Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.101427
Revised: January 29, 2025
Accepted: March 17, 2025
Published online: September 18, 2025
Processing time: 216 Days and 15.3 Hours
Post-pancreaticoduodenectomy (PD) intestinal failure (IF) is rare and associated with poor outcomes. To our knowledge, the role of intestinal transplantation (ITx) as a rescue treatment for this complication has never been reported.
A 42-year-old female with a benign neurilemmoma of the duodenum underwent PD. Her superior mesenteric vein (SMV) was injured during surgery and required reconstruction. She experienced SMV thrombosis and bowel gangrene requiring massive bowel resection. Consequently, she developed short gut syndrome and an enterocutaneous fistula, leading to prolonged hospitalization for wound care and total parenteral nutrition (TPN) support. She was referred to our hospital for ITx evaluation. Upon arrival, she had cholestasis due to IF-associated liver disease. After gastrointestinal (GI) reconstruction to restore GI continuity, she was eligible for multi-visceral transplantation (MVTx). The anticipated allograft included the stomach, small intestine, liver, pancreas, and duodenum. She found a suitable donor after two years of waiting. The MVTx procedure was straightforward with signs of immediate function. Enteral feeding was initiated on postoperative day (POD) 7. TPN weaning was achieved on POD 28, and the patient was discharged on POD 69. Two years post-MVTx, she is healthy with excellent graft function. To our knowledge, this is the first case report on MVTx as the treatment for fatal post-PD complications and also the first reported case of ITx in Southeast Asia.
Post-PD IF is rare and lethal. Intestinal and MVTx might be a rescue treatment for IF after GI surgery in eligible patients.
Core Tip: Herein, we report the case of a patient who developed intestinal failure due to short gut syndrome and complex enterocutaneous fistula after pancreaticoduodenectomy. This patient successfully underwent multi-visceral transplantation as a rescue treatment for this condition. We also described the challenges encountered in preparing the first intestinal transplant candidate in our country. To our knowledge, this is the first intestinal transplantation performed in Southeast Asia.
- Citation: Dumronggittigule W, Kositamongkol P, Sirivatanauksorn Y, Limsrichamrern S, Mahawithitwong P, Tovikkai C, Sangserestid P, Assawasirisin C. Multivisceral transplantation as a rescue treatment for intestinal failure following pancreaticoduodenectomy: A case report. World J Transplant 2025; 15(3): 101427
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/101427.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.101427
Pancreaticoduodenectomy (PD), one of the most challenging gastrointestinal (GI) surgical procedures, is associated with high complication (40%-60%) and mortality (2%-5%) rates[1-3]. The most common causes of mortality following PD include severe postoperative pancreatic fistula, which often leads to sepsis and multi-organ failure syndrome (MOFS)[2,4]. Another fatal adverse event, ischemic complications related to major abdominal vascular occlusion (hepatic artery, superior mesenteric vessels, and portal vein), either due to intraoperative trauma or spontaneous thrombosis, may also result in intestinal gangrene, anastomotic dehiscence, and MOFS[5]. The treatment of these conditions requires a multidisciplinary care team[6,7]. The diagnosis and treatment of specific PD-related complications often require radiologic or endoscopic interventions. Additionally, the prolonged hospitalization-related complications also require the intervention of a multidisciplinary team of doctors to provide intensive care for patients with MOFS, hospital-acquired infection, metabolic derangement, and malnutrition. Moreover, patients with deteriorated physical and mental strength following prolonged hospitalization might need extensive rehabilitation and psychiatric therapy to return to normalcy after surgery. Despite early aggressive treatment, some patients die from sepsis while others develop long-term disability.
Intestinal failure (IF), a condition in which a patient loses gut function and requires permanent total parenteral nutrition (TPN) support, is a rare post-PD complication. Most patients with post-PD IF undergo massive bowel resection due to the sequelae of ischemic complications. The survivors of these devastating complications often experience poor quality of life with long-term TPN dependence[8,9]. Intestinal rehabilitation (IR), which is considered a first-line treatment for IF, is accompanied by home TPN support[10,11]. However, patients who are not eligible for IR or who fail IR treatment should be considered for intestinal transplantation (ITx) evaluation.
Herein, we report a case of IF due to an ischemic complication of PD that required multi-visceral transplantation (MVTx) as a rescue therapy.
A 42-year-old female who presented with short gut syndrome (SGS) and a complex enterocutaneous fistula (ECF) was assessed for the possibility of ITx.
