Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.104109
Revised: December 30, 2024
Accepted: February 10, 2025
Published online: September 18, 2025
Processing time: 129 Days and 14.5 Hours
Liver transplantation (LT) is the only curative, life-saving option for children and adults with end-stage liver disease. Due to the well-known shortage and heterogeneity of grafts, split LT (SLT) is an attractive strategy to expand the donor pool and reduce waitlist times. Given increased risk of cold ischemia time with SLT, machine perfusion represents a promising option to reduce it and optimize tran
A 19-month-old female with biliary atresia after failed Kasai portoenterostomy and a 42-year-old woman with unresectable intrahepatic cholangiocarcinoma were selected as recipients for a SLT from a 17-year-old male donor. The SLT generated a left lateral segment and a right trisectional graft of appropriate volume for both recipients. After a mixed in-situ and ex-situ split, in order to improve logistics, the right trisectional graft was placed on a closed circuit NMP device, following an appropriate vascular reconstruction. Both grafts were implanted with excellent short-term outcomes.
Use of NMP with SLT for preservation prior to implantation allows not only for graft optimization but also for significant improvement of transplant logistics. We propose various models and standardization of logistic options for combining SLT with NMP to optimize graft availability and outcomes.
Core Tip: Due to the well-known shortage and heterogeneity of grafts, split liver transplantation (SLT) is an attractive strategy to expand the donor pool and reduce waitlist times. Given increased risk of cold ischemia time, machine perfusion represents a promising option to optimize transplant logistics and outcomes. This article proposes various models and standardization of logistic options for combining SLT with normothermic machine perfusion to optimize graft availability and outcomes.
- Citation: Baimas-George M, Archie WH, Soltys K, Soto JR, Levi D, Eskind L, Casingal V, Denny R, Attia M, Mazariegos GV, Vrochides D. Optimizing liver utilization for transplantation with partial grafts undergoing normothermic machine perfusion: Two case reports. World J Transplant 2025; 15(3): 104109
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/104109.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.104109
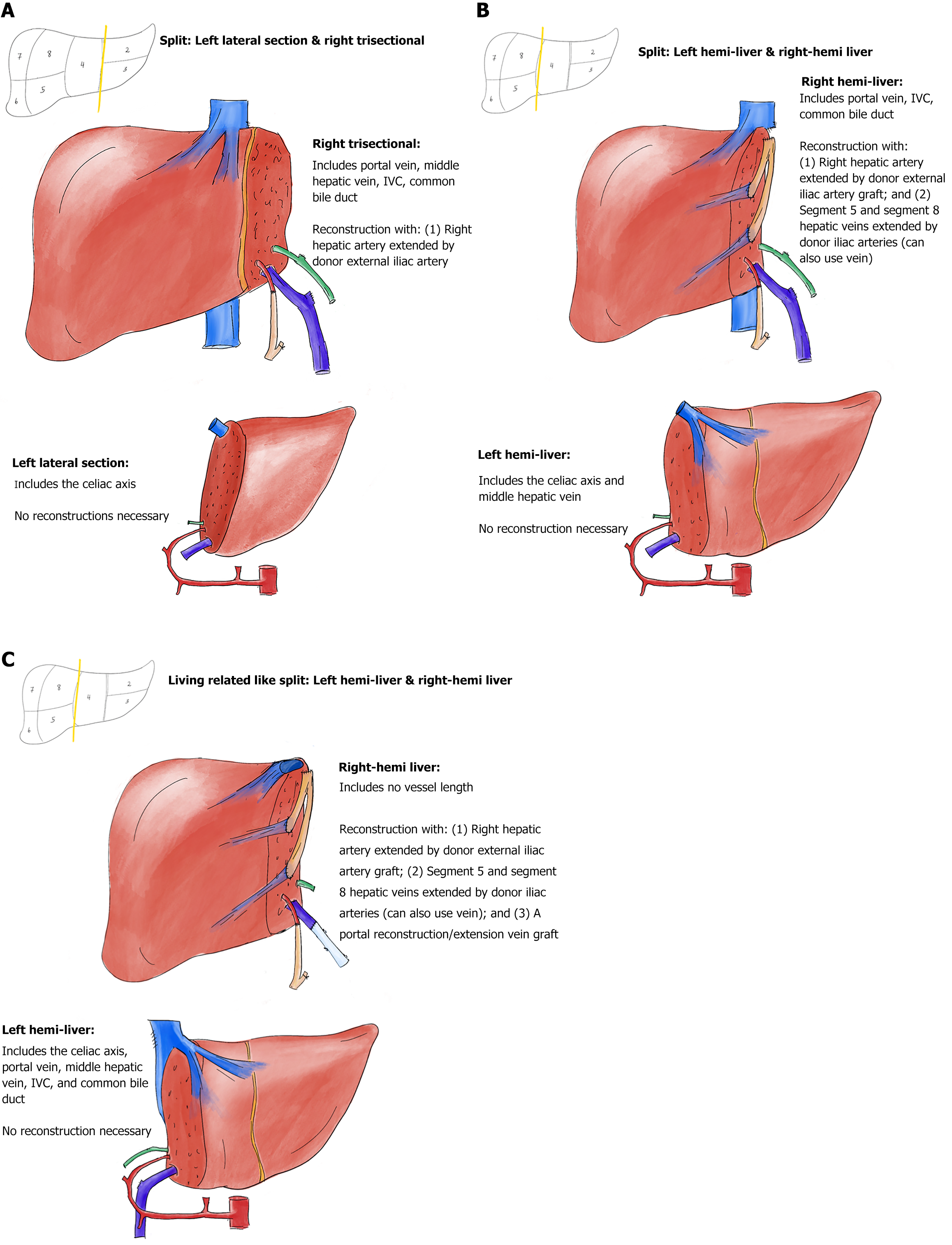
Liver transplantation (LT) offers the only life-saving option for adults and children with end-stage liver disease, but its application is limited by graft availability and heterogeneity amongst other issues. Abdominal domain often limits donor options in children, though a variety of surgical techniques have emerged to reduce the impact of this factor. Split LT (SLT) is one such technique that not only ameliorates some of the issues of graft size for the pediatric recipient of the left lateral segment (LLS) (or monosegment) but expands the donor pool by providing grafts for two recipients from one liver (Figure 1). Acceptance of both grafts not only reduces waitlist time and mortality for pediatric recipients but can offer graft opportunity for expanded transplant indications. Unfortunately, often restricted by center expertise, SLT is utilized infrequently. In fact, less than 2% of all grafts in the United States are split with the possible supply of ‘split-able’ livers far surpassing pediatric waitlist deaths[1]. Another concern lies within the technique itself. SLT creates two extended-criteria organs after a decrease in size and incurred injury with transection[2]. This results in grafts that, when compared to whole grafts, are more susceptible to ischemic reperfusion injury (IRI) and primary nonfunction given significantly prolonged cold ischemic time (CIT), resulting in higher rates of retransplantation in the adult recipient of the right liver allograft[3]. There is hope that improved preservation and resuscitation of split liver allografts with normothermic oxygenated machine perfusion may improve these outcomes.
Machine perfusion has drastically altered the transplantation landscape for marginal grafts and augmented the donor pool[4]. Reducing rates of post-reperfusion syndrome (PRS), IRI and improving outcomes, machine perfusion tempers a procurement’s ischemic insults whilst simultaneously resuscitating the organ[5-8]. Through continuous cycle of nutritional elements and removal of injurious metabolites, it helps to preserve, recuperate, and even evaluate graft function. As such, machine perfusion may be the ideal technique to optimize SLT logistics and ameliorate the injury split grafts are exposed to, thus potentially improving outcomes. First trialed in hypothermic oxygenated perfusion (HOPE), the safety and practicality of this idea began to be established[6,9-11]. An ex-vivo two-step split technique was described in these cases with placement of the graft on the machine and subsequent parenchymal split during HOPE perfusion[11]. With success in early cases, this technique significantly reduced PRS and IRI when compared to static cold storage (SCS) splits[9]. Mitigating the damage seen after SCS SLT, split with HOPE could offer significant benefit.
The other dynamic perfusion concept that has increasingly increased the donor pool supply is normothermic machine perfusion (NMP). Unlike HOPE which seeks a highly oxygenated environment at 8 to 12 degrees C, NMP aims for a physiologic graft haven using a 37 °C blood-based perfusate[4]. Similar to HOPE, NMP has demonstrated a considerable benefit over SCS, with decreased rates of PRS, early allograft dysfunction and ischemic type biliary complications[4]. Systematic appliance of NMP in SLT using Organ-Ox Metra has been previously described at two centers[12,13]. Use of NMP in SLT generously expands the benefits of a LT through transformation into a scheduled surgery under a controlled environment which can avoid unnecessary discards and optimal donor-recipient matching. Further, it helps to avoid prolonged CIT, leading to improved graft outcomes. Difficulty in the feasibility of this approach stems from careful vascular reconstruction and sealing of resection planes such that perfusion pressures do not drop[12]. Use of a closed-circuit system may improve graft perfusion and not require oversewing or careful sealing of cut-surfaces.
This study herein reports the first case of SLT with placement and resuscitation of a right trisectional allograft on NMP using a closed-circuit system. It also seeks to describe a standardization of the multiple logistic possibilities depending on procurement and transportation specifics to allow for successful NMP resuscitation of only one or both split grafts prior to implantation.
Donor information: The donor was a brain dead, 68 kg 17-year-old male with a body mass index of 23 kg/m2 who succumbed to a gunshot wound to the head. There was no relevant medical history or high-risk criteria, liver and renal function was normal limits. Standard whole liver volume was 1500 g (LLS: 300 g and right trisectional: 1200 g). The hepatic vein outflow of the LLS graft had an anatomic variation–a sizeable ventral fissural vein. This would require at least the proximal four centimeters of the middle hepatic vein (MHV) to be included in the pediatric graft to allow for a singular outflow vein for anastomosis. Consequently, the right trisectional graft would require a more complex outflow repair.
Case 1: Our pediatric recipient was a 19-month-old, 11-kilogram female with biliary atresia after failed Kasai procedure.
Case 2: Our adult recipient was a 42-year-old, 90-kilogram female with otherwise unresectable intrahepatic cholangiocarcinoma.
Case 1: The pediatric recipient was listed with an exception for medium percutaneous endoscopic lumbar discectomy at transplant plus three points due to gastrointestinal hemorrhage and sarcopenia.
Case 2: The adult recipient had undergone a prior left hemi-hepatectomy and postoperative radiation.
Case 1: The pediatric recipient had a prior history of biliary atresia status post failed Kasai procedure.
Case 2: The adult recipient had received some treatment for her intrahepatectic cholangiocarcinoma without success–including a prior left hemi-hepatectomy and postoperative radiation.
The family history of two patients was unremarkable for any conditions significant to these cases.
The liver was placed at sequence 12 for our pediatric recipient and at sequence 1422 for our adult recipient. It was likely due to the outflow complexity that the right trisectional graft was quickly rejected by prioritized centers and within less than 20 minutes, it was offered to our center.
Recipients in our center are selected through our Pediatric-Adult Transplant Oncology Recipients couples pathway. In this program, every pediatric recipient allocated a split liver graft from a donation after brain death donor is pre-coupled with a potential adult recipient with an expanded Transplant Oncology indication. This method allows adult recipients with mainly colorectal hepatic metastases or conventionally unresectable intrahepatic cholangiocarcinoma to get lifesaving grafts given they are ineligible for exception points. Recipient coupling is based on blood group and donor weight. For the pediatric recipient, we aim for a suitable LLS or left hemi-liver graft with a graft weight to recipient weight (GWRW) ratio of 1.0 to 4.0. For the adult recipient, we aim for a suitable right trisectional or right hemi-liver graft with a GWRW ratio of 1.0 to 2.0. Once an offer is received for a split pediatric liver graft that is suitable for one patient, our team immediately informs the organ procurement organization (OPO) about our willingness to accept the second graft of the split, were it to become available to our center. We provide this potential adult Transplant Oncology recipient information in an effort to expedite the process.
In this example case, an LLS split graft was offered to a 19-month-old female with biliary atresia after failed Kasai procedure, weighing 11 kg. She was listed with an exception for medium percutaneous endoscopic lumbar discectomy at transplant plus three points due to gastrointestinal hemorrhage and sarcopenia. Calculation of the donor’s standardized liver volume by his body surface area generated an LLS graft of 300 g and a right trisectional graft of 1200 g. Given this information, a suitable Transplant Oncology adult recipient was selected: A 42-year-old female with otherwise unresectable intrahepatic cholangiocarcinoma. She had undergone a prior left hemi-hepatectomy and postoperative radiation. She weighed 90 kg and was at sequence 1422.
Given significant logistical constraints including operating staff and room availability, it was not possible to plan for two simultaneous liver transplants. As such, we planned for an off-label approach with a closed-circuit normothermic perfusion platform (OCS Liver, TransMedics) for an in-situ split and placement of the right trisectional graft on NMP. This would allow preservation of the adult graft such that we could perform the two transplantations sequentially and not in parallel. For this reason, two separate planes were arranged to leave from the donor hospital. One plane was employed to immediately transport the LLS in cold storage to the recipient hospital. The second plane was employed to transplant the right trisectional graft after back table reconstruction and placement on NMP.
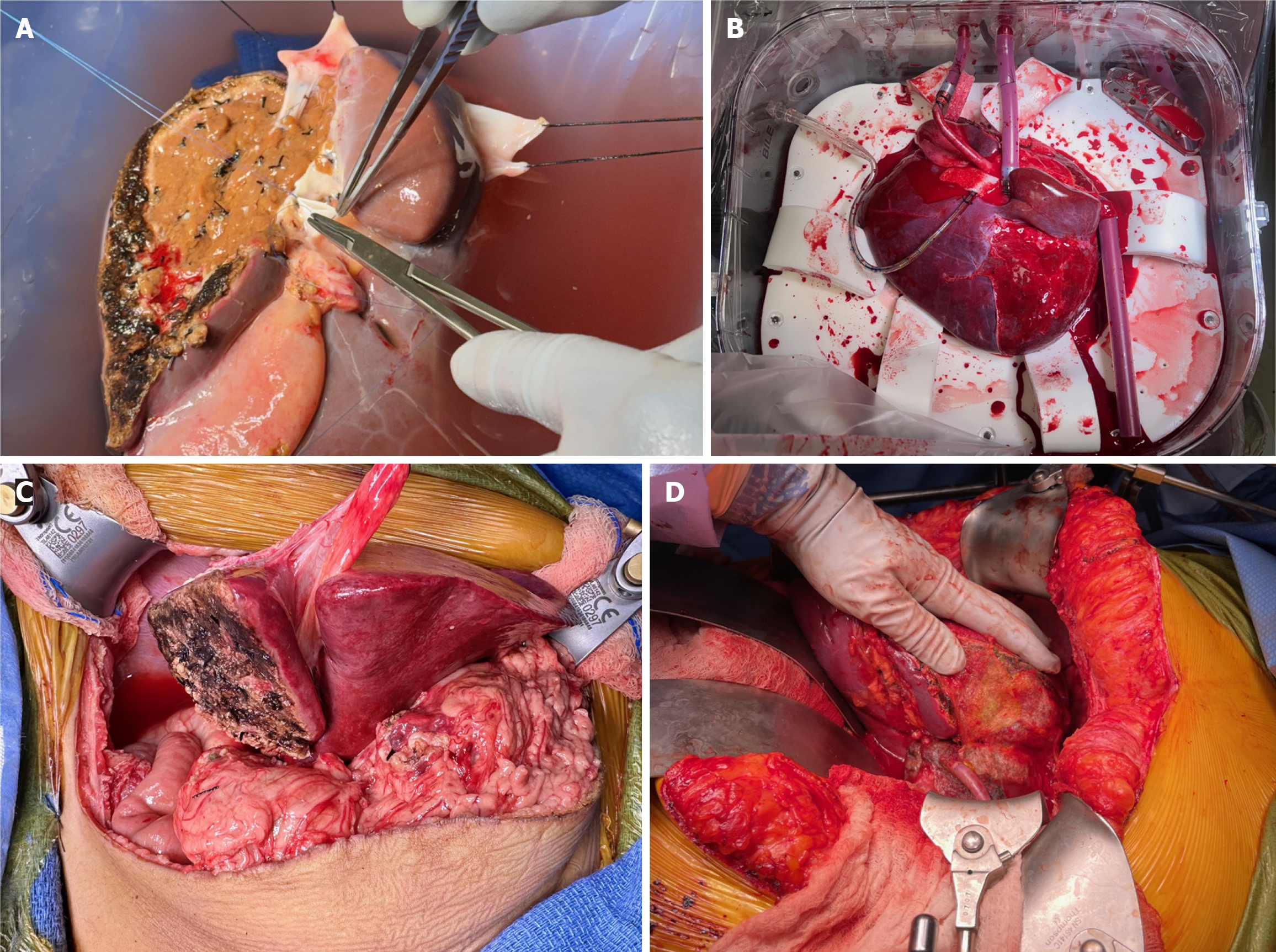
In a pediatric-adult split, we primarily use an in-situ technique, splitting the grafts as depicted in Figure 1. Generally, an LLS and right trisectional split would follow the anatomical separation in Figure 1A. In this case, however, due to the anatomic variation of the left hepatic and MHV outflow, the in-situ component was somewhat limited, involving only 20% of the indented transection surface. The donor also had a large accessory left hepatic artery (HA). The majority of the transection was then performed ex-situ and required a total of two hours of back table preparation in an ice-cold bath. The LLS graft was rapidly separated, within ten minutes, and passed off the back table for immediate packaging and transport with the first plane, resulting in a total CIT of four hours. The right trisectional graft required more back table work. In order to achieve connectivity to the NMP, the following vascular reconstructions and repairs were performed: (1) Right HA extension with a six centimeter vascular graft using donor left iliac artery; (2) Reconstruction of the proximal four centimeters of the MHV using donor left external iliac vein; (3) Transverse repair of the left portal vein ostium of the main portal vein; and (4) Direct repair of the left hepatic duct ostium of the main hepatic duct (with a five millimeter cuff). Of note, while it was desirable to use an arterial conduit for the MHV venous reconstruction, we were unable to do so as the pancreas and small intestine procurement teams took the right arterial grafts. Standard NMP liver cannulas were then placed and secured as follows: (1) A 40 French (Fr) cannula into the main portal vein; (2) A 18 Fr cannula into the arterial conduit of the right HA; (3) A 34 Fr cannula into the supra-hepatic inferior vena cava (IVC); and (4) a 12 Fr cannula into the common bile duct (Figure 2A). Overall, CIT for the right trisectional graft was four hours, with 21 hours of normothermic perfusion, totaling 25 hours of preservation time.
Positioning of the right trisectional graft into the NMP chamber was different than usual. Specifically, due to the short length of the main portal vein from the concomitant pancreatic procurement and the absence of the supporting LLS, the graft required a 90-degree clockwise rotation to assume a neutral position without inflow kinking. This resulted in a very short arterial cannula and a considerably long portal vein cannula. Further, the IVC cannula was unable to be secured in its usual position and was instead left unconnected or ‘floating’ (Figure 2B), which is not a problem for a closed-circuit system such as OCS Liver.
After connection and initiation of the NMP, the first ten minutes were utilized to achieve maximal hemostasis at the transection plane. This was accomplished with sutures for major bleeding sites followed by placement of a matrixed active hemostatic agent. Once the cut surface hemostasis was acceptable, the flow rates were set to a target total flow of 1 mL/minute/gram with one fourth of the flow being arterial. Given a graft weight of 1350 g, we initially targeted 1350 mL/minute of total flow.
Ultimately, as the graft reached a temperature above 34 degrees Celsius, a total flow of 1500 mL/minute was achieved with portal vein pressures less than 10 mmHg and arterial systolic blood pressures less than 50 mmHg. These were set as the NMP transport parameters. Immediately after placement on NMP, the graft started to clear its lactate and after 30 minutes, it began to produce bile.
For the pediatric recipient, the hepatectomy was performed to maximize length of inflow vessels with high dissection of HA and portal vein up to the hilar plate. The prior roux limb was preserved. The LLS was placed into the recipient and the hepatic vein outflow was reconstructed using the spatulated ostia of all three hepatic veins coming off the IVC. The portal vein reconstruction was performed with the graft's left portal vein anastomosed to the recipient’s main portal vein. Finally, the arterial inflow reconstruction was performed with anastomosis of the graft's celiac trunk to the recipient's common HA (Figure 2C). Portal flow was 330 mL/minute and arterial flow was 150 mL/minute and the recipient splenic artery was not ligated.
For the adult recipient, a bilateral subcostal incision was made (Mercedes type). There was no evidence of peritoneal carcinomatosis. It was a challenging hepatectomy given significant adhesions and desmoplastic reaction from her prior surgery and radiation treatments. During mobilization of the caudate lobe, it became apparent that the para-caval portion was densely associated with the previously treated malignancy and unable to be resected free of the IVC. As such, the piggyback approach was aborted and we proceeded to a bicaval approach. The patient did not tolerate test clamp for total vascular isolation, so we performed venovenous bypass cannulations. The hepatectomy was completed with retrohepatic IVC resection. The right trisectional graft was placed into the recipient and the suprahepatic cava was anastomosed to the suprahepatic recipient cuff. Following this, the graft infrahepatic cava was anastomosed to the recipient infrahepatic cava. The portal vein reconstruction was performed with the right portal vein anastomosed to the recipient’s main portal vein with appropriate growth factor. A retrograde blood flush was performed followed by antegrade portal reperfusion. The arterial inflow reconstruction was then performed with anastomosis of the right HA (extended by donor's right iliac artery) to the recipient’s common hepatic and gastroduodenal arteries confluence, creating a spatulated patch (Figure 2D).
Both patients had uneventful postoperative courses and continue to do well. The pediatric recipient was discharged home on postoperative day 14 and the adult recipient was discharged home on postoperative day seven with drain removal on postoperative day four.
Optimal utilization of all suitable deceased donor grafts is critical to minimizing wait list deaths for both adults and children awaiting LT. Despite data suggesting that suitable outcomes with SLT can be achieved for both the adult and pediatric recipient, the use of technical variant grafts varies widely in the United States[14]. Split liver utilization almost always originates with a primary offer to the child and thus collaborative partnerships between adult and pediatric teams are essential and have been shown to increase split liver utilization[15]. Nonetheless, when both offers are ultimately allocated to the same institution, system resources may be strained and optimizing outcomes requires either simul
Machine perfusion represents an ideal technique for optimization of SLT logistics while simultaneously lessening the injury split grafts are exposed to, thus also improving outcomes. Given that the most common reason the second graft from split livers are discarded is due to need for reconstruction and subsequent prolonged CIT, application of NMP offers significant utility by essentially freezing the timer. Initial results from trials with HOPE perfusion are promising, with significant reductions in PRS and IRI when compared with SCS splits[9]. As such, this study seeks to propose a standardization of logistic options for SLT with machine perfusion, using an example with a closed-circuit NMP, in an effort to minimize disposal of high-quality grafts.
Standardization must begin with a key factor in recipient success in SLT which is the consideration of graft vasculature and reconstruction. This planning becomes particularly important when grafts are split prior to placement on NMP as adequate perfusion of the entirety of the split grafts needs to be ensured. The preparation for reconstruction preceding procurement is essential as to allow appropriate discussions with other abdominal teams to facilitate successful cannulation. Our recommendations for reconstruction for placement on NMP are as follows: (1) For an LLS and right trisectional split, the right trisectional should have an arterial reconstruction with donor external iliac artery graft as the LLS includes the celiac axis (Figure 1A); (2) For a hemi-liver split, the right hemi-liver requires both an arterial and venous reconstruction using donor iliac vessels (Figure 1B). The left hemi-liver includes the celiac axis and MHV and thus no reconstruction is necessary; and (3) Finally, when performing a living-related-like split, the right hemi-liver will also need a portal reconstruction/extension vein graft (Figure 1C).
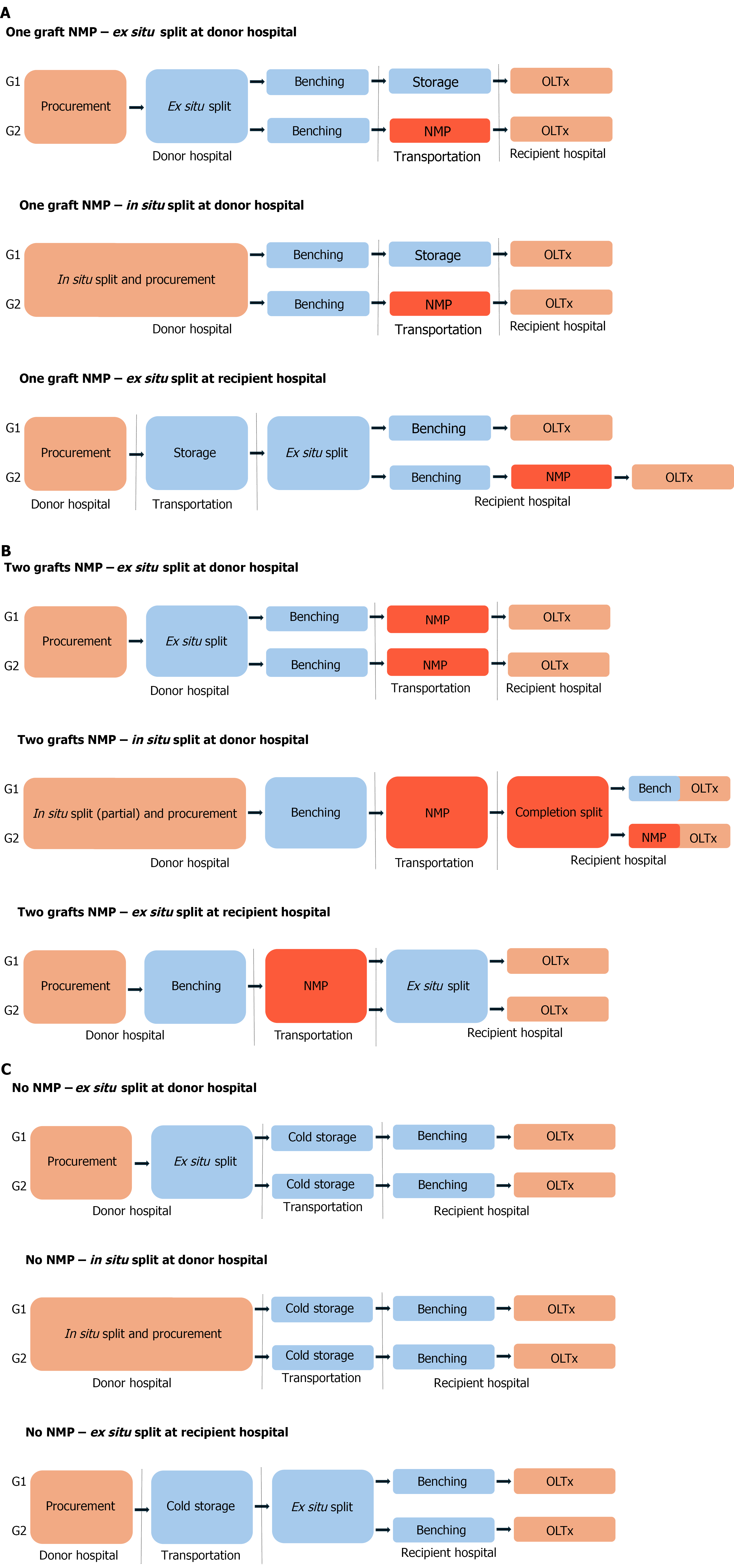
Between procurement and implantation of partial deceased donor grafts, there are three major and discrete steps: (1) Splitting (S); (2) Pumping (P); and (3) Transporting (T). The sequence of these steps not only has direct implications for logistics but likely also for recipient outcomes. For instance, connecting the graft to the NMP at the donor hospital likely will lead to improved recipient outcomes by minimizing CIT whereas connecting at the recipient hospital may improve logistics. Further, the choice of whether one or both grafts should be placed on NMP also has clear logistical implications. Ultimately, depending on the priority of the transplant center for each case, there are variations in the sequence of steps that can be followed (Figure 3).
When placing only one of the two partial grafts on NMP, splitting at the donor hospital (whether ex-situ or in-situ) has the potential in resulting in improved recipient outcomes. An immediate split followed by placement on pump at the donor hospital minimizes CIT for at least one of the grafts (S-P-T sequence, A1, A2) (Figure 3A). The case presented in this communication followed the A1 logistic pattern. A variation of this sequence involves an immediate split but with pump connection performed at the recipient hospital. This variation can compromise recipient outcomes by increasing CIT; however, it may greatly improve center logistics as pump transportation needs are minimized (S-T-P sequence, A1 L, A2 L, not depicted) (Figure 3A). Alternatively, splitting at the recipient hospital (T-S-P sequence, A3) (Figure 3A) greatly improves logistics, however, may further compromise recipient outcomes in correlation with increased CIT.
When placing both partial grafts on NMP, splitting at the donor hospital remains optimal by minimization of CIT, potentially leading to better recipient outcomes. However, there are logistical drawbacks depending on the split technique. When split ex-situ, two pumps are necessary for transportation (S-P-T sequence, B1). When split in-situ, a partial transection facilitates transport with one pump but requires a technically demanding on-pump completion split at the recipient hospital (S-P-T-S sequence, B2). Here, again, there are variations of these sequences, where connection to the pump(s) takes place at the recipient hospital. These variations may compromise recipient outcomes by increasing CIT but improve logistics by minimizing pump transportation needs (S-T-P ± S sequence, B1 L and B2 L, respectively, not depicted). On the other hand, splitting at the recipient hospital (T-S-P sequence, B3) may compromise recipient outcomes as the entirety of the ex-situ split takes place after NMP resuscitation, a fact that may ameliorate the benefits of the pump. Here too, there is a variation of this sequence (S-T-P, B3 L, not depicted) that might improve logistics as it doesn’t require transportation of the NMP pump. It would, however, require two separate pumps but also facilitate active graft evaluation.
Ultimately, the overall effort should be to minimize graft CIT. As such, we believe the best strategy is to transport the graft on the pump from the donor hospital (A1, A2, B1, B2) (Figure 3). The alternate scenarios should be considered when there is a small donor hospital with inadequate operating infrastructure or equipment for a split. Instead, optimization of logistics can be prioritized with graft transportation in SCS and initiating of NMP at the recipient hospital (A1 L, A2 L, A3, B1 L, B2 L) (Figure 3). For OPOs, a ‘pump first–split after’ approach (B3, B3 L) may be ideal. This method evaluates the whole graft and not individual partial graft viability which may represent a good option for “rescuing” livers too difficult to allocate. Here too, B3 is preferrable to B3 L, since it minimizes CIT. Needless to say, the thought process behind all of the aforementioned logistics can be significantly altered if the partial grafts are accepted by two centers instead of just one.
As a side note, the authors’ opinion is that the logistically easier approaches that put partial grafts (and not whole livers) on the pump are the A1 L, A2 L, and A3, with the caveat that CIT is increased by transport time. This might be acceptable in cases where the donor hospital is geographically close, but unacceptable when procurement takes place in remote locations.
This study illustrates the potential of NMP with SLT to not only perform complex surgeries in a time-controlled successive fashion but also to optimize graft allocation without sustaining further CIT. The described logistics offer an array of approaches to allow for the expansion and growth of this strategy while also augmenting graft to recipient matching and facilitating graft access for expanded oncological indications.
| 1. | Perito ER, Roll G, Dodge JL, Rhee S, Roberts JP. Split Liver Transplantation and Pediatric Waitlist Mortality in the United States: Potential for Improvement. Transplantation. 2019;103:552-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (1)] |
| 2. | Dalal AR. Split liver transplantation: What's unique? World J Transplant. 2015;5:89-94. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Andrassy J, Wolf S, Lauseker M, Angele M, van Rosmalen MD, Samuel U, Rogiers X, Werner J, Guba M; Eurotransplant Liver Advisory Committee. Higher retransplantation rate following extended right split-liver transplantation: An analysis from the eurotransplant liver follow-up registry. Liver Transpl. 2018;24:26-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | Markmann JF, Abouljoud MS, Ghobrial RM, Bhati CS, Pelletier SJ, Lu AD, Ottmann S, Klair T, Eymard C, Roll GR, Magliocca J, Pruett TL, Reyes J, Black SM, Marsh CL, Schnickel G, Kinkhabwala M, Florman SS, Merani S, Demetris AJ, Kimura S, Rizzari M, Saharia A, Levy M, Agarwal A, Cigarroa FG, Eason JD, Syed S, Washburn WK, Parekh J, Moon J, Maskin A, Yeh H, Vagefi PA, MacConmara MP. Impact of Portable Normothermic Blood-Based Machine Perfusion on Outcomes of Liver Transplant: The OCS Liver PROTECT Randomized Clinical Trial. JAMA Surg. 2022;157:189-198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 258] [Article Influence: 86.0] [Reference Citation Analysis (0)] |
| 5. | Ravaioli M, Germinario G, Dajti G, Sessa M, Vasuri F, Siniscalchi A, Morelli MC, Serenari M, Del Gaudio M, Zanfi C, Odaldi F, Bertuzzo VR, Maroni L, Laurenzi A, Cescon M. Hypothermic oxygenated perfusion in extended criteria donor liver transplantation-A randomized clinical trial. Am J Transplant. 2022;22:2401-2408. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 86] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 6. | Rossignol G, Muller X, Hervieu V, Collardeau-Frachon S, Breton A, Boulanger N, Lesurtel M, Dubois R, Mohkam K, Mabrut JY. Liver transplantation of partial grafts after ex situ splitting during hypothermic oxygenated perfusion-The HOPE-Split pilot study. Liver Transpl. 2022;28:1576-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 7. | van Rijn R, Schurink IJ, de Vries Y, van den Berg AP, Cortes Cerisuelo M, Darwish Murad S, Erdmann JI, Gilbo N, de Haas RJ, Heaton N, van Hoek B, Huurman VAL, Jochmans I, van Leeuwen OB, de Meijer VE, Monbaliu D, Polak WG, Slangen JJG, Troisi RI, Vanlander A, de Jonge J, Porte RJ; DHOPE-DCD Trial Investigators. Hypothermic Machine Perfusion in Liver Transplantation - A Randomized Trial. N Engl J Med. 2021;384:1391-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 388] [Article Influence: 97.0] [Reference Citation Analysis (0)] |
| 8. | Parente A, Tirotta F, Pini A, Eden J, Dondossola D, Manzia TM, Dutkowski P, Schlegel A. Machine perfusion techniques for liver transplantation - A meta-analysis of the first seven randomized-controlled trials. J Hepatol. 2023;79:1201-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 9. | Rossignol G, Muller X, Ruiz M, Collardeau-Frachon S, Boulanger N, Depaulis C, Antonini T, Dubois R, Mohkam K, Mabrut JY. HOPE Mitigates Ischemia-Reperfusion Injury in Ex-Situ Split Grafts: A Comparative Study With Living Donation in Pediatric Liver Transplantation. Transpl Int. 2024;37:12686. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 10. | Spada M, Angelico R, Grimaldi C, Francalanci P, Saffioti MC, Rigamonti A, Pariante R, Bianchi R, Dionisi Vici C, Candusso M, Maggiore G. The New Horizon of Split-Liver Transplantation: Ex Situ Liver Splitting During Hypothermic Oxygenated Machine Perfusion. Liver Transpl. 2020;26:1363-1367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Mabrut JY, Lesurtel M, Muller X, Dubois R, Ducerf C, Rossignol G, Mohkam K. Ex Vivo Liver Splitting and Hypothermic Oxygenated Machine Perfusion: Technical Refinements of a Promising Preservation Strategy in Split Liver Transplantation. Transplantation. 2021;105:e89-e90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Kathirvel M, Nasralla D, Iype S, Tzerbinis H, Crick K, Cortes Cerisuelo M, Pollok JM. Salvage of a Right-extended Split Liver Graft Using Ex Situ Normothermic Machine Perfusion-A New Therapeutic Horizon. Transplantation. 2022;106:e320-e321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 13. | Krendl FJ, Cardini B, Laimer G, Singh J, Resch T, Oberhuber R, Schneeberger S. Normothermic Liver Machine Perfusion and Successful Transplantation of Split Liver Grafts: From Proof of Concept to Clinical Implementation. Transplantation. 2024;108:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Mazariegos GV, Perito ER, Squires JE, Soltys KA, Griesemer AD, Taylor SA, Pahl E. Center use of technical variant grafts varies widely and impacts pediatric liver transplant waitlist and recipient outcomes in the United States. Liver Transpl. 2023;29:671-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 15. | Vargas PA, Cullen JM, Dalzell C, DiPaola F, Pelletier S, Soltys KA, Mazariegos GV, Oberholzer J, Goldaracena N. Increased use of split liver grafts in adult recipients following implementation of a pediatric liver transplant program. Pediatr Transplant. 2022;26:e14159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |