Published online Sep 18, 2025. doi: 10.5500/wjt.v15.i3.100591
Revised: January 22, 2025
Accepted: February 13, 2025
Published online: September 18, 2025
Processing time: 240 Days and 1.3 Hours
Coronavirus disease 2019 is caused by severe acute respiratory syndrome coronavirus 2 and emerged in Wuhan, China. It affects millions of people all over the world and has caused the deaths of thousands of people. Mortality rates were higher in transplant recipients and patients awaiting transplantation due to social and psychological issues. It also affected candidates who would be transplant providers and caused the transplant chain to be broken worldwide. The corona
Core Tip: The coronavirus disease 2019 pandemic significantly affected solid organ transplantation procedures and led to various changes in protocols and practices to ensure patient safety and increase transplant success.
- Citation: Bozkurt HB, Özdemir Ö. Changes regarding solid organ transplantation during the COVID-19 pandemic. World J Transplant 2025; 15(3): 100591
- URL: https://www.wjgnet.com/2220-3230/full/v15/i3/100591.htm
- DOI: https://dx.doi.org/10.5500/wjt.v15.i3.100591
Since December 2019, the coronavirus disease 2019 (COVID-19) pandemic that emerged in Wuhan, China affected tens of thousands of people worldwide. Healthcare systems in all countries have faced numerous difficulties, and there has been uncompromising prioritization of the capacity of hospital facilities and human resources for patients with COVID-19. The COVID-19 pandemic has severely affected the solid organ transplantation (SOT) chain and both live and deceased donors[1] due to hesitancy of live donor candidates, the possibility of infection in live donor candidates and the difficulties in detecting it, and the lack of intensive care capacity for potential deceased donors because they were occupied by patients with COVID-19. The possible COVID-19 transmission from the donor to the recipient is still questionable. At the beginning of the COVID-19 pandemic, there was a strict screening requirement for the donor.
Moreover, the increase in the risk of post-transplant infection and the increase in mortality among solid organ transplant recipients (SOTRs) is alarming[2,3]. In a study including 9073 SOT patients, the fatality rate was between 7.7%-15.0%[4] Recent publications showed that the clinical progression of this disease in organ recipients was more destructive with a fatality rate of up to 14%-25% and caused more hospitalizations[5]. There was an increased risk of secondary viral, bacterial, and fungal infections. When SOTRs were diagnosed with COVID-19 infection, 75% required hospital admission. Approximately 40% of them required intensive care. Mortality among hospitalized patients in large cohorts of SOTRs with COVID-19 has ranged from 10% to 20%[6-8].
