical techniques and variation in surgical skill and experience can greatly affect the perioperative course of live liver donors[6-8]. In most transplant centers right-lobe hepatectomy is performed for adult-to-adult live donor liver transplantation by removal of 60%-70% of the hepatic mass (right lobe) including middle hepatic vein[9-11].
Post-operative complications, especially during the first few months, include pulmonary embolism with an incidence of 7%, so of which were fatal[12]. Deep venous thrombosis, spleen and portal vein thrombosis have been reported as part of the serious thrombotic complications[12]. Overall, there is underestimation and under-appreciation of the thromboembolic risks in the living donors, a fact that possibly reflects a lack of appreciation of changes in the post-liver resection coagulation profile.
Most of the natural procoagulants and anticoagulants are manufactured in the liver. In addition, other important functions of the liver include removal of activated clotting factors from the blood and thus keeping and maintaining a balance between anticoagulant/procaogulant mediators[13]. Although, during surgery, bleeding is a major concern for both the surgical and anesthesiology teams, the results from recent study showed that living donors progressively developing hypercoagulable state as shown by thromboelastograph (TEG) even in the presence of anti-thrombotic prophylaxis[14].
In spite of the fact that post-operative coagulopathy in living donors can be easily diagnosed by conventional clotting tests (PTT, PT, INR), the incidence of post-surgical bleeding is extremely low. This is true regardless of whether coagulopathy is surgical or medical in origin. In the contrary, the diagnosis of a hypercoagulable state is not a routine part of post-operative care in spite of the several reports of serious thromboembolic complications in this group. The establishment of reliable laboratory tests to diagnose hypercoagulability is urgently needed to predict and diagnose the hypercoagulability in living donors in order to avoid serious thromboembolic complications.
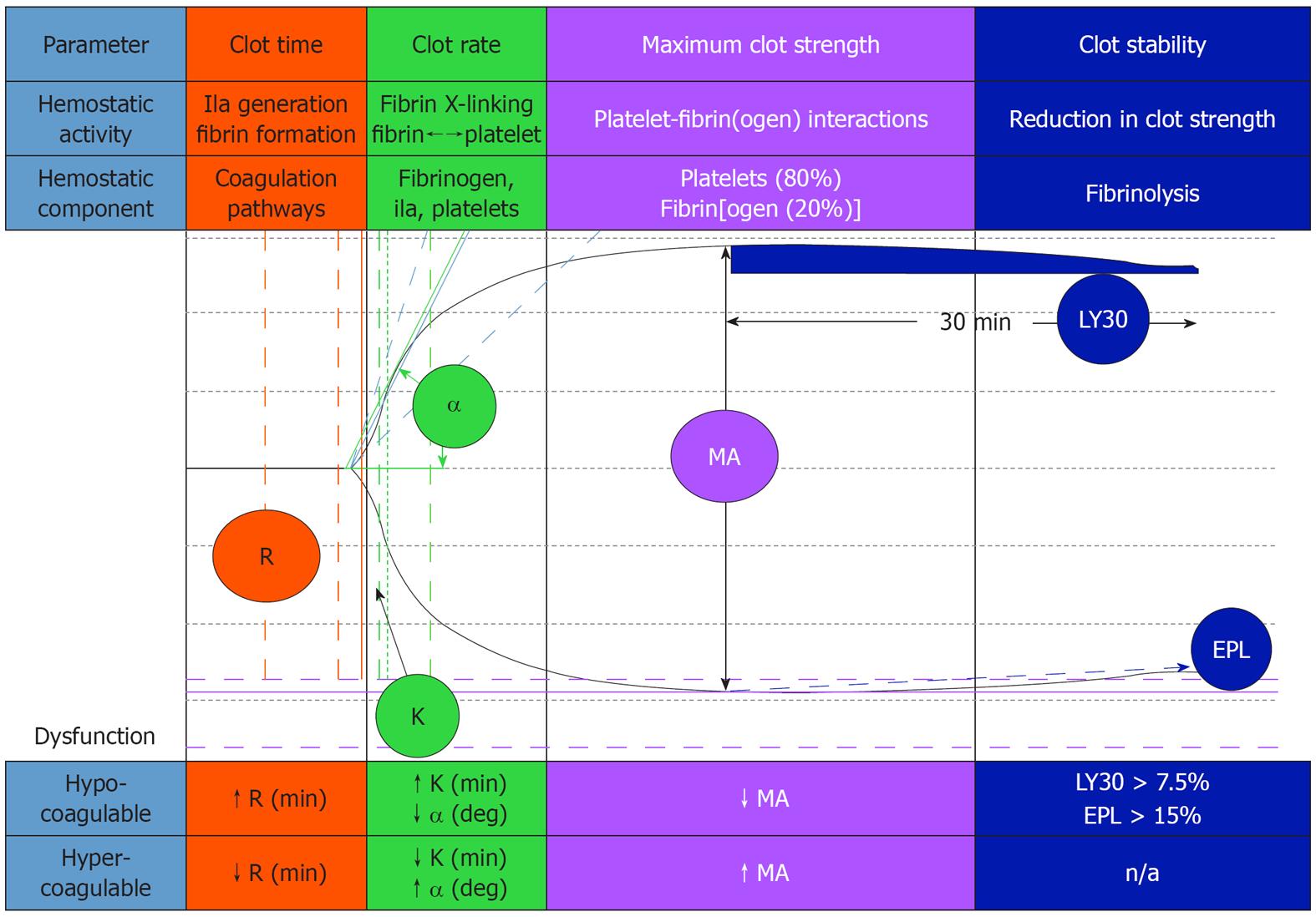
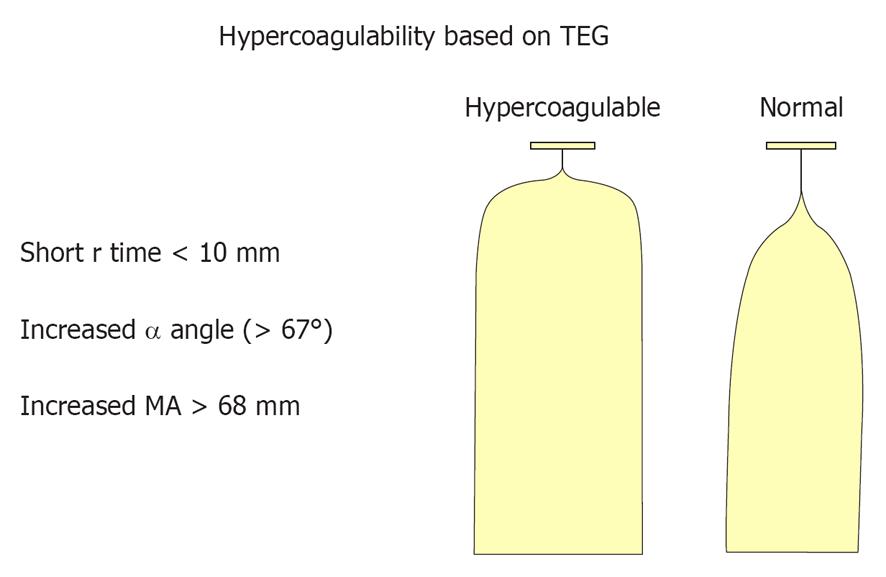
TEG has been used to evaluate acquired and congenital/genetic induced clotting-related problems when compared to healthy reference subjects[14,15]. TEG can demonstrate hypercoagulability by a short R-time, an increased MA and accelerated K-value (Figures 1 and 2). The problem with TEG testing is considerable variability in its accuracy for predicting thromboembolic events[16]. From a review study where TEG was used to monitor coagulation, the conclusion reached was that the TEG, when used alone, did not significantly change the post- from pre-test probabilities of predicting thrombotic complications or its ability to impact decision-making[16]. TEG may have some value when combines with other lab tests. TEG is a global, dynamic test for whole hemostasis, while differential lab assays are more useful in attempting to understand the underlying mechanisms involved and the pathways that are affected.
Such lab tests are protein C (PC), soluble P-selectin (SPS) and antithrombin III (ANTIII), thrombin-antithrombin complex (TAT) and thrombin generation complex (TGC) which are either the natural anticoagulants or indicators for in vivo clotting activation. Protein S is another vitamin-K dependent anticoagulant that is produced in the liver but with a substantial extra-hepatic production and may not be affected that much by hepatic surgery like the rest of anticoagulants[17].
PC is a vitamin k-dependent plasma serine protease zymogen that upon activation by thrombin-thrombomodulin complex, down-regulates the clotting cascades by a feedback loop inhibition mechanism[18]. PC and S will act together to deactivate FVa and FVIIIa, this will shut down the process of thrombin generation through both intrinsic and extrinsic pathways[18]. In addition to the anticoagulant functions of PC, it has anti-inflammatory and cytoprotective functions[19]. In animal experiments, blocking the activation of PC has been shown to convert non-lethal dose of Escherichia coli to lethal phenotype which resulted in multi-organ failure[20]. The clinical application of recombinant human activated PC therapy in sepsis has been recently approved by the Food and Drugs Agency (FDA), however, the scope of anti-inflammatory action of PC is beyond the scope of this review[21].
Inadequate activation or inadequate hepatic production of PC, as in sepsis may play a pivotal role in not only multi-organ failure but in production of pro-thrombotic states as in the initiation of disseminated intravascular coagulation (DIC) in septic patients[22]. Accordingly, we can speculate that patients who are subjected to liver resection as in live donors; PC production may be seriously compromised. This may put patients in real danger of not only thromboembolic complications, but of an increased susceptibility to sepsis, a speculation that needs to be proved by further studies in this patient population.
Another naturally liver-produced anticoagulant which may suffer during hepatic resection is ANTIII. Recombinant human ANTIII has been used to reverse the coagulation abnormalities in sepsis, DIC and hepatic failure. Recent studies have shown that ANTIII may have powerful anti-inflammatory effects. ANTIII has inhibitory effects on endotoxin-induced neutrophil activation and it down-regulates the expression of certain proinflammatory cytokines (TNF-α, IL-6)[23]. It has been used in some of European ICU patients to teat sepsis, but in the USA, it is only FDA approved to treat certain coagulation abnormalities. Low levels of ANTIII may have potential effects on susceptibility to gram-negative infections and/or endotoxemia, not only in critically ill patients, but also in postoperative liver resection patients. The questionable role of ANTIII in postoperative hypercoagulation and sepsis in live liver donors requires serious investigation by all surgical centers that practice this surgical procedure[24].
SPS protein is an adhesive molecule that has a peculiar expression under certain condition by both platelet and vascular endothelium. SPS has been shown to play an essential role in vascular inflammation and injury and links inflammation to thrombosis[25]. Conventional platelet count and platelet activation tests have received major criticism due to the fact that ex vivo studies a part from being operator-dependent might not actually reflect the occurrence of in vivo platelet activation[26]. SPS can be a useful as a specific biomarker for in vivo platelet activation. The mechanisms of SPS expression and cleavage after platelet activation makes this molecule very resistant to ex vivo activation provided that plasma is immediately separated from the cellular elements[27]. Overall, SPS may represent a useful and unique test for in vivo platelet activation and which may be of valuable in understanding changes in coagulation dynamics after major liver resection[28].
TGC, thrombin, the primary enzyme found in the coagulation cascade, plays a pivotal in hemostasis. The measurement of the formation and inhibition of thrombin in plasma relates directly to the patient’s coagulation status. The plasma levels of TGC may give us an excellent picture of what is going in vivo as far as activation of coagulation and it can be very useful tool in monitoring of post-operative changes in coagulation in liver resection patients.
The TAT results when thrombin cleaves a scissile bond near the C-terminal of ANTIII forming a covalent, TAT complex is relatively stable. An elevated levels of TAT indicate ongoing clot activation and can be easily measured by Sandwich-style ELISA test which makes this biomarker a useful in monitoring in vivo changes in coagulation status.
In conclusion, having these natural anticoagulants/procoagulants biomarkers evaluated and combined with the conventional clotting tests and TEG may help in better understanding the pathophysiological changes in coagulation after major liver resection. In live liver donors, monitoring coagulation profiles by this approach may greatly reduce or eliminate the risk of serious thrombotic complications[29]. There are still many questions that need to be answered as far as changes in coagulation and immunological response to stress and sepsis in live liver donors. Further studies are required to better understand this problem and decrease the risk of exposing otherwise healthy patients who do not require surgery to possible life-threatening thrombotic complications. The issues that need to be addressed in any investigations in this group of patients include: what biomarker/biomarkers to monitor, for how long, how frequently, are conventional clotting tests non-diagnostic in this regard, and is use of the TEG enough to monitor the changes in clotting? When we are better able to understand these important changes which occur in these patients, we will be better able to access the risk/benefit ratio with respect to outcomes.
For the time being, the best clinical practice is to fully investigate the potential live liver donors for the possibility of acquired or inherited/congenital coagulation abnormality before considering them as live liver donors. 2nd careful monitoring of their post-surgical coagulation functions with conventional clotting tests, TEG and considering the evaluation of natural anticoagulant biomarkers is vital part of postoperative care. Early postoperative mobilization and anti-thrombotic prophylaxis forms an integral part in the prevention of thrombo-embolic complications.
Peer reviewer: Olivier Detry, MD, PhD, Associate Professor, Department of Abdominal Surgery and Transplantation, University of Liège, CHU Liège, Sart Tilman B35, B4000 Liège, Wallonia, Belgium
S- Editor Wang JL L- Editor A E- Editor Zheng XM
| 1. | Rudow DL, Brown RS, Emond JC, Marratta D, Bellemare S, Kinkhabwala M. One-year morbidity after donor right hepatectomy. Liver Transpl. 2004;10:1428-1431. [PubMed] |
| 2. | Beavers KL, Sandler RS, Shrestha R. Donor morbidity associated with right lobectomy for living donor liver transplantation to adult recipients: a systematic review. Liver Transpl. 2002;8:110-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 3. | Yaprak O, Dayangac M, Demirbas BT, Tabendeh B, Yuzer Y, Tokat Y. Analysis of right lobe living-liver donor complications: a single center experience. Exp Clin Transplant. 2011;9:56-59. [PubMed] |
| 4. | Yuan D, Wei YG, Li B, Yan LN, Wen TF, Zhao JC, Zeng Y, Chen KF. Evaluation outcomes of donors in living donor liver transplantation: a single-center analysis of 132 donors. Hepatobiliary Pancreat Dis Int. 2011;10:480-488. [PubMed] |
| 5. | Sotiropoulos GC, Radtke A, Molmenti EP, Schroeder T, Baba HA, Frilling A, Broelsch CE, Malagó M. Long-term follow-up after right hepatectomy for adult living donation and attitudes toward the procedure. Ann Surg. 2011;254:694-700; discussion 700-1. [PubMed] |
| 6. | Marubashi S, Nagano H, Wada H, Kobayashi S, Eguchi H, Takeda Y, Tanemura M, Doki Y, Mori M. Donor hepatectomy for living donor liver transplantation: learning steps and surgical outcome. Dig Dis Sci. 2011;56:2482-2490. [PubMed] |
| 7. | Cipe G, Tuzuner A, Genc V, Orozakunov E, Ozgencil E, Yılmaz AA, Can OS, Cakmak A, Karayalcin K, Ersoz S. Living-donor hepatectomy. Transplant Proc. 2011;43:888-891. [PubMed] |
| 8. | Ikegami T, Shirabe K, Morita K, Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Kayashima H, Hashimoto N, Maehara Y. Minimal hilar dissection prevents biliary anastomotic stricture after living donor liver transplantation. Transplantation. 2011;92:1147-1151. [PubMed] |
| 9. | Li C, Mi K, Wen TF, Yan LN, Li B. Safety of Patients with a Graft to Body Weight Ratio Less than 0.8% in Living Donor Liver Transplantation using Right Hepatic Lobe without Middle Hepatic Vein. Hepatogastroenterology. 2012;59:469-472. [PubMed] |
| 10. | Eguchi S, Takatsuki M, Soyama A, Hidaka M, Muraoka I, Kanematsu T. Is Preservation of Middle Hepatic Vein Tributaries during Right Hemi-Hepatectomy Beneficial for Live Donor Liver Transplantation? Hepatogastroenterology. 2011;Epub ahead of print. |
| 11. | Marcos A. Right lobe living donor liver transplantation: a review. Liver Transpl. 2000;6:3-20. [PubMed] |
| 12. | Bezeaud A, Denninger MH, Dondero F, Saada V, Venisse L, Huisse MG, Belghiti J, Guillin MC. Hypercoagulability after partial liver resection. Thromb Haemost. 2007;98:1252-1256. [PubMed] |
| 13. | Lambing A, Kuriakose P, Abouljoud MS. Hypercoagulability risks among adult living liver donors. Transplant Proc. 2006;38:3579-3581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Cerutti E, Stratta C, Romagnoli R, Schellino MM, Skurzak S, Rizzetto M, Tamponi G, Salizzoni M. Thromboelastogram monitoring in the perioperative period of hepatectomy for adult living liver donation. Liver Transpl. 2004;10:289-294. [PubMed] |
| 15. | Coakley M, Reddy K, Mackie I, Mallett S. Transfusion triggers in orthotopic liver transplantation: a comparison of the thromboelastometry analyzer, the thromboelastogram, and conventional coagulation tests. J Cardiothorac Vasc Anesth. 2006;20:548-553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 162] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Dai Y, Lee A, Critchley LA, White PF. Does thromboelastography predict postoperative thromboembolic events? A systematic review of the literature. Anesth Analg. 2009;108:734-742. [PubMed] |
| 17. | Schreiber MA, Differding J, Thorborg P, Mayberry JC, Mullins RJ. Hypercoagulability is most prevalent early after injury and in female patients. J Trauma. 2005;58:475-80; discussion 480-1. [PubMed] |
| 18. | Rezaie AR. Regulation of the protein C anticoagulant and antiinflammatory pathways. Curr Med Chem. 2010;17:2059-2069. [PubMed] |
| 19. | Kak V. Mediators of systemic inflammatory response syndrome and the role of recombinant activated protein C in sepsis syndrome. Infect Dis Clin North Am. 2011;25:835-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Taylor FB, Stearns-Kurosawa DJ, Kurosawa S, Ferrell G, Chang AC, Laszik Z, Kosanke S, Peer G, Esmon CT. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680-1686. [PubMed] |
| 21. | Christaki E, Anyfanti P, Opal SM. Immunomodulatory therapy for sepsis: an update. Expert Rev Anti Infect Ther. 2011;9:1013-1033. [PubMed] |
| 22. | Levi M, van der Poll T. Recombinant human activated protein C: current insights into its mechanism of action. Crit Care. 2007;11 Suppl 5:S3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 23. | Leitner JM, Firbas C, Mayr FB, Reiter RA, Steinlechner B, Jilma B. Recombinant human antithrombin inhibits thrombin formation and interleukin 6 release in human endotoxemia. Clin Pharmacol Ther. 2006;79:23-34. [PubMed] |
| 24. | Tapper EB, Tanaka KA, Sarmiento JM. Evaluation of hemostatic factors in patients undergoing major hepatic resection and other major abdominal surgeries. Am Surg. 2011;77:1188-1193. [PubMed] |
| 25. | Ferroni P, Martini F, Riondino S, La Farina F, Magnapera A, Ciatti F, Guadagni F. Soluble P-selectin as a marker of in vivo platelet activation. Clin Chim Acta. 2009;399:88-91. [PubMed] |
| 26. | Blann AD, Lip GY, Beevers DG, McCollum CN. Soluble P-selectin in atherosclerosis: a comparison with endothelial cell and platelet markers. Thromb Haemost. 1997;77:1077-1080. [PubMed] |
| 27. | Fábrega E, Casafont F, Merino J, de la Peña J, Crespo J, Amado JA, Pons-Romero F. Value of plasma P-selectin for vascular complications in liver transplantation. Clin Transplant. 1996;10:261-265. [PubMed] |
| 28. | Wagner DD. New links between inflammation and thrombosis. Arterioscler Thromb Vasc Biol. 2005;25:1321-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 29. | Péters P, Gothot A. [Thrombinography: towards a globalization of coagulation tests]. Rev Med Liege. 2009;64:199-203. [PubMed] |