Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.101975
Revised: January 25, 2025
Accepted: February 17, 2025
Published online: September 18, 2025
Processing time: 196 Days and 20.5 Hours
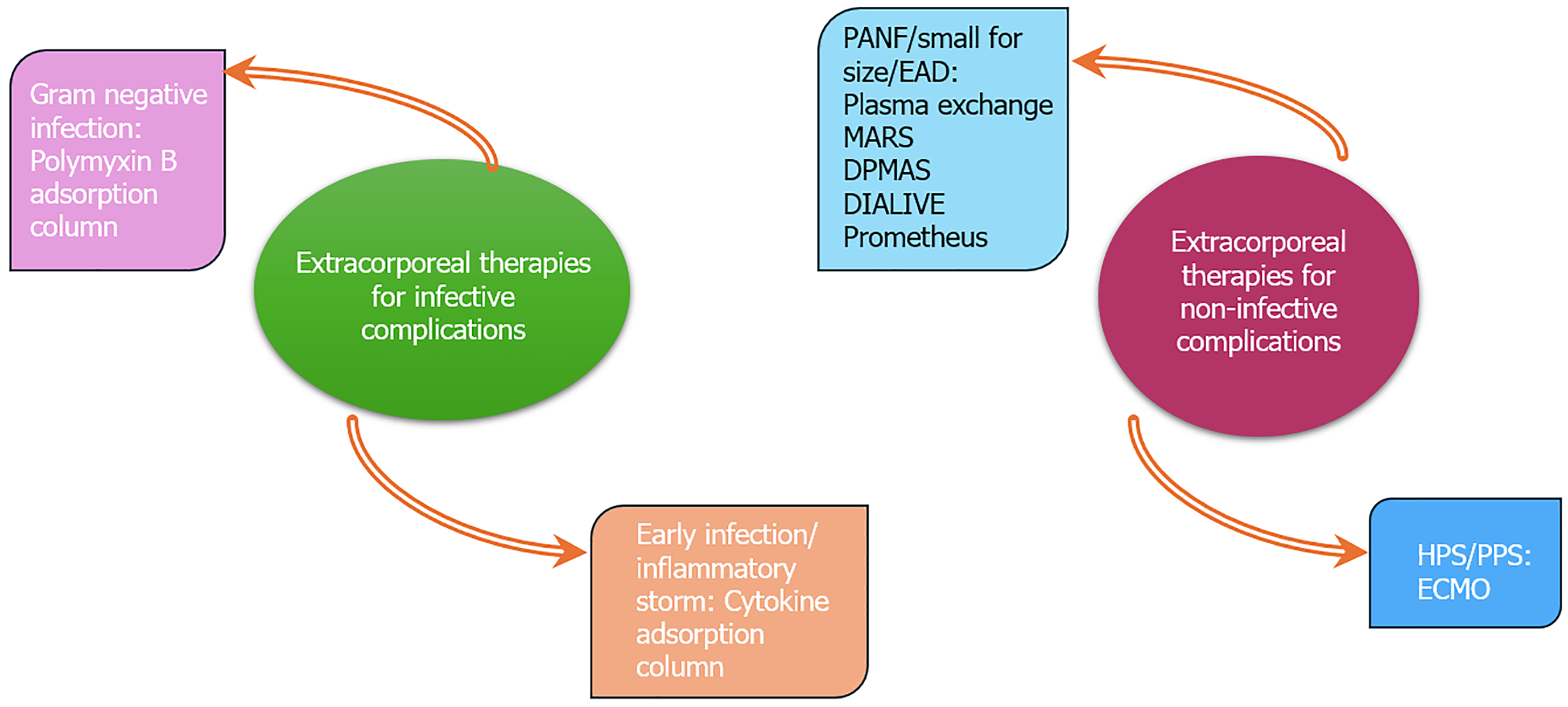
Extracorporeal therapies have a definite role in patients with acute liver failure, acute on-chronic liver failure, and progressive chronic liver disease. They act as a bridge-to-transplant in these patients. With the increasing success of liver transplantation, the immediate postoperative complication spectrum continues to expand. Extracorporeal therapies can play an important role in managing these complications. However, the literature on extracorporeal therapies in the post-liver transplant period is limited. This review article discussed various extracorporeal therapies that are still evolving or marred by limited evidence but can improve patient outcomes. These extracorporeal therapies can be divided into two subgroups: (1) Therapies for infective complications. Endotoxin and cytokine adsorption columns; and (2) Therapies for noninfective complications like small for size syndrome, primary allograft nonfunction, early allograft dysfunction, hyperacute rejection, hepatopulmonary syndrome, etc. (plasma exchange, double plasma molecular adsorption, molecular adsorbent recirculation system, and extracorporeal membrane oxygenation, among others).
Core Tip: With the growing number of liver transplants, both infective and noninfective complications are bound to grow. The discussion on extracorporeal therapies sheds light on innovative approaches that may improve patient outcomes, showcasing advancements in medical technology. By acknowledging the limited literature and ongoing research, the article highlighted the need for further studies to validate the efficacy and safety of these emerging therapies, which is crucial for informed clinical practice.
- Citation: Pachisia AV, Govil D, Jagadeesh K, Patel SJ, Harne R, Pal D, Tyagi P, Pattajoshi S, Brar K, Patel P, Zatakiya R. Extracorporeal therapies for post-liver transplant recipient: The road less traveled. World J Transplant 2025; 15(3): 101975
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/101975.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.101975
Liver transplantation (LT) is now the ultimate treatment option for patients with end-stage liver disease and for many who suffer from acute liver failure. The United Network for organ-sharing data reported that for the first time more than 10000 LTs were performed in 2023 in the United States. With the growing frequency of transplants, complications are inevitable, thereby deeming the role of extracorporeal therapies vital. However, most of these extracorporeal therapies are either still evolving or marred by limited literature and evidence.
The extracorporeal therapies for a patient after LT can be divided into those for infective complications and noninfective complications (Figure 1). The principal mechanism of extracorporeal therapies is described briefly in Table 1[1-7]. Although the effectiveness of modern immunosuppressive drugs has enhanced both graft and patient survival following LT, it has also increased the rate of infections. More than 50% of LT recipients experience infections post-transplant[8]. Over the years, an increased risk of infections from multidrug resistant organisms (MDRO) has been observed. Prolonged use of preoperative broad-spectrum antibiotics, surgical re-explorations, and high model for end-stage disease score (> 25) are associated with an elevated risk of infection from MDRO post-LT[9,10]. These MDROs are resistant to most antibiotics and combinations, making treatment challenging. As a result, timely diagnosis and prompt initiation of appropriate treatments are essential to improving patient outcomes. In this scenario, extracorporeal therapies play a significant role in modulating the sepsis response and improving survival.
| Modality | Mechanism |
| PMX hemoperfusion | Endotoxin, a cell wall component of gram-negative bacilli, is primary reason for generating inflammatory mediators, the emergence of septic shock, and multiple organ failure |
| PMX, a polycationic antibiotic, attaches to lipid A (part of endotoxin), thus neutralizing it | |
| Toraymyxin® (Toray Medical Co., Ltd., Japan) is a PMX-immobilized fiber blood-purification column | |
| PMX is immobilized covalently on the surface of polystyrene-derived, polypropylene-reinforced conjugated carrier fiber for selective endotoxin adsorption from the blood | |
| Cytokine adsorption (Cytosorb®) | Cytokine storms are caused by a high number of circulating cytokines and immune cell hyperactivation |
| One of the most difficult problems in liver transplantation is adverse immunological reactions mediated by cytokines against the allograft, which can cause early malfunction, severe rejection, and chronic damage | |
| Hemoperfusion cartridge containing polymer beads to adsorb proinflammatory and anti-inflammatory cytokines but not endotoxins | |
| Oxiris® hemofilter (cytokine + endotoxin removal) | During manufacture, 4500 UI/m2 heparin is pregrafted onto the oXiris® membrane |
| A layer of PEI, a positively charged molecule that promotes greater biocompatibility, constitutes the membrane surface treatment | |
| PEI grafting has the capacity to adsorb large negatively charged molecules, such as endotoxins, due to its higher concentration of free amino groups, which carry a positive charge | |
| As a result, the oXiris® membrane is composed of three distinct layers | |
| Thanks to this innovative design, one device may perform four functions: Renal support; cytokine removal; endotoxin removal; and local anticoagulant treatment | |
| Therapeutic plasma exchange | Elimination of large molecules from the blood, such as albumin-bound and water-soluble toxins, followed by replacement with plasma and/or albumin |
| Toxins removed include cytokines, endotoxins, bilirubin, bile acids, ammonia, and aromatic amino acids | |
| Molecular adsorbent recirculation system | The system utilizes a dialysate enriched with albumin to aid in the removal of albumin-bound toxins |
| It consists of three separate fluid compartments: A blood circuit, a circuit with 600 mL of 20% human albumin that includes a charcoal column and an anion exchange resin column, and a dialysate circuit | |
| DPMAS | Uses ion exchange resin and neutral macroporous resin to create a new artificial liver model, which may have a better adsorption effect on inflammatory mediators and bilirubin |
| It also reduces the risk of allergic reactions and transmitted diseases, among other risks by avoiding any replacement with plasma or albumin | |
| DPMAS also has drawbacks, like the inability to supplement albumin and coagulation factors | |
| DIALIVE | DIALIVE uses a dual filtration system coupled in series with a renal dialysis machine (Prismaflex, Baxter) |
| The first filter is made up of a membrane that enables albumin and cytokines to be ultrafiltered (Septex, Baxter, United States); the second filter, called oXiris® (Baxter, United States), adsorbs damage-associated molecular patterns and pathogen-associated molecular patterns | |
| 20% bottled albumin is added in equal amounts to replace the lost albumin | |
| Prometheus | The Prometheus system operates based on the principle of plasma separation using a filter that allows albumin, clotting factors, and fibrinogen to pass through |
| It requires an albumin filter (AlbuFlow® AF 01), a neutral resin adsorber (prometh® 01), an adsorber for anion exchange (prometh® 02), and a polysulfone® high-flux dialyzer with the corresponding tubing system | |
| Endogenous albumin is passed through the circuit using an Albuflow filter | |
| Albumin is reactivated in prometh 01 and 02 adsorbers and returned to circulation | |
| Blood then passes through the polysulfone filter, where it is treated by conventional high flux hemodialysis, eventually returning blood to the patient | |
| ECMO | In veno-venous ECMO, deoxygenated blood is drawn from the venous catheter and pumped into an oxygenator |
| This device functions like a synthetic lung, facilitating the exchange of gases by removing carbon dioxide and adding oxygen | |
| Inside the oxygenator, air and oxygen circulate through small, hollow fibers | |
| As blood flows through these fibers, oxygen is transferred into the red blood cells, while carbon dioxide is expelled into the fibers | |
| The carbon dioxide is then eliminated through the exhaust, and the oxygenated blood is returned to the patient via the catheter | |
| In veno-arterial ECMO, blood is drained from the venous side and pumped into the arterial side, bypassing the heart |
In addition to infective complications, post-LT recipients may also encounter immediate noninfective complications, such as primary allograft nonfunction (PANF) right after reperfusion and early allograft dysfunction (EAD) shortly after transplantation or small for size graft. Graft dysfunction often results in several postoperative complications, high mortality, and an increased risk of organ loss[11,12]. Thus, extracorporeal therapies can play a bridging role in supporting the transplanted organ along with conventional management.
This review discussed the literature on the role of extracorporeal membrane oxygenation (ECMO), especially for hepatopulmonary syndrome (HPS) and portopulmonary syndrome in the immediate postoperative period. Herein, a comprehensive literature search was conducted on articles published across the following databases: (1) PubMed; (2) PubMed Central; (3) Cochrane; (4) Elsevier Clinical Key; and (5) Google Scholar. The keyword search included LT, extracorporeal therapies, polymyxin (PMX) B hemoperfusion, cytokine adsorption, EAD, PANF, small for size graft, therapeutic plasma exchange, molecular adsorbent recirculation system, double plasma molecular adsorption system (DPMAS), HPS, and veno-venous ECMO (VV ECMO). The inclusion criteria were publications on post-LT adults (> 18 years) and in the English language, whereas studies conducted in the pediatric population and in languages other than English were excluded.
Patients with end-stage liver disease may benefit greatly from LT, which has a 90% 1-year and 80% 5-year survival rate[13]. One of the major difficulties following LT is managing infections, as the factors contributing to post-transplant infection risk are complex and challenging to control[14]. Hepatic abscesses, cholangitis, infected fluid collections, or peritonitis (intraabdominal infections) account for about half of the early infections post-transplant. Septicemia, pulmonary infection, and wound infection are 34%, 31%, and 10%, respectively[15-17]. Re-transplantation and transplant failure are linked to intraabdominal infections[18,19]. Approximately 67% of infections in the early stages after a transplant are caused by gram-negative bacteria[15,20]. With an estimated frequency of 14% and a fatality rate of up to 54%, septic shock is a significant cause of morbidity and mortality following solid-organ transplantation[21].
PMX B has been shown to protect against endotoxemia and septicemia as assessed in animal models[22-24]. The Early Use of PMX Hemoperfusion in Abdominal Sepsis (EUPHAS) trial was a prospective, multicenter, randomized controlled trial (RCT) that was carried out at ten Italian tertiary care intensive care facilities. A total of 30 patients received conventional therapy, while 34 patients received conventional therapy plus two sessions of PMX B hemoperfusion. The findings revealed that when combined with standard therapy, PMX B hemoperfusion considerably improved organ dysfunction (change in sequential organ failure assessment score, -3.4 vs -0.1; P value < 0.001) and hemodynamics (76–84 mmHg; P value = 0.001). In a targeted population suffering from intraabdominal gram-negative infections that caused septic shock or severe sepsis, it also decreased 28-day mortality [unadjusted hazard ratio (HR) = 0.43; 95% confidence interval (CI): 0.20–0.94; adjusted HR = 0.36; 95%CI: 0.16–0.80][25].
Payen et al[26] focused on the early use of PMX B hemoperfusion in patients with septic shock due to peritonitis, including those requiring urgent surgery for visually confirmed peritonitis. Compared to conventional therapy for peritonitis-induced septic shock, this multicenter RCT showed no significant change in mortality (19.5% vs 27.7%; P value = 0.14), and PMX B hemoperfusion did not lead to any improvement in organ failure (P value = 0.78).
In the EUPHRATES study, Dellinger et al[27] assessed the impact of targeted PMX B hemoperfusion on 28-day mortality in patients with septic shock with high endotoxin levels. A total of 449 critically ill adult patients with septic shock and an endotoxin activity (EA) assay result of 0.60 or higher participated in this multicenter RCT. They inferred that compared to sham treatment with standard medical care, PMX B hemoperfusion treatment did not reduce the 28-day mortality (34.5% vs 37.7%, P value = 0.49) in individuals with septic shock and high EA.
Klein et al[28] conducted a post-hoc analysis of the EUPHRATES trial to examine the effects of PMX B hemoperfusion usage in patients with an EA ranging from 0.60-0.89 and septic shock. Based on post-hoc analysis, they proposed quantifiable responses to variations in mean arterial pressure (P value = 0.04), ventilator-free days (P value = 0.004), and 28-day mortality in patients with septic shock and an EA > 0.60-0.89 (HR = 0.56, 95%CI: 0.33-0.95, P value = 0.03).
Nonetheless, these trials all had limitations. In the Early Use of PMX Hemoperfusion in Abdominal Sepsis trial, there were only 64 patients, and it was terminated early with a considerable risk of type I error. Although 232 patients participated in the ABDOMIX trial, the patients were less sick, and 11% of patients had incomplete PMX B hemoperfusion. Moreover, neither of the two studies had a limitation of not measuring endotoxin levels. In the EUPHRATES trial, the post hoc analysis set an arbitrary cutoff for the endotoxin levels. Another study suggested that these findings should not be taken as evidence of therapeutic efficacy but interpreted as hypothesis generating[29]. Surviving sepsis guidelines 2021 also did not recommend the use of PMX B hemoperfusion[30]. Nonetheless, cartridges with a larger surface area in contact with blood reduce the possibility of system saturation in the presence of high levels of endotoxins. The next step for this therapy might be defining a customized dose of PMX B hemoperfusion and the earliest appropriate application for patients with elevated EA in cases of high inoculum.
The current literature on the use of PMX B hemoperfusion is very limited. A study on the safety of PMX B hemoperfusion in recipients of kidney transplantation and LT recruited 28 (39.5%) patients with an EA > 0.60 to receive the treatment at a blood flow rate of 100 mL/minute for 2 h. PMX B hemoperfusion was administered to each patient until their EA was < 0.40. Consequently, the inflammatory and hemodynamic frameworks were stabilized. All patients were still alive and had minimal levels of EA and satisfactory graft function at the 30-day follow-up. The study concluded that EA had the ability to identify patients who could benefit from targeted treatment. It proved valuable in determining therapeutic dosages by accurately assessing the effects of PMX B hemoperfusion therapy. Additionally, it detected endotoxemia even in the absence of culture evidence of gram-negative infection[31].
In another study, Ruberto et al[1] detected gram-negative bacteria in 10 patients who underwent orthotopic LTs and developed sepsis/septic shock. PMX B hemoperfusion was performed three times in each patient, but no adverse event was reported. The mean arterial pressure improved from 64 mmHg ± 5 mmHg to 89 mmHg ± 4 mmHg between the baseline and the third treatment; in addition, the doses of norepinephrine and dobutamine were lowered from 1.3 µg/kg/minute to 0.001 µg/kg/minute and from 6.4 µg/kg/minute to 1 µg/kg/minute, respectively, whereas the PaO2 to FiO2 ratio rose from 214 mmHg to 291 mmHg.
Sato et al[32] evaluated the impact of hemoperfusion using a PMX B-immobilized fiber column on liver function following ischemic reperfusion injury. A total of 11 pigs were subjected to 120 min of total hepatic vascular exclusion and then monitored for 360 min. The rate pressure product (heart rate × end-systolic arterial blood pressure) and hepatic tissue blood flow were notably high in the PMX B hemoperfusion group. Compared to the control group, the portal venous blood flow in the PMX B hemoperfusion group differed significantly (P value < 0.05) at 360 min after reperfusion and was maintained at about 70% of the flow before ischemia. The serum aspartate aminotransferase levels in both groups increased progressively post-reperfusion; however, after 360 min, the enzyme levels in the PMX B hemoperfusion group were considerably lower (P value < 0.05). In this pig model, PMX B hemoperfusion treatment decreased the hepatic warm ischemia-reperfusion injury produced by total hepatic vascular exclusion.
Recently, a retrospective, multicenter, observational PMX-OTIC study evaluated the optimal time for initiation of PMX B hemoperfusion. A total of 48 patients were analyzed. The results showed that in terms of the timing of PMX B hemoperfusion initiation relative to the noradrenaline equivalent dose peak, the group initiated between -4 hours and 4 hours had the highest survival rate (229/304, 75.3%) compared to those starting ≤ -4 hours (50/75, 66.7%) and ≥ 4 hours (66/101, 65.4%) (P value = 0.085). In multivariable regression analysis, -4 hours to 4 hours was used as the reference and initiation at ≤ -4 hours was found to be associated with an odds ratio of 1.96 (1.07–3.58, P-value = 0.029) for 28-day mortality, while initiation at ≥ 4 hours showed an odds ratio of 1.64 (0.94–2.87, P value = 0.082). Hence, PMX B hemoperfusion induction at the time of the peak of vasopressor dose effectuated low mortality[33].
Thus, PMX B hemoperfusion may play a crucial role in patients experiencing gram-negative sepsis or septic shock after LT in improving the hemodynamic status and probably survival. These effects need to be substantiated in larger and better-planned trials, considering the timing of initiation, endotoxin levels, their clearance, and the number of PMX columns needed for endotoxin clearance. Until then, the limited literature and anecdotal evidence may be the best bet for these patients.
The in vitro studies have revealed clearance rates of > 90%-95% of cytokines using CytoSorb®[34]. Nevertheless, clinical research is still rare and is frequently restricted to case series that present positive findings for blood lactate levels and hemodynamic indices[35]. Despite considerable clearance during sessions, a recent RCT comparing standard therapy to hemoperfusion with CytoSorb® (6 h per day for 7 days) showed no decrease in interleukin-6 plasma levels over time[36].
Another hemoperfusion cartridge commonly used for cytokine and endotoxin removal is oXiris®. In a case report on septic shock after LT, Li et al[37] successfully stabilized the patient with continuous renal replacement therapy (oXiris® hemofilter) and antibiotics.
In a literature review on the clinical utility of extracorporeal cytokine hemadsorption therapy, Bonavia et al[38] concluded that extracorporeal blood purification using cytokine hemadsorption techniques decreased cytokine levels in patients with sepsis and septic shock; however, these benefits have not translated into consistent clinical improvement.
Gaspari et al[39] conducted a retrospective analysis on 7 patients assessing blood purification in hepatic dysfunction after LT or extensive hepatectomy and did not find any potential advantages of CytoSorb® in restoring patients’ clinical and laboratory parameters to a healthy state.
The cytokine storm and cytokine release syndrome represent complex, potentially fatal inflammatory responses that can occur following various triggers, including infections, therapies, and LT. These conditions, characterized by hyperactivation of the immune system and elevated cytokine levels, pose significant challenges in patients undergoing LT and often lead to graft malfunction, severe rejection, or chronic damage.
While extracorporeal blood purification techniques such as CytoSorb® and oXiris® seem promising in adsorbing proinflammatory mediators and improving certain clinical parameters, the clinical evidence remains inconclusive. Despite some positive in vitro findings and case reports indicating beneficial effects on lactate levels, hemodynamics, and septic shock stabilization, the translation of these results into consistent clinical improvement is still lacking. Thus, larger RCTs are needed to definitively establish the safety, efficacy, and clinical benefits of these technologies in managing cytokine-driven complications in LT. Until then, cytokine hemadsorption therapies should be cautiously approached as their role in clinical practice remains uncertain.
Small for size graft is defined by a graft-to-recipient body weight ratio of < 0.8 for living-related LT[40,41]. Acute allograft rejection is still a frequent side effect of LT, accounting for 20%-40% of cases, despite recent advancements in immunosuppressive medication; rejection usually occurs within a month following LT[42,43]. About 5%-10% of LTs result in PANF[44,45]. Certain donor features, including advanced age, steatosis, and prolonged ischemia periods, seem to contribute to the development of graft dysfunction, even if the pathophysiological foundation for PANF remains unclear[46-48]. Shortly after transplantation, a diagnosis is made to rule out the alternative causes of acute graft malfunction, such as vascular thrombosis, hyperacute rejection, or accelerated acute rejection. Currently, immediate re-transplantation has been the standard of care for PANF.
After LT, therapeutic plasma exchange (TPE) has proven to be a crucial adjuvant in treating immune-mediated causes of graft malfunction. For instance, TPE has been used to effectively treat refractory hepatic allograft rejection and ABO-incompatible LTs. In a study by Mandal et al[49], 5 patients diagnosed with PANF underwent TPE. All patients had a restored liver function except 1 patient who died of pulmonary embolism; the other 4 patients who survived had a functional allograft after 1 year. In contrast, 2 patients who underwent re-transplantation for PANF died within 3 months of the transplant.
Evidence for TPE in post-LT small for size graft and allograft rejection is collected primarily from case series and case reports. Khoo et al[50] reported a case series involving 3 patients: 2 patients had small for size syndrome; and 1 patient had biopsy-proven allograft rejection. The number of TPE sessions ranged from one to three, and all patients showed a substantial decrease in bilirubin levels along with evidence of graft recovery.
In a retrospective analysis, Choe et al[51] used TPE in EAD after LT evaluation. The study included 107 patients with EAD who underwent TPE compared to 36 patients with EAD who did not receive TPE over 13 years. EAD was defined as persistent hyperbilirubinemia (approximately 10 mg/dL) without biliary problems within 30 days after LT. The survival rates for the non-TPE group were 58.3% at 1 month and 22.2% at 1 year compared to the TPE group, which showed significantly better survival rates, with 82.2% at 1 month and 53.8% at 1 year. After the last TPE session, the TPE group also demonstrated a statistically significant decrease in both international normalized ratio (INR) and total bilirubin (P value < 0.05). A better prognosis was observed in patients who were responders to TPE and met certain criteria, such as age < 51 years, total bilirubin < 11.1 mg/dL, or INR < 1.15 following the final TPE. Moreover, TPE was associated with a reduced risk of death, while higher INR at EAD onset, male gender, and older age were associated with an increased risk of mortality.
In another retrospective analysis of 67 patients with EAD, 64% were treated with TPE. In the TPE group, 13 (45%) patients were deceased (P value < 0.001), but no deaths occurred in the non-TPE group within 90 days. The TPE group required more postoperative renal replacement therapy and had more complications related to sepsis than the non-TPE group. The authors concluded that the limitations of the retrospective investigation did not allow them to confirm whether TPE contributed to increased premature mortality[52].
In ABO-incompatible LTs, TPE was used to reduce the isoagglutinin titers by using fresh frozen AB plasma. The targeted isoagglutinin titer range was 1:8-1:16[53,54]. TPE sessions may vary depending on the center and the patient’s specific needs. Importantly, TPE is the only technique that can mechanically reduce isoagglutinin titers after transplantation; however, titer rebound requires additional TPE sessions.
Hence, TPE has demonstrated promising results as an adjunctive treatment for immune-mediated complications following LT, particularly in cases of allograft rejection, ABO-incompatible LTs and EAD. While case series and retrospective studies support its efficacy, the lack of large-scale RCTs limits definitive conclusions regarding its widespread use. The clinical response to TPE appears to vary depending on factors such as the patient’s age, bilirubin levels, and INR, underscoring the importance of a customized approach. Although TPE can significantly improve graft function and survival in certain cases, additional studies are required to define its role better and establish standardized protocols for its application in LT.
In a pilot study on the use of molecular adsorbent recirculation system (MARS) for EAD, the criteria for EAD were as follows: (1) Prothrombin time < 40%; (2) Aspartate aminotransferase or alanine aminotransferase > 1000 U/L; (3) Plasma disappearance rate of indocyanine green < 10%/minute within 72 h of reperfusion; and (4) Serum bilirubin > 10 mg/dL. Following MARS treatment, a substantial increase was noted in the plasma disappearance rate of indocyanine green, whereas a significant decrease was detected in serum bilirubin (P value = 0.002), serum creatinine (P value = 0.006), and aspartate aminotransferase (P value = 0.005). On the other hand, platelet count, albumin level, and prothrombin time were constant. Renal, neurological, and mean arterial pressure improvements were noted over time, and MARS therapy offered a secure method of treating EAD without negative consequences[55].
In another study involving 5 patients with EAD and 2 patients with PANF, MARS decreased serum bilirubin levels, bile acids, serum creatinine, and ammonia levels. Four of the five patients with graft dysfunction showed a significant improvement in hepatic encephalopathy and renal and synthetic liver functions, whereas patients with PANF did not show a similar improvement[56].
A retrospective review of catastrophic graft failure (massive exsanguination and liver fracture, portal vein and hepatic artery thrombosis, idiopathic necrosis, necrosis from inadequate donor flushing/primary nonfunction) concluded that timely hepatectomy combined with a portacaval shunt and re-transplantation could result in satisfactory outcomes, especially for patients receiving MARS support[57].
Lee et al[58] compared 15 patients who received 41 sessions of MARS with 16 patients who received 105 sessions of plasmapheresis. The study concluded that both therapies significantly decreased bilirubin levels with a similar overall survival rate.
The literature on the use of DPMAS as a bridge to transplant and bridge to recovery is evolving. However, evidence for its use in patients after transplant is virtually nonexistent. The principle of DPMAS is promising, rendering it an effective option for treating the above-mentioned complications. Nonetheless, additional evidence and knowledge are required before its clinical application in patients after LT.
The extracorporeal liver dialysis machine, DIALIVE, was designed to address the pathophysiological abnormalities that lead to the development of acute on-chronic liver failure (ACLF). DIALIVE may benefit patients in the following ways: (1) In addition to being dysfunctional, the circulating albumin in ACLF can trigger inflammatory responses; and (2) The buildup of damage-associated molecular patterns and pathogen-associated molecular patterns induces organ dysfunction and increases the risk of infections, ultimately leading to systemic inflammation. Thus, DIALIVE seeks to eliminate damage-associated molecular patterns and pathogen-associated molecular patterns and replace albumin[59], albeit the modality is still under investigation.
Prometheus was first described by Falkenhagen et al[60] in 1999. It is based on a combined membrane and adsorbent blood purification technique. Its unique contribution was the development of a polysulfone hollow-fiber filter with a sieving coefficient of 0.89 for human serum albumin but only 0.19 for fibrinogen and 0 for IgM. The HELIOS study, a multicenter RCT comparing Prometheus to standard medical therapy did not find any statistically significant survival benefit in patients with ACLF[61]. Hitherto, various studies have shown MARS to have a better outcome in terms of hemodynamic parameters compared to Prometheus[62,63].
Sentürk et al[64] studied Prometheus in patients with acute liver failure and ACLF and found improvement in biochemical parameters in hepatic encephalopathy. Komardina et al[65] showed hemodynamic and biochemical improvement with Prometheus in patients with alkaline phosphatase but without any difference in survival outcome. Thus, varying conclusions have been derived regarding the clinical benefit of Prometheus. Similar to DPMAS and DIALIVE, Prometheus is also based on sound principles, and its use in post-LT complications should be explored in future trials.
HPS linked to liver disease has emerged as an indication for ECMO. VV ECMO is mainly utilized for severe, acute, reversible respiratory failure after LT, with HPS being the most common etiology. Patients with HPS may undergo excessive pulmonary vasoconstriction, increased ventilation-perfusion mismatch, and hypoxemia due to vasoconstrictors released after LT. ECMO may preserve oxygenation until the patient’s intrapulmonary shunting improves[66].
Several case reports have presented that VV ECMO was utilized to salvage patients who developed severe hypoxia because of HPS after LT[67-69]. In a systematic review of the literature on ECMO for HPS, Wu et al[70] evaluated 16 studies from across four continents on 17 patients who were started on ECMO before, during, or after LT. The median interval between LT and the start of ECMO was 7 days [interquartile range (IQR) = 3-12 days]. The average duration of ECMO treatment was 13 days (IQR = 10-29 days). The average length of stay in the postoperative critical care unit was 40 days (IQR = 37-61 days), whereas the average length of stay in the hospital was 59.5 days (IQR = 42-77 days); 14/17 (82.4%) patients were discharged. Thus, the study concluded that ECMO was a viable option for patients with HPS undergoing LT and seemed to present better results than other reasons for cardiopulmonary failure in LT patients. Eventually, ECMO might play a major role in perioperative care for patients with severe HPS.
Patel et al[71] conducted a single-center, retrospective study of 29 patients who needed peri-LT ECMO support. In the study, 13 patients were supported with ECMO post-LT. Of these, 9 patients required VV ECMO for indications like acute respiratory distress syndrome or aspiration pneumonia, with a median duration of 17 days (IQR = 5-41 days) from LT to ECMO support. All except 1 patient survived at hospital discharge. Veno-arterial ECMO was required for extracorporeal cardiopulmonary resuscitation in 3 patients, while 1 patient required it for portopulmonary hypertension. For veno-arterial ECMO, the median time to initiation after LT was 3.5 days (IQR = 3-8 days). Subsequently, 3 patients were weaned and discharged from the hospital, while 1 patient was deceased due to multiorgan failure. Considering ECMO support for patients after LT presents significant challenges. However, promising outcomes have been reported globally, suggesting potential benefits of ECMO in carefully selected cases. Taken together, the current evidence for the use of VV ECMO in patients with HPS is limited to retrospective data, case reports ,and anecdotal evidence, and no studies have yet been planned in this area of concern in patients after LT.
The landscape of LT is evolving, particularly with the significant increase in procedures performed annually. While this progress offers hope to patients with end-stage liver disease, it also brings forth the challenge of managing infectious and noninfectious complications. Extracorporeal therapies have emerged as a promising avenue to address these complications, ranging from infections like gram-negative sepsis to conditions such as PANF and HPS. Table 2 outlines the advantages and disadvantages of various major extracorporeal therapies in post-LT complications. However, the current evidence supporting these therapies is still in the nascent stage, with many treatments characterized by limited literature and ongoing trials. Although previous results have shown potential benefits, they underscore the need for larger, well-designed RCTs to establish efficacy and safety. Until then, clinicians must navigate the uncertainties and carefully tailor treatment strategies for each patient based on individual risk factors and cost-benefit analysis of the emerging evidence. The journey towards optimizing post-transplant management is ongoing, and continued innovation in this field holds promise for improved survival and quality of life for LT recipients.
| Modality | Advantages | Disadvantages |
| Polymyxin B hemoperfusion | Reduced endotoxemia and potentially improved hemodynamic stability and organ function. Demonstrated positive effects on septic shock caused by gram-negative infections. Can stabilize inflammatory and hemodynamic responses in transplant recipients. Some studies suggest improved survival in specific patient populations | Limited evidence in large, well-designed trials. No consistent reduction in mortality across all trials. Uncertainty around optimal timing for initiation of therapy. High cost and logistical challenges of administering multiple sessions. Not effective for all cases of sepsis, especially when endotoxin levels are low |
| Cytokine adsorption (Cytosorb®) | Removed a broad spectrum of proinflammatory cytokines, potentially preventing or mitigating cytokine storms and graft rejection. Demonstrated high clearance rates of cytokines (> 90%-95%) in vitro. Can be combined with other therapeutic approaches such as continuous renal replacement therapy. May improve hemodynamics and blood lactate levels in some patients | Clinical evidence is limited and inconsistent, with no definitive survival benefit. Primarily used in case reports or small case series, so generalizability is uncertain. Does not target endotoxins, limiting its effectiveness in endotoxin-driven sepsis. Expensive and may require extended treatment (e.g., 6 h per day). No specific cutoff value of interleukin-6 to initiate cytokine adsorption |
| oXiris® hemofilter (cytokine + endotoxin removal) | Multitasking device capable of removing endotoxins and cytokines and providing renal support. It showed promise in managing septic shock and liver dysfunction post-transplant. Can provide anticoagulation during treatment, reducing the need for additional medications | Clinical outcomes are not consistently improved in large trials. Data from case reports and small studies did not establish clear benefits in post-transplant care. More research is needed to confirm its clinical efficacy and safety in the liver transplant population |
| Molecular adsorbent recirculation system | Effectively removed toxins and promoted liver function in cases of liver failure. Showed benefits in early allograft dysfunction and primary allograft nonfunction. Decreased serum bilirubin levels, bile acids, serum creatinine, and ammonia levels | Expensive and resource intensive. Long treatment sessions are required (several hours). Evidence for significant survival benefit remains limited. Not universally available, limiting its application in all settings |
| Therapeutic plasma exchange | Can remove circulating immune complexes, toxins, and cytokines, potentially improving graft survival and reducing inflammation. Established role in managing autoimmune liver diseases, which could benefit patients after transplant. Some studies suggest improvement in post-transplant liver function | Does not directly address the underlying cause of inflammation; only removes circulating mediators. Limited evidence for efficacy in the specific post-transplant setting. Requires multiple sessions, potentially leading to increased costs and complications like transfusion-related acute lung injury, transfusion-associated circulatory overload, etc. |
| Extracorporeal membrane oxygenation | Provides respiratory and circulatory support in patients with hepatopulmonary syndrome, portopulmonary syndrome, and/or respiratory failure. Can improve oxygenation and hemodynamics in critical patients, reducing the need for mechanical ventilation. Acts as a bridge to recovery | High risk of complications, including bleeding, infection, and organ dysfunction. High resource utilization and intensive monitoring required. Does not address liver dysfunction directly; only provides supportive care |
| 1. | Ruberto F, Pugliese F, D'Alio A, Martelli S, Bruno K, Marcellino V, Perrella S, Cappannoli A, Mazzarino V, Tosi A, Novelli G, Rossi M, Ginanni Corradini S, Ferretti G, Berloco PB, Pietropaoli P. Clinical effects of use polymyxin B fixed on fibers in liver transplant patients with severe sepsis or septic shock. Transplant Proc. 2007;39:1953-1955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Shoji H, Tani T, Hanasawa K, Kodama M. Extracorporeal endotoxin removal by polymyxin B immobilized fiber cartridge: designing and antiendotoxin efficacy in the clinical application. Ther Apher. 1998;2:3-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 100] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Assadiasl S, Mooney N, Nicknam MH. Cytokines in Liver Transplantation. Cytokine. 2021;148:155705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Gruda MC, Ruggeberg KG, O'Sullivan P, Guliashvili T, Scheirer AR, Golobish TD, Capponi VJ, Chan PP. Broad adsorption of sepsis-related PAMP and DAMP molecules, mycotoxins, and cytokines from whole blood using CytoSorb® sorbent porous polymer beads. PLoS One. 2018;13:e0191676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 136] [Cited by in RCA: 130] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 5. | Yao J, Li S, Zhou L, Luo L, Yuan L, Duan Z, Xu J, Chen Y. Therapeutic effect of double plasma molecular adsorption system and sequential half-dose plasma exchange in patients with HBV-related acute-on-chronic liver failure. J Clin Apher. 2019;34:392-398. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (1)] |
| 6. | Wan YM, Li YH, Xu ZY, Yang J, Yang LH, Xu Y, Yang JH. Therapeutic plasma exchange versus double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: A prospective study. J Clin Apher. 2017;32:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 7. | Bai W, Yao C, Mao D, Wu J, Wang K, Wei H, Huang Z, Shi Q, Wang N. The Clinical Efficacy of Double Plasma Molecular Absorption System Combined with Plasma Exchange in the Treatment of Acute-on-Chronic Liver Failure: A Systematic Review and Meta-Analysis. J Healthc Eng. 2022;2022:3139929. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 8. | Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007;357:2601-2614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1386] [Cited by in RCA: 1343] [Article Influence: 74.6] [Reference Citation Analysis (0)] |
| 9. | Fernández J, Acevedo J, Castro M, Garcia O, de Lope CR, Roca D, Pavesi M, Sola E, Moreira L, Silva A, Seva-Pereira T, Corradi F, Mensa J, Ginès P, Arroyo V. Prevalence and risk factors of infections by multiresistant bacteria in cirrhosis: a prospective study. Hepatology. 2012;55:1551-1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 434] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 10. | Asensio A, Ramos A, Cuervas-Mons V, Cordero E, Sánchez-Turrión V, Blanes M, Cervera C, Gavalda J, Aguado JM, Torre-Cisneros J; Red de Estudio de la Infección en el Trasplante - Grupo de Estudio de la Infección en el Trasplante. Effect of antibiotic prophylaxis on the risk of surgical site infection in orthotopic liver transplant. Liver Transpl. 2008;14:799-805. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Hudcova J, Scopa C, Rashid J, Waqas A, Ruthazer R, Schumann R. Effect of early allograft dysfunction on outcomes following liver transplantation. Clin Transplant. 2017;31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 12. | Olthoff KM, Kulik L, Samstein B, Kaminski M, Abecassis M, Emond J, Shaked A, Christie JD. Validation of a current definition of early allograft dysfunction in liver transplant recipients and analysis of risk factors. Liver Transpl. 2010;16:943-949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 874] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 13. | Rana A, Ackah RL, Webb GJ, Halazun KJ, Vierling JM, Liu H, Wu MF, Yoeli D, Kueht M, Mindikoglu AL, Sussman NL, Galván NT, Cotton RT, O'Mahony CA, Goss JA. No Gains in Long-term Survival After Liver Transplantation Over the Past Three Decades. Ann Surg. 2019;269:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 14. | van Hoek B, de Rooij BJ, Verspaget HW. Risk factors for infection after liver transplantation. Best Pract Res Clin Gastroenterol. 2012;26:61-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 15. | Vera A, Contreras F, Guevara F. Incidence and risk factors for infections after liver transplant: single-center experience at the University Hospital Fundación Santa Fe de Bogotá, Colombia. Transpl Infect Dis. 2011;13:608-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Blair JE, Kusne S. Bacterial, mycobacterial, and protozoal infections after liver transplantation--part I. Liver Transpl. 2005;11:1452-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Righi E. Management of bacterial and fungal infections in end stage liver disease and liver transplantation: Current options and future directions. World J Gastroenterol. 2018;24:4311-4329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (2)] |
| 18. | Kim SI. Bacterial infection after liver transplantation. World J Gastroenterol. 2014;20:6211-6220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 103] [Cited by in RCA: 127] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 19. | Patel R, Paya CV. Infections in solid-organ transplant recipients. Clin Microbiol Rev. 1997;10:86-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 413] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 20. | Resino E, San-Juan R, Aguado JM. Selective intestinal decontamination for the prevention of early bacterial infections after liver transplantation. World J Gastroenterol. 2016;22:5950-5957. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Fishman JA, Rubin RH. Infection in organ-transplant recipients. N Engl J Med. 1998;338:1741-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1225] [Cited by in RCA: 1098] [Article Influence: 40.7] [Reference Citation Analysis (0)] |
| 22. | Corrigan JJ Jr, Kiernat JF. Effect of polymyxin B sulfate on endotoxin activity in a gram-negative septicemia model. Pediatr Res. 1979;13:48-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Flynn PM, Shenep JL, Stokes DC, Fairclough D, Hildner WK. Polymyxin B moderates acidosis and hypotension in established, experimental gram-negative septicemia. J Infect Dis. 1987;156:706-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 34] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Ingoldby CJ. The value of polymixin B in endotoxaemia due to experimental obstructive jaundice and mesenteric ischaemia. Br J Surg. 1980;67:565-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Cruz DN, Antonelli M, Fumagalli R, Foltran F, Brienza N, Donati A, Malcangi V, Petrini F, Volta G, Bobbio Pallavicini FM, Rottoli F, Giunta F, Ronco C. Early use of polymyxin B hemoperfusion in abdominal septic shock: the EUPHAS randomized controlled trial. JAMA. 2009;301:2445-2452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 534] [Cited by in RCA: 538] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 26. | Payen DM, Guilhot J, Launey Y, Lukaszewicz AC, Kaaki M, Veber B, Pottecher J, Joannes-Boyau O, Martin-Lefevre L, Jabaudon M, Mimoz O, Coudroy R, Ferrandière M, Kipnis E, Vela C, Chevallier S, Mallat J, Robert R; ABDOMIX Group. Early use of polymyxin B hemoperfusion in patients with septic shock due to peritonitis: a multicenter randomized control trial. Intensive Care Med. 2015;41:975-984. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 300] [Cited by in RCA: 256] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 27. | Dellinger RP, Bagshaw SM, Antonelli M, Foster DM, Klein DJ, Marshall JC, Palevsky PM, Weisberg LS, Schorr CA, Trzeciak S, Walker PM; EUPHRATES Trial Investigators. Effect of Targeted Polymyxin B Hemoperfusion on 28-Day Mortality in Patients With Septic Shock and Elevated Endotoxin Level: The EUPHRATES Randomized Clinical Trial. JAMA. 2018;320:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 272] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 28. | Klein DJ, Foster D, Walker PM, Bagshaw SM, Mekonnen H, Antonelli M. Polymyxin B hemoperfusion in endotoxemic septic shock patients without extreme endotoxemia: a post hoc analysis of the EUPHRATES trial. Intensive Care Med. 2018;44:2205-2212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 132] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 29. | Pickkers P, Russell JA. Treatment with a polymyxin B filter to capture endotoxin in sepsis patients: is there a signal for therapeutic efficacy? Intensive Care Med. 2019;45:282-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Evans L, Rhodes A, Alhazzani W, Antonelli M, Coopersmith CM, French C, Machado FR, Mcintyre L, Ostermann M, Prescott HC, Schorr C, Simpson S, Wiersinga WJ, Alshamsi F, Angus DC, Arabi Y, Azevedo L, Beale R, Beilman G, Belley-Cote E, Burry L, Cecconi M, Centofanti J, Coz Yataco A, De Waele J, Dellinger RP, Doi K, Du B, Estenssoro E, Ferrer R, Gomersall C, Hodgson C, Møller MH, Iwashyna T, Jacob S, Kleinpell R, Klompas M, Koh Y, Kumar A, Kwizera A, Lobo S, Masur H, McGloughlin S, Mehta S, Mehta Y, Mer M, Nunnally M, Oczkowski S, Osborn T, Papathanassoglou E, Perner A, Puskarich M, Roberts J, Schweickert W, Seckel M, Sevransky J, Sprung CL, Welte T, Zimmerman J, Levy M. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47:1181-1247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 295] [Cited by in RCA: 2264] [Article Influence: 566.0] [Reference Citation Analysis (0)] |
| 31. | Novelli G, Morabito V, Ferretti G, Poli L, Novelli S, Ruberto F, Pugliese F, Mennini G, Rossi M, Berloco PB. Safety of polymyxin-B-based hemoperfusion in kidney and liver transplant recipients. Transplant Proc. 2012;44:1966-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 32. | Sato H, Oshima K, Kobayashi K, Yamazaki H, Suto Y, Takeyoshi I. Hemoperfusion with polymyxin B-immobilized fiber column improves liver function after ischemia-reperfusion injury. World J Gastroenterol. 2009;15:4571-4575. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Nakamura K, Okazaki T, Tampo A, Mochizuki K, Kanda N, Ono T, Yanagita K, Shimomura T, Murase T, Saito K, Hirayama T, Ito T, Ogawa K, Nakamura M, Oda T, Morishima T, Fukushima T, Yasui H, Akashi N, Oshima K, Kawarazaki H, Akiba T, Uemura S, Honma Y, Nitta K, Okamoto K, Takaki S, Takeda H, Yamashita C. The polymyxin-B direct hemoperfusion OPTimal Initiation timing with Catecholamine PMX-OPTIC study: A multicenter retrospective observational study. Artif Organs. 2025;49:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Malard B, Lambert C, Kellum JA. In vitro comparison of the adsorption of inflammatory mediators by blood purification devices. Intensive Care Med Exp. 2018;6:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 162] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 35. | Houschyar KS, Pyles MN, Rein S, Nietzschmann I, Duscher D, Maan ZN, Weissenberg K, Philipps HM, Strauss C, Reichelt B, Siemers F. Continuous hemoadsorption with a cytokine adsorber during sepsis - a review of the literature. Int J Artif Organs. 2017;40:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 36. | Schädler D, Pausch C, Heise D, Meier-Hellmann A, Brederlau J, Weiler N, Marx G, Putensen C, Spies C, Jörres A, Quintel M, Engel C, Kellum JA, Kuhlmann MK. The effect of a novel extracorporeal cytokine hemoadsorption device on IL-6 elimination in septic patients: A randomized controlled trial. PLoS One. 2017;12:e0187015. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 238] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 37. | Li Y, Zhou L, Yang L, Yuan F. Septic shock after liver transplantation successfully treated with endotoxin and cytokine adsorption continuous renal replacement therapy: a case report and literature review. J Int Med Res. 2020;48:300060520940439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 38. | Bonavia A, Groff A, Karamchandani K, Singbartl K. Clinical Utility of Extracorporeal Cytokine Hemoadsorption Therapy: A Literature Review. Blood Purif. 2018;46:337-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 69] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 39. | Gaspari R, Aceto P, Spinazzola G, Piervincenzi E, Chioffi M, Giuliante F, Antonelli M, Avolio AW. Blood Purification in Hepatic Dysfunction after Liver Transplant or Extensive Hepatectomy: Far from the Best-Case Scenarios. J Clin Med. 2024;13:2853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 40. | Selzner M, Kashfi A, Cattral MS, Selzner N, Greig PD, Lilly L, McGilvray ID, Therapondos G, Adcock LE, Ghanekar A, Levy GA, Renner EL, Grant DR. A graft to body weight ratio less than 0.8 does not exclude adult-to-adult right-lobe living donor liver transplantation. Liver Transpl. 2009;15:1776-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Kiuchi T, Kasahara M, Uryuhara K, Inomata Y, Uemoto S, Asonuma K, Egawa H, Fujita S, Hayashi M, Tanaka K. Impact of graft size mismatching on graft prognosis in liver transplantation from living donors. Transplantation. 1999;67:321-327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 741] [Cited by in RCA: 722] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 42. | Wang YC, Wu TJ, Wu TH, Lee CF, Chou HS, Chan KM, Lee WC. The risk factors to predict acute rejection in liver transplantation. Transplant Proc. 2012;44:526-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 43. | González MG, Madrazo CP, Rodríguez AB, Gutiérrez MG, Herrero JI, Pallardó JM, Ortiz de Urbina J, Paricio PP. An open, randomized, multicenter clinical trial of oral tacrolimus in liver allograft transplantation: a comparison of dual vs. triple drug therapy. Liver Transpl. 2005;11:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Ploeg RJ, D'Alessandro AM, Knechtle SJ, Stegall MD, Pirsch JD, Hoffmann RM, Sasaki T, Sollinger HW, Belzer FO, Kalayoglu M. Risk factors for primary dysfunction after liver transplantation--a multivariate analysis. Transplantation. 1993;55:807-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 804] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 45. | Strasberg SM, Howard TK, Molmenti EP, Hertl M. Selecting the donor liver: risk factors for poor function after orthotopic liver transplantation. Hepatology. 1994;20:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 422] [Cited by in RCA: 411] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 46. | Washburn WK, Johnson LB, Lewis WD, Jenkins RL. Graft function and outcome of older (> or = 60 years) donor livers. Transplantation. 1996;61:1062-1066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 66] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Clavien PA, Harvey PR, Strasberg SM. Preservation and reperfusion injuries in liver allografts. An overview and synthesis of current studies. Transplantation. 1992;53:957-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 614] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 48. | Mor E, Klintmalm GB, Gonwa TA, Solomon H, Holman MJ, Gibbs JF, Watemberg I, Goldstein RM, Husberg BS. The use of marginal donors for liver transplantation. A retrospective study of 365 liver donors. Transplantation. 1992;53:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 192] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 49. | Mandal AK, King KE, Humphreys SL, Maley WR, Burdick JF, Klein AS. Plasmapheresis: an effective therapy for primary allograft nonfunction after liver transplantation. Transplantation. 2000;70:216-220. [PubMed] |
| 50. | Khoo C, Tan E, Koh Y, Goh B, Krishnamoorthy T, Jeyaraj P. Plasma Exchange Is an Effective Adjunct in the Management of Hyperbilirubinemia in Early Allograft Dysfunction and Small for Size Syndrome after Liver Transplant. HPB. 2022;24:S563-S564. [DOI] [Full Text] |
| 51. | Choe W, Kwon SW, Kim SS, Hwang S, Song GW, Lee SG. Effects of therapeutic plasma exchange on early allograft dysfunction after liver transplantation. J Clin Apher. 2017;32:147-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 52. | Hakeem AR, Sandeep J, Subramaniam S, Sachan D, Jothimani D, Rajakumar A, Reddy MS, Rela M. Therapeutic Plasma Exchange for Management of Early Allograft Dysfunction After Living Donor Liver Transplant: An Unmatched Cohort Study. Exp Clin Transplant. 2021;19:1182-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 53. | Song GW, Lee SG, Hwang S, Kim KH, Ahn CS, Moon DB, Ha TY, Jung DH, Park GC, Kim WJ, Sin MH, Yoon YI, Kang WH, Kim SH, Tak EY. ABO-Incompatible Adult Living Donor Liver Transplantation Under the Desensitization Protocol With Rituximab. Am J Transplant. 2016;16:157-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 54. | Salah A, Fujimoto M, Yoshizawa A, Yurugi K, Miyagawa-Hayashino A, Sumiyoshi S, Minamiguchi S, Uemoto S, Maekawa T, Haga H. Application of complement component 4d immunohistochemistry to ABO-compatible and ABO-incompatible liver transplantation. Liver Transpl. 2014;20:200-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 55. | Hetz H, Faybik P, Berlakovich G, Baker A, Bacher A, Burghuber C, Sandner SE, Steltzer H, Krenn CG. Molecular adsorbent recirculating system in patients with early allograft dysfunction after liver transplantation: a pilot study. Liver Transpl. 2006;12:1357-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 56. | Gaspari R, Cavaliere F, Sollazzi L, Perilli V, Melchionda I, Agnes S, Gasbarrini A, Avolio AW. Molecular adsorbent recirculating system (Mars) in patients with primary nonfunction and other causes of graft dysfunction after liver transplantation in the era of extended criteria donor organs. Transplant Proc. 2009;41:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Gaspari R, Avolio AW, Zileri Dal Verme L, Agnes S, Proietti R, Castagneto M, Gasbarrini A. Molecular adsorbent recirculating system in liver transplantation: Safety and efficacy. Transplant Proc. 2006;38:3544-3551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 58. | Lee JY, Kim SB, Chang JW, Park SK, Kwon SW, Song KW, Hwang S, Lee SG. Comparison of the molecular adsorbent recirculating system and plasmapheresis for patients with graft dysfunction after liver transplantation. Transplant Proc. 2010;42:2625-2630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 59. | Agarwal B, Cañizares RB, Saliba F, Ballester MP, Tomescu DR, Martin D, Stadlbauer V, Wright G, Sheikh M, Morgan C, Alzola C, Lavin P, Green D, Kumar R, Sacleux SC, Schilcher G, Koball S, Tudor A, Minten J, Domenech G, Aragones JJ, Oettl K, Paar M, Waterstradt K, Bode-Boger SM, Ibáñez-Samaniego L, Gander A, Ramos C, Chivu A, Stange J, Lamprecht G, Sanchez M, Mookerjee RP, Davenport A, Davies N, Pavesi M, Andreola F, Albillos A, Cordingley J, Schmidt H, Carbonell-Asins JA, Arroyo V, Fernandez J, Mitzner S, Jalan R. Randomized, controlled clinical trial of the DIALIVE liver dialysis device versus standard of care in patients with acute-on- chronic liver failure. J Hepatol. 2023;79:79-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 35] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 60. | Falkenhagen D, Strobl W, Vogt G, Schrefl A, Linsberger I, Gerner FJ, Schoenhofen M. Fractionated plasma separation and adsorption system: a novel system for blood purification to remove albumin bound substances. Artif Organs. 1999;23:81-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 146] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | Kribben A, Gerken G, Haag S, Herget-Rosenthal S, Treichel U, Betz C, Sarrazin C, Hoste E, Van Vlierberghe H, Escorsell A, Hafer C, Schreiner O, Galle PR, Mancini E, Caraceni P, Karvellas CJ, Salmhofer H, Knotek M, Ginès P, Kozik-Jaromin J, Rifai K; HELIOS Study Group. Effects of fractionated plasma separation and adsorption on survival in patients with acute-on-chronic liver failure. Gastroenterology. 2012;142:782-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 268] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 62. | Laleman W, Wilmer A, Evenepoel P, Elst IV, Zeegers M, Zaman Z, Verslype C, Fevery J, Nevens F. Effect of the molecular adsorbent recirculating system and Prometheus devices on systemic haemodynamics and vasoactive agents in patients with acute-on-chronic alcoholic liver failure. Crit Care. 2006;10:R108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 170] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 63. | Dethloff T, Tofteng F, Frederiksen HJ, Hojskov M, Hansen BA, Larsen FS. Effect of Prometheus liver assist system on systemic hemodynamics in patients with cirrhosis: a randomized controlled study. World J Gastroenterol. 2008;14:2065-2071. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 42] [Cited by in RCA: 42] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 64. | Sentürk E, Esen F, Ozcan PE, Rifai K, Pinarbaşi B, Cakar N, Telci L. The treatment of acute liver failure with fractionated plasma separation and adsorption system: Experience in 85 applications. J Clin Apher. 2010;25:195-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 65. | Komardina E, Yaroustovsky M, Abramyan M, Plyushch M. Prometheus therapy for the treatment of acute liver failure in patients after cardiac surgery. Kardiochir Torakochirurgia Pol. 2017;14:230-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 66. | Braun HJ, Pulcrano ME, Weber DJ, Padilla BE, Ascher NL. The Utility of ECMO After Liver Transplantation: Experience at a High-volume Transplant Center and Review of the Literature. Transplantation. 2019;103:1568-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Piltcher-da-Silva R, Chedid MF, Grezzana Filho TJM, Leipnitz I, de Araújo A, Gazzana MB, Saueressig MG, Lorenzi W, Cardoni MG, Bellaver P, Alvares-da-Silva MR, Feier FH, Chedid AD, Kruel CRP. Severe hepatopulmonary syndrome with hypoxemia refractory to liver transplant: Recovery after 67 days of ECMO support. Int J Artif Organs. 2022;45:121-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Sánchez Pérez B, Pérez Reyes M, Aranda Narvaez J, Santoyo Villalba J, Perez Daga JA, Sanchez-Gonzalez C, Santoyo-Santoyo J. New therapeutic strategy with extracorporeal membrane oxygenation for refractory hepatopulmonary syndrome after liver transplant: A case report. World J Transplant. 2024;14:89223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 69. | Kumar L Dr, Balakrishnan D, Varghese R, Surendran S. Extracorporeal membrane oxygenation for post-transplant hypoxaemia following very severe hepatopulmonary syndrome. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Wu WK, Grogan WM, Ziogas IA, Patel YJ, Bacchetta M, Alexopoulos SP. Extracorporeal membrane oxygenation in patients with hepatopulmonary syndrome undergoing liver transplantation: A systematic review of the literature. Transplant Rev (Orlando). 2022;36:100693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 71. | Patel S, Gutmann C, Loveridge R, Pirani T, Willars C, Vercueil A, Angelova-Chee M, Aluvihare V, Heneghan M, Menon K, Heaton N, Bernal W, McPhail M, Gelandt E, Morgan L, Whitehorne M, Wendon J, Auzinger G. Perioperative extracorporeal membrane oxygenation in liver transplantation-bridge to transplantation, intraoperative salvage, and postoperative support: outcomes and predictors for survival in a large-volume liver transplant center. Am J Transplant. 2025;25:396-405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 2.0] [Reference Citation Analysis (0)] |