The patient initially presented with bilious vomiting and abdominal pain. Computed tomography (CT) revealed an occlusive mass in the second part of her duodenum. She underwent PD for the definitive treatment of the duodenal tumor at another hospital. Unfortunately, the superior mesenteric vein (SMV) was injured during the dissection of uncinate process. Although immediate SMV repair was performed before the PD reconstruction phase, she developed peritonitis and severe sepsis on postoperative day (POD) 1. The emergency repeat surgery revealed massive bowel gangrene, including the entire jejuno-ileum and right colon, due to SMV thrombosis. After resecting the non-viable bowel for sepsis control and several repeat laparotomies for further resection of gangrenous tissue and peritoneal toileting, the abdomen was closed with external tube drains from bile and pancreatic ducts, and a closed suction drain was placed. Finally, she developed SGS requiring long-term TPN support.
Following hemodynamic stabilization, the patient’s clinical course was complicated by the development of a complex high-output ECF at the laparotomy wound, necessitating continuous closed suction wound therapy. Due to the challenge with wound care, the patient was unable to receive home TPN, a first-line definitive treatment for SGS. Other complications during the prolonged hospitalization period included multiple episodes of central venous catheter (CVC)-related sepsis, chronic wound pain with opioid dependence, and major depression. The pathological analysis of the PD specimen confirmed the diagnosis of a benign neurilemmoma the absence of invasive cancer. Nine months after PD, our team was consulted regarding the role of ITx, and the patient was referred to our hospital for ITx evaluation.
She was healthy without any underlying disease prior to the PD.
She lived with her husband, two children, and her mother.
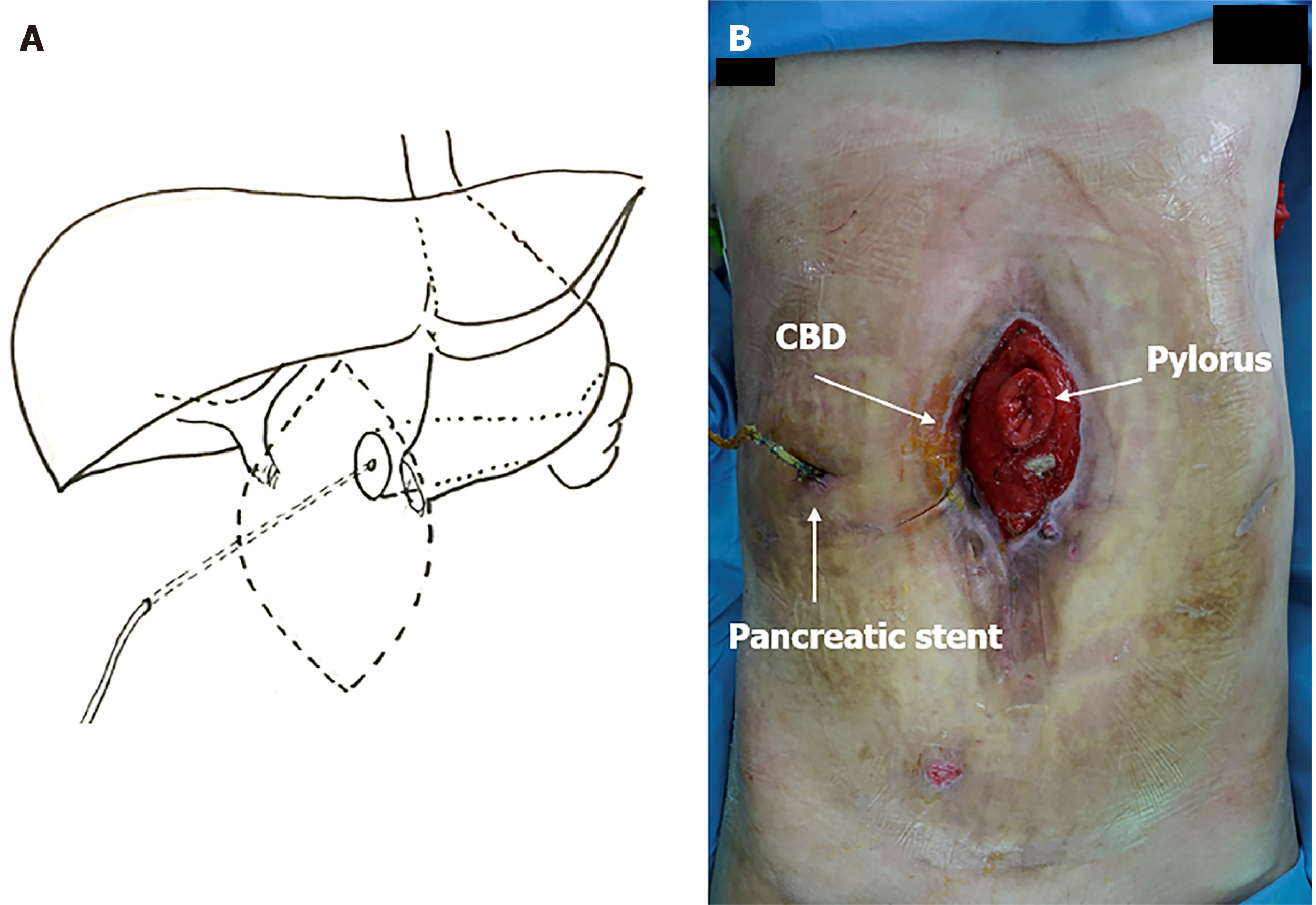
Upon arrival, her hemodynamic parameters were stable. She was receiving TPN via a peripherally inserted central catheter line in her right arm. The abdominal wound contained a complex high-output ECF, involving the opening of the distal end of the stomach and the bile duct. The fistula output was approximately 1000 mL/day. The abdominal wound and a diagram demonstrating GI anatomy are presented in Figure 1.
Liver biochemistry revealed mild cholestasis with elevated alkaline phosphatase titers and increased total bilirubin levels. Other laboratory test results were unremarkable. Her blood group is B, Rh-positive.
The latest abdominal CT scan revealed normal appearances of the liver, remnant pancreas, and spleen. The major abdominal vessels, including the aorta, vena cava, and hepatic vessels, were patent.
The final diagnosis was SGS with a complex high-output ECF.
Upon admission, the appropriate TPN formula was administered to improve her general condition. Negative-pressure wound therapy was initiated to manage the high-output GI fistula. Meanwhile, via a multidisciplinary evaluation, it was determined that the patient had irreversible IF due to SGS and a complex GI fistula. ITx was deemed the best treatment option for her.
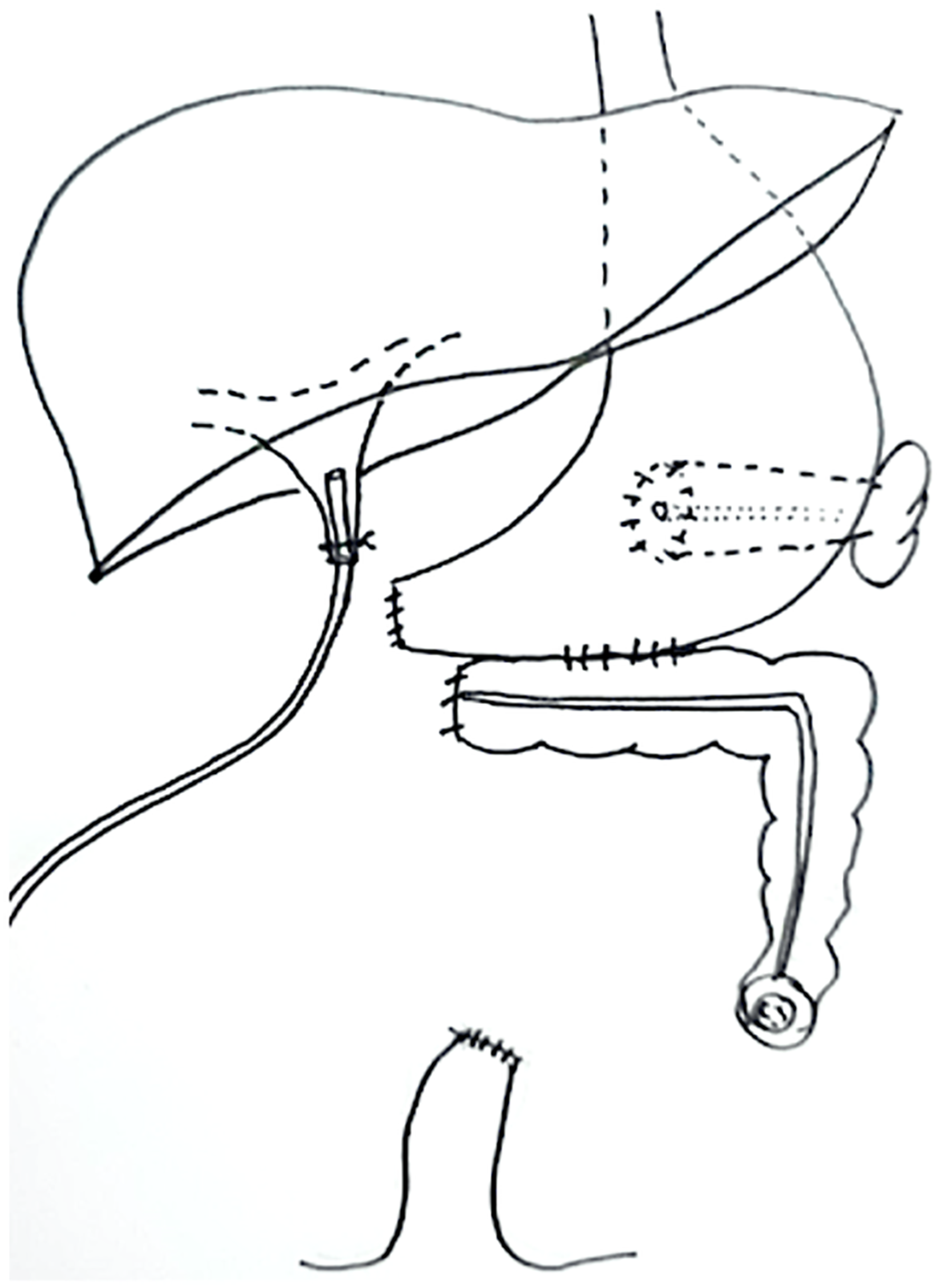
GI reconstructive surgery was performed to restore GI continuity, close the ECF, reduce wound care challenges, and obtain biopsy samples for liver pathology. The procedure included gastric fistula closure, pancreaticogastrostomy, gastrocolostomy, end-sigmoid colostomy, tube biliary drainage, liver biopsy, and abdominal wall closure. The patient’s GI anatomy following GI reconstructive surgery is shown in Figure 2. Liver histology revealed TPN-induced liver injury and early liver fibrosis. The appropriate graft type for her transplantation was determined to include the stomach, duodenum, pancreas, jejuno-ileum, and liver. Therefore, she was listed for MVTx.
Pretransplant human leukocyte antigen (HLA) antibody testing (Luminex technique) revealed a highly sensitized recipient [42% panel reactive antibody (PRA) HLA class 1; mean fluorescence intensity (MFI) 1636-2738, 54% PRA HLA class 2; MFI 3006-13726]. Pretransplant desensitization included plasmapheresis and intravenous immunoglobulin (IVIg) administration. Induction immunosuppression consisted of thymoglobulin and methylprednisolone.
The ideal donor was an ABO-identical donor after brain death, aged 15-40 years old, weighing less than 50 kg, with stable hemodynamics, and requiring low-dose vasopressors. The anticipated total ischemic time was less than 12 hours.
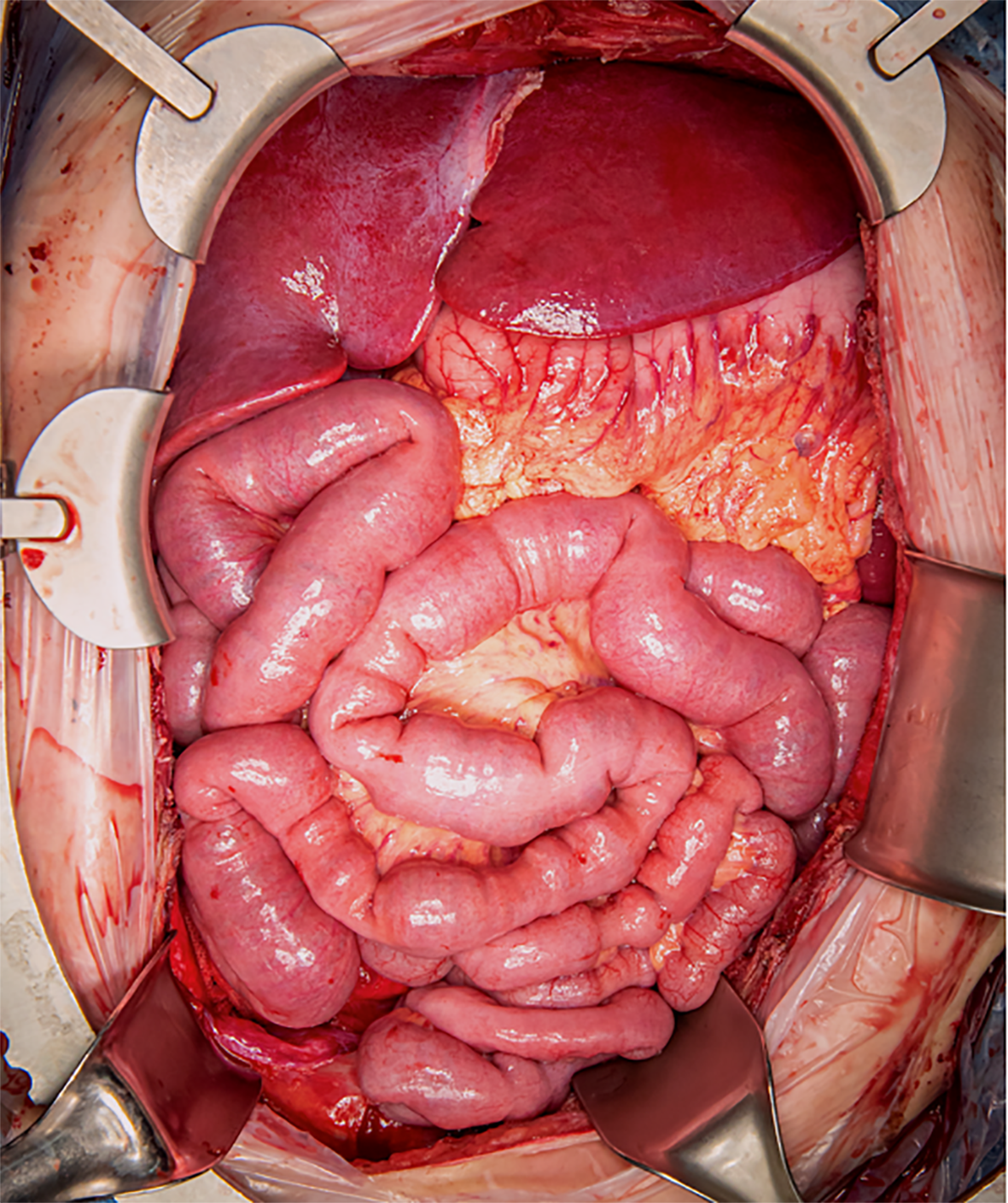
After 26 months on the waiting list (almost three years after the PD), an ideal 17-year-old donor was identified and procured using a University of Wisconsin preservative solution. After confirmation of graft quality adequacy by the donor surgeon, the transplantation procedure commenced with a long midline incision. Extensive adhesiolysis and native organectomy, including the stomach, pancreas, spleen, transverse to sigmoid colon, and liver were performed. The abdominal aorta and inferior vena cava (IVC) were exposed. The donor aortic conduit was anastomosed to the recipient’s infrarenal aorta. Graft implantation began with IVC anastomosis which was performed using the caval replacement technique. After caval anastomosis, graft inflow anastomosis between the conduit and aortic vascular cuff at the graft was performed. Thereafter, 5% cold human albumin was used for graft flushing before complete graft vascular anastomosis. Graft reperfusion was performed without significant reperfusion syndrome. Signs of immediate graft function, including a good