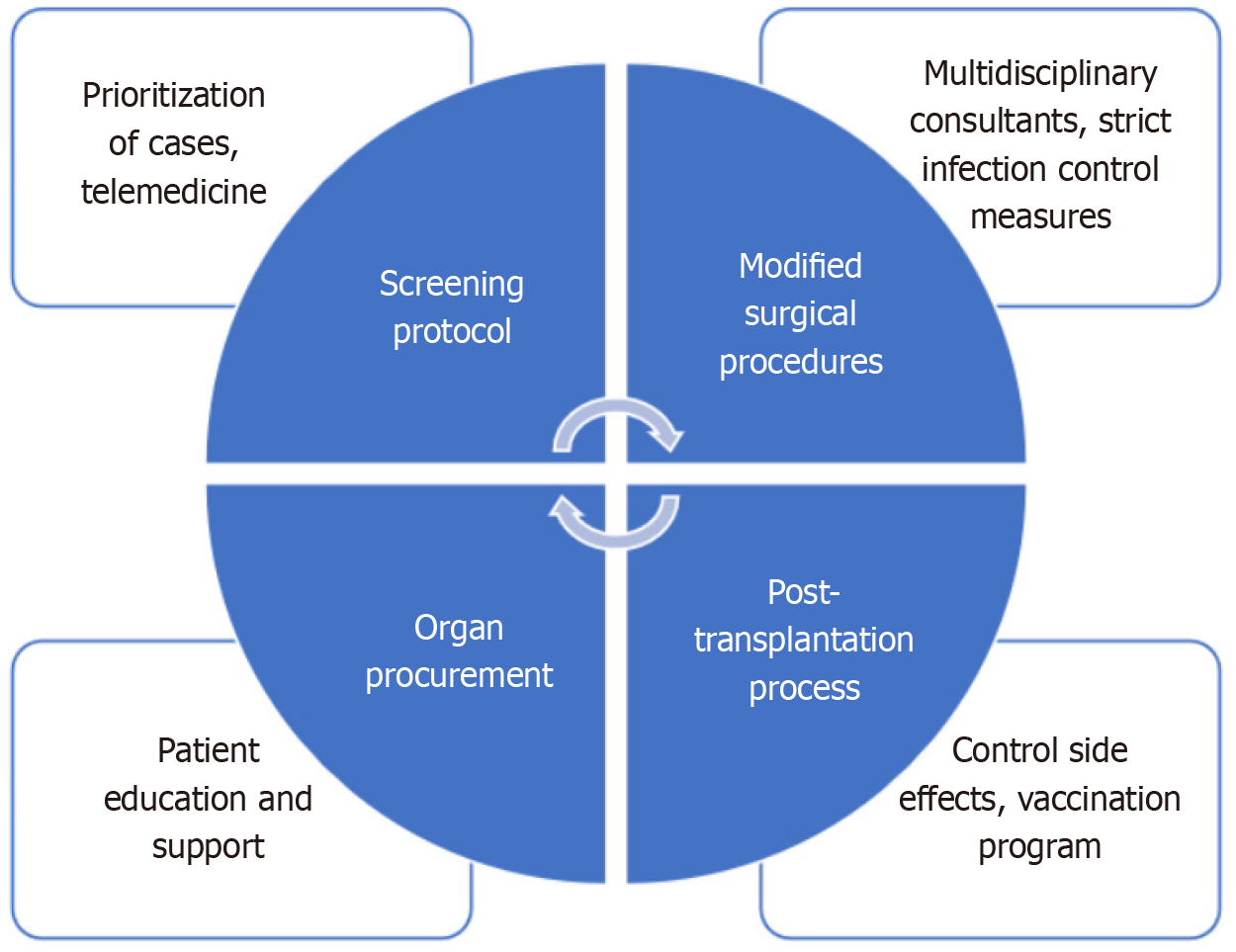
The increase in mortality rates of these patients has revealed the need for new management recommendations on transplantation strategies[7,9,10]. Many experts have evaluated this situation with the current literature and made recommendations to healthcare workers regarding the situation[7,10]. These include challenges in screening protocols, prioritization of cases, telemedicine and virtual consultations, modified surgical procedures, immunosuppression management, post-transplantation process and difficulties controlling side effects, difficulties in organ procurement, and patient education/support[11]. The purpose of this study was to review the changes in SOT during and after the COVID-19 pandemic (Figure 1).
Limitations of screening methods for SOT during the COVID-19 pandemic have evolved to address the heightened risks associated with both the virus and the transplantation process. Due to the existence of asymptomatic patients and the low sensitivity and specificity of the tests and imaging performed, we could not detect real carriers of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)[12] Although COVID-19 could not be detected in tests before death, it has been shown in postmortem autopsies in the lungs, kidney, liver, and heart[13,14].
During this period, the nucleic acid test (NAT) and CT results were used as objective parameters for diagnosis in addition to clinical signs. The nucleic acid test was used for diagnosis but sensitivity rates were low: Nasopharyngeal swabs were 62%; oropharyngeal swabs were 32%; and sputum was 72%[12,15]. Donors were required to have at least two negative results in > 24 h, no abnormal laboratory results (like lymphopenia, thrombocytopenia, etc.), no abnormal CT results (not routine, if exists), and no symptoms, travel history, or SARS-CoV-2 exposure risk[16,17]. The clinician’s examination and experience combined with these test results became very important. If any risk factor existed, transplantation was contraindicated and was delayed[16].
Recipients were tested for COVID-19 multiple times before the transplant surgery, including immediately before the procedure[6]. Screening protocols for SOT during the COVID-19 pandemic were designed to minimize risks associated with SARS-CoV-2 while ensuring the success of the transplant. They involved rigorous testing, infection control practices, and careful monitoring to protect both donors and recipients.
Assessment of urgency was very important for prioritization of cases. Telemedicine and virtual consultations became crucial for a multidisciplinary approach, determination of patient urgency, and detailed evaluation of risk factors during the pandemic period as face-to-face meetings were severely restricted[18]. It was important to determine the risk and benefit status, to provide conditions for the patient to be monitored and closely followed, to record comorbid diseases, COVID-19 status and exposure risk, and patient-doctor cooperation, and to obtain informed consent. In cases of life-threatening urgent transplantation (e.g., heart, lung, liver) needs, it was suggested that transplantation could be performed with informed consent from suitable donors who were negative for COVID-19. In other cases, it was reported that postponement of transplantation should be considered[10,19,20]. Prioritization strategies focused on balancing clinical urgency with COVID-19 risks and resource availability, while telemedicine and virtual consultations were vital in maintaining care continuity, reducing in-person visits, and ensuring patient safety.
After screening protocols and multiple testing, surgical procedures including preoperative isolation, protective equipment, comorbidities management, health assessment, available multidisciplinary consultants, strict infection control measures, protective masks and equipment, and enhanced hygiene protocols were crucial for decreasing mortality and morbidity[10]. Surgeons and operating room staff used enhanced personal protective equipment, including N95 respirators, face shields, and gowns, and the operating room was modified to increase ventilation and to include negative pressure systems. Regular disinfection protocols were necessary to minimize risk[10,21]. Minimally invasive procedures and urgent operations were recommended[10,22]. Many non-urgent elective transplants were postponed to conserve medical resources and reduce the risk of COVID-19 transmission[22,23]. Infection control and resource management formed the basis of surgical procedures.
Immunosuppression management required a careful process. Preventing the development of a rejection reaction in the recipient patient and controlling infection despite immunosuppression is a delicate balancing act. Managing immunosuppression became more complex during the COVID-19 pandemic. Commonly used treatments include intravenous immunoglobulin, steroids, calcineurin inhibitors, or mycophenolic acid, which can cause serious side effects. Coronavirus-related pneumonia, nephrotoxicity, hypertension, hyperlipidemia, and bleeding are some of the serious side effects that may develop[24]. Even when the patient appears to be recovering well, acute lung injury and acute kidney injury can develop during the post-transplant period[25,26].
Studies have shown that the side effects of calcineurin inhibitors and antimetabolites are higher than those of steroids in patients with SOT infected with COVID-19[27,28]. Therefore, it was recommended that other treatments be discontinued in patients with SOT with a positive COVID-19 test and that the steroid dose be reduced or continued in the same manner, taking into account the patient’s clinical condition[26,28]. If the patient was found to be COVID-19 negative and had no clinical symptoms, the dose of other immunosuppressive treatments should be reduced and discontinued along with close clinical follow-up[28].
SOT patients were strongly encouraged to receive COVID-19 vaccinations, especially before the transplantation period[29]. Although vaccine responses might be weaker in immunosuppressed individuals, the benefits of vaccination in preventing severe COVID-19 were substantial[30]. Booster doses were also recommended due to the potential effect of vaccines to decrease mortality and morbidity[31]. It was recommended that infection protection measures were strictly implemented and that each patient-specific process must be managed dynamically[10].
Due to decreased donor availability, increased risk in donors, infection risk, and healthcare system strain, hospitals had to manage both patients with COVID-19 and patients with SOT, which resulted in delay, travel restrictions, and isolation measures. Difficulties in organ procurement included sufficient hospital capacity including intensive care unit beds and ventilators[32]. In Japan in the early period of the COVID-19 pandemic, the number of deceased donor organ donations and transplantations dropped to 61%-69%, and living donor transplantations were affected due to its elective nature and lack of deceased donors[32,33].
Other important problems were increased anxiety, stress, and loss of trust due to social distancing, isolation, and the inability to meet face-to-face[34]. It was very important to communicate with these patients and donors closely in an understanding way[35]. Virtual communication tools, including video conferencing, that the patients with SOT could utilize at any time were frequently used and recommended. Medical psychological support and a more flexible calendar plan provided convenience for patients[10,27,36]. Overall, the pandemic necessitated a rapid shift to virtual and remote methods for patient education and support, with a strong focus on clear communication and comprehensive support and adapting to new challenges.
Navigating SOT during the COVID-19 pandemic required a multidisciplinary approach, close collaboration between transplant teams, and adherence to strict infection control measures to ensure the safety of both transplant recipients and healthcare providers. Despite all the negative effects of the process, strategies continue to be developed to ensure that transplant programs continue.
| 1. | Azzi Y, Bartash R, Scalea J, Loarte-Campos P, Akalin E. COVID-19 and Solid Organ Transplantation: A Review Article. Transplantation. 2021;105:37-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 236] [Article Influence: 59.0] [Reference Citation Analysis (0)] |
| 2. | Pascual J, Mazuecos A, Sánchez-Antolín G, Solé A, Ventura-Aguiar P, Crespo M, Farrero M, Fernández-Rivera C, Garrido IP, Gea F, González-Monte E, González-Rodríguez A, Hernández-Gallego R, Jiménez C, López-Jiménez V, Otero A, Pascual S, Rodríguez-Laiz GP, Ruiz JC, Sancho A, Santos F, Serrano T, Tabernero G, Zarraga S, Delgado JF; Spanish Best Transplant Practices Study Group. Best practices during COVID-19 pandemic in solid organ transplant programs in Spain. Transplant Rev (Orlando). 2023;37:100749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Doná D, Torres Canizales J, Benetti E, Cananzi M, De Corti F, Calore E, Hierro L, Ramos Boluda E, Melgosa Hijosa M, Garcia Guereta L, Pérez Martínez A, Barrios M, Costa Reis P, Teixeira A, Lopes MF, Kaliciński P, Branchereau S, Boyer O, Debray D, Sciveres M, Wennberg L, Fischler B, Barany P, Baker A, Baumann U, Schwerk N, Nicastro E, Candusso M, Toporski J, Sokal E, Stephenne X, Lindemans C, Miglinas M, Rascon J, Jara P; ERN TransplantChild. Pediatric transplantation in Europe during the COVID-19 pandemic: Early impact on activity and healthcare. Clin Transplant. 2020;34:e14063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 4. | Søfteland JM, Li H, Magnusson JM, Leach S, Friman V, Gisslén M, Felldin M, Schult A, Karason K, Baid-Agrawal S, Wallquist C, Nyberg F. COVID-19 Outcomes and Vaccinations in Swedish Solid Organ Transplant Recipients 2020-2021: A Nationwide Multi-Register Comparative Cohort Study. Viruses. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 5. | Yılmaz EA, Özdemir Ö. Solid organ transplantations and COVID-19 disease. World J Transplant. 2021;11:503-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Sahota A, Tien A, Yao J, Dong E, Herald J, Javaherifar S, Neyer J, Hwang J, Lee R, Fong TL. Incidence, Risk Factors, and Outcomes of COVID-19 Infection in a Large Cohort of Solid Organ Transplant Recipients. Transplantation. 2022;106:2426-2434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 26] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 7. | Overvad M, Koch A, Jespersen B, Gustafsson F, Krause TG, Hansen CH, Ethelberg S, Obel N. Outcomes following SARS-CoV-2 infection in individuals with and without solid organ transplantation-A Danish nationwide cohort study. Am J Transplant. 2022;22:2627-2636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 34] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Trapani S, Masiero L, Puoti F, Rota MC, Del Manso M, Lombardini L, Riccardo F, Amoroso A, Pezzotti P, Grossi PA, Brusaferro S, Cardillo M; Italian Network of Regional Transplant Coordinating Centers Collaborating group; Italian Surveillance System of Covid-19, Italian Society for Organ Transplantation (SITO), The Italian Board of Experts in Liver Transplantation (I-BELT) Study Group, Italian Association for the Study of the Liver (AISF), Italian Society of Nephrology (SIN), SIN-SITO Study Group. Incidence and outcome of SARS-CoV-2 infection on solid organ transplantation recipients: A nationwide population-based study. Am J Transplant. 2021;21:2509-2521. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 71] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 9. | Rendina M, Barone M, Trapani S, Masiero L, Trerotoli P, Puoti F, Lupo L, Agnes S, Grieco A, Andorno E, Marenco S, Baccarani U, Toniutto P, Carraro A, Colecchia A, Cescon M, Morelli M, Cillo U, Burra P, Angeli P, Colledan M, Fagiuoli S, De Carlis L, Belli L, De Simone P, Carrai P, Di Benedetto F, De Maria N, Ettorre G, Giannelli V, Gruttadauria S, Volpes R, Mazzaferro V, Bhoori S, Romagnoli R, Martini S, Rossi G, Donato F, Rossi M, Ginanni Corradini S, Spada M, Maggiore G, Tisone G, Lenci I, Vennarecci G, Di Costanzo G, Vivarelli M, Svegliati Baroni G, Zamboni FO, Mameli L, Tafuri S, Simone S, Gesualdo L, Cardillo M, Di Leo A. SARS-CoV-2 infection in liver transplantation is associated with favorable outcomes: an Italian transplant registry study. Dig Liver Dis. 2022;54:S14. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Weiss MJ, Hornby L, Foroutan F, Belga S, Bernier S, Bhat M, Buchan CA, Gagnon M, Hardman G, Ibrahim M, Luo C, Luong ML, Mainra R, Manara AR, Sapir-Pichhadze R, Shalhoub S, Shaver T, Singh JM, Srinathan S, Thomas I, Wilson LC, Wilson TM, Wright A, Mah A. Clinical Practice Guideline for Solid Organ Donation and Transplantation During the COVID-19 Pandemic. Transplant Direct. 2021;7:e755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Visentin A, Pickavance E, San-Juan R, Grossi PA, Manuel O, Aguado JM. Current management of SARS-CoV-2 infection in solid organ transplant recipients: Experience derived from an ESGICH-ESOT survey. Transpl Infect Dis. 2024;26:e14252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 12. | Wang W, Xu Y, Gao R, Lu R, Han K, Wu G, Tan W. Detection of SARS-CoV-2 in Different Types of Clinical Specimens. JAMA. 2020;323:1843-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1894] [Cited by in RCA: 2658] [Article Influence: 531.6] [Reference Citation Analysis (0)] |
| 13. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5781] [Article Influence: 1156.2] [Reference Citation Analysis (2)] |
| 14. | Yao XH, Li TY, He ZC, Ping YF, Liu HW, Yu SC, Mou HM, Wang LH, Zhang HR, Fu WJ, Luo T, Liu F, Guo QN, Chen C, Xiao HL, Guo HT, Lin S, Xiang DF, Shi Y, Pan GQ, Li QR, Huang X, Cui Y, Liu XZ, Tang W, Pan PF, Huang XQ, Ding YQ, Bian XW. [A pathological report of three COVID-19 cases by minimal invasive autopsies]. Zhonghua Bing Li Xue Za Zhi. 2020;49:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 461] [Reference Citation Analysis (0)] |
| 15. | Mohanty A, Kabi A, Mohanty AP, Kumar N, Kumar S. Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges: An Indian Perspective. J Adv Med Med Res. 2020;32:10-17. [DOI] [Full Text] |
| 16. | Zhang H, Dai H, Xie X. Solid Organ Transplantation During the COVID-19 Pandemic. Front Immunol. 2020;11:1392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 17. | Xiao AT, Tong YX, Zhang S. False negative of RT-PCR and prolonged nucleic acid conversion in COVID-19: Rather than recurrence. J Med Virol. 2020;92:1755-1756. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 366] [Article Influence: 73.2] [Reference Citation Analysis (0)] |
| 18. | Kayser MZ, Valtin C, Greer M, Karow B, Fuge J, Gottlieb J. Video Consultation During the COVID-19 Pandemic: A Single Center's Experience with Lung Transplant Recipients. Telemed J E Health. 2021;27:807-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Dhand A, Okumura K, Nabors C, Nishida S. Solid organ transplantation from COVID positive donors in the United States: Analysis of United Network for Organ Sharing database. Transpl Infect Dis. 2023;25:e13925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 20. | Vinson AJ, Agarwal G, Dai R, Anzalone AJ, Lee SB, French E, Olex A, Madhira V, Mannon RB. COVID-19 in Solid Organ Transplantation: Results of the National COVID Cohort Collaborative. Transplant Direct. 2021;7:e775. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 21. | Yi SG, Rogers AW, Saharia A, Aoun M, Faour R, Abdelrahim M, Knight RJ, Grimes K, Bullock S, Hobeika M, McMillan R, Mobley C, Moaddab M, Huang HJ, Bhimaraj A, Ghobrial RM, Gaber AO. Early Experience With COVID-19 and Solid Organ Transplantation at a US High-volume Transplant Center. Transplantation. 2020;104:2208-2214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 22. | Kniepeiss D, Jantscher L, Al-Sharafy S, Sendlhofer G, Schemmer P. Framework for Solid-Organ Transplantation During COVID-19 Pandemic in Europe. Risk Manag Healthc Policy. 2021;14:2421-2433. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Husain I, Luo X. Novel Approaches to Immunomodulation for Solid Organ Transplantation. Annu Rev Med. 2024;75:369-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Parlakpinar H, Gunata M. Transplantation and immunosuppression: a review of novel transplant-related immunosuppressant drugs. Immunopharmacol Immunotoxicol. 2021;43:651-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 88] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 25. | Mohammed AH, Blebil A, Dujaili J, Rasool-Hassan BA. The Risk and Impact of COVID-19 Pandemic on Immunosuppressed Patients: Cancer, HIV, and Solid Organ Transplant Recipients. AIDS Rev. 2020;22:151-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 26. | Laracy JC, Miko BA, Pereira MR. The solid organ transplant recipient with SARS-CoV-2 infection. Curr Opin Organ Transplant. 2021;26:412-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Moosavi SA, Mashhadiagha A, Motazedian N, Hashemazar A, Hoveidaei AH, Bolignano D. COVID-19 clinical manifestations and treatment strategies among solid-organ recipients: A systematic review of cases. Transpl Infect Dis. 2020;22:e13427. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 28. | Udomkarnjananun S, Kerr SJ, Townamchai N, Susantitaphong P, Tulvatana W, Praditpornsilpa K, Eiam-Ong S, Avihingsanon Y. Mortality risk factors of COVID-19 infection in kidney transplantation recipients: a systematic review and meta-analysis of cohorts and clinical registries. Sci Rep. 2021;11:20073. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 29. | Heldman MR, Limaye AP. SARS-CoV-2 Vaccines in Kidney Transplant Recipients: Will They Be Safe and Effective and How Will We Know? J Am Soc Nephrol. 2021;32:1021-1024. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Tripodi D, Dominici R, Sacco D, Santorelli G, Rivera R, Acquaviva S, Marchisio M, Brambilla P, Battini G, Leoni V. Antibody Response after 3-Dose Booster against SARS-CoV-2 mRNA Vaccine in Kidney Transplant Recipients. Vaccines (Basel). 2024;12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 31. | Caillard S, Chavarot N, Bertrand D, Kamar N, Thaunat O, Moal V, Masset C, Hazzan M, Gatault P, Sicard A, Chemouny JM, Rerolle JP, Colosio C, Francois H, Bamoulid J, Bouvier N, Duveau A, Anglicheau D, Blancho G; French Society of Transplantation. Occurrence of severe COVID-19 in vaccinated transplant patients. Kidney Int. 2021;100:477-479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 96] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 32. | Yamanaga S, Shimata K, Ohfuji S, Yoshikawa M, Natori Y, Hibi T, Yuzawa K, Egawa H; Japan Society for Transplantation COVID-19 Registry Study Group. Excess mortality in COVID-19-affected solid organ transplant recipients across the pandemic. Am J Transplant. 2024;24:1495-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Reference Citation Analysis (0)] |
| 33. | Ito T, Kenmochi T, Ota A, Kuramitsu K, Soyama A, Kinoshita O, Eguchi S, Yuzawa K, Egawa H. National survey on deceased donor organ transplantation during the COVID-19 pandemic in Japan. Surg Today. 2022;52:763-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 34. | Yıldırım M, Akgül Ö, Geçer E. The Effect of COVID-19 Anxiety on General Health: the Role of COVID-19 Coping. Int J Ment Health Addict. 2022;20:1110-1121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 42] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 35. | Forner-Puntonet M, Castell-Panisello E, Quintero J, Ariceta G, Gran F, Iglesias-Serrano I, Gisbert-Gustemps L, Daigre C, Ibañez-Jimenez P, Delgado M, Español-Martín G, Parramon G, Pont T, Ramos-Quiroga JA. Impact of COVID-19 on Families of Pediatric Solid Organ Transplant Recipients. J Pediatr Psychol. 2021;46:927-938. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 36. | Nimmo A, Gardiner D, Ushiro-Lumb I, Ravanan R, Forsythe JLR. The Global Impact of COVID-19 on Solid Organ Transplantation: Two Years Into a Pandemic. Transplantation. 2022;106:1312-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 20.7] [Reference Citation Analysis (0)] |