Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.102378
Revised: March 12, 2025
Accepted: March 21, 2025
Published online: September 18, 2025
Processing time: 180 Days and 0.8 Hours
Living donor liver transplantation (LDLT) is a crucial alternative to deceased donor transplantation, especially in regions with limited access to cadaveric or
To compare two venous anastomosis techniques—direct polytetrafluoroethylene (PTFE) grafting of V5-V8 veins to the IVC vs triangulation to the right hepatic vein (RHV)—in terms of graft viability and postoperative outcomes.
A retrospective analysis was conducted on 96 patients who underwent LDLT with right lobe grafts between 2014 and 2023. Patients were divided into three groups: (1) No venous outflow reconstruction; (2) PTFE graft direct anastomosis to the IVC; and (3) PTFE graft anastomosis using triangulation to the RHV. Perioperative and postoperative outcomes, including bile duct complications, alanine aminotransferase/aspartate aminotransferase levels, and graft perfusion, were compared across groups.
Group 3 (triangulation to RHV) showed significantly improved venous outflow, fewer complications, and faster normalization of liver function tests. Bile duct complications were highest in group 1 (12.8%) and lowest in group 3 (7%). Doppler ultrasonography revealed better graft perfusion in group 3 compared to groups 1 and 2.
Triangulation to the RHV improves graft viability, reduces biliary complications, and enhances early postoperative outcomes compared to direct PTFE grafting to the IVC.
Core Tip: This study compares two venous anastomosis techniques—direct polytetrafluoroethylene grafting of V5-V8 veins to the inferior vena cava vs triangulation to the right hepatic vein (RHV). It is shown that triangulation to the RHV improves graft viability, reduces biliary complications, and enhances early postoperative outcomes.
- Citation: Beridze D, Mikeladze L, Tomadze G, Kordzaia D, Kashibadze K. Peculiarities of implantation of the right graft veins into the inferior vena cava during living donor liver transplantation. World J Transplant 2025; 15(3): 102378
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/102378.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.102378
In living donor liver transplantation (LDLT), particularly in adult-to-adult cases using right lobe grafts, ensuring adequate hepatic venous outflow is a critical determinant of graft success. The middle hepatic vein (MHV) plays a crucial role in draining the anterior segments (liver segments V and VIII)[1-3]. The inclusion of the MHV trunk in an extended right liver graft (RLG) helped resolve the issue of graft congestion. However, the reduced size of the remaining liver poses a risk to the donor's safety. When the MHV is excluded from the right lobe graft, there is a significant risk of venous congestion, which can lead to graft dysfunction, sepsis, or even graft failure[4,5]. Each hepatic vein has its own drainage territory, making complete reconstruction of these outflow veins essential to prevent hepatic venous congestion[6,7].
As a result, venous outflow reconstruction becomes essential to maintain graft hemodynamics and ensure long-term viability. Various strategies have been proposed to address this challenge, including venous graft reconstruction techni
The decision to reconstruct the MHV has been the subject of ongoing debate, as including the MHV in the graft increases the complexity of surgery and may pose higher risks to the donor[4,8]. Without reconstruction, venous con
Venous outflow reconstruction is the most important procedure for successful implantation of a RLG in LDLT[9,10]. A wide outflow orifice also appears to be crucial for optimal graft performance[11].
This study aims to evaluate the clinical outcomes of venous outflow reconstruction techniques in LDLT using right lobe grafts without the MHV. By examining patency rates, graft regeneration, and patient outcomes, we aim to refine the surgical approach to venous drainage reconstruction in LDLT. These findings are intended to contribute to optimizing venous outflow management, ultimately improving early postoperative outcomes and long-term graft survival in com
In accordance with the objectives of this research, we evaluated 96 patients who underwent LDLT using right lobe grafts from 2014 to 2023. All operations followed the established surgical methodologies, utilizing only artificial PTFE material, as cadaveric transplantation is not currently performed in Georgia. Therefore, our study exclusively focuses on LDLT, comparing venous outflow reconstruction with and without PTFE prostheses (Gore-Tex). Anatomical studies have shown that accessory hepatic veins with diameters less than 3 mm are common and often do not require reconstruction due to their minimal contribution to overall hepatic drainage. Therefore, setting a threshold at 3.5 mm allows for a balance between ensuring adequate venous outflow-in our experience, veins above 3.5 mm with good outflow during back table flush is often reconstructed especially when the graft-to-recipient weight ratio (GRWR) is borderline, but most impor
During donor surgery, primarily non-marginal donors were selected, ensuring that the remnant liver volume was at least 33%, and the GRWR met the minimum requirement of 0.7. All donor hepatectomies were performed using the Cavitron Ultrasonic Surgical Aspirator, with 70% of cases using Olympus and 30% using Integra devices. Subsequently, the grafts were flushed on the bench with 2000 mL of Custodiol-HTK solution. The branches of segments V-VIII, tributaries of the MHV, were reconstructed using PTFE grafts, with the graft diameter being at least 1.5 times to 2 times larger than the diameter of the vein.
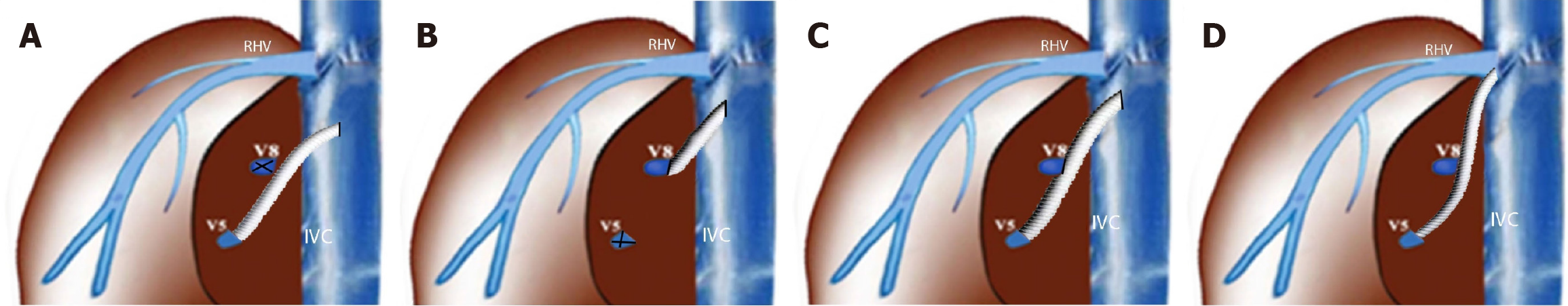
Patients were divided into three groups based on the vein diameter and reconstruction approach: (1) Group 1 (12 patients): No reconstruction was performed as the V5-V8 vein diameters were less than 3.5 mm; (2) Group 2 (44 patients): Implantation was performed using interposition grafting directly into the inferior vena cava (IVC): V5 + V8 veins in 15 patients, only V5 in 17 patients, and only V8 in 12 patients; and (3) Group 3 (40 patients): Reconstruction was performed via right hepatic vein (RHV)-triangulation: V5 + V8 veins in 11 patients, only V5 in 18 patients, and only V8 in 11 patients (Figure 1 and Table 1).
| Parameter | Group 1 (no venous reconstruction) | Group 2 (V5/V8 PTFE graft to inferior vena cava) | Group 3 (V5/V8 PTFE graft to right hepatic vein) | P value |
| Number of patients (n) | 12 | 44 | 40 | Not applicable |
| Male (female) | 9 (3) | 29 (15) | 28 (12) | 0.38 |
| Age (years) | 46.44 | 48.76 | 47.56 | 0.57 |
| HCV | 8 | 19 | 22 | 0.12 |
| HBV | 2 | 6 | 4 | 0.69 |
| HCV + HBV | 0 | 3 | 4 | 0.67 |
| HBV + delta | 1 | 4 | 2 | 0.68 |
| Other | 1 | 12 | 8 | 0.06 |
| Model for end-stage liver disease score | 19.7 | 19.8 | 20.4 | 0.24 |
| Donor age (years) | 37.2 ± 9.6 | 39.1 ± 11.2 | 38.5 ± 12.3 | 0.79 |
| Graft-to-recipient weight ratio | 0.81 ± 0.17 | 0.85 ± 0.19 | 0.87 ± 0.21 | 0.43 |
| Graft weight (g) | 770 ± 112 | 750 ± 140 | 780 ± 135 | 0.53 |
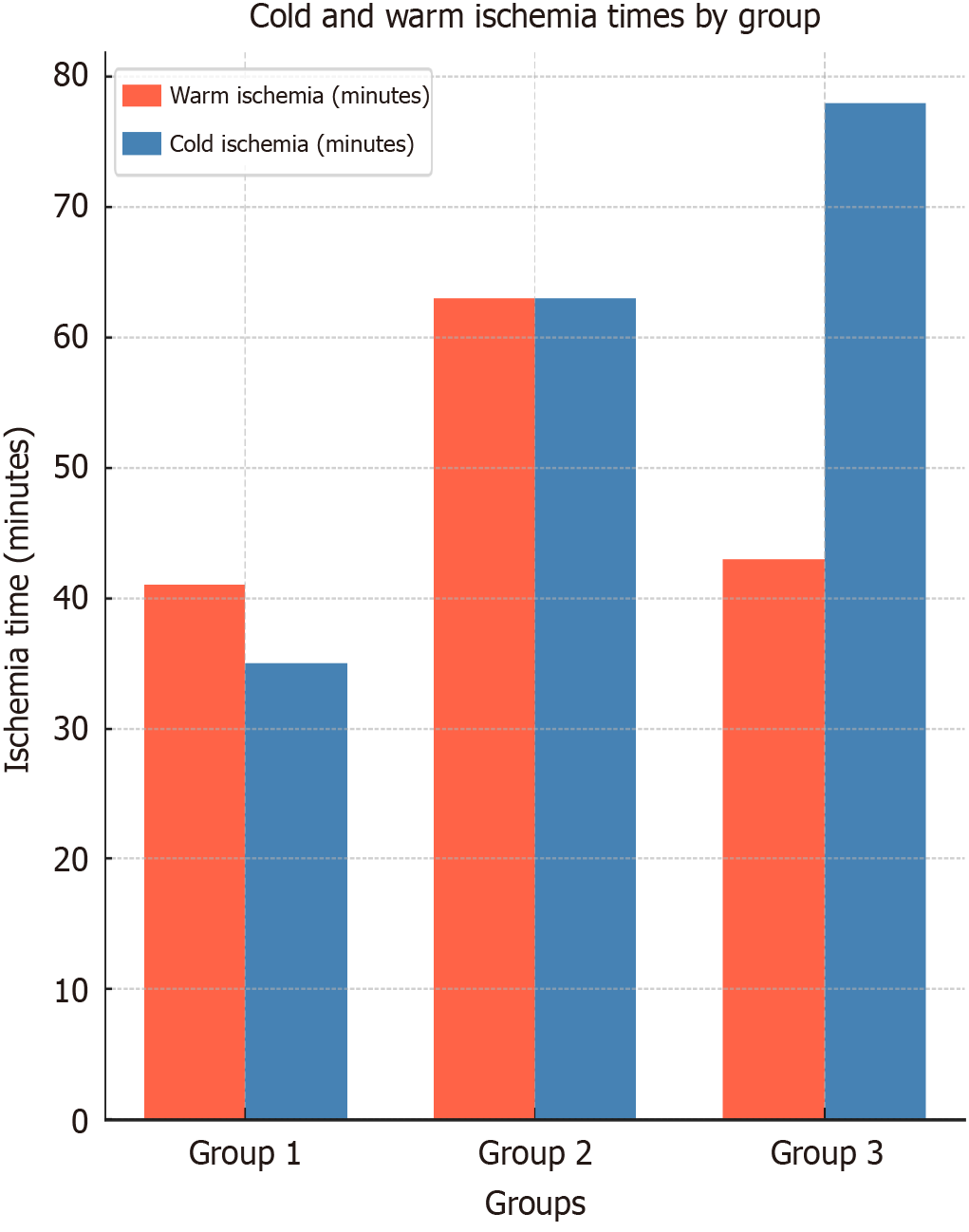
| Warm ischemia time (minutes) | 41 ± 3.5 | 63 ± 7.2 | 43 ± 6.1 | < 0.01 |
| Cold ischemia time (minutes) | 35 ± 3.8 | 63 ± 8.2 | 78 ± 7.3 | < 0.01 |
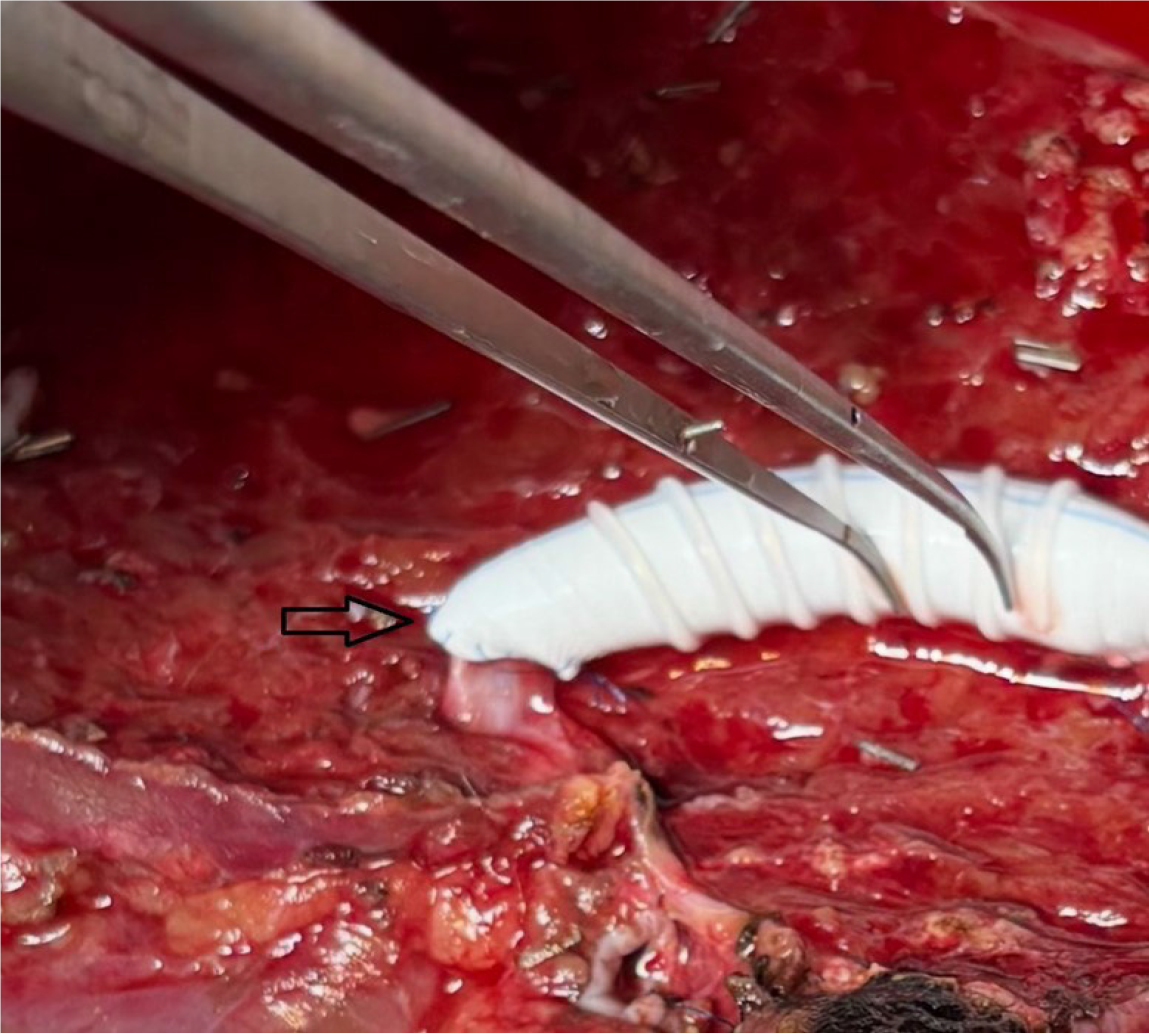
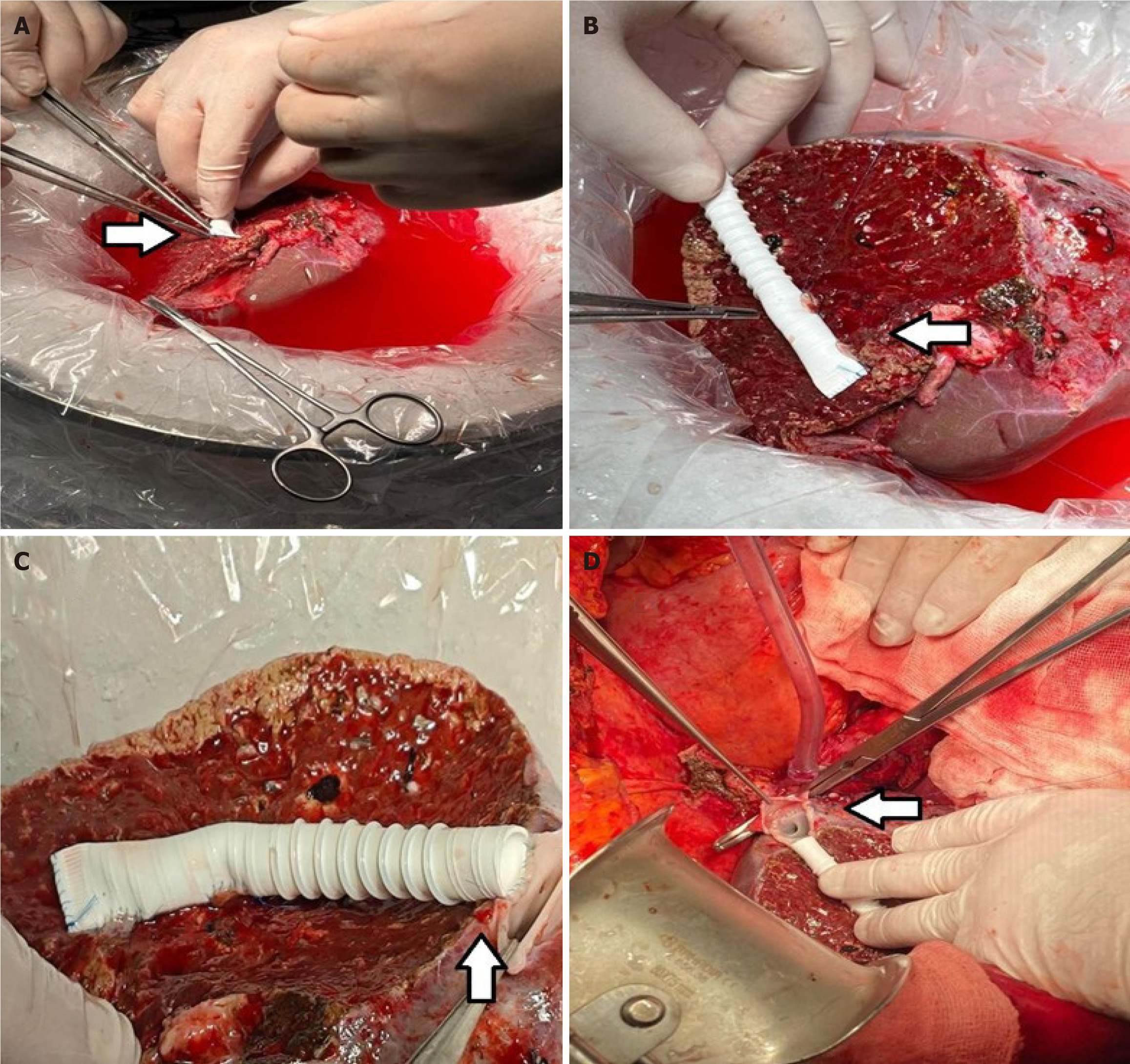
We used ringed PTFE grafts (GORE-TEX; W.L. Gore and Associates, Newark, DE, United States) with an internal diameter of 10 mm. After creating a small niche to enlarge the orifices of V5 and V8 (branches of the hepatic veins in 5th and 8th segments), an intervening allograft patch was applied to facilitate the end-to-end anastomosis of MHV branch V5 (Figure 2) and the end-to-side anastomosis to branch V8 (Figure 3). This PTFE graft was then anastomosed to the recipient’s IVC, either directly or using triangulation with the RHV. The triangulation technique was not modified and it was kept standard in all group 3 patients. Non-absorbable monofilament sutures made from expanded PTFE were used to minimize needle-hole bleeding from the graft (Figure 3).
The liver graft was implanted using 6-0 polypropylene sutures in cases of direct implantation into the IVC, or 5-0 polypropylene continuous sutures for triangulation. Before reperfusion, the graft was flushed through this area to test the patency and flow intensity of the reconstructed veins. Graft implantation was performed with full IVC clamping in 80% of cases, and with partial clamping (piggyback technique) in 20%.
Reperfusion was initiated by removing the upper IVC clamp, allowing blood inflow through the reconstructed hepatic veins into the graft. After one minute, the lower IVC clamp was removed, fully connecting the hepatic venous system to the circulation. After warming the liver for two minutes, the portal clamp was opened, and full reperfusion began. Doppler ultrasonography (Phillips 8) was used to assess vascular flow postoperatively, and daily assessments were conducted to monitor flow characteristics and liver perfusion.
Cold and warm ischemia times, the nature of blood vessels in the implanted liver, and liver perfusion in the early postoperative period were key factors in this study. We evaluated the amount of ascites, liver function tests, coagulation factors [international normalized ratio (INR)], and lactate levels daily for two weeks, as well as at one-month and three-months post-transplant. Additionally, at three months, we compared the graft volume with its original size. The results of the daily biochemical analysis were evaluated. Congestion and liver perfusion parameters were evaluated, maintaining a central venous pressure (CVP) of 5-7 mmHg in all cases. No further studies were performed when CVP exceeded this range. Continuous variables were expressed as medians and compared with the student’s t test or Mann-Whitney U test as appropriate. Differences between proportions derived from categorical data were compared with the χ2 test. Given that some variables did not follow a normal distribution, we opted for the Mann-Whitney U test for non-parametric com
Cold ischemia includes the period from the removal of the liver graft from the donor's body to its preservation in an ice-cold solution on the back table. Prolonged cold ischemia time is known to impair graft function and survival[13,14]. In all 96 transplants performed, the removed grafts were flushed with Custodiol-HTK solution before implantation and placed in an ice bowl, reducing the graft temperature to 2-3 °C. For grafts weighing 500 g, we used 1000 mL of Custodiol-HTK solution; for grafts weighing 500-800 g, we used up to 2000 mL, and for grafts weighing over 800 g, 3000 mL of Custodiol-HTK was used.
The ischemia duration depended on the number of veins to be sutured, not on the graft size, which was similar across all three groups: (1) Group 1: 770 g ± 112 g; (2) Group 2: 750 g ± 140 g; and (3) Group 3: 780 g ± 135 g, with an average of 768.72 g. The number of veins to be sutured was a significant factor influencing ischemia time. Group 1, where no veins were sutured, had the shortest cold ischemia time (35 minutes ± 3.8 minutes), spent on flushing, suturing the bile duct, and preparation for implantation. In contrast, groups 2 and 3 required more time for venous reconstruction. Group 2 involved V5 (17 patients), V8 (12 patients), and V5 + V8 (15 patients), while group 3 involved V5 (18 patients), V8 (11 patients), and V5 + V8 (11 patients). In group 2, the distal end of the PTFE graft was sutured to the segmental veins, and the proximal end was sutured directly to the IVC, with an average ischemia time of 78 minutes ± 7.3 minutes. Group 3 used RHV-triangulation. While concealed information test varied among groups, all values remained below the critical threshold where any ischemic damage would be expected.
Warm ischemia includes the period from when the graft is removed from the ice-cold liquid and placed into the abdominal cavity for implantation, to the start of reperfusion (release of portal and IVC flows), also referred to as anastomosis or implantation time[15,16]. In group 1, where no prosthetic grafts were used and only the RHV was reconstructed, the warm ischemia time was 41 minutes ± 3.5 minutes. Similarly, group 3, where the proximal part of the graft was reconstructed into the RHV using triangulation, had a warm ischemia time of 43 minutes ± 6.1 minutes. Group 2, where the proximal end of the vein graft was implanted directly into the IVC, required more time, extending the warm ischemia to 63 minutes ± 7.2 minutes (Figure 4).
After graft implantation, Doppler ultrasonography was used intraoperatively to assess flow in the hepatic artery, portal vein, and hepatic veins. This qualitative Doppler ultrasound assessment helps detect immediate graft complications[17,18]. Portal and arterial inflow were satisfactory across all three groups, with average portal vein velocity (PSV) of 34.5 cm/second ± 3 cm/second and arterial PSV of 30.25 cm/second ± 4.6 cm/second. However, discrepancies between inflow and outflow were observed, particularly in right partial grafts, potentially leading to congestion and postoperative complications. Studies suggest that venous congestion in the anterior sector of RLGs increases the risk of septic complications and graft dysfunction[19,20]. In our cases, group 3, using the triangulation method, had the good outflow (25.7 cm/second ± 3.1 cm/second), Outflow in group 2 (29.73 cm/second ± 4.3 cm/second) was also satisfactory, while highest outflow was observed in group 1, where no MHV tributaries were reconstructed (36.47 cm/second ± 5.12 cm/second), that could cause disturbance in inflow/outflow balance, leading partial or complete congestion.
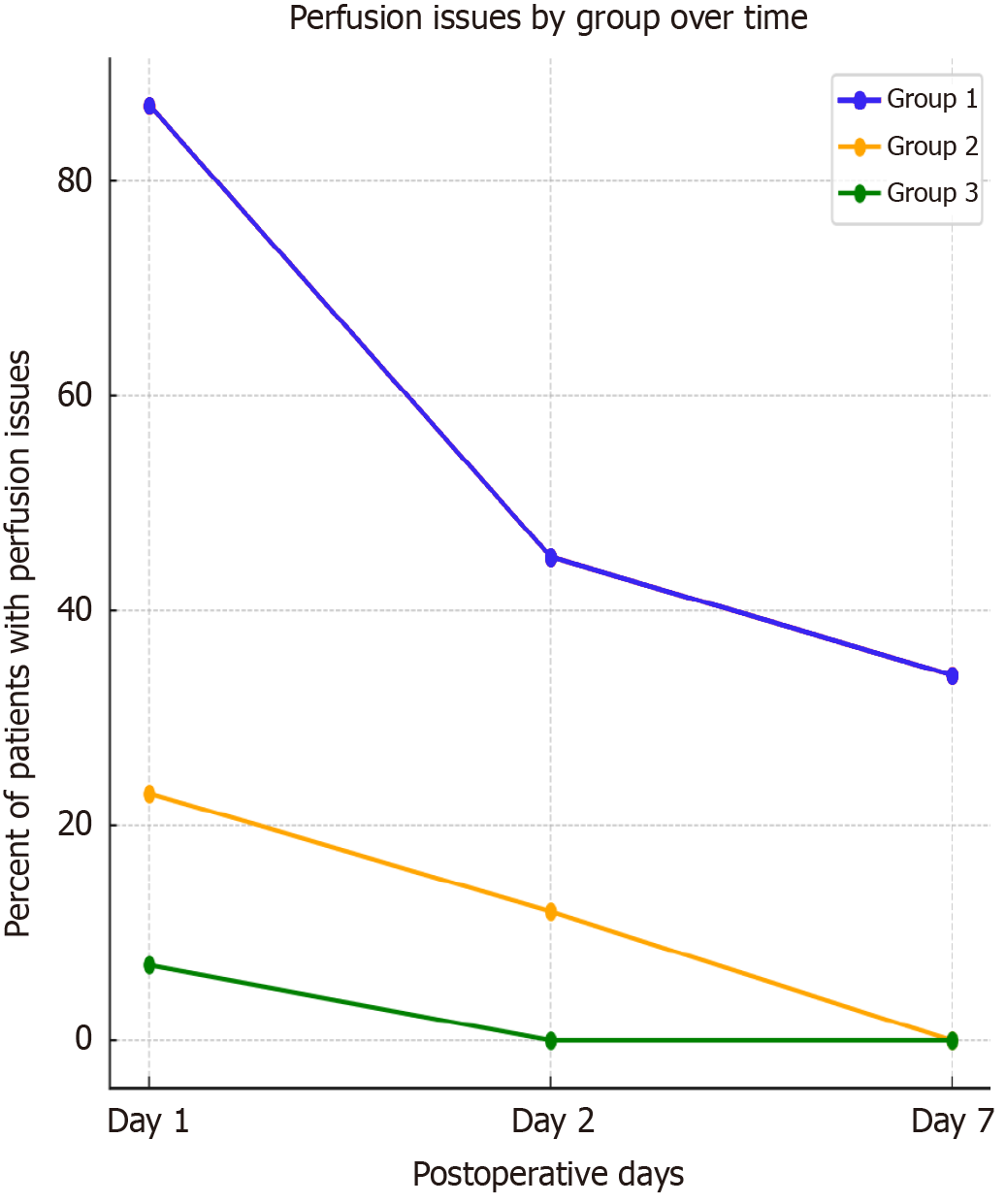
On postoperative days 12-14, routine ultrasonographic investigations showed no flow in the prosthetic venous graft in 32% of patients from group 2, compared to only 17% in group 3. Doppler assessments of liver graft perfusion on days 1 and 2 postoperatively revealed hypoperfused regions in 87% of group 1 patients, compared to 23% in group 2 and only 7% in group 3. By postoperative days 6-7, hypoperfusion was no longer seen in groups 2 and 3, but persisted in 34% of group 1 (Figure 5).
Postoperative discharge was most prevalent and prolonged in group 1, averaging 2200 mL/24 hours ± 280 mL/24 hours and lasting for 7-10 days until the drainage tube was removed when discharge was less than 700 mL. In groups 2 and 3, the average discharge was 1500 mL ± 170 mL within 24 hours and decreased gradually. The drainage tube was removed on day 3 in 52% of patients based on total bilirubin (T-Bil) levels, and in other cases, by days 7-10 when discharge was less than 700 mL.
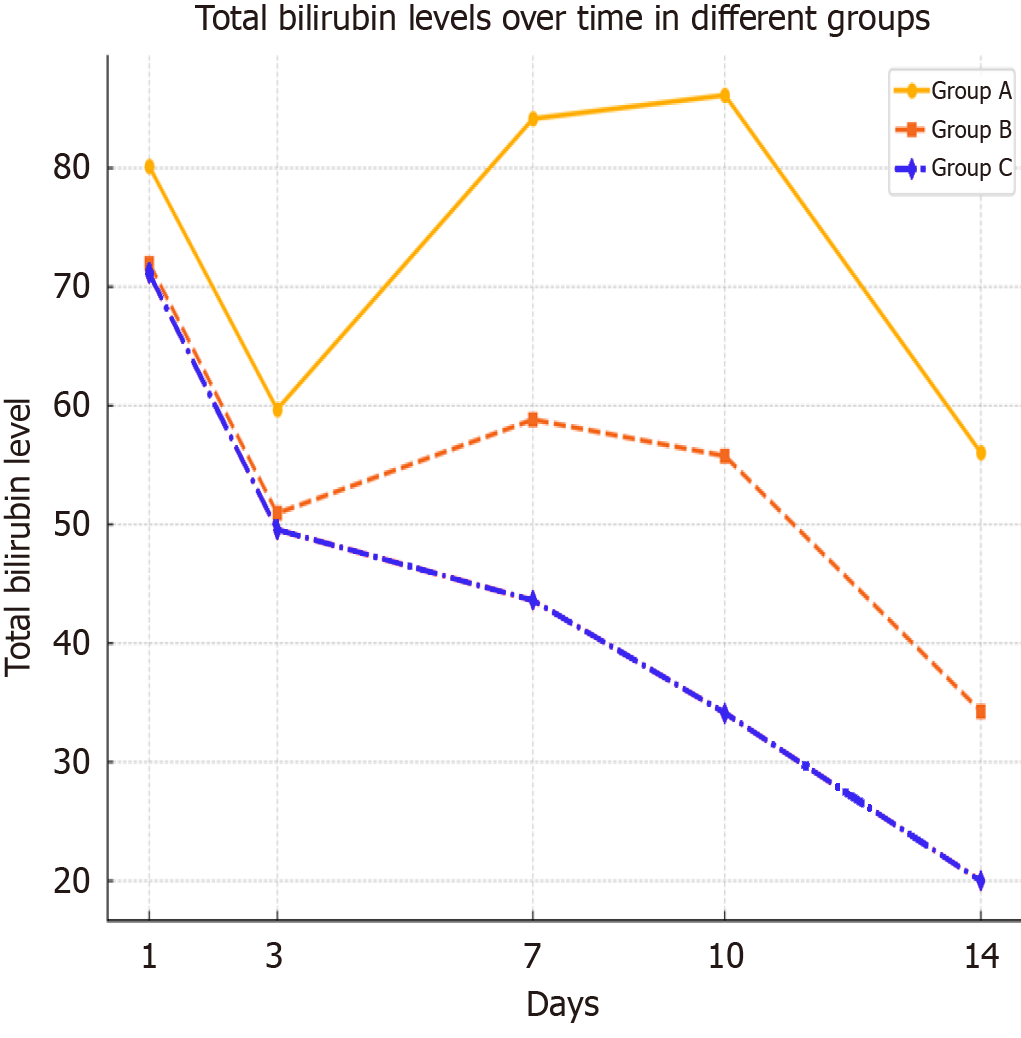
Blood laboratory tests were performed daily across all groups. T-Bil levels on postoperative day 1 averaged 80.17 mg/dL ± 4.9 mg/dL in group 1, compared to 72.15 mg/dL ± 3.6 mg/dL and 71.14 mg/dL ± 2.84 mg/dL in groups 2 and 3, respectively. By day 3, bilirubin levels dropped to 59.7 mg/dL ± 2.94 mg/dL in group 1, and to 50.94 mg/dL ± 2.73 mg/dL and 49.56 mg/dL ± 2.84 mg/dL in groups 2 and 3. By day 7, levels in group 1 increased to 84.17 mg/dL ± 3.4 mg/dL, while group 2 had 58.83 mg/dL ± 4.61 mg/dL, and group 3 had 43.6 mg/dL ± 2.76 mg/dL. On day 14, bilirubin levels were 56.04 mg/dL ± 4.31 mg/dL in group 1, compared to 34.27 mg/dL ± 3.76 mg/dL in group 2 and 19.97 mg/dL ± 2.01 mg/dL in group 3 (Figure 6).
INR levels on the first postoperative day were 2.29 ± 0.21 in group 1, 2.08 ± 0.17 in group 2, and 2.05 ± 0.19 in group 3. By day 7, INR increased to 2.44 ± 0.29 in group 1, while groups 2 and 3 had levels of 2.21 ± 0.24 and 2.17 ± 0.29, respectively. On day 14, INR levels were 2.04 ± 0.63 in group 1, 1.7 ± 0.42 in group 2, and 1.63 ± 0.52 in group 3.
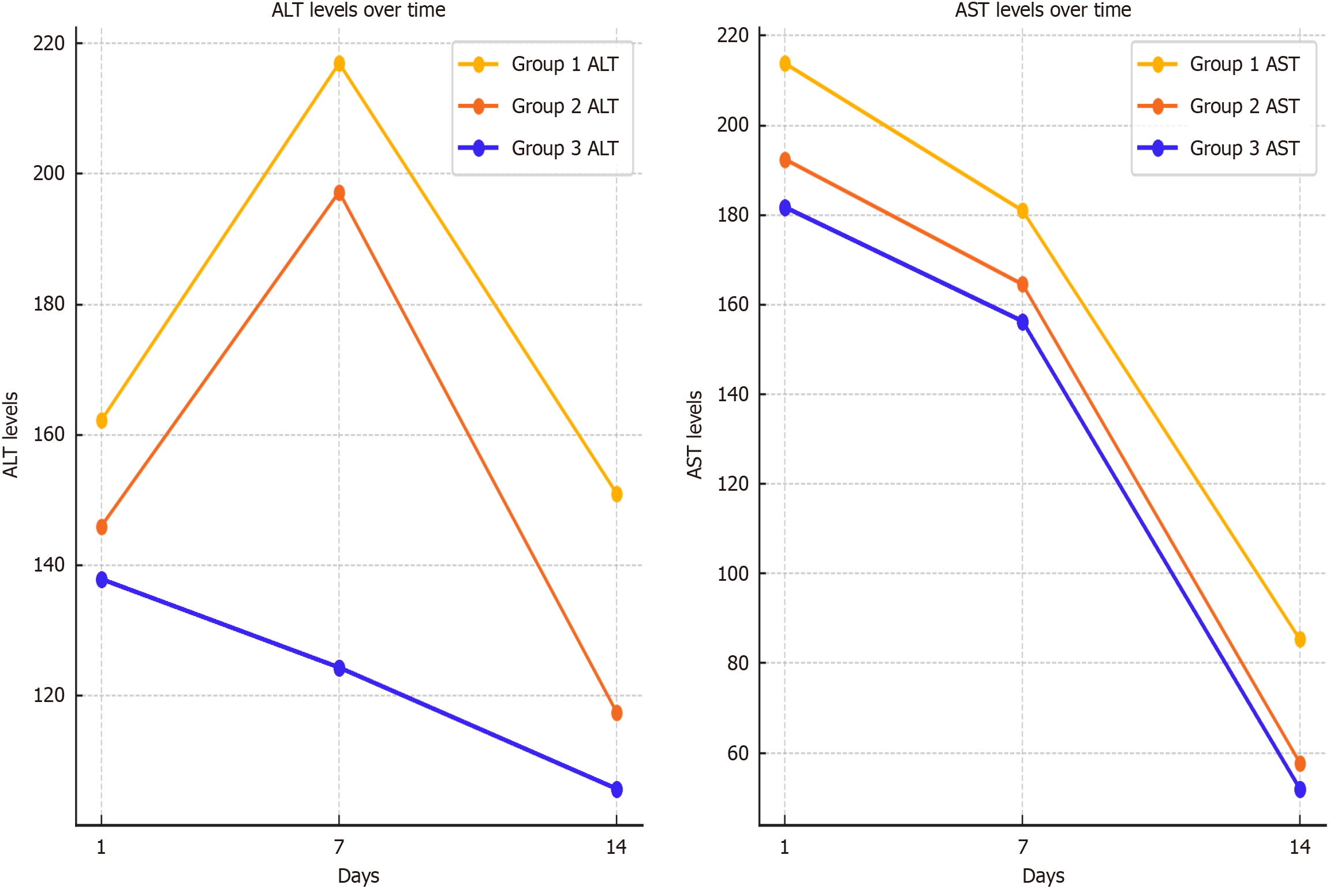
Alanine aminotransferase (ALT)/aspartate aminotransferase (AST) values on the first postoperative day were 162.25/213.86 in group 1, compared to 146.02/192.47 in group 2 and 137.91/181.78 in group 3. By day 7, ALT/AST levels were 216.94/180.99 in group 1, 197.22/164.54 in group 2, and 124.36/156.31 in group 3. By day 14, ALT/AST levels were 151.04/85.32 in group 1, 117.53/57.77 in group 2, and 105.78/51.99 in group 3 (Figure 7). Postoperatively, INR levels showed a slight increase from day 1 to day 7 across all groups, with group 1 exhibiting the highest values throughout the period. By day 14, INR levels gradually declined in all groups, with group 2 and group 3 demonstrating a more pronounced decrease compared to group 1. Lactate levels followed a different pattern, initially higher in group 2 on day 1. However, a steady decline was observed in all groups from day 1 to day 3, with group 2 maintaining the highest levels during this period. The decline was more significant in groups 1 and 3, leading to a convergence of values by day 3. Overall, INR levels peaked around day 7 before decreasing, while lactate levels showed a continuous downward trend, particularly in groups 1 and 3 (Table 2).
| Day | INR group 1 (mean ± SD) | INR group 2 (mean ± SD) | INR group 3 (mean ± SD) | Lactate group 1 (mean ± SD) | Lactate group 2 (mean ± SD) | Lactate group 3 (mean ± SD) |
| Postoperative day 1 | 2.29 ± 0.21 | 2.08 ± 0.17 | 2.05 ± 0.19 | 4.57 ± 0.48 | 6.07 ± 0.64 | 4.64 ± 0.57 |
| Day 2 | 3.96 ± 0.37 | 5.46 ± 0.47 | 4.12 ± 0.51 | |||
| Day 3 | 2.58 ± 0.37 | 3.98 ± 0.56 | 2.70 ± 0.42 | |||
| Day 7 | 2.44 ± 0.29 | 2.21 ± 0.24 | 2.17 ± 0.29 | |||
| Day 14 | 2.04 ± 0.63 | 1.7 ± 0.42 | 1.63 ± 0.52 |
The analysis of the obtained data revealed that although cold ischemia time was prolonged in group 3, it did not have a statistically significant impact on post-transplantation dynamics or complications. Group 1, which had the shortest cold and warm ischemia periods, exhibited the highest outflow rate but also had multiple areas of non-uniform liver perfusion postoperatively. This was reflected in the increased amount of postoperative ascitic fluid and its prolonged presence, likely due to the higher combined gradient of portal and arterial flow compared to the outflow via the RHV alone. This imbalance contributed to a delay in the normalization of biochemical parameters, and biliary complications reached 12.8% by the third postoperative month. hepatic venous congestion leads to: Intrahepatic biliary ischemia Reduced arterial buffer response Increased susceptibility to bile duct injury studies demonstrating venous congestion-induced biliary damage (e.g., Sugawara et al[19], Marcos et al[20]). Despite the important findings of this study, several limitations should be acknowledged.
The study involved a retrospective analysis of 96 patients across three groups, which, although meaningful, limits the statistical power of the conclusions. A larger sample size could provide more robust and generalizable results, especially when considering the comparison between different venous reconstruction techniques. but given the real-world constraints of LDLT studies, our approach provides a valid and methodologically sound means of addressing this issue.
This study primarily focused on short-term to medium-term outcomes such as immediate postoperative complications, liver function tests, and Doppler ultrasonography results. The current study lacks data on graft and patient survival rates beyond the initial 3 months, limiting the ability to evaluate the sustained efficacy of the transplantation and the durability of PTFE grafts. We recognize the necessity of extended follow-up to assess the long-term patency and functionality of PTFE grafts. Future studies will include regular imaging assessments: (1) Utilizing Doppler ultrasonography; and (2) Contrast-enhanced imaging at 6 months and 12 months post-transplant to monitor graft patency.
While this study highlighted the differences in venous outflow reconstruction techniques and their impact on early graft function, it did not extensively investigate other potential complications such as rejection, infections, or long-term biliary complications, which could also influence the overall success of the transplantation.
Although Doppler ultrasonography was used to assess liver perfusion (only one radiologist has been measuring hemodynamic values using Doppler ultrasonography, due to this fact inter-observer variability is absent) it has certain limitations in detecting subtle changes in hepatic blood flow. More advanced imaging modalities, such as magnetic resonance imaging or computed tomography angiography, could provide a more comprehensive understanding of venous flow and graft perfusion.
We hypothesize that these complications stemmed from the imbalance between inflow and outflow, leading to intrahepatic perfusion disturbances and, ultimately, ischemic damage to the bile ducts in 12.8% of cases. When comparing groups 2 and 3, it is evident that group 3 had a shorter warm ischemia time and a longer cold ischemia time, while group 2 exhibited the opposite pattern. Additionally, venous reconstruction in group 3 required less time, resulting in earlier reperfusion. However, this earlier reperfusion did not significantly affect postoperative outcomes. While the inflow and outflow dynamics in group 2 were satisfactory, they were not as optimal as in group 3, where the triangulation method ensured a better balance between inflow and outflow. No areas of congestion or perfusion disruptions were observed in group 3, unlike in group 1, where 87% of cases had impaired perfusion on the first and second postoperative days, compared to 23% in group 2 and only 7% in group 3. By the sixth and seventh postoperative days, areas of hypoperfusion had nearly disappeared in groups 2 and 3, while they persisted in 34% of group 1 cases.
Postoperative discharge volumes were not significantly different between the groups, and the timing of drainage tube removal did not vary statistically (P < 0.35). However, the T-Bil levels were a key marker of recovery, with group 3 showing a continuous trend toward normalization, while groups 1 and 2 experienced increases in T-Bil levels on days 7-10, which only began to normalize by day 14. T-Bil levels on day 1 were 80.17 mg/dL ± 4.9 mg/dL in group 1, compared to 72.15 mg/dL ± 3.6 mg/dL and 71.14 mg/dL ± 2.84 mg/dL in groups 2 and 3, respectively. By day 7, T-Bil levels in group 1 had risen to 84.17 mg/dL ± 3.4 mg/dL, while in group 2 they were 58.83 mg/dL ± 4.61 mg/dL, and in group 3 they were 43.6 mg/dL ± 2.76 mg/dL. By day 14, T-Bil levels had dropped to 56.04 mg/dL ± 4.31 mg/dL in group 1, while groups 2 and 3 showed significantly lower levels of 34.27 mg/dL ± 3.76 mg/dL and 19.97 mg/dL ± 2.01 mg/dL, respectively.
As for INR levels, there was no significant difference between groups 2 and 3, but group 1 consistently had higher values. Similarly, transaminase (ALT/AST) levels showed better trends in groups 2 and 3 compared to group 1. On the first postoperative day, the average ALT/AST values in group 1 were 162.25/213.86, while in groups 2 and 3 they were 146.02/192.47 and 137.91/181.78, respectively. By day 7, ALT/AST levels in group 1 were 216.94/180.99, compared to 197.22/164.54 in group 2 and 187.36/156.31 in group 3.
Lactate levels were also an important indicator of recovery. On the first postoperative day, groups 1 and 3 had similar lactate levels of 4.57 ± 0.48 and 4.64 ± 0.57, respectively, while group 2 had a higher level of 6.07 ± 0.64, likely due to the longer warm ischemia period.
Although the initial laboratory data across groups did not differ significantly, ALT/AST levels in groups 1 and 2 began to rise after the first week. This prompted further investigation to rule out early complications. In group 1, the increase was five times more frequent in patients with perfusion disorders, while in group 2, it was associated with obstructed or thrombosed PTFE grafts as detected by Doppler ultrasonography. Normalization of these parameters occurred later in all three groups, but group 3 showed statistically significantly better outcomes. Biliary complications were highest in group 1 and were more frequent in group 2 than in group 3, although this difference was not statistically significant.
The results of this study highlight that the primary difference between the three groups was the degree of perfusion disturbance as revealed by Doppler imaging on the first and second postoperative days. Group 1 showed the most significant perfusion disturbances, while groups 2 and 3 fared much better. Additionally, postoperative ascites was more prevalent in group 1, and biochemical analyses in the second week after transplantation showed elevated ALT/AST and bilirubin levels (excluding cases of rejection), which were associated with perfusion disorders in group 1 and with venous graft patency issues in group 2.
Based on our research, we conclude that when the outflow of the implanted liver is provided solely by the RHV, and the inflow/outflow gradient is disrupted, the risk of postoperative biliary complications increases. Graft implantation using the triangulation technique, where the proximal end of the venous graft is connected to the RHV, reduces warm ischemia time, enhances outflow, and improves the long-term efficacy of the graft. These findings support a shift in clinical practice, particularly in preoperative vascular assessment, intraoperative venous reconstruction, and postoperative surveillance protocols. By integrating these insights into diagnostic, surgical, and management strategies, we can optimize long-term graft survival and patient outcomes in LDLT. We can say that this study highlights gaps that future research should target to improve patient outcomes.
With these results, we can suggest: (1) Prioritize triangulated PTFE grafts to RHV over direct IVC implantation for optimal venous outflow; (2) Implement preoperative venous congestion risk stratification based on imaging and flow assessments; and (3) Establish structured Doppler monitoring and extended anticoagulation protocols for PTFE recipients.
| 1. | Couinaud C. Schema general de la distribution intra-hepatique. In: Couinaud C, editor. Le foie.Etudes anatomiques et chirurgicales. Paris: Massaon and Cie, 1957: 9-12. |
| 2. | Park KM, Lee SG, Lee YJ, Hwang S, Nam CW, Choi KM, Nam CH, Choi DN, Kim KH, Choi KT, Ko KS, Min PC. Adult-to-adult living donor liver transplantation at Asian Medical Center, Seoul, Korea. Transplant Proc. 1999;31:456-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Nakamura S, Tsuzuki T. Surgical anatomy of the hepatic veins and the inferior vena cava. Surg Gynecol Obstet. 1981;152:43-50. [PubMed] |
| 4. | Wu J, Wang W, Zhang M, Shen Y, Liang T, Yu P, Xu X, Yan S, Zheng S. Reconstruction of middle hepatic vein in living donor liver transplantation with modified right lobe graft: a single center experience. Transpl Int. 2008;21:843-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Sakamoto K, Ogawa K, Matsui T, Utsunomiya T, Honjo M, Ueno Y, Tamura K, Inoue H, Nakamura T, Watanabe J, Takai A, Tohyama T, Takada Y. Reconstruction of Middle Hepatic Vein Tributaries With Artificial Vascular Grafts in Living Donor Liver Transplant Using Right Lobe Grafts: A Case Series. Transplant Proc. 2019;51:1506-1510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Hwang S, Lee SG, Park KM, Kim KH, Ahn CS, Lee YJ, Sung KB, Moon DB, Ha TY, Cho SH, Oh KB, Han JM, Kim MH. Hepatic venous congestion in living donor liver transplantation: preoperative quantitative prediction and follow-up using computed tomography. Liver Transpl. 2004;10:763-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Lee SG. Techniques of reconstruction of hepatic veins in living-donor liver transplantation, especially for right hepatic vein and major short hepatic veins of right-lobe graft. J Hepatobiliary Pancreat Surg. 2006;13:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Hwang S, Jung DH, Ha TY, Ahn CS, Moon DB, Kim KH, Song GW, Park GC, Jung SW, Yoon SY, Namgoong JM, Park CS, Park YH, Park HW, Lee HJ, Lee SG. Usability of ringed polytetrafluoroethylene grafts for middle hepatic vein reconstruction during living donor liver transplantation. Liver Transpl. 2012;18:955-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 9. | Uchida K, Taniguchi M, Shimamura T, Suzuki T, Yamashita K, Ota M, Kamiyama T, Matsushita M, Furukawa H, Todo S. Three-dimensional computed tomography scan analysis of hepatic vasculatures in the donor liver for living donor liver transplantation. Liver Transpl. 2010;16:1062-1068. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 10. | Varotti G, Gondolesi GE, Goldman J, Wayne M, Florman SS, Schwartz ME, Miller CM, Sukru E. Anatomic variations in right liver living donors. J Am Coll Surg. 2004;198:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 107] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 11. | Sano K, Makuuchi M, Miki K, Maema A, Sugawara Y, Imamura H, Matsunami H, Takayama T. Evaluation of hepatic venous congestion: proposed indication criteria for hepatic vein reconstruction. Ann Surg. 2002;236:241-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 219] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Tashiro H, Ohdan H, Itamoto T, Fudaba Y, Amano H, Oshita A, Ishiyama K, Ushitora Y, Irei T, Ohira M, Tahara H, Banshoudani M, Tanimoto Y, Ishufuro M, Asahara T. Using recipient's middle hepatic vein for drainage of the right paramedian sector in right liver graft. Transplantation. 2008;86:1565-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, Greenstein SM, Merion RM. Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 2006;6:783-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1435] [Cited by in RCA: 1486] [Article Influence: 78.2] [Reference Citation Analysis (0)] |
| 14. | Burroughs AK, Sabin CA, Rolles K, Delvart V, Karam V, Buckels J, O'Grady JG, Castaing D, Klempnauer J, Jamieson N, Neuhaus P, Lerut J, de Ville de Goyet J, Pollard S, Salizzoni M, Rogiers X, Muhlbacher F, Garcia Valdecasas JC, Broelsch C, Jaeck D, Berenguer J, Gonzalez EM, Adam R; European Liver Transplant Association. 3-month and 12-month mortality after first liver transplant in adults in Europe: predictive models for outcome. Lancet. 2006;367:225-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 15. | Villa R, Fondevila C, Erill I, Guimerà A, Bombuy E, Gómez-Suárez C, Sacristán JC, García-Valdecasas JC. Real-time direct measurement of human liver allograft temperature from recovery to transplantation. Transplantation. 2006;81:483-486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 16. | Jochmans I, Fieuws S, Tieken I, Samuel U, Pirenne J. The Impact of Implantation Time During Liver Transplantation on Outcome: A Eurotransplant Cohort Study. Transplant Direct. 2018;4:e356. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Okeke RI, Bettag J, Wells R, Wycoff M, Hallcox T, Lok J, Phocas A, Annakie DL, Shoela R, Nazzal M. Intraoperative Doppler Ultrasound for Detection of Early Postoperative Vascular Complications in Orthotopic Liver Transplants. Cureus. 2022;14:e26077. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 18. | Cheng YF, Huang TL, Chen CL, Lee TY, Chen TY, Chen YS, Liu PP, Chiang YC, Eng HL, Wang CC, Cheung HK, Jawan B, Goto S. Intraoperative Doppler ultrasound in liver transplantation. Clin Transplant. 1998;12:292-299. [PubMed] [DOI] [Full Text] |
| 19. | Sugawara Y, Makuuchi M, Sano K, Imamura H, Kaneko J, Ohkubo T, Matsui Y, Kokudo N. Vein reconstruction in modified right liver graft for living donor liver transplantation. Ann Surg. 2003;237:180-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 20. | Marcos A, Orloff M, Mieles L, Olzinski AT, Renz JF, Sitzmann JV. Functional venous anatomy for right-lobe grafting and techniques to optimize outflow. Liver Transpl. 2001;7:845-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 102] [Article Influence: 4.3] [Reference Citation Analysis (0)] |