appearance, normal peristalsis, and the presence of intestinal juice, were observed. Proximal GI anastomosis (gastro-gastrostomy) was performed. Distal GI anastomosis, side-to-end ileocolostomy, and chimney terminal ileostomy were completed. Temporary abdominal closure was applied to prevent compartment syndrome and she was scheduled for a second-look operation in two days. The surgery duration was seven hours and there was no major intraoperative complication. The allograft after transplantation is shown in Figure 3. The second-look operation revealed a viable allograft with excellent function. Finally, the abdomen was closed with a mesh derived from human cadaveric skin [AlloDerm® Regenerative Tissue Matrix (LifeCell Corporation, Branchburg, NJ)] to ensure adequate peritoneal space for the allograft and mitigate the risk of infection related to a conventional abdominal wall mesh.
The patient was discharged from the intensive care unit on POD 3 without experiencing any major complications. A liquid enteral diet was initiated on POD 7, and solid food was introduced on POD 14. The patient achieved enteral autonomy through an enteral diet and was completely weaned off TPN on POD 28. Discharge planning was prepared throughout her hospital stay. She underwent daily rehabilitation including muscular exercises to regain physical strength. Balance and walk training was also performed to ensure normal daily activity after discharge. Chronic pain with opioid addiction was successfully managed by tapering off opioid use after laparotomy wound healing while administering a few other analgesics. The patient’s depression improved significantly with medication prescribed and adjusted by a psychiatrist. The patient’s family received education and support to prepare for her long-term care needs. She was discharged from the hospital on POD 69 (a total of 34 months since the initial PD).
Maintenance immunosuppressive medications at discharge included tacrolimus (target trough level 8-10 mcg/mL), mycophenolate sodium 540 mg twice daily, and prednisolone 10 mg daily. The graft surveillance protocol included fecal calprotectin and endoscopic random graft mucosal biopsy via the chimney ileostomy weekly during the first month, every other week during the second and third months, and monthly for 3-6 months post-transplantation. To date, there has been no evidence of graft rejection. After hospital discharge, she was readmitted twice during the first year due to bacterial enteritis, which was successfully treated with antimicrobial medications. Currently, more than two years post-transplantation, the graft function remains excellent. The patient has achieved remission of her depressive disorder and opioid use cessation.
While ischemic post-PD complications constitute a major determinant of PD-related mortality, they rarely result in IF requiring long-term TPN support. Post-PD SMV thrombosis is uncommon; however, its prevalence is as high as 10%-20% among patients undergoing PD with portal vein resection and reconstruction[12-14]. To date, there has been no reported case of post-PD IF requiring ITx as a rescue therapy. Nevertheless, there has been a growing number of reports of ITx as a rescue treatment for IF following porto-mesenteric vein thrombotic complications after surgery for benign diseases, particularly bariatric surgery, one of the most common operations in the United States[15,16]. This concept is applicable to this patient, who developed IF after PD was performed for a benign tumor.
Generally, the first-line therapy for IF includes parenteral nutrition support and IR, medical and surgical rehabilitation, to promote intestinal adaptation and facilitate TPN weaning. In this case, there was no residual small bowel in the GI tract after surgery due to extensive SMV thrombosis and massive bowel resection. Therefore, she was not a suitable candidate for successful IR. Additionally, the complex high-output gastro-cutaneous and choledocho-cutaneous fistula at the laparotomy wound required continuous negative-pressure wound therapy. This not only caused chronic wound pain and opioid dependence but also prohibited hospital discharge. Furthermore, she experienced multiple episodes of CVC septicemia during the TPN use. Moreover, liver biochemistry revealed early cholestasis as a result of prolonged TPN use. Therefore, MVTx was deemed the best treatment option for her multifaceted problems. During the pre-transplant work-up, autologous GI reconstruction was performed to close ECF, facilitating wound care and reducing chronic wound pain.
To the best of our knowledge, MVTx has never been performed in a Southeast Asian country. This necessitated a multidisciplinary team effort to achieve the best outcome. According to the results of HLA antibody testing, the patient was a highly sensitized recipient who required pre-transplant desensitization. In addition, the anticipated donor needed to be smaller than the recipient to prevent large-for-size issues. Therefore, we decided to desensitize the patient when we received an appropriate donor offer. Our desensitization protocol included plasmapheresis and IVIg before induction immunosuppression by using thymoglobulin and methylprednisolone.
The post-MVTx outcomes in this case are excellent. The allograft has demonstrated normal function ever since the early postoperative period. Selecting an appropriate donor (young age, good hemodynamics, normal laboratory profiles, and a donor hospital located not too far away) and choosing the liver-inclusive graft type were major contributing factors[17-19]. These factors were crucial to avoiding graft-related complications and reducing graft rejection rates. In addition, a multidisciplinary team, comprised of a psychiatrist, a clinical nutritionist, a rehabilitation physician, and physical therapists providing care for both physical and psychosocial dimensions, can help the patient cope with suffering, including depression, while awaiting ITx.
Furthermore, family support is another key factor contributing to the patient’s quality of life after ITx[20]. This support helps the patient in managing stress and coping with depression while waiting for an organ donor and also facilitates safe societal reintegration.
In terms of long-term outcomes of intestinal transplant patients, graft survival has been improving significantly over the last decade due to the effectiveness of immunosuppressive drugs and the increasing availability of options to treat graft-related complications, especially rejection and infection[21]. Although the success of treatment might be a good example to demonstrate the benefit of MVTx for IF following GI surgery, the major limitation of this study is that it is a case report whose findings are not generalizable.
To our knowledge, this is the first reported case of ITx as a life-saving procedure for IF following ischemic complications of PD. The exceptional outcome of this case underscores the potential of ITx as a rescue therapy for IF after complex operations in carefully selected patients. Despite our limited experience with ITx, a strong multidisciplinary team approach, appropriate patient preparation, and a well-defined operative plan resulted in an excellent outcome of the first ITx performed in the region.
| 1. | Braga M, Capretti G, Pecorelli N, Balzano G, Doglioni C, Ariotti R, Di Carlo V. A prognostic score to predict major complications after pancreaticoduodenectomy. Ann Surg. 2011;254:702-7; discussion 707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (1)] |
| 2. | Smits FJ, Verweij ME, Daamen LA, van Werkhoven CH, Goense L, Besselink MG, Bonsing BA, Busch OR, van Dam RM, van Eijck CHJ, Festen S, Koerkamp BG, van der Harst E, de Hingh IH, Kazemier G, Klaase JM, van der Kolk M, Liem M, Luyer MDP, Meerdink M, Mieog JSD, Nieuwenhuijs VB, Roos D, Schreinemakers JM, Stommel MW, Wit F, Zonderhuis BM, de Meijer VE, van Santvoort HC, Molenaar IQ; Dutch Pancreatic Cancer Group. Impact of Complications After Pancreatoduodenectomy on Mortality, Organ Failure, Hospital Stay, and Readmission: Analysis of a Nationwide Audit. Ann Surg. 2022;275:e222-e228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 50] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 3. | van Rijssen LB, Zwart MJ, van Dieren S, de Rooij T, Bonsing BA, Bosscha K, van Dam RM, van Eijck CH, Gerhards MF, Gerritsen JJ, van der Harst E, de Hingh IH, de Jong KP, Kazemier G, Klaase J, van der Kolk BM, van Laarhoven CJ, Luyer MD, Molenaar IQ, Patijn GA, Rupert CG, Scheepers JJ, van der Schelling GP, Vahrmeijer AL, Busch ORC, van Santvoort HC, Groot Koerkamp B, Besselink MG; Dutch Pancreatic Cancer Group. Variation in hospital mortality after pancreatoduodenectomy is related to failure to rescue rather than major complications: a nationwide audit. HPB (Oxford). 2018;20:759-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 89] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 4. | Giuliani T, Marchegiani G, Di Gioia A, Amadori B, Perri G, Salvia R, Bassi C. Patterns of mortality after pancreatoduodenectomy: A root cause, day-to-day analysis. Surgery. 2022;172:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Gaujoux S, Sauvanet A, Vullierme MP, Cortes A, Dokmak S, Sibert A, Vilgrain V, Belghiti J. Ischemic complications after pancreaticoduodenectomy: incidence, prevention, and management. Ann Surg. 2009;249:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 6. | Giuliani T, Perri G, Kang R, Marchegiani G. Current Perioperative Care in Pancreatoduodenectomy: A Step-by-Step Surgical Roadmap from First Visit to Discharge. Cancers (Basel). 2023;15:2499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Reference Citation Analysis (0)] |
| 7. | Lermite E, Sommacale D, Piardi T, Arnaud JP, Sauvanet A, Dejong CH, Pessaux P. Complications after pancreatic resection: diagnosis, prevention and management. Clin Res Hepatol Gastroenterol. 2013;37:230-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 8. | Sowerbutts AM, Jones D, Lal S, Burden S. Quality of life in patients and in family members of those receiving home parenteral support with intestinal failure: A systematic review. Clin Nutr. 2021;40:3210-3220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 9. | Baxter JP, Fayers PM, Bozzetti F, Kelly D, Joly F, Wanten G, Jonkers C, Cuerda C, van Gossum A, Klek S, Boudreault MF, Gilbert A, Jobin M, Staun M, Gillanders L, Forbes A, O'Callaghan M, Faedo CM, Brunelli C, Mariani L, Pironi L; Home Artificial Nutrition and Chronic Intestinal Failure Special Interest Group of the European Society for Clinical Nutrition and Metabolism (ESPEN). An international study of the quality of life of adult patients treated with home parenteral nutrition. Clin Nutr. 2019;38:1788-1796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 61] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 10. | Torres C, Sudan D, Vanderhoof J, Grant W, Botha J, Raynor S, Langnas A. Role of an intestinal rehabilitation program in the treatment of advanced intestinal failure. J Pediatr Gastroenterol Nutr. 2007;45:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 11. | Kaufman SS, Avitzur Y, Beath SV, Ceulemans LJ, Gondolesi GE, Mazariegos GV, Pironi L. New Insights Into the Indications for Intestinal Transplantation: Consensus in the Year 2019. Transplantation. 2020;104:937-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 82] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 12. | Roch AM, Kilbane EM, Nguyen T, Ceppa EP, Zyromski NJ, Schmidt CM, Nakeeb A, House MG. Portal Vein Thrombosis After Venous Reconstruction During Pancreatectomy: Timing and Risks. J Gastrointest Surg. 2022;26:2148-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Mohammed S, Mendez-Reyes JE, McElhany A, Gonzales-Luna D, Van Buren G, Bland DS, Villafane-Ferriol N, Pierzynski JA, West CA, Silberfein EJ, Fisher WE. Venous thrombosis following pancreaticoduodenectomy with venous resection. J Surg Res. 2018;228:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 14. | Raptis DA, Sánchez-Velázquez P, Machairas N, Sauvanet A, Rueda de Leon A, Oba A, Groot Koerkamp B, Lovasik B, Chan C, Yeo CJ, Bassi C, Ferrone CR, Kooby D, Moskal D, Tamburrino D, Yoon DS, Barroso E, de Santibañes E, Kauffmann EF, Vigia E, Robin F, Casciani F, Burdío F, Belfiori G, Malleo G, Lavu H, Hartog H, Hwang HK, Han HS, Poves I, Rosado ID, Park JS, Lillemoe KD, Roberts KJ, Sulpice L, Besselink MG, Abuawwad M, Del Chiaro M, de Santibañes M, Falconi M, D'Silva M, Silva M, Abu Hilal M, Qadan M, Sell NM, Beghdadi N, Napoli N, Busch ORC, Mazza O, Muiesan P, Müller PC, Ravikumar R, Schulick R, Powell-Brett S, Abbas SH, Mackay TM, Stoop TF, Gallagher TK, Boggi U, van Eijck C, Clavien PA, Conlon KCP, Fusai GK. Defining Benchmark Outcomes for Pancreatoduodenectomy With Portomesenteric Venous Resection. Ann Surg. 2020;272:731-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 66] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 15. | Dumronggittigule W, Marcus EA, DuBray BJ, Venick RS, Dutson E, Farmer DG. Intestinal failure after bariatric surgery: Treatment and outcome at a single-intestinal rehabilitation and transplant center. Surg Obes Relat Dis. 2019;15:98-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Abu-Elmagd KM, Costa G, McMichael D, Khanna A, Cruz RJ, Parekh N, Fujiki M, Hashimoto K, Quintini C, Koritsky AD, Kroh MD, Sogawa H, Kandeel A, da Cunha-Melo JR, Steiger E, Kirby D, Matarese L, Shatnawei A, Humar A, Walsh RM, Schauer PR, Simmons R, Billiar T, Fung J. Autologous Reconstruction and Visceral Transplantation for Management of Patients With Gut Failure After Bariatric Surgery: 20 Years of Experience. Ann Surg. 2015;262:586-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Farmer DG, Venick RS, Colangelo J, Esmailian Y, Yersiz H, Duffy JP, Cortina GR, Artavia K, Ngo K, McDiarmid SV, Busuttil RW. Pretransplant predictors of survival after intestinal transplantation: analysis of a single-center experience of more than 100 transplants. Transplantation. 2010;90:1574-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 18. | Vianna R, Gaynor JJ, Selvaggi G, Farag A, Garcia J, Tekin A, Tabbara MM, Ciancio G. Liver Inclusion Appears to Be Protective Against Graft Loss-Due-to Chronic But Not Acute Rejection Following Intestinal Transplantation. Transpl Int. 2023;36:11568. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 19. | Fischer-Fröhlich CL, Königsrainer A, Schaffer R, Schaub F, Pratschke J, Pascher A, Steurer W, Nadalin S. Organ donation: when should we consider intestinal donation. Transpl Int. 2012;25:1229-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Abu-Elmagd KM, Kosmach-Park B, Costa G, Zenati M, Martin L, Koritsky DA, Emerling M, Murase N, Bond GJ, Soltys K, Sogawa H, Lunz J, Al Samman M, Shaefer N, Sindhi R, Mazariegos GV. Long-term survival, nutritional autonomy, and quality of life after intestinal and multivisceral transplantation. Ann Surg. 2012;256:494-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 21. | Horslen SP, Smith JM, Weaver T, Cafarella M, Foutz J. OPTN/SRTR 2020 Annual Data Report: Intestine. Am J Transplant. 2022;22 Suppl 2:310-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |